Halide-free deep eutectic solvents with low viscosity and corrosion for efficient SO2 capture and conversion under environmental conditions
Tao
Yang
and
Tianxiang
Zhao
 *
*
Key Laboratory of Green Chemical and Clean Energy Technology, School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, P. R. China. E-mail: txzhao3@gzu.edu.cn
First published on 13th November 2025
Abstract
Eight halogen-free deep eutectic solvents (DESs) with low viscosity were synthesized for the capture and subsequent catalytic conversion of SO2. The resulting DESs exhibit excellent SO2 solubility (up to 1.2514 g g−1 at 20 °C and 1 bar) and high catalytic activity in the cycloaddition between SO2 and epoxides. Spectroscopic and theoretical investigations elucidate the mechanisms of SO2 capture and conversion, revealing a synergistic effect between hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs).
Sulfur dioxide (SO2), a well-known respiratory irritant and corrosive gas, presents substantial environmental and public health hazards when emitted directly, leading to ecosystem deterioration and serious health issues in humans.1–3 Conventional flue gas desulfurization (FGD) technologies, encompassing dry scrubbing, wet limestone processes, and biological treatment systems, present significant limitations despite their widespread application. These methods are often accompanied by major challenges such as the formation of secondary pollutants, severe equipment corrosion, and ongoing difficulties in wastewater treatment.4,5 Therefore, the development of efficient SO2 capture techniques is essential for environmental protection.6
Solvent-based systems show promise for SO2 capture, achieving high removal efficiencies while ensuring operational stability and exhibiting strong resistance to impurities under complex flue gas conditions.7 As emerging green solvents, the DESs have advantages including low cost, high thermal stability, straightforward synthesis without purification, and minimal ecotoxicity.8–10 Their tuneable properties through strategic selection of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) enable tailored designs for specific applications, and consequently they have been widely applied in various fields.11 For example, Wang et al.12 developed a 1-ethyl-3-methylimidazole chloride (EmimCl) and pyridine derivative deep eutectic solvent, where the EmimCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-NH2Py (7
2-NH2Py (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system demonstrates rapid absorption rate, high selectivity, and superior recyclability. Despite notable progress in SO2 capture using DESs, the majority of these systems require energy-intensive high-temperature regeneration processes.13–15 Direct conversion of absorbed SO2 into value-added chemicals could simultaneously mitigate regeneration energy demands and simplify downstream processing.16 Our prior work17 established an EmimCl-Trtz (2
1) system demonstrates rapid absorption rate, high selectivity, and superior recyclability. Despite notable progress in SO2 capture using DESs, the majority of these systems require energy-intensive high-temperature regeneration processes.13–15 Direct conversion of absorbed SO2 into value-added chemicals could simultaneously mitigate regeneration energy demands and simplify downstream processing.16 Our prior work17 established an EmimCl-Trtz (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DES system that concurrently absorbed SO2 and catalysed its cycloaddition with epoxides into cyclic sulfites, effectively addressing both absorbent regeneration and value-added product synthesis. Nevertheless, the high viscosity and toxic, corrosive halogen components of the DESs hinder SO2 absorption efficiency and compromise green sustainable conversion processes.18,19 Hence, the rational design of low-viscosity, halogen-free dual-functional DESs that synergistically combine efficient SO2 capture with catalytic epoxide conversion is imperative to advance sustainable and clean chemical processes.
1) DES system that concurrently absorbed SO2 and catalysed its cycloaddition with epoxides into cyclic sulfites, effectively addressing both absorbent regeneration and value-added product synthesis. Nevertheless, the high viscosity and toxic, corrosive halogen components of the DESs hinder SO2 absorption efficiency and compromise green sustainable conversion processes.18,19 Hence, the rational design of low-viscosity, halogen-free dual-functional DESs that synergistically combine efficient SO2 capture with catalytic epoxide conversion is imperative to advance sustainable and clean chemical processes.
Inspired by the polyhydroxycarboxylic acid ionic liquid-catalysed CO2 cycloaddition reaction,20 we developed halogen-free DESs for efficient SO2 capture and subsequent conversion. As illustrated in Fig. 1, eight DESs were prepared using 1-ethyl-3-methylimidazolium acetate (EmimOAc) and N-alkylimidazolium acetate proton ionic liquids (PILs) as HBAs, paired with 2-aminopyridine (2-AmPy) and imidazole (Im) as HBDs, at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The nuclear magnetic resonance hydrogen spectrum (1H NMR) indicates that ethyl imidazole and acetic acid have undergone a complete neutralization reaction, forming EimOAc as the HBA (Fig. S1). Thereafter, the physicochemical properties of these DESs were evaluated using thermogravimetric analysis (TGA, Fig. S2), viscometry, and densitometry, while structural characteristics were analysed by NMR (Fig. S3–S10) and Fourier transform infrared (FTIR, Fig. S11) spectroscopy. Among these DESs, [EmimOAc][lm] demonstrates the best thermal stability, with an initial decomposition temperature of 120.8 °C. Notably, the viscosities of the six PIL-based DESs are all below 20 cP (Table S1), with [EimOAc][Im] exhibiting the lowest viscosity of 11.8 cP at 20 °C, which facilitates SO2 absorption.
1 molar ratio. The nuclear magnetic resonance hydrogen spectrum (1H NMR) indicates that ethyl imidazole and acetic acid have undergone a complete neutralization reaction, forming EimOAc as the HBA (Fig. S1). Thereafter, the physicochemical properties of these DESs were evaluated using thermogravimetric analysis (TGA, Fig. S2), viscometry, and densitometry, while structural characteristics were analysed by NMR (Fig. S3–S10) and Fourier transform infrared (FTIR, Fig. S11) spectroscopy. Among these DESs, [EmimOAc][lm] demonstrates the best thermal stability, with an initial decomposition temperature of 120.8 °C. Notably, the viscosities of the six PIL-based DESs are all below 20 cP (Table S1), with [EimOAc][Im] exhibiting the lowest viscosity of 11.8 cP at 20 °C, which facilitates SO2 absorption.
Initially, we evaluated the absorption capacity of eight DESs at 20 °C by introducing SO2 into 1.0 g of DES. As depicted in Fig. 2a, all DESs rapidly absorbed SO2 within 5 min and reached absorption equilibrium in approximately 10 min. The absorption capacities of these DESs all exceeded 0.9 g g−1, with [EmimOAc][Im] demonstrating a superior absorption capacity of 1.2514 g g−1, which is comparable to or surpasses those of previously reported DESs (Table S2). The influence of HBAs on absorption performance was further investigated. Fig. 2b demonstrates that the SO2 solubility follows this descending order: [EmimOAc][Im] > [MimOAc][Im] > [EimOAc][Im] > [BimOAc][Im], demonstrating that non-protonic ionic liquid exhibits significantly higher SO2 absorption capacity than PILs. Furthermore, a shorter alkyl chain in the cation of PILs is beneficial for SO2 absorption. Im demonstrates stronger alkalinity than 2-AmPy, thereby enhancing SO2 uptake performance in all HBD-based DESs except [BimOAc][Im]. Nevertheless, the uptake variations among these DESs remain relatively minor.
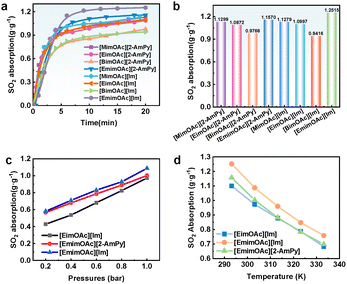 | ||
| Fig. 2 (a) and (b) Comparison of SO2 absorption in eight DESs at 20 °C and 1.0 bar. (c) the effects of temperature at 1.0 bar, and (d) SO2 partial pressure at 30 °C on SO2 absorption. | ||
The influence of temperature and pressure on SO2 absorption by the three DESs was investigated. The results shown in Fig. 2c and d reveal that the solubility of SO2 in all three DESs decreases as the temperature increases and pressure decreases. For instance, the SO2 absorption capacity of [EmimOAc][Im] decreased from 1.2514 g g−1 at 20 °C to 0.7588 g g−1 at 60 °C under 1.0 bar. This decline can be attributed to the fact that an increase in temperature not only impedes the physical solubilization of SO2, as described by Henry's law, but also weakens the acid–base interactions between the DESs and SO2, ultimately resulting in reduced solubility of SO2. Notably, SO2 absorption shows a distinct nonlinear pattern at low pressures (0–0.2 bar), typically attributed to chemical absorption. Beyond 0.2 bar, absorption increases nearly linearly, indicating physical absorption as the dominant process. At a partial pressure of 0.2 bar SO2, [EmimOAc][Im] exhibits an absorption capacity of 0.5812 g g−1, demonstrating significant SO2 uptake even at lower pressures. To further investigate the absorption capacity of [EimOAc][Im] at low SO2 concentrations, we evaluated their performance at 6000 ppm and 1000 ppm, achieving absorption capacities of 0.336 and 0.073 g g−1 (Fig. S12). The regeneration performance of DESs is crucial in the process of SO2 capture. We thus conducted absorption-desorption cycling experiments at temperatures of 30 °C and 80 °C to evaluate the DESs. The results shown in Fig. S13 demonstrate that [EimOAc][Im] retains its efficient SO2 capture capacity after five cycles.
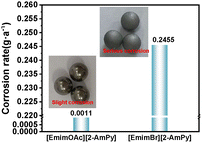
Corrosion tests (weight loss method) using Q235 iron spheres show that the halogen-free [EmimOAc][2-AmPy] causes significantly less corrosion than [EmimBr][2-AmPy] (Fig. 3 and Table S3). Q235 iron spheres in [EmimBr][2-AmPy] exhibited substantial mass loss (corrosion rate: 0.2455 g a−1) and visible surface changes, while those in [EmimOAc][2-AmPy] remained largely unaffected (corrosion rate: 0.0011 g a−1). These results demonstrate that halogen-free DESs reduce equipment corrosion, making them more suitable for practical applications.
After evaluating the SO2 absorption, DESs were used as catalysts for the cycloaddition of SO2 and styrene oxide (SO) (Table 1). These DESs demonstrated exceptional catalytic performance, achieving yields exceeding 90% for eight DESs at 60 °C and 1 bar SO2 (entries 1–8). The synergistic catalytic effect of HBA and HBD in the DESs results in enhanced catalytic activity relative to either HBA or HBD alone, as well as their corresponding precursor compounds (Table S4). To systematically optimize the reaction parameters, [MimOAc][2-AmPy], which demonstrated excellent catalytic activity, was selected as the model catalyst. Time-dependent evaluation at 30 °C revealed incremental yield improvements from 84% (8 h) to 85% (12 h), reaching 92% at the optimal duration of 24 h (entries 9–11). Consequently, the optimal conditions were established as 30 °C and 24 h, under which three DESs with 2-AmPy as the hydrogen bond donor (HBD) achieved yields ranging from 84% to 91%, respectively (entries 12-14). Significantly, [EmimOAc][2-AmPy] exhibited the lowest catalytic activity among the three DESs, with alkyl chain elongation demonstrating an inverse correlation with catalytic efficiency. Given the limited thermal stability of [MimOAc][Im], [EimOAc][Im] and [BimOAc][Im] were evaluated (entries 15 and 16), attaining 95% and 93% yields, which may be attributed to Im having a stronger interaction with SO2 than that of 2-AmPy. The results indicate that [EimOAc][Im] has the best catalytic effect. To evaluate the effect of water content on catalytic performance, we tested [EimOAc][Im] containing 10wt% H2O (entry 17). The results indicate a minimal impact on catalytic efficiency.
| Entry | Catalyst | T (°C) | t (h) | Yieldb (%) | Sel.b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: SO (3 mmol), catalyst (0.3 mmol), SO2 (1.0 bar). b Determined by gas chromatography. c 10 wt% H2O. | |||||
| 1 | [MimOAc][2-AmPy] | 60 | 5 | 95 | >99 |
| 2 | [EimOAc][2-AmPy] | 60 | 5 | 95 | >99 |
| 3 | [BimOAc][2-AmPy] | 60 | 5 | 95 | >99 |
| 4 | [EmimOAc][2-AmPy] | 60 | 5 | 92 | >99 |
| 5 | [MimOAc][Im] | 60 | 5 | 95 | >99 |
| 6 | [EimOAc][Im] | 60 | 5 | 95 | >99 |
| 7 | [BimOAc][Im] | 60 | 5 | 94 | >99 |
| 8 | [EmimOAc][Im] | 60 | 5 | 93 | >99 |
| 9 | [MimOAc][2-AmPy] | 30 | 8 | 84 | 96 |
| 10 | [MimOAc][2-AmPy] | 30 | 12 | 85 | 97 |
| 11 | [MimOAc][2-AmPy] | 30 | 24 | 92 | 96 |
| 12 | [EimOAc][2-AmPy] | 30 | 24 | 91 | 95 |
| 13 | [BimOAc][2-AmPy] | 30 | 24 | 85 | 94 |
| 14 | [EmimOAc][2-AmPy] | 30 | 24 | 84 | 94 |
| 15 | [EimOAc][Im] | 30 | 24 | 95 | >99 |
| 16 | [BimOAc][Im] | 30 | 24 | 93 | 98 |
| 17c | [EimOAc][Im] | 30 | 24 | 92 | >99 |
Under the optimized conditions, the [EimOAc][Im]-catalyzed cycloaddition of diverse epoxides with SO2 achieved yields spanning 54% to 98% (2a–2h), demonstrating broad substrate compatibility. Fig. 4a shows the [EimOAc][Im]-catalyzed cycloadditions, where epichlorohydrin and 1,2-epoxyoctane with SO2 delivered yields of 89% and 98% (2a and 2b). Notably, even epoxides with bulky substituents on the carbon atoms, including allyl glycidyl ether, styrene oxide, or benzyl glycidyl ether, maintained exceptional efficiency (≥90% yields, 2d, 2e, 2g). Furthermore, cyclohexene oxides exhibited reduced reactivity due to their structural constraints as internal epoxides compounded by significant steric hindrance at the oxirane ring, ultimately resulting in a diminished yield of 54% (2h). The results indicated that the [EimOAc][Im] catalyst displays excellent catalytic activity.
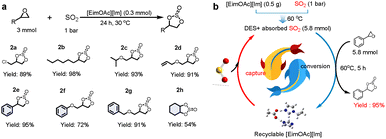 | ||
| Fig. 4 (a) The cycloaddition of SO2 with various epoxides. (b) SO2 capture and fixation for the synthesis of 2e in [EimOAc][Im]. | ||
Upon completion of the reaction, water and ethyl acetate were added to the reaction solution. The [EimOAc][Im] catalyst could then be isolated by removing water from the aqueous phase, allowing for the recovered catalyst to be utilized directly in the subsequent cycle of the reaction. As shown in Fig. S14, the activity of the catalyst decreased by almost 10% in yield after each cycle. The reduced catalytic performance is attributed to partial dissolution of [EimOAc][Im] in ethyl acetate. To improve integration between absorption and conversion processes, we investigated the in situ transformation of absorbed SO2 into cyclic sulfite. At 60 °C, [EimOAc][Im] achieved SO2 absorption equilibrium with a capacity of 5.8 mmol, followed by the addition of an equimolar amount of SO (1e). Upon completion of the 5 h reaction, cyclic sulfite 2e was isolated in 95% yield (Fig. 4b). After separation from the product, [EimOAc][Im] can be reused in the SO2 capture and conversion cycle. The cycling performance of DES was evaluated in situ (Fig. S15). The results show that the conversion efficiency remained above 90% over three cycles. Notably, however, the viscosity of the recovered [EimOAc][Im] increased markedly after the third cycle, likely due to accumulated unreacted cyclic sulfites. This change may impair the mass transfer properties of [EimOAc][Im] and consequently reduce its absorption performance. The above results provide new insights into the coupling of SO2 capture and conversion processes.
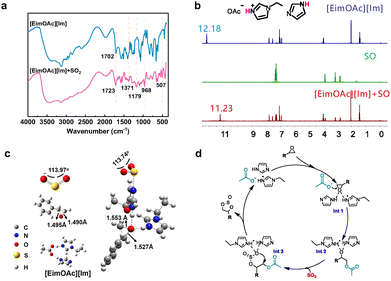
FTIR and NMR spectra of [EimOAc][Im] before and after the absorption of SO2 were analyzed to investigate the absorption mechanism. As can be seen in Fig. S16, the chemical shifts of the three H atoms of imidazole in [EimOAc][Im] changed from 7.04 (h and i) and 7.70 (g) ppm to 7.52 and 8.75 ppm after absorption of SO2, indicating that there is an acid–base interaction between SO2 and Im.21 The protons (c–f) on [EimOAc] exhibit downfield shifts after absorption of SO2, suggesting that [EimOAc] interacts with SO2 as well. The difference is that the chemical shift of H(1) in [EimOAc][Im] shifted from 10.49 ppm to 9.93 ppm, which is attributed to the absorption of SO2 affecting the original hydrogen bonding interactions of the DES molecules.22Fig. 5a displays the FTIR spectra of [EimOAc][Im] before and after SO2 absorption, revealing four new peaks at 1371, 1179, 968, and 507 cm−1. Among them, the peaks at 1371, 1179 and 507 cm−1 correspond to asymmetrical telescopic vibration, symmetrical telescopic vibration, and bending vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.23,24 The bond at 968 cm-1 originates from N–S coordination between the imidazole (Im) group and SO2.25 Additionally, the carboxyl group peak shifts from 1702 cm−1 to 1723 cm−1 due to the electrophilic environment induced by SO2, indicating an interaction between the oxygen atoms in the acetate ion's –COO− group and SO2.26 These spectral signatures confirm a dual trapping mechanism involving physical and chemical absorption in the DES system.
O, respectively.23,24 The bond at 968 cm-1 originates from N–S coordination between the imidazole (Im) group and SO2.25 Additionally, the carboxyl group peak shifts from 1702 cm−1 to 1723 cm−1 due to the electrophilic environment induced by SO2, indicating an interaction between the oxygen atoms in the acetate ion's –COO− group and SO2.26 These spectral signatures confirm a dual trapping mechanism involving physical and chemical absorption in the DES system.
In order to clarify the catalytic mechanism, we investigated the interaction between epoxide and [EimOAc][Im] using 1H NMR spectroscopy. As shown in Fig. 5b, when [EimOAc][Im] and styrene oxide (SO) were mixed in equimolar ratios, 1H NMR analysis revealed a marked upfield shift of the hydrogen-bonded proton H(1) in the DES from 12.18 to 11.23 ppm; however, the chemical shifts of hydrogen atoms in the SO epoxide group remained largely unchanged, suggesting weak hydrogen-bonding interactions between the DES and SO. This differential behavior implies that [EimOAc][Im] likely activates the epoxide through selective hydrogen-bonding interactions with the oxygen atom while minimal electronic perturbation occurs in the SO.
Theoretical calculations were further performed to gain insight into the activation of epoxides and SO2 by DESs (Fig. 5c). In [EimOAc][Im], after the N atoms on the imidazole ring interacted with SO2, the bond angle of SO2 was bent from 113.97° to 113.74°, indicating that SO2 was effectively activated by the N sites. When SO interacted with proton hydrogens in DES and N-H on the imidazole ring to form hydrogen bonds, the C–O bond length of SO increased from 1.495 Å to 1.527 Å and 1.490 Å to 1.553 Å, respectively. The C–O bond in SO was activated and lengthened accordingly, which was favourable for the ring-opening step during OAc− anion attack. Theoretical calculations demonstrate that the DES facilitates epoxide ring-opening through hydrogen bonding while simultaneously activating SO2 molecules.
According to both experimental and computational findings, a plausible reaction mechanism is proposed (Fig. 5d). The mechanism initiates with hydrogen bond-mediated activation of the epoxide by DES, inducing polarization of the C–O bond (Int 1). This activation facilitates a regioselective nucleophilic attack by the acetate anion at the electrophilic carbon centre, leading to the formation of an oxyanion intermediate (Int 2). Subsequent insertion of SO2 into the activated intermediate produces a sulfur-containing adduct (Int 3). This is followed by an intramolecular cyclization step that releases the acetate anion, yielding the target product and allowing for catalyst regeneration.
In summary, we have developed a series of low-viscosity, halogen-free DESs for the simultaneous capture and conversion of SO2 into cyclic sulfites. These DESs effectively capture SO2 through Lewis acid–base interactions and charge transfer with acetate, reversibly capturing up to 1.2514 g g−1 (1 bar SO2 at 20 °C). Remarkably, [EimOAc][Im] demonstrates excellent activity as a catalyst for cycloaddition reactions with various epoxides, yielding moderate to high conversions (54–98%) under mild reaction conditions. Spectroscopic characterization and theoretical calculations revealed a multi-site absorption mechanism and synergistic catalytic process. Our findings not only provide a green and efficient pathway for SO2 management but also offer insights into the rational design of functional solvents for sustainable chemical processes.
This work was supported by the National Natural Science Foundation of China (no. 22208070 and 22168012).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental section and characterization information of DESs. See DOI: https://doi.org/10.1039/d5cc05880e.Notes and references
- P. Zhang, G. Xu, M. Shi, Z. Wang, Z. Tu, X. Hu, X. Zhang and Y. Wu, Sep. Purif. Technol., 2022, 286, 120489 CrossRef CAS
.
- J. L. Obeso, C. V. Flores, R. A. Peralta, M. Viniegra, N. Martín-Guaregua, M. T. Huxley, D. Solis-Ibarra, I. A. Ibarra and C. Janiak, Chem. Soc. Rev., 2025, e00997 Search PubMed
.
- H. Wang, P. Wu, C. Li, J. Zhang and R. Deng, ACS Sustainable Chem. Eng., 2022, 10, 4451–4461 CrossRef CAS
.
- J. Yan, J. Zhu, S. Tong, Q. Wang and Z. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 56181–56190 CAS
.
- 5 F. Liu, J. Yu, A. B. Qazi, L. Zhang and X. Liu, Environ. Sci. Technol., 2021, 55, 1419–1435 CrossRef CAS
.
- R. Zhang, D. Hu, Y. Fu, Q. Feng, C. Mu, K. Gao, H. Ma, M. Liu and M. Zhang, Aggregate, 2024, 5, e408 CrossRef CAS
.
- S. Foorginezhad, G. Yu and X. Ji, Front. Chem., 2022, 10, 951951 CrossRef CAS PubMed
.
- H. Zhu, Y. Zhong, L. Yan, H. Zhang, Y. Shen, Z. Le, Q. Fan and Z. Xie, Green Chem., 2024, 26, 1387–1392 RSC
.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC
.
- W. Xiong, Y. Lu, C. Li, J. Geng, Y. Wu and X. Hu, Green Chem., 2023, 25, 1898–1907 RSC
.
- H. Qin, X. Hu, J. Wang, H. Cheng, L. Chen and Z. Qi, Green Energy Environ., 2020, 5, 8–21 CrossRef
.
- C. Wang, H. Wu, J. Li, J. Zhang, J. Zhang, J. Ding, H. Li, H. Li and W. Zhu, Chem. Eng. J., 2023, 471, 144394 CrossRef CAS
.
- H. Wu, W. Xiong, S. Wen, X. Zhang and S. Zhang, Chem. Commun., 2022, 58, 7801–7804 CAS
.
- Z.-M. Li, W.-Q. Gong, J.-F. Li, S.-X. Zhu, D.-J. Tao and Y. Zhou, J. Mol. Liq., 2022, 367, 120521 CAS
.
- Y. Zhou, X.-Y. Sang, H. Guan, X.-J. Shu, Z.-H. Xu, M.-S. Sun and M.-Z. Cai, J. Mol. Liq., 2023, 389, 122921 CrossRef CAS
.
- P. Li, X. Wang, T. Zhao, C. Yang, X. Wang and F. Liu, Chem. Eng. J., 2021, 422, 130033 CAS
.
- G. Long, C. Yang, X. Yang, T. Zhao, F. Liu and J. Cao, ACS Sustainable Chem. Eng., 2020, 8, 2608–2613 CAS
.
- A. W. Kleij, Curr. Opin. Green Sustainability, 2020, 24, 72–81 Search PubMed
.
- J. Li, C. Wang, H. Wu, J. Zhang, M. Zhang, Q. Zhu, Y. Fan, H. Liu and W. Zhu, Energy Fuels, 2023, 37, 8051–8056 CAS
.
- Y. Jiang, D. Wang, B. Guo, J. Zhao, Z. Zhou, L. Jin and Y. Lei, Mol. Catal., 2023, 551, 113664 CAS
.
- X. Li, L. Meng, F. Yang, Z. Yang, J. Li, Y. Chen and X. Ji, Chem. Commun., 2024, 60, 10560–10563 RSC
.
- X. Yang, Y. Zhang, F. Liu, P. Chen, T. Zhao and Y. Wu, Sep. Purif. Technol., 2020, 250, 117273 CrossRef CAS
.
- N. Scaglione, L. Wylie, A. Padua and M. Costa Gomes, ACS Sustainable Chem. Eng., 2024, 12, 10486–10497 CrossRef CAS
.
- P. Liu, K. Cai, X. Zhang and T. Zhao, AIChE J., 2022, 68, 17596 CrossRef
.
- C. Wang, G. Cui, X. Luo, Y. Xu, H. Li and S. Dai, J. Am. Chem. Soc., 2011, 133, 11916–11919 CrossRef CAS PubMed
.
- J. Zhao, S. Ren, Y. Hou, K. Zhang and W. Wu, Ind. Eng. Chem. Res., 2016, 55, 12919–12928 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |