Radial illumination enables the concentration, dispersion, lateral transport, and sorting of photocatalytic TiO2 microspheres
Ruijun
Lin†
a,
Lingshan
Fu†
a,
Fengyi
Yang
b,
Kai
Lou
*b and
Wei
Wang
 *a
*a
aSchool of Materials Science and Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen, Guangdong 518055, China. E-mail: weiwangsz@hit.edu.cn
bShenzhen KAYJA-OPTICS Technology Co., Ltd., Shenzhen, Guangdong 518000, China. E-mail: loukai@kayjaopt.com
First published on 28th November 2025
Abstract
The precise manipulation of a large number of colloidal particles in lateral (xy) dimensions is important for various applications. We introduce a simple way of concentrating, dispersing, moving, and sorting a large group of isotropic, photocatalytic TiO2 microspheres in 2D by moving the optical condenser of a microscope up and down. This operation varies the vertical (z) position of the beam waist of the illumination light and causes the light beam to be radially oblique, and the nonuniform photocatalysis on the surface of TiO2 microspheres causes them to move away from light via diffusiophoresis/osmosis.
Colloidal particles, particularly colloidal spheres, serve as building blocks for functional materials and devices used in a wide range of applications.1 These applications require precise and dynamic control over the concentration, dispersion, in-plane displacement, and sorting of large numbers of colloids. Over the years, several techniques have been developed to address this need, including optical tweezers,2 optoelectronic (OET) tweezers,3 and acoustic tweezers.4 A common theme for these techniques is the presence of a potential energy landscape, in which a colloid moves.
An alternative to forcing a colloid to move in a predefined energy field is to enable it to move autonomously. These are known as “active colloids”.5–7 Due to their self-propulsion, pair-wise interactions, and interactions with the environment, they can concentrate, disperse, migrate collectively, or sort independently.8–10 One limitation of this “motility-induced manipulation”, however, is the lack of control over the exact shape, size, and time evolution of the assembly or the precise location of a large population's movement. This limitation can be somewhat mitigated by exposing boundaries or other forms of energy landscapes,11–14 but the nature of self-propulsion endows a very large effective diffusivity, which makes precise control challenging.
By combining the chemical activity of colloidal particles and the high controllability of an external gradient, researchers have reported that titanium dioxide (TiO2) microspheres can collectively migrate in oblique ultraviolet illumination.15–19 In these studies, the microspheres migrate away from the incident UV light due to a shadow effect that breaks the fore-aft symmetry of the chemical activity on the TiO2 surface (see below for details).20 Similar operations have also been achieved by the optothermal effect.18,21 These studies manipulated a large number of TiO2 microspheres by taking advantage of their chemical activity and the resulting pairwise interactions.
Building upon the principle of light-induced TiO2 manipulation, we demonstrate that a large population of photocatalytic TiO2 microspheres can be concentrated, dispersed, and collectively moved. This is achieved by slightly adjusting the optical condenser installed on a standard inverted optical microscope (see Fig. S1 for a photo of the microscope), which vertically moves the beam waist of the illumination light and leads to oblique illumination on the TiO2 microspheres. This manipulation only requires a slight turning of the condenser adjustment knob on the microscope and does not require additional lamps, optical components, structured illumination, or adjustments to a standard commercial optical microscope, offering a minimalistic yet effective alternative to conventional tweezing techniques.
The operating principle of our manipulation technique consists of two elements. First is a manipulation of the illumination light beam (Fig. 1a). To elaborate, the UV LED light source emits a near-collimated beam that is focused by the optical condenser to generate a radially symmetric biconical beam. This beam converges toward the focus and then diverges symmetrically with a divergence half-angle of ∼28° (see Fig. S2 for the simulated light path and cone shapes). After properly adjusted, the beam waist typically sits on the sample plane to provide uniform, uncollimated illumination. However, by vertically displacing the optical condenser, the beam waist can be moved vertically away from the sample for it to receive radially oblique illumination. Specifically, in a typical inverted microscope with a top-mounted condenser, the sample receives convergent illumination when the condenser is moved downward, and divergent illumination when the condenser is moved upward.
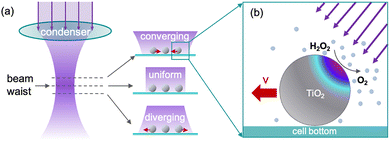 | ||
| Fig. 1 Operating principle of lateral manipulation of TiO2 microspheres via radial illumination. (a) Cartoon schematic showing the biconical light beam after passing through the optical condenser (left) and the angles at which the light beam irradiates the TiO2 microspheres at different planes of the light beam (right). From top to bottom: convergent, uniform, and divergent illumination. (b) A magnified cartoon view of the asymmetric photo-decomposition of H2O2 into O2 and the resulting chemical gradient on the surface of a TiO2 microsphere due to oblique illumination. UV light can only penetrate part of the TiO2 microsphere, which is highlighted in purple. All figures are for illustrative purposes only and are not to scale. (b) is adapted from ref. 20 with permission. Copyright John Wiley & Sons, Inc., 2016. | ||
Second, TiO2 microspheres are known to move away from the direction of incoming UV light (Fig. 1b).20 To elaborate, TiO2 microspheres under UV catalyze the decomposition of H2O2 on the colloidal surface, producing O2 and water. It has been proposed20 that TiO2 microspheres move away from a high concentration of O2via diffusiophoresis and/or diffusioosmosis. Although the exact chemical that is responsible for such transport, or why TiO2 microspheres move down rather than up the O2 gradient, remains poorly understood, the fact that TiO2 microspheres move away from each other when photo-activated (Fig. 6, ref. 22) lends qualitative support to this mechanism. Importantly, as first proposed in ref. 20, a TiO2 microsphere under an oblique illumination is photoexcited more on the side closer to the light than the other side, due to the limited penetration depth of UV inside TiO2 (Fig. 1b). This asymmetry in reactivity generates a chemical gradient across its body so that a TiO2 microsphere moves away from the illuminated side, and thus away from the incoming light.23
As a result, when the optical condenser is moved downward, light beam becomes convergent, and TiO2 microspheres move radially toward the beam center and become concentrated in the convergent illumination. Conversely, TiO2 microspheres move outward and disperse when the condenser is moved upward. Below, we describe the experimental results confirming this proposed technique and its tunability.
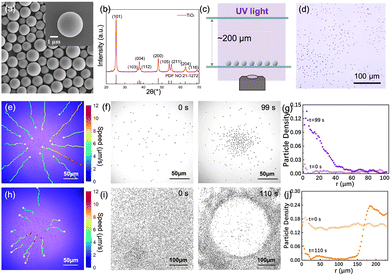
TiO2 microspheres of 2.4 ± 0.6 µm in diameter (ζ potential = −22.9 ± 2.0 mV) were chemically synthesized and details are given in the SI. See Fig. 2a and Fig. S6 for the scanning electron micrograph and Fig. S4 for size distributions. They are of anatase phase, confirmed by XRD in Fig. 2b. As commonly reported in the literature,24 these TiO2 microspheres absorb UV light and generate electron–hole pairs. UV-vis spectrum in Fig. S5 shows clear absorption of light below 380 nm, corresponding to a bandgap of 3.1 eV. In a typical experiment (see Fig. 2c for setup), TiO2 microspheres were mixed with H2O2 aqueous solutions of 0.5–1 v/v% and sedimented to the bottom of an experimental cell. 2D packing fraction of TiO2 microspheres of ∼1.5–5.0% was typically used in our experiments, but qualitatively the same observations were made for packing as low as 0.36% or as high as 9.83%. Ultraviolet light of 365 nm and a maximum power density of 1460 mW cm−2 was used to illuminate the sample from above, and the sample was observed below with an objective lens.
H2O2 react with the photoexcited electrons and holes and the overall reaction is its photocatalytic decomposition into water and O2 on the surface of the TiO2 microspheres (Fig. 1b). The photocatalytic activity of these TiO2 microspheres is supported by their spontaneous dispersion under uniform UV illumination, shown in Fig. 2d and Fig. S3. Such dispersion has been observed in the literature,19 and was attributed to their phoretic/osmotic repulsion because of the chemical gradients from photocatalysis.19
Before laterally manipulating TiO2 microspheres, we first performed an alignment technique called Köhler illumination to ensure the beam waist coincides with the sample plane to begin with, and that TiO2 microspheres received uniform illumination. This position of the condenser is hereafter referred to as the Köhler position (i.e., a displacement of 0). We then moved the condenser slightly downward away from the Köhler position by <1 mm, so the sample received convergent illumination at a divergence half-angle of ∼28°. The TiO2 microspheres that originally distributed randomly moved toward the center of the field of view in a roughly linear trajectory (Fig. 2e). The speed was on the order of 10 µm s−1 at a condenser displacement of −0.6 mm (minus means moving downward, and all subsequent displacement is relative to the Köhler position) and a UV intensity of 1102 mW cm−2.
Because of moving radially inward, TiO2 microspheres were then essentially concentrated to the center, and a cluster of TiO2 grew radially outward as more TiO2 microspheres joined (Fig. 2f, g and Video S1). Several features of such a TiO2 cluster are worth noting. First, qualitatively similar concentration and clustering was observed for TiO2 microspheres of diameters ranging from 0.5–4.5 µm. Second, the cluster was mostly in 2D, consistent with the density mismatch between TiO2 and water. However, a few layers could develop near the cluster center over time (see Fig. S7 for an example). Third, the cluster size appears to be limited by the number of particles available and the duration of illumination, and clusters as large as 2.5 × 104 µm2 were formed under UV of 1460 mW cm−2 and over 266 s (Fig. S7). Fourth, the cluster was of a roughly circular shape and often loose, because of the phoretic/osmotically induced pairwise repulsion of TiO2 microspheres. Finally, the formed clusters were highly dynamic, but this feature is beyond the scope of this study and will be extensively explored in a subsequent study.
TiO2 microspheres can also be dispersed from the center by moving the condenser upward, so that the TiO2 microspheres receive divergent illumination. In this case, TiO2 microspheres move radially outward from the center of view, also in a roughly linear trajectory. An example is given in Fig. 2h–j and Video S2. As a result of such dispersion, the center of view was in the end excluded of TiO2 microspheres. This exclusion zone can be as large as 3.8 × 104 µm2 at a condenser displacement of 0.2 mm and UV light of 1460 mW cm−2 (Fig. S8). To quantitatively distinguish this light-induced dispersion from passive Brownian motion, the mean square displacement (MSD) of microspheres in the absence of UV illumination and those in divergent illumination was measured and compared in Fig. S10. The parabolic MSD under divergent illumination confirms the directional transport of TiO2 during the dispersion.
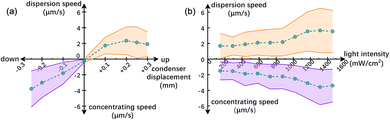
The speed of TiO2 microspheres is tunable, regardless of being concentrated or dispersed. Specifically, Fig. 3a shows that such speeds increased when the condenser was moved farther away from the Köhler position. However, moving away from the beam waist also reduces the light power density received by the sample. See Fig. S2 for the simulated power density at different xy planes along the optical axis. Further displacement of the condenser therefore undermines photocatalysis and, consequently, reduces the dispersing speed. On the other hand, Fig. 3b shows that increasing the light intensity increased the speeds of TiO2 microspheres being either concentrated or dispersed. However, at large light intensities (e.g. beyond 1300 mW cm−2) the increase in particle speeds plateaus. This is likely because high intensity light beam could penetrate the TiO2 microspheres more, thereby reducing the asymmetry in the chemical activity on the two sides of a TiO2 microsphere. This reduction in asymmetry then leads to slower transport. Moreover, we made the empirical observation that larger TiO2 microspheres moved faster than smaller ones, possibly because of a more pronounced asymmetry in the generated chemical gradient. However, the dependence of concentrating speeds as a function of particle sizes was not studied systematically.
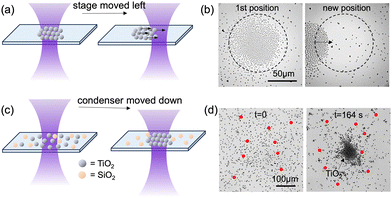
In addition to being concentrated or dispersed, a population of TiO2 microspheres can be collectively transported by convergent or divergent UV light. To elaborate, the concentration center is determined by the light beam, whose horizontal position on the xy plane is fixed by the microscope. Therefore, moving the sample stage laterally away from the light spot causes the TiO2 cluster, now moved away with the stage from the light spot, to return to the center of view (Fig. 4a). This provides a simple yet effective way of laterally transporting a large cluster of TiO2 microspheres in a controlled manner. See Fig. 4b and Video S3 for an example. Here, a cluster of approximately 1200 TiO2 microspheres with a diameter of 1–2 µm followed the lateral displacement of the sample stage, and swarmed at a speed of 18 µm s−1 under UV light of 1170 mW cm−2 and a condenser displacement of −0.2 mm.
Such a collective migration of photoresponsive TiO2 microspheres can also be exploited to sort them from photo-inert particles of similar sizes and shapes. For example, Fig. 4c, d and Video S4 show that TiO2 microspheres can be sorted from inert, SiO2 microspheres (i.e. tracers, zeta potential −30.6 ± 0.2 mV) of 5 µm in diameter from a well-mixed population, because only TiO2 microspheres were concentrated under convergent UV light while SiO2 spheres remained largely Brownian. The fact that TiO2 and SiO2 microspheres were separated also excludes convection, possibly arising from local heating or liquid drifting, as the dominant mechanism for the transport of TiO2 microspheres in a radial light beam.
To summarize, we have demonstrated a technique to concentrate, disperse, transport, and sort large numbers of TiO2 microspheres by convergent or divergent UV light beams. This is achieved by moving the optical condenser of an optical microscope up or down to displace the waist of the biconical beam above or below the sample plane, respectively. In either case, the speed of concentration or dispersion is positively related to the condenser displacement and light intensity. Using the same principle, TiO2 microspheres can be collectively moved or sorted from photo-inert SiO2 microspheres.
The key features of our demonstrated technique include simplicity (by just turning a knob on a commercial microscope), versatility (multiple manipulations are possible), and tunability. Compared with optical tweezers and optoelectronic tweezers, our technique requires a much less sophisticated setup, and can manipulate 103–104 particles. Furthermore, although our demonstrations used a microscope, the operating principle of the optical manipulation of TiO2 colloids is general and can be applied to a range of optical setups beyond microscopy. The simplicity, versatility, and tunability of our approach suggest that it could be extended beyond laboratory settings. In particular, large-scale, on-site applications such as photocatalytic water treatment, air purification, formulation of cosmetic and coating products, or catalytic reactors may benefit from the ability to dynamically concentrate, disperse, or sort TiO2 particles simply by adjusting the incidence angle of light. Importantly, concentration, dispersion, transport, and sorting, although seemingly distinct, are all manifestations of the same underlying mechanism: TiO2 microspheres exhibit negative phototaxis under oblique UV illumination.
This project is financially supported by the National Natural Science Foundation of China (T2322006), the China Manned Space Engineering Program (Project number: KJZ-YY-NLT0502), and the Shenzhen Science and Technology Program (RCYX20210609103122038).
Conflicts of interest
A Chinese patent (No. 2025116339752) based on this work has been filed on Nov. 10, 2025.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06293d.References
- F. Li, D. P. Josephson and A. Stein, Angew. Chem., Int. Ed., 2011, 50, 360–388 CrossRef CAS PubMed
.
- A. C. H. Coughlan, Z. Mao, M. A. Bevan and Y. Zheng, Sci. Adv., 2017, 3, e1700458 CrossRef
.
- X. Hong, B. Xu, G. Li, F. Nan, X. Wang, Q. Liang, W. Dong, W. Dong, H. Sun, Y. Zhang, C. Li, R. Fu, Z. Wang, G. Shen, Y. Wang, Y. Yao, S. Zhang and J. Li, Sci. Adv., 2024, 10, eadn7582 CrossRef CAS PubMed
.
- Y. Xie, C. Zhao, Y. Zhao, S. Li, J. Rufo, S. Yang, F. Guo and T. J. Huang, Lab Chip, 2013, 13, 1772 RSC
.
- W. Wang, X. Lv, J. L. Moran, S. Duan and C. Zhou, Soft Matter, 2020, 16, 3846–3868 RSC
.
- X. Chen, C. Zhou and W. Wang, Chem. – Asian J., 2019, 14, 2388–2405 CrossRef CAS PubMed
.
- I. S. Aranson, Phys.-Usp., 2013, 56, 79–92 Search PubMed
.
- J. Zhang, E. Luijten, B. A. Grzybowski and S. Granick, Chem. Soc. Rev., 2017, 46, 5551–5569 RSC
.
- Y. Huang, C. Wu, J. Chen and J. Tang, Angew. Chem., Int. Ed., 2024, 136, e202313885 CrossRef
.
- S. A. Mallory, C. Valeriani and A. Cacciuto, Annu. Rev. Phys. Chem., 2018, 69, 59–79 CrossRef CAS PubMed
.
- A. E. Patteson and A. G. Paulo, Curr. Opin. Colloid Interface Sci., 2016, 21, 86–96 CrossRef CAS
.
- Z. Xiao, M. Wei and W. Wang, ACS Appl. Mater. Interfaces, 2018, 11, 6667–6684 CrossRef
.
- M. N. Popescu and W. E. Uspal, Acc. Chem. Res., 2018, 51, 2991–2997 CrossRef CAS
.
- X. Chen, B. Ran, H. Wu and G. Zeng, Adv. Funct. Mater., 2025, e04234 CrossRef
.
- J. Zhang, F. Mou, Z. Wu, J. Song, J. E. Kauffman, A. Sen and J. Guan, Nanoscale Adv., 2021, 3, 6157–6163 RSC
.
- F. Mou, J. Zhang, Z. Wu, S. Du, Z. Zhang, L. Xu and J. Guan, iScience, 2019, 19, 415–424 CrossRef
.
- S. Che, J. Zhang, F. Mou, X. Guo, J. E. Kauffman, A. Sen and J. Guan, Research, 2022, 1, 9816562 Search PubMed
.
- J. Zhang, A. Laskar, J. Song, O. E. Shklyaev, F. Mou, J. Guan, A. C. Balazs and A. Sen, ACS Nano, 2022, 17, 251–262 CrossRef
.
- X. Guo, Y. Wang, F. Mou, Q. Xie, S. Su, C. Chen and J. Guan, J. Mater. Chem., 2022, 10, 5079–5087 CAS
.
- C. Chen, F. Mou, L. Xu, S. Wang, J. Guan, Z. Feng, Q. Wang, L. Kong, W. Li, J. Wang and Q. Zhang, Adv. Mater., 2016, 29, 1603374 CrossRef PubMed
.
- B. M. Tansi, M. L. Peris, O. E. Shklyaev, A. C. Balazs and A. Sen, Angew. Chem., Int. Ed., 2019, 58, 2295–2299 CrossRef CAS
.
- P. Xu, S. Duan, Z. Xiao, Z. Yang and W. Wang, Soft Matter, 2020, 16, 6082–6090 RSC
.
- J. L. Moran and J. D. Posner, Annu. Rev. Fluid Mech., 2017, 49, 511–540 CrossRef
.
- R. Dong, Y. Cai, Y. Yang, W. Gao and B. Ren, Annu. Rev. Fluid Mech., 2018, 51, 1940–1947 CAS
.
Footnote |
| † Contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |