Reversible twisting-induced crystalline–polycrystalline transformation in cyanoacrylate crystals
Thiyagaraj
Parthasarathy
a,
Aritra
Bhowmik
 bc,
Biswajit
Bhattacharya
bc,
Biswajit
Bhattacharya
 d,
Manish Kumar
Mishra
d,
Manish Kumar
Mishra
 *bc and
Soumyajit
Ghosh
*bc and
Soumyajit
Ghosh
 *a
*a
aDepartment of Chemistry, SRM Institute of Science and Technology, Kattankulathur 603 203, Tamil Nadu, India. E-mail: soumyajitghosh89@gmail.com
bPhysical and Materials Chemistry Division, CSIR-National Chemical Laboratory, Pune 411008, India. E-mail: mishra_mani07@yahoo.in; mk.mishra.ncl@csir.res.in
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
dBAM Federal Institute for Materials Research and Testing, Richard-Willstätter-Str. 11, 12489 Berlin, Germany
First published on 26th November 2025
Abstract
We report cyanoacrylate-based organic crystalline materials that exhibit reversible hand-induced helical twisting through the combined action of 1D elasticity and 2D plasticity. These crystals twist and untwist without lattice damage, retaining their elastic integrity through π slip-stacking and Cl⋯Cl, C–H⋯Cl, and Me⋯Me interactions. Their unique integration of elastic recovery, plastic bending, and reversible twisting highlights their potential as mechanically adaptive materials for flexible and responsive devices.
Flexible crystalline materials have emerged as fascinating materials due to their mechanical adaptability including elasticity,1 plasticity,2 and twisting,3 could provide an opportunity to develop crystalline materials with tailored mechanical properties for diverse applications such as wearable devices,4 molecular machines,5 and next-generation optoelectronic devices.6 Unlike polymers7 and metals,8 molecular crystalline materials offer unique advantages, including high crystallinity, molecular-level precision, and the ability to engineer specific properties through rational crystal design assisted by crystal engineering approaches.9 The brittle nature of such crystals has limited their use in flexible applications, motivating recent advances in flexible molecular crystals. Elastic deformation in molecular crystals is reversible, arising from isotropic or corrugated packing that allows lattice recovery after stress.1 In contrast, plastic deformation is permanent, associated with anisotropic packing and slidable layers enabling dislocations.2 Twisting, a rare yet versatile mechanical deformation mode, enhances structural flexibility and induces optical effects such as polarization rotation and light modulation,10 enabling optical waveguides11 and enantiosensitive sensors.12 Although often achieved via controlled crystallization, manual twisting of organic crystals remains scarcely reported.13
Recent studies have revealed key structure–property relationships in mechanically twisted crystals, emphasizing the role of weak interactions such as π⋯π stacking, hydrogen and halogen bonding, and synthon modularity. The interplay of σ-hole and π-hole interactions is critical for hand-twistable crystals. Saha and Desiraju demonstrated hand-twistable crystals stabilized by hydrogen bonds,13a while Zhang et al. reported crystals combining plastic twisting, elastic bending, and polarization rotation.10b McMurtrie et al. showed that reversible molecular rotations enable elastic bending and twisting in copper(II) acetylacetonate crystals,13b and Barbour et al. showed that rigid molecules can form hand-twistable plastic crystals.13c Hongxun et al. recently developed 2D elastic and twistable crystals for optical applications.13d Despite these advances, the mechanisms of twisting remain unclear, and examples combining elasticity, plasticity, and twisting are extremely rare, making their rational design a continuing challenge. Here, we present a study of molecular crystals exhibiting simultaneous elasticity, plasticity, and hand-twistability (Fig. 1). Two cyanoacrylate-based crystals, ethyl (E)-3-(3,4-dichlorophenyl)-2-cyanoacrylate (crystal 1) and ethyl (E)-3-(4-chlorophenyl)-2-cyanoacrylate (crystal 2) exhibit 1D elasticity, 2D plasticity, and reversible mechanical twisting, returning to their original shape upon reverse twisting without fatigue. Plastic twisting temporarily converts the single crystal into a polycrystalline state through molecular rotation and π-column gliding, which reverses upon untwisting, as confirmed by XRD, Raman, and DSC. These findings advance understanding of mechanical flexibility in crystals and suggest new routes for designing flexible materials with enhanced mechanical and optical performance.14a
Two cyanoacrylate derivatives were synthesized using a Knoevenagel reaction,14b as shown in SI, Scheme S1, and confirmed by 1H NMR spectroscopy (Fig. S1 and S2). Long needle-shaped crystals 1 and 2, with 0.5 mm to 7.5 cm in length and 0.05 to 0.1 mm in width, were obtained by slow evaporation from the mixture of methanol and acetonitrile in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature (Table S1 and Fig. S3). As shown in Videos S1 and S2, crystals 1 and 2 showed elastic bending along the (
1 at room temperature (Table S1 and Fig. S3). As shown in Videos S1 and S2, crystals 1 and 2 showed elastic bending along the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face under a three-point bending test, forming loops under stress and recovering their shape upon release (Fig. 1b for crystal 1; Fig. 1g for crystal 2). The calculated maximum elastic strain for crystals 1 and 2 were found to be ∼4.3% and ∼4.1%, respectively, based on the Euler–Bernoulli bending theory (Fig. S4).
01) face under a three-point bending test, forming loops under stress and recovering their shape upon release (Fig. 1b for crystal 1; Fig. 1g for crystal 2). The calculated maximum elastic strain for crystals 1 and 2 were found to be ∼4.3% and ∼4.1%, respectively, based on the Euler–Bernoulli bending theory (Fig. S4).
Elastic bending was observed from one face (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) of both the crystals 1 and 2, pointing to 1D elastic bending behavior of the crystals.1,15 However, when mechanical stress is applied perpendicular to (
01) of both the crystals 1 and 2, pointing to 1D elastic bending behavior of the crystals.1,15 However, when mechanical stress is applied perpendicular to (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face from short range, both crystals 1 and 2 exhibited irreversible plastic bending (Fig. 1c and h). Apart from plastic bending from (
01) face from short range, both crystals 1 and 2 exhibited irreversible plastic bending (Fig. 1c and h). Apart from plastic bending from (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face, both the crystals demonstrated plastic bending up to a smaller extent from the (101) face, albeit with the presence of striations observed on the surface of the crystal (Fig. 1d and i). Hence, both crystals 1, and 2 can be considered as 1D elastic and 2D plastic (Fig. 1).
01) face, both the crystals demonstrated plastic bending up to a smaller extent from the (101) face, albeit with the presence of striations observed on the surface of the crystal (Fig. 1d and i). Hence, both crystals 1, and 2 can be considered as 1D elastic and 2D plastic (Fig. 1).
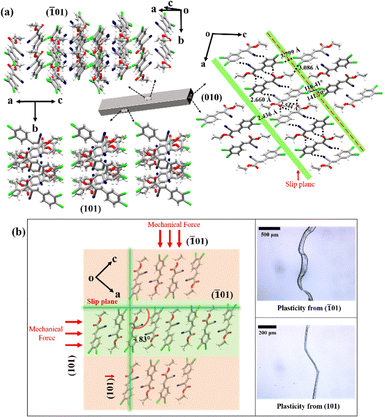
To understand the mechanical flexibility of crystals 1 and 2, their crystal packing were analysed. Since crystal structures 1 and 2 are isostructural, the packing analysis is discussed here for crystal 1, while the analysis for crystal 2 is provided in the SI (Fig. S5). Crystals of 1 grew with space group P21/n with Z = 4. In crystal 1, molecules form centrosymmetric dimers via bifurcated C–H⋯O interactions (2.54(3), 2.46(3) Å), which link through C–H⋯N interactions (2.66(3), 2.87(3) Å) into zigzag 1D tapes along [101]. These tapes stack through π⋯π interactions along b to form 2D corrugated sheets in the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) plane, further stabilized by Cl⋯Cl (3.60(1) Å), C–H⋯Cl (3.08(5) Å), and Me⋯Me interactions (Fig. 2a).
01) plane, further stabilized by Cl⋯Cl (3.60(1) Å), C–H⋯Cl (3.08(5) Å), and Me⋯Me interactions (Fig. 2a).
Weak and dispersive interactions in crystal 1 generate low-energy slip planes parallel to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) plane, forming lubricating or buffering zones that enable smooth molecular motion and 2D plasticity (Fig. 2a).1,2 The overall packing is anisotropic, with corrugated 2D sheets arranged in a criss-cross fashion across the slip planes. This arrangement allows short-range stretching of molecules, giving rise to elasticity along the (
01) plane, forming lubricating or buffering zones that enable smooth molecular motion and 2D plasticity (Fig. 2a).1,2 The overall packing is anisotropic, with corrugated 2D sheets arranged in a criss-cross fashion across the slip planes. This arrangement allows short-range stretching of molecules, giving rise to elasticity along the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face. Hirshfeld surface analysis (Fig. S6) confirms that while the π-stacked columns remain intact, weak interactions such as C–H⋯O, C–H⋯N, Cl⋯Cl, and Me⋯Me play a central role in maintaining lattice cohesion. Upon applying stress, the π⋯π distances along the b-axis increase in the outer arc (extension) and decrease in the inner arc (compression). These subtle, reversible adjustments, together with weak interlayer interactions (Cl⋯Cl, C–H⋯Cl, Me⋯Me), facilitate elastic recovery by allowing smooth sliding across layers and restoring the original crystal shape (Fig. S7–S8).
01) face. Hirshfeld surface analysis (Fig. S6) confirms that while the π-stacked columns remain intact, weak interactions such as C–H⋯O, C–H⋯N, Cl⋯Cl, and Me⋯Me play a central role in maintaining lattice cohesion. Upon applying stress, the π⋯π distances along the b-axis increase in the outer arc (extension) and decrease in the inner arc (compression). These subtle, reversible adjustments, together with weak interlayer interactions (Cl⋯Cl, C–H⋯Cl, Me⋯Me), facilitate elastic recovery by allowing smooth sliding across layers and restoring the original crystal shape (Fig. S7–S8).
In addition to 1D elasticity, crystals 1 and 2 exhibit 2D plasticity from the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) and (101) faces, respectively. The angles between these planes are 83(2)° for crystal 1 and 88.2(3)° for crystal 2 (Fig. 2b and Fig. S9). When stress is applied perpendicular to the (
01) and (101) faces, respectively. The angles between these planes are 83(2)° for crystal 1 and 88.2(3)° for crystal 2 (Fig. 2b and Fig. S9). When stress is applied perpendicular to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face, the crystal undergoes plastic deformation due to slippage of corrugated sheets along slip planes, facilitated by Cl⋯Cl, C–H⋯Cl, and Me⋯Me interactions. The deformation is irreversible, with the bent shape retained after stress release. Under higher stress, delamination occurs, producing acute bending without disturbing the π-stacked layers. In contrast, stress applied along the (101) face causes plastic deformation with surface striations, indicating directional layer migration and disruption of weak interactions. These observations confirm that the mode of 2D plasticity depends on crystal orientation and interaction directionality.2a,3,13
01) face, the crystal undergoes plastic deformation due to slippage of corrugated sheets along slip planes, facilitated by Cl⋯Cl, C–H⋯Cl, and Me⋯Me interactions. The deformation is irreversible, with the bent shape retained after stress release. Under higher stress, delamination occurs, producing acute bending without disturbing the π-stacked layers. In contrast, stress applied along the (101) face causes plastic deformation with surface striations, indicating directional layer migration and disruption of weak interactions. These observations confirm that the mode of 2D plasticity depends on crystal orientation and interaction directionality.2a,3,13
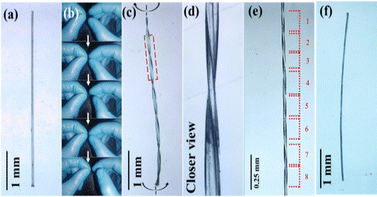
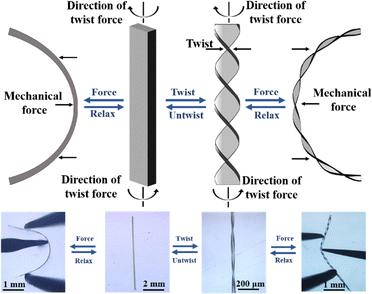
Apart from 1D elasticity and 2D plasticity, crystals 1 and 2 also show hand-induced twisting behavior (Fig. 1e, j and 3). When a tangential force is applied to the crystal cross-section, it undergoes a plastic twist transition, forming a helical shape.10,13 The degree of twisting depends on the applied force, greater force yields more twist pitches. Remarkably, the twisted crystals retain elasticity, showing reversible deformation with elastic strains of ∼3.4% and ∼2.8% for crystals 1 and 2, respectively (Fig. S10). Controlled twisting allows up to eight turns within 1 mm (Fig. 3e and Fig. S11, S12). Upon reversing the twisting direction, the crystals return to their original configuration, demonstrating fully reversible hand-twisting (Videos S3, S4).
Unlike typical twistable crystals showing only 2D plasticity,13 crystals 1 and 2 combine 1D elasticity with 2D plasticity, enabling controlled short-range molecular motion that leads to twisting (Fig. 3 and Fig. S13). Since both are isostructural, crystal 1 was examined for structure–property correlation. Weak interactions such as Cl⋯Cl, C–H⋯Cl, and Me⋯Me across slip planes facilitate sliding of π-stacked columns along the b-axis. When twisting forces act tangentially to this axis, these interactions are disrupted, allowing gliding and partial rotation of the π-columns. The π⋯π interactions accommodate stress while maintaining column integrity, ensuring cohesion and structural continuity. This interplay of weak interactions and π-slip stacking stabilizes the helically twisted form without permanent damage (Fig. 4 and Fig. S14).
Restoring non-covalent interactions in crystals 1 and 2 enable efficient torsional energy dissipation across low-energy barriers, allowing plastic twisting without permanent deformation. The balance between orthogonal weak interactions and π⋯π stacking facilitates short-range molecular rotation, leading to controllable twisting.13 These crystals exhibit up to eight twist pitches per millimeter, with the number of pitches governed by the applied force. Remarkably, they display exceptional torsional reversibility, returning to their original straight form upon untwisting without structural damage. Even after 30 twist–untwist cycles, the crystals remain intact, showing no cracks or degradation, as confirmed by FESEM images that reveal complete surface recovery from the helical to straight form (Fig. 1k–m and Fig. S15). The restoring nature of the interactions allows the molecules to return to their thermodynamically stable positions, ensuring full reversibility and mechanical robustness. The twisted crystals 1 and 2 show remarkable elastic deformation while retaining structural integrity. Upon stress release, they recover fully, as seen in Videos S5 and S6. Twisting induces rotation of π-stacked columns into helical arrangements, while the retention of π⋯π interactions along the b-axis ensures elasticity. These crystals exhibit elastic strains of ∼3.4% and ∼2.8% for 1 and 2, respectively. The slight reduction in elasticity after twisting arises from partial loss of weak intermolecular interactions and minor molecular rearrangements. The reversible behavior is governed by the restoring nature and directionality of Cl⋯Cl, C–H⋯Cl, Me⋯Me, and π⋯π interactions within the lattice.
Here, the plastic twisting in crystals is governed by three factors: the directionality of weak and dispersive interactions, slidable/rotatable π-stacked columns with accommodative π⋯π interactions, and short-range molecular movement along the twisting force. During twisting, single crystals convert into a polycrystalline state due to molecular rotation and π-column gliding. Upon untwisting, molecules return to their original positions, making a smooth transition from the polycrystalline state to the single-crystalline state.16 Single-crystal X-ray diffraction confirmed this behavior: untwisted crystals showed sharp diffraction spots, which became elongated and unindexable upon twisting, indicating molecular sliding and disruption of packing. Diffraction patterns reverted to their original sharpness upon reversal. Crystal 2 exhibited similar behavior to crystal 1 (Fig. S16). Powder X-ray diffraction (PXRD) of straight and twisted crystals also showed identical profiles (Fig. S17), confirming that fundamental packing remains intact during the single-crystalline to polycrystalline transformation. Differential scanning calorimetry (DSC) of mechanically twisted crystals 1 and 2 showed a broad endothermic peak, indicating transition to a less-ordered polycrystalline state (Fig. 5a). Upon untwisting, the original thermal profile was fully restored, demonstrating a reversible single-crystal-to-polycrystalline-to-crystalline transition and highlighting the dynamic adaptability of these materials.
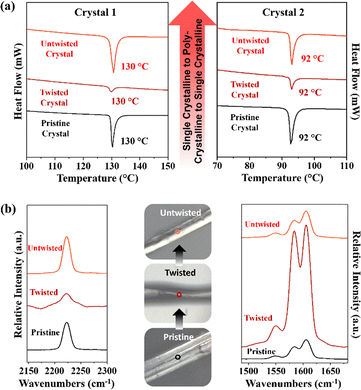 | ||
| Fig. 5 (a) DSC thermogram of pristine, twisted and untwisted crystal 1 and crystal 2, and (b) micro-focus Raman spectra of pristine, twisted, and untwisted crystal 1 and crystal 2. | ||
Micro-focus Raman spectroscopy of pristine, twisted, and untwisted crystals of compounds 1 and 2 (Fig. 5b and Fig. S18, S19) further supported the reversible phase behavior. The nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stretching band at ∼2222 cm−1 broadened upon twisting, indicating increased vibrational disorder and local lattice heterogeneity due to disruption of weak C–H⋯N hydrogen bonds. Upon untwisting, the peak regained sharpness, suggesting partial restoration of the hydrogen-bonding network. Twisting also enhanced aromatic C
N) stretching band at ∼2222 cm−1 broadened upon twisting, indicating increased vibrational disorder and local lattice heterogeneity due to disruption of weak C–H⋯N hydrogen bonds. Upon untwisting, the peak regained sharpness, suggesting partial restoration of the hydrogen-bonding network. Twisting also enhanced aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands at 1548, 1583, and 1604 cm−1, reflecting altered π-system polarizability and closer π⋯π interactions; these features partly persisted after untwisting, indicating temporary structural reorganization. Together with DSC and SCXRD data, these results confirm a mechanically induced, reversible single-crystal-to-polycrystalline-to-crystalline transformation.
C stretching bands at 1548, 1583, and 1604 cm−1, reflecting altered π-system polarizability and closer π⋯π interactions; these features partly persisted after untwisting, indicating temporary structural reorganization. Together with DSC and SCXRD data, these results confirm a mechanically induced, reversible single-crystal-to-polycrystalline-to-crystalline transformation.
In conclusions, we designed two flexible crystals that exhibit 1D elastic bending, 2D plastic bending, and handheld twisting. Elasticity originates from criss-cross and corrugated packing stabilized by weak interactions, while plasticity arises from slippage of π-stacked columns and sheet migration along specific faces. Hand-twisting integrates these mechanisms, producing helical configurations through short-range molecular movement and rotatable π-columns. Upon release, crystals revert to their original structure, as confirmed by SCXRD, PXRD, DSC, and Raman spectroscopy. Twisted crystals retain elasticity due to the integrity of π-stacked columns, highlighting the crucial role of intermolecular interactions in dynamic crystal adaptability and the potential for twistable materials in flexible optoelectronics and optical waveguides.
S. G. thanks DST-SERB (CRG/2020/000885) and CSIR (01(3078)/21/EMR-II) for research support. T. P. thanks SRMIST for a fellowship. A. B. thanks CSIR and the Centre of Analytical Facility (CAF), CSIR-NCL. M. K. M. acknowledges DST-SERB/ANRF (SRG/2022/000543) for a start-up grant. We thank the Nanotechnology Research Centre (NRC), SRMIST, for access to the Bruker D8 Quest SCXRD facility.
Conflicts of interest
There are no conflicts to declare.Data availability
The supporting data has been included in the supplementary information (SI). Supplementary information: synthesis, crystal preparation, SCXRD, PXRD, DSC details, etc. See DOI: https://doi.org/10.1039/d5cc05852j.CCDC 2446156 and 2446157 contain the supplementary crystallographic data for this paper.17a,b
Notes and references
- (a) S. Ghosh and C. M. Reddy, Angew. Chem., Int. Ed., 2012, 51, 10319–10323 CrossRef CAS; (b) P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS; (c) A. J. Thompson, B. S. K. Chong, E. P. Kenny, J. D. Evans, J. A. Powell, M. A. Spackman, J. C. McMurtrie, B. J. Powell and J. K. Clegg, Nat. Mater., 2025, 24, 356–360 CrossRef CAS PubMed.
- (a) S. Takamizawa, Y. Takasaki, T. Sasaki and N. Ozaki, Nat. Commun., 2018, 9, 3984 CrossRef PubMed; (b) D. Manoharan, M. S. Nisha, M. S. Kiran, F. Emmerling, B. Bhattacharya and S. Ghosh, Cryst. Growth Des., 2023, 23, 657–661 CrossRef CAS; (c) J. R. Wang, M. Li, Q. Yu, Z. Zhang, B. Zhu, W. Qin and X. Mei, Chem. Commun., 2019, 55, 8532–8535 RSC; (d) L. Zhu, R. O. Al-Kaysi and C. J. Bardeen, J. Am. Chem. Soc., 2011, 133, 12569–12575 CrossRef CAS.
- (a) R. Rai, B. P. Krishnan and K. M. Sureshan, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 2896–2901 CrossRef CAS PubMed; (b) A. G. Shtukenberg, Y. O. Punin, A. Gujral and B. Kahr, Angew. Chem., Int. Ed., 2014, 53, 672–699 CrossRef CAS; (c) S. Hu, M. K. Mishra and C. C. Sun, Chem. Mater., 2019, 31, 3818–3822 CrossRef CAS; (d) Z. Wang, W. Han, B. Fu, H. Kang, P. Cheng, J. Guan, Y. Zheng, R. Shi, J. Xu and X. H. Bu, J. Am. Chem. Soc., 2025, 147, 2766–2775 CrossRef CAS.
- X. Pan, L. Lan, Q. Di, X. Yang and H. Zhang, Wearable Electron., 2024, 1, 111–118 CrossRef CAS.
- L. Lan, X. Pan, P. Commins, L. Li, L. Catalano, D. Yan, H. Xiong, C. Wang, P. Naumov and H. Zhang, CCS Chem., 2024, 6, 111–118 Search PubMed.
- (a) Y. Wang, L. Sun, C. Wang, F. Yang, X. Ren, X. Zhang, H. Dong and W. Hu, Chem. Soc. Rev., 2019, 48, 1492–1530 RSC; (b) M. Annadhasan, S. Basak, N. Chandrasekhar and R. Chandrasekar, Adv. Opt. Mater., 2020, 8, 2000959 CrossRef CAS.
- A. Matsumoto, T. Odani, K. Sada, M. Miyata and K. Tashiro, Nature, 2000, 405, 328–330 CrossRef CAS PubMed.
- A. Sorrenti, L. Jones, S. Sevim, X. Cao, A. J. DeMello, C. Martí-Gastaldo and J. Puigmartí-Luis, J. Am. Chem. Soc., 2020, 142, 9372–9381 CrossRef CAS PubMed.
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952–9967 CrossRef CAS.
- (a) M. Rohullah, V. V. Pradeep, S. Singh and R. Chandrasekar, Nat. Commun., 2024, 15, 4040 CrossRef CAS; (b) S. Tang, K. Ye and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202210128 CrossRef CAS.
- X. Yang, L. Lan, X. Pan, X. Liu, Y. Song, X. Yang, Q. Dong, L. Li, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 7874 CrossRef CAS PubMed.
- X. Wu, X. Han, Q. Xu, Y. Liu, C. Yuan, S. Yang, X. Liu, Y. Deng, F. Xu and Y. Cui, J. Am. Chem. Soc., 2019, 141, 7081–7089 CrossRef CAS PubMed.
- (a) S. Saha and G. R. Desiraju, J. Am. Chem. Soc., 2017, 139, 1975–1983 CrossRef CAS; (b) A. Worthy, A. Grosjean, M. C. Pfrunder, Y. Xu, C. Yan, G. Edwards, J. K. Clegg and J. C. McMurtrie, Nat. Chem., 2018, 10, 65–69 CrossRef CAS PubMed; (c) L. O. Alimi, P. Lama, V. J. Smith and L. J. Barbour, Chem. Commun., 2018, 54, 2994–2997 RSC; (d) K. Chen, J. Wang, H. Shan, H. Fan, Y. Hao, M. Taiwaikuli, N. Wang, X. Huang, T. Wang and H. Hao, Sci. China Mater., 2023, 66, 2418–2428 CrossRef CAS.
- (a) X. Liu, H. Li, M. Tao, Y. Yu, Z. Zhu, D. Wu, J. Wang, L. Zhang, Q. Sun, R. Yang and Y. Chen, Adv. Mater. Technol., 2025, 10, 2400661 CrossRef CAS; (b) H. Yasuda and H. Midorikawa, Bull. Chem. Soc. Jpn., 1966, 39, 1754–1759 CrossRef CAS.
- (a) S. Ghosh, M. K. Mishra, S. B. Kadambi, U. Ramamurty and G. R. Desiraju, Angew. Chem., Int. Ed., 2015, 54, 2674–2678 CAS; (b) S. Ghosh and M. K. Mishra, Cryst. Growth Des., 2021, 21, 2566–2580 CrossRef CAS; (c) A. Bhowmik, S. Bamane and M. K. Mishra, CrystEngComm, 2024, 26, 5694–5698 CAS; (d) M. K. Mishra, S. B. Kadambi, U. Ramamurty and S. Ghosh, Chem. Commun., 2018, 54, 9047–9050 CAS.
- (a) B. H. Frodal, S. Thomesen, T. Børvik and O. S. Hopperstad, Int. J. Plast., 2021, 142, 102996 CrossRef CAS; (b) G. Yang and S. J. Park, Materials, 2019, 12, 2003 CrossRef CAS.
- (a) CCDC 2446156: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2n3f8h; (b) CCDC 2446157: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2n3f9j.
| This journal is © The Royal Society of Chemistry 2026 |