Anion–π catalysis with halogen bonding
Bingqian
Shi†
,
Qianmu
Xu†
,
Kaiyang
Fan
,
Jie
Zhang
,
Hongling
Wang
and
Xiang
Zhang
 *
*
Shaanxi Key Laboratory of Natural Products & Chemical Biology, College of Chemistry & Pharmacy, Northwest A&F University, 22 Xinong Road, Yangling 712100, Shaanxi, P. R. China. E-mail: xiang.zhang@nwafu.edu.cn
First published on 25th November 2025
Abstract
Anion–π catalysis with halogen bonding has been successfully applied in decarboxylative Michael addition reactions. The synergistic strategy exhibits significant advantages over each individual interaction in terms of yield, selectivity and anti-interference ability.
As a recently discovered non-covalent interaction, anion–π interaction primarily exists between anions and the π-acidic surfaces of electron-deficient aromatic rings (the quadrupole moment Qzz > 0, Fig. 1A).1 Since the proposal of this concept, an increasing amount of evidence has confirmed the occurrence of such anion binding in the solid and solution state with functional relevance in ion channels,2 materials,3 ion recognition,4 and molecular assembly.5
In fact, anion–π interactions can further be harnessed to stabilize anionic transition states (TS) or intermediates (Int) on π-acidic surfaces, thereby facilitating the realization of specific catalytic transformations.6 Typical examples include the Kemp eliminations,7 Michael additions,8 Diels–Alder cyclizations9 and epoxide ring-opening reactions.10 Moreover, external factors and systems such as electric fields,11 π–π stacking,12 fullerenes13 and carbon nanotubes14 have also been successfully employed in such catalytic reactions, significantly enhancing reactivity and selectivity. Recently, calixarenes and molecular cages, developed by Wang and coworkers, were also applicable to catalysing decarboxylative Mannich reactions in excellent yields with high selectivity.15
It is evident that anion–π interactions, owing to their unique surface activation mode, can exhibit excellent catalytic properties and advantages in certain reactions, thus attracting increasing attention from synthetic chemists. However, anion–π catalysis also faces several critical challenges. First, most catalysts possess a rigid planar or curved structure, which results in poor solubility, such as naphthalimide (NDI) and fullerene derivatives. Second, the strength of anion–π interactions is relatively weaker than that of other noncovalent bonds, thereby offering potential for the enhancement of catalytic performance. In addition, anion–π catalysis is susceptible to interference from counter anions, leading to antagonistic effects. Therefore, the development of novel anion–π catalysts is highly necessary.
Compared with anion–π catalysis (π-hole), relevant reports on σ–hole catalysis, e.g., halogen bonding (XB),16 chalcogen bonding (ChB)17 and pnictogen bonding (PnB),18 generally emerged earlier and have been widely applied in catalysing various types of organic reactions (Fig. 1B). Taking halogen bonding as an example, there are various publications describing XB-catalyzed halide abstractions,19 Michael additions,20 Diels–Alder cyclizations21 and enantioselective Mukaiyama aldol reactions,22etc. Since the aforementioned weak interactions also facilitate the catalytic process by stabilizing anionic transition states or intermediates, we herein attempt to combine π–hole interaction with σ–hole catalysis, aiming to enhance catalytic efficiency or selectivity through synergistic catalysis in this protocol (Fig. 1C).
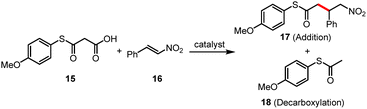
Based on the above analysis, in this study, we first designed and synthesized anion–π catalysts (1–8), halogen-bonding catalysts (9–12), anion–π–halogen-bonding cooperative catalysts (13) and control catalysts (14). The synthetic routes and characterization data can be found in the SI. The decarboxylative Michael addition reaction of malonic acid half thioester (MAHT) was selected as the model reaction for synergistic catalysis.
The catalytic activity was determined in a mixture of deuterated chloroform (CDCl3)/deuterated tetrahydrofuran (THF-d8) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 200 mM MAHT substrate 15 and 2.0 M enolate acceptor 16 at 20 °C (Table 1 and Fig. 3). First of all, a single anion–π interaction could promote the reaction, but exhibited a limited effect on improving the reaction selectivity, with A/D ratios of approximately 1.7 to 2.0, which was attributed to the restricted π-surface of the phenyl moiety (Table 1, entries 1 and 2). Although the improvement range was small, NH-type catalysts could indeed enhance the reaction selectivity, indicating that the potential hydrogen-bonding interactions could synergize with the anion–π interactions (Table 1, entries 3 and 4). Consistent with expectations, catalysts 5–8 with dual and triple anion–π interactions displayed better selectivity, resulting in A/D ratios ranging from 5.1 to 8.1 (Table 1, entries 5–8). Intriguingly, the reactivities for these transformations were relatively lower. For catalysts 7 and 8, it is presumably attributed to their poor solubility.
1) containing 200 mM MAHT substrate 15 and 2.0 M enolate acceptor 16 at 20 °C (Table 1 and Fig. 3). First of all, a single anion–π interaction could promote the reaction, but exhibited a limited effect on improving the reaction selectivity, with A/D ratios of approximately 1.7 to 2.0, which was attributed to the restricted π-surface of the phenyl moiety (Table 1, entries 1 and 2). Although the improvement range was small, NH-type catalysts could indeed enhance the reaction selectivity, indicating that the potential hydrogen-bonding interactions could synergize with the anion–π interactions (Table 1, entries 3 and 4). Consistent with expectations, catalysts 5–8 with dual and triple anion–π interactions displayed better selectivity, resulting in A/D ratios ranging from 5.1 to 8.1 (Table 1, entries 5–8). Intriguingly, the reactivities for these transformations were relatively lower. For catalysts 7 and 8, it is presumably attributed to their poor solubility.
| Catb | c(Cat)c | Sd | η (%) | A/Df | |
|---|---|---|---|---|---|
| a Reaction conditions: Substrates 14 (200 mM) and 15 (2.0 M) and catalyst (1–20 mol%), see columns Cat and c (Cat) in a solvent at 20 °C for 24 h, unless otherwise stated. b Catalysts, see Fig. 2. c Catalyst loading (mol%). d Solvent. e Conversion of 15 (%) based on 1H NMR analysis. f The ratio of addition (17) and decarboxylation (18) products. g TBANO3 was added as an inhibitor (1.0, 2.0 and 4.0 eq for entries 20, 21 and 22, respectively). | |||||
| 1 | 1 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1.7 |
| 2 | 2 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
82 | 2.0 |
| 3 | 3 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
91 | 3.7 |
| 4 | 4 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
91 | 2.9 |
| 5 | 5 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68 | 5.8 |
| 6 | 6 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
38 | 5.1 |
| 7 | 7 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
61 | 7.9 |
| 8 | 8 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48 | 8.1 |
| 9 | 9 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
94 | 1.9 |
| 10 | 10 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
96 | 2.6 |
| 11 | 11 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68 | 5.2 |
| 12 | 12 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
56 | 7.3 |
| 13 | 13 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 10.9 |
| 14 | 14 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 8.3 |
| 15 | 13 | 1 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
98 | 2.1 |
| 16 | 13 | 5 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
88 | 9.2 |
| 17 | 13 | 20 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 25.2 |
| 18 | 13 | 10 | CDCl3 | 84 | 11.3 |
| 19 | 13 | 10 | THF-d8 | 72 | 3.8 |
| 20g | 13 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
97 | 10.8 |
| 21g | 13 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
99 | 10.7 |
| 22g | 13 | 10 | CDCl3/THF-d8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
97 | 10.8 |
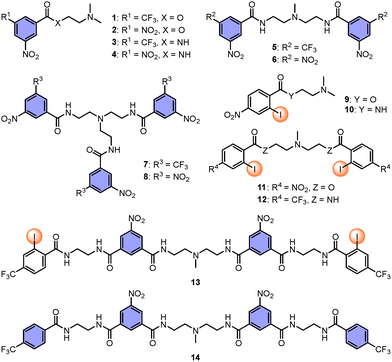 | ||
| Fig. 2 Structure of catalysts 1–14 made to elaborate on synergistic anion–π catalysis with halogen bonding. | ||
 | ||
| Fig. 3 (A) Proposed intermediate and transition states for the generation of addition (A) and decarboxylation (D) products. (B) Diagnostic peaks in the 1H NMR spectra of the product mixtures obtained with 15 (200 mM) and 16 (2.0 M) catalyzed by (a) 1 mol%, (b) 5 mol%, (c) 10 mol%, and (d) 20 mol% of 13; 3.07 ppm 17; and 2.38 ppm 18 (Table 1, entries 13–16). (C) Conversion (left, pink) and the A/D ratio (right, cyan) of the reaction in the presence of inhibitor TBANO3. | ||
In the halogen-bonding catalytic system, the selectivity was unsatisfactory with either single halogen-bonding catalysis or the cooperative catalysis of halogen bonding and hydrogen bonding (Table 1, entries 9 and 10). However, dual halogen-bonding catalysis with 11 and 12 resulted in deceleration of the reaction and increased selectivity, which could increase the A/D ratio of the reaction to 5.2 and 7.3, respectively (Table 1, entries 11 and 12). To our delight, the cooperative catalyst 13 exhibited optimal catalytic efficiency and selectivity, achieving not only quantitative yield but also an A/D ratio of 10.9 (Table 1, entry 13). The initial velocities were also determined from the plotting of concentration of products against time and linear (see Fig. S2 in the SI for details). Besides, catalyst 14 without the iodide groups was also designed and synthesized as a control of 13. Although the reaction yielded the target compounds quantitatively, the A/D ratio declined to 8.3 (Table 1, entry 14). The above results confirm that the synergistic effect between halogen-bonding catalysis and anion–π interactions is practically feasible and significant to maintain efficient conversion and high selectivity.
As a significant template, the benchmark reaction has been utilized to assess anion–π catalysts.23 A feasible mechanism was proposed based on the previous studies of the Matile group.6b The first reactive intermediate of the MAHT reaction exists in two tautomeric forms: the planar, charge-delocalized enol tautomer and the bent, charge-localized keto tautomer. The increasing π-acidity of aromatic surfaces can change the chemoselectivity of the reaction in favor of the biologically and chemically relevant enolate addition (A) product 17 compared to the simple but less relevant decarboxylation (D) product 18. Stabilized by multiple noncovalent interactions, the anionic intermediate (Int A) or transition state (TS-A) can undergo directional transformation to yield more addition product 17 (Fig. 3A).
In consideration of the effect of catalyst concentration, we also investigated the MAHT reaction under different catalyst loadings (Table 1 and Fig. 3B). The results showed that 1 mol% of 13 was sufficient for catalysing this reaction, affording the products in high to quantitative yields. However, significant differences were observed in the reaction selectivity (Table 1, entry 14). Catalyst at an extremely low concentration barely improved the A/D ratio. In contrast, when the loading of 13 exceeded 5 mol%, the catalytic selectivity increased remarkably (Table 1, entries 13 and 15). In addition, increasing the catalyst loading of 13 to 20 mol% improved the addition selectivity to A/D = 25.2 (Table 1, entry 15), which represents a prominent enhancement compared to the control catalyst triethylamine (TEA).12a
Further investigation revealed that the solvent is another crucial factor. When CDCl3 was used as the solvent, the reaction yield decreased slightly to 84%, while the selectivity increased marginally to A/D = 11.3 (Table 1, entry 17). In the case of THF-d8, despite the improved solubility of the catalyst, both the reaction yield and the A/D selectivity experienced a significant decline (Table 1, entry 18). The experimental results demonstrated that mixed solvents were significant and favourable for the assurance of both efficient conversions and high addition selectivity.
Given that anion–π interactions, halogen bonds, and hydrogen bonds all enable catalytic transformation through anion recognition, we attempted to inhibit this catalytic process using exogenous counter anions (Table 1, entries 19–21). Surprisingly, the aforementioned synergistic catalysis remained almost unaffected, even in the presence of 4.0 equivalents of inhibitor tetrabutylammonium nitrate (TBANO3), with both the yield and selectivity being comparable to those of the model reaction (Fig. 3C). This result indicates that synergistic catalysis can resist interference from external anions without compromising its intrinsic catalytic activity in contrast to single-interaction catalysis.
Inspired by the aforementioned results, we further attempted to apply the synergistic catalyst in several different reactions (Fig. 4). In the Michael reaction involving nonadjacent stereocenters,24 catalyst 13 exhibited relatively lower catalytic activity compared with the MAHT reaction. However, the catalytic behaviour differs from that of conventional alkaloid catalysts, yielding more disfavoured products (Fig. 4A and Fig. S12, S13). As for the Diels–Alder reaction of 22 and 23,9 the endo-24 product was obtained almost exclusively in the presence of 10 mol% of catalyst 13, without detectable exo-24 (Fig. 4B and Fig. S14 and S15). Notably, the synergistic strategy was also effective in catalyzing the [3+2] cycloaddition reaction,25 affording the bicyclic products 26 and 26d in excellent yield with 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. (Fig. 4C and Fig. S16, S17). These results further corroborated the concept of cooperative supramolecular catalysis.
1 d.r. (Fig. 4C and Fig. S16, S17). These results further corroborated the concept of cooperative supramolecular catalysis.
 | ||
| Fig. 4 Catalyst evaluation in other types of reactions: (A) the Michael addition reaction (focus on nonadjacent stereocenters), (B) the Diels–Alder reaction, and (C) the [3+2] cycloaddition reaction. | ||
In summary, a novel synergistic catalytic system, involving anion–π interactions and halogen bonding, was designed and successfully applied to the decarboxylative Michael addition reaction of MAHT substrate 15 and nitroolefin 16. Systematic studies have demonstrated that the synergistic catalysis based on π-holes and σ-holes is practically feasible. Reaction yields and selectivity by synergistic catalysis are superior to those of catalysis relying solely on anion–π interactions or halogen bonding. Furthermore, this catalytic system exhibits strong anti-interference ability against the exogenous nitrate inhibitor at high concentrations. With the readily available and highly soluble properties, the cooperative catalyst is expected to overcome the limitations of currently existing catalysts, thereby providing more options and possibilities for the development of anion–π catalysis. Further investigations are currently ongoing in our laboratory and will be available soon for all interested colleagues.
B. Shi and Q. Xu: synthesis, characterization and evaluation of catalysts; K. Fan and J. Zhang: synthesis of intermediates; H. Wang: data curation; X. Zhang: writing – review and editing, resources, project administration, funding acquisition, and conceptualization.
We are grateful for financial support from the Natural Science Foundation of Shannxi Province (2022JQ-144).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: general methods and synthetic procedures for all compounds. Characterisation data and NMR spectra for all new compounds. See DOI: https://doi.org/10.1039/d5cc05369b.Notes and references
- (a) D. Quiñonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa and P. M. Deyà, Angew. Chem., Int. Ed., 2002, 41, 3389 Search PubMed; (b) M. Mascal, A. Armstrong and M. D. Bartberger, J. Am. Chem. Soc., 2002, 124, 6274 CAS; (c) I. Alkorta, I. Rozas and J. Elguero, J. Am. Chem. Soc., 2002, 124, 8593 CrossRef CAS PubMed; (d) A. Vargas Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791 CrossRef CAS PubMed.
- (a) R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Motenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533 CrossRef CAS; (b) L. S. Adriaenssens, C. Esarellas, A. V. Jentzsch, M. Belmonte, S. Matile and P. Ballester, J. Am. Chem. Soc., 2013, 135, 8324 CrossRef CAS PubMed.
- J. Wang, X. Gu, P. Zhang, X. Huang, X. Zheng, M. Chen, H. Feng, R. T. Kwok, J. W. Y. Lam and B. Tang, J. Am. Chem. Soc., 2017, 139, 16974 CrossRef CAS PubMed.
- (a) Y. Chen, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Chem. Commun., 2011, 47, 8112 CAS; (b) D. X. Wang and M. X. Wang, Acc. Chem. Res., 2020, 53, 1364 CAS; (c) Q. P. Hu, H. Zhou, T. Y. Huang, Y.-F. Ao, D.-X. Wang and Q.-Q. Wang, J. Am. Chem. Soc., 2022, 144, 6180 CrossRef CAS PubMed.
- (a) D.-X. Wang, S.-X. Fa, Y. Liu, B.-Y. Hou and M.-X. Wang, Chem. Commun., 2012, 48, 11458 RSC; (b) S. Guha and S. Saha, J. Am. Chem. Soc., 2010, 132, 17674 CrossRef CAS; (c) Q. He, Y. Ao, Z. Huang and D. Wang, Angew. Chem., Int. Ed., 2015, 54, 11785 CrossRef CAS; (d) R. Xu, Q. Wang, Y. Ao, Z. Li, Z. Huang and D. Wang, Org. Lett., 2017, 19, 738 CrossRef CAS PubMed; (e) S. Saha, Acc. Chem. Res., 2018, 51, 2225 CrossRef CAS.
- (a) M. A. Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685 CrossRef CAS PubMed; (b) Y. Zhao, Y. Cotelle, L. Liu, J. Lopez-Andarias, A. Bornhof, M. Akamatsu, N. Sakai and S. Matile, Acc. Chem. Res., 2018, 51, 2255 CrossRef CAS; (c) X. Kan, H. Liu, Q. Pan, Z. Li and Y. Zhao, Chin. Chem. Lett., 2018, 29, 261 CrossRef CAS.
- (a) Y. Zhao, Y. Domoto, E. Orentas, C. Beuchat, D. Emery, J. Mareda, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2013, 52, 9940 CrossRef CAS PubMed; (b) Y. Zhao, C. Beuchat, Y. Domoto, J. Gajewy, A. Wilson, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2014, 136, 2101 CrossRef CAS PubMed.
- (a) Y. Zhao, S. Benz, N. Sakai and S. Matile, Chem. Sci., 2015, 6, 6219 RSC; (b) C. Wang, F. N. Miros, J. Mareda, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2016, 55, 14422 CrossRef CAS PubMed.
- L. Liu, Y. Cotelle, A.-B. Bornhof, C. Besnard, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2017, 56, 13066 CrossRef CAS PubMed.
- (a) X. Zhang, X. Hao, L. Liu, A.-T. Pham, J. López-Andarias, A. Frontera, N. Sakai and S. Matile, J. Am. Chem. Soc., 2018, 140, 17867 CrossRef CAS PubMed; (b) M. Á. Gutiérrez López, M.-L. Tan, A. Frontera and S. Matile, JACS Au, 2023, 3, 1039 CrossRef PubMed; (c) M. Paraja, X. Hao and S. Matile, Angew. Chem., Int. Ed., 2020, 59, 15093 CrossRef CAS.
- (a) M. Akamatsu, N. Sakai and S. Matile, J. Am. Chem. Soc., 2017, 139, 6558 CrossRef CAS; (b) M. Á. Gutiérrez López, R. Ali, M.-L. Tan, N. Sakai, T. Wirth and S. Matile, Sci. Adv., 2023, 9, eadj5502 CrossRef PubMed; (c) M. Á. Gutiérrez López, A. Marsalek, N. Sakai and S. Matile, Chem. Sci., 2025, 16, 11264 RSC.
- (a) A.-B. Bornhof, A. Bauzá, A. Aster, M. Pupier, A. Frontera, E. Vauthey, N. Sakai and S. Matile, J. Am. Chem. Soc., 2018, 140, 4884 CrossRef CAS PubMed; (b) M.-L. Tan, M. Á. Gutiérrez López, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2023, 62, e202310393 CrossRef CAS.
- (a) J. López-Andarias, A. Frontera and S. Matile, J. Am. Chem. Soc., 2017, 139, 13296 CrossRef; (b) J. López-Andarias, A. Bauza, N. Sakai, A. Frontera and S. Matile, Angew. Chem., Int. Ed., 2018, 57, 10883 CrossRef PubMed.
- (a) A. Jozeliūnaitė, S.-Y. Guo, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2024, 63, e202417333 Search PubMed; (b) A.-B. Bornhof, M. Vázquez-Nakagawa, L. Rodríguez-Pérez, M. Á. Herranz, N. Sakai, N. Martín, S. Matile and J. López-Andarias, Angew. Chem., Int. Ed., 2019, 58, 16097 CrossRef CAS.
- (a) D.-H. Tuo, W. Liu, X.-Y. Wang, X.-D. Wang, Y.-F. Ao, Q.-Q. Wang, Z.-Y. Li and D.-X. Wang, J. Am. Chem. Soc., 2019, 141, 1118 CrossRef CAS PubMed; (b) N. Luo, Y.-F. Ao, D.-X. Wang and Q.-Q. Wang, Angew. Chem., Int. Ed., 2021, 60, 20650 CrossRef CAS PubMed.
- (a) G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478 CrossRef CAS PubMed; (b) D. Jovanovic, M. P. Mohanan and S. M. Huber, Angew. Chem., Int. Ed., 2024, 63, e202404823 CrossRef CAS; (c) A. Liu and Y.-W. Yang, Coord. Chem. Rev., 2025, 530, 216488 CrossRef CAS.
- (a) N. Biot and D. Bonifazi, Coord. Chem. Rev., 2020, 413, 213243 CrossRef CAS; (b) Z. Zhao and Y. Wang, Acc. Chem. Res., 2023, 56, 608 CrossRef CAS; (c) G. Sekar, V. V. Nair and J. P. Zhu, Chem. Soc. Rev., 2024, 53, 586 RSC; (d) W. Wang, H. Zhu, S. Liu, Z. Zhao, L. Zhang, J. Hao and Y. Wang, J. Am. Chem. Soc., 2019, 141, 9175 CrossRef CAS.
- (a) S. Benz, A. I. Poblador-Bahamonde, N. Low-Ders and S. Matile, Angew. Chem., Int. Ed., 2018, 57, 5408 CrossRef CAS; (b) J. Y. C. Lim and P. D. Beer, Chemistry, 2018, 4, 731 CrossRef CAS; (c) S. Jena, J. Dutta, K. D. Tulsiyan, A. K. Sahu, S. S. Choudhury and H. S. Biswal, Chem. Soc. Rev., 2022, 51, 426 RSC; (d) G. Renno, D. Chen, Q.-X. Zhang, R. M. Gomila, A. Frontera, N. Sakai, T. R. Ward and S. Matile, Angew. Chem., Int. Ed., 2024, 63, e2024113 CrossRef PubMed.
- (a) F. Kniep, L. Rout, S. M. Walter, H. K. V. Bensch, S. H. Jungbauer, E. Herdtweck and S. M. Huber, Chem. Commun., 2012, 48, 9299 RSC; (b) S. H. Jungbauer and S. M. Huber, J. Am. Chem. Soc., 2015, 137, 12110 CrossRef CAS; (c) F. Heinen, E. Engelage, A. Dreger, R. Weiss and S. M. Huber, Angew. Chem., Int. Ed., 2018, 57, 3830 CrossRef CAS; (d) A. Dreger, E. Engelage, B. Mallick, P. D. Beer and S. M. Huber, Chem. Commun., 2018, 54, 4013 RSC.
- (a) P. Wonner, A. Dreger, L. Vogel, E. Engelage and S. M. Huber, Angew. Chem., Int. Ed., 2019, 58, 16923 CrossRef CAS; (b) R. L. Sutar and S. M. Huber, ACS Catal., 2019, 9, 9622 CrossRef CAS.
- S. H. Jungbauer, S. M. Walter, S. Schindler, L. Rout, F. Kniep and S. M. Huber, Chem. Commun., 2014, 50, 6281 RSC.
- R. L. Sutar, E. Engelage, R. Stoll and S. M. Huber, Angew. Chem., Int. Ed., 2020, 59, 6806 CrossRef CAS PubMed.
- (a) D.-X. Wang and M.-X. Wang, Chimia, 2011, 65, 939 CrossRef CAS PubMed; (b) P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435 CrossRef CAS PubMed; (c) H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894 CrossRef CAS; (d) M. M. Watt, M. S. Collins and D. W. Johnson, Acc. Chem. Res., 2013, 46, 955 CrossRef CAS.
- (a) Y. Wang, X. Liu and L. Deng, J. Am. Chem. Soc., 2006, 128, 3928–3930 CrossRef CAS; (b) X. Zhang, L. Liu, J. López-Andarias, C. Wang, N. Sakai and S. Matile, Helv. Chim. Acta, 2018, 101, e1700288 CrossRef.
- (a) M. Rueping, A. Kuenkel and R. Frohlich, Chem. – Eur. J., 2010, 16, 4173 CrossRef CAS; (b) L. Liu, Y. Cotelle, J. Klehr, N. Sakai, T. R. Ward and S. Matile, Chem. Sci., 2017, 8, 3770 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |