Boosting methane dry reforming via enhanced CO2 adsorption over TiN-supported Ni catalysts
Zhuoshi
Zheng†
,
Yu
Zhang†
,
Yanxi
Zhao
 *,
Chengchao
Liu
,
Aihua
Lin
,
Yuhua
Zhang
and
Jinlin
Li
*,
Chengchao
Liu
,
Aihua
Lin
,
Yuhua
Zhang
and
Jinlin
Li
 *
*
Key Laboratory of Catalysis and Energy Materials Chemistry of Ministry of Education, Hubei Key Laboratory of Catalysis and Materials Science, South-Central Minzu University, Wuhan 430074, China. E-mail: zhaoyx@scuec.edu.cn; jinlinli@aliyun.com
First published on 26th November 2025
Abstract
Herein, titanium nitride (TiN) is employed as a support for nickel (Ni) catalysts in the dry reforming of methane. TiN exhibits strong metal–support interactions that remarkably enhance Ni dispersion. In addition, TiN possesses strong CO2 and H2 adsorption properties and electron-donating capability. Collectively, these properties inhibit carbon buildup and enhance catalytic reactivity. A synergistic interaction with CaO, a secondary defence layer, dramatically boosts catalyst stability. At a similar initial conversion rate, the 5Ni–3CaO/TiN catalyst showed outstanding performance, achieving CH4 and CO2 conversion rates of 81.6% and 87.9%, respectively. The deactivation rates of DCH4 and DCO2 were 0.018 h−1 and 0.028 h−1, respectively, in the 20-hour stability test.
CH4 and CO2 are two major greenhouse gases. Therefore, the efficient utilisation and conversion of these gases into valuable chemicals or clean energy sources is a current hotspot in energy and environmental research.1 Dry reforming of methane (DRM) is a highly promising technology for converting CH4 and CO2 into syngas (H2 and CO), a vital feedstock for producing methanol, Fischer–Tropsch synthetic oil and other chemicals. However, DRM is highly exothermic and occurs under extreme conditions (generally requires temperatures of 700–1000 °C) and suffers from problems such as catalyst deactivation, thus limiting its industrial application.2 Among metals with DMR activity, nickel-based catalysts are promising candidates owing to their high activity and low cost. However, they are prone to sintering and carbon accumulation at high temperatures, resulting in decreased activity and low stability. Therefore, developing nickel (Ni)-based catalyst systems that are highly active and stable has become a key area of research.3 One of the preferred strategies is the selection and modification of the support. For example, Xiao et al. exploited the strong hydrogen spillover effect of HZSM-5 to promote CO2 reduction and enhance the performance of DRM.4 Wei et al. improved the stability of DRM by tuning the synergistic effect at the interface of the Ir@CeO2−x catalyst.1 Luo et al. engineered a dual-constraint microenvironment for Ni-based catalysts—combining spatial confinement and metal–hydroxylated carrier anchoring. It dramatically enhanced DMR stability.5
Common supports used in DMR include SiO2, γ-Al2O3, MgO, CeO2, ZnO, etc. SiO2-supported catalysts possess high initial activity but weak metal–support interactions (MSI), which results in poor stability. γ-Al2O3-supported catalysts have stronger MSI, which help them form and stabilise small Ni nanoclusters, and improve catalyst stability; however, the activity is lower. MgO exhibits even stronger MSI than γ-Al2O3, along with strong alkalinity and better CO2 capture ability. Consequently, MgO-supported catalysts are more active and stable than other catalysts. Therefore, enhancing CO2 adsorption by incorporating promoters, such as alkali and alkaline earth metals oxide as well as rare earth metals oxide, into the supports can effectively improve catalytic performance.6,7 CaO is one of the commonly used CO2 adsorbents, characterized by its low cost, easy availability, and high efficiency. It is widely used in calcium looping processes for CO2 capture and is increasingly being integrated into DRM systems.8 TiN is a semiconducting ceramic material which exhibits stronger MSI and CO2 adsorption capacity than traditional supports. For example, Esposito et al.'s studies suggest that Ni forms stronger MSI with TiN than with TiO2.9 TiN also interacts strongly with metals such as Ru, Pt, Fe and Co, which can considerably improve their dispersion and enhance stability.10–12 Adding TiN to Ni/SBA-15 catalysts significantly increases their CO2 adsorption capacity. TiN-supported In, Pt, and Cu–Ni catalysts have demonstrated outstanding performance in the hydrogenation of CO2 to produce valuable products.5,13,14 In this work, we prepared 5Ni/TiN and 5Ni/TiO2 catalysts via the impregnation method using TiN as support and further introduced CaO to investigate their synergistic enhancement of CO2 adsorption and electron transfer.
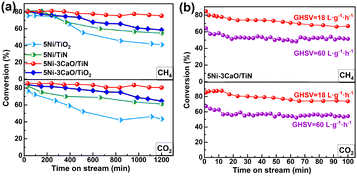
The DRM performance of these catalysts was evaluated at 700 °C (Fig. 1a). The average activity and stability of the 5Ni/TiN catalyst exceed those of the 5Ni/TiO2 catalyst. The stability of the 5Ni–3CaO/TiN and 5Ni–3CaO/TiO2 catalysts was further enhanced with the addition of CaO. The 5Ni–3CaO/TiN catalyst showed outstanding performance with CH4 and CO2 conversion rates of 81.6% and 87.9%, respectively (Table S1). The deactivation rates of DCH4 and DCO2 were the lowest, being 0.018 h−1 and 0.028 h−1, respectively, in the stability test at 20 h (Fig. S1). The H2/CO ratio remained close to 1.0 throughout the reaction (Fig. S2), indicating minimal side reactions.15 Conversely, the H2/CO ratio of the 5Ni/TiO2 catalyst first increased and then decreased. In the initial stage (before 400 min), the conversion of CH4 was higher than that of CO2, which indicated that the catalyst was more active in methane cracking and started producing carbon deposits. No change of this kind was observed in the 5Ni–3CaO/TiO2 catalyst following the addition of CaO. In the later stage, the water–gas shift reaction was enhanced and the selectivity of hydrogen decreased. The long-term stability of the 5Ni–3CaO/TiN catalyst was evaluated over 100 h on stream, as presented in Fig. 1b. At GHSV values of 18 and 60 L gcat−1 h−1, the CO2 conversions (from initial to final values) were 85–73% and 67–54%, respectively. Compared with catalysts featuring an identical nickel loading and reaction temperature, such as Ni–MgO/Y2TiO7, Ni/MgO–TiO2, Ni/Al2O3–TiO2, Ni/TiO2–Al2O3, and Ni–Ce/TiO2–Al2O3 (Table S2), the 5Ni–3CaO/TiN catalyst exhibits outstanding activity and long-term reaction stability.
CH4-TPD analysis (Fig. 2a) shows that the methane desorption temperature for the 5Ni/TiN (161.1 °C) catalyst is lower than that for the 5Ni/TiO2 catalyst (258.6 °C). With the addition of CaO, the desorption temperature shifts to a lower temperature. The lowest temperature is observed for the 5Ni–3CaO/TiN catalyst (133.6 °C), which is lower than for the 5Ni–3CaO/TiO2 catalyst (144.3 °C). The high desorption temperature of the 5NiO/TiO2 catalyst indicates strong interactions between methane and the catalyst. This leads to excessive methane cracking and an increased H2/CO ratio at the onset of the reaction. Ultimately, this resulting in enhanced carbon accumulation on the catalyst and reduced stability.
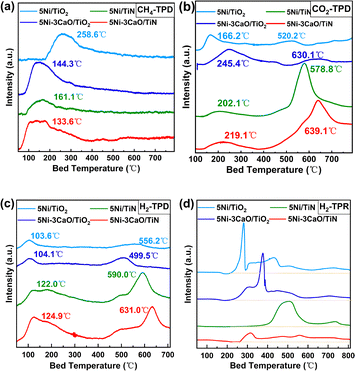 | ||
| Fig. 2 (a) CH4-TPD, (b) CO2-TPD, (c) H2-TPD and (d) H2-TPR profiles of the fresh 5Ni/TiN, 5Ni/TiO2, 5Ni–3CaO/TiO2 and 5Ni–3CaO/TiN catalysts. | ||
CO2-TPD (Fig. 2b and Fig. S3) reveals that the 5Ni/TiN catalyst exhibited a stronger CO2 adsorption capacity than the 5Ni/TiO2 catalyst. The CO2-TPD profile of the 5Ni/TiN catalyst displays two notable CO2 desorption peaks. The low-temperature desorption peak (<350 °C) corresponds to the desorption peak of CO2 adsorbed at the weakly basic and moderately strongly basic sites of the catalyst. The high-temperature desorption peak (>400 °C) corresponds to the desorption peak of CO2 adsorbed at the strongly basic sites. These strongly basic sites originate from the Ti–NH1–2 formed during the reduction process (Fig. S4).5,16 The CO2 desorption temperature of the 5Ni/TiO2 catalyst at low temperature was 166.2 °C, which was lower than that of the 5Ni/TiN catalyst (202.1 °C). Following the addition of CaO, the desorption temperatures of the 5Ni–3CaO/TiN and 5Ni–3CaO/TiO2 catalysts were further increased to 219.1 °C and 245.4 °C, respectively. The 5Ni/TiN catalyst showed a higher desorption peak in the high-temperature region and a higher desorption temperature than that of the 5Ni/TiO2 catalyst. It indicates that the 5Ni/TiN catalyst has stronger basicity and a greater number of basic sites than that in the 5Ni/TiO2 catalyst (Table S3). The addition of CaO further enhanced the basicity of the 5Ni–3CaO/TiN catalyst and slightly increased the number of basic sites. On the 5Ni/TiN and 5Ni–3CaO/TiN catalysts, a significant portion of CO2 is adsorbed onto the support. By comparison, CO2 is adsorbed more strongly onto the support of the 5Ni/TiN catalyst than onto that of the 5Ni/TiO2 catalyst. The enhanced CO2 adsorption capacity, as evidenced by CO2-TPD (Fig. 2b), is thought to contribute to CO2 activation and reduce the activation energy of its dissociation. This is expected to provide additional reactive oxygen species, which could effectively inhibit carbon buildup,17 which could be a reason for the observed improvement in catalyst stability. TG-MS analysis of the spent 5Ni–3CaO/TiN catalyst in a CO2 atmosphere (Fig. S5) reveals significant weight loss beginning at 700 °C, as CO2 reacts with carbon deposits on the spent catalyst to produce CO. Weight loss reaches approximately 30% at 800 °C. By contrast, under an N2 atmosphere, the weight loss rate at 800 °C was only 4%, indicating that CO2 plays a significant role in removing coke deposits.
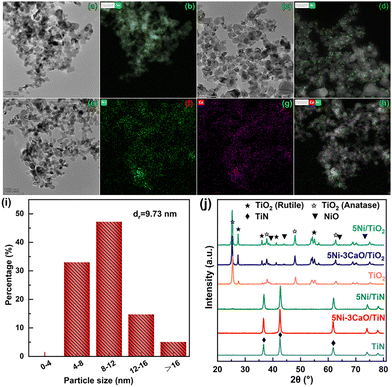
Two sets of H2 desorption peaks are presented in the H2-TPD (Fig. 2c) profiles, with the low-temperature desorption peaks (<300 °C) attributed to H2 chemisorbed on the surface of the Ni nanoparticles18 and the high-temperature desorption peaks (>400 °C) formed by H2 spillover onto the catalysts.19 The total amounts of H2 adsorbed by the four catalysts were 31.984, 43.223, 68.279 and 88.282 µmol g−1 (Table S4). The H2 desorption amount of the 5Ni/TiN catalyst was markedly higher than that of the 5Ni/TiO2 catalyst, while the H2 desorption temperature and desorption amount of the 5Ni–3CaO/TiN catalyst were further increased. Evidently, the TiN-supported catalysts possessed stronger H2 spillover ability, as determined by measuring the H2 spillover ability of the 5Ni/TiN and 5Ni/TiO2 catalysts using WO3 as a chromogenic agent (Fig. S6). This helps eliminate carbon deposits and enhances the stability of the 5Ni–3CaO/TiN catalyst. TG analysis (Fig. S5b) and calcination experiments (Fig. S7) under a H2 atmosphere of the spent 5Ni–3CaO/TiN catalyst indicated that H2 facilitates the removal of carbon deposits. The calculated nickel dispersions of the 5Ni/TiN and 5Ni–3CaO/TiN catalysts were 16.0% and 20.7%, respectively, which were considerably higher than those of the 5Ni/TiO2 (7.5%) and 5Ni–3CaO/TiO2 (10.1%) catalysts. This was further confirmed by TEM and EDS mapping (Fig. 3 and Fig. S8). The Ni species in the 5Ni/TiO2 and 5Ni–3CaO/TiO2 catalysts showed a granular distribution, with a particle size of ∼9.73 nm and ∼8.23 nm, respectively (Fig. 3c, d, I and Fig. S8b, e). By contrast, the Ni as well as the calcium species were uniformly and highly dispersed on the TiN surface in the 5Ni/TiN and 5Ni–3CaO/TiN catalysts, with much smaller Ni cluster sizes (Fig. 3a, b and e–h). The XRD patterns of the 5Ni/TiO2 and 5Ni–3CaO/TiO2 catalysts (Fig. 3j) show diffraction peaks at 37.3°, 43.3°, 62.9° and 75.4°, corresponding to the characteristic peaks of the cubic NiO phase (PDF#47-1049). However, no obvious characteristic diffraction peaks of NiO were detected on either the 5Ni/TiN or 5Ni–3CaO/TiN catalysts.
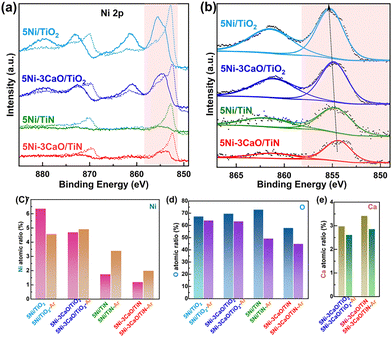
H2-TPR (Fig. 2d) was used to investigate the interaction between the metal and the support. The 5Ni/TiO2 catalyst exhibited a sharp reduction peak at ∼275 °C, suggesting that NiO particles are larger and that larger Ni particles form upon reduction. The 5Ni/TiN catalyst showed a broader reduction peak at temperatures between 400–500 °C. This suggests that the interaction between Ni and the TiN support is stronger than that between Ni and TiO2. Ni species have a smaller particle size and are more difficult to reduce. Following the introduction of CaO, the 5Ni–3CaO/TiN catalyst showed an insignificant reduction peak at high temperatures. The reduction peak at temperatures between 250 °C and 350 °C was attributed to the addition of CaO, which is evident from the comparison of the H2-TPR profile of the 5Ni–3CaO/TiO2 catalyst. The introduction of CaO may partially cover the surface of the highly dispersed small-sized NiO nanoclusters, which inhibited the reduction of NiO. Therefore, the catalyst was characterized by XPS to investigate the chemical state of Ni and its elemental surface composition before and after Ar+ etching (Fig. 4).
Following Ar+ etching, the Ni 2p peak area increased substantially for the 5Ni/TiN, 5Ni–3CaO/TiN and 5Ni–3CaO/TiO2 catalysts, while it changed little for the 5Ni/TiO2 catalyst (Fig. 4a). This indicates that the finely dispersed NiO particles on the 5Ni/TiN and 5Ni–3CaO/TiN surfaces possess strong adsorption capacity, resulting in coverage by airborne oxygenated species (and CaO in the case of 5Ni–3CaO/TiN and 5Ni–3CaO/TiO2). Fig. 4(c)–(e) presents the variation in Ni, O and Ca contents on the catalyst surface before and after etching. The content of Ni and O elements decreased after etching of the 5Ni/TiO2 catalyst. Contrastingly, the Ni content increased from 1.7% to 3.4% and the O content decreased from 72.9% to 49.2% for the 5Ni/TiN catalyst. The Ni content of the 5Ni–3CaO/TiN catalyst increased from 1.2% to 2.0%, and the O content decreased from 57.9% to 44.9%, while the Ca content decreased from 3.4% to 2.9%, and the combined Ca and O content dropped from 61.3% to 47.8%. This analysis shows that the 5Ni/TiN catalyst has a strong adsorption capacity, and the surface is covered by oxygenated species following exposure to air. The 5Ni–3CaO/TiN catalyst is partially covered by CaO, which collectively inhibits the reduction of NiO. These CaO particles will adsorb CO2 during the reaction process, which will further enhance the CO2 concentration on the Ni particle surface and improve the CO2 conversion rate. Additionally, they can also restrict the migration and agglomeration of nickel particles, thereby enhancing the long-term reaction stability of the 5Ni–3CaO/TiN catalyst. The Ni 2p binding energy of the 5Ni/TiN catalyst (Fig. 4b) is lower than that of the 5Ni/TiO2 catalyst, which indicates that TiN has a stronger electron-donating capability. Upon adding Ca, the Ni 2p binding energy of the 5Ni–3CaO/TiN catalyst further shifts to a lower binding energy. This is caused by the CaO coating on the surface of the NiO and TiN that provides electrons to the Ni elements, either directly or indirectly via the support. An increased electron density on the Ni elements weakens CH4 adsorption but enhances CO2 adsorption, thereby inhibiting graphitic carbon buildup and improving stability.6
The TG curves of the spent catalysts (Fig. S9a) show that the percentage mass losses for the 5Ni/TiO2, 5Ni–3CaO/TiO2, 5Ni/TiN and 5Ni–3CaO/TiN catalysts are 74.9%, 71.1%, 56.5% and 43.3%, respectively. The 5Ni/TiN catalyst showed lower carbon accumulation than that in the 5Ni/TiO2 catalyst. Adding Ca to the 5Ni/TiO2 and 5Ni/TiN catalysts further reduced carbon accumulation. The IG/ID value (Fig. S9b) of 1.11 for the 5Ni/TiN catalyst indicates a lower degree of graphitisation of the carbon accumulated on the surface than that of the 5Ni/TiO2 catalyst (IG/ID value of 1.48). The IG/ID value of 0.79 for the 5Ni–CaO/TiN catalyst is the lowest. The XRD patterns (Fig. S10) of the spent catalysts show that the diffraction peaks are attributed to graphitic carbon in the (002) and (101) facets, which appear at 26.2° and 44.4°(PDF#75-1621). The graphitic carbon diffraction peak intensities of the spent 5Ni/TiN and 5Ni–3CaO/TiN catalysts were weaker than that of the spent 5Ni/TiO2 and 5Ni–3CaO/TiO2 catalysts. This indicates that less graphitic carbon forms on the catalysts supported by TiN. The TEM image of the spent 5Ni/TiO2 and 5Ni–3CaO/TiO2 catalysts (Fig. S11a and d) shows a large number of coarse carbon nanotubes (CNTs) and more severely sintered NiO particles. Contrastingly, the CNTs in the spent 5Ni/TiN and 5Ni–3CaO/TiN catalysts are relatively fewer in number (Fig. S11b and c). Although NiO particles are present in the catalysts, they are relatively small in size (Fig. S11e–h).
In summary, TiN exhibits strong MSI that remarkably improve the dispersion of Ni species. The electron-donating ability of TiN increases the electron density of Ni, which together with the strong basicity of TiN improves the adsorption of CO2 and H2 and decreases the adsorption of CH4. Collectively, these effects inhibit carbon deposition and improve the stability. The addition of CaO further enhanced the activity and stability of DMR. As well as increasing CO2 adsorption to a certain extent, CaO also restricts the migration and agglomeration of Ni particles to some extent. These effects improve the catalyst's stability collectively. The results of this study provide a new strategy for developing Ni-based DMR catalysts with higher catalytic reaction performance.
This work was supported by the National Key Research and Development Program of China (2022YFB4101201), the National Natural Science Foundation of China (U22A20394, 21902187, 21203253), the Fundamental Research Funds for the Central Universities of South-Central Minzu University (CZZ24008), the Hubei Provincial Department of Education Research Program (B2024259), and Hubei Provincial Key Laboratory of Green Materials for Light Industry (202007A03).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: associated with this article contains additional supporting data, figures, and experimental details. See DOI: https://doi.org/10.1039/d5cc05014f.Notes and references
- H. Wang, G. Cui, H. Lu, Z. Li, L. Wang, H. Meng, J. Li, H. Yan, Y. Yang and M. Wei, Nat. Commun., 2024, 15, 3765 CAS.
- D. He, Y. Zhang, T. Li, D. Chen, H. Wang, L. Zhang, J. Liu, X. Cao, J. Lu and Y. Luo, Adv. Funct. Mater., 2025, 35, 2412895 CAS.
- J. Tian, B. Ma, S. Bu, Q. Yuan and C. Zhao, Chem. Commun., 2018, 54, 13993–13996 CAS.
- Q. Zhu, H. Zhou, L. Wang, L. Wang, C. Wang, H. Wang, W. Fang, M. He, Q. Wu and F.-S. Xiao, Nat. Catal., 2022, 5, 1030–1037 CAS.
- Y. Zou, C. Mao, M. Xu, C. Xing, R. Wang, G. A. Ozin and L. Ling, Nat. Commun., 2024, 15, 10604 CrossRef CAS PubMed.
- D. Guo, M. Li, Y. Lu, Y. Zhao, M. Li, Y. Zhao, S. Wang and X. Ma, ACS Catal., 2022, 12, 316–330 CrossRef CAS.
- L. Meng, Y. Jia and S. Wu, Chem. Commun., 2025, 61, 2301–2304 RSC.
- S. Sun, Y. Wang, Y. Xu, H. Sun, X. Zhao, Y. Zhang, X. Yang, X. Bie, M. Wu, C. Zhang, Y. Zhu, Y. Xu, H. Zhou and C. Wu, Appl. Catal., B, 2024, 348, 123838 CrossRef CAS.
- S. H. Gage, C. Ngo, V. Molinari, M. Causà, R. M. Richards, F. S. Gentile, S. Pylypenko and D. Esposito, J. Phys. Chem. C, 2018, 122, 339–348 CrossRef CAS.
- J. Zhao, R. Urrego-Ortiz, N. Liao, F. Calle-Vallejo and J. Luo, Nat. Commun., 2024, 15, 6391 CAS.
- S. Yang, J. Kim, Y. J. Tak, A. Soon and H. Lee, Angew. Chem., Int. Ed., 2016, 55, 2058–2062 CAS.
- Y. Zhao, D. Wu, C. Long, C. Liu, Y. Liang, S. Yang, Z. Li, Y. Zhang, X. Yi, J. Li and H. Xiong, ACS Catal., 2025, 15, 16165–16175 CAS.
- H. Pan and C. J. Barile, Sustainable Energy Fuels, 2020, 4, 5654–5664 RSC.
- N. T. Nguyen, T. Yan, L. Wang, J. Y. Y. Loh, P. N. Duchesne, C. Mao, P.-C. Li, F. M. Ali, M. Xia, M. Ghoussoub, N. P. Kherani, Z.-H. Lu and G. A. Ozin, Small, 2020, 16, 2005754 CrossRef CAS.
- H. Kaneko, Y. Cho, T. Sugimura, A. Hashimoto, A. Yamaguchi and M. Miyauchi, Chem. Commun., 2024, 60, 10406–10409 RSC.
- C. Mao, J. Wang, Y. Zou, Y. Shi, C. J. Viasus, J. Y. Y. Loh, M. Xia, S. Ji, M. Li, H. Shang, M. Ghoussoub, Y.-F. Xu, J. Ye, Z. Li, N. P. Kherani, L. Zheng, Y. Liu, L. Zhang and G. A. Ozin, J. Am. Chem. Soc., 2023, 145, 13134–13146 CrossRef CAS PubMed.
- A. M. Manabayeva, P. Mäki-Arvela, Z. Vajglová, M. Martinéz-Klimov, O. Yevdokimova, A. Peuronen, M. Lastusaari, T. Tirri, S. A. Tungatarova, T. S. Baizhumanova, K. Kassymkan, G. N. Kaumenova, M. Zhumabek, D. A. Zhumadullaev, D. Shoganbek and D. Y. Murzin, Catal. Today, 2025, 453, 115261 CrossRef CAS.
- J. Liu, C. Li, F. Wang, S. He, H. Chen, Y. Zhao, M. Wei, D. G. Evans and X. Duan, Catal. Sci. Technol., 2013, 3, 2627–2633 RSC.
- J. Ren, H. Li, Y. Jin, J. Zhu, S. Liu, J. Lin and Z. Li, Appl. Catal., B, 2017, 201, 561–572 CrossRef CAS.
Footnote |
| † These authors are co-first authors of this work. |
| This journal is © The Royal Society of Chemistry 2026 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: