Electroreduction cross-coupling of thiosulfonates with (hetero)aryl boronic acids to access thioethers
Yaqin
Zhou
a,
Jiang
Lei
b,
Meimei
Chen
a,
Chi
Zhang
a,
Zhiheng
Zhao
a and
Lijun
Gu
 *a
*a
aState Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou 550025, China. E-mail: gulijun2005@126.com
bZhejiang Apeloa Jiayuan Pharmaceutical Co. Ltd., Dongyang, Zhejiang 322118, P. R. China
First published on 1st December 2025
Abstract
Herein, we present a robust electrochemically driven, 1-iodonaphthalene-enabled cross-coupling of thiosulfonates with arylboronic acids for the synthesis of thioether molecules. This reaction is characterized by exceptional selectivity, gentle reaction conditions, a broad substrate scope, and significant functional group tolerance that extends to late-stage modifications of biologically relevant molecules.
Organosulfur compounds have attracted tremendous attention due to their extensive applications in agrochemicals, pharmaceuticals, and materials science.1 Thioethers, in particular, possess a high degree of structural diversity and exhibit a variety of biological activities, including anti-Alzheimer, anti-HIV, and anti-viral properties. They are also used as herbicides, ligands, and synthetic building blocks.2 Given their structural diversity and remarkable biological functions, the synthesis of thioethers has been and continues to be a focal point of research interest for synthetic organic chemists.
General methodologies for thioether synthesis typically involve transition metal-catalyzed coupling reactions between thiolation reagents (such as thiols, disulfides, thiol metal salts, sulfenyl chlorides, and sulfonyl compounds) and halides or non-halide reagents (Fig. 1a).3,4 However, the reliance on transition metals, strong acids or bases, stoichiometric oxidants or reductants, and specific ligands remains an impediment to these methods. Alternatively, such compounds could also be synthesized through cross coupling of radical precursors with thiolation reagents under visible-light irradiation (Fig. 1b).5 Nevertheless, these photo-induced methods face some limitations, such as the necessity for prefunctionalized starting materials, bases or acids and a narrow substrate scope resulting in somewhat restricted synthetic applications. Furthermore, the limited availability of thiols significantly constrains the structural diversity of thioether products. Among all the aforementioned strategies, there is an absence of a general modular approach capable of constructing aryl–aryl, aryl–alkyl, and aryl–alkynyl thioethers through a single set of catalysts and substrates without necessitating transition metal catalysts, ligands, external redox agents, acids, or bases. Complementary catalytic methods that facilitate the efficient synthesis of structurally diverse thioethers are highly sought after.
As an alternative and environmentally friendly technique, electrochemical organic transformation has gained significant attention compared to conventional synthetic methods.6 By utilizing inexpensive and sustainable electricity as an electron source, this approach circumvents the need for stoichiometric amounts of oxidants or reductants, thereby eliminating waste production. Generally, electrochemical organic synthesis can be categorized into direct or indirect electrolysis.7 The past decade has witnessed rapid advancements in numerous direct electrolysis technologies for the synthesis of structurally complex molecules.8 However, due to the adsorption of reactants onto electrode surfaces, certain sensitive molecules or functional groups may not withstand high potentials, thus limiting the applicability and substrate scope of these reactions. Consequently, indirect electrolysis driven by electrochemical redox mediators presents a promising alternative for producing redox-active species. With the rapid development of indirect electrolysis in organic synthesis, a variety of electrochemical mediators, such as phthalimide N-oxyl compounds,9 halogens,10 and transition metals,11 have been well developed in recent years. Compared with well-established mediator-initiated electrooxidation processes, organo-initiated electroreduction reactions remain relatively underexplored.12 Over the last few years, some applications involving organo-mediator-enabled electroreduction reactions have been developed towards the construction and modification of complex organic molecules.13 Since organo-mediator-enabled electroreduction for C–S bond formation to access thioethers has not been reported to date, we herein report the successful development of an electroreduction cross-coupling of thiosulfonates with boronic acids for the synthesis of thioethers, using 1-iodonaphthalene as an efficient organic mediator (Fig. 1c). This method is both convenient and efficient, demonstrating compatibility with various functional groups without requiring a transition metal catalyst, ligand, external redox, acid or base.
The electroreduction cross-coupling reaction between p-phenylboronic acid 1a and S-phenyl benzenesulfonothioate 2a was chosen as a model system to optimize the electrolysis conditions. Following extensive screening of the reaction parameters, we were pleased to find that the desired coupling product 3aa could be obtained in 81% yield alongside a by-product yield of 13% phenyl disulfide when the model reaction was performed using a combination of 30 mol% 1-iodonaphthalene and 2 equivalents n-Bu4NBF4 in MeCN solvent under constant current at 30 mA for 1.5 hours in an undivided cell (Table 1, entry 1). Screening of solvent effects demonstrated MeCN as the optimal choice (Table 1, entry 2). The use of 0.3 equivalents of 1-iodonaphthalene proved optimal, as decreased yields were observed when smaller amounts were employed (Table S4, see the SI). In addition, alternative organo-mediators including naphthalene, 1-methyl-naphthalene, 1-bromonaphthalene, and 1-naphthyl-benzene were examined, but demonstrated inferior efficacy compared to that achieved with 1-iodonaphthalene (Table S5, see the SI). Either decreasing or increasing the electric current led to lower yields (Table 1, entry 3 and 4). Other supporting electrolytes, such as n-Bu4NPF6, n-Bu4NI, and Et4NBF4, were also evaluated; however, they showed less efficiency than n-Bu4NBF4 (Table 1, entry 5). Alterations in reaction time—whether shortening or prolonging, did not improve the desired product yield either (Table 1, entry 6 and 7). Furthermore, changing the cathode or anode electrode to alternative materials resulted in a reduced product yield (Table 1, entry 8 and 9). Unsurprisingly, both 1-iodonaphthalene and electrical current are necessary for this transformation (Table 1, entries 11 and 12). The gram-scale reaction was demonstrated by the facile preparation of 0.68 g of product 3aa, highlighting the robustness of this method (Table 1, entry 13).
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: GF anode (5.25 × 0.8 × 0.2 cm3), Pb cathode (5.25 × 0.8 × 0.2 cm3), 1a (0.2 mmol), 2a (0.26 mmol), 1-iodonaphthalene (30 mol%), n-Bu4NBF4 (0.4 mmol), MeCN (4.0 mL), undivided cell, r.t., 30 mA, and 1.5 h. b Isolated yield. c For details, see the SI. GF = graphite felt. | ||
| 1 | None | 81 |
| 2 | DMF, MeOH, DMSO instead of MeCN | 0, 14, 23 |
| 3 | 25 mA instead of 30 mA | 46 |
| 4 | 35 mA instead of 30 mA | 54 |
| 5 | n-Bu4NPF6, n-Bu4NI, Et4NBF4 instead of n-Bu4NBF4 | 58, 41, 60 |
| 6 | 1.2 h instead of 1.5 h | 62 |
| 7 | 2 h instead of 1.5 h | 81 |
| 8 | C(+)|Pb(−) instead of GF(+)|Pb(−) | 22 |
| 9 | GF(+)|Ni(−) instead of GF(+)|Pb(−) | 0 |
| 10 | Phenylboronic acid pinacol ester instead of phenylboronic acid | 44 |
| 11 | Without 1-iodonaphthalene | 0 |
| 12 | No current | 0 |
| 13c | Large-scale reaction | 63 |
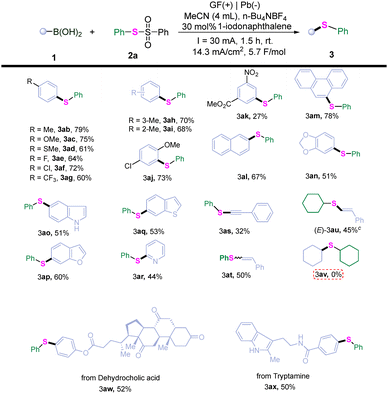
With the optimal reaction conditions established, the substrate scope of this electroreduction cross-coupling reaction was investigated. As summarized in Table 2, a variety of (hetero)arylboronic acids were compatible with the reaction. The desired thioethers were formed in moderate to good yields in the presence of both electron-rich groups (3ab–3ad) and electron-deficient groups (3ae–3ag) at the para position on the phenyl ring of the arylboronic acid substrates. Even sterically hindered phenylboronic acids could be readily converted into their corresponding products (3ah, 3ai). The application of poly-substituted arylboronic acid gave the desired products in 73% yield (3aj). In contrast, an arylboronic acid bearing two strong electron-withdrawing groups on the phenyl ring produced a product with only a yield of 27% (3ak). Arylboronic acids featuring fused rings were also tolerated during this transformation (3al, 3am). Furthermore, heteroaryl-substituted substrates successfully underwent this electroreduction cross-coupling reaction producing the desired thioethers in good to moderate yields (3an–3ar).
To further explore the potential of this electroreduction cross-coupling reaction, 2-phenyl-1-ethynylboronic acid pinacol ester and (Z/E)-styrylboronic acid were investigated, respectively. Encouragingly, aryl-alkynyl (3as) and aryl-alkenyl (3at) thioethers were obtained in 32% and 50% yield, respectively. However, when reacting (E)-styrylboronic acid with S-cyclohexyl benzenesulfonothioate under standard conditions, no alkyl–alkenyl thioether product (E-3au) was obtained. Notably, alkyl–alkenyl thioether (E-3au) could be generated in 45% yield by substituting 1-iodonaphthalene with 9,10-anthracenedicarbonitrile (for details, see the SI). Unfortunately, no alkyl–alkyl thioether product (3av) was produced under the standard reaction conditions. To showcase the application of this methodology, two arylboronic acids featuring complex scaffolds were then subjected to the standard reaction conditions. The desired thioethers (3aw, 3ax) were successively achieved in 52% and 50% yields, respectively.
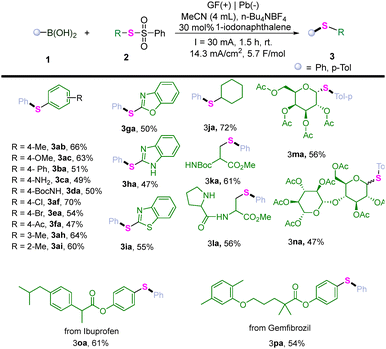
Next, we examined the generality of this electrocatalytic protocol with respect to a range of thiosulfonates (Table 3). Interestingly, the electroreduction cross-coupling method showed robust compatibility with S-phenyl benzenesulfonothioate regardless of the electronic properties associated with substituents on the S-phenyl ring. The positions of these substituents had negligible effects on both the yield and selectivity, whether ortho-, meta- or para-substituted (3ab, 3ac, 3ba, 3af, 3ah, 3ai). Moreover, NH2, BocNH, Br14 and acetyl groups (3ca–3fa), which hardly survive in traditional approaches, were tolerated under these conditions resulting in moderate yields of their corresponding thioethers. Additionally, benzenesulfonothioate-containing heteroaryl moieties were also employed in this reaction yielding satisfactory products (3ga–3ia). Notably, our approach facilitated access to an alkyl-tethered thioether from S-alkyl benzenesulfonothioate (3ja). Additionally, the electrocatalytic method expanded the range of applications by tolerating functional groups, including protected amino acids, dipeptides and glycosides (3ka–3na). Furthermore, employing this method, S-alkyl benzenesulfonothioates based on natural products or bioactive molecules smoothly furnished the desired products in moderate yields (3oa, 3pa).
To shed light on the possible reaction pathway of this electroreduction cross-coupling reaction, cyclic voltammetry (CV) experiments were then conducted (Fig. S2). First, 1-iodonaphthalene showed two reduction peaks: one at −1.85 V (vs. Ag/AgCl) and the other at −2.59 V (vs. Ag/AgCl) (Fig. S2, curve d). In addition, a reversible peak of 1-iodonaphthalene occurred at −2.38 V (vs. Ag/AgCl), which demonstrated that a 1-iodonaphthalene radical anion could be oxidized to iodonaphthalene. Second, in the thiosulfonate case, the initial reduction potential was observed at −1.06 V (vs. Ag/AgCl). Plausibly, this could be attributed to the formation of the thiyl radical, which could recombine to form a symmetric disulfide.15 Additionally, an additional reduction potential was found at −1.60 V (vs. Ag/AgCl) (Fig. S2, curve c). Surprisingly, the reduction potential of the thiosulfonate is lower than that of 1-iodonaphthalene. This can likely be attributed to the formation of a reactive layer, distinct from the electrode, coupled with the equilibrium between the diffusion of thiosulfonates from the electrolytic solution and the diffusion of highly reducing mediator radical anions from the cathode. This dynamic effectively precludes the substrate from infiltrating the electrode. This phenomenon signifies that the mediation pathway predominantly governs the transition process.16
When the CV of 1-iodonaphthalene was performed under the same conditions in the presence of 0.1 mmol 2a, an increase in the reduction peak current was observed from −0.18 mA to −0.73 mA, and the oxidation peak current changed from −0.09 mA to −0.36 mA (Fig. S3, curve d, see the SI). When 2a was added further, the reduction peak current changed to −1.16 mA and the oxidation peak current changed to −0.89 mA (Fig. S3, curve e, see the SI). Clearly, these results demonstrate that 1-iodonaphthalene works as an electron transfer mediator when thiyl radicals are electro-reductively generated from thiosulfonate 2a. Furthermore, a series of CV tests of 1-iodonaphthalene with increasing amounts of 2a were conducted (Fig. S4, see the SI). It was found that the oxidation peak of the 1-iodonaphthalene radical anion disappeared when the amount of 2a increased. This result further indicated that 2a would undergo electron transfer with a 1-iodonaphthalene radical anion.
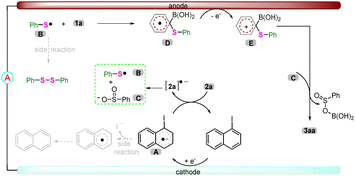
Based on the above observations and previous studies,17 the plausible mechanism for the electroreduction cross-coupling reaction was proposed (Scheme 1). At the cathode, 1-iodonaphthalene is reduced to afford radical anion A, followed by a SET process with thiosulfonate 2a to produce the corresponding radical anion [2a]˙− and regenerated 1-iodonaphthalene. The resulting radical anion [2a]˙− can generate a thiyl radical B and a sulfinate anion Cvia S–S bond cleavage.18 After that, the radical addition of thiyl radical B with substrate 1a yields another radical species D, which could be oxidized at the anode to furnish the cation intermediate E. With the aid of sulfinate anion C, the key aryl cation E would be converted to the desired product 3aa.
In conclusion, we have developed an electrochemical reduction cross-coupling of thiosulfonates with arylboronic acids using 1-iodonaphthalene as an efficient organic mediator. Featuring simple and mild conditions, this electroreduction cross-coupling exhibits a broad substrate scope. This research not only expands the repertoire of methodologies for the preparation of thioethers, but also caters to the increasing necessity for more sustainable and eco-friendly approaches within the realm of organic synthesis.
We are grateful for the financial support from the Guizhou Provincial Basic Research Program (Natural Science) (No. QianKeHeJiChuZK [2024]YiBan086).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc05687j.Notes and references
- (a) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1206 CrossRef CAS; (b) M. Lu, L.-L. Jiang, Y.-M. Xu, S. Li, Q.-X. Tong and J.-J. Zhong, Chin. J. Chem., 2024, 42, 2751–2756 CrossRef CAS.
- M. Wang and X. Jiang, ACS Sustainable Chem. Eng., 2022, 10, 671–677 CrossRef CAS.
- (a) M. Kosugi, T. Shimizu and T. Migita, Chem. Lett., 1977, 6, 1423–1424 CrossRef; (b) D. Liu, H. X. Ma, P. Fang and T. S. Mei, Angew. Chem., Int. Ed., 2019, 58, 5033–5037 CrossRef CAS; (c) Y.-Z. Yang, Y. Li, G.-F. Lv, D.-L. He and J.-H. Li, Org. Lett., 2022, 24, 5115–5119 CrossRef CAS PubMed; (d) Y. Li, J. Pu and X. Jiang, Org. Lett., 2014, 16, 2692–2695 CrossRef CAS PubMed.
- X. Zhou, D. Pyle, Z. Zhang and G. Dong, Angew. Chem., Int. Ed., 2023, 62, e202213691 CrossRef CAS PubMed.
- S. Wu, T. H.-F. Wong, P. Righi and P. Melchiorre, J. Am. Chem. Soc., 2024, 146, 2907–2912 CrossRef CAS PubMed.
- (a) Z.-Y. Li, N. Chen and H.-C. Xu, Chin. J. Chem., 2025, 43, 1167–1172 CrossRef CAS; (b) C. Ma, P. Fang, Z. R. Liu, S. S. Xu, K. Xu, X. Cheng, A. Lei, H. C. Xu, C. Zeng and T. S. Mei, Sci. Bull., 2021, 66, 2412–2429 CrossRef CAS; (c) X. Cheng, A. Lei, T.-S. Mei, H.-C. Xu, K. Xu and C. Zeng, CCS Chem., 2022, 4, 1120–1152 CrossRef CAS; (d) Q. Wan, R.-X. Liu, Z. Zhang, X.-D. Wu, Z.-W. Hou and L. Wang, Chin. J. Chem., 2024, 42, 1913–1928 CrossRef CAS.
- S. Liang and C.-C. Zeng, Curr. Opin. Electrochem., 2020, 24, 31–43 CrossRef CAS.
- (a) K. Yang, T. Feng and Y. Qiu, Angew. Chem., Int. Ed., 2023, 62, e202312803 CrossRef CAS; (b) H.-C. Xu and K. D. Moeller, J. Org. Chem., 2021, 86, 15845–15846 CrossRef CAS; (c) J. Zhu, Z. Chen, M. He, D. Wang, L. Li, J. Qi, R. Shi and A. Lei, Org. Chem. Front., 2021, 8, 3815–3819 RSC; (d) C. Ge, L. Qiao, Y. Zhang, K. Sun, J. An, M. Peng, X. Chen, L. Qu and B. Yu, Chin. J. Chem., 2024, 42, 1679–1685 CrossRef CAS.
- M. A. Hoque, J. Twilton, J. Zhu, M. D. Graaf, K. C. Harper, E. Tuca, G. A. DiLabio and S. S. Stahl, J. Am. Chem. Soc., 2022, 144, 15295–15302 CrossRef CAS.
- (a) Z. Zhao, H. Yan, Y. Zhou, W. Xue, L. Gu and S. Zhang, Chin. J. Chem., 2024, 42, 2049–2055 CrossRef CAS; (b) Z. Zhao, H. Zhang, H. Yan, X. Yu, L. Gu and S. Zhang, Org. Lett., 2024, 26, 6114–6119 CrossRef CAS PubMed; (c) Z. Zhao, H. Yan and L. Gu, Chem. Commun., 2025, 61, 3868–3871 RSC.
- C. Ma, P. Fang, D. Liu, K. J. Jiao, P. S. Gao, H. Qiu and T. S. Mei, Chem. Sci., 2021, 12, 12866–12873 RSC.
- P. Li, Y. Wang, H. Zhao and Y. Qiu, Acc. Chem. Res., 2025, 58, 113–129 CrossRef CAS.
- W. Zeng, Y. Wang, C. Peng and Y. Qiu, Chem. Soc. Rev., 2025, 54, 4468–4501 RSC.
- In this case, the debromination of product 3ea as the side reaction could be observed.
- N. W. Ang and L. Ackermann, Chem. – Eur. J., 2021, 27, 4883–4887 CrossRef CAS PubMed.
- X.-Q. Zhou, P.-B. Chen, Q. Xia, T.-K. Xiong, X.-J. Li, Y.-M. Pan, M.-X. He and Y. Liang, Org. Chem. Front., 2023, 10, 2039–2044 RSC.
- L. F. T. Novaes, J. Liu, Y. Shen, L. Lu, J. M. Meinhardt and S. Lin, Chem. Soc. Rev., 2021, 50, 7941–8002 RSC.
- For radical trapping experiments, see the SI.
| This journal is © The Royal Society of Chemistry 2026 |