Interlayer bismuthene as a charge-transporter improves the photoelectrochemical water oxidation activity of a bismuth vanadate metal–organic framework
Deepak
Kumar
,
Sagnik
Mukherjee
and
Arindam
Indra
 *
*
Department of Chemistry, Indian Institute of Technology (BHU), Varanasi, UP-221005, India. E-mail: arindam.chy@iitbhu.ac.in
First published on 18th November 2025
Abstract
The introduction of bismuthene as a charge transport layer between BiVO4 and a cocatalyst (Ni-metal–organic framework, Ni-MOF) improves the photoelectrochemical water oxidation activity. The photoanode BiVO4/Bi/Ni-MOF produces a photocurrent of 4.71 (±0.2) mA cm−2 (at 1.23 V vs. RHE), which is 2.5 and 1.25 times higher than those of bare BiVO4 and BiVO4/Ni-MOF, respectively.
Photoelectrochemical (PEC) water oxidation using semiconductors and sunlight offers a sustainable route for energy conversion.1 The development of an efficient photoanode for the maximum utilization of visible light and to minimize the kinetic barrier of the sluggish oxygen evolution reaction (OER) is crucial for PEC water splitting.2 BiVO4 has garnered significant attention as a photoanode for the OER because of its suitable valence band maximum (VBM = 2.15 eV) and narrow band gap (2.42 eV).3 Additionally, the low cost and high chemical stability of BiVO4 are suitable for practical applications.4,5 However, the use of BiVO4 for PEC-OER is limited by its sluggish water oxidation kinetics and poor charge carrier mobility (0.04 cm2 V−1 s−1).6 Various approaches, including heteroatom doping, heterostructure formation, cocatalyst loading, etc., have been investigated to improve the water oxidation activity of BiVO4.5,7
Among these, cocatalyst loading has been extensively studied to modify the charge-transfer dynamics, to improve the water oxidation kinetics, transfer of holes, and access to active sites, and to increase the stability of the photoanode.8 The loading of cocatalysts such as metal oxides, hydroxides, metal–organic frameworks (MOFs), etc., on BiVO4 has been found to improve the PEC activity of the photoanode.9 In this context, the use of MOFs as the cocatalyst offers extra advantages, such as providing abundant active sites, a high electrochemically active surface area, and improved charge carrier dynamics.10 For example, the fabrication of a CoNi-MOF on BiVO4 was reported to achieve an increase in the photocurrent density compared to the bare BiVO4 photoanode.11 Similarly, the integration of Zn-MOF,7 Co-MOF,12 CoFe-MOF,13etc., on BiVO4 to improve its OER performance has been explored.
Additionally, heterostructure formation via layer-by-layer deposition of BiVO4 and an electron/hole conductor has been found to improve the efficiency of the photoanode. In particular, the use of 2D materials such as graphene, phosphorene, bismuthene, etc., to attain high PEC activity has been explored.6,14–16 Qiao et al. demonstrated the use of 2D phosphorene to enhance the charge separation efficiency of BiVO4.14 The Park group reported that 2D phosphorene layers between BiVO4 and NiOOH improved the PEC-OER kinetics.15 A functional interlayer of bismuthene nanosheets between BiVO4 and NiFeOOH was also reported to enhance the PEC-OER activity.6 The construction of BiVO4–reduced graphene oxide led to a 10-fold increase in PEC-OER efficiency compared to pure BiVO4.16
The above studies inspired us to explore the combined effect of heterostructure formation and the use of Ni-MOF as the cocatalyst to improve the PEC water oxidation activity of the BiVO4 photoanode. The interlayer deposition of bismuthene nanosheets as an electron-transporter between BiVO4 and Ni-MOF was found to improve the PEC water oxidation activity.
Bismuthene with atomic-level thickness can improve charge separation and carrier mobility.6 The charge transfer between BiVO4 and bismuthene results in upward band bending in the former and a shift of the CB to a more negative position. Consequently, the band gap of BiVO4 expands, and the Fermi level relocates, leading to a reduction in the charge recombination. The partially oxidized bismuthene increases the density of oxygen vacancies on the photoanode surface, improves the n-type characteristics, and enhances charge separation and transport.6
Herein, physicochemical and (photo)electrochemical characterizations reveal the complementary roles of bismuthene and Ni-MOF to improve the PEC activity of BiVO4. The deposition of Ni-MOF over the bismuthene layer is important for charge transfer and for increasing the number of surface active sites.
Therefore, this work highlights the crucial role of bismuthene as a charge-transporter between the semiconductor and cocatalyst to enhance the performance of the photoanode. The role of bismuthene as the charge transport layer is prominent, as the photoanode BiVO4/Bi/Ni-MOF produces a photocurrent density of 4.71 (±0.2) mA cm−2 (at 1.23 V vs. RHE), which is significantly higher than that of BiVO4/Ni-MOF.
BiVO4 was first deposited on a fluorine-doped tin oxide (FTO)-coated glass film, followed by the layer-by-layer deposition of bismuthene and Ni-MOF to form BiVO4/Bi/Ni-MOF (Table S1, SI). The PXRD patterns revealed the monoclinic phase of BiVO4 (JCPDS 014-0688) (Fig. 1a).13 With the loading of bismuthene and/or Ni-MOF on BiVO4, the characteristic peaks of rhombohedral Bi with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group (JCPDS 05-0519) and Ni-MOF with the space group P3
m space group (JCPDS 05-0519) and Ni-MOF with the space group P3![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) m were observed (Fig. 1a).17,18 In the attenuated total reflection Fourier-transformed infrared (ATR-FTIR) spectrum of the nickel-chloranilate framework Ni-MOF, the C
m were observed (Fig. 1a).17,18 In the attenuated total reflection Fourier-transformed infrared (ATR-FTIR) spectrum of the nickel-chloranilate framework Ni-MOF, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of the chloranilate ligand was detected at 1652 cm−1 (Fig. S1, SI).18
O vibration of the chloranilate ligand was detected at 1652 cm−1 (Fig. S1, SI).18
The scanning electron microscopy (SEM) image of BiVO4 showed a wormlike morphology with a porous structure (Fig. 1b). The SEM image of Ni-MOF revealed flower-like nanosheets (Fig. S2a, SI). In BiVO4/Bi/Ni-MOF, the flower-like structure of Ni-MOF and the nanosheets of bismuthene were observed together (Fig. 1c and Fig. S2b, SI). The energy-dispersive X-ray (EDX) and elemental mapping of BiVO4/Bi/Ni-MOF confirmed the elements present and their distribution (Fig. S3 and S4, SI).
The transmission electron microscopy (TEM) image of BiVO4/Bi/Ni-MOF revealed transparent ultrathin nanosheets of bismuthene and nanosheets of Ni-MOF (Fig. 1d). High-resolution TEM (HR-TEM) detected the formation of the heterostructure (Fig. 1e). The HR-TEM revealed a lattice spacing of 0.21 nm,18 corresponding to the Ni-MOF (31![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) plane (Fig. 1e1).18 A lattice spacing (0.33 nm) corresponding to the bismuthene (012) plane was also detected via HR-TEM (Fig. 1e2).17 The (110) plane of BiVO4 showed a lattice spacing of 0.47 nm (Fig. 1e3).13 These results confirm the coexistence of the three different phases with intimate interfaces, which can enhance interfacial charge transfer and thus is beneficial for PEC performance.
) plane (Fig. 1e1).18 A lattice spacing (0.33 nm) corresponding to the bismuthene (012) plane was also detected via HR-TEM (Fig. 1e2).17 The (110) plane of BiVO4 showed a lattice spacing of 0.47 nm (Fig. 1e3).13 These results confirm the coexistence of the three different phases with intimate interfaces, which can enhance interfacial charge transfer and thus is beneficial for PEC performance.
The Raman spectrum of pure bismuthene showed peaks at 119.0 cm−1 and 87.8 cm−1 corresponding to A1g (out-of-plane mode) and Eg (in-plane mode) vibrations, respectively. The considerably higher wavenumber values for the Eg and A1g vibrations compared to those of bulk Bi indicate the lowering of the dimensionality of the metal to form atomic-level thin nanosheets (Fig. 2a).6,17 Further, the peak intensity ratio of A1g to Eg was calculated to be 0.87, indicating the formation of few-layer bismuthene (Fig. 2a).6,17
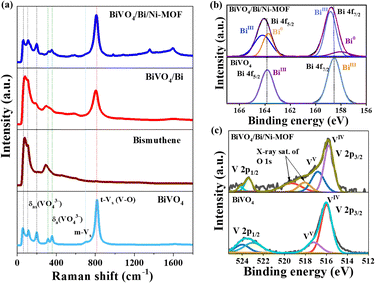 | ||
| Fig. 2 (a) Raman spectra of BiVO4, BiVO4/Bi, and BiVO4/Bi/Ni-MOF. (b) Bi 4f XPS of BiVO4/Bi/Ni-MOF, showing the peaks of the BiIII and Bi0 species. (c) V 2p XPS of BiVO4 and BiVO4/Bi/Ni-MOF. | ||
The band at 306.0 cm−1 corresponding to Bi–O indicates that the bismuthene is partially oxidized.6 The Raman spectra of Bi/BiVO4 and BiVO4/Bi/Ni-MOF showed a slight change in the Raman shifts of the bismuthene-related peaks compared to those of pure bismuthene due to charge transfer (Fig. 2a).
In the BiVO4/Bi/Ni-MOF spectrum, the two peaks at 564 cm−1 and 484 cm−1 corresponded to the characteristic A1g stretching and Eg bending modes of the Ni–O bond in Ni-MOF.19 Peaks corresponding to the C–C and C–O stretches of Ni-MOF were observed at 1351 and 1586 cm−1, respectively.20 Other peaks in the Raman spectra were assigned to the various asymmetric and symmetric stretching vibration modes of the [VO4]3− unit in BiVO4 (Fig. 2a).
The Bi 4f X-ray photoelectron spectrum (XPS) of BiVO4 was fitted into two peaks corresponding to Bi 4f7/2 (158.9 eV) and Bi 4f5/2 (164.3 eV), indicating the presence of only Bi(III) species. In BiVO4/Bi/Ni-MOF, the Bi 4f7/2 and Bi 4f5/2 peaks were broadened due to the presence of both Bi(0) and Bi(III) species.17 Further, the Bi(III) peak was shifted to a higher binding energy (by 0.32 eV) than that of bare BiVO4 due to electron transfer to Bi through the interface. The 4f7/2 peaks at 158.8 eV and 157.6 eV were attributed to Bi–O and metallic Bi(0) species, respectively (Fig. 2b).6,17
The V 2p XPS of BiVO4 was fitted to V5+ and V4+ species (Fig. 2c). In BiVO4/Bi/Ni-MOF, a significant decrease in the ratio of V5+/V4+ was observed because of the electron transfer from bismuthene to BiVO4.6,21
The O 1s XPS spectrum of BiVO4 was fitted to three peaks (Fig. S5a, SI). The peaks at 531.2, 530.45, and 532.5 eV were attributed to oxygen vacancies (OV), lattice oxygen (OL), and chemisorbed oxygen species (OC) from water molecules, respectively.6 The XPS spectrum of BiVO4/Bi/Ni-MOF was fitted to three peaks with binding energies of 528.97 eV, 530.80 eV, 531.08 eV, and 532.11 eV corresponding to Ni–O, Bi–O, oxygen vacancies (Ov), and –CO groups,6 respectively (Fig. S5a, SI).
The Ni 2p XPS of BiVO4/Bi/Ni-MOF showed two peaks at 854.93 eV and 872.34 eV, corresponding to Ni 2p3/2 and Ni 2p1/2, respectively. The peak at 854.93 eV confirmed the presence of Ni2+, whereas the peaks marked with an asterisk (*) were assigned as its satellites (Fig. S5b, SI).22
UV-visible diffuse reflectance spectra (UV-vis DRS) were recorded to analyse the optical properties of the catalysts. The absorption band of pristine BiVO4 was observed at 464 nm, and the loading of the Ni-MOF and bismuthene slightly changed the peak features (Fig. S6a, SI). A new peak (397 nm) corresponding to the metal-to-ligand charge transfer (MLCT) in Ni-MOF was observed in BiVO4/Bi/Ni-MOF (Fig. S6a, SI), while bismuthene showed a broad absorption in the visible-light region (Fig. S6b, SI).
The Tauc plot revealed a slight change in the bandgap after heterostructure formation (2.53 eV for BiVO4/Bi/Ni-MOF, 2.44 eV for BiVO4/Bi, and 2.46 eV for BiVO4) (Fig. S6c, SI). Bismuthene showed a narrow bandgap of 0.62 eV, supporting extended near-infrared absorption (Fig. S6d, SI).23
The improvement in the charge transport in BiVO4/Bi/Ni-MOF was confirmed by the photoluminescence (PL) study, as the PL-emission intensity of BiVO4/Ni-MOF was found to be lower than that of BiVO4/Bi and bare BiVO4 (Fig. S7a, SI). The deposition of bismuthene on BiVO4 improves the charge transport and hence minimizes the radiative charge carrier recombination, as reflected by the decrease in the PL intensity. Furthermore, Ni-MOF facilitates the interface electron transfer from bismuthene to the electrode surface for improved PEC activity (Fig. S7b, SI). A slight change in the emission peak λmax was also observed with the loading of bismuthene and Ni-MOF on BiVO4 because of the strong electronic interaction between the components.
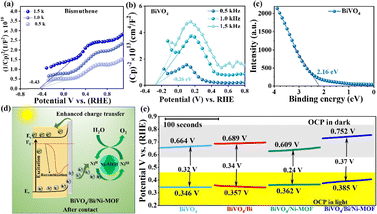
A more comprehensive understanding of the band structure of the photoanodes was realized via Mott–Schottky (M–S) studies. BiVO4 and bismuthene exhibited positive slopes in the M–S plots, indicating n-type semiconductor properties.23 The flat band potentials of BiVO4 and bismuthene were measured to be −0.26 V and −0.43 V vs. RHE, respectively. Hence, the CBM values of BiVO4 and bismuthene were calculated to be −0.36 eV and −0.53 eV, respectively (Fig. 3a and b). The VBM of BiVO4 was determined from the XPS valence band spectra to be 2.16 eV (Fig. 3c). Therefore, the bandgap calculated to be 2.52 eV closely matches the Tauc plot value (2.48 eV). Furthermore, the band alignment confirms the electron transfer from bismuthene to BiVO4 (Fig. S6e).
The M–S plot confirmed the electron transfer from bismuthene to BiVO4, which facilitates charge separation. This also results in an upward band bending and a shift of the CB of BiVO4 to a more negative potential (Fig. 3d and e). As a result, the band gap of BiVO4 widens, accompanied by repositioning of the Fermi level and suppression of charge recombination. The open-circuit potential (OCP) measurements under dark and light conditions revealed a pronounced shift in the OCP values of the bismuthene-modified photoanodes in the dark and similar values under light-saturated conditions (+0.36 ± 0.02 V vs. RHE), confirming that bismuthene modulates the interfacial charge transport properties (Fig. 3e).
The PEC water oxidation activity of the photoanodes was measured in phosphate buffer in the presence of visible light (Fig. 4a). Bare BiVO4 showed the lowest photocurrent response (1.91 ± 0.2 mA cm−2 at 1.23 V vs. RHE). After the deposition of bismuthene or Ni-MOF on BiVO4, the photocurrent density increased. The best activity was recorded for BiVO4/Bi/Ni-MOF, which achieved a photocurrent density of 4.71 ± 0.2 mA cm−2 at 1.23 V vs. RHE. The linear sweep voltammetry (LSV) profiles detected poor current density in the dark, while under light irradiation, the photoanodes generated a significant photocurrent. Comparison of the PEC water oxidation activity of BiVO4/Bi/Ni-MOF with that of the reported BiVO4-based photoanodes revealed that it had higher activity than most of the reported catalysts (Table S2, SI). The loading of other Ni-based cocatalysts (NiO, Ni(OH)2) on BiVO4/Bi resulted in PEC activity superior to that of BiVO4/Bi but inferior to that of BiVO4/Bi/Ni-MOF, demonstrating the importance of the MOF as a cocatalyst (Fig. S8a and b, SI).
The transient photocurrent response provided insights into the charge separation behavior of photogenerated electron–hole pairs (Fig. 4b).7 The potential-dependent photocurrent also demonstrated the superior performance of the BiVO4/Bi/Ni-MOF photoanode compared to the other synthesized catalysts (Fig. S9, SI). The applied bias photon-to-current efficiency (ABPE) of BiVO4/Bi/Ni-MOF photoanode exhibited a peak of 2.01% at 0.59 V vs. RHE (Fig. 4c), which was significantly higher than that of pure BiVO4 (0.73% at 0.582 V vs. RHE).
The BiVO4/Bi/Ni-MOF photoanode showed remarkably improved incident photon-to-current conversion efficiency (IPCE) across the full 300–620 nm wavelength range (Fig. 4d), achieving a peak of 62.17% at 420 nm, which is 1.72 times greater than that of bare BiVO4. As the light absorption capacity remained nearly constant after bismuthene and Ni-MOF loading, the substantial increase in the IPCE can be attributed to improved charge carrier transport and enhanced surface reaction kinetics (Fig. 4d).
The deposition of interlayer bismuthene enhances the electron transport properties, as revealed by electrochemical impedance spectroscopy (Fig. S10, SI). The loading of bismuthene on BiVO4 reduced the charge-transfer resistance (Rct) of BiVO4, and BiVO4/Bi/Ni-MOF showed the lowest Rct value compared to other photoanodes.
Further, the electrical double-layer capacitance (Cdl) was determined to examine the electrochemically active surface area (which is proportional to the Cdl) (Fig. S11a–d, SI). The highest Cdl was observed for BiVO4/Bi/Ni-MOF.
In addition, a 24 h photo-stability test at 1.23 V vs. RHE showed that the BiVO4/Bi/Ni-MOF photoanode maintained a stable photocurrent, indicating that the introduction of bismuthene and the Ni-MOF layer significantly enhanced the stability of BiVO4 (Fig. S12, SI).
In summary, we report the BiVO4/Bi/Ni-MOF photoanode, in which bismuthene functions as an interlayer charge-transporter between BiVO4 and the Ni-MOF cocatalyst, achieving a pronounced improvement in the PEC water oxidation activity. The effective electron transfer from bismuthene to BiVO4 facilitates better charge carrier separation and suppresses recombination, while Ni-MOF offers abundant active sites for efficient hole extraction, leading to an enhancement in the charge separation and hence, the PEC-OER activity. The combined effect exerted by bismuthene and Ni-MOF results in an OER photocurrent of 4.76 mA cm−2 at 1.23 V vs. RHE (ABPE of 2.01% at 0.59 V vs. RHE, and IPCE of 62.17% at 420 nm), which is far better than that of BiVO4 and BiVO4/Ni-MOF. The improved ABPE and IPCE establish a new interlayer-engineering paradigm for high-performance BiVO4-based solar water-splitting systems.
This work is financially supported by ANRF, India (DST/C3E/MI2.0/CCUS/2K23/CALL/2023/71(G)/2). D. K. acknowledges UGC for providing the senior research fellowship.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: synthesis and characterization of the catalysts, details of the experiments. See DOI: https://doi.org/10.1039/d5cc04966k.References
- S. Zhao, B. Liu, K. Li, S. Wang, G. Zhang, Z. J. Zhao, T. Wang and J. Gong, Nat. Commun., 2024, 15, 2970 CrossRef CAS PubMed
.
- I. J. Teh, L. K. Putri, C. S. Yaw, W. C. Ng, M. N. Chong and S. P. Chai, Coord. Chem. Rev., 2025, 541, 216773 CrossRef CAS
.
- X. Li, J. Wu, C. Dong, Y. Kou, C. Hu, J. Zang, J. Zhu, B. Ma, Y. Li and Y. Ding, Appl. Catal., B, 2024, 353, 124096 CrossRef CAS
.
- J. Chi, Z. Jiang, J. Yan, A. Larimi, Z. Wang, L. Wang and W. Shangguan, Mater. Today Chem., 2022, 26, 101060 CrossRef CAS
.
- Y. An, C. Lin, C. Dong, R. Wang, J. Hao, J. Miao, X. Fan, Y. Min and K. Zhang, ACS Energy Lett., 2024, 9, 1415–1422 CAS
.
- J. Cui, M. Daboczi, M. Regue, Y. C. Chin, K. Pagano, J. Zhang, M. A. Isaacs, G. Kerherve, A. Mornto, J. West, S. Gimenez, J. S. Kim and S. Eslava, Adv. Funct. Mater., 2022, 32, 2207136 CAS
.
- H. Bai, F. Wang, Z. You, D. Sun, J. Cui and W. Fan, Colloids Surf., A, 2022, 640, 128412 CAS
.
- S. Wang, D. Cui, W. Hao and Y. Du, Energy Fuels, 2022, 36, 11394–11403 CrossRef CAS
.
- Y. Zhang, B. Liu, L. Xu, Z. Ding, R. Yan and S. Wanag, ChemSusChem, 2025, 18, e202401420 CrossRef CAS PubMed
.
- M. Jia, W. Xiong, Z. Yang, J. Cao, Y. Zhang, Y. Xiang, H. Xu, P. Song and Z. Xu, Coord. Chem. Rev., 2021, 434, 213780 CrossRef CAS
.
- S. Zhou, K. Chen, J. Huang, L. Wang, M. Zhang, B. Bai, H. Liu and Q. Wang, Appl. Catal., B, 2020, 266, 118513 CrossRef CAS
.
- S. Zhong, B. Kang, X. Cheng, P. Chen and B. Fang, ACS Sustainable Chem. Eng., 2024, 12, 1233–1246 CrossRef CAS
.
- X. Y. Yang, Z. W. Chen, X. Z. Yue, X. Du, X. H. Hou, L. Y. Zhang, D. L. Chen and S. S. Yi, Small, 2023, 19, 2205246 CrossRef CAS PubMed
.
- J. Qiao, X. Kong, Z. X. Hu, F. Yang and W. Ji, Nat. Commun., 2014, 5, 4475 CrossRef CAS PubMed
.
- K. Zhang, B. Jin, C. Park, Y. Cho, X. Song, X. Shi, S. Zhang, W. Kim, H. Zeng and J. H. Park, Nat. Commun., 2019, 10, 2001 CrossRef
.
- Y. H. Ng, A. Iwase, A. Kudo and R. Amal, J. Phys. Chem. Lett., 2010, 1, 2607–2612 CrossRef CAS
.
- D. Kumar, L. Gu, A. D. Chowdhury and A. Indra, Inorg. Chem., 2025, 64, 11610–11617 CrossRef CAS PubMed
.
- A. Yadav, T. Ansari, P. Mannu, B. Singh, A. K. Singh, Y. C. Huang, V. Kumar, S. Singh, C. L. Dong and A. Indra, J. Mater. Chem. A, 2024, 12, 29072–29080 RSC
.
- S. Ghosh, D. Bagchi, I. Mondal, T. Sontheimer, R. V. Jagadeesh and P. W. Menezes, Adv. Energy Mater., 2024, 14, 2400696 CrossRef CAS
.
- J. A. DeGayner, I. R. Jeon, L. Sun, M. Dincă and T. D. Harris, J. Am. Chem. Soc., 2017, 139, 4175–4184 CrossRef CAS PubMed
.
- H. T. T. Nguyen, D. Jung, C. Y. Park and D. J. Kang, Mater. Chem. Phys., 2015, 165, 19–24 CrossRef CAS
.
- A. Indra, A. Acharjya, P. W. Menezes, C. Merschjann, D. Hollmann, M. Schwarze, M. Aktas, A. Friedrich, S. Lochbrunner, A. Thomas and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 1653–1657 CrossRef CAS
.
- Z. Niu, W. Shan, X. Wang, X. Zhang, A. Shi, Y. Zhang and X. Niu, J. Phys. Chem. Lett., 2025, 16, 4057–4065 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |