Selective strain-promoted reactivity of a fluorene-derived [6]cycloparaphenyleneacetylene carbon nanohoop
Robert J.
Herman
 a,
Said
Jalife
a,
Said
Jalife
 b,
Abigail G.
LeBlanc
a,
Muhammad Usama Gul
Khan
b,
Abigail G.
LeBlanc
a,
Muhammad Usama Gul
Khan
 b,
Marvin L.
Stewart
a,
Sheila W.
Njoroge
b,
Marvin L.
Stewart
a,
Sheila W.
Njoroge
 a,
Sajila Riman
Tanha
a,
Sajila Riman
Tanha
 a,
Frank R.
Fronczek
a,
Frank R.
Fronczek
 a,
Judy
Wu
a,
Judy
Wu
 b and
Semin
Lee
b and
Semin
Lee
 *a
*a
aDepartment of Chemistry, Louisiana State University, Baton Rouge, Louisiana 70803, USA. E-mail: seminlee@lsu.edu
bCollege of Natural Science and Mathematics, University of Houston, Houston, Texas 77204, USA
First published on 24th November 2025
Abstract
The modular synthesis of a fluorene-based nanohoop containing six strained alkynes is described herein. We demonstrate its scalability using alkyne metathesis as the macrocyclization method and its reactivity with azides using strain-promoted azide–alkyne cycloaddition. The nanohoop undergoes two cycloadditions selectively on two oppositely located alkynes on the macrocyclic backbone.
Carbon nanohoops1 have strain along their backbone due to para-linked phenylene and/or acetylene groups bending to form a circular structure.2 Their chemical reactivity, involving the release of strain energy, has been explored since their inception. Cycloparaphenyleneacetylene3 (CPPA) carbon nanohoops, with alkynes embedded along the backbone, have been investigated by Kawase and Oda.4 Highly strained [4]CPPA underwent an in situ [4+2] cycloaddition with two furan molecules on opposite sides of the nanohoop (Fig. 1a). When Lee and Moore5 performed strain-promoted azide–alkyne cycloaddition (SPAAC)6 with [3]CPP3A nanohoops, only isomers of the triple-SPAAC macrocycles were isolated. Yamago and researchers observed that [6]cycloparaphenylene (CPP) nanohoops undergo selective strain-promoted bromination7 and C–C bond activation with Pt0 complexes8 on opposing phenylene groups in a similar fashion. Michinobu and coworkers demonstrated regioselective double-SPAAC on an octadehydrobenzo[12]annulene derivative.9 The subsequent relief of strain across these backbones did not allow any further reactions. Jasti and coworkers10,11 have developed a family of CPP derivatives containing a single strained alkyne. Upon SPAAC, they display a change in structure, adopting a teardrop-like shape with increased strain on the opposing phenylene groups.11,12 They have also discovered that strained alkynes within the CPP backbone are prone to undergo rapid Pd-catalyzed cyclotrimerization.13
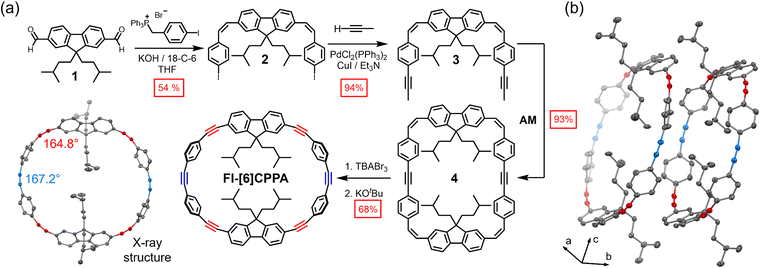
Herein, we designed and synthesized a CPPA carbon nanohoop with two dialkylated fluorenes, four phenylenes, and six strained alkynes (Fl-[6]CPPA, Fig. 1b). We examined its structure, photophysical properties, and reactivity in SPAAC. One could posit that Fl-[6]CPPA, containing multiple strained alkynes, would behave similarly to [4]CPPA, reacting with two alkyne groups on opposite sides of the nanohoop (Fig. 1a).
Our previous synthesis of an [8]CPPA derivative relied on a tetrameric macrocyclization of a 4,4′-dipropynyl-(Z)-stilbene.14 To improve the solubility and modularity of the monomer, we placed a dialkylated fluorene group in the center with two (Z)-alkenes on either side. This U-shaped geometry (Fig. 2) allows simple dimeric macrocyclization via alkyne metathesis. We envisioned that the fluorene group could be easily interchanged with other arene groups in the future, potentially providing a modular synthetic method.
2,7-Dibromofluorene was subjected to dialkylation and diformylation, resulting in 2,7-diformyl-9,9-di-iso-pentylfluorene (1, Fig. 2a, see SI). A cis-selective Wittig reaction15 afforded 2 as a (Z,Z)-isomer, isolable via column chromatography in 54% yield. Sonogashira cross-coupling with propyne formed the U-shaped dipropynyl monomer 3 in an excellent yield (94%). Mo(VI)-catalyzed alkyne metathesis16 of 3 afforded the dimeric macrocycle 4 in 93% yield. An alkene-selective bromination of 4 was followed by dehydrobromination to reveal Fl-[6]CPPA in 68% yield (two steps combined). Compared to a similarly sized [8]CPPA derivative14 previously synthesized in our group, Fl-[6]CPPA was stable under atmospheric conditions. Fl-[6]CPPA is bench-stable for more than 30 days, making its shelf life comparable to larger CPPA analogs ([3]CPP4A and [3]CPP5A) previously synthesized in our group.17
Single crystals of Fl-[6]CPPA suitable for X-ray diffraction (XRD) were grown by slow diffusion of pentane into a concentrated solution of Fl-[6]CPPA in THF (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group). The resolution limit of the data was somewhat low (0.83 Å), but sufficient to define the connectivity and conformations of the molecules. The solid-state structure contained two staggered, ellipsoidal, alternating conformations of itself along the xz plane with a central inversion center in the unit cell. Two iso-pentyl groups, one from each fluorene unit, occupy the inner cavity. The fluorene groups were leaning in towards the center by 45° and 51° (Fig. S35) due to the contracting angle between two phenylenes caused by the five-membered ring. Such structural aspects were also observed in fluorenone-containing [9] and [11]CPP carbon nanohoops reported by Jasti and coworkers.18Fl-[6]CPPA showed an oval shape, with one hoop being slightly more ovate (Fig. 2b, left, 16.416 Å/14.846 Å) than the other (Fig. 2b, right, 16.019 Å/15.364 Å, Fig. S33). While the average alkyne angles (∠C–C
space group). The resolution limit of the data was somewhat low (0.83 Å), but sufficient to define the connectivity and conformations of the molecules. The solid-state structure contained two staggered, ellipsoidal, alternating conformations of itself along the xz plane with a central inversion center in the unit cell. Two iso-pentyl groups, one from each fluorene unit, occupy the inner cavity. The fluorene groups were leaning in towards the center by 45° and 51° (Fig. S35) due to the contracting angle between two phenylenes caused by the five-membered ring. Such structural aspects were also observed in fluorenone-containing [9] and [11]CPP carbon nanohoops reported by Jasti and coworkers.18Fl-[6]CPPA showed an oval shape, with one hoop being slightly more ovate (Fig. 2b, left, 16.416 Å/14.846 Å) than the other (Fig. 2b, right, 16.019 Å/15.364 Å, Fig. S33). While the average alkyne angles (∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) were 165.59°, fluorene-adjacent alkyne and diphenylacetylene alkyne angles were 164.80° and 167.17°, respectively (Fig. S33).
C) were 165.59°, fluorene-adjacent alkyne and diphenylacetylene alkyne angles were 164.80° and 167.17°, respectively (Fig. S33).
To gauge the selective reactivity of the alkyne groups within Fl-[6]CPPA, SPAAC was performed with 2.4 equivalents of benzyl azide (5, Scheme 1). The cycloaddition required heating at 40 °C for five days to react fully. The 1H NMR chemical shift of methylene protons (4.35 ppm) on benzyl azide showed a downfield shift upon formation of a 1,2,3-triazole. The emergence of two new singlets at 5.60 and 5.66 ppm indicated the formation of multiple SPAAC species (Fig. S17). The major double-SPAAC products 5/5′ (77%) and minor triple-SPAAC products (20%) were isolated via column chromatography. Their formations were confirmed using MALDI-MS. It should be noted that MALDI-MS of the crude reaction mixture showed minor traces of quadruple-SPAAC products (Fig. S29). Attempts to isolate the potential regioisomers (Fig. S2) of the double and triple click products (Fig. S3) via column chromatography and HPLC were unsuccessful. Additionally, complete structural analysis was unsuccessful due to the overlapping peaks in the aromatic and aliphatic regions in the 1H, 13C NMR spectra.
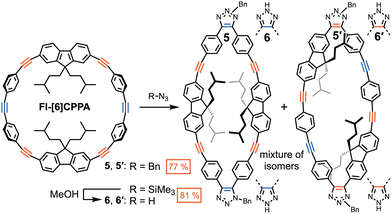 | ||
| Scheme 1 SPAAC products of Fl-[6]CPPA with BnN3 (5, 5′) and TMSN3 (6, 6′). Isolated yields are reported. | ||
Slow diffusion of pentane into a concentrated solution of the double-SPAAC compounds in CHCl3 formed clear yellow crystals. Out of five potential regioisomers (Fig. S2), the crystal suitable for XRD revealed 5 (P21/c space group) with two oppositely faced 1-benzyl-1,2,3-triazole rings between two phenylene groups (Fig. 3a). The resolution of diffraction data obtained from solid-state 5 is low (0.90 Å), yet sufficient to confirm overall connectivity. The overall oval shape of 5 is significantly more elongated (20.866 Å/11.085 Å) compared to Fl-[6]CPPA. The phenylene and fluorene groups are arranged more flatly along the plane of the macrocycle. The remaining four alkynes were more linear with an average alkyne angle (∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) of 170.62°, rendering them inactive for SPAAC. To substantiate the inactivity of these alkyne groups, we subjected a solution of 5 and 5′ to another SPAAC with 2.4 equivalents of BnN3. After 5 days of heating at 40 °C, no reaction was observed (Fig. S20). This confirmed that the triple-SPAAC products did not stem from 5 or 5′. The proposed routes for double-click and triple-click products and their regioisomers are depicted in the SI (Fig. S1–S3).
C) of 170.62°, rendering them inactive for SPAAC. To substantiate the inactivity of these alkyne groups, we subjected a solution of 5 and 5′ to another SPAAC with 2.4 equivalents of BnN3. After 5 days of heating at 40 °C, no reaction was observed (Fig. S20). This confirmed that the triple-SPAAC products did not stem from 5 or 5′. The proposed routes for double-click and triple-click products and their regioisomers are depicted in the SI (Fig. S1–S3).
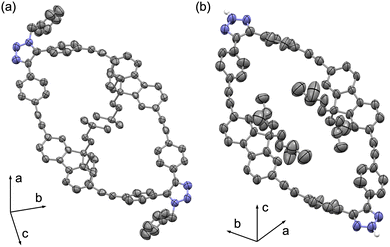 | ||
| Fig. 3 Solid-state structures of (a) 5 and (b) 6, displayed at 50% ellipsoid probability. C–H hydrogen atoms are omitted for clarity. | ||
In order to reduce the number of regioisomers and simplify their spectroscopic analysis, we prepared products with 2H-1,2,3-triazoles (6 and 6′, Scheme 1) through SPAAC with trimethylsilyl azide (TMSN3). Due to the steric bulk of the trimethylsilyl group, this reaction required heating at 75 °C for 14 days. Treating the product mixture with methanol19 during the workup revealed 6 and 6′ in good yield (81%). Only double-SPAAC products (6/6′) containing two 2H-1,2,3-triazoles were isolated. Compared to the five potential regioisomers of 5 and 5′ (Fig. S2), only two isomers exist for 6 and 6′. Still, we could not separate 6 from 6′via column chromatography.
Due to the poor solubility of the 6/6′ mixture in common deuterated solvents (CDCl3, CD2Cl2, DMSO-d6, acetone-d6, CD3CN, CD3OD, C6D6), the NMR (700 MHz) analysis relied on using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CDCl3 and CD3OD at 40 °C. 2D NMR analysis of the double-SPAAC product revealed iso-pentyl groups in two separate spin systems (Fig. S24–S27). Most notably, the terminal methyl groups on 6 displayed a doublet (δ = 0.68 ppm) while 6′ displayed a pair of upfield-shifted, diastereotopic doublets (δ = 0.58 ppm, 0.53 ppm) in 1H-NMR (Fig. S21). Computed proton chemical shifts (Fig. S49) suggest that the terminal methyl groups on 6′ are more upfield shifted than 6 due to their proximity to the phenylene groups on the opposite side of the nanohoop. 2D TOCSY, HSQC, and HMBC NMR experiments confirmed the presence of two separate species, 6 and 6′, in a 1
1 mixture of CDCl3 and CD3OD at 40 °C. 2D NMR analysis of the double-SPAAC product revealed iso-pentyl groups in two separate spin systems (Fig. S24–S27). Most notably, the terminal methyl groups on 6 displayed a doublet (δ = 0.68 ppm) while 6′ displayed a pair of upfield-shifted, diastereotopic doublets (δ = 0.58 ppm, 0.53 ppm) in 1H-NMR (Fig. S21). Computed proton chemical shifts (Fig. S49) suggest that the terminal methyl groups on 6′ are more upfield shifted than 6 due to their proximity to the phenylene groups on the opposite side of the nanohoop. 2D TOCSY, HSQC, and HMBC NMR experiments confirmed the presence of two separate species, 6 and 6′, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 ratio (Fig. S21).
0.8 ratio (Fig. S21).
Slow evaporation of a solution of double-SPAAC compounds (6 and 6′) in ethyl acetate resulted in yellow crystals. Crystals suitable for XRD revealed the solid-state structure of 6 (Fig. 3b) in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Much like XRD of 5, the resolution of diffraction data obtained from solid-state 6 is low (0.99 Å) but sufficient to determine connectivity and conformation. The overall structure was similar to 5, with an elongated oval shape (21.025 Å/11.019 Å). The two newly formed 2H-1,2,3-triazole rings are located between two phenylene groups, identical to 5. The average angle of the four remaining alkynes (∠C–C
. Much like XRD of 5, the resolution of diffraction data obtained from solid-state 6 is low (0.99 Å) but sufficient to determine connectivity and conformation. The overall structure was similar to 5, with an elongated oval shape (21.025 Å/11.019 Å). The two newly formed 2H-1,2,3-triazole rings are located between two phenylene groups, identical to 5. The average angle of the four remaining alkynes (∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) was 171.03°.
C) was 171.03°.
UV-vis absorbance, fluorescence emission, and quantum yield (Φ) were measured for Fl-[6]CPPA and the double-SPAAC products (Fig. 4). Fl-[6]CPPA displays a maximum absorption at 362 nm and emission at 468 nm (Φ = 0.56). 5/5′ and 6/6′ show slightly blue-shifted absorbance at 347 nm and 344 nm, respectively. On the other hand, the emission was significantly blue-shifted for 5/5′ (398/420 nm, Φ = 0.64) and 6/6′ (395/418 nm, Φ = 0.59). In contrast, Jasti and coworkers reported that carbon nanohoops with one alkyne group showed almost identical fluorescence wavelength before and after a single-SPAAC reaction.10,11 We attribute this difference to the near-total loss of curvature in the nanohoop backbone after two SPAAC reactions; Jasti's single-SPAAC product maintains its curved backbone opposite of the triazole. The calculated HOMO–LUMO energy levels of Fl-[6]CPPA and their SPAAC products revealed the increased band gap upon double-SPAAC reactions (Fig. S51), which agrees with the blue-shifted absorbance and emission that is observed.
 | ||
| Fig. 4 UV-vis absorption (abs) and normalized fluorescence emission (em) spectra of Fl-[6]CPPA (λex = 362 nm), 5/5′ (λex = 347 nm), and 6/6′ (λex = 344 nm) in CH2Cl2. | ||
Density functional theory (DFT) calculations were carried out to investigate the double-SPAAC on Fl-[6]CPPA. Gibbs free energy profiles for Fl-[6]CPPA reacting with TMSN3 at the alkyne positions between two phenylene groups (averaged ∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C angle = 166.1°) were computed at ωB97XD/def2-SVP employing Gaussian16. The two consecutive SPAAC reactions exhibit modest free energy barriers (
C angle = 166.1°) were computed at ωB97XD/def2-SVP employing Gaussian16. The two consecutive SPAAC reactions exhibit modest free energy barriers (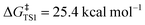 ,
, 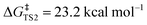 , Fig. 5). Upon the first cycloaddition with TMSN3, 7 adopts a teardrop shape, with the opposing alkyne position now bearing the most strain (note the smaller averaged alkyne ∠C–C
, Fig. 5). Upon the first cycloaddition with TMSN3, 7 adopts a teardrop shape, with the opposing alkyne position now bearing the most strain (note the smaller averaged alkyne ∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C angle, 164.7°). Increased strain at the opposing alkyne position lowers the activation energy of the second SPAAC by 2.2 kcal mol−1. Reacting with a second equivalent of TMSN3 at this position gives 8, a narrow ellipsoidal product. Overall, the double-SPAAC is highly exergonic (ΔG = −139.4 kcal mol−1). Similar results were observed by Hosoya and coworkers20 using Sondheimer diyne21 and by Michinobu and coworkers using octadehydrodibenzo[12]annulene.9 The first cycloaddition was the rate-limiting step, followed by a near-instantaneous second cycloaddition due to increased strain energy on the opposing alkyne. Experimentally, when Fl-[6]CPPA was treated with equimolar benzyl azide, the single-SPAAC intermediate could only be seen in trace amounts via MALDI-MS (Fig. S31).
C angle, 164.7°). Increased strain at the opposing alkyne position lowers the activation energy of the second SPAAC by 2.2 kcal mol−1. Reacting with a second equivalent of TMSN3 at this position gives 8, a narrow ellipsoidal product. Overall, the double-SPAAC is highly exergonic (ΔG = −139.4 kcal mol−1). Similar results were observed by Hosoya and coworkers20 using Sondheimer diyne21 and by Michinobu and coworkers using octadehydrodibenzo[12]annulene.9 The first cycloaddition was the rate-limiting step, followed by a near-instantaneous second cycloaddition due to increased strain energy on the opposing alkyne. Experimentally, when Fl-[6]CPPA was treated with equimolar benzyl azide, the single-SPAAC intermediate could only be seen in trace amounts via MALDI-MS (Fig. S31).
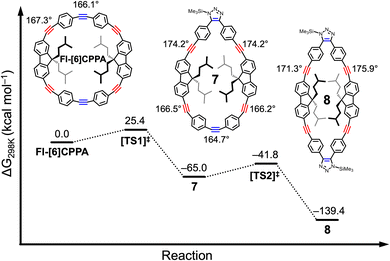 | ||
Fig. 5 Computed free energy profiles for Fl-[6]CPPA reacting with two equivalents of TMSN3 at the diphenyleneacetylene alkyne positions and averaged ∠C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C angles at each of the alkyne positions. C angles at each of the alkyne positions. | ||
A conformational search identified 2870 competitive structures within 3.19 kcal mol−1 relative to the optimized minimum structure of the Fl-[6]CPPA macrocycle. Of the 2870 structures considered, 18.1% of the structures exhibited distorted oblong shapes where strain increases in alkynes positioned between two phenylene groups (Fig. S49). This explains the observed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 product ratio of 6 and 6′; the alkyne position between two phenylene groups is more reactive when Fl-[6]CPPA adopts a distorted oblong shape.
0.8 product ratio of 6 and 6′; the alkyne position between two phenylene groups is more reactive when Fl-[6]CPPA adopts a distorted oblong shape.
In conclusion, Fl-[6]CPPA was synthesized in a good yield using alkyne metathesis macrocyclization. Incorporating two 2,7-fluorene units into the CPPA scaffold allows for the modular diversification of CPPA derivatives with tunable properties. Fl-[6]CPPA displays remarkable benchtop stability and selective strain-promoted reactivity. Two SPAACs on opposite sides of the nanohoop compress the ellipsoidal topology of Fl-[6]CPPA, relieving strain and preventing further reactions on the remaining alkynes. This reactivity could be utilized in ring-opening alkyne metathesis polymerization22 (ROAMP) to prepare linear conjugated polymers with high molecular weights. Furthermore, Pd-catalyzed cyclotrimerization13 of CPPA derivatives could lead to 2D framework-type materials.
This research was supported by the National Science Foundation (SL: CHE-1956302, JW: CHE-2303851). The authors would like to thank Senuri Jayawardana Arachchige for assistance with UV-vis and quantum yield studies, Dr Fengli Zhang and Dr Thomas Weldeghiorghis for assistance with 1D and 2D NMR experiments, and Dr Fabrizio Donnarumma from the Louisiana State University Mass Spec Facility for assistance with MALDI analysis.
Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article (synthetic methods, NMR spectra, mass spectrometry data, X-ray crystallography, computational calculations) and full reference for the Gaussian software are included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5cc05493a.CCDC 2392289 (6), 2392290 (5) and 2392291 (Fl-[6]CPPA) contain the supplementary crystallographic data for this paper.23a–c
References
- C. E. Colwell, T. W. Price, T. Stauch and R. Jasti, Chem. Sci., 2020, 11, 3923–3930 CAS.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646–17647 CrossRef CAS.
- K. Miki and K. Ohe, Chem. – Eur. J., 2020, 26, 2529–2575 CrossRef CAS PubMed.
- T. Kawase, H. R. Darabi and M. Oda, Angew. Chem., Int. Ed. Engl., 1996, 35, 2664–2666 CrossRef CAS.
- S. Lee, E. Chénard, D. L. Gray and J. S. Moore, J. Am. Chem. Soc., 2016, 138, 13814–13817 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CAS.
- E. Kayahara, R. Qu and S. Yamago, Angew. Chem., Int. Ed., 2017, 56, 10428–10432 CAS.
- E. Kayahara, T. Hayashi, K. Takeuchi, F. Ozawa, K. Ashida, S. Ogoshi and S. Yamago, Angew. Chem., Int. Ed., 2018, 57, 11418–11421 CAS.
- S. Fukushima, M. Ashizawa, S. Kawauchi and T. Michinobu, Helv. Chim. Acta, 2019, 102, e1900016 CrossRef.
- T. A. Schaub, J. T. Margraf, L. Zakharov, K. Reuter and R. Jasti, Angew. Chem., Int. Ed., 2018, 57, 16348–16353 CrossRef CAS PubMed.
- J. M. Fehr, N. Myrthil, A. L. Garrison, T. W. Price, S. A. Lopez and R. Jasti, Chem. Sci., 2023, 14, 2839–2848 RSC.
- T. A. Schaub, A. Zieleniewska, R. Kaur, M. Minameyer, W. Yang, C. M. Schüßlbauer, L. Zhang, M. Freiberger, L. N. Zakharov, T. Drewello, P. O. Dral, D. M. Guldi and R. Jasti, Chem. – Eur. J., 2023, 29, e202300668 CrossRef CAS PubMed.
- T. D. Clayton, J. M. Fehr, T. W. Price, L. N. Zakharov and R. Jasti, J. Am. Chem. Soc., 2024, 146, 30607–30614 CrossRef CAS.
- X. Zhou, H. Kwon, R. R. Thompson, R. J. Herman, F. R. Fronczek, C. J. Bruns and S. Lee, Chem. Commun., 2021, 57, 10887–10890 RSC.
- M. Linseis, S. Záliš, M. Zabel and R. F. Winter, J. Am. Chem. Soc., 2012, 134, 16671–16692 CrossRef CAS PubMed.
- J. Heppekausen, R. Stade, R. Goddard and A. Furstner, J. Am. Chem. Soc., 2010, 132, 11045–11057 CrossRef CAS.
- X. Zhou, R. R. Thompson, F. R. Fronczek and S. Lee, Org. Lett., 2019, 21, 4680–4683 CrossRef CAS.
- V. Bliksted Roug Pedersen, T. W. Price, N. Kofod, L. N. Zakharov, B. W. Laursen, R. Jasti and M. Brondsted Nielsen, Chemistry, 2024, 30, e202303490 CrossRef CAS PubMed.
- K. Niedermann, N. Früh, R. Senn, B. Czarniecki, R. Verel and A. Togni, Angew. Chem., Int. Ed., 2012, 51, 6511–6515 CrossRef CAS PubMed.
- I. Kii, A. Shiraishi, T. Hiramatsu, T. Matsushita, H. Uekusa, S. Yoshida, M. Yamamoto, A. Kudo, M. Hagiwara and T. Hosoya, Org. Biomol. Chem., 2010, 8, 4051–4055 RSC.
- H. N. C. Wong, P. J. Garratt and F. Sondheimer, J. Am. Chem. Soc., 1974, 96, 5604–5605 CrossRef CAS.
- S. von Kugelgen, I. Piskun, J. H. Griffin, C. T. Eckdahl, N. N. Jarenwattananon and F. R. Fischer, J. Am. Chem. Soc., 2019, 141, 11050–11058 CrossRef CAS.
- (a) CCDC 2392289: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2l9cmx; (b) CCDC 2392290: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2l9cny; (c) CCDC 2392291: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2l9cpz.
| This journal is © The Royal Society of Chemistry 2026 |