Frontiers in photodynamic therapy: type I NIR-II photosensitizers with aggregation-induced emission features
Ye
Tong
a,
Xue
Li
 *a,
Dong
Wang
*a,
Dong
Wang
 *a and
Ben Zhong
Tang
*a and
Ben Zhong
Tang
 *b
*b
aCenter for AIE Research, Shenzhen Key Laboratory of Polymer Science and Technology, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, China. E-mail: persist2803@126.com; wangd@szu.edu.cn
bGuangdong Basic Research Center of Excellence for Aggregate Science, School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen, Guangdong 518172, China. E-mail: tangbenz@cuhk.edu.cn
First published on 24th November 2025
Abstract
Photodynamic therapy (PDT) is a minimally invasive treatment modality characterized by high spatiotemporal precision and controllability, showcasing extensive potential applications in the biomedical field in recent years. However, conventional type II photosensitizers, which rely heavily on oxygen availability, exhibit limited therapeutic efficacy in hypoxic microenvironments such as solid tumors. Type I photosensitizers with their superior hypoxia tolerance offer an effective solution to this challenge. In particular, aggregation-induced emission (AIE)-active type I photosensitizers, especially those operating in the second near-infrared window (NIR-II, 1000–1700 nm), have attracted extensive research interest due to their unique advantages, including enhanced fluorescence emission in the aggregated state, efficient generation of free radical reactive oxygen species (ROS), and excellent tissue penetration capability. This review systematically discusses molecular design strategies for NIR-II AIE photosensitizers, focusing on enhancing intersystem crossing (ISC) and introducing high electron-affinity groups to promote the type I photodynamic process. Furthermore, it comprehensively summarizes the potential applications of these materials in tumor therapy, antibacterial treatment, and antiviral therapy, providing new perspectives for addressing the limitations of conventional PDT and advancing precision treatment strategies.
1. Introduction
Thousands of years ago, it was observed that certain plants (such as psoralea) could induce photosensitive reactions in the skin when exposed to sunlight, and were employed to treat conditions like vitiligo. This marked the ancient recognition that light could be harnessed for therapeutic purposes1,2 Modern scientific exploration of photodynamic therapy (PDT) commenced as early as the 18th century.3,4 A pivotal milestone was reached in 1993 when Canada first approved the photosensitizer Porfimer sodium (Photofrin®) for bladder cancer treatment; subsequent approvals by the U.S. FDA (1995) and European EMA (1999) followed for esophageal and lung cancers, respectively. The commercialization of Photofrin marked PDT's formal recognition as an authoritative therapeutic strategy for oncology.5 Since then, PDT has continued to evolve, and its applications have expanded beyond oncology into various other disease areas. As an innovative and promising therapeutic approach, PDT has achieved significant progress in treating numerous diseases, sparking considerable research interest.6,7 For instance, the efficacy of PDT in treating skin cancer, cervical cancer, superficial bladder cancer, as well as infectious diseases caused by pathogens8–10 (such as Cutibacterium acnes and human papillomavirus11,12), has been experimentally or clinically validated.The therapeutic mechanism of PDT primarily relies on the generation of reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide anion (O2•−), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). These ROS not only directly induce oxidative damage to cellular structures, leading to the death of cancer cells or pathogens, but also elicit vascular damage and activate local and systemic immune responses, thereby synergistically enhancing antitumor outcomes.13 During photodynamic processes, photosensitizers (PSs) generate ROS mainly through two types of photochemical reactions: type I and type II.14 Specifically, type I reactions proceed via hydrogen atom or electron transfer, yielding radicals such as •OH or O2•−, whereas type II reactions involve energy transfer from the excited triplet state of the PSs to ground-state molecular oxygen, resulting in the generation of 1O2.15
Despite these advantages, the advancement of PDT, particularly in the near-infrared window II (NIR-II, 1000–1700 nm) region, faces considerable challenges. The low photon energy in this spectral range is often insufficient to meet the energy threshold required for producing 1O2,16,17 fundamentally limiting the efficiency of type II reactions, Moreover, according to the energy gap law, molecules absorbing long-wavelength light exhibit small energy gaps between excited and ground states, facilitating non-radiative decay (e.g., thermal dissipation) over radiative pathways or ROS generation, thereby predisposing them to photothermal therapy (PTT).18,19 At the molecular design level, strong donor–acceptor architectures adopted to achieve NIR-II absorption often result in reduced triplet energy levels, further impairing ROS generation and predisposing the molecules to aggregation-caused quenching (ACQ) of both fluorescence and ROS.20 Furthermore, the deeply penetrating NIR-II light ideally targets solid tumors, which are often characterized by a highly hypoxic microenvironment, thereby severely compromising the oxygen-dependent efficacy of PDT.21 This hypoxia drastically diminishes the efficacy of oxygen-dependent PDT. Consequently, developing efficient NIR-II photodynamic systems requires overcoming multiple physical and physiological constraints.
The emergence of aggregation-induced emission luminogens (AIEgens) offers a promising solution to these challenges. Their unique mechanism involves restricting intramolecular motion to suppress non-radiative decay, thereby promoting radiative transitions and ROS generation.22 In contrast to conventional ACQ photosensitizers, AIEgens exhibit enhanced fluorescence emission and ROS production in the aggregated state, along with excellent photostability and biocompatibility, making them highly attractive for fluorescence-guided PDT/PTT applications.23–25 While ACQ dyes often suffer from fluorescence and ROS quenching due to strong π–π stacking, AIEgens effectively circumvent this through aggregation-enhanced emission.26–28 Recently, various phototherapeutic systems involving AIEgens have been meticulously designed and widely applied in the field of NIR-II FLI-guided PDT.29,30
Leveraging the distinctive advantages of AIEgens as ideal photosensitizers, an increasing number of type I AIE PSs have been rationally designed and have demonstrated highly efficient PDT in various disease models.31–33 This review aims to systematically summarize recent advances in type I NIR-II AIE photosensitizers, with an emphasis on their molecular design strategies and underlying mechanisms. We begin by outlining the fundamental photophysical and photochemical processes involved in type I photoreactions.34–36 Subsequently, we discuss rational design strategies for high-performance type I AIE PSs, focusing on two primary approaches: introducing strong electron-accepting units and enhancing intramolecular charge transfer (ICT) effects. We then showcase the broad biological applications of type I NIR-II AIE PSs in photodynamic therapy. Finally, the current limitations, challenges, and future development prospects of type I NIR-II AIE PSs are discussed (Scheme 1).
 | ||
| Scheme 1 Schematic diagram of an AIE luminogen-based system for photoacoustic imaging-guided combined photodynamic and photothermal therapy. | ||
2. The mechanism promoting the generation of type I ROS
Unlike the type II reaction, which generates 1O2 through direct energy transfer to triplet-state molecular oxygen, the type I process involves the formation of hydrogen peroxide (H2O2) and radical species (e.g., O2•−, •OH) through a series of electron-transfer and hydrogen-abstraction steps.1 More specifically, the type I pathway typically initiates with a one-electron reduction of the triplet-state photosensitizer (3PS*), yielding a photosensitizer radical anion (PS•−) (reaction 1). This anion can subsequently donate an electron to molecular oxygen, forming O2•− (reaction 2). Through either superoxide dismutase (SOD)-catalyzed disproportionation (reaction 3) or an additional one-electron reduction by PS•− (reaction 4), O2•− is converted into H2O2.37 The resulting H2O2 can then be transformed into highly oxidative hydroxyl radicals (•OH) via either the Haber–Weiss reaction (reaction 5) or the Fenton reaction (reaction 6) in the presence of Fe2+.38 Notably, the Fenton reaction can be enhanced through recycling: Fe3+ produced during the process is reduced back to Fe2+ by O2•− (reaction 7).39 By leveraging disproportionation, Haber–Weiss, and Fenton reactions, type I PDT has demonstrated enhanced therapeutic efficacy under hypoxic conditions compared to type II PDT. This advantage stems from several factors: (1) O2•− has a substantially longer half-life (several seconds) than 1O2 (∼ 10−5 s), allowing it to diffuse over greater distances within biological environments;40–42 (2) •OH is among the most reactive oxygen species, exhibiting high oxidative capacity that enables direct and severe damage to essential biomacromolecules, thereby amplifying PDT outcomes;43–45 (3) in contrast to the type II pathway, which consumes significant amounts of oxygen, the type I mechanism facilitates oxygen recycling through its reaction cascade, making efficient use of limited oxygen supplies and thus showing better performance under hypoxia.463. Design strategies for type I NIR-II AIE photosensitizer
Driven by the exceptional advantages of NIR-II AIE molecules and the significant practical potential of type I PSs, type I NIR-II AIE PSs have rapidly emerged over the past five years. As illustrated in Fig. 1, ISC is the primary step in ROS generation. Therefore, a high ISC rate, ensuring sufficient population of triplet excited states, is considered a prerequisite for enhancing the photosensitizing performance of PSs. To date, numerous studies have demonstrated strategies to promote the ISC process in PSs based on the ISC rate equation (Fig. 1). AIE molecules, owing to their distinctive donor (D)–acceptor (A) structural engineering, enable minimization of the energy gap (ΔES1–T1) and facilitate spin–orbit coupling (SOC) enhancement. However, specific strategies for selectively generating type I ROS over type II ROS remain relatively underdeveloped. The inherently low triplet energy of NIR-II molecules makes them less suitable for type II ROS generation, thereby providing a rationale for combining NIR-II AIE luminogens with type I ROS-generating PSs. Based on the fundamental principle of enhancing SOC, this section summarizes current approaches for constructing type I NIR-II AIE PSs into two main aspects: strengthening the electron donor–acceptor (D–A) architecture to promote ICT, thereby enabling more efficient type I ROS generation.3.1. Enhancing electron affinity for efficient electron transfer
A deeper understanding of the type-I photodynamic mechanism reveals that the triplet photosensitizer (3PS*) must first capture electrons from its surrounding environment before it can transfer them to oxygen, generating O2•−. Therefore, incorporating functional groups with high electron affinity facilitates this process by serving as effective electron-transfer mediators. However, the strong electron-accepting units employed in recent years—primarily limited to benzobisthiadiazole (BBTD) and 6,7-diphenylthiadiazoloquinoxaline (DPTQ)—exhibit a trend toward structural homogeneity. This has led to a convergence in design paradigms, which severely restricting the structural diversity and functional expandability of NIR-II materials.47–50To address this limitation, Tang et al.51 To address this limitation, Tang et al.51 rationally designed a series of PSs based on BBTD, DPTQ, and a novel acceptor unit—indandione-fused thiadiazolo[3,4-g]quinoxaline (ITQ) (Fig. 2A). In this system, two tert-butyl-substituted triphenylamine (TPA) groups serve not only as electron donors but also as efficient molecular rotors due to their highly twisted conformations and abundant σ-bonds, enabling the PSs to exhibit both a narrow energy gap and AIE characteristics. The thiadiazolo[3,4-g]quinoxaline moiety in ITQ confers high electron affinity, enhancing the intramolecular push–pull effect. Moreover, the additional carbonyl-containing indandione group extends the π-conjugation of the acceptor framework and contributes to SOC. This SOC effect benefits from the hybrid electronic configuration involving both n and π orbitals of the carbonyl group, facilitating transitions between singlet and triplet states.52,53 Experimental studies employing electron spin resonance (ESR) spectroscopy and specific fluorescent probes (e.g., DCFH) confirmed the excellent ROS generation capability of these PSs, with a propensity for producing superoxide anion (O2•−) and hydroxyl radical (•OH) via a type-I mechanism (Fig. 2C). Aggregation significantly enhanced ROS yields: TITQ exhibited typical AIE behavior and its ROS production in the nanoparticle state was markedly higher than in solution. This is attributed to aggregation-induced ISC, which suppresses non-radiative decay and channels more energy into the ISC process and subsequent ROS generation.
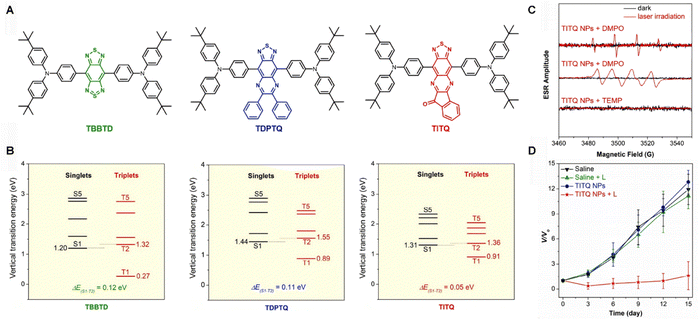 | ||
| Fig. 2 (A) Schematic diagram of the molecular structures. (B) Total ROS generation levels detected using the DCFH-DA probe. (C) ESR signals of radicals in TITQ nanoparticles captured by DMPO and TEMP under dark conditions and upon 808 nm laser irradiation. (D) Time-dependent tumor growth curves of tumor-bearing mice with various treatments.51 Reproduced from ref. 51 with permission from Springe, copyright 2024. | ||
A key contribution of this work is the proposal of a multi-level, synergistic strategy for enhancing ROS generation. The introduction of the novel ITQ acceptor was particularly crucial: its carbonyl unit effectively enhanced the SOC effect, and its combination with strong donor units reduced the singlet–triplet energy gap (ΔEST) to as low as 0.05 eV (Fig. 2B). These factors synergistically promoted efficient ISC. Furthermore, since the first triplet energy (T1) of this molecule is below 0.98 eV—insufficient for energy transfer to singlet oxygen 1O2—electron transfer became the dominant pathway. This enables thermodynamically spontaneous reactions (ΔG < 0) with oxygen or biomolecules, a particularly advantageous feature in hypoxic environments. As depicted in Fig. 2D, subsequent experiments evaluating TITQ molecules using the same tumour model revealed that tumour volumes in the control group increased rapidly during the study period, with no significant differences observed between the groups. This indicates that neither phototherapy alone nor TITQ NPs administered alone inhibited tumour growth. Encouragingly, laser-irradiated TITQ-NPs demonstrated a marked inhibitory effect on tumour growth.
In summary, this study significantly improved ROS generation by enhancing ISC efficiency, steering the reaction toward the type I pathway, and leveraging the AIE enhancement effect. It successfully established a design strategy for NIR-II type I AIE PSs based on high-electron-affinity acceptors, providing an important photochemical foundation for type-I photodynamic therapy.
3.2. Incorporation of metal complexes
Iridium, as a heavy atom, induces a significant SOC effect due to its strong nuclear charge. This effect mixes electron spin and orbital angular momentum, effectively relaxing the spin-forbidden nature of ISC and thereby greatly promoting the transition from the singlet excited state (S1) to the T1 in photosensitizing molecules. This process lays a critical foundation for efficient generation of ROS.54 Currently, several theranostic molecules based on Ru(II) complexes have demonstrated potential in integrating in vivo NIR-II imaging with NIR-light-cooperative therapy.55 As a group congenor, iridium(III) possesses a higher spin–orbit coupling constant than Ru(II) (4430 cm−1 for Ir3+vs. 990 cm−1 for Ru2+), which can further accelerate the ISC process, leading to enhanced ROS generation in PDT.56–59You et al.60 reported a novel iridium(III) complex, DPTPzIr (Fig. 3A), which exhibits exceptional ROS generation efficiency through a multi-factor synergistic design strategy, offering new insights for the development of metallophotosensitizers. The core mechanism originates primarily from the strong SOC effect of the iridium(III) metal center. Calculations revealed that DPTPzIr possesses a reduced singlet–triplet energy gap (ΔEST), significantly promoting ISC and enabling efficient conversion of singlet excitons into long-lived triplet excitons. This process is a crucial prerequisite for subsequent photophysical reactions and the main reason for its superior performance compared to the pure organic ligand DPTPz (Fig. 3B). DPTPzIr primarily undergoes a hypoxia-tolerant type I photodynamic process. Unlike the type II mechanism, which relies on energy transfer to generate 1O2, detection using DCFH and HPF probes confirmed that its triplet excitons mainly interact with surrounding substrates or oxygen via electron transfer, ultimately producing type-I ROS (Fig. 3C). Further verification using DMPO and TEMP as spin traps in electron paramagnetic resonance (ESR) studies under dark conditions and 808 nm laser irradiation confirmed that ROS generation by DPTPzIr nanoparticles follows predominantly a type-I mechanism. This mechanism reduces reliance on oxygen concentration, extending its therapeutic applicability within the tumor microenvironment. The D–A–D (donor–acceptor–donor) type ligand design adopted in the complex not only effectively narrows the energy gap—enabling absorption matched to an 808 nm laser and NIR-II emission—but also induces significant charge separation in the excited state, forming a polarized electron distribution that facilitates electron transfer. Further experiments demonstrated that DPTPzIr nanoparticles effectively target lysosomes and exhibit low dark toxicity towards normal tissue cells such as 3T3, LO2, and bEnd. 3 normal tissue cells under dark conditions (Fig. 3D) and demonstrated remarkable PDT therapeutic efficacy against 4T1 tumour cells under light irradiation (Fig. 3E), attributed to efficient intracellular type I ROS generation. Subsequent in vivo tumour model experiments revealed that DPTPzIr NPs exhibited favourable biocompatibility (Fig. 3F) and outstanding antitumour efficacy (Fig. 3G) in FLI-guided PDT. This mechanism reduces dependence on oxygen concentration, broadening its therapeutic applicability within the tumour microenvironment In summary, DPTPzIr achieves highly efficient ROS generation through a synergistic strategy of “metal-center driving–AIE enhancement–type I mechanism dominance”, providing a valuable paradigm for developing integrated metallophotosensitizers for the diagnosis and treatment of deep-seated tumors.
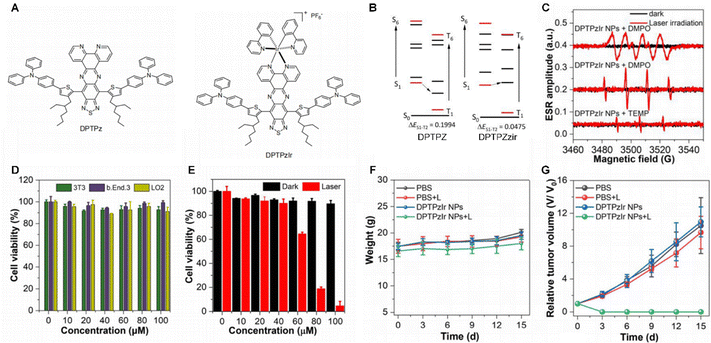 | ||
| Fig. 3 (A) Molecular structures of DPTPzIr and DPTPz. (B) Calculated excitation energies of low-lying excited states and ΔEST for DPTPzIr and DPTPz, obtained at the TDA-B3LYP level of theory. (C) ESR signals identifying the ROS generated by DPTPzIr nanoparticles under dark conditions and 808 nm laser irradiation, using DMPO and TEMP as spin traps. (D) Cell viability of 3T3, b.End.3 and LO2 cell lines incubated with DPTPzIr NPs by varying concentrations from 0 to 100 µM under dark conditions (mean ± SD, n = 3). (E) 4T1 cells viability incubated with DPTPzIr NPs in the presence and absence of 808 nm laser (mean ± SD, n = 3). (F) Body weights and (G) tumor growth profiles of mice from different treatment groups for 15 days (5 mice in each group) (mean ± SD, n = 5).60 Reproduced from ref. 60 with permission from American Chemical Society, copyright 2025. | ||
3.3. Acceptor planarization
In addition to enhancing the electron-donating ability of the donor in AIEgens, Chen et al.61 designed a novel pyrazine-based planar electron acceptor to construct a D–A–D structured molecule named Py-NIR (Fig. 4A), which significantly improved photodynamic performance. Its high efficiency in generating ROS stems from synergistic effects among multiple mechanisms. The core of this molecule's performance lies in its sophisticated structural design. The developed pyrazine-based planar electron acceptor possesses strong electron affinity. When coupled with two triphenylamine donor units via a unique “V-shaped” linkage that induces strong inter-donor repulsion, a highly twisted and conformationally flexible D–A–D structure is formed. This structure leads to significant spatial separation between the HOMO and LUMO orbitals and compresses the singlet–triplet energy gap (ΔEST) to an exceptionally low value of 0.14 eV (Fig. 4B). Such a small energy gap greatly promotes the intersystem crossing (ISC) process, enabling efficient conversion of singlet excitons into long-lived triplet excitons and providing ample triplet energy for subsequent photochemical reactions. Subsequent evaluation using the DCFH probe confirmed a ROS generation yield exceeding 400-fold (Fig. 4C). Furthermore, Py-NIR primarily undergoes a hypoxia-tolerant type I photodynamic mechanism. Validation using multiple specific fluorescent probes (such as HPF and DHR123) and ESR spectroscopy demonstrated that upon light irradiation, Py-NIR mainly produces highly cytotoxic hydroxyl radicals (•OH) rather than oxygen concentration-dependent 1O2 (Fig. 4D and E). This type I mechanism, proceeding via an electron transfer pathway, significantly reduces dependence on the oxygen level in the tumor microenvironment, broadening its therapeutic applicability and offering potential to overcome the limitations of conventional photodynamic therapy. It is noteworthy that the planar electron acceptor in this design plays a crucial “dual regulatory” role: its strong electron affinity and conjugated planar structure not only enhance ICT, enabling NIR-II emission, but also moderately restrict intramolecular motion, balancing the dissipation pathways of excited-state energy. Subsequent cellular experiments demonstrated Py-NIR NPs exhibit significant cytotoxic effects against 4T1 tumour cells under illumination (Fig. 4F). In an in situ mouse model of breast cancer, Py-NIR NPs also exhibited pronounced tumour suppression. Crucially, mice injected with Py-NIR NPs showed no significant weight difference compared to controls throughout the treatment period (Fig. 4G and H).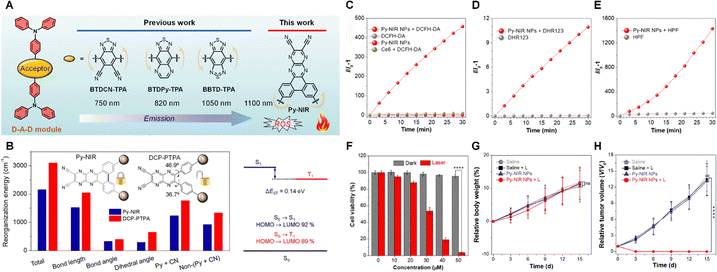 | ||
| Fig. 4 (A) Evolution of BTD-related electron acceptor structures in this study. (B) Proportional contributions of the pyrazine and cyano units (Py + CN) in Py-NIR and DCP-PTPA to the reorganization energy, along with bond lengths, bond angles, and dihedral angles and energy levels of the S1 and T1 states. (C–E) Changes in relative fluorescence emission intensity of (C) DCFH-DA, (D) DHR123, and (E) HPF in the presence of Py-NIR NPs. (F) cell viability of 4T1 cells with different treatments (mean ± SD, n = 6), ****p < 0.0001. (G) Changes in body weight of mice with treatment of saline, saline + L, Py-NIR NPs, and Py-NIR NPs + L, respectively during the 15-day study duration (mean ± SD, n = 6), ns: not significant. (H) Changes in tumor volume with different treatments during the 15-day study duration (mean ± SD, n = 5), ****p < 0.0001.61 Reproduced from ref. 61 with permission from Royal Society of Chemistry, copyright 2024. | ||
Additionally, Py-NIR exhibits distinct AIE characteristics, further enhancing its theranostic performance. In the aggregated state within nanoparticles (NPs), restricted intramolecular motion (RIM effect) not only enhances fluorescence emission but also further improves ROS generation efficiency.
3.4. Donor and π-bridge co-modulation strategy
Yang et al.62 constructed D–A photosensitising molecules by introducing methoxy-modified triphenylamine (MeO-TPA) as a strong electron donor and linking it via a thiophene π-bridge (Fig. 5A). Within these molecules, the thiazole and cyano-pyridine moieties function as potent electron-withdrawing groups, significantly enhancing intermolecular charge transfer effects to achieve long-wavelength absorption and emission. In MDPA-P-TCP and MDPA-T-TCP, the introduction of methoxy groups not only increases molecular free volume—facilitating structural deformation and molecular motion—but also amplifies thermogenic potential.63,64 Concurrently, the selection of phenyl and thiophene units as π-bridges further fine-tuned the compounds' ICT behaviour and degree of conjugation. The incorporation of this strong donor structure is pivotal for achieving high photodynamic performance: its potent electron-donating capacity induces a pronounced ICT effect. Calculations indicate that MDPA-T-TCP exhibits a higher spin–orbit coupling constant ξ(S1, Tn) between the S1 state and the excited triplet state compared to DPA-P-TCP and MDPA-P-TCP, suggesting superior ROS generation potential and effective promotion of the ISC process. This provides a rich triplet exciton source for ROS generation (Fig. 5A). To further characterise the generated ROS species, ABDA, DHR123, and HPF were employed to detect 1O2, O2˙−, and ˙OH generation using ABDA, DHR123, and HPF, respectively. The results reveal that, with the introduction of the methoxy group, MDPA-T-TCP and MDPA-P-TCP generate negligible amounts of 1O2 compared to DPA-P-TCP, indicating a shift towards generating type I ROS. However, upon introducing a thiophene ring to replace the benzene ring as the π-bridge, MDPA-T-TCP generates significantly higher levels of type I ROS than MDPA-P-TCP (Fig. 5B–D). In an in situ mouse breast cancer model, MDPA-T-TCP NPs similarly demonstrated significant tumour suppression effects (Fig. 5E). Crucially, throughout the treatment period, there was no significant difference in body weight between mice injected with MDPA-T-TCP nanoparticles and the control group (Fig. 5F). In summary, this study optimised photophysical processes at the light source via a ‘donor modulation’ strategy, successfully establishing a highly efficient diagnostic and therapeutic platform excitable by a single laser. This platform integrates near-infrared II imaging with type I photoluminescence. | ||
| Fig. 5 (A) Schematic diagram of the molecular design strategy for NIR-II photosensitizers and the singlet–triplet energy gap (ΔEST) for DPA-P-TCP, MDPA-P-TCP, and MDPA-T-TCP. (B) Production of 1O2 under the same conditions. (C) Detection results of O2•− generation. (D) Time-dependent generation curve of •OH by the nanoparticles under 660 nm laser irradiation. (E) Tumor growth of mice with different days under treatment. (F) Changes in body weight of mice in different remedy groups.62 Reproduced from ref. 62 with permission from Wiley-VCH, copyright 2025. | ||
3.5. Introduction of cationic moieties
As previously discussed, the type I photodynamic process typically initiates with the triplet-state photosensitizer (3PS) capturing a single electron from surrounding biological substrates. It can thus be inferred that providing an electron-rich microenvironment for 3PS under light irradiation would facilitate the capture of external electrons, thereby initiating the type I photoreaction. Based on this concept, researchers have developed a series of cationic-π-type AIE photosensitizers. By introducing cationic groups to construct a localized electron-rich environment, efficient type I ROS generation has been achieved.65–67 Under electron-rich conditions, if a photosensitizer exhibits significant ICT characteristics, it becomes more favorable for the type I mechanism—enhanced ICT can accelerate ISC, thereby improving ROS generation efficiency. Previous studies have confirmed that cationic photosensitizers with strong ICT properties can more effectively generate ROS via the type I pathway.68–70Zhuang et al.71 through ingenious molecular design, developed a novel NIR-II photosensitizer, TPEQM-DMA, whose mechanism for enhanced ROS generation represents a significant advancement in the field. This molecular design integrates AIE characteristics, a strong push–pull electronic effect, mitochondrial targeting capability, and a type I photochemical pathway into a single system (Fig. 6A). Specifically, the structure employs tetraphenylethylene (TPE) as the electron donor and AIE-active unit, combined with a strong electron-withdrawing quinolinium group. This not only achieves an emission redshift into the NIR-II region (>1000 nm), enhancing tissue penetration, but also demonstrates typical AIE behavior. More importantly, this design significantly reduces the singlet–triplet energy gap (ΔEST) (Fig. 6B), increases ROS production, and effectively promotes the electron transfer process (over energy transfer) under light irradiation, leading to the dominant generation of O2˙− and ˙OH (Fig. 6C). Furthermore, the lipophilic cationic structure at the molecular terminus enables specific targeting and accumulation within the mitochondria of cancer cells. Leveraging the high mitochondrial membrane potential, it facilitates an in situ burst of ROS, significantly enhancing oxidative stress damage. Further in vivo studies revealed favourable biosafety (Fig. 6D). Under laser irradiation, TTFMN-NPs induced highly efficient tumour ablation (Fig. 6E).
 | ||
| Fig. 6 (A) Chemical structure and design schematic of TPEQM-DMA. (B) Excited-state relaxation pathways and calculated S1 and T1 energy level distributions for TPEPM-DMA and TPEQM-DMA. (C) Detection results of O2•− generation and Detection results of •OH generation and EPR spectra obtained using DMPO and TEMP as spin traps under different conditions, in the presence or absence of TPEQM-DMA. The profiles of (D) tumor volume changes and (E) average weight loss of CT26 tumor-bearing mice during the whole period of TPEQM-DMA-mediated type-I phototheranostics.71 Reproduced from ref. 71 with permission from American Chemical Society, copyright 2023. | ||
This multi-level synergistic strategy—ranging from molecular energy level modulation to subcellular organelle localization—enables TPEQM-DMA to efficiently generate free radicals even within the hypoxic tumor microenvironment, demonstrating excellent therapeutic potential.
4. Applications of type I AIE PS
As indicated by the mechanisms described above, type I PSs, with their relatively low dependence on external oxygen, exhibit significant theranostic potential across various biomedical applications.4.1. Antitumor applications
The aggressive proliferation of cancer cells coupled with inadequate blood supply often leads to hypoxia within the tumor microenvironment of solid tumors. This severely impedes the production of type II ROS, which is highly dependent on ambient O2 concentration. In contrast, type I PDT demonstrates considerable potential for ablating hypoxic tumors, benefiting from its inherently lower O2 requirement. Consequently, AIE PSs capable of generating type I ROS represent ideal candidates for effective PDT, offering superior therapeutic outcomes.Zheng et al.79 developed a novel NIR-II AIEgen, 4TPQ, and its nanoformulation (4TPQ NPs), which demonstrated exceptional multi-modal theranostic efficacy in a model of breast cancer bone metastasis. Through systematic in vitro and in vivo evaluation, this system successfully achieved NIR-II FLI-guided synergistic PDT/PTT, exhibiting potent tumor suppression and favorable biosafety. In in vivo experiments, mice bearing 4T1 bone metastatic tumors, after intravenous injection of 4TPQ NPs, showed excellent tumor targeting and imaging performance. NIR-II FLI revealed that the strongest tumor signal was achieved at 24 hours, providing an optimal time window for precise intervention (Fig. 7A and B). Photothermal imaging (PTI) further confirmed its photothermal conversion efficacy: under 660 nm laser irradiation, the local tumor temperature rose to 54.7 °C within 10 min, far exceeding the threshold temperature required for PTT (∼ 42 °C), with the temperature increase being dependent on laser power and NP concentration (Fig. 7C and D). In terms of therapeutic efficacy, a single injection of 4TPQ NPs followed by one laser irradiation session (660 nm, 0.3 W cm−2, 10 min) significantly inhibited tumor growth. After 21 days of treatment, the 4TPQ NPs + Laser group exhibited the smallest tumor volume and weight, with bioluminescence signals significantly lower than those of other control groups (PBS, PBS + L, 4TPQ NPs without laser) (Fig. 7E–G). Furthermore, the mice maintained stable body weight throughout the treatment, and no pathological damage was observed in major organs, indicating good systemic safety (Fig. 7H). In summary, 4TPQ NPs, leveraging their efficient NIR-II imaging capability and excellent phototherapeutic performance, achieved precise image-guided synergistic therapy in a breast cancer bone metastasis model, offering a promising candidate for clinical translation.
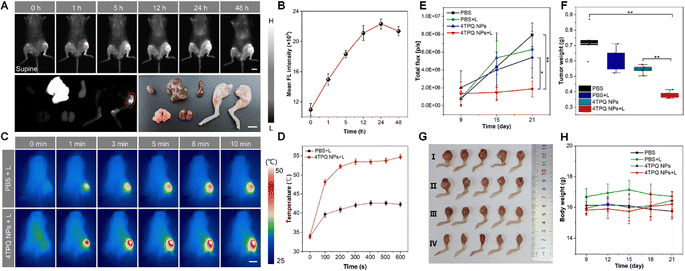 | ||
| Fig. 7 (A) FLI of the tumor region in 4T1 tumor-bearing mice at different time points after intravenous injection of 4TPQ nanoparticles (1 mg mL−1, 200 µL). Scale bar: 1 cm. (B) Quantitative analysis of the NIR-II fluorescence intensity in the tumor region corresponding to the time points in (A). (C) In vitro NIR-II fluorescence and bright-field images of the tumor and major organs harvested 48 hours post-injection. The tumor is circled in red. Scale bar: 1 cm. (D) The real-time temperature of tumor tissues in (C). (E) Quantified tumor bioluminescence levels of 4T1 breast cancer bone metastasis mice in each group (n = 5 biologically independent samples). Data are presented as mean ± SD. (F) Tumor-weight changes in mice (n = 5 biologically independent samples). (G) Photo images of the tumors for different groups at the end of the treatment. (I) PBS; (II) PBS + L; (III) 4TPQ NPs; (IV) 4TPQ NPs + L. Data are presented as mean ± SD. (H) Body-weight changes in mice (n = 5 biologically independent samples). Data are presented as mean ± SD79 Reproduced from ref. 79 with permission from Elsevier, copyright 2025. | ||
Among various cancers, pancreatic cancer, characterized by severe hypoxia, high invasiveness, and difficulty in diagnosis, shows limited response to conventional treatments, necessitating the development of novel theranostic strategies.80–82 Phototheranostics, which integrates diagnostic and therapeutic functions, provides a new paradigm for precision oncology. However, traditional PSs suffer from limitations such as insufficient tissue penetration depth, severe oxygen dependence, and heat shock protein (HSP)-mediated thermotolerance, which significantly restrict their efficacy in deep-seated, hypoxic tumors like pancreatic cancer.83–85 Recently, Li et al.86 provided a breakthrough solution through ingenious molecular engineering. They developed a NIR-II emissive AIEgen, DCTBT, and constructed a targeted liposomal nanoplatform, simultaneously achieving NIR-II FLI-guided highly efficient synergistic type I PDT and PTT, representing a major advance in the field of multi-modal theranostics (Fig. 8A). The core advantage of this system lies in its multi-modal synergy and imaging-guided therapeutic capability. Firstly, the excellent NIR-II emission properties of DCTBT liposomes (emission peak > 1000 nm) laid the foundation for high-resolution, deep-penetration real-time fluorescence imaging. The NIR-II window greatly reduces photon scattering and autofluorescence in biological tissues, enabling clear visualization of the precise contours and boundaries of subcutaneous and even orthotopic pancreatic tumors at the in vivo level, providing crucial spatial guidance for precise light irradiation therapy. More importantly, this study moved beyond the traditional type II (oxygen-dependent) PDT mechanism. By enhancing ICT, DCTBT efficiently generates O2•− and •OH via a type I photoreaction pathway under 808 nm laser excitation. This mechanism significantly reduces dependence on oxygen concentration in the tumor microenvironment, allowing it to maintain potent cytotoxic effects even in the typical hypoxic environment of pancreatic cancer. Crucially, this work profoundly demonstrates the synergistic enhancement between type I PDT and PTT. On one hand, the local hyperthermia (> 60 °C) generated by the PTT process effectively promotes blood perfusion in the tumor area, thereby alleviating tumor hypoxia and replenishing the “fuel” (oxygen) for the subsequent PDT process, breaking the oxygen limitation bottleneck of PDT (Fig. 8C). On the other hand, the ROS produced by type I PDT can directly damage organelles and downregulate HSP expression, thereby weakening the tumor cells' defense against thermotherapy and reversing thermotolerance in PTT. This bidirectional positive feedback mechanism allows the two therapies to complement each other, forming a powerful synergistic antitumor arsenal. In vitro and in vivo results showed that this synergistic treatment strategy under the precise guidance of NIR-II imaging (with in vitro NIR-II FLI showing maximum drug accumulation at 24 h, Fig. 8B) achieved significant tumor suppression effects (inhibition rate up to 87.5%) in both subcutaneous and orthotopic pancreatic cancer models, with negligible systemic toxicity (Fig. 8D–F). In conclusion, this study not only provides innovative molecular design ideas for developing a new generation of tumor phototheranostic agents but also validates the great clinical potential of imaging-guided multi-mode synergistic therapy through practice, paving a new way to overcome refractory malignancies such as pancreatic cancer.
 | ||
| Fig. 8 (A) Schematic diagram of the molecular design strategy for the NIR-II photosensitizer. (B) NIR-II fluorescence images of subcutaneous PANC-1 tumor-bearing mice at different monitoring times after intravenous injection of lip-DCTBT NPs, (a) nonTarget-NPs, (b) target-NPs. (C) Thermal images, heating temperatures (at tumor sites) of tumor-bearing mice during continuous 808 nm laser irradiation at 8 h postinjection of lip-DCTBT NPs. (D) Relative tumor volume changes for various treatment groups. **P < 0.01, ***P < 0.001. (E) Tumor images harvested at day 17 after different treatments. Scale bar: 1 cm. (F) The average tumor weights of each group recorded on day 17. **P < 0.01.86 Reproduced from ref. 86 with permission from Elsevier, copyright 2022. | ||
 | ||
| Fig. 9 (A) Schematic diagram of NIR-II photosensitizer Z1/Z1 NPs and its bio-applications. (B) Photoacoustic imaging (PAI) signals acquired at different time points from tumor-bearing mice after injection of Z1 nanoparticles. (C) Corresponding NIR-II fluorescence intensity signals. (D) Tumor volume and (E) body weight measurements of various groups of 4T1 tumor-bearing mice after treatment (n = 5). (F) Photographs of dissected tumor tissues from various groups after 14 days of treatment.87 Reproduced from ref. 87 with permission from Wiley-VCH, copyright 2024. | ||
Beyond its diagnostic prowess, Z1 NPs demonstrate remarkable therapeutic efficacy by predominantly generating O2•− and •OH via a type-I photochemical pathway, which effectively circumvents oxygen dependence. This ROS burst induces severe oxidative stress within mitochondria, leading to irreversible dysfunction and promoting dual cell-death mechanisms. Such a synergistic approach not only potentiates tumor cell apoptosis but also mitigates the risk of treatment resistance often associated with single-pathway therapies. Extensive in vitro and in vivo evaluations in a 4T1 tumor model confirmed the high targeting specificity and prolonged retention of Z1 NPs at the tumor site. Following phototherapeutic intervention, significant tumor suppression was observed, accompanied by minimal pathological changes in major organs, underscoring the system's high biocompatibility and therapeutic safety (Fig. 9D–F). This study provides profound molecular-level insights into the design of organelle-specific type-I photosensitizers and highlights the transformative potential of multimodal imaging in guiding synergistic cancer treatment regimens. It establishes a compelling framework for the future development of precision photo-immunotherapies and offers valuable directions for clinical translation in oncological treatment.
4.2. Antibacterial application
Bacteria are a common class of pathogenic microorganisms, with certain bacterial infections posing a grave threat to human health. These frequently cause severe illnesses such as foodborne diseases, tuberculosis, septicaemia, meningitis, and pneumonia. The emergence of antibiotic-resistant superbugs is exacerbating this situation.88,89 Given this severe challenge, PDT has become a promising candidate for antibacterial applications, including the inactivation of multidrug-resistant (MDR) microbial species. ROS can attack microorganisms without the PS needing to fully enter the microbe, potentially avoiding the development of microbial resistance.90 In this context, AIE-active phototheranostic agents have recently made significant breakthroughs in the diagnosis of infections and inflammation due to their inherent controllable and non-invasive characteristics.Xu et al.91 developed a NIR-II multifunctional theranostic probe, ZSY-TPE, based on the AIE characteristic, providing an innovative solution to this problem. It was successfully used for NIR-II FLI-guided PDT/PTT in a Staphylococcus aureus (S. aureus) infected mouse model. In vitro tests and cellular assays demonstrated powerful ROS generation capability (Fig. 10A and B). Subsequent work focused on demonstrating the imaging and therapeutic application of this probe in a bacterial infection model. In in vivo imaging experiments, after intravenous injection of ZSY-TPE dots, a gradually enhanced fluorescence signal over time could be clearly observed at the infection site via NIR-II FLI (Fig. 10C). The signal peaked at 24 hours with a signal-to-background ratio (SBR) as high as 9.6, significantly higher than that in tumor models, demonstrating excellent imaging contrast and infection-targeting ability (Fig. 10D and E). In vitro distribution experiments further confirmed that ZSY-TPE dots were mainly enriched in infected tissue with almost no retention in normal skin, indicating good targeting and biosafety (Fig. 10F). For treatment, infected mice were divided into four groups (PBS, PBS + Laser, ZSY-TPE dots, ZSY-TPE dots + Laser) and received a single laser irradiation (808 nm, 1.0 W cm−2, 5 min). Results showed that only the “ZSY-TPE dots + laser” group exhibited significant wound healing within 5 days, with a markedly reduced wound area (Fig. 10G and H), while other control groups developed suppuration and worsened conditions. This result indicated that ZSY-TPE dots, upon laser activation, could efficiently clear bacteria and promote tissue repair through synergistic PDT/PTT effects. In summary, this study successfully applied ZSY-TPE dots for NIR-II FLI-guided combined PTT/PDT in a bacterial infection model, demonstrating high-contrast imaging capability and significant therapeutic effects, providing a new strategy for the integration of visualization and phototherapy of deep-tissue infections.
 | ||
| Fig. 10 (A) Fluorescence spectra of a mixture containing ZSY-TPE nanoparticles and DCFH under 808 nm laser irradiation for 5 minutes. (B) Detection of ROS generation in 4T1 cells incubated with ZSY-TPE nanodots for 2 hours, treated with DCFH-DA for 30 minutes, and then irradiated with an 808 nm laser. Scale bar: 25 µm. (C) NIR-II fluorescence imaging of a Staphylococcus aureus-infected mouse after tail vein injection of ZSY-TPE nanodots. The infected region is marked by a yellow dashed line. (D) In vitro bright-field and fluorescence images. (E) Statistical analysis of the normalized fluorescence intensity and signal-to-background ratio (SBR) from the signals in (C) (n = 4). (F) Comparison of in vitro bright-field and fluorescence images between the infected area and normal skin 48 hours post-injection of ZSY-TPE nanodots. (G) Representative photographs of wound areas from different treatment groups in the S. aureus infection model. Scale bar: 10 mm. (H) Changes in the relative wound area over time (days 1, 3, and 5) for each group (n = 3).91 Reproduced from ref. 91 with permission from Elsevier, copyright 2020. | ||
4.3. Antiviral application
Besides bacterial infections, viruses are another important class of pathogens. Compared to bacteria, viruses are more infectious and lethal due to their smaller size and ability to destroy host cells by hijacking their machinery for replication. Similar to antibiotic resistance, resistance to antiviral drugs is also a current concern.92 Therefore, phototherapy is an alternative tool for antiviral treatment.Wang et al.93 developed a biomimetic nanoplatform for Mpox (monkeypox) theranostics, achieving efficient virus clearance and transmission blocking through the synergistic effect of PTT and type I PDT. This study was the first to propose a multi-modal theranostic strategy based on macrophage membrane-biomimetic nanoparticles (PN-AIE MØ) for efficient diagnosis, treatment, and transmission blockade of the Mpox virus via NIR-II FLI-guided synergistic PTT and PDT. The core of the research was the construction of a biomimetic nanosystem with viral targeting capability. This system used macrophage membranes pre-stimulated with vaccinia virus (a surrogate for Mpox virus) as a carrier to encapsulate the AIE photosensitizer TPE-BT-DCTBT, forming PN-AIE MØ. These nanoparticles not only possessed excellent NIR-II fluorescence emission ability but also exhibited efficient type I photodynamic effects and photothermal conversion performance (photothermal conversion efficiency of 34.1%), enabling fluorescence-photothermal dual-modal imaging and synergistic therapy both in vitro and in vivo (Fig. 11A). Subsequent experiments demonstrated the multi-modal imaging performance of PN-AIE MØ in a mouse tail model with Mpox-like skin lesions. After intravenous injection, PN-AIE MØ showed the strongest NIR-II fluorescence signal and the longest retention time (exceeding 72 hours) at the infection site, significantly superior to the untreated group and other control groups (AIE cores and N-AIE MØs). Under 808 nm laser irradiation, the PN-AIE MØ group showed a significant enhancement in infrared thermal signals, indicating its excellent photothermal imaging capability and targeted aggregation effect (Fig. 11B). This result was attributed to the upregulation of virus recognition receptors (such as CCR2, CD206, CD14) on the pre-stimulated macrophage membrane, enabling specific targeting and enrichment at the viral foci. Further studies validated the in vivo therapeutic efficacy of PN-AIE MØ. Under 808 nm laser irradiation, the PN-AIE MØ treatment group significantly promoted scab detachment and wound healing, almost completely clearing the viral load, while control groups (e.g., PBS + Laser, N-AIE MØ + Laser) showed only limited efficacy (Fig. 11C). Histological analysis (immunohistochemistry and H&E staining) showed that the PN-AIE MØ treatment group had significantly reduced viral antigen density, significantly reduced inflammatory infiltration and tissue necrosis area, and repaired epidermal layer. This indicated that PN-AIE MØ achieved effective virus killing and tissue repair through photothermal-photodynamic synergy. Furthermore, this study confirmed that PN-AIE MØ could effectively block virus transmission. Homogenates of lesion tissue taken from treated mice were reinoculated into healthy mice. The PN-AIE MØ + Laser group barely caused new lesions, and the viral load and inflammatory factor levels were significantly lower than those in the control groups, highlighting its potential in preventing Mpox transmission. In summary, this study, by constructing an intelligent biomimetic nanoplatform, achieved NIR-II FLI-guided synergistic PTT/PDT and multi-modal imaging for Mpox, not only providing a new strategy for the precise diagnosis and treatment of Mpox but also offering important references for the design of therapeutics for other viral diseases.
 | ||
| Fig. 11 (A) Pre-treated AIE-active nano-macrophages (PN-AIE MØs) for achieving NIR-II-triggered PDT and PTT of virus and prevention of its transmission. (B) NIR-II fluorescence intensity and corresponding statistical analysis at different time points for the PBS group, AIE core group, N-AIE MØs group, and PN-AIE MØs group. (C) Representative appearance of mouse tail wounds before and after treatment under different conditions. Scale bar: 3 cm.93 Reproduced from ref. 93 with permission from Cell Press, copyright 2024. | ||
5. Conclusion and perspectives
Benefiting from the AIE characteristics and ROS generation capabilities of AIE PSs, as well as the significant advantages demonstrated by type I PDT in overcoming the limitations of traditional type II PDT, AIE-active type I photosensitizers have gradually emerged as a burgeoning research hotspot after years of development, showing great potential in promoting the future of PDT. Based on the latest research progress of novel NIR-II AIE-type I PSs, this review has systematically summarized the photophysical and photochemical processes involved in the type I reaction mechanism, corresponding molecular design strategies, and their practical applications in the biological field. Beyond a high ISC rate ensuring sufficient triplet excitons to participate in subsequent electron or energy transfer processes, it is particularly crucial to develop strategies that preferentially initiate the type I electron transfer pathway over the type II energy transfer pathway when constructing type I PSs. Currently, based on the type I reaction cascade, there are two main molecular design approaches to promote type I ROS generation: first, introducing functional groups with high electron affinity into the acceptor moiety to capture and stabilize external electrons; second, enhancing the ICT intensity through donor engineering or acceptor planarization. Guided by these strategies, various type I AIE PSs have been successfully developed and applied in type I ROS-mediated tumor and pathogen eradication, as well as in antibacterial and antiviral applications. Furthermore, leveraging their advantages such as fluorescence enhancement in the aggregated state, abundant intramolecular motion units, and ease of structural functionalization, researchers have also realized the construction of FLI-guided type-I PDT and even FLI-guided synergistic type-I PDT/PTT theranostic platforms by rationally regulating the excited-state energy dissipation pathways of type-I AIE PSs.Despite the promising prospects, the further development of type I AIE PSs still faces several challenges and opportunities. Firstly, although several design strategies have been proposed to promote the ISC process and enhance the performance of AIE PSs, authoritative methods capable of specifically activating the type I reaction path are still relatively lacking. Although the aforementioned two types of strategies have achieved some success, their practical application cases are still limited, and their universality needs to be verified by more experiments. Therefore, there is an urgent need for more in-depth mechanistic studies on the photochemistry of type I reactions to establish clearer design principles and develop more ingenious construction routes. Secondly, to accurately evaluate the ROS generation ability of type-I AIE PSs, avoid misjudgment, and promote their gradual development, it is imperative to develop quantitative analysis methods capable of precisely detecting type I ROS (O2•− and •OH). Most existing detection methods can only provide qualitative results, making direct quantitative comparison of the performance of different PSs difficult, which to some extent limits the systematic development and optimization of such materials.
Author contributions
Ye Tong: responsible for the overall writing and structural design of the paper. Xue Li: research and literature search. Dong Wang: conceived the research framework and led the literature review, providing theoretical support and innovative directions for the writing of the review. Ben Zhong Tang: conceived the research framework, providing theoretical support and innovative directions for the writing of the review.Conflicts of interest
The authors declare no conflict of interest.Data availability
No new data were created or analyzed in this study. All information is derived from cited publications.Acknowledgements
This work was partially supported by the National Key Research and Development Program of China (2024YFA1212100), National Natural Science Foundation of China (52573204), the Science and Technology Foundation of Shenzhen City (JCYJ20241202124423032, 20220809130438001), the Pearl River Talent Recruitment Program (2019QN01Y103), Medical-Engineering Interdisciplinary Research Foundation of Shenzhen University (2023YG021), and Research Team Cultivation Program of Shenzhen University (2023QNT003)Notes and references
- C. N. Lee, R. Hsu, H. Chen and T. W. Wong, Molecules, 2020, 25, 5195 CrossRef CAS.
- D. Mitton and R. Ackroyd, Photodiagn. Photodyn. Ther., 2008, 5, 103–111 CrossRef CAS PubMed.
- M. R. Hamblin, Photochem. Photobiol., 2020, 96, 506–516 CrossRef CAS PubMed.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Pharmaceutics, 2021, 13, 1332 CrossRef CAS PubMed.
- T. Hu, Z. Wang, W. Shen, R. Liang, D. Yan and M. Wei, Theranostics, 2021, 11, 3278–3300 CrossRef CAS PubMed.
- F. Cieplik, D. Deng, W. Crielaard, W. Buchalla, E. Hellwig, A. Al-Ahmad and T. Maisch, Crit. Rev. Microbiol., 2018, 44, 571–589 CrossRef CAS.
- M. R. Hamblin, Curr. Opin. Microbiol., 2016, 33, 67–73 CrossRef CAS PubMed.
- G. Ambreen, L. Duse, I. Tariq, U. Ali, S. Ali, S. R. Pinnapireddy, M. Bette, U. Bakowsky and R. Mandic, Cancers, 2020, 12, 3278 CrossRef CAS.
- P. Wang, H. Tang and P. Zhang, Nanomedicine, 2018, 13, 2629–2636 CrossRef PubMed.
- Y. D. Pertiwi, T. Chikama, K. Sueoka, J.-A. Ko, Y. Kiuchi, M. Onodera and T. Sakaguchi, Photodiagn. Photodyn. Ther., 2019, 28, 166–171 CrossRef CAS.
- H. Bai, W. He, J. H. C. Chau, Z. Zheng, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Biomaterials, 2021, 268, 120598 CrossRef CAS PubMed.
- S. Shen, J. Feng, X. Song and W. Xiang, Photodiagn. Photodyn. Ther., 2022, 39, 102913 CrossRef CAS.
- J. M. Dabrowski and L. G. Arnaut, Photochem. Photobiol. Sci., 2015, 14, 1765–1780 CrossRef CAS.
- C. S. Foote, Photochem. Photobiol., 1991, 54, 659 CrossRef CAS PubMed.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kędzierska, K. Knap-Czop, J. Kotlińska, O. Michel, K. Kotowski and J. Kulbacka, Biomed. Pharmacother., 2018, 106, 1098–1107 CrossRef.
- G. Feng, G. Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- H. Gao, X. Duan, D. Jiao, Y. Zeng, X. Zheng, J. Zhang, H. Ou, J. Qi and D. Ding, Angew. Chem., Int. Ed., 2021, 60, 21047–21055 CrossRef CAS PubMed.
- Y. Yuan, G. Feng, W. Qin, B. Z. Tang and B. Liu, Chem. Commun., 2014, 50, 8757 RSC.
- F. Hu, S. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350 CrossRef.
- M. Kang, Z. Zhang, N. Song, M. Li, P. Sun, X. Chen, D. Wang and B. Z. Tang, Aggregate, 2020, 1, 80–106 CrossRef.
- Z. Zhang, W. Xu, M. Kang, H. Wen, H. Guo, P. Zhang, L. Xi, K. Li, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, 2003210 CrossRef CAS.
- W. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 7476–7487 CrossRef CAS.
- C. Xu, R. Ye, H. Shen, J. W. Y. Lam, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2022, 61, e202204604 CrossRef CAS PubMed.
- Y. Duo, G. Luo, W. Zhang, R. Wang, G. G. Xiao, Z. Li, X. Li, M. Chen, J. Yoon and B. Z. Tang, Chem. Soc. Rev., 2023, 52, 1024–1067 RSC.
- Z. Fan, Y. Liu, Y. Ye and Y. Liao, Aggregate, 2024, 5, e620 CrossRef CAS.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS.
- M. Gao and B. Z. Tang, Coord. Chem. Rev., 2020, 402, 213076 CrossRef CAS.
- L. Chen, S. Chen, Y. Yuan, X. Leng, X. Xu, J. Chen, J. Shi, K. Qian, Y. Xie, Q. Ding, Z. Cheng and M. Gu, Aggregate, 2024, 5, e657 CrossRef CAS.
- J. Li, Z. Zhuang, Z. Zhao and B. Z. Tang, View, 2022, 3, 20200121 CrossRef CAS.
- S. Liu, Y. Pei, Y. Sun, Z. Wang, H. Chen, D. Zhu, M. R. Bryce, B. Z. Tang and Y. Chang, Aggregate, 2024, 5, e547 CrossRef CAS.
- H. Wen, Z. Zhang, M. Kang, H. Li, W. Xu, H. Guo, Y. Li, Y. Tan, Z. Wen, Q. Wu, J. Huang, L. Xi, K. Li, L. Wang, D. Wang and B. Z. Tang, Biomaterials, 2021, 274, 120892 CrossRef CAS PubMed.
- W. Zhang, X. Li, M. Kang, Z. Zhang, Y. Pei, M. Fan, D. Yan, Y. Zhang, C. Yang, G. Xu, D. Wang, Z. Xu and B. Z. Tang, ACS Materials Lett., 2024, 6, 2174–2185 CrossRef CAS.
- S. Song, Y. Zhao, M. Kang, Z. Zhang, Q. Wu, S. Fu, Y. Li, H. Wen, D. Wang and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2107545 CrossRef CAS.
- W. Zhu, M. Kang, Q. Wu, Z. Zhang, Y. Wu, C. Li, K. Li, L. Wang, D. Wang and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2007026 CrossRef CAS.
- Y. Chen, D. Li, X. Chen, D. Wang, Y. Huang, Y. Gao, F. Liu, X. Lin, D. Zhao, J. Ji, D. Wang and Q. Jin, ACS Nano, 2025, 19, 16147–16162 CrossRef CAS PubMed.
- D. Li, P. Liu, Y. Tan, Z. Zhang, M. Kang, D. Wang and B. Z. Tang, Biosensors, 2022, 12, 722 CrossRef CAS PubMed.
- Q. Wan, R. Zhang, Z. Zhuang, Y. Li, Y. Huang, Z. Wang, W. Zhang, J. Hou and B. Z. Tang, Adv. Funct. Mater., 2020, 30, 2002057 CrossRef CAS.
- H. Ding, H. Yu, Y. Dong, R. Tian, G. Huang, D. A. Boothman, B. D. Sumer and J. Gao, J. Controlled Release, 2011, 156, 276–280 CrossRef CAS PubMed.
- Z. Zhuang, J. Dai, M. Yu, J. Li, P. Shen, R. Hu, X. Lou, Z. Zhao and B. Z. Tang, Chem. Sci., 2020, 11, 3405–3417 RSC.
- M. Li, Y. Shao, J. H. Kim, Z. Pu, X. Zhao, H. Huang, T. Xiong, Y. Kang, G. Li, K. Shao, J. Fan, J. W. Foley, J. S. Kim and X. Peng, J. Am. Chem. Soc., 2020, 142, 5380–5388 CrossRef CAS PubMed.
- M. Li, T. Xiong, J. Du, R. Tian, M. Xiao, L. Guo, S. Long, J. Fan, W. Sun, K. Shao, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2019, 141, 2695–2702 CrossRef CAS PubMed.
- Y. Xu, Y. Xie, Q. Wan, J. Tian, J. Liang, J. Zhou, M. Song, X. Zhou and M. Teng, Aggregate, 2024, 5, e612 CrossRef CAS.
- H. Sies, Eur. J. Biochem., 1993, 215, 213–219 CrossRef CAS.
- S. Marklund, J. Biol. Chem., 1976, 251, 7504–7507 CrossRef CAS.
- M. Garcia-Diaz, Y. Y. Huang and M. R. Hamblin, Methods, 2016, 109, 158–166 CrossRef CAS.
- Y. Wang, K. Ma, M. Kang, D. Yan, N. Niu, S. Yan, P. Sun, L. Zhang, L. Sun, D. Wang, H. Tan and B. Z. Tang, Chem. Soc. Rev., 2024, 53, 12014–12042 RSC.
- S. Song, Y. Wang, Y. Zhao, W. Huang, F. Zhang, S. Zhu, Q. Wu, S. Fu, B. Z. Tang and D. Wang, Matter, 2022, 5, 2847–2863 CrossRef CAS.
- B. Li, M. Zhao and F. Zhang, ACS Materials Lett., 2020, 2, 905–917 CrossRef CAS.
- J. Mu, M. Xiao, Y. Shi, X. Geng, H. Li, Y. Yin and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202114722 CrossRef CAS.
- D. Yan, W. Xie, J. Zhang, L. Wang, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 26769–26776 CrossRef CAS.
- J. Li, N. Niu, D. Wang, X. Liu, Y. Qin, L. Wang, B. Z. Tang and D. Wang, Sci. China: Chem., 2024, 67, 2647–2660 CrossRef CAS.
- Z. Yang, Z. Zhang, Y. Sun, Z. Lei, D. Wang, H. Ma and B. Z. Tang, Biomaterials, 2021, 275, 120934 CrossRef CAS PubMed.
- Z. Yang, Z. Zhang, Z. Lei, D. Wang, H. Ma and B. Z. Tang, ACS Nano, 2021, 15, 7328–7339 CrossRef CAS PubMed.
- Y. Xu, C. Li, J. An, X. Ma, J. Yang, L. Luo, Y. Deng, J. S. Kim and Y. Sun, Sci. China: Chem., 2023, 66, 155–163 CrossRef CAS.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Nat. Commun., 2022, 13, 2009 CrossRef CAS.
- S. Monro, K. L. Colón, H. Yin, J. Roque, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron and S. A. McFarland, Chem. Rev., 2019, 119, 797–828 CrossRef CAS PubMed.
- L. Tu, C. Li, X. Xiong, J. Hyeon Kim, Q. Li, L. Mei, J. Li, S. Liu, J. Seung Kim and Y. Sun, Angew. Chem., Int. Ed., 2023, 62, e202301560 CrossRef CAS.
- Y. Fan, C. Li, S. Bai, X. Ma, J. Yang, X. Guan and Y. Sun, Small, 2022, 18, 2201625 CrossRef CAS PubMed.
- Y. Liu, Q. Li, M. Gu, D. Lu, X. Xiong, Z. Zhang, Y. Pan, Y. Liao, Q. Ding, W. Gong, D. S. Chen, M. Guan, J. Wu, Z. Tian, H. Deng, L. Gu, X. Hong and Y. Xiao, J. Med. Chem., 2022, 65, 2225–2237 CrossRef CAS PubMed.
- C. You, L. Tian, J. Zhu, L. Wang, B. Z. Tang and D. Wang, J. Am. Chem. Soc., 2025, 147, 2010–2020 CrossRef CAS PubMed.
- M. Chen, Z. Zhang, R. Lin, J. Liu, M. Xie, X. He, C. Zheng, M. Kang, X. Li, H.-T. Feng, J. W. Y. Lam, D. Wang and B. Z. Tang, Chem. Sci., 2024, 15, 6777–6788 RSC.
- X. Yang, D. Wang, J. Zhu, D. Yan, J. Tong, Y. Ding, D. Wang, G. Shan and B. Z. Tang, Adv. Funct. Mater., 2025, e07262 CrossRef CAS.
- H. Gao, S. Shi, S. Wang, Q. Tao, H. Ma, J. Hu, H. Chen and J. Xu, Aggregate, 2024, 5, e394 CrossRef CAS.
- D. Zhou, G. Zhang, J. Li, Z. Zhuang, P. Shen, X. Fu, L. Wang, J. Qian, A. Qin and B. Z. Tang, ACS Nano, 2024, 18, 25144–25154 CrossRef CAS PubMed.
- W. Xu, M. M. S. Lee, J. Nie, Z. Zhang, R. T. K. Kwok, J. W. Y. Lam, F. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9610–9616 CrossRef CAS PubMed.
- X. Zhao, Y. Dai, F. Ma, S. Misal, K. Hasrat, H. Zhu and Z. Qi, Chem. Eng. J., 2021, 410, 128133 CrossRef CAS.
- D. Yan, D. Wang and B. Z. Tang, Nat. Rev. Bioeng., 2025, 3, 976–991 CrossRef.
- S. Wang, C. Chen, J. Wu, J. Zhang, J. W. Y. Lam, H. Wang, L. Chen and B. Z. Tang, Sci. China: Chem., 2022, 65, 870–876 CAS.
- W. Lv, Z. Zhang, K. Y. Zhang, H. Yang, S. Liu, A. Xu, S. Guo, Q. Zhao and W. Huang, Angew. Chem., Int. Ed., 2016, 55, 9947–9951 CrossRef CAS.
- L. Ke, F. Wei, L. Xie, J. Karges, Y. Chen, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2022, 61, e202205429 CrossRef CAS PubMed.
- J. Zhuang, B. Wang, H. Chen, K. Zhang, N. Li, N. Zhao and B. Z. Tang, ACS Nano, 2023, 17, 9110–9125 CrossRef CAS PubMed.
- Z. Zhang, W. Xu, P. Xiao, M. Kang, D. Yan, H. Wen, N. Song, D. Wang and B. Z. Tang, ACS Nano, 2021, 15, 10689–10699 CrossRef CAS PubMed.
- Z. Zhang, X. Li, H. Yang, C. You, K. Ren, Z. Yang, M. Kang, D. Wang and B. Z. Tang, Aggregate, 2025, 6, e70102 CrossRef CAS.
- J. Li, M. Kang, Z. Zhang, X. Li, W. Xu, D. Wang, X. Gao and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202301617 CrossRef CAS PubMed.
- S. Liu, Y. Li, J. Zhang, H. Zhang, Y. Wang, C. Chuah, Y. Tang, J. W. Y. Lam, R. T. K. Kwok, H. Ou, D. Ding and B. Z. Tang, Mater. Today Bio., 2021, 10, 100087 CrossRef CAS PubMed.
- Y. Qin, X. Chen, Y. Gui, H. Wang, B. Z. Tang and D. Wang, J. Am. Chem. Soc., 2022, 144, 12825–12833 CrossRef CAS PubMed.
- Z. Sun, H. Wen, Z. Zhang, W. Xu, M. Bao, H. Mo, X. Hua, J. Niu, J. Song, M. Kang, D. Wang and B. Z. Tang, Biomaterials, 2023, 301, 122276 CrossRef CAS PubMed.
- S. Yang, J. Zhang, Z. Zhang, R. Zhang, X. Ou, W. Xu, M. Kang, X. Li, D. Yan, R. T. K. Kwok, J. Sun, J. W. Y. Lam, D. Wang and B. Z. Tang, J. Am. Chem. Soc., 2023, 145, 22776–22787 CrossRef CAS PubMed.
- P. Zheng, X. Yan, J. Zhu, Y. Liu, L. Wang, H. Su, D. Wang and B. Z. Tang, Biomaterials, 2025, 317, 123105 CrossRef CAS PubMed.
- R. L. Siegel, K. D. Miller, H. E. Fuchs and A. Jemal, Ca-Cancer J. Clin., 2021, 71, 7–33 CrossRef PubMed.
- M. Falasca, M. Kim and I. Casari, Biochim. Biophys. Acta, Rev. Cancer, 2016, 1865, 123–132 CrossRef CAS.
- M. A. Tempero, J. Berlin, M. Ducreux, D. Haller, P. Harper, D. Khayat, H.-J. Schmoll, A. Sobrero and E. Van Cutsem, Ann. Oncol., 2011, 22, 1500–1506 CrossRef CAS.
- J. Chen, T. Fan, Z. Xie, Q. Zeng, P. Xue, T. Zheng, Y. Chen, X. Luo and H. Zhang, Biomaterials, 2020, 237, 119827 CrossRef CAS.
- J. Du, T. Shi, S. Long, P. Chen, W. Sun, J. Fan and X. Peng, Coord. Chem. Rev., 2021, 427, 213604 CrossRef CAS.
- Z. Zhou, J. Song, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- D. Li, X. Chen, D. Wang, H. Wu, H. Wen, L. Wang, Q. Jin, D. Wang, J. Ji and B. Z. Tang, Biomaterials, 2022, 283, 121476 CrossRef CAS.
- B. Wang, H. Zhou, L. Chen, Y. Ding, X. Zhang, H. Chen, H. Liu, P. Li, Y. Chen, C. Yin and Q. Fan, Angew. Chem., Int. Ed., 2024, 63, e202408874 CrossRef CAS.
- S. R. Singh, Environ. Microbiol., 2014, 2(4), 106–115 Search PubMed.
- N. D. Wolfe, C. P. Dunavan and J. Diamond, Nature, 2007, 447, 279–283 CrossRef CAS PubMed.
- M. Kang, C. Zhou, S. Wu, B. Yu, Z. Zhang, N. Song, M. M. S. Lee, W. Xu, F.-J. Xu, D. Wang, L. Wang and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 16781–16789 CrossRef CAS PubMed.
- Y. Xu, Y. Zhang, J. Li, J. An, C. Li, S. Bai, A. Sharma, G. Deng, J. S. Kim and Y. Sun, Biomaterials, 2020, 259, 120315 CrossRef CAS PubMed.
- G. Reina, S. Peng, L. Jacquemin, A. F. Andrade and A. Bianco, ACS Nano, 2020, 14, 9364–9388 CrossRef CAS PubMed.
- W. Wang, B. Li, Y. Wu, M. Li, S. Ma, D. Yan, D. Li, J. Zhang, X. Li, Q. Gao, L. Zhao, Z. Hu, Y. Jiang, Z. Liu, K. Liu, Y. Yan, Y. Feng, J. Zheng, B. Shu, J. Wang, H. Wang, L. He, S. Zhou, D. Wang, C. Shen, B. Z. Tang and Y. Liao, Matter, 2024, 7, 1187–1206 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





