Construction of a Ru@WO3−x–W catalyst with hydrogen spillover effect toward ampere-level hydrogen evolution in acidic solution
Hailin
Ye
,
Yuefei
Xin
,
Zhengjie
Zhang
,
Shichun
Cheng
,
Ruyi
Zhang
,
Mingming
Wang
,
Yuchan
Zhu
 * and
Zhandong
Ren
* and
Zhandong
Ren
 *
*
Hubei Key Laboratory of Agricultural Waste Resource Utilization, School of Chemical and Environmental Engineering, Wuhan Polytechnic University, Wuhan, 430023, P. R. China. E-mail: renzhandong@163.com; zhuyuchan@163.com
First published on 18th November 2025
Abstract
A Ru@WO3−x–W/TM catalyst with WO3−x(surface)–W(bulk) structure was carefully designed and prepared. Compared with W or WO3 support, the presence of WO3−x–W modifies the electronic structure, optimizes H adsorption/desorption and promotes hydrogen spillover with Ru. The Ru@WO3−x–W/TM catalyst can generate an industrial HER current density of 1204 mA cm−2 in an acidic system.
As a hydrogen production technology with zero carbon emission, research on electrocatalytic water splitting catalysts has been widely undertaken. Pt is undoubtedly the best catalyst for the hydrogen evolution reaction (HER), but its high cost and scarce reserves limit its large-scale application. Ru has a hydrogen adsorption energy (65 kcal mol−1) similar to that of Pt (62 kcal mol−1) and is considered a promising candidate as a substitute for Pt. The HER activity of Ru can be improved by optimizing its electronic structure and morphology, including alloying, engineering defects, phase transformation, strain engineering, heteroatom doping and functionalized support. At present, the design of efficient HER catalysts using the “hydrogen spillover strategy from metal to support” has attracted wide attention. In the process of hydrogen spillover, H atoms are adsorbed and desorbed at two different active sites, which can overcome the limitations of the Sabatier principle.
Some progress has been made in Ru-based HER catalysts designed with hydrogen spillover in mind. The commonly used supports are reducible metal oxides, such as WO3, CeO2, MoO2 and TiO2,1–3 which exhibit hydrogen spillover effects with Ru. To further improve the efficiencies of hydrogen spillover, metal oxide supports rich in oxygen defects, such as WO3−x, MoOx, NiMoO4−x and LaCeOx, were studied.4–8 In addition, various supports, such as metal phosphides,9–12 metal carbides,13–16 metal sulfides,17,18 metal selenides,19 metal hydroxides,20 transition metal (oxy)hydroxides,21 MXene,22 layered double hydroxides23 and functional carbon supports,24,25 have also been combined with metal Ru to induce efficient hydrogen spillover. In addition to the improvement of the support, the hydrogen spillover efficiency can be further improved by replacing metal Ru with metal Ru composites, such as RuMo alloy,5 Ru2P,11 Ru1Fe112 and RuSx.17
With the deepening understanding of hydrogen spillover, it has been found that there is also a significant reverse spillover effect in HER catalysts. The reverse hydrogen spillover pathway is that the adsorbed hydrogen overflows from the support to the metal catalyst. Reverse hydrogen spillover can also greatly improve HER performance, especially in neutral and alkaline solutions. For the Ru/WO3−x electrocatalyst,4 the existence of the reverse spillover effect (H overflow from WO3−x to Ru) was demonstrated through in situ Raman spectroscopy and DFT calculations. In the Ru/W2C heterogeneous catalyst,26 H2O dissociates on the W2C support, and the H intermediate thus formed overflows to the adjacent interface Ru site for H–H coupling and H2 release. In addition, significant reverse hydrogen spillover also occurs for Ru/NiMoO4−x,6 LaCeOx@NGr/Ru1,7 Ru/ac-ZrO2,8 Ru–Mo2C,13 Ru/WCx,14 and Ru1–Mo2C16 catalysts, which significantly improves their HER performances. Meanwhile, some researchers have put forward the principle of minimum work function difference8,12,18,19,23,27 and crystal surface matching26 in the design of catalysts with obvious hydrogen spillover.
Ru-based hydrogen spillover catalysts still have the following two problems. (1) The conductivity of reducible oxide support is poor, and the preparation process of support with a defect structure is relatively complicated. (2) There has been little research into acidic systems (Table S2, SI), and the current density rarely reaches the ampere level. In this paper, a Ru@WO3−x–W/TM catalyst with WO3−x(surface)–W(bulk) phase structure is designed for the first time, and its preparation process is simple, as shown in Scheme 1 (detailed description is presented in the SI). The presence of the WO3−x–W phase modifies the electronic structure of the catalyst, increases the conductivity, optimizes the H adsorption/desorption performance, and leads to an improvement of hydrogen spillover efficiency. The current density of Ru@WO3−x–W/TM reaches 1204 mA cm−2, which meets the requirements of industrial application.
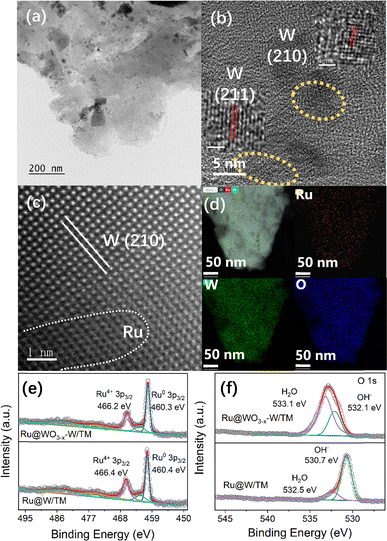
The morphology of Ru@WO3−x–W/TM was analyzed by transmission electron microscopy (TEM). In Fig. 1a and S1a (SI), it presents a thin-film structure. The particle size distribution is uniform within the range of 2–5 nm in Fig. S1b (SI). In the high-resolution TEM (HRTEM) image, W (211) and (210) planes with interplanar spacings of 0.206 and 0.230 nm were observed (Fig. 1b). In Fig. S1c and d (SI), W nanoparticles in an amorphous state can also be observed. Moreover, typical dark dots—corresponding to Ru atoms distributed as atomic clusters—can be observed on the lattice of W in the aberration-corrected high-angle annular dark-field scanning TEM (AC-HAADF-STEM) image (Fig. 1c).28 The corresponding elemental mapping in Fig. 1d confirms that Ru, W and O are evenly distributed. In the XRD patterns of Ru@W/TM and Ru@WO3−x–W/TM (Fig. S2a, SI), because the diffraction peaks of W and Ti are very close, only the diffraction peaks of the TM support were observed. However, in Fig. S2b (SI), if the TM support is replaced by an indium–tin oxide (ITO) support, the diffraction peaks of W(210) and WO3−x(110) can be observed. In addition, the composition analysis results of Ru@WO3−x–W are listed in Table S1 (SI).
XPS analysis of Ru@WO3−x–W/TM was carried out to investigate the modification of its electronic structure. For the W in Ru@W, the binding energy (B.E.) peaks at 33.8 and 31.3 eV belong to the 4f orbitals of W0, and the B.E. peaks at 38.0 and 35.8 eV belong to the 4f orbitals of W6+ in Fig. S3 (SI). For Ru@WO3−x–W, the situation is different. The B.E. peaks of the W6+ 4f orbital have an obvious positive shift, which are located at 39.0 and 36.9 eV. This is the result of electrochemical oxidation. Meanwhile, a weak B.E. peak of the W 4f orbital was observed at 35.2 eV, and its valence state was between 0 and 6+. The above analysis proves that the WO3−x–W phase is formed. In Fig. 1e, the B.E. peaks of 466.4 and 460.4 eV can be attributed to the spin-splitting peaks of Ru4+ and Ru0 3p orbitals in Ru@W/TM. Compared with those of Ru@W/TM, the B.E. peaks of the Ru 3p orbital of Ru@WO3−x–W/TM are negatively shifted. There are two forms of adsorbed oxygen in the O 1s peaks of Ru@W/TM, namely OH− (530.7 eV) and H2O (532.5 eV) in Fig. 1f. However, for Ru@WO3−x–W/TM, they are located at 532.1 and 533.1 eV. The obvious positive shift of B.E peaks of O 1s indicates the formation of a RuO(OH)2 phase on the surface of Ru@WO3−x–W/TM. The theoretical calculation results of Karlsson et al.29 had indicated that, after 1–6H atom is modified to the surface of RuO2, the peak of O 1s for OH− will move by 1.7–2.6 eV, while the peak of O1s without H will move by 0.3–0.8 eV. Detailed analysis is presented in Table S3 (SI).
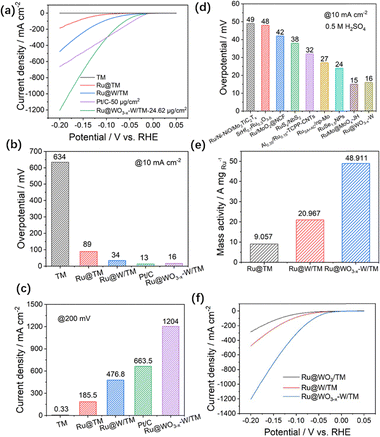
The HER activity of a Ru@WO3−x–W/TM electrode is shown in Fig. 2a. At the same time, the HER activities of Ru/TM, Ru@W/TM and commercial Pt/C electrodes are also compared. First, the titanium mesh (TM) support has a porous structure, but it has almost no HER activity. The Ru/TM electrode obtained by simply electrodepositing Ru on the TM support shows a certain HER activity, but it is not ideal. The overpotential at 10 mA cm−2 (η10) of the Ru/TM electrode is 89 mV in Fig. 2b. However, for the Ru@W/TM electrode, the situation changes greatly, and its η10 decreased to 34 mV. The electrode with the best HER activity is Ru@WO3−x–W/TM, and its η10 is reduced to 16 mV, which is similar to that of Pt/C (η10 = 13 mV). From the perspective of current density, Ru@WO3−x–W/TM reaches 1204 mA cm−2 at an overpotential of 200 mV (j200) in Fig. 2c, reaching a level suitable for industrial production. The j200 of Ru@WO3−x–W/TM is not only 6.5 and 2.5 times that of Ru/TM and Ru@W/TM respectively, but also 1.8 times that of Pt/C. At present, Ru-based electrocatalysts have been extensively studied in alkaline systems and have demonstrated satisfactory HER activities (Table S4, SI). However, in acidic systems, Ru-based electrocatalysts have not been studied enough (Table S2, SI), and HER activities need to be further improved. In this paper, the Ru@WO3−x–W/TM catalyst has achieved HER activity corresponding to ampere-level current, which is superior to that of most reported Ru-based catalysts in acidic systems (Fig. 2d). Mass activities (MAs) of Ru/TM and Ru@W/TM are 9.1 and 21.0 A mg−1, respectively (Fig. 2e). The MA of Ru@WO3−x–W/TM is 48.9 A mg−1, which is 5.4 times higher than that of Ru/TM and 2.3 times than that of Ru@W/TM. This suggests that the utilization rate of Ru has been improved in Ru@WO3−x–W/TM. In Fig. S4, the electrochemically active surface area (ECSA) of Ru@WO3−x–W/TM is the largest, which is beneficial for improved HER activity (detailed description is shown in the SI). At the same time, the specific activity and turnover frequency of Ru@WO3−x–W/TM are the largest in Fig. S5 (SI), which indicates that the intrinsic activity is also improved.
The formation of WO3−x–W on the surface plays an important role in improving HER activity. This is related to the scan rate during electro-oxidation. The slower the scan rate, the more efficient the oxidation. With the decrease of scan rate, the η10 of Ru@WO3−x–W/TM decreases from 32 to 16 mV, and the j200 increases from 817.5 to 1204 mA cm−2 (Fig. S6, SI). The MA of Ru@WO3−x–W/TM increases from 37.4 to 48.9 A mg−1 (Fig. S7, SI), and its ECSA increases from 134.7 to 195.5 cm2 (Fig. S8, SI). In Fig. 2f and Fig. S9 (SI), the HER activity of Ru@WO3/TM (bulk oxidation, as proved by XRD in Fig. S10, SI) is obviously lower than that of Ru@WO3−x–W/TM (surface oxidation), even lower than that of Ru@W/TM. Meanwhile, the MA and ECSA of Ru@WO3/TM are also lower than those of Ru@WO3−x–W/TM (Fig. S11 and S12, SI). This indicates that bulk oxidized WO3 is not as effective in promoting HER activity as surface oxidized WO3−x.
The increase in HER activity of Ru@WO3−x–W/TM may come from the change of its H adsorption and desorption properties. In Fig. S13 (SI), the H adsorption/desorption charges of Ru/TM, Ru@W/TM and Ru@WO3−x–W/TM are obtained by integrating the hydrogen region areas. Whether it is H adsorption or desorption charge, they both exhibit the following changing trend: Ru@WO3−x–W/TM > Ru@W/TM > Ru/TM. An increase in H adsorption charge facilitates the Volmer step in the HER process, and an increase in H desorption charge promotes the Tafel or Heyrovsky step in the HER process. In addition, H adsorption and desorption potentials can also reflect the change of electrochemical properties of the electrode. In Fig. S14a and b (SI), the potential of Had of Ru@WO3−x–W/TM is the highest, which indicates that it is most beneficial to the H adsorption process. At the same time, the potential of Hdes of Ru@WO3−x–W/TM is the lowest, indicating that it is also the most beneficial to H desorption. In Fig. S14c (SI), by comparing the CVs of W/TM and WO3−x–W/TM, it can be concluded that WO3−x–W/TM is more effective in activating and adsorbing H (from the charge and potential of Had). Therefore, the change in the characteristics of Ru@WO3−x–W/TM comes from the formation of the WO3−x–W phase and the resulting hydrogen spillover effect between WO3−x–W and Ru. In addition, the changes of Had/Hdes properties of Ru@WO3−x–W/TM with different oxidation degrees (Fig. S15, SI) and Ru@WO3/TM (Fig. S16, SI) are further discussed in the SI.
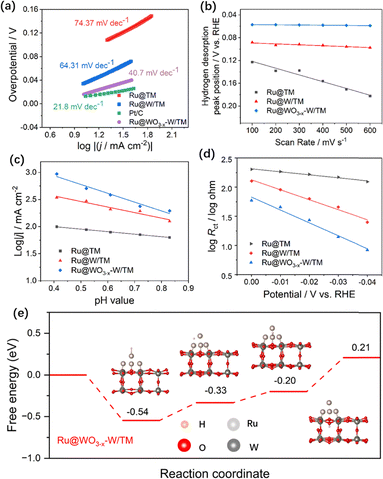
In order to study the dynamics of the HER process, Tafel analyses are performed, as shown in Fig. 3a. The Tafel slope of Ru/TM is very high (74.37 mV dec−1), indicating slow reaction kinetics. The Tafel slope of Ru@W/TM decreases to 64.31 mV dec−1, indicating the acceleration of reaction kinetics. The fastest kinetics of the HER reaction is exhibited by Ru@WO3−x–W/TM, and its Tafel slope has been reduced to 40.70 mV dec−1, which is close to that of Pt/C (21.80 mV dec−1). Moreover, the values of Tafel slope indicate that the mechanism of HER on a Ru@WO3−x–W/TM electrode is consistent with the Volmer–Heyrovsky pathway, in which the Heyrovsky step is a rate-determining one. Tafel slopes of Ru@WO3−x–W/TM electrodes with different surface oxidation degrees were analyzed, as shown in Fig. S17 (SI). In addition, the Nyquist diagrams of Ru/TM, Ru@W/TM and Ru@WO3−x–W/TM, recorded at an overpotential of 50 mV by electrochemical impedance spectroscopy (EIS) measurements, are shown in Fig. S18. The EIS measurements of Ru@WO3−x–W/TM electrodes with different surface oxidation degrees are shown in Fig. S19 (SI). The results of long-term stability tests of Ru@WO3−x–W/TM are shown in Fig. S20 (SI). XPS, XRD and composition analysis results after stability tests are shown in Fig. S21, S22 and Table S1 (SI), respectively.
In order to further quantitatively prove the influence of hydrogen spillover of Ru@WO3−x–W/TM on H adsorption/desorption kinetics, the following analyses were made. In the potential range of 0–0.5 V, CVs were performed at different scan rates in Fig. S23 (SI). With the increase of scan rate, the Hdes potentials of Ru@W/TM and Ru@WO3−x–W/TM do not move forward obviously in Fig. 3b, which means that their hydrogen desorption kinetics are faster. Second, the effect of [H+] on HER activity kinetics was investigated, as shown in Fig. S24 (SI). In Fig. 3c, the slope for Ru@WO3−x–W/TM is 1.63, which is obviously higher than that for Ru/TM (1.02) and Ru@W/TM (0.47). The slope for Ru@WO3−x–W/TM is close to 2, which indicates that there is obvious hydrogen spillover. EIS measurements were conducted on Ru/TM, Ru@W/TM and Ru@WO3−x–W/TM at different overpotentials as shown in Fig. S25 (SI). The hydrogen adsorption kinetics can be quantified by plotting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rctvs. potential in Fig. 3d. Ru@WO3−x–W/TM exhibits a lower Tafel slope, indicating a higher hydrogen adsorption rate, which may be due to enhanced hydrogen spillover. As shown in Fig. 3e, the Hads Gibbs free energy (ΔGH*) at site-A (Ru-top) is −0.54 eV, which is beneficial for the occurrence of H adsorption. ΔGH* is 0.21 eV for site-D (O–WO3−x support), which is beneficial for H desorption. Moreover, ΔGH* at site-A is closer to zero, which can balance the H adsorption and desorption for enhancing HER activity. In addition, the transfer between active sites has a small migration energy barrier. ΔGH* between site-C (Ru–O bridge) and site-D is 0.41 eV, implying a lower energy barrier for the migration of Hads from Ru to WO3−x.
Rctvs. potential in Fig. 3d. Ru@WO3−x–W/TM exhibits a lower Tafel slope, indicating a higher hydrogen adsorption rate, which may be due to enhanced hydrogen spillover. As shown in Fig. 3e, the Hads Gibbs free energy (ΔGH*) at site-A (Ru-top) is −0.54 eV, which is beneficial for the occurrence of H adsorption. ΔGH* is 0.21 eV for site-D (O–WO3−x support), which is beneficial for H desorption. Moreover, ΔGH* at site-A is closer to zero, which can balance the H adsorption and desorption for enhancing HER activity. In addition, the transfer between active sites has a small migration energy barrier. ΔGH* between site-C (Ru–O bridge) and site-D is 0.41 eV, implying a lower energy barrier for the migration of Hads from Ru to WO3−x.
In summary, there is a significant hydrogen spillover effect between Ru and WO3−x–W, resulting in an industrial-level current density of 1204 mA cm−2 in acidic systems. The η10 of Ru@WO3−x–W/TM is 16 mV, which is obviously lower than that of Ru/TM, Ru@W/TM and Ru@WO3/TM, and close to that of Pt/C.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details and addition data. See DOI: https://doi.org/10.1039/d5cc05104e.Notes and references
- K. Shun, K. Mori, S. Masuda, N. Hashimoto, Y. Hinuma, H. Kobayashi and H. Yamashita, Chem. Sci., 2022, 13, 8137–8147 RSC.
- S. Zhou, H. Jang, Q. Qin, L. Hou, M. G. Kim, S. Liu, X. Liu and J. Cho, Angew. Chem., Int. Ed., 2022, 1, e202212196 Search PubMed.
- Y. Chen, Y. Liu, L. Li, T. Sakthivel, Z. Guo and Z. Dai, Adv. Funct. Mater., 2024, 34, 2401452 CrossRef CAS.
- J. Chen, C. Chen, M. Qin, B. Li, B. Lin, Q. Mao, H. Yang, B. Liu and Y. Wang, Nat. Commun., 2022, 13, 5382 CrossRef CAS PubMed.
- Z. Zhao, J. Sun, X. Li, S. Qin, C. Li, Z. Zhang, Z. Li and X. Meng, Nat. Commun., 2024, 15, 7475 CrossRef CAS PubMed.
- X. Liu, X. Wang, K. Li, J. Tang, J. Zhu, J. Chi, J. Lai and L. Wang, Angew. Chem., Int. Ed., 2024, 63, e202316319 CrossRef CAS.
- V. Dao, H. Choi, S. Yadav, J. D. Jiménez, C. Kim, T. V. Nguyen, K. Chen, P. Uthirakumar, Q. V. Le, S. D. Senanayake, H. Y. Kim and I.-H. Lee, Appl. Catal., B, 2024, 343, 12345 CrossRef.
- J. Niu, H. Duan, T. Sun, Z. Zhi, D. Li, X. Fan, L. Zhang and D. Yang, Small, 2025, 2410436 CrossRef.
- S. Liu, Z. Li, Y. Chang, M. G. Kim, H. Jang, J. Cho, L. Hou and X. Liu, Angew. Chem., Int. Ed., 2024, 63, e202400069 CrossRef CAS.
- K. Ji, S. Wang, S. Yao, Y. Ji, J. Li, X. Wang, L. Shi, G. Wang, W. Ren, J. Wang, F. Zhang, J. Xie, Z. Yang and Y.-M. Yan, Adv. Mater., 2025, 37, 2503182 CrossRef CAS.
- Y. Hong, S. Jeong, J. H. Seol, T. Kim, S. C. Cho, T. K. Lee, C. Yang, H. Baik, H. S. Park, E. Lee, S. J. Yoo, S. U. Lee and K. Lee, Adv. Energy Mater., 2024, 14, 2401426 CrossRef CAS.
- J. Li, Y. Tan, M. Zhang, W. Gou, S. Zhang, Y. Ma, J. Hu and Y. Qu, ACS Energy Lett., 2022, 7, 1330–1337 CrossRef CAS.
- D. K. Sam, Z. Tang and Y. Cao, J. Colloid Interface Sci., 2025, 699, 138213 CrossRef CAS PubMed.
- J. Zhao, M. Kou, Q. Yuan, Y. Yuan and J. Zhao, Small, 2025, 21, 2406022 CrossRef CAS.
- J. Sun, Z. Wang, Y. Wang, Y. Song, Y. Pei, W. Yan, R. Xiong, Y. Liu, B. Lin, X. Wang, X. Zhang, J. Chen and L. Zhang, Small, 2025, 21, 2411975 CrossRef CAS.
- T. Chao, W. Xie, Y. Hu, G. Yu, T. Zhao, C. Chen, Z. Zhang, X. Hong, H. Jin, D. Wang, W. Chen, X. Li, P. Hu and Y. Li, Energy Environ. Sci., 2024, 17, 1397–1406 RSC.
- H. Yue, Z. Guo, Z. Zhou, X. Zhang, W. Guo, S. Zhen, P. Wang, K. Wang and W. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202409465 CrossRef CAS PubMed.
- J. Chen, J. Ni, H. Xu, X. Qian, Y. Liu, L. Jin, K. Wang, G. He and H. Chen, Int. J. Hydrogen Energy, 2025, 139, 621–629 CrossRef CAS.
- C. Mu, H. Xin, Q. Luo, Y. Li and F. Ma, J. Mater. Chem. A, 2023, 11, 7016–7024 RSC.
- R. Tang, P. Yan, Y. Zhou and X.-Y. Yu, EES Catal., 2024, 2, 932–940 RSC.
- H. Lei, W. Yang, S. Hu, L. Yi, C. Ma, C. Hu, F. Qian, M. Zhao, L. Liu, G. Yang and Q. Chen, Angew. Chem., Int. Ed., 2025, 64, e202503871 CrossRef CAS PubMed.
- J. Tang, X. Liu, X. Wang, S. Wu, J. Zhu, X. Wang, T. Wang, J. Chi, Z. Wu and L. Wang, Small, 2024, 21, 2409675 CrossRef.
- J. Zhu, J. Chi, X. Wang, T. Cui, L. Guo, B. Dong, X. Liu and L. Wang, Nano Energy, 2024, 121, 109249 CrossRef CAS.
- X. Liu, F. Xie, Q. Xi, Y. Wang, Y. Wang, H. Li, R. Li, C. Fan, J. Liu and J. Wang, J. Colloid Interface Sci., 2025, 694, 137738 CrossRef CAS.
- Q. Li, F. Huang, S. Li, H. Zhang and X.-Y. Yu, Small, 2021, 18, 2104323 CrossRef.
- J.-Z. Jiang, S. Liu, Z. Li, M. G. Kim, H. Jang, X. Liu and L. Hou, Adv. Energy Mater., 2025, 15, 2405546 CrossRef CAS.
- Y. Liu, H. Li, X. Liu, Y. Wang, L. Wang, T. Yang, A. R. Jadhav, J. Zhang, Y. Wang, M. Wu, J. Y. Lee, M. G. Kim and H. Lee, ACS Nano, 2024, 18, 874–884 CrossRef CAS PubMed.
- H. Liu, G. Tan, M. Li, Z. Zhang, M. G. Sendeku, Y. Li, Y. Kuang and X. Sun, Chem. Eng. J., 2023, 458, 141414 CrossRef CAS.
- R. K. B. Karlsson, A. Cornell and L. G. M. Pettersson, J. Phys. Chem. C, 2016, 120, 7094–7102 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |