Mn-doping method boosts Se doping concentration in Cu2S towards high thermoelectric performance†
Chen
Gong
,
Zhiwei
Zeng
,
Xiaoling
Sun
,
Chengran
Luo
and
Hongyi
Chen
 *
*
College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: hongyichen@csu.edu.cn
First published on 19th October 2024
Abstract
Copper chalcogenides have garnered attention as promising thermoelectric materials, owing to their environmental benignity, cost-effectiveness, and superior thermoelectric performance. Anionic doping within the same group is an effective method to improve the thermoelectric performance of copper chalcogenides, but Se-doped Cu2S contains a large fraction of the secondary phase leading to poor thermoelectric properties. In this study, we refined the thermoelectric properties of Cu2S by extending the concentration limit of Se-doping concentration. Our findings reveal that the incorporation of 2% Mn significantly elevates the concentration limit of Se from 1.5% to 5%. The increased Se doping effectively narrows the band gap, augmenting the carrier concentration to an optimal level. Furthermore, it also adjusts the electronic structure, enabling additional bands to contribute to electron transport, which results in elevated weighted mobility, and consequently, enhanced electrical conductivity performance. Additionally, the Se doping introduces dislocations and lattice distortions that bolster alloy scattering, thereby diminishing lattice thermal conductivity. As a result, the zT of Cu1.96Mn0.02S0.95Se0.05 reaches a peak of 0.97 at 723 K, which is 3.8 times that of Cu2S, and at a high level compared with the reported Cu2S-based materials. Hence, our research underscores the efficacy of co-doping as a strategy to expand the Se-doping concentration limit, and anionic doping within the same group can optimize the thermoelectric properties of Cu2S-based materials.
1. Introduction
Thermoelectric (TE) materials represent a burgeoning class of energy conversion materials, capable of directly transforming thermal energy into electrical energy, and are poised for significant development in the face of dwindling natural resources.1–3 The thermoelectric efficiency of TE materials hinges on a dimensionless figure of merit, zT, defined as zT = α2σT/κ, where α is the Seebeck coefficient, σ is the electrical conductivity, κ is the thermal conductivity, and T is the absolute temperature in Kelvin.4–6 Researchers have discovered a variety of thermoelectric materials with excellent properties, such as PbTe,7–9 GeTe,10–12 SnTe,13,14 BiTe15,16 and so on. Copper chalcogenides, characterized by liquid-like properties, exhibit low lattice thermal conductivity and good thermoelectric performance, positioning them as promising candidates for thermoelectric applications.17–20Anionic doping within the same group in copper chalcogenide has been demonstrated to diminish lattice thermal conductivity and modulate carrier concentration, thereby effectively ameliorating the TE performance of these materials.21–23 For instance, 8% S doping in Cu2Se has been shown to optimize carrier concentration, augmenting the power factor (PF) to 11 μW cm−1 K−2 at 1000 K.24 30% Te doping in Cu2Se has been reported to curtail lattice thermal conductivity, thereby elevating the zT value to 1.4 at 1000 K.25 Se or S doping in Cu2Te has similarly been observed to enhance TE performance.26 However, Se-doping in Cu2S is less effective due to a low doping concentration limit, leading to suboptimal TE performance. Wang et al. detected a substantial fraction of Se-rich secondary phases in the energy-dispersive spectroscopy (EDS) analysis of Cu1.98S0.985Se0.015, alongside an additional phase transition peak in differential scanning calorimetry (DSC), suggesting that the doping concentration limit of Se less than 1.5%.27 Recent studies have indicated that anionic and cationic co-doping methodologies can extend the anionic doping limit, exemplified by Cu1.8Ag0.2Se0.9S0.128 and Pb0.9875Sb0.0125Te1−xSx.29 Thus, co-doping emerges as a promising avenue to escalate the doping concentration limit of Se in Cu2S and to optimize its TE properties.
In this study, we optimized the TE properties of Cu2S by increasing the Se-doping concentration limit. Mn doping weakens the Cu–S bond and stabilizes the high-temperature cubic phase, which increases the doping concentration limit of Se. Therefore, we involved 2% Mn in Cu2S, successfully elevating the doping concentration limit of Se from 1.5% to 5%. Furthermore, Se doping was found to narrow the band gap, thereby increasing the carrier concentration to an optimal value. Additionally, it adjusted the electronic structure, facilitating the participation of an extra band in electron transport, which led to enhanced weighted mobility, and a consequent increase in the power factor to 7.74 μW cm−1 K−2 at 723 K. Moreover, the Se doping induced alloy scattering, which served to reduce lattice thermal conductivity, thereby suppressing it to its minimum value in the Cu1.96Mn0.02S0.925Se0.075. Ultimately, the zT value for Cu1.96Mn0.02S0.95Se0.05 achieved a peak of 0.97 at 723 K, marking a 3.8-fold improvement over that of pristine Cu2S. Our experimental findings corroborate that co-doping is an efficacious strategy for augmenting the Se-doping concentration limit and further optimizing the TE properties of Cu2S-based materials.
2. Experimental
2.1. Sample synthesis
A series of Cu2S1−xSex (0.015, 0.025, 0.05, 0.1) and Cu1.96Mn0.02S1−xSex (x = 0.025, 0.05, and 0.075) samples were fabricated via a high-temperature melting-annealing-hot pressing technique. High-purity elements, including copper (Cu, shots, 99.9999%, AlfaAesar), manganese (Mn, pieces, 99.8%, AlfaAesar), sulfur (S, pieces, 99.999%, AlfaAesar), and selenium (Se, shots, 99.9999%, AlfaAesar), were weighed according to the stoichiometric proportions, mixed, and loaded into a quartz tube. The tube was then sealed in a vacuum environment. The mixture was transferred to a muffle furnace, heated to 1373 K, and held for 12 hours to ensure a complete reaction of all elements. Subsequently, the samples were annealed at 873 K for 5 days to prevent the precipitation of copper. The resulting initial samples were manually milled into powders and loaded into a hot-pressing mold. Utilizing a hot press machine, the powders were compacted for 10 minutes under a program of 713 K and 60 MPa, yielding cylindrical samples with a diameter of 10 mm and a height of 12 mm.2.2. Characterization
The phase purity and crystalline structure of Cu2S and Cu1.96Mn0.02S1−xSex (x = 0.025, 0.05 and 0.075) samples were examined using room temperature X-ray diffraction (XRD, Cu Kα, Rint 2000, Rigaku, Japan) at a scanning speed of 10° min−1 over a range of 5–80°. The surface microstructure of the polished samples was visualized using scanning electron microscopy (SEM, JEOL/JSM-7610FPlus), and elemental distribution was analyzed by energy dispersive spectroscopy (EDS, JED-2300T). The valence states of the Cu1.96Mn0.02S0.975Se0.025 sample were investigated using X-ray photoelectron spectroscopy (XPS, Thermo Scientific Nexsa). Diffuse reflectance measurements were performed on the powdered samples using a UV-vis-NIR spectrophotometer (Cary 5000, Varian, USA). The microstructure of the Cu1.96Mn0.02S0.975Se0.025 sample was elucidated using a transmission electron microscope (TEM, Philips Tecnai F20). The carrier concentration (nH) and mobility (μH) at room temperature were determined using a Hall measurement system. Electrical conductivity (σ) and Seebeck coefficient (α) were measured in an argon atmosphere using the Ulvac ZEM-3 system over a temperature range from room temperature to 723 K. The total thermal conductivity κ was calculated as κ = Cp × ρ × D, where the thermal diffusion coefficient D was measured using the laser flash method (Netzsch, LFA-457), and the density ρ was determined. The heat capacity Cp was measured using the Archimedes method and calculated based on the Dulong–Petit law.2.3. Calculations
The first-principles calculations were carried out using the Vienna ab initio Simulation Package (VASP).30 Based on the density functional theory (DFT), the projector augmented-wave (PAW) approach with the generalized gradient approximation (GGA) method of Perdew–Burke–Ernzerhof (PBE) was introduced to calculate the electronic and phonon structures. The electron correlation and exchange of 3d orbitals of Cu are described by the Hubbard-type correction Ueff (Ueff = U − J) (4 eV).31 520 eV is the plane-wave energy cutoff point, and 10−5 eV is the energy convergence criterion, calculated using the phonon spectrum and electric band structure, respectively.3. Results and discussion
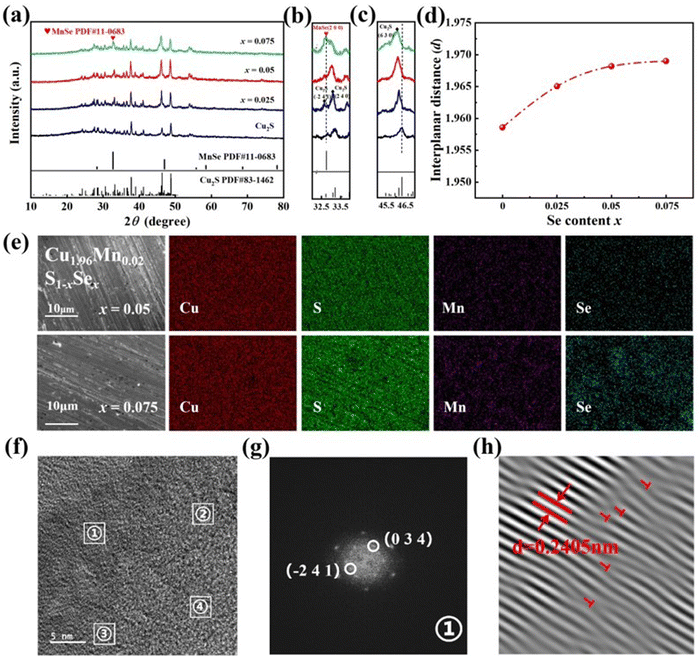
The phase composition of Cu2S and Cu1.96Mn0.02S1−xSex (x = 0.025, 0.05, and 0.075) at room temperature was examined using X-ray diffraction (XRD), as presented in Fig. 1a. The diffraction pattern of the Cu2S sample aligns with the monoclinic Cu2S phase (PDF#83-1462). Upon slight Mn and Se co-doping, the XRD peak remains unaltered, with no discernible impurity phase peaks. The local amplification of the XRD pattern at 32–34° is shown in Fig. 4b. It reveals that the main peak of Cu2S was at 33.02°, which suggests that it is the (2 4 0) peak of Cu2S. When the doping concentration of Se reaches 0.25 and 0.05, the peak is consistent with that of Cu2S. But a strong peak appears next to the (2 4 0) peak of Cu1.96Mn0.02S0.925Se0.075, which corresponds to the (2 0 0) characteristic peak of MnSe (PDF#11-0683), suggesting that Se has reached its doping concentration limit. Local amplification of the XRD pattern (Fig. 1c) indicates that the (6 3 0) characteristic peak of Cu1.96Mn0.02S1−xSex near 2θ = 45.933° shifts towards a lower 2θ angle with Se doping, due to the larger ionic radius of Se compared to that of S.32 We calculated the interplanar spacing of the (6 3 0) plane, as shown in Fig. 1d, which increases and then exhibits minimal change with Se doping, implying a Se doping limit of less than 7.5%.The impact of Mn doping on the solid solubility of Se was further investigated by energy-dispersive X-ray spectroscopy (EDS) mapping, as depicted in Fig. S1 (ESI†) and Fig. 1c. The Se enrichment in Cu2S0.985Se0.015 (Fig. S1, ESI†), indicates that the doping limit of Se in Cu2S is less than 1.5%. However, upon the introduction of 2% Mn, Cu, S, Mn, and Se are uniformly dispersed across the characterization region in Cu1.96Mn0.02S0.95Se0.05, without any element aggregation. This uniform distribution suggests that a 5% Se-doping can form a homogeneous solid solution. The Mn doping is inferred to enhance configurationally entropy and induce lattice distortion,33 facilitating the incorporation of Se into the Cu2S lattice. Consequently, co-doping is demonstrated to effectively elevate the doping limit of Se.
Further investigation of the microstructure of Cu1.96Mn0.02S0.975Se0.025 was performed using a high-magnification transmission electron microscope (TEM). EDS mapping confirms the uniform distribution of Cu, Mn, S, and Se (Fig. S2, ESI†). Fast Fourier transform (FFT) was performed on four distinct regions within the TEM image (Fig. 2d), with the results displayed in Fig. 2e. The four regions exhibit the same diffraction pattern, albeit with varying diffraction intensities. The marked diffraction points correspond to the (0 3 4) and (−2 4 1) planes of Cu2S, with the lattice spacing of (0 3 4) being 0.2405 nm.34 Inverse FFT (Fig. 2f) reveals that Mn and Se doping have induced lattice distortion and dislocations (marked with red “T”). The abundance of dislocations and the bending of lattice fringes contribute to phonon scattering,35 which effectively reduces the lattice thermal conductivity of the co-doped samples.
Fig. S3a (ESI†) presents the X-ray photoelectron spectroscopy (XPS) analysis of Cu1.96Mn0.02S0.975Se0.025. The two spin-orbital peaks of Cu in Fig. S3b (ESI†) suggest that the Cu 2p peak predominantly corresponds to Cu+.36 The binding energy observed in the XPS spectrum for S 2p in Fig. S3c (ESI†) indicates the presence of sulfur in the form of S2−. Due to the spin–orbit coupling, Mn exhibits two peaks in the binding energy spectrum (Fig. S3d, ESI†), with an additional satellite peak for Mn2+ at 2P3/2 appearing at a binding energy of 647.18 eV.37 The Mn 3s core state spectrum (Fig. S3e, ESI†) further corroborates that Mn ions are predominantly in the Mn2+ state.38–40 The Se 3d spectrum consists of 3d5/2 at 53.8 eV and 3d3/2 at 54.5 eV, which are characteristic of Se2−.35
According to previous studies, doping has a great influence on band structure, such as band degeneracy,13,14,41,42 resonance level,11,43,44 the Rashba effect,45,46 classical and quantum size effect.47 We conducted UV-visible diffuse reflectance studies and density functional theory (DFT) calculations to investigate the electronic structure. Utilizing the Tauc plot method (with the calculation formula detailed in the ESI†),48,49Fig. 2a–d illustrate that the band gap of Cu2S was 1.011 eV but gradually reduced to 0.884 eV with Se doping, signifying that Se doping narrows the band gap of Cu2S. Furthermore, the DFT calculations, depicted in Fig. 2e and Fig. S4 (ESI†), and the schematic representation of the band structure in Fig. 2f, reveal that Se doping elevates the valence band and narrows the band gap, which is in agreement with the results from the UV-visible diffuse reflectance measurements. The valence band of Cu2S is anti-bond, composed of the Cu 3d orbital and S 3p orbital,35 and the energy of the Se 4p orbital is higher than that of the S 3p orbital.50 Thus, Se doping increases the energy of the anti-bond. Additionally, Se doping weakens the Cu–S bond, leading to a narrower band gap (as shown in Fig. S5, ESI†). Moreover, the increase in the valence band at the M point is more pronounced than at the gamma point following Se doping, resulting in a gradual decrease in the energy difference between the M points and the gamma point (as illustrated in Fig. 2f), which is consistent with the pDOS in Fig. S6 (ESI†), After Se doping, the atomic orbital of Se is higher than that of S, so the energy of the anti-bond of Se is increased, leading to the increase in the energy of the M point in the band structure, and the electrical performance is improved. As a result, Se doping enhances the contribution of the band at the M point to electron transport, thereby increasing the effective mass and the Seebeck coefficient.
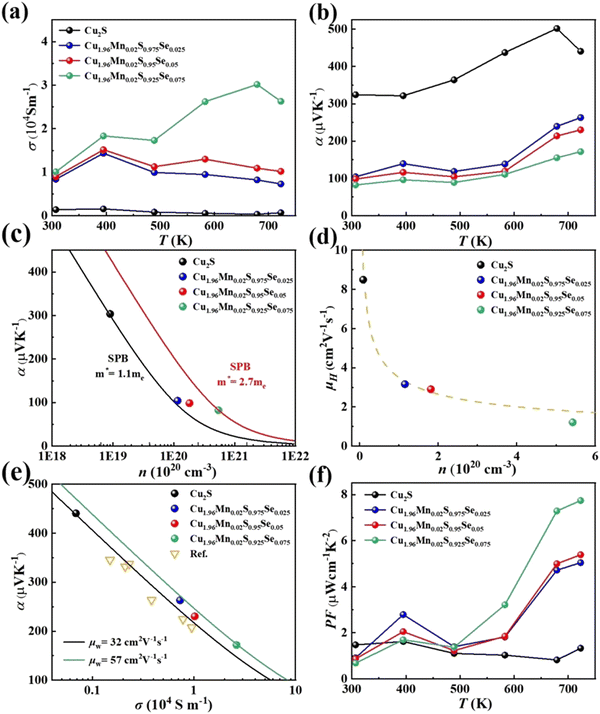
The temperature-dependent electrical transport properties of Cu1.96Mn0.02S1-xSex are illustrated in Fig. 3. Se doping enhances electrical conductivity, but concurrently diminishes the Seebeck coefficient, as depicted in Fig. 3a and b.27 This behavior is primarily attributed to the effects on carrier concentration (n) and effective mass (m*). The introduction of Se weakens Cu–S bonds and narrows the band gap, thereby increasing the carrier concentration, as shown in Fig. 3c. Given that Cu2S typically exhibits a suboptimal carrier concentration, appropriate Se doping can optimize this parameter, thereby enhancing electrical performance.
The effective mass (m*) was estimated using a single parabolic band (SPB) model, with detailed formulas provided in the ESI.†51,52 The effective mass is initially 1.1me for pristine Cu2S (black curve) and progressively increases to 2.7me (red curve) with Se doping (Fig. 3c). This increase in m* is due to Se doping, which encourages additional bands to participate in the electron transport, thereby improving the electrical transport properties.
Furthermore, Fig. 3d presents the carrier mobility (μH) for all samples. The reduced carrier mobility in Cu1.96Mn0.02S0.925Se0.075 is attributed to the Se-rich secondary phase, which induces alloy scattering and thus decreases mobility. However, for the carrier mobility of other samples, a decrease in mobility with an increase in carrier concentration aligns with the μH ∝ 1/n relationship, indicating that electron–phonon scattering is the predominant scattering mechanism.53 Since Se and S are from the same group in the periodic table, Se doping in Cu2S does not introduce significant additional scattering mechanisms such as alloy scattering,54 or ionized impurity scattering,55 which helps maintain carrier mobility.
As a result, due to the increased effective mass and reduced scattering, the weighted mobility (μw) increased from 32 cm2 V−1 S−1 of Cu2S to 57 cm2 V−1 S−1 of Cu1.96Mn0.02S0.925Se0.075 with Se doping (Fig. 3e). This leads to a significant enhancement in the power factor (PF), with Cu1.96Mn0.02S0.925Se0.075 achieving a maximum PF of 7.74 μW cm−1 K−2 at 723 K, nearly four times greater than that of Cu2S. Due to the phase transition, PF fluctuates significantly at 400 K. Thus, co-doping with elements from the same group is an effective approach to optimize the electrical transport properties of Cu2S.
Se doping not only refines the electrical properties but also displays a significant impact on thermal conductivity, as illustrated in Fig. 4a. The total thermal conductivity (κ) escalates with Se doping, mirroring the trend observed in electrical conductivity. The total thermal conductivity (κ) is the sum of lattice thermal conductivity (κL) and electronic thermal conductivity (κe). The electronic thermal conductivity (κe) is estimated using the Wiedemann–Franz formula, κe = LσT, where L is the Lorentz constant, which can be approximated from the single parabolic band (SPB) model (as shown in Fig. S8, ESI†).56 Sedoping engenders substantial lattice distortion and dislocations, which augment phonon scattering and consequently lead to a reduction in the lattice thermal conductivity of the doped samples (Fig. 4a). Comparative analysis with the lattice thermal conductivity of Te-doped in Cu2S,57 S doped in Cu2Se,24,58 Te doped in Cu2Se and Se doped in Cu2Te,25,26 it reveals a consistent trend: the lattice thermal conductivity of the doped samples decreases with increasing doping concentration. This suggests that doping with elements from the same group not only boosts the power factor (PF) but also serves as an effective strategy to diminish the lattice thermal conductivity of copper chalcogenides. Consequently, if the Se-doping concentration limit could be further extended, the lattice thermal conductivity would be expected to continue to decrease.
The dimensionless figure of merit (zT) values for all doped samples are significantly higher than those of pristine Cu2S (as depicted in Fig. 4c), due to the enhanced electrical and thermal transport properties following co-doping with Mn and Se. On the one hand, Se doping enhances the weighted mobility, thereby optimizing the electrical transport properties. On the other hand, Se doping diminishes the lattice's thermal conductivity, optimizing the thermal transport properties. Ultimately, the zT value for Cu1.96Mn0.02S0.95Se0.05 peaks at 0.97 at 723 K, and when juxtaposed with the reported Cu2S-based materials and other class of performing copper-based materials containing multiple dopants, the zT value of Cu1.96Mn0.02S0.95Se0.05 is notably high (as shown in Fig. 4d and Fig. S10, ESI†).36,57,59–61
4. Conclusions
In summary, we have demonstrated that the increased Se-doping concentration limit can optimize Cu2S thermoelectric properties. The co-doping of 2% Mn and Se effectively raises the doping concentration limit of Se from 1.5% to 5%. This elevated level of Se-doping has not only solely increased the carrier concentration to an optimal range but also reconfigured the electronic structure. The inclusion of additional bands in electron transport engendered a heightened weighted mobility, thereby significantly enhancing the electrical transport properties. This enhancement propelled the power factor (PF) of the Cu1.96Mn0.02S0.95Se0.05 to reach 7.74 μW cm−1 K−2 at 723K. Moreover, extra alloy scattering generated by Se doping results in its lattice thermal conductance below Cu2S. Finally, the zT value of the Cu1.96Mn0.02S0.95Se0.05 reaches 0.97 at 723 K, which is 3.8 times that of pristine Cu2S. Therefore, it is an effective strategy to increase the concentration limit of Se through co-doping and optimize the thermoelectric properties with anionic doping within the same group, which has the potential for broad applications to other thermoelectric materials.Author contributions
Chen Gong: investigation, methodology, writing – original draft preparation. Zhiwei Zeng: methodology. Xiaoling Sun: methodology. Chengran Luo: methodology. Hongyi Chen: supervision, project administration, conceptualization, writing – reviewing and editing.Data availability
The data supporting this article have been included in the manuscript and ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by Excellent Youth Funding of the Natural Science Foundation of Hunan Province (Grant No. 2022JJ20062), the National Natural Science Foundation of China (Grant No. 52002406), the National Natural Science Foundation of China-Hunan Joint Funds (Grant No. U20A20247). We are grateful to the High-Performance Computing Center of Central South University for partial support of this work.Notes and references
- J. Mao, Z. Liu, J. Zhou, H. Zhu, Q. Zhang, G. Chen and Z. Ren, Adv. Phys., 2018, 67, 69–147 CrossRef.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed.
- Q. Yang, S. Yang, P. Qiu, L. Peng, T.-R. Wei, Z. Zhang, X. Shi and L. Chen, Science, 2022, 377, 854–858 CrossRef CAS PubMed.
- X. Li, Y. Lou, K. Jin, L. Fu, P. Xu, Z. Shi, T. Feng and B. Xu, Angew. Chem., Int. Ed., 2022, 61, e202212885 CrossRef CAS.
- P. Qiu, X. Shi and L. Chen, Energy Storage Mater., 2016, 3, 85–97 CrossRef.
- Y. Yu, D. Yang, J. Li, M. Zhang, H. Luo, Q. Liang, H. Ye, Q. Zhang, X. Tang and J. Wu, Adv. Funct. Mater., 2022, 32, 2107284 CrossRef CAS.
- Z. Chen, Z. Jian, W. Li, Y. Chang, B. Ge, R. Hanus, J. Yang, Y. Chen, M. Huang, G. J. Snyder and Y. Pei, Adv. Mater., 2017, 29, 1606768 CrossRef PubMed.
- R. Hanus, M. T. Agne, A. J. E. Rettie, Z. Chen, G. Tan, D. Y. Chung, M. G. Kanatzidis, Y. Pei, P. W. Voorhees and G. J. Snyder, Adv. Mater., 2019, 31, 1900108 CrossRef.
- Y. Pei, N. A. Heinz, A. LaLonde and G. J. Snyder, Energy Environ. Sci., 2011, 4, 3640–3645 RSC.
- X. Zhang, Z. Bu, S. Lin, Z. Chen and Y. Pei, Joule, 2020, 4, 986–1003 CrossRef CAS.
- U. S. Shenoy, G. K. D and D. K. Bhat, J. Alloys Compd., 2022, 921, 165965 CrossRef CAS.
- J. Li, X. Zhang, Z. Chen, S. Lin, W. Li, J. Shen, I. T. Witting, A. Faghaninia, Y. Chen, A. Jain, L. Chen, G. J. Snyder and Y. Pei, Joule, 2018, 2, 976–987 CrossRef CAS.
- U. S. Shenoy, G. K. D and D. K. Bhat, Mater. Adv., 2022, 3, 5941–5946 RSC.
- U. S. Shenoy and D. K. Bhat, J. Alloys Compd. Commun., 2024, 1, 100001 Search PubMed.
- M. Samanta, K. Pal, U. V. Waghmare and K. Biswas, Angew. Chem., Int. Ed., 2020, 59, 4822–4829 CrossRef CAS.
- Y. Li, S. Bai, Y. Wen, Z. Zhao, L. Wang, S. Liu, J. Zheng, S. Wang, S. Liu, D. Gao, D. Liu, Y. Zhu, Q. Cao, X. Gao, H. Xie and L.-D. Zhao, Sci. Bull., 2024, 69, 1728–1737 CrossRef CAS PubMed.
- K. Zhao, P. Qiu, X. Shi and L. Chen, Adv. Funct. Mater., 2020, 30, 1903867 CrossRef CAS.
- Y. He, T. Day, T. Zhang, H. Liu, X. Shi, L. Chen and G. J. Snyder, Adv. Mater., 2014, 26, 3974–3978 CrossRef CAS PubMed.
- H. Liu, X. Shi, F. Xu, L. Zhang, W. Zhang, L. Chen, Q. Li, C. Uher, T. Day and G. J. Snyder, Nat. Mater., 2012, 11, 422–425 CrossRef CAS.
- H. Chen, Z. Yue, D. Ren, H. Zeng, T. Wei, K. Zhao, R. Yang, P. Qiu, L. Chen and X. Shi, Adv. Mater., 2019, 31, 1806518 CrossRef.
- F. Shen, Y. Zheng, L. Miao, C. Liu, J. Gao, X. Wang, P. Liu, K. Yoshida and H. Cai, ACS Appl. Mater. Interfaces, 2020, 12, 8385–8391 CrossRef CAS PubMed.
- T. Xing, C. Zhu, Q. Song, H. Huang, J. Xiao, D. Ren, M. Shi, P. Qiu, X. Shi, F. Xu and L. Chen, Adv. Mater., 2021, 33, 2008773 CrossRef CAS.
- H. Xu, H. Wan, R. Xu, Z. Hu, X. Liang, Z. Li and J. Song, J. Mater. Chem. A, 2023, 11, 4310–4318 RSC.
- K. Zhao, A. B. Blichfeld, H. Chen, Q. Song, T. Zhang, C. Zhu, D. Ren, R. Hanus, P. Qiu, B. B. Iversen, F. Xu, G. J. Snyder, X. Shi and L. Chen, Chem. Mater., 2017, 29, 6367–6377 CrossRef CAS.
- K. Zhao, M. Guan, P. Qiu, A. B. Blichfeld, E. Eikeland, C. Zhu, D. Ren, F. Xu, B. B. Iversen, X. Shi and L. Chen, J. Mater. Chem. A, 2018, 6, 6977–6986 CAS.
- R. Rajkumar, J. Mani, S. Radha, M. Arivanandhan, R. Jayavel and G. Anbalagan, J. Mater. Sci.: Mater. Electron., 2023, 34, 1041 CrossRef CAS.
- Y. Wang, Z. Long, Y. Cheng, M. Zhou, H. Chen, K. Zhao and X. Shi, Mater. Today Phys., 2023, 32, 101028 CrossRef CAS.
- M. Guan, K. Zhao, P. Qiu, D. Ren, X. Shi and L. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 13433–13440 CAS.
- G. Tan, C. C. Stoumpos, S. Wang, T. P. Bailey, L.-D. Zhao, C. Uher and M. G. Kanatzidis, Adv. Energy Mater., 2017, 7, 1700099 Search PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CAS.
- A. Jain, G. Hautier, S. P. Ong, C. J. Moore, C. C. Fischer, K. A. Persson and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 045115 Search PubMed.
- Y. Yao, B.-P. Zhang, J. Pei, Y.-C. Liu and J.-F. Li, J. Mater. Chem. C, 2017, 5, 7845–7852 CAS.
- X. Zhang, M. Huang, H. Li, J. Chen, P. Xu, B. Xu, Y. Wang, G. Tang and S. Yang, J. Mater. Chem. A, 2023, 11, 8150–8161 CAS.
- X. Lu, P. Lu, Y. Wei, C. Zhu, H. Su, X. Qiu, S. Feng, P. Qiu, X. Shi, L. Chen and F. Xu, Nano Lett., 2024, 24, 2853–2860 CrossRef CAS PubMed.
- Z. Long, Y. Wang, X. Sun, Y. Li, Z. Zeng, L. Zhang and H. Chen, Adv. Mater., 2023, 35, 2210345 CrossRef CAS PubMed.
- X. Liang, D. Jin and F. Dai, Adv. Electron. Mater., 2019, 5, 1900486 CrossRef CAS.
- J. Liu, M. P. Hanson, J. A. Peters and B. W. Wessels, ACS Appl. Mater. Interfaces, 2015, 7, 24159–24167 CrossRef CAS.
- G. C. Allen, S. J. Harris, J. A. Jutson and J. M. Dyke, Appl. Surf. Sci., 1989, 37, 111–134 CrossRef CAS.
- N. Andreu, D. Flahaut, R. Dedryvère, M. Minvielle, H. Martinez and D. Gonbeau, ACS Appl. Mater. Interfaces, 2015, 7, 6629–6636 CrossRef CAS PubMed.
- V. A. M. Brabers, F. M. van Setten and P. S. A. Knapen, J. Solid State Chem., 1983, 49, 93–98 CrossRef CAS.
- J. Li, X. Zhang, X. Wang, Z. Bu, L. Zheng, B. Zhou, F. Long, Y. Chen and Y. Pei, J. Am. Chem. Soc., 2018, 140, 16190–16197 CrossRef CAS PubMed.
- U. S. Shenoy, G. K. Ddd and D. K. Bhat, J. Alloys Compd., 2022, 905, 164146 CrossRef CAS.
- S. Perumal, M. Samanta, T. Ghosh, U. S. Shenoy, A. K. Bohra, S. Bhattacharya, A. Singh, U. V. Waghmare and K. Biswas, Joule, 2019, 3, 2565–2580 CrossRef CAS.
- U. S. Shenoy and D. K. Bhat, Mater. Adv., 2021, 2, 6267–6271 RSC.
- U. S. Shenoy and D. K. Bhat, ACS Sustainable Chem. Eng., 2021, 9, 13033–13038 CrossRef CAS.
- M. Hong, W. Lyv, M. Li, S. Xu and Z. G. Chen, Joule, 2020, 4, 2030–2043 CrossRef CAS.
- J. He and T. M. Tritt, Science, 2017, 357, eaak9997 CrossRef PubMed.
- Y. Li, W. Qi, Y. Li, E. Janssens and B. Huang, J. Phys. Chem. C, 2012, 116, 9800–9804 CrossRef CAS.
- R. Marshall and S. S. Mitra, J. Appl. Phys., 1965, 36, 3882–3883 CrossRef CAS.
- Y. Zhang, Y. Wang, L. Xi, R. Qiu, X. Shi, P. Zhang and W. Zhang, J. Chem. Phys., 2014, 140, 074702 CrossRef PubMed.
- Y. Xiao, H. Wu, W. Li, M. Yin, Y. Pei, Y. Zhang, L. Fu, Y. Chen, S. J. Pennycook, L. Huang, J. He and L.-D. Zhao, J. Am. Chem. Soc., 2017, 139, 18732–18738 CrossRef CAS PubMed.
- A. F. May, E. S. Toberer, A. Saramat and G. J. Snyder, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 125205 CrossRef.
- A. F. May, J. P. Fleurial and G. J. Snyder, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 125205 CrossRef.
- R. Gurunathan, R. Hanus and G. J. Snyder, Mater. Horiz., 2020, 7, 1452–1456 RSC.
- C. Hu, K. Xia, C. Fu, X. Zhao and T. Zhu, Energy Environ. Sci., 2022, 15, 1406–1422 RSC.
- H. J. Goldsmid, Conversion Efficiency and Figure-of-Merit, CRC Handbook of Thermoelectrics, 1995 Search PubMed.
- Y. Yao, B.-P. Zhang, J. Pei, Q. Sun, G. Nie, W.-Z. Zhang, Z.-T. Zhuo and W. Zhou, ACS Appl. Mater. Interfaces, 2018, 10, 32201–32211 CrossRef CAS.
- K. Zhao, A. B. Blichfeld, E. Eikeland, P. Qiu, D. Ren, B. B. Iversen, X. Shi and L. Chen, J. Mater. Chem. A, 2017, 5, 18148–18156 RSC.
- S. Zhao, H. Chen, X. Zhao, J. Luo, Z. Tang, G. Zeng, K. Yang, Z. Wei, W. Wen, X. Chen and Y. Sun, Mater. Today Phys., 2020, 15, 100271 CrossRef.
- H. Kim, S. K. Kihoi, U. S. Shenoy, J. N. Kahiu, D. H. Shin, D. K. Bhat and H. S. Lee, J. Mater. Chem. A, 2023, 11, 8119–8130 RSC.
- Y.-H. Zhao, Z.-H. Shan, W. Zhou, R. Zhang, J. Pei, H.-Z. Li, J.-F. Li, Z.-H. Ge, Y.-B. Wang and B.-P. Zhang, ACS Appl. Energy Mater., 2022, 5, 5076–5086 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03056g |
| This journal is © The Royal Society of Chemistry 2025 |