Open Access Article
Open Access ArticleModular molecular design of polymerized pro-estrogen materials enables controlled astrocyte response†
Devan L.
Puhl‡
ab,
Alexis
Ziemba‡
 ab,
Samuel A. T.
Ellman‡
c,
Alex
Hsu
c,
Jayant
Saksena
abc,
Penelope Phillips
Falcone
c,
Bailey
Balouch
b,
Deniz
Rende
d,
Tanner
Fink
ae,
R. Helen
Zha
ab,
Samuel A. T.
Ellman‡
c,
Alex
Hsu
c,
Jayant
Saksena
abc,
Penelope Phillips
Falcone
c,
Bailey
Balouch
b,
Deniz
Rende
d,
Tanner
Fink
ae,
R. Helen
Zha
 ae,
Ryan J.
Gilbert
*ab and
Edmund F.
Palermo
ae,
Ryan J.
Gilbert
*ab and
Edmund F.
Palermo
 *abcd
*abcd
aCenter for Biotechnology & Interdisciplinary Studies, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA. E-mail: palere@rpi.edu; gilber2@rpi.edu
bBiomedical Engineering, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA
cMaterials Science & Engineering, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA
dCenter for Materials Devices & Integrated Systems, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA
eChemical & Biological Engineering, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA
First published on 15th April 2025
Abstract
The hormone 17β-estradiol (estrogen or “E2”) has demonstrated robust neuroprotective properties in countering oxidative stress-induced neurotoxicity, as well as strong neurotrophic properties to promote axonal growth, following injury to the central nervous system (CNS). However, oral or injected E2 is a suboptimal drug, as systemic administration fails to achieve a therapeutic dose at the injury site, in addition to being contraindicated in most male patients. Polymerized pro-drug biomaterials can mitigate these issues by locally releasing small quantities of drug over vastly extended timescales. We sought to study the effect of the biomaterial properties of poly(pro-E2) on astrocyte-surface interactions because astrocytes are the most abundant cell type in the central nervous system, play vital roles in neuron support and traumatic injury, and express estrogen receptors. Herein, we sought to study the effect of novel poly(pro-E2) films on astrocyte behavior, as well as to gauge the biomaterial properties that would lead to optimal astrocyte functionality. We synthesized pro-E2 as carbonate and ester derivatives and copolymerized each of these monomers with either oligoethylene glycol dithiol (EG) or hexylene dithiol (Hex) linkers to generate four unique poly(pro-E2) materials with tunable physiochemical and mechanical properties. We found that films of polymer with Hex-linkers supported sustained astrocyte adhesion, whereas the EG-linked analogs did not. To explain this behavior, we investigated the physical and chemical surface properties that may influence cell attachment. SEM images for films incubated in buffer showed marked surface roughness with micro- and nano-scale topography for the polymers with Hex-linkers, whereas those with EG-linkers appeared smooth. This result suggests that astrocytes preferentially adhere to rougher surfaces. Hex-linked polymer surfaces also demonstrated more negative zeta potentials compared to EG-linked polymer surfaces – indicating favorable electrostatic interactions for astrocyte adhesion. Finally, while all polymers exhibited hysteresis during mechanical testing, films with Hex-linkers demonstrated greater dissipation, suggesting more pronounced viscoelasticity. Taken together, these results indicate that a combination of physiochemical surfaces properties, which arise from subtle differences in chemical composition, can exert marked effects on astrocyte adhesion and spreading.
Introduction
There is an urgent need for interventions that can improve functional recovery and quality of life following traumatic injury to the central nervous system (CNS).1 Astrocytes are a key cell type involved in the injury response; their role as a principal neuronal support cell shifts to leading a process known as astrogliosis.2 Astrogliosis leads to the formation of the glial scar, which is prohibitive of neural regeneration, and is thus a critical target for nervous system injuries.3 One way to target astrocytes and induce neuroprotective mechanisms is through exposure to the steroid hormone, 17β-estradiol (“estrogen” or “E2”). E2 has been shown to be neuroprotective in nervous system injury and disease, and estrogen receptors (ER) are expressed on astrocytes.4,5 ERα is highly expressed in astrocytes with nuclear localization,6 with increased expression following injury.7 Nanoparticles containing estrogen have shown promise in improving recovery from SCI in animal models.8–10 Further, studies using a neuroautoimmune mouse model have shown that activation of ERα on astrocytes is neuroprotective.11,12 However, systemic delivery of E2 fails to achieve therapeutic doses locally at the site of the lesions and causes adverse effects as a result of hepatic metabolism, in addition to being contraindicated in most male patients.13,14 Further development of estrogen biomaterials that can locally deliver estrogen offers a solution to these challenges.Prodrugs are chemically modified derivatives of drug molecules intended to improve their pharmacological performance. These prodrugs undergo chemical transformations in vivo to release the active parent drug, which then exerts the desired biological effect, typically over longer timescales.15,16 Polymerized pro-drugs extend such small molecules into long chain macromolecules, in which every repeating unit in the polymer chain contains a pro-drug embedded in the structure.17 The advantages of polymerized drugs include higher drug loading and longer-lasting release profiles relative to soluble small molecule prodrugs. Additionally, polyprodrugs can be processed into biomaterials with a wide variety of geometries and mechanical properties to serve as implantable depots of drug that will eventually fully resorb. Consequently, development of E2 prodrugs has generated considerable interest.18–21 In this context, our laboratories have been interested in developing E2 polyprodrugs as biomaterials that modulate response to injuries of the central nervous system.22–25 For spinal cord injury, the chronic progression of diseases proceeds on the timescale of many years, requiring much slower release rates than soluble small-molecule prodrugs can typically achieve. Moreover, local release at the site of the injury is desired to avoid off-target effects associated with systemic administration of E2. Hence, we sought to develop polymerized poly(pro-E2) thermoplastic materials capable of local implantation and slow degradation. In our previous work, we developed a polycarbonate alternating copolymer of E2 and oligoethylene glycol, which showed neurotrophic and neuroprotective effects in vitro,22 and promising preliminary neuroprotective effects in vivo.26
While our previous work on E2 polycarbonates demonstrated promise for modestly increasing neuron density at the lesion site, there remains a need to precisely tune the polyprodrug properties to minimize the inhibitory and toxic injury response of various CNS cell types following CNS injuries. The rate of E2 release from the polyprodrug scaffold is controlled primarily by the hydrolytic stability and the hydrophobicity. Hence, we postulated that the E2 release rate from this polymer can be precisely tuned by modifying the species E2 is copolymerized with and linkages between these species to provide a broad range of thermomechanical properties, degradation rates, and consequently modulated cell-biomaterial interactions. In this paper, we leveraged a modular molecular design platform to tailor the physiochemical attributes of polymerized E2-based materials. First, two pro-drugs of E2 were synthesized: homobifunctionalized with either allyl chloroformate to form the carbonate-linked bis-allyl species 1 (Fig. S1, ESI†) or with pentenoic acid to form the ester-linked bis-alkene 2 (Fig. S4, ESI†). Next, each of these prodrugs was then copolymerized with either 1,2-bis(2-mercaptoethoxy)ethane, a flexible dithiol based on an ethylene glycol unit, or 1,6-hexenedithiol, a somewhat less flexible but putatively more hydrophobic linker. Thus, we generated a series of four E2 polymers (Fig. 1). The four polymer types were then processed into films, characterized for their physical and thermomechanical material properties, tested for cytotoxicity with primary cortical cultures, and investigated for their influence on the behavior of primary cortical astrocytes.
 | ||
| Fig. 1 Structure of poly(pro-E2) as polycarbonates or polyesters containing either EG or Hex type linker groups, in alternating sequence, prepared by photoinitiated thiol–ene radical addition. | ||
Results and discussion
Monomer and polymer synthesis
Two hydrolytically labile pro-drugs of 17β-estradiol (E2), either as the carbonate (1) or ester (2) derivative bearing pendant alkenes, were copolymerized with either a dithiol linker (3) or a hydrophobic 1,6-hexylenedithiol (4) linker, via UV-photoinitiated thiol–ene radical addition chemistry in concentrated THF solution (Fig. S7, S10, S13 and S16, ESI†), following our previously published method22 with minor modifications. Details of all synthetic procedures are given in the Methods section and characterization data are given in the ESI.† Owing to the step-growth nature of this polymerization, careful control of the stoichiometric equivalence was of paramount importance. This resulted in a library of poly(pro-E2) polymers: PC-EGi.e. poly(1-alt-3), PC-Hexi.e. poly(1-alt-4), PE-EGi.e. poly(2-alt-3), and PE-Hexi.e. poly(2-alt-4). The polymer structures are shown in Fig. 1.Polymer characterization
Polymer structures were confirmed by NMR and are consistent with established precedent.22 The 1H and 13C NMR spectra for each monomer and alternating copolymer are given in the ESI† (Fig. S8, S11, S14 and S17). It is clear from the resonances in the region of ∼5–6 ppm that the alkene groups of the monomer (1 or 2) are mostly consumed in the polymerization, consistent with a high degree of conversion, >95% in all cases. The molecular weight distributions (MWD) were estimated by GPC in THF at 40 °C, relative to polystyrene standards. We obtained four distinct alternating copolymers with comparable Mw in the range of 40–60 kDa and a peak molecular weight Mp in the range of 20–40 kDa. For all four samples, the MWDs span a broad range encompassing ∼103–105 g mol−1 with high dispersities (Đ ∼ 3–4), which is unsurprising due to the inherently stochastic nature of step-growth processes. Specific characterization data are summarized Table 1.| Polymer | M p (kDa) | Đ | T g (°C) | E (GPa) | θ (t = 0) |
|---|---|---|---|---|---|
| PE-EG | 28.1 | 2.72 | 3 | 0.86 | 94° |
| PE-Hex | 38.4 | 4.20 | 10 | 7.4 | 74° |
| PC-EG | 21.0 | 3.34 | 24 | 0.44 | 86° |
| PC-Hex | 24.1 | 4.03 | 44 | 4.6 | 78° |
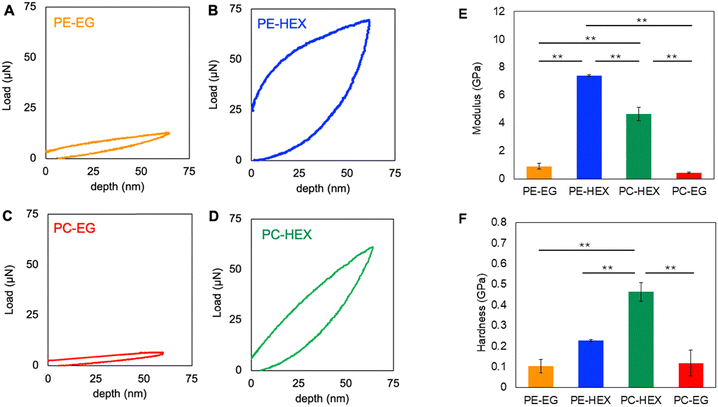
Finally, nanoindentation data clearly indicate that while all polymers exhibited hysteresis during mechanical testing, Hex-linked polymers demonstrated greater energy dissipation, as indicated by the areas of their elastic hysteresis loops, with PC-Hex showing 7× and PE-Hex showing 8× greater energy dissipation compared to their counterparts, PC-EG and PE-EG, respectively, suggesting pronounced viscoelastic creep.30 Viscoelasticity is generally a favorable material property for improved adhesion and function of cells, including astrocytes31,32 and spinal tissue itself is notably viscoelastic in nature.33
Polymer film degradation
With the four representative polymers in hand, we proceeded to examine their degradation behavior as drop-cast films submerged in phosphate buffered saline (PBS) for extended periods of time (up to 6 weeks). Films were solution drop-cast onto glass slides, rigorously dried, and biopsy punched to 12 mm round disks. Disk dimensions and film thickness were finely controlled such that the surface:volume ratio and total initial mass were nearly identical across all four films. The films had an initial thickness of ∼100 μm and weighed ∼18 mg total starting mass. The as-cast films appear optically clear, colorless and smooth in the initial dry state, to the naked eye. We monitored their erosion in terms of mass loss as a function of incubation time, changes in surface appearance by optical microscopy, changes in thickness by profilometry, and changes to the microscopic surface roughness by SEM. Finally, changes in the MWD were quantified by GPC on the remaining intact polymer films as well as methanolic extracts of any soluble intermediary degradation products (presumably including oligomers and small molecule pro-E2 fragments). | ||
| Fig. 7 SEM images of the four polymer films as-cast and after 1- and 4-weeks incubation (37 °C, PBS). In all images, the scale bar is 250 μm. | ||
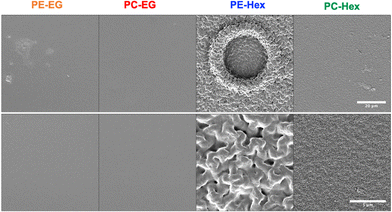
At still higher magnification in the SEM, microscopic roughness was revealed in the polymers containing Hex linkers, but not for those containing EG linkers (Fig. 8). The heterogenous erosion observed in Hex-linked polymers might be due to microscale phase separation that occurs upon partial hydrolysis of the polymer chains. Though initially these materials are indeed hydrophobic, ester or carbonate hydrolysis converts relatively non-polar linkages into more hydrophilic hydroxy, phenol and carboxylic acid moieties, which in turn admit further water penetration and thus could locally accelerate the hydrolysis process.
Acidic byproducts, in particular, have been shown to have an autocatalytic effect on degradation leading to significant decreases in MWD.34,35 Local regions of hydrophilic material could then phase separate from intact, hydrophobic polymer, leading to the rough textured surfaces that are evident in the SEM micrographs for Hex-linked polymers, but not so for their EG-linked counterparts. For those polymers with a Tg well below 37 °C (all but PC-Hex), we expect the phase separation to be facile. The increased roughness of PE-Hex could be attributed to greater phase separation between the hydrophilic and hydrophobic components of the polyester material.36–40 Since PC-Hex is the harder, higher-Tg material, the polymer has limited mobility, and the spatial extent of phase segregation appears limited to the sub-micron length scale.
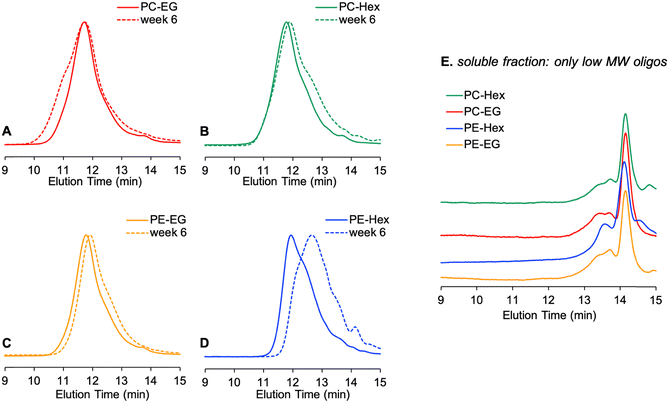 | ||
| Fig. 9 Changes in the MWD for films of poly(pro-E2) incubated at 37 °C. (A)–(D) Methanol-insoluble films; (E) methanol-soluble fraction extracted from the remaining intact films. | ||
The GPC traces of the MeOH-insoluble intact polymer films reveal some interesting trends. The hydrophobic polyester PE-Hex, which showed the most pronounced mass loss, also shows the most pronounced reduction in average molecular weight. After 6 weeks incubation at 37 °C in PBS, the entire MWD shifts to later elution time (smaller size) and new oligomeric peak grows in at about 14.2 min in the chromatograph. The Mw of PE-Hex is reduced by about 50% of its initial value. The hexylene-linked polyester PE-EG, on the other hand, shows a more modest shift in MWD to later elution times and the Mw drops to about 82% of its initial value. For the polycarbonates, marked differences are evident: the MWD of PC-Hex broadens only on the low-MW side while the high end remains relatively unchanged, resulting in a slight reduction of the average Mw value. In stark contrast, PC-EG appears to increase in MW due to broadening on the high MW side of the distribution, to about 151% of its initial value. Combined with the knowledge that this polymer loses mass, the increase in average Mw of PC-EG could be due to selective cleavage of shorter chains near the surface, preferentially leaving behind the higher MW subpopulation. Other possibilities include aggregation in THF, crosslinking between chains during degradation, or perhaps oxidation of the thioethers to the sulfoxide or sulfone, expanding the hydrodynamic volume and thus GPC elution time, even in the absence of changes in chain length. Although the precise mechanism of crosslinking remains unknown at present, we suspect some crosslinking mechanism may be operative because a small subfraction of the MeOH-insoluble fraction was also THF-insoluble, leaving behind a trace quantity of THF-swollen, gel-like substance.
Considering that the PC-EG films did not appreciably swell, we speculate that PC-EG films of this ∼100 μm thickness may undergo surface-limited erosion. In our prior study on electrospun fibers (approximately 1 μm diameter) made from a different batch of the same material, we observed predominately bulk erosion at 80 °C.22 The much thicker film geometry in this work appears to undergo surface erosion at 37 °C, on the other hand. Whereas both diffusion and hydrolysis kinetics are accelerated at higher temperature, greater thickness favors surface-limited erosion.41
Next, we examined the GPC traces of the methanolic extracts. Intact polymer is not methanol soluble, but short oligomers and small molecules that did not dissolve into the PBS may be extracted from the intact polymer in this manner. All four extracts show the same result: a multimodal distribution at late elution times corresponding to a MW in the range of ∼500–2000 g mol−1 (relative to PS standards). These are presumably short oligomers or pro-drugs of E2 containing one or two pendant linkers which are MeOH-soluble but not readily released into PBS buffer. When pure E2 in THF is evaluated by GPC for comparison, a peak appears at ∼17 minutes, which is outside the calibration range for our PS standards. All the polymer samples displayed some feature in this region as well, but it is obscured by a much larger negative RI peak which may be related to residual salt carryover from the PBS. Therefore, some amount of pure E2 is retained within the remaining intact films and does not get released immediately into PBS buffer, presumably due to limited aqueous solubility of E2 and hydrophobic interactions between E2 and the remaining poly(pro-E2) films. We speculate that at much later degradation times, when little solid polymer remains, such residues would eventually get resorbed. Transport of cleaved drug from remaining polymer film into the surrounding media encompasses both the desorption of E2 or pro-E2 from the intact polymer film and then diffusion via percolation pathways to escape into free aqueous solution. While autocatalytic degradation mechanisms such as ester hydrolysis can accelerate degradation locally, size- and solubility-dependent drug transport may also strongly influence drug release kinetics.34 Note, however, that these are incubations were done under static conditions in vitro. The release of soluble fragments from films in vivo would be less prone to such retention issues, where fluid flow and clearance are constantly at play. Therefore, we predict that release of soluble drug would occur faster in the case of dynamic clearance, all else being equal.
Cell-biomaterial interactions
In order to probe these effects, we cultured astrocytes on various polymer surfaces for 1 and 7 days, and then assessed the ability of cells to adhere and spread on the surfaces. As controls for comparison, we have included astrocytes on bare glass alone (see ESI†), glass coated with a garden-variety polyester containing no drug (PLLA films), as well as PLLA films with exogenous E2 added to the culture media. Astrocytes hardly adhere to bare glass (negative control) but they stick well to PLLA coatings (positive control). This comparison shows the effect of switching from a hard inorganic surface to a softer polymer material in the absence of drug. The samples that have PLLA + exogenous E2 show the role of free estrogen, if any, on the adhesion events; this simulates what would be the case for a partially degraded polyestrogen that has released some free E2 into the surrounding media.
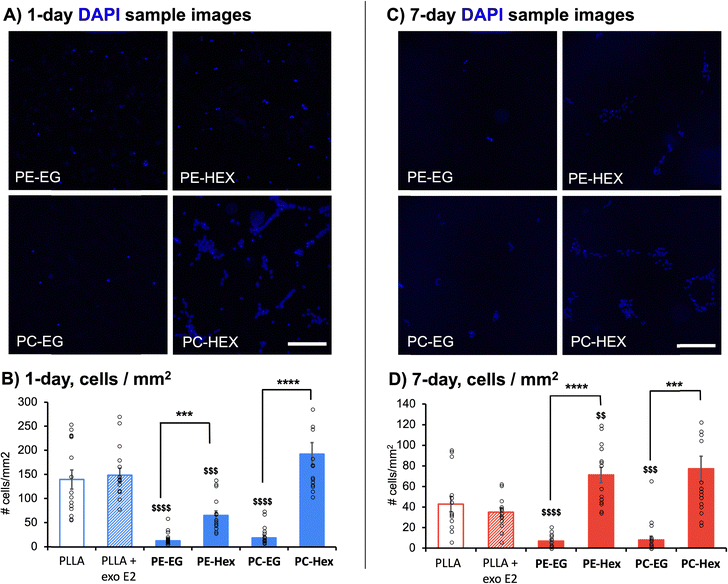
Because astrocytes express ERα and are key targets to mediate neuroprotective activity,5,7,11,12 primary rat cortical astrocytes were cultured directly onto each polymer substrate for 1 day or 7 days, and cell adhesion and spreading were assessed at each time point (Fig. 12 and 13, Table 2, Tables S3 and S4, ESI†). Although both the PE-EG and PC-EG films were not cytotoxic to mixed cortical cultures, astrocyte adhesion was significantly reduced on EG-linked polymer films. Both the PE-Hex and PC-Hex films showed significantly greater cell adhesion compared to their EG-linked counterparts following both 1 day (F7,47.10 = 32.83; PE: p ≤ 0.001; PC: p ≤ 0.0001) and 7 days (F7,51.46 = 20.08; PE: p ≤ 0.0001; PC: p ≤ 0.001) in culture directly on the surface of untreated polymer films (Fig. 12). Additionally, both the PE-Hex and PC-Hex films showed significantly greater cell spreading compared to their EG-linked counterparts following both 1 day (F7,43.53 = 45.24; PE: p ≤ 0.01; PC: p ≤ 0.0001) and 7 days (F7,50.55 = 14.28; PE: p ≤ 0.0001; PC: p ≤ 0.001) in culture directly on the surface of untreated polymer films (Fig. 13). PE-EG and PC-EG also showed reduced cell adhesion compared to the PLLA + Exo. E2 control at both 1 day (F7,47.10 = 32.83; PE: p ≤ 0.0001; PC: p ≤ 0.0001) and 7 days (F7,51.46 = 20.08; PE: p ≤ 0.0001; PC: p ≤ 0.001). However, PE-EG and PC-EG only showed a significant reduction in cell spreading compared to the PLLA + Exo. E2 control at 1 day (F7,43.53 = 45.24; PE: p ≤ 0.0001; PC: p ≤ 0.0001). By 7 days, cell spreading on both PE-EG and PC-EG was comparable to the PLLA + Exo. E2 control. This indicates that although the PLLA + Exo. E2 control supported improved initial adhesion and spreading compared to both EG-containing substrates, it did not support spreading long-term in serum-free media conditions. However, the Hex-containing films resulted in superior astrocyte adhesion and spreading long-term compared to the PLLA + Exo. E2 control. PE-Hex films initially showed reduced cell adhesion (F7,47.10 = 32.83, p ≤ 0.001) and spreading (F7,43.53 = 45.24, p ≤ 0.0001) compared with the PLLA + Exo. E2 control at 1 day; however, PE-Hex showed significantly greater cell adhesion (F7,51.46 = 20.08, p ≤ 0.01) and spreading (F7,50.55 = 14.28, p ≤ 0.0001) compared with the PLLA + Exo. E2 control at 7 days. PC-Hex showed similar levels of cell adhesion compared to the PLLA + Exo. E2 control at both 1-day and 7-day time points. PC-Hex also showed similar levels of cell spreading compared with the PLLA + Exo. E2 control at 1 day; however, PC-Hex supported significantly greater cell spreading compared with the PLLA + Exo. E2 control at 7 days (F7,50.55 = 14.28, p ≤ 0.01).
| Material | Adhesion (cells per mm2) | Spreading (% surface area covered) | ||
|---|---|---|---|---|
| 1 day | 7 day | 1 day | 7 day | |
| PLLA + Exo. E2 | 149.52 ± 14.31 | 34.93 ± 4.20 | 7.09 ± 0.78 | 1.25 ± 0.31 |
| PE-EG | 12.72 ± 3.63 | 6.89 ± 1.56 | 0.18 ± 0.06 | 0.33 ± 0.19 |
| PE-HEX | 65.47 ± 9.39 | 71.35 ± 7.30 | 1.26 ± 0.23 | 6.93 ± 0.80 |
| PC-EG | 18.98 ± 5.47 | 8.00 ± 3.63 | 0.04 ± 0.02 | 0.47 ± 0.25 |
| PC-HEX | 192.65 ± 23.92 | 77.00 ± 12.30 | 9.46 ± 1.13 | 8.29 ± 1.50 |
The reduced cell adhesion and spreading that was observed on both the PE-EG and PC-EG compared to the Hex-containing polymers and PLLA + Exo. E2 control may be due to repulsive interactions between the cellular membrane and the EG linkers. Astrocytes have a highly negative resting membrane potential.44 Such potential arises from accumulation of negatively-charged ions on the intracellular side of the membrane and positively-charged ions on the extracellular side of the membrane.45 The electronegative oxygen atoms within ethylene glycol linkages in the polymer chains are also known to attract positively-charged ions in solution.46–48 The association of cations in medium surrounding the astrocytes with EG is thought to, in part, lead to repulsive interactions between the astrocytes and the EG-containing polymers, ultimately reducing cell adhesion to EG-containing substrates.48 To investigate the interaction of ions in solution with the polymer films, we conducted zeta potential measurements on each of the polymer groups (Fig. 14). The results indeed show that both the PE-EG and PC-EG have significantly less negative zeta potentials (−46.87 ± 11.68 and −58.44 ± 10.32 mV, respectively) compared to their Hex-linked counterparts (PE-Hex: −61.02 ± 11.74 mV; PC-Hex: −92.65 ± 4.13 mV) (F4,40 = 32.31; PE: p ≤ 0.01; PC: p ≤ 0.0001). The PE-EG group also showed a less negative zeta potential compared to the PLLA control (−67.10 ± 2.23 mV) (F4,40 = 32.31; PE: p ≤ 0.001). Additionally, PC-Hex showed a significantly more negative zeta potential compared to the PLLA control (F4,40 = 32.31; PE: p ≤ 0.0001). In summary, the trends in zeta potential reflect the trends observed in the initial astrocyte adhesion and spreading data; the collection of a greater number of cations at the surface of the substrates, most notably at the surface of the EG-containing poly(pro-E2) materials, potentially contributes to increased repulsion of astrocytes with negative membrane potentials that have attracted cations around the cell surface.
Because only the textured polymerized estrogen films in this work showed markedly higher adhesion and spreading, compared to PLLA + exogenous E2 controls, it is clear that specific some characteristics of the Hex type materials must be mediating their interactions with astrocytes at the interface. The improved astrocyte interactions with the PE-Hex and PC-Hex substrates over the longer-term may be due to the combination of less cationic surface charge and increased surface roughness over time. Images of the poly(pro-E2) films show that the films initially appear smooth and transparent; however, upon 1 week of incubation in PBS at 37 °C, the PE-Hex and PC-Hex films become turbid. Based on SEM images of the film surfaces, we observed the formation of microscopic roughness on the surface of the PE-Hex and PC-Hex substrates. On the other hand, PE-EG and PC-EG remained locally smooth on the micro-scale throughout the 5-week incubation period, despite heterogeneities on longer ∼mm length scales. Surface roughness caused by nanoscale topographical features is shown to positively influence cell adhesion and spreading.49–52 For example, Ganguly et al. showed that nanoporous anodic aluminum oxide surfaces significantly improved rat cortical astrocyte adhesion compared to the nonporous aluminum control. In part, this effect may be due to the increased formation of focal adhesion sights on the nanoporous substrate compared to the smooth control.52 Focal adhesion sites are formed through the mechanical linkage of cell transmembrane integrin proteins and extracellular ligands, and it is well established that an increase in these sites leads to increase cell adhesion strength and cell spreading.53,54
Further, free thiols are known to bond with integrins on the cell surface to improve the formation of cell adhesion sites and, in turn, improve cell adhesion and spreading.55–57 Since all of the poly(pro-E2) materials were synthesized with dithiol type linkers, it was expected that these polymers would likely contain some amount of unreacted free thiols at the chain ends. Thus, we investigated whether a difference in concentration of free thiols could contribute to the changes in cell adhesion and spreading observed on the different substrates (Fig. S19, ESI†). Using Ellman's test, all polyestrogens were found to have a significantly greater concentration of free thiols (PE-EG: 51.02 ± 1.05 nM; PE-Hex: 46.61 ± 1.22 nM; PC-EG: 52.43 ± 0.49 nM; PC-Hex: 47.18 ± 3.10 nM) compared to the PLLA control that should have no thiols (26.37 ± 0.47 nM) (F5,12 = 160.00; p ≤ 0.0001 for all). We also observed a significantly greater concentration of free thiols in the PE-EG and PC-EG substrates compared to their Hex-linked counterparts (F5,12 = 160.00; PE: p ≤ 0.05; PC: p ≤ 0.01). This observation could be due to the increased ability of aqueous solutions to penetrate within the ethylene glycol-containing substrates compared to those containing hexyl linkers, resulting in a greater number of reactions between the Ellman's reagent and free thiols to occur. Although these observations do not strictly indicate interactions at the film surface, the short incubation time of 15 minutes likely precludes contributions from deep within the bulk. Given that the EG-linked polymers showed markedly lower astrocyte adhesion/spreading, despite containing slightly higher free thiol content, we reject the notion that the presence of free thiols in the polyestrogen films substantially impacts the astrocyte adhesion and spreading.
Finally, it has been demonstrated that viscoelastic microenvironments that lead to favorable biomechanical properties in astrocytes for promoting neural regeneration in the CNS under inflammatory conditions.30–32,58 Since the Hex-linked polymers exhibit the greatest degree of hysteresis and surface roughness compared to other polymer analogues in this work, this may also contribute to astrocyte longer-term adhesion and spreading on Hex-linked polymers.
Conclusions
Estrogen is known for its neurotrophic and neuroprotective actions, and estrogen variants have been studied extensively for the treatment of stroke, Alzheimer's, Parkinson's, and CNS injury.59 Astrocytes are a promising target for estrogen therapeutics due to their expression of ERα, which has been shown to mediate neuroprotective activity. Previous studies by our group found poly(pro-E2) films to be neuroprotective against oxidative stress and to enhance neurite outgrowth of dorsal root ganglia neurons in vitro,22 which suggested that polymerized forms of estrogen can maintain estrogen bioactivity and could be particularly beneficial for CNS injuries that require localized treatment over an extended period (months to years).In this work, we developed a series of poly(pro-E2) films with tunable thermal and mechanical properties and demonstrate a range of different erosion mechanisms in vitro. This work provides an innovative and tunable synthetic approach to yield poly(pro-estrogens) biomaterials with varying linkers and degradable units, reveals how linker chemistry in polyprodrugs influences thermal/mechanical properties, degradability, and surface properties, and demonstrates poly(pro-drug) materials with the ability to promote specific astrocyte behaviors. Interestingly, we found that increased micro/nano-scale surface roughness and more negative zeta potential, as well as increased mechanical hysteresis, appear to correlate with robust astrocyte adhesion and spreading. Owing to their lack of cytotoxicity and ability to modulate astrocyte response, our novel library of tunable poly(pro-E2) polymers can be utilized to target CNS injuries and disorders more effectively. For future in vivo and clinical translation of our novel poly(pro-E2) slow-eluting materials, we anticipate that the films, which encourage astrocyte adhesion and spreading, would be ideal for applications involving placement of scaffolds or conduits to encourage nerve regrowth, while those poly(pro-E2) materials that discourage astrocyte adhesion and spreading i.e., the EG-linked polymers, would be useful as coatings for stents or shunts (to drain excess fluid), structural implants and microelectrodes, to record neural activity or deliver electrical stimulation to assist with nerve regrowth but resist cell attachment.
Materials and methods
Synthesis and characterization
Polymerized estrogen materials were prepared following our previously described route with minor modifications. All detailed procedures are given in the ESI.† The 1H and 13C NMR are given in the ESI.† Spectra were recorded using 500 MHz Agilent NMR spectrometer at 25 °C. NMR chemical shifts were reported in parts per million (ppm, δ) and referenced to tetramethylsilane ((CH3)4Si, 0.00 ppm). Residual solvent signals for 1H NMR: CDCl3 (δ 7.26), DMSO-d6 (δ 2.50) and 13C NMR: CDCl3 (δ 77.0), DMSO-d6 (δ 39.5).Gel permeation chromatography (GPC) performed on an Agilent Technologies 1260 Infinity GPC with THF as the mobile phase (100 μL injection volume, 1 mL min−1 flow rate). Polymer MW was then determined using monodisperse polystyrene standards. High resolution mass spectroscopy (HRMS) was performed on Thermo LTQ Orbitrap XL instrument at resolution 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (at m/z 400) and mass accuracy better than 3 ppm. Samples were injected in the ESI† source using Agilent 1200 HPLC system in methanol as a mobile phase at flow rate 50 μl min−1.
000 (at m/z 400) and mass accuracy better than 3 ppm. Samples were injected in the ESI† source using Agilent 1200 HPLC system in methanol as a mobile phase at flow rate 50 μl min−1.
 | (1) |
Cell assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells/78.5-mm2 coverslip in DMEM + 10% FBS, 10% F-12 nutrient supplement, and 1% PS. After 24 h at 37 °C/5% CO2, cells were rinsed once with HBSS then media was replaced with neurobasal medium + L-glutamine (200 mM), B-27 (2%), and PS (1%) for 12 days. On day 13, media was replaced with 2.25 mL of media to fully submerge a 15 × 15 mm coverslip with drop cast polymer. Cell incubation conditions were as follows: media only (no treatment), media + exogenous E2 (Exo. E2) (100 nM), PE-EG film, PE-Hex film, PC-EG film, and PC-Hex film. Cells were incubated with the polymers for 7 days at which point the supernatant was collected to conduct a lactate dehydrogenase (LDH) assay, and the cells were stained with propidium iodide (PI) and Hoechst 33342 for imaging (n = 3 biological replicates from different animals).
000 cells/78.5-mm2 coverslip in DMEM + 10% FBS, 10% F-12 nutrient supplement, and 1% PS. After 24 h at 37 °C/5% CO2, cells were rinsed once with HBSS then media was replaced with neurobasal medium + L-glutamine (200 mM), B-27 (2%), and PS (1%) for 12 days. On day 13, media was replaced with 2.25 mL of media to fully submerge a 15 × 15 mm coverslip with drop cast polymer. Cell incubation conditions were as follows: media only (no treatment), media + exogenous E2 (Exo. E2) (100 nM), PE-EG film, PE-Hex film, PC-EG film, and PC-Hex film. Cells were incubated with the polymers for 7 days at which point the supernatant was collected to conduct a lactate dehydrogenase (LDH) assay, and the cells were stained with propidium iodide (PI) and Hoechst 33342 for imaging (n = 3 biological replicates from different animals).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per 225 mm2 coverslips and cultured for 1 day or 7 days in serum-free DMEM + 1% glutamax + 1% PS containing no phenol red or FBS; a 2.25 mL volume/12-well plate well was used to avoid media changes during the incubation periods. Astrocytes were cultured directly on the following substrates: glass coverslip only (glass), glass coverslip + Exo. E2 (100 nM), poly-L-lactic acid film (PLLA), PLLA + Exo. E2 (100 nM), PE-EG, PE-Hex, PC-EG, and PC-Hex. Following the 1 day or 7 days culture, the cells were fixed and stained to assess astrocyte adhesion and spreading (n = 3 biological replicates from different animals).
000 cells per 225 mm2 coverslips and cultured for 1 day or 7 days in serum-free DMEM + 1% glutamax + 1% PS containing no phenol red or FBS; a 2.25 mL volume/12-well plate well was used to avoid media changes during the incubation periods. Astrocytes were cultured directly on the following substrates: glass coverslip only (glass), glass coverslip + Exo. E2 (100 nM), poly-L-lactic acid film (PLLA), PLLA + Exo. E2 (100 nM), PE-EG, PE-Hex, PC-EG, and PC-Hex. Following the 1 day or 7 days culture, the cells were fixed and stained to assess astrocyte adhesion and spreading (n = 3 biological replicates from different animals).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), diluted in 0.5% BSA and 0.1% Tween-20 in PBS overnight at 4 °C. Following three washes with 0.1% Tween-20 in PBS to remove residual primary antibody, cells were incubated with Alexa Fluor 594 goat anti-rabbit secondary antibody (1
1000), diluted in 0.5% BSA and 0.1% Tween-20 in PBS overnight at 4 °C. Following three washes with 0.1% Tween-20 in PBS to remove residual primary antibody, cells were incubated with Alexa Fluor 594 goat anti-rabbit secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) to label GFAP and Alexa Fluor 488 phalloidin (1
1000) to label GFAP and Alexa Fluor 488 phalloidin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) to label f-actin in 0.5% BSA and 0.1% Tween-20 in PBS for 2 hours at room temperature. Nuclei were then counterstained by incubating with DAPI (1
1000) to label f-actin in 0.5% BSA and 0.1% Tween-20 in PBS for 2 hours at room temperature. Nuclei were then counterstained by incubating with DAPI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) in PBS for 10 minutes. Cells were washed 3 times with PBS and then stored in PBS at 4 °C until imaged.
1000) in PBS for 10 minutes. Cells were washed 3 times with PBS and then stored in PBS at 4 °C until imaged.
Cells were imaged using an Olympus IX-81 confocal microscope with a 10× objective (five fields of view per n = 3 biological replicate coverslips), and maximum intensity projections were obtained from the z-stacks. DAPI-stained nuclei were counted using the ImageJ multi-point tool to quantify cell adhesion, and the phalloidin-stained images were analyzed with the automated thresholding ImageJ plugin to quantify the percent area covered by astrocytes in each 10× field of view. Astrocyte adhesion data are represented as the mean number of cells per mm2 ± standard error of the mean and astrocyte spreading data are represented by the mean percent area covered by cells ± standard error of the mean (n = 3 biological replicates for both cell adhesion and spreading).
Data availability
All raw data files were uploaded to Open Science Framework and can be accessed publicly at the following link: Palermo, E. F. (2025, February 7). Polyestrogen Astrocyte paper. Retrieved from osf.io/ybsw2.Conflicts of interest
S. A. T. E., E. F. P. and R. J. G. are co-inventors on US Patent #11,202,834 which pertains to the polymerized estrogen chemistry used in this manuscript. The patent was filed on October 16, 2019.Acknowledgements
This work was supported by the National Science Foundation, grant #2217513 and NIH NIBIB grant 1R03EB034482.References
- C. S. Ahuja, J. R. Wilson, S. Nori, M. R. N. Kotter, C. Druschel, A. Curt and M. G. Fehlings, Traumatic Spinal Cord Injury, Nat. Rev. Dis. Primers, 2017, 3(1), 1–21, DOI:10.1038/nrdp.2017.18.
- A. R. Filous and J. Silver, Targeting Astrocytes in CNS Injury and Disease: A Translational Research Approach, Prog. Neurobiol., 2016, 144, 173–187, DOI:10.1016/j.pneurobio.2016.03.009.
- S. Y. Ng and A. Y. W. Lee, Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets, Front. Cell. Neurosci., 2019, 13, 528, DOI:10.3389/FNCEL.2019.00528.
- K. M. Dhandapani and D. W. Brann, Role of Astrocytes in Estrogen-Mediated Neuroprotection, Exp. Gerontol., 2007, 42(1–2), 70–75, DOI:10.1016/J.EXGER.2006.06.032.
- E. Acaz-Fonseca, R. Sanchez-Gonzalez, I. Azcoitia, M. A. Arevalo and L. M. Garcia-Segura, Role of Astrocytes in the Neuroprotective Actions of 17β-Estradiol and Selective Estrogen Receptor Modulators, Mol. Cell. Endocrinol., 2014, 389(1–2), 48–57, DOI:10.1016/J.MCE.2014.01.009.
- D. Kata, I. Gróf, Z. Hoyk, E. Ducza, M. A. Deli, I. Zupkó and I. Földesi, Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System, Int. J. Mol. Sci., 2022, 23(16), 8946, DOI:10.3390/IJMS23168946/S1.
- D. García-Ovejero, S. Veiga, L. M. García-Segura and L. L. Doncarlos, Glial Expression of Estrogen and Androgen Receptors after Rat Brain Injury, J. Comp. Neurol., 2002, 450(3), 256–271, DOI:10.1002/CNE.10325.
- A. Cox, M. Capone, D. Matzelle, A. Vertegel, M. Bredikhin, A. Varma, A. Haque, D. C. Shields and N. L. Banik, Nanoparticle-Based Estrogen Delivery to Spinal Cord Injury Site Reduces Local Parenchymal Destruction and Improves Functional Recovery, J. Neurotrauma, 2021, 38(3), 342–352, DOI:10.1089/NEU.2020.7047.
- S. Samantaray, E. A. Sribnick, A. Das, N. P. Thakore, D. Matzelle, S. P. Yu, S. K. Ray, L. Wei and N. L. Banik, Neuroprotective Efficacy of Estrogen in Experimental Spinal Cord Injury in Rats, Ann. N. Y. Acad. Sci., 2010, 1199, 90–94, DOI:10.1111/J.1749-6632.2009.05357.X.
- E. A. Sribnick, J. M. Wingrave, D. D. Matzelle, S. K. Ray and N. L. Banik, Estrogen as a Neuroprotective Agent in the Treatment of Spinal Cord Injury, Ann. N. Y. Acad. Sci., 2003, 993, 125–133, DOI:10.1111/J.1749-6632.2003.TB07521.X.
- R. D. Spence, M. E. Hamby, E. Umeda, N. Itoh, S. Du, A. J. Wisdom, Y. Cao, G. Bondar, J. Lama, Y. Ao, F. Sandoval, S. Suriany, M. V. Sofroniew and R. R. Voskuhl, Neuroprotection Mediated through Estrogen Receptor-α in Astrocytes, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(21), 8867–8872, DOI:10.1073/PNAS.1103833108/SUPPL_FILE/PNAS.201103833SI.PDF.
- R. D. Spence, A. J. Wisdom, Y. Cao, H. M. Hill, C. R. L. Mongerson, B. Stapornkul, N. Itoh, M. V. Sofroniew and R. R. Voskuhl, Estrogen Mediates Neuroprotection and Anti-Inflammatory Effects during EAE through ERα Signaling on Astrocytes But Not through ERβ Signaling on Astrocytes or Neurons, J. Neurosci., 2013, 33(26), 10924–10933, DOI:10.1523/JNEUROSCI.0886-13.2013.
- P. E. Ludwig, A. A. Patil, A. J. Chamczuk and D. K. Agrawal, Hormonal Therapy in Traumatic Spinal Cord Injury, Am. J. Transl. Res., 2017, 9(9), 3881–3895 CAS.
- A. Coyoy-Salgado, J. Segura-Uribe, H. Salgado-Ceballos, T. Castillo-Mendieta, S. Sánchez-Torres, X. Freyermuth-Trujillo, C. Orozco-Barrios, S. Orozco-Suarez, I. Feria-Romero, R. Pinto-Almazán, G. Moralí de la Brena and C. Guerra-Araiza, Evaluating Sex Steroid Hormone Neuroprotection in Spinal Cord Injury in Animal Models: Is It Promising in the Clinic?, Biomedicines, 2024, 12(7), 1478, DOI:10.3390/biomedicines12071478.
- M. Markovic, S. Ben-Shabat and A. Dahan, Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products, Pharmaceutics, 2020, 12(11), 1–12, DOI:10.3390/pharmaceutics12111031.
- J. Rautio, N. A. Meanwell, L. Di and M. J. Hageman, The Expanding Role of Prodrugs in Contemporary Drug Design and Development, Nat. Rev. Drug Discovery, 2018, 17(8), 559–587, DOI:10.1038/nrd.2018.46.
- F. Seidi, Y. Zhong, H. Xiao, Y. Jin and D. Crespy, Degradable Polyprodrugs: Design and Therapeutic Efficiency, Chem. Soc. Rev., 2022, 51(15), 6652–6703, 10.1039/d2cs00099g.
- W. Elger, R. Wyrwa, G. Ahmed, F. Meece, H. B. Nair, B. Santhamma, Z. Kileen, B. Schneider, R. Meister, H. Schubert, H. Schubert and K. Nickisch, Estradiol Prodrugs (EP) for Efficient Oral Estrogen Treatment and Abolished Effects on Estrogen Modulated Liver Functions, J. Steroid Biochem. Mol. Biol., 2017, 165, 305–311, DOI:10.1016/j.jsbmb.2016.07.008.
- L. Prokai, V. Nguyen, S. Szarka, P. Garg, G. Sabnis, H. A. Bimonte-Nelson, K. J. McLaughlin, J. S. Talboom, C. D. Conrad, P. J. Shughrue, P. Koulen and K. Prokai-Tatrai, The Prodrug DHED Selectively Delivers 17β-Estradiol to the Brain for Treating Estrogen-Responsive Disorders, Sci. Transl. Med., 2015, 7(297), 297ra113, DOI:10.1126/scitranslmed.aab1290.
- J. A. Pollock and H. K. Parker, Advances in the Development of Prodrugs as Selective Modulators of Estrogen Receptors, J. Endocr. Soc., 2022, 6(12), bvac158, DOI:10.1210/jendso/bvac158.
- A. E. Salinero, C. Abi-Ghanem, H. Venkataganesh, A. Sura, R. M. Smith, C. A. Thrasher, R. D. Kelly, K. M. Hatcher, V. NyBlom, V. Shamlian, D. G. Zuloaga and K. L. Zuloaga, Treatment with Brain Specific Estrogen Prodrug Ameliorates Cognitive Effects of Surgical Menopause in Mice, Horm. Behav., 2024, 164, DOI:10.1016/j.yhbeh.2024.105594.
- A. R. D’Amato, D. L. Puhl, S. A. T. Ellman, B. Balouch, R. J. Gilbert and E. F. Palermo, Vastly Extended Drug Release from Poly(pro-17β-Estradiol) Materials Facilitates in Vitro Neurotrophism and Neuroprotection, Nat. Commun., 2019, 10, 4830, DOI:10.1038/s41467-019-12835-w.
- A. M. Ziemba and R. J. Gilbert, Biomaterials for Local, Controlled Drug Delivery to the Injured Spinal Cord, Front. Pharmacol., 2017, 8, 245, DOI:10.3389/FPHAR.2017.00245/BIBTEX.
- A. M. Ziemba, M. C. C. Woodson, J. L. Funnell, D. Wich, B. Balouch, D. Rende, D. N. Amato, J. Bao, I. Oprea, D. Cao, E. F. Palermo and R. J. Gilbert, Development of a Slow-Degrading Polymerized Curcumin Coating for Intracortical Microelectrodes, ACS Appl. Bio Mater., 2023, 6(2), 806–818, DOI:10.1021/acsabm.2c00969.
- R. Chen, J. Funnell, G. Quinones, M. Bentley, J. Capadona, R. Gilbert and E. Palermo, Poly(pro-Curcumin) Materials Exhibit Dual Release Rates and Prolonged Antioxidant Activity as Thin Films and Self-Assembled Particles, Biomacromolecules, 2022, 24(1), 294–307, DOI:10.1021/acs.biomac.2c01135.
- M. K. Gottipati, S. A. T. Ellman, D. L. Puhl, Z. Guan, P. G. Popovich, E. F. Palermo and R. J. Gilbert, Acute Dose-Dependent Neuroprotective Effects of Poly(pro-17β-Estradiol) in a Mouse Model of Spinal Contusion Injury, ACS Chem. Neurosci., 2021, 12(6), 959–965, DOI:10.1021/acschemneuro.0c00798.
- F. Andriani and T. Fuoco, Statistical Enchainment of Ester/Ether and Carbonate Cleavable Bonds to Control Copolymers’ Erosion Rate and Trigger Environment-Specific Degradation, Eur. Polym. J., 2022, 178, 111457, DOI:10.1016/J.EURPOLYMJ.2022.111457.
- X. Wang, Z. Chen and Z. Shen, Dynamic Behavior of Polymer Surface and the Time Dependence of Contact Angle, Sci. China, Ser. B:Chem., 2005, 48(6), 553–559, DOI:10.1360/042004-22.
- J. Diani and P. Gilormini, Molecular Mobility with Respect to Accessible Volume in Monte Carlo Lattice Model for Polymers, Phys. A, 2017, 468, 825–831, DOI:10.1016/j.physa.2016.11.088.
- Y. Li and M. Xu, Hysteresis Loop and Energy Dissipation of Viscoelastic Solid Models, Mech. Time-Depend. Mater., 2007, 11(1), 1–14, DOI:10.1007/s11043-007-9027-4.
- J. G. Roth, M. S. Huang, R. S. Navarro, J. T. Akram, B. L. LeSavage and S. C. Heilshorn, Tunable Hydrogel Viscoelasticity Modulates Human Neural Maturation, Sci. Adv., 2023, 9, eadh8313, DOI:10.1126/SCIADV.ADH8313.
- Y.-B. Lu, K. Franze, G. Seifert, C. Steinhäuser, F. Kirchhoff, H. Wolburg, J. Guck, P. Janmey, E.-Q. Wei, J. Käs, J. Käs and A. Reichenbach, Viscoelastic Properties of Individual Glial Cells and Neurons in the CNS, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(47), 17759–17764, DOI:10.1073/pnas.0606150103.
- O. Neumann, H. V. Surana, S. Melly, P. Steinmann and S. Budday, Mechanical Characteristics of Spinal Cord Tissue by Indentation, J. Mech. Behav. Biomed. Mater., 2025, 163, 106863, DOI:10.1016/J.JMBBM.2024.106863.
- L. N. Woodard and M. A. Grunlan, Hydrolytic Degradation and Erosion of Polyester Biomaterials, ACS Macro Lett., 2018, 7(8), 976–982, DOI:10.1021/acsmacrolett.8b00424.
- H.-M. Chang, C.-C. Huang, V. R. Parasuraman, J.-J. Jhu, C.-Y. Tsai, H.-Y. Chao, Y.-L. Lee and H.-C. Tsai, In Vivo Degradation of Poly (ε-Caprolactone) Films in Gastro Intestinal (GI) Tract, Mater. Today Commun., 2017, 11, 18–25, DOI:10.1016/j.mtcomm.2017.01.006.
- S. K. Saha and H. Tsuji, Effects of Molecular Weight and Small Amounts of D-Lactide Units on Hydrolytic Degradation of Poly(l-Lactic Acid)s, Polym. Degrad. Stab., 2006, 91(8), 1665–1673, DOI:10.1016/j.polymdegradstab.2005.12.009.
- H. Tsuji, A. Mizuno and Y. Ikada, Properties and Morphology of Poly(L-Lactide). III. Effects of Initial Crystallinity on Long-Term in Vitro Hydrolysis of High Molecular Weight Poly(L-Lactide) Film in Phosphate-Buffered Solution, J. Appl. Polym. Sci., 2000, 77(7), 1452–1464, DOI:10.1002/1097-4628(20000815)77:7<1452::AID-APP7>3.0.CO;2-S.
- R. Auras, L.-T. Lim, S. E. M. Selke and H. Tsuji, Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications, 2010 DOI:10.1002/9780470649848.
- E. A. Schmitt, D. R. Flanagan and R. J. Linhardt, Importance of Distinct Water Environments in the Hydrolysis of Poly(Dl-Lactide-Co-Glycolide, Macromolecules, 1994, 27(3), 743–748, DOI:10.1021/ma00081a019.
- H. K. Makadia and S. J. Siegel, Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier, Polymers, 2011, 3(3), 1377–1397, DOI:10.3390/polym3031377.
- F. Von Burkersroda, L. Schedl and A. Göpferich, Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion, Biomaterials, 2002, 23(21), 4221–4231, DOI:10.1016/S0142-9612(02)00170-9.
- M.-N. Kim, B.-Y. Lee, I.-M. Lee, H.-S. Lee and J.-S. Yoon, Toxicity and Biodegradation of Products from Polyester Hydrolysis, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2001, 36(4), 447–463, DOI:10.1081/ESE-100103475.
- A. Verkhratsky, A. Butt, B. Li, P. Illes, R. Zorec, A. Semyanov, Y. Tang and M. V. Sofroniew, Astrocytes in Human Central Nervous System Diseases: A Frontier for New Therapies, Signal Transduction Targeted Ther., 2023, 8, 396, DOI:10.1038/s41392-023-01628-9.
- J. McNeill, C. Rudyk, M. E. Hildebrand and N. Salmaso, Ion Channels and Electrophysiological Properties of Astrocytes: Implications for Emergent Stimulation Technologies, Front. Cell. Neurosci., 2021, 15, 644126, DOI:10.3389/FNCEL.2021.644126/BIBTEX.
- M. Stȩpniewski, A. Bunker, M. Pasenkiewicz-Gierula, M. Karttunen and T. Róg, Effects of the Lipid Bilayer Phase State on the Water Membrane Interface, J. Phys. Chem. B, 2010, 114(36), 11784–11792, DOI:10.1021/jp104739a.
- M. Stepniewski, M. Pasenkiewicz-Gierula, T. Rog, R. Danne, A. Orlowski, M. Karttunen, A. Urtti, M. Yliperttula, E. Vuorimaa and A. Bunker, Study of PEGylated Lipid Layers as a Model for PEGylated Liposome Surfaces: Molecular Dynamics Simulation and Langmuir Monolayer Studies, Langmuir, 2011, 27(12), 7788–7798, DOI:10.1021/la200003n.
- A. Magarkar, E. Karakas, M. Stepniewski, T. Róg and A. Bunker, Molecular Dynamics Simulation of PEGylated Bilayer Interacting with Salt Ions: A Model of the Liposome Surface in the Bloodstream, J. Phys. Chem. B, 2012, 116(14), 4212–4219, DOI:10.1021/jp300184z.
- S. Rissanen, M. Kumorek, H. Martinez-Seara, Y.-C. Li, D. Jamróz, A. Bunker, M. Nowakowska, I. Vattulainen, M. Kepczynski and T. Róg, Effect of PEGylation on Drug Entry into Lipid Bilayer, J. Phys. Chem. B, 2014, 118(1), 144–151, DOI:10.1021/jp4105745.
- M. Lampin, R. Warocquier-Clérout, C. Legris, M. Degrange and M. F. Sigot-Luizard, Correlation between Substratum Roughness and Wettability, Cell Adhesion, and Cell Migration, J. Biomed. Mater. Res., 1997, 36(1), 99–108, DOI:10.1002/(SICI)1097-4636(199707)36:1<99::AID-JBM12>3.0.CO;2-E.
- M. J. P. Biggs, R. G. Richards and M. J. Dalby, Nanotopographical Modification: A Regulator of Cellular Function through Focal Adhesions, Nanomedicine, 2010, 6(5), 619–633, DOI:10.1016/j.nano.2010.01.009.
- T.-W. Chung, D.-Z. Liu, S.-Y. Wang and S.-S. Wang, Enhancement of the Growth of Human Endothelial Cells by Surface Roughness at Nanometer Scale, Biomaterials, 2003, 24(25), 4655–4661, DOI:10.1016/S0142-9612(03)00361-2.
- D. Ganguly, C. D. L. Johnson, M. K. Gottipati, D. Rende, D.-A. Borca-Tasciuc and R. J. Gilbert, Specific Nanoporous Geometries on Anodized Alumina Surfaces Influence Astrocyte Adhesion and Glial Fibrillary Acidic Protein Immunoreactivity Levels, ACS Biomater. Sci. Eng., 2018, 4(1), 128–141, DOI:10.1021/acsbiomaterials.7b00760.
- K. K. Elineni and N. D. Gallant, Regulation of Cell Adhesion Strength by Peripheral Focal Adhesion Distribution, Biophys. J., 2011, 101(12), 2903–2911, DOI:10.1016/j.bpj.2011.11.013.
- C. Selhuber-Unkel, T. Erdmann, M. López-García, H. Kessler, U. S. Schwarz and J. P. Spatz, Cell Adhesion Strength Is Controlled by Intermolecular Spacing of Adhesion Receptors, Biophys. J., 2010, 98(4), 543–551, DOI:10.1016/j.bpj.2009.11.001.
- C. Galli, L. Parisi, L. Elviri, A. Bianchera, A. Smerieri, P. Lagonegro, S. Lumetti, E. Manfredi, R. Bettini and G. M. Macaluso, Chitosan Scaffold Modified with D-(+) Raffinose and Enriched with Thiol-Modified Gelatin for Improved Osteoblast Adhesion, Biomed. Mater., 2016, 11(1), 015004, DOI:10.1088/1748-6041/11/1/015004.
- E. A. Mun, A. C. Williams and V. V. Khutoryanskiy, Adhesion of Thiolated Silica Nanoparticles to Urinary Bladder Mucosa: Effects of PEGylation, Thiol Content and Particle Size, Int. J. Pharm., 2016, 512(1), 32–38, DOI:10.1016/j.ijpharm.2016.08.026.
- O. Hegedus, D. Juriga, E. Sipos, C. Voniatis, A. Juhász, A. Idrissi, M. Zrínyi, G. Varga, A. Jedlovszky-Hajdú and K. S. Nagy, Free Thiol Groups on Poly(Aspartamide) Based Hydrogels Facilitate Tooth-Derived Progenitor Cell Proliferation and Differentiation, PLoS One, 2019, 14(12), e0226363, DOI:10.1371/journal.pone.0226363.
- L. J. Dooling, M. E. Buck, W. B. Zhang and D. A. Tirrell, Programming Molecular Association and Viscoelastic Behavior in Protein Networks, Adv. Mater., 2016, 28(23), 4651–4657, DOI:10.1002/adma.201506216.
- E. Brotfain, S. E. Gruenbaum, M. Boyko, R. Kutz, A. Zlotnik and M. Klein, Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury, Curr. Neuropharmacol., 2016, 14(6), 641–653, DOI:10.2174/1570159X14666160309123554.
- W. C. Oliver and G. M. Pharr, Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments, J. Mater. Res., 1992, 7(6), 1564–1580 CrossRef CAS.
- T.-H. Fang and W.-J. Chang, Nanoindentation Characteristics on Polycarbonate Polymer Film, Microelectron. J., 2004, 35(7), 595–599, DOI:10.1016/j.mejo.2004.02.004.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb00285k |
| ‡ These authors contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2025 |