Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coordination-driven safer and sustainable energetic materials†
Jatinder
Singh
 a,
Richard J.
Staples
a,
Richard J.
Staples
 b and
Jean'ne M.
Shreeve
b and
Jean'ne M.
Shreeve
 *a
*a
aDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu; Fax: +1-208-885-5173
bDepartment of Chemistry, Michigan State University, East Lansing, Michigan 48824, USA
First published on 17th March 2025
Abstract
The convergence of performance optimization and environmental stewardship has positioned coordination-driven approaches at the forefront of energetic material innovation. Now, using 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazine and N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide as precursors (P1 and P4), we have synthesized various coordination-driven polymeric energetic frameworks with potential applications as energetic materials, pyrotechnics, and solid propellants. Unique temperature-controlled reactivity of N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide with bases such as ammonia and alkali metal hydroxides are now reported, which results in the synthesis of new materials in a straightforward manner. These frameworks exhibit high thermal stability, controllable sensitivity, and tunable energy densities, making them versatile candidates for modern energetic applications. Furthermore, coordination-driven syntheses allow for precise structural control, enabling the fine-tuning of properties to meet specific requirements. The environmentally conscious design of these materials emphasizes the reduction of toxic byproducts which align with global sustainability goals.
1 Introduction
To promote the sustainability of energetic materials, azole-based salts with performance-advantaged properties have emerged as a focal point of research, offering potential breakthroughs in efficiency and environmental compatibility.1–5 However, challenges remain in achieving precise control over structural properties, scaling up production processes, and ensuring cost-effectiveness, all of which are critical for the practical implementation of these advanced materials. Coordination-driven polymeric energetic frameworks (CPEs) offer a promising solution to address these challenges.6–10 By utilizing coordination chemistry principles, molecules or metal ions are strategically organized into well-defined, stable structures. Researchers are now exploring innovative approaches to fine-tune these arrangements, aiming to optimize their performance and expand their applications in sustainable technologies.11–15 This approach allows for precise control over the properties of the energetic materials, such as sensitivity, thermal stability, and energy output.21–25 Furthermore, coordination complexes can incorporate eco-friendly ligands and renewable materials, reducing the environmental footprint of energetic compounds. As research continues to explore the potential of CPEs, these materials are poised to redefine the field of energetics.16–20Recent advances in the design of potassium-azole salts have demonstrated the potential to bridge the gap between high-performance and sustainable development. These materials not only exhibit enhanced thermal stability and controlled sensitivity but also enable the integration of green chemistry principles, such as the use of biodegradable ligands and light metals. To date, several representative potassium derivatives with environmentally friendly characteristics, such as K2DNABT, DTAT-K, K2DNAT, and K2DNAAzT, offer a promising platform for primary initiators (Fig. 1A).21–23 Notably, the four-nitrogen-containing tetrazole backbone has gained prominence in energetics, pyrotechnics, and propellants due to the high heats of formation and ease of syntheses (Fig. 1B).
 | ||
| Fig. 1 (A) Azole-based salts with performance-advantaged properties. (B) Application of metal-azole salts. | ||
Similar to the tetrazole ring, the tetrazine ring, distinguished by its high nitrogen content and unique reactivity, serves as an environmentally friendly, and versatile ligand for the design of advanced energetic salts.24–30 The ability of the ring nitrogens to form coordination bonds with various metal ions makes it an ideal candidate for creating new coordination materials. By designing tetrazine-based CPEs, researchers can enhance the energy density, thermal stability, and sensitivity of energetic salts while simultaneously reducing their environmental impact. Moreover, the ease of synthesis of tetrazine enables the development of less hazardous energetic materials, aligning with the broader goals of sustainability in the field of energetics. This dual functionality of the tetrazine ring as both a performance-enhancing and eco-conscious component highlights its critical role in the next generation of energetic materials. In this study, we have synthesized new CPEs using easily accessible precursors, 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazine (P1) and N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide (P4). Precursor P4 exhibits distinct reactivity with bases such as ammonia and metal hydroxides. At room temperature, it reacts to form typical salts. However, at elevated temperatures, one of the nitramine groups acts as a leaving group, resulting in the formation of two asymmetrically substituted anions (Fig. 2A).
 | ||
| Fig. 2 (A) Reactions of N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide. (B) Effect of salt formation and various interactions on thermal stability. | ||
2 Results and discussion
2.1 Synthesis
Precursors P1, P2 and P3 were prepared following literature procedures.31 The small-scale synthesis of P3 from P2 was observed to proceed with relative ease. Reaction of diamino tetrazine (P3) with freshly distilled nitric acid (red fuming) results in the formation of N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide (P4) as a yellow solid (Scheme 1). Precursor P4 upon reaction with metal carbonates in ethanol (95%) at room temperature resulted in formation of CPEs 1–5 in quantitative yields. | ||
| Scheme 1 Reactions of 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazine (P1) and temperature-based reactivity of N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide (P4). | ||
Interestingly, the reaction of P4 with aqueous ammonia in ethanol at 60 °C yields diammonium (3,5-dinitropyrazine-2,6-diyl)bis(nitroamide) (P5) through substitution with an amine, accompanied by the formation of the ammonium salt (Scheme 1). CPEs 6–10 were prepared by neutralizing P5 with 10% H2SO4, followed by reaction with metal carbonates at room temperature. Then P4 was reacted with potassium and sodium carbonates at 60 °C, which produced potassium nitro(6-oxido-1,2,4,5-tetrazin-3-yl)amide (11) and sodium nitro(6-oxido-1,2,4,5-tetrazin-3-yl)amide (12), respectively. Compound 13 was obtained by the neutralization of 12 with 10% H2SO4. Both reactions serve as notable examples of a base substituting one nitramine while facilitating salt formation with another nitramine in the same molecule. Compound 14 was synthesized from precursors P1, P6, or P7. Reaction of P7 with two equivalents of hydrazine resulted in the formation of compound 14.
2.2 Characterization
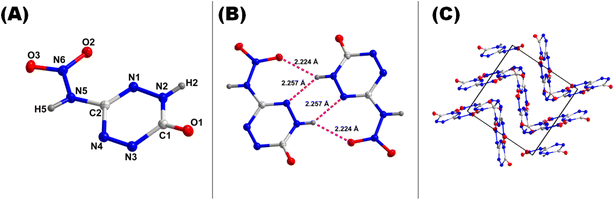
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Each unit cell contains two chemical formula units (Fig. 5A). The calculated density for compound 4·4H2O is 2.191 g cm−3 at 100 K. The Sr2+ ion is coordinated to four water molecules and O and N atoms of nitramine groups, along with the nitrogen atoms of the tetrazine ring (Fig. 5B). Packing diagram of the compound 4·4H2O shows polymeric nature through extended coordination in three-dimensional space (Fig. 5B). The coordination bond between an O-atom and the Sr2+ ranges from 2.558 Å to 2.921 Å. Compound 6 crystallizes in monoclinic space group P21/c (Fig. 6A). Each unit cell contains four chemical formula units. The calculated density for compound 6 is 1.990 g cm−3 at 100 K. In the crystal packing of 6, the potassium ion (K1) is coordinated to O-atoms of the nitramine groups along with the nitrogen atoms of the tetrazine ring (Fig. 6B). Similarly to compound 1, compound 6 also shows extended coordination in three-dimensional space, which increases the overall density of the material (Fig. 6C).
. Each unit cell contains two chemical formula units (Fig. 5A). The calculated density for compound 4·4H2O is 2.191 g cm−3 at 100 K. The Sr2+ ion is coordinated to four water molecules and O and N atoms of nitramine groups, along with the nitrogen atoms of the tetrazine ring (Fig. 5B). Packing diagram of the compound 4·4H2O shows polymeric nature through extended coordination in three-dimensional space (Fig. 5B). The coordination bond between an O-atom and the Sr2+ ranges from 2.558 Å to 2.921 Å. Compound 6 crystallizes in monoclinic space group P21/c (Fig. 6A). Each unit cell contains four chemical formula units. The calculated density for compound 6 is 1.990 g cm−3 at 100 K. In the crystal packing of 6, the potassium ion (K1) is coordinated to O-atoms of the nitramine groups along with the nitrogen atoms of the tetrazine ring (Fig. 6B). Similarly to compound 1, compound 6 also shows extended coordination in three-dimensional space, which increases the overall density of the material (Fig. 6C).
 | ||
| Fig. 4 (A) Labeling scheme for 1·H2O (thermal ellipsoid at 50%). (B) Crystal packing of 1. (C and D) Coordination modes of K+. | ||
The coordination bond between an O-atoms (O1, O2) and K1 ranges from 2.652 Å to 2.858 Å. The nitrogen atoms of the tetrazine ring (N1–N4) and the nitramine group (N5, N6) are coordinated to the potassium atoms with bond distances ranging between 2.934 Å to 3.287 Å. Compound 9·3H2O crystallizes in the monoclinic space group P2/c (Fig. 7A). Each unit cell contains two chemical formula units. The calculated density for compound 9·3H2O is 1.993 g cm−3 at 100 K. In the crystal packing of 9·3H2O, the Sr2+ atoms are coordinated to O and N atoms of the nitramine groups and two water molecules (Fig. 7B).
As a result, compound 9·3H2O shows extended coordination in one dimensional space (Fig. 7C). The coordination bond between an O-atom and the Sr atom ranges from 2.458 Å to 2.666 Å. The nitrogen atoms (N5 and N6) of the nitramine group are coordinated to the Sr atoms with bond distances ranging between 2.847 Å to 3.201 Å. Compound 13 crystallizes in orthorhombic space group Pbca (Fig. 8A). Each unit cell contains eight chemical formula units. The calculated density for compound 13 is 1.976 g cm−3 at 100 K. The unusual high density of compound 13 is attributed to the presence of strong hydrogen bond networks with distances ranging from 1.81 Å to 2.26 Å (Fig. 8B). The crystal packing shows the presence of wave-like packing and π-stacking interactions.
2.3 Physicochemical properties
| T d (°C) | ISb (J) | FSc (N) | ρ (g cm−3) | ΔHfe kJ mol−1 | D v (m s−1) | P (GPa) | |
|---|---|---|---|---|---|---|---|
| a Temperature (onset at 5 °C min−1) of decomposition. b Sensitivity to impact (IS). c Sensitivity to friction (FS). d Density at 25 °C using gas pycnometer. e Molar enthalpy of formation calculated using isodesmic reactions with the Gaussian 03 suite of programs (revision D.01). f Velocity. g Pressure (calculated using EXPLO5 version 7.01.01). h h 50 = 24 cm/2.5 kg hammer (ref. 39) | |||||||
| 1 | 242 | 3 | 40 | 2.06 | 161.8 | 7703 | 25.4 |
| 2 | 253 | 4 | 40 | 2.01 | 275.1 | 8833 | 28.8 |
| 3 | 223 | 5 | 80 | 1.85 | 422.7 | 8878 | 33.6 |
| 4 | 251 | 12 | 80 | 2.22 | 2281.0 | 9017 | 44.8 |
| 5 | 251 | 12 | 80 | 2.53 | 1888.0 | 8496 | 43.3 |
| 6 | 229 | 20 | 240 | 1.86 | 372.3 | 7644 | 23.5 |
| 7 | 257 | 20 | 240 | 1.98 | 434.9 | 9114 | 33.7 |
| 8 | 272 | 20 | 240 | 1.81 | 524.2 | 8947 | 32.2 |
| 9 | 232 | >20 | 360 | 2.19 | 1395.2 | 8145 | 33.6 |
| 10 | 212 | >20 | 360 | 2.55 | 985.6 | 8636 | 40.8 |
| 11 | 293 | 5 | 240 | 2.14 | −230.3 | 6945 | 18.8 |
| 12 | 279 | >20 | 240 | 1.98 | −104.0 | 7387 | 18.8 |
| 13 | 98 | 1 | 5 | 1.92 | 255.3 | 9214 | 36.8 |
| 14 | 136 | 4 | 40 | 1.83 | 472.7 | 7900 | 25.0 |
| RDX | 204 | 7.5/5.6h | 120 | 1.80 | 92.6 | 8795 | 34.9 |
Based upon peak decomposition temperatures measured by differential scanning calorimetry (DSC) at different heating rates of 5, 10, 15, and 20 °C min−1, the activation energies (Ek and E0), pre-exponential constant A, and linear correlation coefficients (Rk and R0) were calculated using the methods of Kissinger40 and Ozawa41 (See ESI†). The results show that the activation energy calculated by either method is essentially the same for compound 1 (Ek = 196.11 kJ mol−1; E0 = 194.82 kJ mol−1). Compound 1 exhibits a low activation energy, comparable to that of certain reported primary initiators.42 Similarly, the activation energy for compound 2 was calculated (Ek = 168.97 kJ mol−1; E0 = 168.97 kJ mol−1). For both compounds, impact and friction sensitivities, measured using the standard BAM fall hammer and BAM friction tester, respectively, further indicate high sensitivity, suggesting that they can be readily triggered to undergo intense chemical reactions.
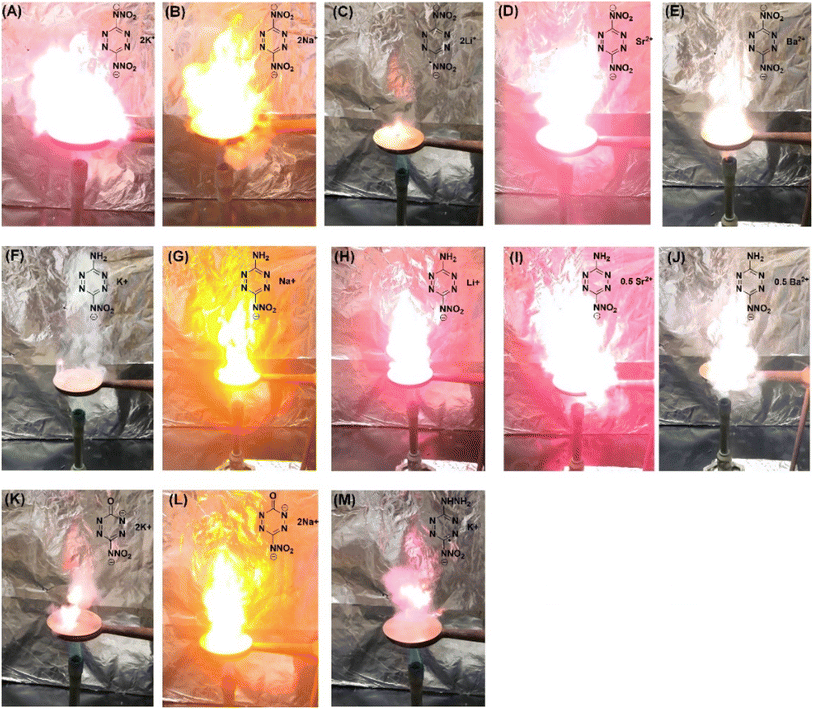
Experiments such as the “red-hot needle” and “heated plate” tests were carried out to determine the energetic behavior of compounds 1 and 2. Generally, achieving detonation in any of the tests is essential, indicating a rapid deflagration to detonation transition (DDT), which is an important characteristic of a primary initiators. For both compounds, detonation was observed in the red-hot needle test (Fig. 11A). In the heated plate test of compounds 1 and 2, two frames within the 10 millisecond differences have been captured, where intense deflagration (Fig. 11B) to detonation was observed which indicates that both of these materials behave as primary initiators.
| I sp [s]a,x | I sp [s]b,x | I sp [s]c,x | I sp [s]d,x | I sp [s]e,x | |
|---|---|---|---|---|---|
| a I sp – specific impulse of neat compound (monopropellant). b I sp – specific impulse at 88% compound and 12% Al as fuel additive. c I sp – specific impulse at 78% compound, 12% Al, and 10% HTPB as a binder. d I sp – specific impulse at 80% compound, 20% AP. e I sp – specific impulse at 42% compound, 20% AP, 20% HTPB and 18% Al. xSpecific impulse calculated at an isobaric pressure of 70 bar and initial temperature of 3300 K using EXPLO5 V7.01. | |||||
| 13 | 266 | 270 | 252 | 266 | 235 |
| 14 | 248 | 246 | 233 | 259 | 233 |
| RDX | 267 | 278 | 259 | 268 | 242 |
3 Conclusion
In this study, we have demonstrated the potential of coordination-driven polymeric energetic frameworks (CPEs) synthesized from 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazine and N,N′-(1,2,4,5-tetrazine-3,6-diyl)dinitramide (P4) as precursors. These frameworks exhibit enhanced thermal stability, tunable sensitivity, and high energy densities, making them promising candidates for various energetic applications, including primary initiators, pyrotechnics and solid propellants. The unique reactivity of precursor P4 with bases underscores the versatility and ease of synthesis of these materials. Our findings highlight the advantages of using coordination-driven approaches to achieve precise structural control, enabling the fine-tuning of material properties to meet specific application requirements. Importantly, the incorporation of eco-friendly ligands and the reduction of toxic byproducts align these materials with global sustainability goals.Data availability
All data relevant to the work described here are available in the ESI† or from the CCDC.Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.Acknowledgements
The diffractometer (Rigaku Synergy S) for SC-XRD was purchased with support from the National Science Foundation (MRI program) under grant no. 1919565. We are grateful to the Fluorine-19 fund for support.References
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418–20422 RSC.
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- A. J. Bennett, L. M. Foroughi and A. J. Matzger, J. Am. Chem. Soc., 2024, 146, 1771–1775 CrossRef CAS PubMed.
- X. Yu, J. Tang, C. Lei, C. Xue, H. Yang, C. Xiao and G. Cheng, J. Mater. Chem. A, 2024, 12, 29638–29644 RSC.
- P. Bhatia, P. Das and D. Kumar, ACS Appl. Mater. Interfaces, 2024, 16, 64846–64857 CrossRef CAS PubMed.
- M. Jujam, R. Rajak and S. Dharavath, Adv. Funct. Mater., 2025, 35, 2412638 CrossRef CAS.
- J. Singh, A. K. Chinnam, R. J. Staples and J. M. Shreeve, Inorg. Chem., 2022, 61, 16493–16500 CrossRef CAS PubMed.
- S. Li, Y. Wang, C. Qi, X. Zhao, J. Zhang, S. Zhang, S. Pang, S. Li, Y. Wang, C. Qi, X. Zhao, J. Zhang, S. Pang and S. Zhang, Angew. Chem., Int. Ed., 2013, 52, 14031–14035 CrossRef CAS PubMed.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Angew. Chem., Int. Ed, 2014, 53, 8172–8175 CrossRef CAS PubMed.
- J. Zhang, Y. Du, K. Dong, H. Su, S. Zhang, S. Li and S. Pang, Chem. Mater., 2016, 28, 1472–1480 CrossRef CAS.
- K. Pandey, A. Tiwari, J. Singh, P. Bhatia, P. Das, D. Kumar and J. M. Shreeve, Org. Lett., 2024, 26, 1952–1958 CrossRef CAS PubMed.
- L. Liang, Y. Zhong, J. Chen, J. Zhang, T. Zhang and Z. Li, Inorg. Chem., 2022, 61, 14864–14870 CrossRef CAS PubMed.
- S. Zhang, Q. Yang, X. Liu, X. Qu, Q. Wei, G. Xie, S. Chen and S. Gao, Coord. Chem. Rev., 2016, 307, 292–312 CrossRef CAS.
- R. Rajak, N. Kumar, V. D. Ghule and S. Dharavath, ACS Appl. Mater. Interfaces, 2024, 16, 20670–20680 CAS.
- Y. Liu, P. Yi, L. Gong, X. Yi, P. He, T. Wang and J. Zhang, Inorg. Chem., 2023, 62, 3186–3194 CrossRef CAS PubMed.
- Q. Sun, X. Li, Q. Lin and M. Lu, J. Mater. Chem. A, 2019, 7, 4611–4618 RSC.
- M. Benz, M. S. Gruhne, T. M. Klapötke, N. Krüger, T. Lenz, M. Lommel and J. Stierstorfer, Eur. J. Org. Chem., 2021, 2021, 4388–4392 CAS.
- J. Singh, R. J. Staples and J. M. Shreeve, ACS Appl. Mater. Interfaces, 2021, 13, 61357–61364 CrossRef CAS PubMed.
- W. Hu, J. Tang, X. Ju, Z. Yi, H. Yang, C. Xiao and G. Cheng, ACS Cent. Sci., 2023, 9, 742–747 CrossRef CAS PubMed.
- C. Li, S. Wang, S. Li, H. Yin, Q. Ma and F. X. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 35232–35244 CAS.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Angew. Chem., Int. Ed., 2014, 53, 8172–8175 CAS.
- Y. Feng, J. Zhang, W. Cao, J. Zhang and J. M. Shreeve, Nat. Commun., 2023, 141, 1–8 Search PubMed.
- D. Kumar and A. J. Elias, Resonance, 2019, 24, 1253–1271 Search PubMed.
- D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Org. Lett., 2004, 6, 2889–2891 CAS.
- M. H. V. Huynh, M. A. Hiskey, D. E. Chavez, D. L. Naud and R. D. Gilardi, J. Am. Chem. Soc., 2005, 127, 12537–12543 CAS.
- D. E. Chavez and M. A. Hiskey, J. Heterocycl. Chem., 1998, 35, 1329–1332 CAS.
- X. Zhang, Y. Wang, Y. Liu, Q. Zhang, L. Hu, C. He and S. Pang, ACS Appl. Mater. Interfaces, 2022, 14, 37975–37981 CAS.
- Y. Liu, G. Zhao, Y. Tang, J. Zhang, L. Hu, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2019, 7, 7875–7884 RSC.
- D. M. Bystrov, A. N. Pivkina and L. L. Fershtat, Molecules, 2022, 27, 5891 CrossRef CAS PubMed.
- M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K. -Y Lee and D. G. Ott, J. Heterocycl. Chem., 1991, 28, 2049–2050 CrossRef CAS.
- M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K. -Y Lee and D. G. Ott, J. Heterocycl. Chem., 1991, 28, 2049 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. L. Caricato, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Revision D.01, Gaussian, Inc., Wallingford, CT, 2003 Search PubMed.
- R. Mayer, J. Köhler and A. Homburg, Explosives, Wiley-VCH, 2007 Search PubMed.
- M. Tang, X. Wang, X. Xu, Z. Zeng, C. Chen, Y. Liu, W. Huang and Y. Tang, J. Mater. Chem. A, 2024, 12, 13081–13085 RSC.
- T. M. Klapötke, Chemistry of High-Energy Materials, De Gruyter, 6th edn, 2022 Search PubMed.
- D. Luca, Chemical Rocket Propulsion, Springer Aerospace Technology, 2017 Search PubMed.
- C. B. Storm, J. R. Stine and J. F. Kramer, Chem. Phys. Energ. Mater., 1990, 605–639 CAS.
- K. Yang, F. Bi, Q. Xue, H. Huo, C. Bai, J. Zhang and B. Wang, Dalt. Trans., 2021, 50, 8338–8348 RSC.
- T. Ozawa, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- D. J. Whelan, R. J. Spear and R. W. Read, Thermochim. Acta, 1984, 80, 149–163 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compounds, isodesmic reactions, characterization data. CCDC 2417561 (6); 2417562 (1); 2417563 (9); 2417564 (13) and 2417565 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ta01130b |
| This journal is © The Royal Society of Chemistry 2025 |