Open Access Article
Open Access ArticleSustainable biogas production through anaerobic co-digestion of Ulva lactuca (Chlorophyta) and cow manure: a kinetic and process optimization study
Obie
Farobie
 *ab,
Veni Anggita
Sari
b,
Edy
Hartulistiyoso
ab,
Widya
Fatriasari
c,
Asep Bayu Dani
Nandiyanto
d,
Apip
Amrullah
e,
Lusi
Ernawati
f and
Misbahuddin
g
*ab,
Veni Anggita
Sari
b,
Edy
Hartulistiyoso
ab,
Widya
Fatriasari
c,
Asep Bayu Dani
Nandiyanto
d,
Apip
Amrullah
e,
Lusi
Ernawati
f and
Misbahuddin
g
aDepartment of Mechanical and Biosystem Engineering, IPB University (Bogor Agriculture University), Bogor, West Java 16680, Indonesia. E-mail: obiefarobie@apps.ipb.ac.id
bSurfactant and Bioenergy Research Center (SBRC), IPB University (Bogor Agriculture University), Bogor, West Java 16680, Indonesia
cResearch Center for Biomass and Bioproducts, National Research and Innovation Agency (BRIN), Indonesia
dUniversitas Pendidikan Indonesia, Jl. Dr Setiabudi No. 229, Bandung 40154, Indonesia
eDepartment of Mechanical Engineering, Lambung Mangkurat University, Banjarmasin, South Kalimantan, Indonesia
fDepartment of Chemical Engineering, Institut Teknologi Kalimantan, Indonesia
gElectrical Engineering Department, University of Mataram, Mataram, Indonesia
First published on 24th June 2025
Abstract
Energy derived from biomass is increasingly appealing due to escalating energy demand and the urgent need to mitigate greenhouse gas emissions. However, to ensure the sustainability of bioenergy, the diversification of feedstocks, including marine biota, is essential. Among the various marine biota, harnessing U. lactuca for biogas production remains scarcely explored in the literature. This study aims to fill this gap by examining the synergistic effects of U. lactuca and cow manure in anaerobic co-digestion to optimize methane yield. The novelty of this study lies in its comprehensive kinetic analysis of biogas production from U. lactuca, offering valuable insights into the digestion process and providing optimal conditions for maximizing methane yield. Anaerobic co-digestion was conducted in a semi-continuous reactor with varying algae-to-cow manure ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under controlled conditions for over 30 days. The results showed that a 2
1) under controlled conditions for over 30 days. The results showed that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio resulted in the maximum methane yield of 325.75 mL per g VS. Kinetic evaluation using first-order, logistic, transference, and modified Gompertz models revealed that the modified Gompertz model most accurately represented the experimental data, showing a high coefficient of determination (R2 = 0.999). RSM revealed that the fermentation time and substrate ratio significantly influenced methane production. These findings highlight the effectiveness of the anaerobic co-digestion of U. lactuca and cow manure, emphasizing its potential as a viable strategy for sustainable bioenergy generation.
1 ratio resulted in the maximum methane yield of 325.75 mL per g VS. Kinetic evaluation using first-order, logistic, transference, and modified Gompertz models revealed that the modified Gompertz model most accurately represented the experimental data, showing a high coefficient of determination (R2 = 0.999). RSM revealed that the fermentation time and substrate ratio significantly influenced methane production. These findings highlight the effectiveness of the anaerobic co-digestion of U. lactuca and cow manure, emphasizing its potential as a viable strategy for sustainable bioenergy generation.
Sustainability spotlightAs global energy demands rise and the effects of climate change intensify, there is an urgent need to transition from fossil fuels to sustainable energy sources. This research addresses this issue by exploring the potential of Ulva lactuca, a marine macroalga, in producing biogas through anaerobic co-digestion with cow manure. The sustainable advancement of this work lies in utilizing marine biota as an alternative feedstock for bioenergy production, thus diversifying feedstocks and enhancing the sustainability of biogas generation. This study contributes to the circular economy, reduces waste, and mitigates greenhouse gas emissions. Aligning with the United Nations Sustainable Development Goals (SDGs), this work directly supports SDG 7 (Affordable and Clean Energy) and SDG 13 (Climate Action), offering a sustainable solution to renewable energy generation while promoting environmental stewardship. |
1 Introduction
Energy is essential for sustaining daily life, powering industries, and supporting technological advancement. However, reliance on fossil fuels to meet global energy demands has led to significant environmental challenges, including climate change, greenhouse gas emissions, and resource depletion.1 To mitigate these issues, there is a pressing need to transition to renewable energy sources that are both sustainable and environmentally friendly. One such promising solution is the production of biogas through anaerobic digestion (AD), a process that generates renewable energy and helps manage organic waste.2 Biogas is a viable bioenergy source that supports the concept of a circular economy.3 This facilitates sustainable resource recovery by transforming biodegradable waste into energy, reducing landfill usage, and decreasing the methane emissions resulting from waste decomposition. This well-established technology has been effectively utilized worldwide, showcasing the best practices for biogas production. These practices have demonstrated the potential to convert excess organic materials, previously considered waste, into renewable energy, thus supporting a more sustainable and clean energy future.4The circular economy concept plays a pivotal role in enhancing biogas production, as it encourages the integration of waste into the production cycle and reduces environmental impacts.5 By utilizing organic waste materials, including agricultural residues, food processing by-products, and marine macroalgae, biogas production offers a sustainable pathway for managing local waste streams while contributing to local energy systems. The implementation of biogas production can complement the existing waste management infrastructure by offering an alternative to landfilling and incineration, turning waste into a valuable resource. Furthermore, biogas production contributes to local energy systems by supplying renewable energy that can be used to meet local energy demands, thereby reducing the dependence on non-renewable sources. In particular, the integration of marine macroalgae, such as U. lactuca, into biogas systems could provide new solutions for coastal regions, where these algae are often considered waste or nuisance. By processing them into bioenergy, not only is waste reduced, but local energy production is also enhanced, supporting a circular economy that promotes sustainability at both the environmental and community levels.
The sustainability of biogas production relies heavily on the availability of a suitable feedstock. Various biomass sources, including agricultural wastes such as wheat straw, corncobs, bagasse, corn stalks, rice straw, and olive husks, have been successfully used for biogas generation.6 However, one of the challenges associated with terrestrial biomass is its high lignin content. This hinders microbial degradation and limits the efficiency of the AD process.7 Recent studies have demonstrated the potential of dry anaerobic digestion as a robust alternative for solid biomass such as brewer's spent grains (BSG), which not only offers efficient methane recovery (10.53 L CH4 per kg TVS) but also contributes to the circular economy by supplying a portion of the energy demand in industrial processes such as brewing.8 Similarly, valorization of fruit processing residues like apple pomace has shown promise in semi-continuous AD systems. Apple pomace, a lignocellulosic by-product with significant biogas potential, yielded 36.61 L CH4 per kg TVS_removed in dry AD systems and enabled avoided GHG emissions while supporting partial energy self-sufficiency in processing facilities.9 These examples emphasize the versatility of anaerobic digestion in utilizing diverse agro-industrial wastes within biorefinery frameworks aimed at circular bioeconomy development.
Marine macroalgae, particularly those with a low or negligible lignin content, offer an alternative feedstock that can overcome these limitations. These algae are abundant, fast-growing, and have the added benefit of absorbing nutrients from their surrounding environment, thus helping to reduce the overfertilization of water bodies.10,11 However, when overgrown, some macroalgal species can cause eutrophication, leading to issues such as smell, carbon emissions, and disruption of coastal ecosystems.12,13 Processing algae into bioenergy through anaerobic digestion is a promising approach to address these issues and transform environmental concerns into opportunities for sustainable energy generation.
Recently, the use of marine macroalgae as a feedstock for biogas production has gained increasing attention. Studies have explored various species of macroalgae, including Gracilaria sp. and U. lactuca, with Barbot et al.14 reporting methane yields ranging from 200 to 480 L CH4 per kg VS. Further research by Chikani-Cabrera et al.15 yielded 387 ± 3.09 L CH4 per kg VS from Sargassum spp. in Mexico, while Farobie et al.16 investigated anaerobic digestion of brown macroalgae Sargassum plagiophylum, achieving a maximum cumulative methane yield of 266.18 L per kg VS. Recently, Aigbe et al.17 expanded this area of study by focusing on Ulva intestinalis Linnaeus and utilizing statistical regression and machine learning approaches to optimize and predict biogas production.
Despite these advancements, there remains a gap in our understanding of the biogas production potential of U. lactuca, particularly concerning the effects of the feedstock ratio and fermentation time on methane yield. Moreover, comprehensive kinetic studies of biogas production from U. lactuca have yet to be conducted. Determining the reaction kinetics is crucial because it provides insights into the efficiency of the digestion process and can help optimize biogas production.
U. lactuca, known for its widespread availability, fast growth, and ability to absorb organic pollutants and heavy metals, has been identified as a promising feedstock for bioenergy generation.18–21 Species from the genus Ulva are easy to cultivate and can grow up to five times faster than conventional crops such as corn, making them a competitive candidate for bioenergy applications.22 Additionally, the production of U. lactuca in West Nusa Tenggara is approximately 758![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714.4 tons per year, ensuring its year-round availability and large-scale sustainability at the collection site.23 These characteristics make U. lactuca a compelling option for further research on biogas production.
714.4 tons per year, ensuring its year-round availability and large-scale sustainability at the collection site.23 These characteristics make U. lactuca a compelling option for further research on biogas production.
Most earlier investigations involving Ulva sp. used batch systems or focused solely on pretreatment strategies without applying kinetic or optimization modeling. For example, Akila (2019)24 utilized Ulva sp. mixed with cow dung in batch mode for biogas and biofertilizer production but did not explore reaction kinetics or process optimization. Ben Yahmed (2017)25 employed solid-state fermentation as a pretreatment to enhance biogas yield but omitted kinetic evaluations. Hassaan (2021)26 investigated ozonation pretreatment for Ulva sp. collected from the Mediterranean coast but similarly lacked kinetic and optimization studies.
This study addresses these gaps through three key novelties. First, we adopted a semi-continuous anaerobic digestion setup that offers advantages over batch systems in terms of higher throughput and improved process efficiency. Second, we conducted a comprehensive kinetic analysis of biogas production from U. lactuca by applying multiple kinetic models (first-order, transference, logistic, and modified Gompertz models) to understand the dynamics of methane generation. Third, we implemented response surface methodology (RSM) to optimize the process parameters, particularly feedstock ratios and digestion time, an approach not previously reported in U. lactuca studies. These contributions provide new insights into both theoretical and applied aspects of macroalgae-based bioenergy production. The objective of this study was to assess the optimal conditions for biogas generation utilizing U. lactuca, determine the reaction kinetics, and apply RSM to optimize the process.
2 Experimental
2.1. Biomass preparation and analysis
Fresh cow manure was collected from a cow farm in Bogor, Indonesia. The green algae U. lactuca utilized in this study was collected from Ekas Beach in Lombok, East Nusa Tenggara, Indonesia. The feedstock was thoroughly cleaned using tap water to remove surface impurities. After cleaning, the algae were left to air dry under sunlight for approximately 8 h to reduce moisture content. Once dried, the algae were packed in sacks and stored at a controlled temperature (3 °C) in a refrigerator until further analysis. This procedure ensured the stability of the biomass before chemical and biochemical assessments were conducted.Various analyses were performed to evaluate the chemical composition of U. lactuca. The protein content was determined using a Kjeltec 8400 automated Kjeldahl system (Foss, Denmark). This system measures nitrogen content accurately and then converts it to protein content. For proximate analysis, the feedstock moisture, volatile matter, ash, and fixed carbon were analyzed using the ASTM E1131-08 standard method. This process was carried out using a thermogravimetric analyzer (TGA 4000, PerkinElmer, USA). The ultimate analysis was performed using CHN analyzers (Leco CHN628 and CHN632). These values are essential for understanding the elemental composition of the biomass and its potential for methane production. Additionally, the structural and surface morphologies of the algae were examined using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX). This analysis was conducted using a Hitachi SU 3500 SEM. The instrument provided high-resolution images and elemental composition data.
2.2. Preparation of substate and inoculum
To prepare the substrate and inoculum for biogas production, the green macroalgae U. lactuca was initially processed into macroalgal juice by blending it with water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. Specifically, 500 g U. lactuca was mixed with 1000 g water. This mixture was combined with cow manure for acclimatization. The effect of varying the algae-to-cow manure ratios was evaluated by testing three different proportions: 1
2 ratio. Specifically, 500 g U. lactuca was mixed with 1000 g water. This mixture was combined with cow manure for acclimatization. The effect of varying the algae-to-cow manure ratios was evaluated by testing three different proportions: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (algae-to-cow manure by weight). Each mixture was thoroughly stirred for 15 min to ensure homogeneous blending of macroalgal juice and cow manure. This preparation step was crucial for maintaining consistent conditions across the experiments, facilitating an accurate assessment of biogas yield and methane production potential from each substrate formulation.
1 (algae-to-cow manure by weight). Each mixture was thoroughly stirred for 15 min to ensure homogeneous blending of macroalgal juice and cow manure. This preparation step was crucial for maintaining consistent conditions across the experiments, facilitating an accurate assessment of biogas yield and methane production potential from each substrate formulation.
2.3. Acclimatization process
A semi-continuous reactor with a total capacity of 5 L was used for acclimatization, as shown in Fig. 1. The reactor was loaded with 2000 g of a prepared inoculum-substrate mixture consisting of Ulva lactuca and cow manure in three different ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Every two days, approximately 5% of the total slurry (approximately 100 g) was extracted from the outlet to monitor essential parameters, such as volatile solids (VS), pH, and chemical oxygen demand (COD). Concurrently, 5% fresh feedstock consisting of macroalgal juice (a blend of U. lactuca and water at a 1
1. Every two days, approximately 5% of the total slurry (approximately 100 g) was extracted from the outlet to monitor essential parameters, such as volatile solids (VS), pH, and chemical oxygen demand (COD). Concurrently, 5% fresh feedstock consisting of macroalgal juice (a blend of U. lactuca and water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) was introduced into the reactor to maintain a semi-continuous feeding regimen. The biogas production experiments were conducted in duplicate to ensure data reliability. In cases where outlier data points were observed, the experiments were repeated to confirm the reproducibility and ensure the accuracy of the results.
2 ratio) was introduced into the reactor to maintain a semi-continuous feeding regimen. The biogas production experiments were conducted in duplicate to ensure data reliability. In cases where outlier data points were observed, the experiments were repeated to confirm the reproducibility and ensure the accuracy of the results.
 | ||
| Fig. 1 A schematic representation of the reactor used for the biogas production from co-digestion of U. lactuca and cow manure. | ||
Gas production during the acclimatization period was measured by recording the volume of gas produced every two days. The gas was collected and transferred to a 500 mL gas bag for further analysis using gas chromatography (GC). The acclimatization process spanned 30 days, ensuring that the microbial communities were adjusted to the substrate conditions and stabilized the reactor performance for subsequent methane yield experiments.
2.4. Anaerobic biodegradation
Following acclimatization, anaerobic biodegradation was initiated using the same semi-continuous reactor. The substrate for this stage consisted of fresh macroalgal juice, whereas the inoculum was derived from the acclimatization phase. Every two days, approximately 5% of the total slurry (approximately 100 g) was removed from the reactor outlet to monitor volatile solids (VS), pH, and chemical oxygen demand (COD). Simultaneously, 5% fresh feedstock consisting of macroalgal juice (U. lactuca combined with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) was added to the vessel to sustain anaerobic degradation. The biogas produced was measured every two days by recording its volume. It was subsequently collected in 500 mL gas bags for further analysis using gas chromatography (GC). The anaerobic biodegradation process was sustained for 30 days, ensuring that the system operated under optimal anaerobic conditions to assess methane yield and substrate degradation efficiency.
2 ratio) was added to the vessel to sustain anaerobic degradation. The biogas produced was measured every two days by recording its volume. It was subsequently collected in 500 mL gas bags for further analysis using gas chromatography (GC). The anaerobic biodegradation process was sustained for 30 days, ensuring that the system operated under optimal anaerobic conditions to assess methane yield and substrate degradation efficiency.
2.5. Analysis of product
Slurry samples were collected every two days and analyzed for volatile solids (VS), pH, and total chemical oxygen demand (COD). The pH of the slurry samples was measured using a Mettler Toledo pH meter to monitor acidity or alkalinity levels throughout the process. Total COD was determined using a standard kit from Hach Lange, Germany, which measures the amount of organic matter present in the slurry. APHA standard procedure was used to examine volatile solids.27 This provided insights into the organic content available for biodegradation.The gaseous products generated during the anaerobic biodegradation process were analyzed using gas chromatography (GC), specifically the HP 6890 series. The GC was equipped with an HP-PLOT Q column (0.53 mm × 30 m i.d., 40 μm, part no. 19095P-Q04).It was operated in a temperature-controlled environment to ensure accurate separation of the gas components. Two types of detectors were used: a thermal conductivity detector (TCD) for identifying CO2 and CO and a flame ionization detector (FID) for measuring CH4, C2H4, and C2H6. Helium served as the carrier gas at a pressure of 9.0 psi and temperature of 60 °C for FID detection, while the TCD was operated with helium at 250 °C. Calibration was performed prior to gas sample analysis. The standard gases were purchased from PT. Air Liquide, Jakarta. Multiple injections of standard gas (0.1–0.4 mL) were performed in duplicate and calibration curves were generated based on the peak areas.
Gas sample analysis followed a specific temperature program. It started at 60 °C for 5 min, followed by an incremental increase of 20 °C per min until reaching 200 °C, where it was held for 1 min. After calibration, 0.1 mL of the collected gas sample from the gas bag into the GC system. Each sample was analyzed three times, and the gas composition was identified by comparing the peak areas of the gas components against a standard calibration curve to quantify the content of each gas in mL.
2.6. Response surface methodology (RSM)
In this study, RSM was employed using Design-Expert software, version 13.0.5.0 (Stat-Ease Inc., Minneapolis, MN, USA) to optimize the anaerobic digestion process of U. lactuca with the aim of enhancing methane yield. The software's default settings for the Box–Behnken design (BBD) and quadratic model fitting were used in the analysis, and no modifications were made to the model-fitting algorithm or the calculation settings. Fermentation time and algae-to-cow manure ratio were delineated by A and B, respectively. The independent variables were varied within a range of −1 to +1 based on the initial experimental analysis. The statistical metrics used to assess the polynomial degree for the regression models include the sequential p-value, lack-of-fit p-value, adjusted R2, and predicted R2.3 Results and discussion
3.1. Characteristics of U. Lactuca and inoculum
The chemical composition of Ulva lactuca and the inoculum play a crucial role in determining the efficiency of the feedstock in anaerobic digestion, influencing both biogas production and its quality. The chemical composition, and proximate and ultimate analyses of U. lactuca are presented in Table 1. Proximate analysis revealed significant components, including proteins, carbohydrates, and lipids, that are essential for determining the potential of biomass for biogas production. The carbohydrate content in U. lactuca collected from this region was 40.66 ± 0.13 wt%. This relatively high value suggests its suitability for biogas production due to its ease of degradation by bacteria during anaerobic processes. Compared to previous studies, this carbohydrate value is lower than the 61.5 ± 2.3 wt% reported by Ortiz et al.28 for U. lactuca from Northern Chile and 59.1 ± 0.37 wt% by Rohani-Ghadikolaei et al.,29 for U. lactuca from the Persian Gulf. However, it was closer to the 48.40 wt% reported by Djoh et al.30 for Ulva reticula from East Sumba Island, Indonesia. These variations in carbohydrate content can be linked to differences in geographical origin and environmental conditions, such as water temperature, pH, and salinity, which significantly influence the biochemical composition of macroalgae.31,32| Parameters | U. lactuca |
|---|---|
| Chemical constituent (wt%) | |
| Carbohydrates | 40.66 ± 0.13 |
| Proteins | 22.92 ± 0.17 |
| Lipids | 1.32 ± 0.04 |
| Others | 35.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Proximate analysis (wt%) | |
| Moisture | 12.33 ± 0.03 |
| Ash content | 22.77 ± 0.19 |
| Fixed carbon | 7.01 ± 0.01 |
| Volatile matter | 57.89 ± 0.15 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Ultimate analysis (wt%) | |
| C | 39.10 ± 0.05 |
| H | 6.20 ± 0.05 |
| N | 1.46 ± 0.02 |
| S | 7.28 ± 0.03 |
| O | 45.96 ± 0.05 |
| C/N ratio | 26.78 |
The protein level in U. lactuca from Ekas Beach was found to be 22.92 ± 0.17 wt%, indicating a moderate protein level in the biomass. The moderate protein content in U. lactuca is advantageous for biogas production. Ganesh Saratale et al.33 reported that higher protein levels could enhance the release of ammonia during anaerobic digestion. This ammonia release may inhibit methanogenic bacteria,as also supported by Kovács et al.34 The lipid content in U. lactuca in this study was 1.32 ± 0.04 wt%, which is within the acceptable range for biogas production substrates. The lipid content of macroalgae is critical for biogas production, and according to Cirne et al.,35 substrates with lipid contents above 30% can inhibit biogas production. The lipid content of U. lactuca in this study was far below this threshold, making it a suitable substrate for anaerobic digestion and subsequent biogas production.
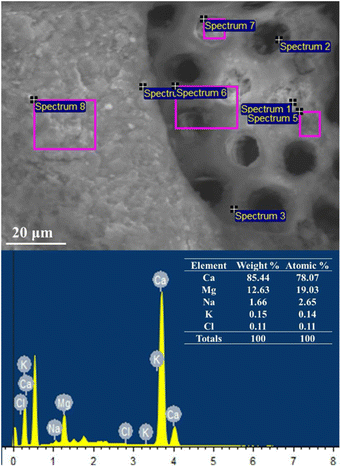
SEM-EDX analysis (Fig. 2) indicated the predominant presence of Ca, Mg, and Na. These elements help explain the high ash content of U. lactuca. These macronutrients are crucial for the metabolic activity of methanogens and are essential for AD.36 Moreover, the presence of Na can enhance biodigester stability, although excessive Na levels can inhibit methanogen proliferation.37,38
Additionally, the characteristics of the inoculum used in this study are presented in Table 2. The inoculum mixture had a C/N ratio of 23.61 and a pH of 8.2. Thompson et al.36 reported that the optimal C/N ratio for methane production ranges between 20 and 30, confirming the suitability of the inoculum for anaerobic digestion.
| Parameter | Value |
|---|---|
| pH | 8.2 |
| C/N ratio | 23.61 |
| TS [mg L−1] | 2245 |
| VS [mg L−1] | 183.2 |
3.2. The production of biogas and methane during the acclimatization phase
The acclimatization process plays a crucial role in enhancing the efficiency of biogas production, particularly when the microbial source for anaerobic digestion originates from cow manure.39 Cow manure was chosen because of its affordability and effectiveness as a microbial source, rich in diverse anaerobic microorganisms essential for breaking down organic materials.40 However, because cows primarily consume terrestrial plants and not marine macroalgae, it is essential to acclimatize the inoculum to the marine environment to ensure that the microbial community adapts to the substrate. This acclimatization ensures that the microorganisms become effective at digesting U. lactuca, a marine macroalgae, thereby optimizing biogas production. The acclimatization process gradually introduced microorganisms into the unique biochemical composition of the marine algae. Without this step, microorganisms may not efficiently convert algae into biogas because of their specialization in breaking down terrestrial organic matter. The adaptation period allows the microbial community to adjust to the higher salinity, different carbohydrate compositions, and other distinct properties of marine macroalgae.The gas composition during the acclimatization process, as shown in Fig. 3, highlights the effect of varying the algae-to-cow manure ratio on biogas production. Ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were evaluated to determine their effects on the composition of methane and carbon dioxide.
1 were evaluated to determine their effects on the composition of methane and carbon dioxide.
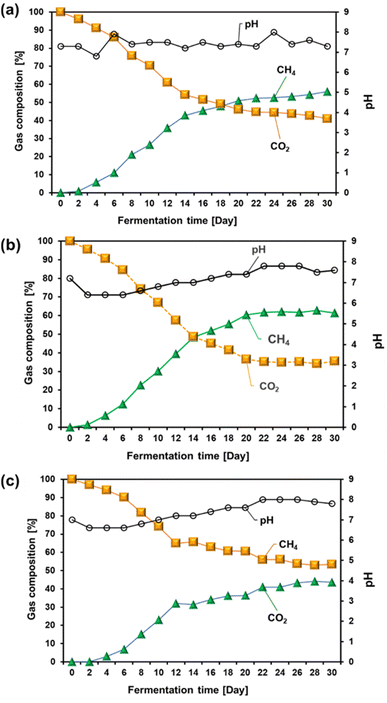 | ||
Fig. 3 Effect of algae-to-cow manure ratio on gas composition during the acclimatization process: algae-to-cow manure ratios of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 2 1, (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and (c) 3 1, and (c) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Methane production increased as acclimatization progressed, demonstrating the growth efficiency of the adapted microorganisms in digesting marine macroalgae. In contrast, the carbon dioxide levels initially spiked but eventually stabilized, indicating that the digestion process shifted toward more efficient methane production.
At an algae-to-cow manure ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, initially on day 0, the biogas composition was entirely carbon dioxide (CO2), with no methane (CH4) detected. This is expected, as the microbial community had not yet acclimated to the marine substrate, and anaerobic digestion had not commenced. By day 2, small amounts of methane began to appear (0.85%), with CO2 dominating the biogas composition. Over the next 30 days, the methane concentration increased steadily, reaching 55.99% by day 30, whereas the CO2 levels declined proportionally, dropping to 41.01%. This trend suggests that as the microorganisms adapted, their efficiency in breaking down marine macroalgae improved, shifting the biogas composition toward higher methane yields. By day 10, the methane content increased significantly to 10.97%, with CO2 reducing to 86.03%, indicating that the microbial populations had begun to effectively digest organic matter from U. lactuca. The methane content continued to increase, reaching 55.99% by day 30, reflecting the increasing dominance of methanogenic activity as the microbial community became better suited to the marine algae substrate.16
1, initially on day 0, the biogas composition was entirely carbon dioxide (CO2), with no methane (CH4) detected. This is expected, as the microbial community had not yet acclimated to the marine substrate, and anaerobic digestion had not commenced. By day 2, small amounts of methane began to appear (0.85%), with CO2 dominating the biogas composition. Over the next 30 days, the methane concentration increased steadily, reaching 55.99% by day 30, whereas the CO2 levels declined proportionally, dropping to 41.01%. This trend suggests that as the microorganisms adapted, their efficiency in breaking down marine macroalgae improved, shifting the biogas composition toward higher methane yields. By day 10, the methane content increased significantly to 10.97%, with CO2 reducing to 86.03%, indicating that the microbial populations had begun to effectively digest organic matter from U. lactuca. The methane content continued to increase, reaching 55.99% by day 30, reflecting the increasing dominance of methanogenic activity as the microbial community became better suited to the marine algae substrate.16
At an algae-to-cow manure ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, methane content was consistently higher than that at a ratio of 1
1, methane content was consistently higher than that at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For example, by day 10, methane production reached approximately 30.03% at a 2
1. For example, by day 10, methane production reached approximately 30.03% at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, whereas it was still 26.53% for the 1
1 ratio, whereas it was still 26.53% for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. This difference can be attributed to the increased availability of algal substrates in the 2
1 ratio. This difference can be attributed to the increased availability of algal substrates in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The higher content of organic material, rich in polysaccharides, such as ulvan, supports this result. These polysaccharides are efficiently broken down by specialized microbial consortia, leading to higher methane yields.24 Furthermore, the cow manure in this mixture likely provides sufficient essential microbial diversity, including methanogens, to maintain a robust anaerobic digestion process while benefiting from the energy-rich algal substrate. Under these conditions, the methane content peaked at 60.41% on day 20, stabilizing as the fermentation progressed. This stability reflects an optimal balance between substrate availability and microbial activity, where microbes have effectively adapted to the marine feedstock and converted into biogas at a steady rate.
1 ratio. The higher content of organic material, rich in polysaccharides, such as ulvan, supports this result. These polysaccharides are efficiently broken down by specialized microbial consortia, leading to higher methane yields.24 Furthermore, the cow manure in this mixture likely provides sufficient essential microbial diversity, including methanogens, to maintain a robust anaerobic digestion process while benefiting from the energy-rich algal substrate. Under these conditions, the methane content peaked at 60.41% on day 20, stabilizing as the fermentation progressed. This stability reflects an optimal balance between substrate availability and microbial activity, where microbes have effectively adapted to the marine feedstock and converted into biogas at a steady rate.
Conversely, at an algae-to-cow manure ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the methane concentration was lower than that at both 1
1, the methane concentration was lower than that at both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. One potential reason for this could be the reduced proportion of cow manure in the mixture.41,42 Cow manure is a crucial source of methanogenic bacteria and other microbial species necessary for effective biomass degradation. With less manure in the 3
1 ratios. One potential reason for this could be the reduced proportion of cow manure in the mixture.41,42 Cow manure is a crucial source of methanogenic bacteria and other microbial species necessary for effective biomass degradation. With less manure in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, there may be an insufficient microbial consortium to decompose the multifaceted organic material present in the algae. This reduction in microbial diversity and population size could slow the digestion process, leading to lower methane yields. Cow manure not only provides the necessary microbes, but also acts as a buffering agent that stabilizes the digestion environment, prevents acidification, and maintains optimal conditions for methane production.
1 ratio, there may be an insufficient microbial consortium to decompose the multifaceted organic material present in the algae. This reduction in microbial diversity and population size could slow the digestion process, leading to lower methane yields. Cow manure not only provides the necessary microbes, but also acts as a buffering agent that stabilizes the digestion environment, prevents acidification, and maintains optimal conditions for methane production.
The pH during acclimatization is a crucial factor for maintaining optimal microbial activity. Although the pH fluctuated slightly, it remained within a range suitable for methanogenic activity. In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 algae-to-cow manure ratio, the pH started at 7.3 and fluctuated between 7.3 and 7.6, stabilizing at 7.3 by day 30. This range is ideal for methane-producing archaea that thrive in slightly neutral to mildly alkaline environments. Deviations outside this range can negatively affect methane production by disrupting microbial function. According to Hilkiah Igoni et al.,43 microorganisms involved in anaerobic digestion perform most efficiently when the pH is maintained between 6 and 8, further supporting the importance of maintaining these conditions.
1 algae-to-cow manure ratio, the pH started at 7.3 and fluctuated between 7.3 and 7.6, stabilizing at 7.3 by day 30. This range is ideal for methane-producing archaea that thrive in slightly neutral to mildly alkaline environments. Deviations outside this range can negatively affect methane production by disrupting microbial function. According to Hilkiah Igoni et al.,43 microorganisms involved in anaerobic digestion perform most efficiently when the pH is maintained between 6 and 8, further supporting the importance of maintaining these conditions.
Overall, methane production peaked when the algae-to-cow manure ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaching 60.41% on day 20, and stabilizing as the fermentation time progressed. This indicates that a 2
1, reaching 60.41% on day 20, and stabilizing as the fermentation time progressed. This indicates that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio provides the most favorable conditions for methane generation. The optimal combination of substrate proportions, consistent gas composition trends, and a stable pH environment allowed methanogenic microbes to thrive, resulting in more efficient conversion of marine macroalgae into biogas. These results align with the findings of Feng et al.,44 who studied the anaerobic digestion of cow manure and rice straw at various ratios (0
1 ratio provides the most favorable conditions for methane generation. The optimal combination of substrate proportions, consistent gas composition trends, and a stable pH environment allowed methanogenic microbes to thrive, resulting in more efficient conversion of marine macroalgae into biogas. These results align with the findings of Feng et al.,44 who studied the anaerobic digestion of cow manure and rice straw at various ratios (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). Feng et al.,44 demonstrated that a 2
0). Feng et al.,44 demonstrated that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rice straw-to-cow manure ratio yielded the highest methane production at 194.91 L per kg VS.
1 rice straw-to-cow manure ratio yielded the highest methane production at 194.91 L per kg VS.
The cumulative biogas and methane production trends showed significant variations across different algae-to-cow manure ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
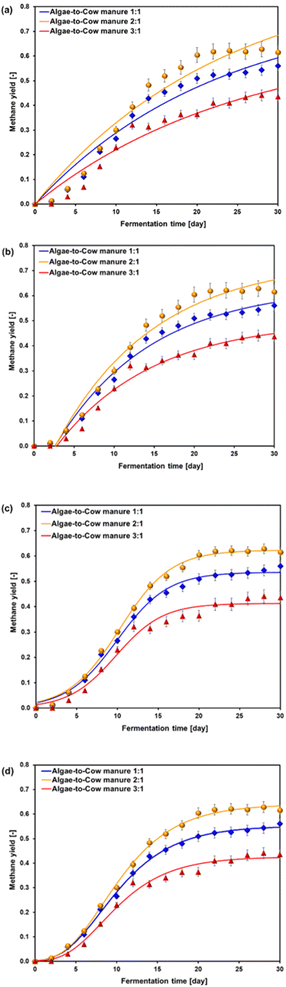
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), highlighting the impact of feedstock composition on biogas yield. Fig. 4 presents the average cumulative volumes of biogas and methane in relation to COD during the acclimatization phase under various algae-to-cow manure ratios.
1), highlighting the impact of feedstock composition on biogas yield. Fig. 4 presents the average cumulative volumes of biogas and methane in relation to COD during the acclimatization phase under various algae-to-cow manure ratios.
 | ||
Fig. 4 Effect of algae-to-cow manure ratio on cumulative methane and biogas volume during the acclimatization process: algae-to-cow manure ratios of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 2 1, (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and (c) 3 1, and (c) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
For the algae-to-cow manure ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4(a)), the cumulative biogas production showed a steady increase, reaching 200.02 mL per g VS by day 30. Methane production followed a similar trend, with cumulative methane reaching 111.99 mL per g VS by the end of the acclimatization period, accounting for approximately 56% of the total biogas volume. The balanced nutrient composition of algae and cow manure likely promoted stable microbial activity and optimized biogas and methane generation. The co-digestion of algae and manure in equal proportions may have provided a balanced carbon-to-nitrogen (C/N) ratio, which is crucial for microbial metabolism during anaerobic digestion. The COD values decreased from 17.79 g L−1 on day 0 to 2.10 g L−1 on day 30, reflecting efficient organic matter degradation.
1 (Fig. 4(a)), the cumulative biogas production showed a steady increase, reaching 200.02 mL per g VS by day 30. Methane production followed a similar trend, with cumulative methane reaching 111.99 mL per g VS by the end of the acclimatization period, accounting for approximately 56% of the total biogas volume. The balanced nutrient composition of algae and cow manure likely promoted stable microbial activity and optimized biogas and methane generation. The co-digestion of algae and manure in equal proportions may have provided a balanced carbon-to-nitrogen (C/N) ratio, which is crucial for microbial metabolism during anaerobic digestion. The COD values decreased from 17.79 g L−1 on day 0 to 2.10 g L−1 on day 30, reflecting efficient organic matter degradation.
For the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 algae-to-cow manure ratio (Fig. 4(b)), a slightly higher cumulative biogas volume was observed, reaching 214.714 mL per g VS by day 30. The methane volume also increased, with the cumulative methane production peaking at 132.0326 mL per g VS, constituting approximately 61.5% of the total biogas output. A higher proportion of algae contributed to an increase in readily biodegradable substrates, resulting in enhanced methane production. However, an imbalance in the C/N ratio may have led to suboptimal conditions for microbial growth at certain stages, potentially limiting the biogas yield. COD decreased from 20.29 g L−1 on day 0 to 2.35 g L−1 by day 30. This suggests an efficient degradation, although it was slightly less effective than the 1
1 algae-to-cow manure ratio (Fig. 4(b)), a slightly higher cumulative biogas volume was observed, reaching 214.714 mL per g VS by day 30. The methane volume also increased, with the cumulative methane production peaking at 132.0326 mL per g VS, constituting approximately 61.5% of the total biogas output. A higher proportion of algae contributed to an increase in readily biodegradable substrates, resulting in enhanced methane production. However, an imbalance in the C/N ratio may have led to suboptimal conditions for microbial growth at certain stages, potentially limiting the biogas yield. COD decreased from 20.29 g L−1 on day 0 to 2.35 g L−1 by day 30. This suggests an efficient degradation, although it was slightly less effective than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
At the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of algae-to-cow manure (Fig. 4(c)), cumulative biogas production reached 91.05 mL per g VS by day 30, with methane production peaking at 39.68 mL per g VS, which is about 43.6% of the total biogas. A larger proportion of algae might have contributed to the formation of more volatile solids and organic matter. However, a higher organic load might have caused inhibitory effects such as ammonia accumulation. This condition can suppress methane-forming archaea and reduce the methane yield efficiency. The COD levels dropped from 36.41 g L−1 on day 0 to 2.34 g L−1 by day 30, indicating that while the organic matter was degraded, the system's efficiency in biogas production was lower than that of other ratios.
1 ratio of algae-to-cow manure (Fig. 4(c)), cumulative biogas production reached 91.05 mL per g VS by day 30, with methane production peaking at 39.68 mL per g VS, which is about 43.6% of the total biogas. A larger proportion of algae might have contributed to the formation of more volatile solids and organic matter. However, a higher organic load might have caused inhibitory effects such as ammonia accumulation. This condition can suppress methane-forming archaea and reduce the methane yield efficiency. The COD levels dropped from 36.41 g L−1 on day 0 to 2.34 g L−1 by day 30, indicating that while the organic matter was degraded, the system's efficiency in biogas production was lower than that of other ratios.
3.3. The biodegradability under anaerobic condition post-acclimatization
Post-acclimatization anaerobic biodegradability was further examined by introducing fresh Ulva lactuca macroalgae into the inoculum. This step is essential for observing the response of the system to new organic inputs following acclimatization, allowing us to understand the capacity of the inoculum to effectively degrade macroalgae under anaerobic conditions. An algae-to-cow manure ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen for this study because it yielded optimal conditions during acclimatization. Fig. 5(a) illustrates the gas composition following the acclimatization process, whereas Fig. 5(b) illustrates the total volumes of biogas and methane during anaerobic digestion after acclimatization using the selected ratio.
1 was chosen for this study because it yielded optimal conditions during acclimatization. Fig. 5(a) illustrates the gas composition following the acclimatization process, whereas Fig. 5(b) illustrates the total volumes of biogas and methane during anaerobic digestion after acclimatization using the selected ratio.
From Fig. 5(a), it is clear that methane (CH4) constitutes the major component of the gas produced, accounting for approximately 65% of the total gas composition, with carbon dioxide (CO2) accounting for approximately 30%. These results indicate a well-balanced anaerobic digestion process. Scientifically, this aligns with the expectations of a semi-continuous reactor system. Methane production becomes predominant after acclimatization, indicating effective degradation of organic matter. The relatively low percentage of CO2 supports the notion of a stable methane-producing environment. Furthermore, the pH value after acclimatization was approximately 7.5, which is within the optimal range for methane production (6.8–7.8). This pH range is crucial for maintaining the activity of methanogenic bacteria. These bacteria thrive under slightly alkaline conditions and support efficient biogas production.
As shown in Fig. 5(b), the cumulative biogas and methane volumes followed a steady upward trend after acclimatization, indicating sustained biogas production. After 30 days, the cumulative methane volume reached approximately 325.75 mL per g VS, which aligns with the expected methane yields for macroalgal substrates under anaerobic digestion. Biogas production showed a similar trend, with the cumulative biogas volume exceeding 400 mL per g VS by the end of the digestion period. This steady increase suggests that the inoculum was successfully acclimatized to the macroalgal feedstock, enabling effective transformation of organic material into methane.
Moreover, chemical oxygen demand (COD) levels decreased substantially during this period, indicating a significant degradation of organic matter. The reduction in COD correlates with increased methane production, because the breakdown of complex organic compounds into simpler molecules is a precursor to methane formation. The observed trends in COD reduction and methane yield suggest that the anaerobic system was highly effective in converting the available organic content into biogas, confirming the success of the acclimatization process. This demonstrated the efficiency of the reactor in utilizing the algae-to-cow manure ratio for optimal methane production under anaerobic conditions.
The methane production in this study, which used U. lactuca as feedstock, was compared with the results of previous studies involving different macroalgal species (Table 3). This comparison allowed for a clearer understanding of the variability in the methane yield across macroalgal species from different regions. In this study, U. lactuca from Lombok, Indonesia, yielded 325.75 mL per g VS. This value falls within the range reported by Barbot et al.14 for U. lactuca from Faralhão, Portugal, producing 200–480 mL per g VS. The wide variation in methane yield might be attributed to the differing environmental conditions where the algae were harvested. Habitat plays a critical role in determining biochemical composition. It has been reported by Abusweireh (2023)45 that environmental factors, such as freshwater and seawater conditions, deep oceans, and rocky coasts, significantly influence the chemical composition of macroalgae. Additionally, seasonal variations can cause changes in the chemical makeup of seaweeds, which may lead to variations in the concentration of inhibitory substances. Consequently, these factors could have affected the methane yield if the process was conducted using U. lactuca sourced from different environmental conditions.
| Macroalgae species | Macroalgae origin | Methane production [mL per g VS] | References |
|---|---|---|---|
| Codium tomentosum | Brittany, France | 158 | Jard et al.46 |
| Gigartina spp. | North coast of Portugal | 266 | Maia et al.47 |
| Gracilaria | Faralhão, Portugal | 280–400 | Barbot et al.14 |
| Laminaria ochroleuca | North coast of Portugal | 472 | Maia et al.47 |
| Palmaria palmate | Brittany, France | 279 | Jard et al.46 |
| Saccharina latissima | North coast of Portugal | 425 | Maia et al.47 |
| Saccorhizapolyschides | Brittany, France | 232 | Jard et al.46 |
| Sargassum plagiophyllum | Lombok, Indonesia | 266.18 | Farobie et al.16 |
| Sargassum spp. | Puerto Morelos, Quintana Roo, Mexico | 387 | Chikani-Cabrera et al.15 |
| Ulva lactuca | Lombok, Indonesia | 325.75 | This study |
| Ulva lactuca | Faralhão, Portugal | 200–480 | Barbot et al.14 |
| Undaria pinnatifida | Brittany, France | 283 | Jard et al.46 |
Other species, such as Laminaria ochroleuca, with a yield of 472 mL per g VS, and Saccharina latissima, producing 425 mL per g VS,47 outperformed U. lactuca. The higher methane production from these species could be attributed to their biochemical composition. A higher carbohydrate content is easily converted to methane during anaerobic digestion. In contrast, species such as Codium tomentosum from Brittany, France, produced a much lower methane yield of 158 mL per g VS,46 indicating significant variability between species, likely influenced by differences in algal structure and environmental factors.
When comparing macroalgae with terrestrial plants, the yield of methane from AD of terrestrial feedstock such as pine wood (0.02 m3 per kg VS) and corn stover (0.107–0.241 m3 per kg VS) reported by Xu et al.48 is generally lower. This difference could be explained by the complex lignocellulosic composition of terrestrial plants, which hinders the microbial breakdown during digestion. In contrast, macroalgae, as indicated by Zabed et al.,49 contain less lignin, making them more susceptible to decomposition. Furthermore, Ganesh Saratale et al.33 noted that the absence of significant lignin content in macroalgae facilitates faster conversion to biogas compared to terrestrial plants. Terrestrial biomass requires more intensive pretreatment to break down lignocellulosic materials.
3.4. Kinetic modelling of methane generation
Kinetic modeling of methane production from algae-to-cow manure at different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) provided insight into the reaction kinetics under various conditions. Kinetic analysis was performed using four models: first-order, logistic, transference, and modified Gompertz models, each offering a unique perspective on the methane generation process.
1) provided insight into the reaction kinetics under various conditions. Kinetic analysis was performed using four models: first-order, logistic, transference, and modified Gompertz models, each offering a unique perspective on the methane generation process.
The first-order kinetic model aids in predicting methane production based on the assumption that the reaction follows first-order kinetics. This model calculates the reaction rate constant, reflecting hydrolysis efficiency, as shown in eqn (1). The model successfully predicted methane production, highlighting the significant role of hydrolysis rate in the overall methane yield.50–52
| Mt = Mm[1 − exp(−kt)] | (1) |
 | (2) |
The logistic model, with its characteristic sigmoidal curve, captured the time-dependent behavior of methane generation. This reflects an initial exponential increase, followed by a gradual deceleration as methane production reaches a saturation point.54,55 The ability of the logistic model to describe this dynamic process is demonstrated by eqn (3), showing good agreement with the experimental data.
 | (3) |
The modified Gompertz model shown in eqn (4) provides a detailed description of the methane generation process, particularly for cases with noticeable lag phases. This model accounts for the exponential phase and deceleration in methane production as the system stabilizes at the maximum yield.54,56 It is widely used to describe biological growth processes, including methane generation.
 | (4) |
The kinetic constants of the models, i.e., μ (maximum specific methane rate), Mm (methane potential), and λ (lag phase time) were determined using the least-squares-error (LSE) approach, ensuring a robust fit between the experimental and predicted data. An analysis comparing the experimental results and the four models for different feedstock ratios is shown in Fig. 6.
 | ||
| Fig. 6 Kinetic modeling employing (a) first-order, (b) transference, (c) logistic, and (d) modified Gompertz models for methane yield. | ||
This figure shows that the models effectively described methane production, with each model capturing different aspects of the process. The accuracy of each model was assessed using the coefficient of determination (R2), as shown in Fig. 7.
 | ||
| Fig. 7 Parity plots comparing the experimental data with the kinetic model using (a) first-order, (b) ternsference, (c) logistic, and (d) modified Gompertz model. | ||
The parity plots in this figure compare the experimental data with the predictions from each model. Based on parity plots comparing the experimental data with the models, the modified Gompertz model most accurately represented the experimental data, with the highest R2 value of 0.999, indicating its superior ability to capture the complex kinetic behavior of the system. The logistic model followed, with an R2 of 0.9981, showing a strong correlation owing to its effectiveness in modeling systems with growth and saturation effects. The transference model exhibited a slightly lower R2 value of 0.9956, reflecting its relevance in systems where diffusion or material transfer plays a significant role but not as accurately as the logistic or Gompertz models. The first-order model had the lowest R2 value of 0.9879, suggesting that while it captures basic kinetic trends, it is insufficient for modeling more complex systems with multiple influencing factors.
The results of the calculations for kinetic modeling using the first-order, transference, logistic, and modified Gompertz models are summarized in Table 4. The kinetic parameters, namely the maximum methane potential (Mm), rate constant (k), lag phase (λ), and specific methane production rate (μ), were obtained for different algae-to-cow manure ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In comparison, the first-order kinetic model yielded the highest values of Mm at a 2
1). In comparison, the first-order kinetic model yielded the highest values of Mm at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (0.9477), but demonstrated a lower rate constant (k), decreasing from 0.0442 per day at a 1
1 ratio (0.9477), but demonstrated a lower rate constant (k), decreasing from 0.0442 per day at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to 0.0399 per day at a 3
1 ratio to 0.0399 per day at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, indicating that while the methane potential increased with a higher algae ratio, the reaction slowed slightly.
1 ratio, indicating that while the methane potential increased with a higher algae ratio, the reaction slowed slightly.
| Kinetic model | Kinetic parameter | Algae-to-cow manure ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Algae-to-cow manure ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Algae-to-cow manure ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|---|---|---|---|---|
| First-order | M m [—] | 0.8079 | 0.9477 | 0.6670 |
| k [per day] | 0.0442 | 0.0427 | 0.0399 | |
| Transference | M m [—] | 0.6377 | 0.7430 | 0.5016 |
| λ [days] | 2.6047 | 2.6574 | 2.8185 | |
| μ [mL g−1] | 0.0534 | 0.0605 | 0.0417 | |
| Logistic | M m [—] | 0.5366 | 0.6225 | 0.4128 |
| λ [days] | 3.7789 | 3.9918 | 4.0578 | |
| μ [mL g−1] | 0.0432 | 0.0496 | 0.0358 | |
| Modified | M m [—] | 0.2030 | 0.2353 | 0.1569 |
| Gompertz | λ [days] | 8.0982 | 8.3369 | 8.1571 |
| μ [mL g−1] | 0.0157 | 0.0181 | 0.0128 |
In contrast, the modified Gompertz model predicted significantly lower methane potential values across all ratios, with Mm values ranging from 0.2030 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to 0.1569 for the 3
1 ratio to 0.1569 for the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. This model also provides a longer lag phase (λ) with values of approximately 8.09 to 8.34 days. A longer lag phase suggests a delayed onset of methane production. This observation aligns with previous research by Pardilhó et al.,57 who reported a similar range of lag phase periods (6.6 to 9.8 days) for the AD of macroalgal waste.
1 ratio. This model also provides a longer lag phase (λ) with values of approximately 8.09 to 8.34 days. A longer lag phase suggests a delayed onset of methane production. This observation aligns with previous research by Pardilhó et al.,57 who reported a similar range of lag phase periods (6.6 to 9.8 days) for the AD of macroalgal waste.
A trend was observed across all models when examining the effect of the algae-to-cow manure ratio on kinetic parameters. Higher algae ratios (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) generally led to reduced methane potential and production rates (μ), particularly in the logistic and modified Gompertz models. This could be attributed to the varying biodegradability of algae compared to cow manure, where an increase in algae content may introduce more complex organic matter, leading to a slower degradation rate, and consequently, lower methane yields. The transference model showed a moderate decrease in methane potential and production rate with increased algae content, but maintained lower lag phases than the logistic or modified Gompertz models, suggesting a relatively quicker adaptation phase for methanogenic activity.
1) generally led to reduced methane potential and production rates (μ), particularly in the logistic and modified Gompertz models. This could be attributed to the varying biodegradability of algae compared to cow manure, where an increase in algae content may introduce more complex organic matter, leading to a slower degradation rate, and consequently, lower methane yields. The transference model showed a moderate decrease in methane potential and production rate with increased algae content, but maintained lower lag phases than the logistic or modified Gompertz models, suggesting a relatively quicker adaptation phase for methanogenic activity.
While the coefficient of determination (R2) is commonly used to evaluate the model fit, the kinetic parameters derived from each model offer deeper insights into reactor design and operational strategies, which are crucial for optimizing biogas production. For example, in the first-order model, the rate constant (k) provides valuable information on the hydrolysis efficiency and speed at which the feedstock degrades. A higher k indicates faster degradation, making it suitable for high-rate reactors that require shorter hydraulic retention times (HRTs).58 Lower k values, observed with higher algae ratios, suggest slower degradation, which would favor reactors with longer HRTs or batch operations, where slower processes can occur. In the transference model, the lag phase (λ) and maximum specific methane rate (μ) indicate the reactor startup and loading strategies. A shorter lag phase and higher μ indicate that a system can quickly adapt to varying feedstock conditions, making it more suitable for reactors with variable feeding schedules or those transitioning between different feedstocks. Conversely, a longer lag phase may require careful attention to feedstock loading and temperature control to avoid inefficiency during start-up. A logistic model, with its characteristic sigmoidal curve reflecting the growth and saturation phases, is valuable for reactor design. In continuous reactors, knowing the saturation points helps to adjust the organic loading rate (OLR) to maintain the reactor in the exponential growth phase for maximum biogas production, avoiding the plateau phase that can lead to a decrease in production efficiency. The time to reach saturation also informs decisions regarding the frequency of the feeding cycles and mixing. Finally, the modified Gompertz model, incorporating the lag phase and exponential growth phase, provides important insights into reactor startup, stabilization, and the maximum possible methane yield. The modified Gompertz model is particularly well suited for a continuous stirred tank reactor (CSTR) because it accurately captures the dynamics of both the start-up phase and the continuous nature of the digestion process, where methane production increases exponentially before stabilizing. This model's parameters establish a direct relationship between maximum production rates and operational settings, allowing for fine-tuning reactor performance to achieve optimal yield.5
3.5. Response surface methodology
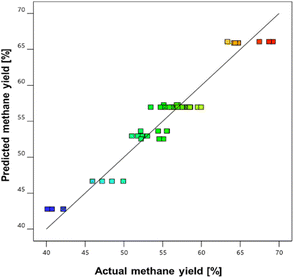
Response Surface Methodology (RSM) was employed to optimize the anaerobic digestion process of U. lactuca with the aim of enhancing methane yield. To assess the impact of two critical factors, fermentation time (A) and the algae-to-cow manure ratio (B), on methane production, a Box–Behnken design (BBD) was utilized. This experimental design facilitated the exploration of interaction effects and development of a quadratic regression model to predict outcomes.RSM analysis led to the creation of a robust quadratic model, providing insights into how these variables interact to influence the methane yield. The relationship between actual and predicted methane yields is shown in Fig. 8. The plot demonstrates a close agreement between the predicted and experimental data, indicating that the model accurately represents the system, as evidenced by the R2 value of 0.9414. This high R2 value signifies the strong predictive ability of the model and confirms its suitability for optimizing the methane production from U. lactuca under the tested conditions.
The analysis of variance (ANOVA) results for the anaerobic digestion of Ulva lactuca inoculated with cow manure are summarized in Table 5. ANOVA provides insights into the influence of different variables on methane yield, highlighting the key factors that significantly influence the outcome. The total sum of squares of the model (1976.04) indicates that the overall variability in the methane yield can be largely interpreted by the factors analyzed. The high F-value of 212.28, combined with a p-value of less than 0.0001, confirmed the statistical significance of the model, suggesting that the experimental design effectively captured the relationships between variables.
| Source | Sum of square | Df | Mean square | F-Value | p-Value | Note |
|---|---|---|---|---|---|---|
| Model | 1976.0 | 1 | 395.2 | 212.3 | <0.0001 | Significant |
| A-Fermentation time | 1507.1 | 5 | 1507.1 | 809.6 | <0.0001 | |
| B-Algae-to-cow manure ratio | 4.8 | 1 | 4.8 | 2.58 | 0.115 | |
| AB | 352.8 | 1 | 352.8 | 189.5 | <0.0001 | |
| A 2 | 2.6 | 1 | 2.6 | 1.42 | 0.2399 | |
| B 2 | 110.7 | 1 | 110.7 | 59.44 | <0.0001 | |
| Residual | 91.2 | 49 | 1.9 | |||
| Lack of fit | 4.0 | 3 | 1.3 | 0.708 | 0.552 | Significant |
| Pure error | 87.2 | 46 | 1.9 | |||
| Cor total | 2125.9 | 55 | ||||
| Std. dev. | 1.4 | |||||
| Mean | 55.7 | |||||
| C.V.% | 2.5 | |||||
| R 2 | 0.956 | |||||
| Adjusted R2 | 0.951 | |||||
| Predicted R2 | 0.94 | |||||
| Adeq precision | 52.518 |
In the one-way ANOVA, fermentation time (factor A) emerged as a crucial variable, with a sum of squares of 1507.14 and a notably high F-value of 809.55 (p < 0.0001), indicating its dominant role in methane production. This suggests that extending or varying the fermentation time significantly affects the methane yield, likely due to enhanced microbial activity over longer digestion periods. The algae-to-cow manure ratio (factor B), although showing some variability (sum of squares = 4.79), was not statistically significant (F-value = 2.58, p = 0.1150). This suggests that, within the range tested, changes in the ratio had a minimal impact on methane yields. This may indicate a saturation point or an optimal balance between algae and cow manure for anaerobic digestion.
The interaction between fermentation time and the algae-to-cow manure ratio (AB) demonstrated a significant effect (sum of squares = 352.78, F-value = 189.49, p < 0.0001). This interaction indicates that the combined influence of these two variables is more pronounced than their individual effects, possibly suggesting synergistic mechanisms at certain combinations that enhance the methane production efficiency. Additionally, the nonlinear effects of both factors were assessed, with B2 (the squared term of the algae-to-cow manure ratio) showing statistical significance (F-value = 59.44, p < 0.0001). In contrast, A2 (the squared term of the fermentation time) was not significant (F-value = 1.42, p = 0.2399). This emphasizes that deviations in the algae-to-cow manure ratio have a more complex, nonlinear effect on methane yields than the fermentation time. The residual error was relatively low (91.22), and the coefficient of determination of the model (R2 = 0.9559) indicated a strong fit, meaning that 95.59% of the variability in methane yield could be explained by the model. The adjusted R2 (0.9514) and predicted R2 (0.9414) values further confirm the robustness and predictive accuracy of the model. The Adeq precision value of 52.5176 indicates a strong signal-to-noise ratio. This supports the reliability of the experimental design.
Fig. 9 illustrates the three-dimensional surface and contour plots depicting the interaction between fermentation time and algae-to-cow manure ratio in relation to methane yield. The plots revealed that methane production was influenced by both the parameters. The three-dimensional surface plot showed that increasing the algae-to-cow manure ratio initially enhanced methane yield. However, further increases beyond a certain point led to a decline. This behavior suggests the presence of an optimal ratio that maximizes the synergy between the organic content of algae and cow manure, promoting efficient fermentation. Additionally, the plot shows that extending the fermentation time generally improved methane production. However, the rate of increase diminishes after reaching an optimum period, indicating that prolonged fermentation may lead to substrate depletion or the accumulation of inhibitory by-products. While this study does not directly measure or analyze the concentrations of known inhibitors, such as ammonia or volatile fatty acids (VFAs), it is important to note that the accumulation of these by-products could contribute to the observed reduction in methane production. As reported by Li et al. (2023),59 longer fermentation times can lead to an increase in ammonia concentration, which is known to inhibit methanogenic activity and reduce methane yield. The contour plot complements these observations by providing a clearer view of interactive effects. This shows regions where the methane yield remains high, helping to identify the optimal conditions for biogas production. Specifically, the highest yields were concentrated around intermediate values of the algae-to-cow manure ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and moderate fermentation times (24 days), reflecting a balance that facilitates effective microbial activity.
1) and moderate fermentation times (24 days), reflecting a balance that facilitates effective microbial activity.
 | ||
| Fig. 9 (a) Three-dimensional surface and (b) contour plots of methane yield against fermentation time and algae-to-cow manure ratio. | ||
3.6. Economic analysis
A techno-economic evaluation was conducted to assess the financial feasibility of biogas production from the co-digestion of U. lactuca and cow manure at a mixing ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The analysis employed several standard financial indicators including Net Present Value (NPV), Internal Rate of Return (IRR), Net Benefit-Cost Ratio (Net B/C), and Payback Period (PBP). Table 6 lists the assumptions used in the analysis.
1. The analysis employed several standard financial indicators including Net Present Value (NPV), Internal Rate of Return (IRR), Net Benefit-Cost Ratio (Net B/C), and Payback Period (PBP). Table 6 lists the assumptions used in the analysis.
| Item | Value | Unit |
|---|---|---|
| Project period | 10 | Year |
| Biogas production | 0.1945 | m3 kg−1 |
| Reactor capacity | 7 | m3 |
| Starter volume | 0.67 | |
| Starter biomass | 30% | |
| Initial biomass requirements | 2100 | kg |
| Biomass content per day | 10% | |
| Biomass requirements per day | 210 | kg |
Macroalgae![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cow manure ratio cow manure ratio |
2 | |
| Macroalgae requirements | 140 | kg per day |
| Cow manure requirements | 70 | kg per day |
| Water requirements | 280 | per day |
| Biogas production | 15.16 | m3 per day |
| Biomass shrinkage | 20% | |
| Biogas waste | 168 | kg per day |
| Sludge shrinkage | 10% | |
| Organic fertilizer | 151.2 | kg per day |
| Organic fertilizer price | 600 | IDR per kg |
| Biogas prize | 1000 | IDR per m3 |
| Inflation | 5% | |
| Interest rate | 14% | |
| Macroalgae price | 100 | IDR per kg |
| Cow manure price | 100 | IDR per kg |
The project was modeled over a 10-year operational horizon with a daily reactor capacity of 7 m3, aligned with the conditions typically observed in small-scale decentralized biogas installations in the Lombok region. The chosen substrate mix consisted of 140 kg per day macroalgae and 70 kg per day cow manure, with an additional water requirement of 280 L per day to ensure optimal anaerobic digestion conditions. The selling prices for biogas and fertilizer were set at IDR 1000 per m3 and IDR 600 per kg, respectively, based on the local market values in Lombok. On the cost side, the raw material price was assumed to be IDR 100 per kg for both macroalgae and cow manure. The inflation and interest rates were fixed at 5% and 14%, respectively, in line with the prevailing economic conditions in Indonesia.
The establishment of a macroalgae processing unit for biogas production involves an initial capital investment that contributes to the project's fixed cash outflows. Table 7 summarizes the components of this investment, including their quantities, unit prices, total costs, estimated economic life, residual values, and the corresponding annual depreciation values. The total investment cost required for the biogas production facility is IDR 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910
910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, which includes land acquisition, installation of the biogas reactor, and procurement of necessary operational equipment. The largest investment component is biogas installation, priced at IDR 45
000, which includes land acquisition, installation of the biogas reactor, and procurement of necessary operational equipment. The largest investment component is biogas installation, priced at IDR 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for a 7 m3 digester sourced from PT. Swen is a reputable Indonesian provider of renewable energy technologies. This unit is expected to operate for 10 years. The land cost, listed at IDR 5
000 for a 7 m3 digester sourced from PT. Swen is a reputable Indonesian provider of renewable energy technologies. This unit is expected to operate for 10 years. The land cost, listed at IDR 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 10 m2, was based on the government-determined property value (NJOP) for the Lombok region. The total residual value of all capital items (excluding land appreciation) is IDR 14
000 for 10 m2, was based on the government-determined property value (NJOP) for the Lombok region. The total residual value of all capital items (excluding land appreciation) is IDR 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865
865![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, while the aggregate annual depreciation is calculated at IDR 5
000, while the aggregate annual depreciation is calculated at IDR 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 247
247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. This depreciation cost is essential for accurately estimating operational profitability and is integrated into the project's annual expense projections for financial modeling and cash flow analysis.
000. This depreciation cost is essential for accurately estimating operational profitability and is integrated into the project's annual expense projections for financial modeling and cash flow analysis.
| No | Component | Quantity | Unit | Price/unit (IDR) | Total cost (IDR) | Economic life (Years) | Residual value (IDR) | Annual depreciation (IDR) |
|---|---|---|---|---|---|---|---|---|
| 1 | Land | 10 | m2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0 |
| 2 | Biogas installation | 1 | unit | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 3 | Sack sewing machine | 1 | Unit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 4 | Digital weighing scale | 1 | Unit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 5 | Water Pump | 1 | Unit | 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 6 | Water pipeline installation | 30 | m | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 7 | Production equipment (scoop, waste stirrer, and hoe) | 1 | Unit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2 | 0 | 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Total (IDR) | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865 865![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 247 247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
The operational feasibility of the macroalgal biogas processing unit was assessed through a detailed breakdown of the first-year operating costs, as summarized in Table 8. These costs were categorized into variable and fixed costs, each of which directly contributed to the net cash outflows and profitability of the project.
| No | Description | Quantity | Unit | Price/unit (IDR) | Total (IDR) | |
|---|---|---|---|---|---|---|
| I | Variable costs | |||||
| 1 | Macroalgae | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
kg | 100 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 2 | Manure | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
kg | 100 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 3 | Starter culture | 1 | Liter | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 4 | Sacks | 2722 | Unit | 1500 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 083![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Sub total | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| II | Fixed costs | |||||
| 1 | Employee salary | 1 | Person | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 2 | Maintenance | 12 | Month | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 3 | Electricity | 12 | Month | 0 | 0 | |
| 4 | Phone | 12 | Month | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Sub total | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| Total (IDR) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963 963![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
As mentioned above, the financial feasibility of the biogas production unit from the co-digestion of macroalgae (U. lactuca) and cow manure was evaluated using NPV, IRR, Net B/C, and PBP, with an assumed interest rate of 14% based on the prevailing rate at Bank Rakyat Indonesia (BRI). The calculation results for financial feasibility are presented in Table 9. The analysis yielded an NPV of IDR 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 216
216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749, indicating that the project would generate a net gain in the present value over its 10-year lifespan. This positive NPV confirms that the business is financially viable and expected to provide economic benefits beyond the initial investment. The IRR was calculated at 40.71%, which is significantly higher than the 14% discount rate, suggesting that the return on investment from this biogas system is substantially better than the conventional bank savings or lending rates. The Net B/C ratio of 2.52 means that for every IDR 1 invested, the project is expected to return IDR 2.52 in benefits, demonstrating the strong cost-effectiveness of the operation. Moreover, the payback period of 2.68 years shows that the initial investment will be recovered relatively quickly, well within the project duration, leaving several years of profit generation. Overall, these financial indicators strongly support the conclusion that the macroalgae biogas project is a profitable and sustainable venture suitable for implementation, particularly in regions with abundant organic waste and marine biomass resources.
749, indicating that the project would generate a net gain in the present value over its 10-year lifespan. This positive NPV confirms that the business is financially viable and expected to provide economic benefits beyond the initial investment. The IRR was calculated at 40.71%, which is significantly higher than the 14% discount rate, suggesting that the return on investment from this biogas system is substantially better than the conventional bank savings or lending rates. The Net B/C ratio of 2.52 means that for every IDR 1 invested, the project is expected to return IDR 2.52 in benefits, demonstrating the strong cost-effectiveness of the operation. Moreover, the payback period of 2.68 years shows that the initial investment will be recovered relatively quickly, well within the project duration, leaving several years of profit generation. Overall, these financial indicators strongly support the conclusion that the macroalgae biogas project is a profitable and sustainable venture suitable for implementation, particularly in regions with abundant organic waste and marine biomass resources.
| Investment criteria | Value |
|---|---|
| NPV | IDR 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 216 216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 749 |
| IRR | 40.71% |
| Net B/C | 2.52 |
| PBP | 2.68 years |
4 Conclusion
This study highlights the potential of U. lactuca as a viable feedstock for anaerobic co-digestion with cow manure, reinforcing its role in sustainable biogas production. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 algae-to-cow manure ratio provided the highest methane yield, reaching 325.75 mL per g VS, and the cumulative biogas yield exceeded 400 mL per g VS, indicating an efficient substrate conversion. The modified Gompertz model best represented the methane production process and achieved a high coefficient of determination (R2 = 0.999). The model also indicated that under optimal conditions, the shortest duration required to generate methane (λ) was 8.3 days, reflecting the efficient adaptation of the microbial community to the substrate. Response surface methodology (RSM) analysis also identified fermentation time and substrate ratio as the key parameters influencing methane yield. These findings support the integration of marine biomass into anaerobic systems, thereby advancing circular bioeconomy strategies in the wastewater treatment sector.
1 algae-to-cow manure ratio provided the highest methane yield, reaching 325.75 mL per g VS, and the cumulative biogas yield exceeded 400 mL per g VS, indicating an efficient substrate conversion. The modified Gompertz model best represented the methane production process and achieved a high coefficient of determination (R2 = 0.999). The model also indicated that under optimal conditions, the shortest duration required to generate methane (λ) was 8.3 days, reflecting the efficient adaptation of the microbial community to the substrate. Response surface methodology (RSM) analysis also identified fermentation time and substrate ratio as the key parameters influencing methane yield. These findings support the integration of marine biomass into anaerobic systems, thereby advancing circular bioeconomy strategies in the wastewater treatment sector.
Data availability
Data will be made available on request.Author contributions
Obie Farobie: writing – original draft, conceptualization, resources, methodology, data curation, formal analysis, funding acquisition, project administration. Veni Anggita Sari: data curation, investigation. Edy Hartulistiyoso: conceptualization, resources. Widya Fatriasari: writing – review and editing, resources. Asep Bayu Dani Nandiyanto: conceptualization, methodology, writing – review and editing. Apip Amrullah: formal analysis, conceptualization, methodology. Lusi Ernawati: writing – review and editing, conceptualization. Misbahuddin: resources, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We would like to express our gratitude to the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology of Indonesia, for funding this research through the Strategic Research Collaboration (Kolaborasi Penelitian Strategis–KATALIS), under grant number: 001/E5/PG.02.00/PL.BATCH.2/2024.References
- D. Singh, M. Tembhare, A. K. Dikshit, M. B. Dangi and S. Kumar, Process Saf. Environ. Prot., 2024, 185, 392–407 CrossRef CAS.
- F. Sher, N. Smječanin, H. Hrnjić, A. Karadža, R. Omanović, E. Šehović and J. Sulejmanović, Process Saf. Environ. Prot., 2024, 188, 834–859 CrossRef CAS.
- S. C. Iweka, K. C. Owuama, J. L. Chukwuneke and O. A. Falowo, Heliyon, 2021, 7, e08255 CrossRef CAS PubMed.
- N. A. S. Tjutju, J. Ammenberg and A. Lindfors, Renewable Sustainable Energy Rev., 2024, 201, 114631 CrossRef CAS.
- K. Al bkoor Alrawashdeh, L. Al-Sameraie, A. Al Bsoul, A. Khasawneh and J. Al-Tabbal, Int. J. Low-Carbon Technol., 2024, 19, 1501–1515 CrossRef CAS.
- M. Keerthana Devi, S. Manikandan, M. Oviyapriya, M. Selvaraj, M. A. Assiri, S. Vickram, R. Subbaiya, N. Karmegam, B. Ravindran, S. W. Chang and M. K. Awasthi, Bioresour. Technol., 2022, 363, 127871 CrossRef CAS PubMed.
- M. U. Khan and B. K. Ahring, Biomass Bioenergy, 2019, 128, 105325 CrossRef CAS.
- W. G. Sganzerla, J. M. Costa, M. Tena-Villares, L. S. Buller, S. I. Mussatto and T. Forster-Carneiro, Fermentation, 2023, 9(1), 2 CrossRef CAS.
- L. C. Ampese, W. G. Sganzerla, H. Di Domenico Ziero, J. M. Costa, G. Martins and T. Forster-Carneiro, Biomass Convers. Biorefin., 2024, 14, 14843–14857 CrossRef CAS.
- O. Farobie, A. Amrullah, N. Syaftika, A. Bayu, E. Hartulistiyoso, W. Fatriasari and A. B. Dani Nandiyanto, ACS Omega, 2024, 9, 16665–16675 CrossRef CAS PubMed.
- L. O. Ohlsson, S. Karlsson, K. Rupar-Gadd, E. Albers and U. Welander, Biomass Bioenergy, 2020, 140, 105670 CrossRef CAS.
- O. Farobie, N. F. Santosa, W. Fatriasari, A. Karimah, A. Amrullah, S. H. Suseno, A. B. D. Nandiyanto and E. Hartulistiyoso, Bioresour. Technol. Rep., 2024, 25, 101768 CrossRef CAS.
- C. F. H. Joniver, A. Photiades, P. J. Moore, A. L. Winters, A. Woolmer and J. M. M. Adams, Algal Res., 2021, 58, 102407 CrossRef.
- Y. N. Barbot, H. Al-Ghaili and R. Benz, Mar. Drugs, 2016, 14, 120 CrossRef PubMed.
- K. D. Chikani-Cabrera, P. M. B. Fernandes, R. Tapia-Tussell, D. L. Parra-Ortiz, G. Hernández-Zárate, R. Valdez-Ojeda and L. Alzate-Gaviria, Life, 2022, 12(8), 1214 CrossRef CAS PubMed.
- O. Farobie, A. Amrullah, L. A. Anis, E. Hartulistiyoso, N. Syaftika, G. Saefurahman and A. Bayu, Bioresour. Technol. Rep., 2023, 22, 101403 CrossRef CAS.
- U. O. Aigbe, K. E. Ukhurebor, A. O. Osibote, M. A. Hassaan and A. El Nemr, Renewable Energy, 2024, 235, 121347 CrossRef CAS.
- A. Amrullah, O. Farobie, A. Bayu, N. Syaftika, E. Hartulistiyoso, N. R. Moheimani, S. Karnjanakom and Y. Matsumura, Sustainability, 2022, 14, 1–14 CrossRef.
- G. Gao, A. S. Clare, C. Rose and G. S. Caldwell, GCB Bioenergy, 2018, 10, 39–51 CrossRef CAS.
- J. Liu, Y. Tong, J. Xia, Y. Sun, X. Zhao, J. Sun, S. Zhao, M. Zhuang, J. Zhang and P. He, Mar. Pollut. Bull., 2022, 174, 113243 CrossRef CAS PubMed.
- R. F. Pari, Uju, A. T. Wijayanta, W. Ramadhan, S. D. Hardiningtyas, K. A. Kurnia, M. L. Firmansyah, A. Hana, M. N. Abrar, R. Wakabayashi, N. Kamiya and M. Goto, Fish. Sci., 2024, 90, 795–808 CrossRef CAS.
- M. M. Nielsen, A. Bruhn, M. B. Rasmussen, B. Olesen, M. M. Larsen and H. B. Møller, J. Appl. Phycol., 2012, 24, 449–458 CrossRef.
- R. Adiansyah, I. T. Asfar, M. Rianti, I. Andriani, A. C. Malina, Kasmiati, M. I. A. Asfar, A. Nurannisa and Lideman, Rumput Laut Ulva sp., Diversifikasi Produk Olahan, 2024. Search PubMed.
- V. Akila, A. Manikandan, D. Sahaya Sukeetha, S. Balakrishnan, P. M. Ayyasamy and S. Rajakumar, Biocatal. Agric. Biotechnol., 2019, 18, 101035 CrossRef.
- N. Ben Yahmed, H. Carrere, M. N. Marzouki and I. Smaali, Algal Res., 2017, 27, 206–214 CrossRef.
- M. A. Hassaan, A. El Nemr, M. R. Elkatory, A. Eleryan, S. Ragab, A. El Sikaily and A. Pantaleo, Energies, 2021, 14, 1–16 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA, 2005 Search PubMed.
- J. Ortiz, N. Romero, P. Robert, J. Araya, J. Lopez-Hernández, C. Bozzo, E. Navarrete, A. Osorio and A. Rios, Food Chem., 2006, 99, 98–104 CrossRef CAS.
- K. Rohani-Ghadikolaei, E. Abdulalian and W. K. Ng, J. Food Sci. Technol., 2012, 49, 774–780 CrossRef CAS PubMed.
- E. F. Kale Djoh, F. Meiyasa, S. Ndahawali and N. Tarigan, Biodiversitas, 2024, 25, 2943–2949 Search PubMed.
- S. M. Mohy El-Din, Int. J. Environ. Sci. Technol., 2019, 16, 5783–5796 CrossRef CAS.
- L. E. Rioux, S. L. Turgeon and M. Beaulieu, Carbohydr. Polym., 2007, 69, 530–537 CrossRef CAS.
- R. Ganesh Saratale, G. Kumar, R. Banu, A. Xia, S. Periyasamy and G. Dattatraya Saratale, Bioresour. Technol., 2018, 262, 319–332 CrossRef CAS PubMed.
- E. Kovács, R. Wirth, G. Maróti, Z. Bagi, G. Rákhely and K. L. Kovács, PLoS One, 2013, 8, 1–18 CrossRef.
- D. G. Cirne, X. Paloumet, L. Björnsson, M. M. Alves and B. Mattiasson, Renewable Energy, 2007, 32, 965–975 CrossRef CAS.
- T. M. Thompson, B. R. Young and S. Baroutian, Fuel, 2020, 279, 118527 CrossRef CAS.
- Y. Chen, J. J. Cheng and K. S. Creamer, Bioresour. Technol., 2008, 99, 4044–4064 CrossRef CAS PubMed.
- D. M. Wall, E. Allen, B. Straccialini, P. O'Kiely and J. D. Murphy, Bioresour. Technol., 2014, 172, 349–355 CrossRef CAS PubMed.
- L. M. Ningsih, U. Hasanudin and H. Roubík, Renewable Energy, 2024, 236, 121519 CrossRef CAS.
- S. Agustina, K. G. Wiryawan, S. Suharti and A. Meryandini, Biodiversitas, 2024, 25, 107–115 CrossRef.
- H. Hadiyanto, F. M. Octafalahanda, J. Nabila, A. K. Jati, M. Christwardana, K. Kusmiyati and A. Khoironi, Int. J. Renewable Energy Dev., 2023, 12, 390–395 CrossRef CAS.
- A. Sackey D, Int. J. Hydrol., 2018, 2, 567–571 Search PubMed.
- A. Hilkiah Igoni, M. J. Ayotamuno, C. L. Eze, S. O. T. Ogaji and S. D. Probert, Appl. Energy, 2008, 85, 430–438 CrossRef.
- Z. Feng, W. Yao, S. Yongming, K. Xiaoying, Y. Zhenhong and Z. Xinshu, Nat. Environ. Pollut. Technol., 2017, 16, 837–842 CAS.
- R. S. Abusweireh, N. Rajamohan, C. Sonne and Y. Vasseghian, Heliyon, 2023, 9, e17757 CrossRef CAS PubMed.
- G. Jard, H. Marfaing, H. Carrère, J. P. Delgenes, J. P. Steyer and C. Dumas, Bioresour. Technol., 2013, 144, 492–498 CrossRef CAS PubMed.
- M. R. G. Maia, A. J. M. Fonseca, H. M. Oliveira, C. Mendonça and A. R. J. Cabrita, Sci. Rep., 2016, 6, 1–10 CrossRef PubMed.
- F. Xu, Z. W. Wang and Y. Li, Bioresour. Technol., 2014, 173, 168–176 CrossRef CAS PubMed.
- H. M. Zabed, S. Akter, J. Yun, G. Zhang, Y. Zhang and X. Qi, Renewable Sustainable Energy Rev., 2020, 117, 109503 CrossRef CAS.
- R. Karki, W. Chuenchart, K. C. Surendra, S. Sung, L. Raskin and S. K. Khanal, Bioresour. Technol., 2022, 343, 126063 CrossRef CAS PubMed.
- A. M. Rahmani, V. K. Tyagi, B. Ahmed, A. A. Kazmi, C. S. P. Ojha and R. Singh, Environ. Res., 2022, 212, 113382 CrossRef CAS PubMed.
- Y. F. Lim, Y. J. Chan, Y. A. Abakr, V. Sethu, A. Selvarajoo, A. Singh, J. Lee and M. Gareth, Environ. Technol., 2022, 43, 2492–2509 CrossRef CAS PubMed.
- M. J. Fernández-Rodríguez, D. de la Lama-Calvente, A. Jiménez-Rodríguez, R. Borja and B. Rincón-Llorente, Process Saf. Environ. Prot., 2019, 128, 167–175 CrossRef.
- L. S. Avinash and A. Mishra, Fuel, 2024, 367, 131545 CrossRef CAS.
- M. Hakimi, M. D. Manogaran, R. Shamsuddin, S. A. Mohd Johari, M. Abdalla M Hassan and T. Soehartanto, Heliyon, 2023, 9, e17096 CrossRef CAS PubMed.
- Y. Cai, D. Gallegos, Z. Zheng, W. Stinner, X. Wang, J. Pröter and F. Schäfer, Bioresour. Technol., 2021, 337, 125328 CrossRef CAS PubMed.
- S. Pardilhó, J. C. Pires, R. Boaventura, M. Almeida and J. Maia Dias, Bioresour. Technol., 2022, 359, 127473 CrossRef PubMed.
- S. R. Basak, S. A. Chowdhury, R. Khan, A. H. Nury, M. J. Bin Alam and M. I. Kabir, Waste Manag. Bull., 2025, 3, 271–292 CrossRef.
- Y. Li, J. Zhu, Y. Tang, X. Shi, S. Anwar, J. Wang, L. Gao and J. Zhang, Agriculture, 2023, 13(8), 1645 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |