 Open Access Article
Open Access ArticleA review on breast cancer diagnostic techniques
Parikshana
Mathur
 a,
Saakshi
Dhanekar
a,
Saakshi
Dhanekar
 *abc and
B. D.
Malhotra
*abc and
B. D.
Malhotra
 de
de
aSensekriti Technology Solutions Pvt. Ltd., Technology Innovation and Start-up Centre (TISC), IIT Jodhpur, NH 62, Nagaur Road, Karwar, Jodhpur 342030, Rajasthan, India. E-mail: saakshi@iitj.ac.in
bInter Disciplinary Research Division – Smart Healthcare, IIT Jodhpur-AIIMS Jodhpur, NH 62, Nagaur Road, Karwar, Jodhpur 342030, Rajasthan, India
cDepartment of Electrical Engineering, IIT Jodhpur, NH 62, Nagaur Road, Karwar, Jodhpur 342030, Rajasthan, India
dEnvironmental Sciences & Biomedical Metrology, CSIR-National Physical Laboratory, Dr K.S. Krishnan Road, New Delhi 110012, India
eDepartment of Biotechnology, Delhi Technological University, Main Bawana Road, Delhi 110042, India
First published on 19th May 2025
Abstract
Breast cancer occurs when cells grow abnormally and form tumors. It is currently one of the most prevalent cancers in women, and it is known to cause serious detrimental effects if not detected on time. Thus, early detection and screening may tremendously contribute to a patient's medical treatment. The boom in cancer diagnostics resulted from the demand to overcome the limitations of bulky and time-consuming conventional detection methods. The new and advanced methods are simpler, faster and easily deployable. This review elucidates various techniques used for breast cancer detection, which include optical, electrochemical, mechanical, electrical, thermal and color- and breath-based methods. An overview of different techniques is presented with additional information related to the available commercial options. This review also presents the integration of artificial intelligence and Internet of Things into futuristic diagnostic techniques. The unmet needs and challenges are also discussed. Overall, this review is a comprehensive package for researchers who want to dive into the advances of breast cancer diagnostics.
1. Introduction
Breast cancer (BrC) is a type of tumor that generally occurs in the inner lining of epithelial tissues, milk ducts, and/or lobules. The reason for its occurrence is linked to several factors, such as inheritance of mutations in the genes, past exposure to radiation, and hormonal imbalance. It has now become the foremost cause for cancer-linked illnesses and mortality in females.1 According to the WHO reports of 2024, it was the most common cancer in women with 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths reported worldwide in recent times.2 Early screening and diagnosis may improve the treatment efficiency. However, majority of the tests carried out in hospital laboratories are time-consuming and expensive.3 The tests recommended for the detection of BrC at an early stage require professionally trained personnel to operate on bulky equipment, such as those used for ultrasounds, MRI, and mammograms, which are presently not easily affordable by the mass population. Further, the presence of specific BrC biomarkers (e.g., HER2, ER, PR, CA15-3, and CD44) formidably demands precise detection.4 Owing to these limitations and an urgent need for improvement in the current diagnostics, the research focus has shifted towards the development of detection techniques that are relatively less painful, more accurate, affordable, rapid, and easy to use with a low limit of detection. In addition to this, with the advent of nanotechnology, the use of nanoparticles has become prominent in various BrC detecting methods owing to their small size and unique physiochemical properties.5
000 deaths reported worldwide in recent times.2 Early screening and diagnosis may improve the treatment efficiency. However, majority of the tests carried out in hospital laboratories are time-consuming and expensive.3 The tests recommended for the detection of BrC at an early stage require professionally trained personnel to operate on bulky equipment, such as those used for ultrasounds, MRI, and mammograms, which are presently not easily affordable by the mass population. Further, the presence of specific BrC biomarkers (e.g., HER2, ER, PR, CA15-3, and CD44) formidably demands precise detection.4 Owing to these limitations and an urgent need for improvement in the current diagnostics, the research focus has shifted towards the development of detection techniques that are relatively less painful, more accurate, affordable, rapid, and easy to use with a low limit of detection. In addition to this, with the advent of nanotechnology, the use of nanoparticles has become prominent in various BrC detecting methods owing to their small size and unique physiochemical properties.5
This review will take readers through a wide variety of recent techniques that are currently used for BrC diagnostics and their advantages and disadvantages (Table 1). Different aspects of sensing, commercial kits and future perspectives with an essence of AI and IoT methods used for BrC detection are covered.
| Technique | Advantage | Limitation | Ref. |
|---|---|---|---|
| LFIA: lateral flow immunoassay; SERS: surface enhanced Raman spectroscopy; ELISA: enzyme-linked immunosorbent assay; 1H-MRS: proton magnetic resonance spectroscopy; EIT: electrical impedance tomography; MEMS: microelectromechanical systems; PMUTs: piezoelectric micromachined ultrasonic transducers; SAW: surface acoustic waves; QCM: quartz crystal microbalance; DOI/OM: diffuse optical imaging/optical mammography. | |||
| LFIA | Cost-effective, point of care | Semi-quantitative with possibility of cross-reactivity | 6 |
| Flow cytometry | High sensitivity with multiparameter analysis | Complex sample preparation | 7 |
| SERS | Highly sensitive and specific | Reproducibility and standardization of the SERS signal | 8 |
| ELISA | Can be observed with naked eye | Less sensitive | 9 |
| Fluorescence-based techniques | Chromosomal aberration at single cell level can be analysed | Provide semi-quantitative results | 9 |
| 1H-MRS | Detailed soft tissue imaging without ionizing radiation exposure | Time consuming, expensive, lack of anatomical information | 10 |
| EIT | Generates tomographic image using non-ionizing radiation | Poor spatial resolution | 11 |
| Electrochemical techniques | Real-time monitoring, and cost-effective | Less sensitive | 12 |
| Breath-based techniques | Easy sample collection | Time-consuming data processing, less specificity | 13 |
| MEMS | Highly sensitive real time measurement with minimal sample consumption | Lack of standardization, complex fabrication leading to increased cost | 14 |
| PMUTs | Reduced size, enhanced bandwidth and sensitivity, lower power consumption | Lower signal-to-noise ratio | 15 |
| SAW | Real time quantification of cells in 2D and 3D cultures | Analyte range is design limited | 16 |
| QCM | Detect very small (nanogram) mass variations | Low reproducibility | 17 |
| Infrared thermography | Non-invasive, fast imaging time | Highly prone to false-negative and false-positive results depending on size of tumour and body temperature | 18 |
| DOI/OM | Generates 2D and 3D images when combined with other imaging modalities | Less spatial resolution when used alone | 19 |
| Microwave-based technique | Non-ionizing radiation, non-invasive, inexpensive, comfortable | Not available in clinic or hospital | 20 |
2. Techniques in breast cancer (BrC) screening and diagnosis
2.1 Color-based techniques
Conventionally, gold nanoparticles have been used as labels. However, considering their cost, other materials such as graphenes have also been explored.23 The most significant advantage of this method is that it provides real-time information, can be read by the naked eye and is extremely rapid in delivering results.
The traditional LFIAs can be used for the naked observation of qualitative or semi-quantitative results for samples having concentration above that of the assay. For quantitative analysis of the targets, the advancements in LFIA techniques have emerged, which are employed with portable optical readers. Ye et al. have developed a sandwich LFIA-based HER2 immunosensor, wherein HER2-ECD protein in serum was detected with a limit of detection (LOD) of 1.7 ng mL−1, and detection in the range of 1.7 to 400 ng mL−1 using a portable sensing method.24 Another such advancement is fluorescent LFIA presented by Deng et al. they have synthesized second near-infrared (NIR-II) Ag2Se polystyrene beads that can be used as a fluorescent probe in LFIA for fast (within 15 min) and accurate detection of BrC markers (CEA and CA153) with a very low limit of detection (CEA: 0.768 ng mL−1, CA153: 1.192 U mL−1) and high recoveries (93.7% ± 6.2% to 108.8% ± 4%). This method is suitable for clinical and quantitative detection.25
Wang and co-workers created an optofluidic metasurface composed of silicon nanoposts (SNP) for lateral flow-through identification of BrC biomarker ErbB2. It consists of a top thin silicon layer, with a coating of graphene oxide (GO) nanosheets functionalized with anti-ErbB2 (Fig. 1a). The GO coating enhances the loading capacity of anti-ErbB2 molecules due to the presence of oxygenated groups like –COOH and –CHO, which assist in covalent bond formation with anti-ERbB2 through EDC-NHS coupling. The analyte-ligand binding is monitored from the shift in the wavelength as observed in the reflectance spectra. The shift was apparently displaced in case of bare SNP to SNP with anti-ErbB2 (Fig. 1b). The resonance dip shifts to longer wavelengths with the increase in ErbB2 concentration (Fig. 1c). The dose–response curve and reflectance spectra, as shown in Fig. 1d and e, indicate that the ErbB2 free samples form a very small shift (<1 nm) in their resonance wavelength, whereas ErbB2-conjugated samples show a wavelength shift of 6 nm. It can be concluded that the device selectively targets ErbB2 antigen. The results of the study (Fig. 1f) are coinciding in simulation and experiment for ErbB2 and anti-ErbB2 binding. The LOD of the SNP-based biosensor is 0.7 ng mL−1 with a sensitivity of 2 nm nM−1 for ErbB2. It is also suggested that the lateral flow-through feature, along with optical bound states, supports the increase in the sensitivity of molecule-bond changes in refractive index.26
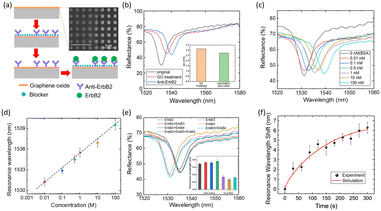 | ||
| Fig. 1 (a) Schematic of the label-free assay and SEM of the SNPs. (b) Reflection spectra of the bare sensor, GO layer, and anti-ErbB2 antibody coating. (c) Reflection spectra in the presence of ErbB2. (d) Dose–response curve for the detection of ErbB2. (e) Reflection spectra for seven different combinations of ErbB2, ErbB3, and ErbB4 antigens. Inset: resonance wavelength in presence of antigens and interfering molecules. (f) Simulation and experimental results for the binding of ErbB2 and anti-ErbB2 antibodies. Reproduced from ref. 26 with permission from Elsevier, copyright 2018. | ||
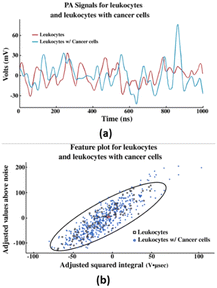 | ||
| Fig. 2 (a) PA waveforms obtained from the PAFC test from centrifuged blood. (b) Feature plot of each PA waveform from leukocytes and leukocytes with cancer cells. Reproduced from ref. 29. Published under the terms and conditions of the Creative Commons Attribution 4.0 Unported license. | ||
2.2 Optical techniques
The authors in ref. 32 report an evaluation of 253 total serum samples from unaffected subjects and patients for differential diagnosis of breast, oral, lung, ovarian, and colorectal cancer. Forty-two patients are diagnosed with BrC using SERS-based detection. The SERS spectra of deproteinized serum were obtained using a 532 nm laser, and the SERS substrates were presented by hydroxylamine-reduced silver nanoparticles (AgNPs). Further estimation was performed using principal component analysis-linear discriminant analysis (PCA-LDA) model for identifying the specific cancer of each sample. The PCA-LDA model depicted 76% efficacy level for BrC. The results indicate that the differential expression of the metabolites in the SERS signal is more profound in breast, ovarian, and oral cancers than that of colorectal and lung cancers. A similar technique has been employed,33 wherein BrC was diagnosed from the urine sample. For this, urine samples from 53 BrC patients and 22 as control were collected. The SERS spectra were recorded using Ag NPs obtained by reduction with hydroxylamine hydrochloride and further activated with Ca2+. This further promoted the adsorption of the anionic purines including uric acid, xanthine and hypoxanthine. By employing PCA-LDA and SERS, the samples from BrC patients were classified with 81% sensitivity and 95% specificity with an overall accuracy of 88%. Both the studies 32,33 suggest the translation of SERS in the clinical setting and emphasize the potential of SERS as a novel and prominent diagnosis strategy for the detection of BrC.
Another work39 depicts the synthesis of a fluorogenic material functionalized with peptides using self-assembly between molybdenum disulfide (MoS2) and a fluorescent peptide probe for fluorescence detection. The developed material presented for the detection of protein C receptor (PROCR), which is a transmembrane biomarker for triple-negative BrC (TNBC) cells and their imaging. The study used TAMRA (tetramethyl-rhodamine, a fluorescent label) to bind with AN33 (peptide), to form TAMRA-AN33 followed by the synthesis of TAMRA-AN33/MoS2 composites. To evaluate the fluorescence imaging ability of the synthesized composite, a TNBC cell line MDA-MB-231 (high PROCR expression) and an MCF-7 BrC cell line (low PROCR expression) were used. It was observed that upon increase in TAMRA-AN33 concentration, the fluorescence in MDA-MB-231 cells increased proportionally, whereas, not much change was observed in the fluorescence of MCF-7 cells (Fig. 3a–c). Similarly, upon incubation of TAMRA-AN33/MoS2, a fluorescence increment was seen in MDA-MB-231 when MoS2 concentration was increased up to 20 μg mL−1, but MCF-7 cells remained unaffected (Fig. 3d and e). This indicates that MoS2 enhances the peptide probe binding with the membrane-bound PROCR by clustering effects.40 Additionally, the cell proliferation assay suggested good compatibility of the peptide probe for pharmacological investigations. The targeting capability of the composite was also investigated and it presented with a strong imaging signature for the BrC cells.
 | ||
| Fig. 3 (a) Fluorescence imaging, (b) quantification of MDA-MB-231 and MCF-7 cell lines treated with TAMRA-AN33, (c) relative mRNA level of PROCR in MDA-MB-231 and MCF-7 cells using RT-qPCR method, (d) fluorescence imaging, and (e) quantification of MDA-MB-231 and MCF-7 treated with TAMRA-AN33 and MoS2. Reproduced from ref. 39. Published under the terms and conditions of the Creative Commons Attribution 3.0 Unported license. | ||
2.3 Electrical techniques
Electrical impedance tomography (EIT) also known as electrical impedance scanning is used to estimate various electrical properties of breast tissue. It is a method where there is no or zero exposure to any form of radiation, and the breast tissue can be safely examined without facing any hazardous conditions.46 This is completely a non-invasive and user-friendly method with facilities of mobile screening. The mechanism followed by this method is the measurement of impedances using dense electrodes to be placed on the surface of the patient's body. The result is further generated as reconstructed tomographic images, 2D or 3D. Here, impedance mainly involves both conductivity and/or permittivity, and as it changes, the EIT imaging starts its activity. The important mechanisms it follows are data acquisition and image reconstruction.47Lee et al.48 designed and proposed an 8-channel 10 MHz wide bandwidth EIT-AFE-IC having a wide dynamic range, reconfigurable front-end architecture, and a phase compensation loop for high precision detection of BrC. This wide bandwidth EIT system could go up to 10 MHz and portrayed a small phase error of 4.32 degree and could detect an object of 0.5 cm. The results were confirmed using phantom experiments.
A mobile-based EIT demonstrated by Hong et al. has a brassiere shape and EIT IC is integrated using a fabric-based board, which consists of an EIT electrode array, electronic circuitry and imaging device.49 This IC has a chip size of 0.18 μm size 1P6M CMOS process with a consumption of 53.4 mW. The system offers 4.9 mΩ sensitivity and detects a 5 mm cancer mass (0.1% breast volume) within an agar test phantom. The complete time taken for EIT measurement is less than 10 s. in a recent study by Wan et al.,50 HER2 and CA15-3 biomarkers were included into commercially accessible disposable strips, which are functionalized to detect BrC. These strips resemble the widely used glucose detection strips. The findings showed that the limits of detection for these two biomarkers were as low as 1 fg mL−1, which is significantly lower than the range of 1–4 ng mL−1 for the traditional enzyme-linked immunosorbent test. The detected signal was obtained by applying 10 1.2 ms voltage pulses to the transistor's drain electrode and sensing strip electrode using a synchronized double-pulse method. The average of the 10 digital output readings that corresponded to those 10 voltage pulses was the detected signal. For HER2 and CA15-3, the sensor sensitivity values were attained at about 70/dec and 30/dec, respectively.
2.4 Electrochemical techniques
Electrochemical sensors are simple, sensitive, and cost-effective devices for cancer detection. The electrochemical techniques detect the change in electrochemical parameters such as resistivity, current, and redox reactions at the electrode and interpret them for diagnostic results.51 Zare et al.52 report the synthesis of an electrochemical biosensor showing prominent electrochemical activity, porosity, and dispersity, along with a large surface area for better functionalization. The biosensor is made of a haemoglobin-capped Ag nanocluster conjugated with a Cu(II)-porphyrin-metal organic framework in a graphene-incorporated nanohybrid probe. The Hb-AgNCs@MOF-G nanocomposite immobilizes anti-HER2 on a glassy carbon electrode to detect the presence of HER2-positive BrC cells using electrochemical impedance spectroscopy (EIS) “signal on” and square wave voltammetry (SWV) “signal off” techniques. It reportedly detects 2 cells per mL using EIS “signal on” and 16 cells per mL by SWV “signal off”. The sensor demonstrates 15–16 fold selectivity with a recovery efficiency in the range of 94.8 to 106% by the EIS method and 95.4 to 111% via the SWV method.Another multiplexed electrochemical biosensor has been fabricated using screen-printed carbon electrodes functionalized with gold nanoparticles in conjugation with graphene quantum dots and graphene oxide (AuNPs/GQDs/GO) to detect BrC-related microRNAs (miRNA-21, miRNA-155, and miRNA-210). It further involves the use of anthraquinone, methylene blue, and polydopamine as redox indicators, which hybridize with the complementary targets, miRNA-21, miRNA-155, and miRNA-210, respectively. The qualitative and quantitative estimation of each miRNA is identified by its respective peaks and their intensity. The developed biosensor demonstrated efficient sensing of miRNA. It renders a wide linear range of 0.001–1000 pM with low detection limits of 0.04 (miRNA-21), 0.33 (miRNA-155), and 0.28 fM (miRNA-210).53
A similar electrochemical sensor has been demonstrated using a GO-IL-AuNP (graphene oxide-ionic liquid-gold nanoparticles) hybrid nanocomposite on a glassy carbon electrode to detect the presence of a BrC biomarker, CD44 antigen. The functionalization of IL with GO helped in tremendously increasing the conductivity of GO. The biocompatibility of IL augments the loading capacity of the anti-CD44 molecule and prohibits the lumping of the nanocomposite materials improving their stability. The AuNPs in the nanocomposite increase the surface area, which favors the immobilization of anti-CD44 antibodies, and also improve the conductivity of the nanocomposite. All the components result in the synthesis of a highly specific and sensitive device for CD44 detection with a wide range of detection of 5.0 fg mL−1 to 50.0 μg mL−1 and an LOD of 2.80 fg mL−1.54Table 2 summarizes a few electrochemical diagnostics for BrC with respect to the biomarkers, techniques and quantifiable parameters.
| Biomarker | Technique | Transducer | LOD | LR | Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| 8 × SPE: eight screen-printed electrochemical cells, AuE: gold electrode, AuNPs: gold nanoparticles, Au@PdAg DBNR: Au@PdAg dog-bone-like nanorod, CILE: carbon ionic liquid electrode, CV: cyclic voltammetry, DPV: differential pulse voltammetry, EIS: electrochemical impedance spectroscopy, GCE: glassy carbon electrode, GSPE: graphite screen-printed electrode, HER2: human epidermal growth factor receptor, IDμEs: interdigitated gold micro-electrode arrays on silicon/silicon oxide wafers, LOD: limit of detection, LR: linear range, LSV: linear sweep voltammetry, SPCE: screen-printed carbon electrode. | ||||||
| HER2 | Differential pulse voltammetry (DPV) | 8 × SPE | 1.8–2.6 ng mL−1 | 0–20 ng mL−1 | Validated | 55 |
| AuE | 0.995 pg mL−1 | 0.01–10 ng mL−1, 10–100 ng mL−1 | 5.921 μA mL ng−1 | 56 | ||
| SPCE | 6.0 ng mL−1 | 0–30 ng mL−1 | 6 ng mL−1 | 57 | ||
| Au@PdAg DBNR/GCE | 0.25 pg mL−1 | — | 58 | |||
| Electrochemical impedance spectroscopy (EIS) | CILE | 7.4 ng mL−1 | 10–110 ng mL−1 | Validated | 59 | |
| GSPE | 6.0 μg mL−1 | 0–40 μg mL−1 | — | 60 | ||
| Au/AuNP | 5.0 ng mL−1 | 10−5–102 ng mL−1 | — | 61 | ||
| IDμEs | 1 pM L−1 | 1 pM–100 nM | 0.035 μF/log([HER2] pM) | 62 | ||
| Linear sweep voltammetry (LSV) | SPCE | 4.4 ng mL−1 | 15–100 ng mL−1 | — | 63 | |
| Amperometry | SPCE | 1.0 μg mL−1 | 1–200 μg mL−1 | — | 64 | |
| CA15-3 | DPV | GCE | 0.015 U mL−1 | 2.0–25.0 U mL−1, 0.05–2.0 U mL−1 | — | 65 |
| 0.012 U mL−1 | 0.1–20 U mL−1 | — | 66 | |||
| 5 × 10−6 U mL−1 | 2 × 10−5–40 U mL−1 | — | 67 | |||
| Cyclic voltammetry (CV) | GCE | 0.040 U mL−1 | 0.1–160 U mL−1 | — | 68 | |
| AuE | 0.640 U mL−1 | 2.0–240 U mL−1 | — | 69 | ||
| 0.6 ng mL−1 | 1.0–240 ng mL−1 | 70 | ||||
Bioimpedance is an electrochemical-based technique in which current is passed to the object through electrodes, and the electrical impedance generated gives an impedance score based on its electrical characteristics. The impedance value can be used to differentiate between cancerous (low impedance) and normal tissues (higher impedance).71 In the study by Mansouri and group,72 a low-frequency current of 1 kHz, 0.9 mA is introduced through a device to measure the differences in the extracellular resistance (from 0.6 g l−1) between the breasts. It is suggested to visit the doctor if the difference is more than 50 Ω. The breast afflicted by the cancer can be determined by looking at the resistance with the lowest value.
2.5 Breath-based techniques
BrC can alter the metabolic constitution and synthesis of volatile metabolites in the breath of patients, which can serve as a non-invasive diagnostic marker. The ER, PR and HER2 biomarker receptors exhibit unique metabolomic expression in BrC patients.73 There are different methods to identify breath VOCs. One is traditional, GC-MS, and the other is advanced, electronic nose. The latter uses an array of sensors and machine learning algorithm to map the breath print to breath VOCs.74 Types of breath tests for BrC analysis have been presented by Yang et al.75 based on volatile compounds found in the exhaled alveolar breath, with an electronic nose (E-nose) made of an array of carbon nanotube sensors. The volatile organic compounds (VOCs) interact with the array and generate a change in their electric resistance. Further, machine learning was employed to create prediction models for BrC detection and its molecular phenotyping. The breath samples were analyzed using HPPI-TOFMS (high-pressure photon ionization-time-of-flight mass spectrometry). The E-nose system can execute rapid breath biopsy, (in 30 min), which generally requires 7 days. The test reports 86% sensitivity, 97% specificity, and 91% prediction accuracy using the random forest model for 899 volunteers. Another study was conducted on 5047 women based on VOC markers, wherein 465 (9.21%) participants were identified with BrC. It employed similar breathomics and forest algorithm techniques, as reported in the previous study. The results indicated 96.97% detection rates and 87.70% specificity for ductal carcinoma.76The profiling of BrC with 276 subjects using VOCs is portrayed77 in Fig. 4, wherein the breath analysis was done by GC-MS and nanoarray technology. Twenty-three compounds including alkanes, methylated alkanes, chlorinated alkanes, ethers aldehydes, alcohols, ketones, acetic acid and benzene derivatives were identified as belonging to various molecular sub-types of BrC. These VOCs were found similar to the previously reported results of breast-derived cell lines.78 Particularly, 2-ethyl-1-hexanol was identified as a potential biomarker for BrC progression. Furthermore, certain VOCs were found to be the products of DNA damage caused by the action of reactive oxygen species (ROS) present in tumor cells. ROS also induces the upregulation of cytochrome p-450 enzyme, which further leads to the peroxidation of polyunsaturated fatty acids in the cell membranes. This results in the overexpression of volatile alkanes and alkane-derivatives in the breath.79 The discriminant function analysis (DFA) of the nanoarray recognized specific differences in the volatolomics between affected and non-affected cells, and different molecular sub-types with 82–87% accuracy, 76–96% specificity and 81–88% sensitivity. Another work presented by Herman-Saffar et al. demonstrated that commercial electronic noses and GC-MS can be used to detect initial stages of BrC. They processed exhaled breath samples from 48 BrC patients and followed artificial neural networks for statistical analysis. The model showed up to 95.2% accuracy for the classification of BrC patients.80 In another study, 443 exhaled breath samples, having 262 diagnosed with BrC, were collected. The results indicated that the breath prints of BrC patients differed from those of healthy women, with a valid classification rate of 98% and a range up to 98.8%. The ROC curve indicates that the sensitivity, specificity, negative predictive value, and positive predictive value were all 100%. This study shows that the electronic nose can distinguish between BrC patients and healthy individuals.81
 | ||
| Fig. 4 (A) Patient exhales into a collection bag. (B) Collection and concentration of breath on Tenax® (C) exposure of the Tenax® tube to GC-MS for detecting the presence of specific compounds. (D) Exposure to the AI nanoarray for volatolomic signature of BrC genetic mutations. Reproduced from ref. 77 published under the terms and conditions of the Creative Commons Attribution license. | ||
2.6 Mechanical techniques
Khosla87 has presented a novel nano electrode array-based electroimpedance tomography for non-invasive and radiation-free medical imaging. It has been reported as the first device employing a nano-electrode array integrated to a flexible textile sensor fabricated using MEMS. It can scan through a wide range of frequencies and allow for simultaneous scanning of both the breasts. Further, it is reported as the first sensor that parallelly scans lymph nodes present under the arm. These features can assist in tracking cancer progression and treatment.
A RapidET:MEMS-based platform has been evidenced that can quickly demarcate between normal breast and biopsy tissue. The technique is label free and the microchip is based on electrothermal sensing. This MEMS platform contains a platinum microheater, interdigitated electrodes (IDEs), and resistance temperature detectors (RTDs). Fig. 5a depicts the design of the microchip with other elements fabricated on a SiO2 wafer. The optical photograph and SEM are shown in Fig. 5b and c, respectively. A thermal isolation trench of 350 μm depth was designed around the microheater for maintaining a uniform temperature around the microheater. This also reduces the temperature dissipation to other elements such as RTDs. This trench was first simulated in COMSOL to assess its effects.88Fig. 5d displays the sample holder that comes into contact with the sensor holder (Fig. 5e). The detailed picture of the sample holder is shown in Fig. 5f, and the enlarged image of microchip is shown in Fig. 5g and h.
 | ||
| Fig. 5 (a) Microchip design, (b) optical photograph of the fabricated microchip, (c) SEM image of the active area of the microchip, (d) sample loaded in the system, (e) subsystem with the PCBs, (f) microchip packaged into the sensor holder structure through slide-fit contacts, (g and h) rendered and actual image of the microchip connected to the slide-fit contacts. Reproduced from ref. 88 published under the terms and conditions of Creative Commons Attribution 4.0 International license. | ||
The parameters bulk resistivity, surface resistivity and thermal conductivity were measured, and a pathologist confirmed whether the tissue is normal or tumorous. The biopsy tissues were deparaffinized formalin-fixed paraffin-embedded (FFPE) tissues and formalin-fixed fresh tissues. Using this RapidET system, the microheater provided different temperatures to the tissues and both bulk (ρB) and surface resistivity (ρS) were measured.
It was observed that both these values were higher for tumor tissues at higher temperatures. A similar trend was found for both formalin fixed and deparaffinized tissue samples. The mean ρB was measured to be 4-fold for tumor tissues in comparison to the 3-fold change in case of normal tissues. A lower thermal conductivity (K) was exhibited by tumor tissues than normal tissues. The mean K of the formalin-fixed tumor tissues was a smaller value of 0.309 ± 0.02 W m−1 K−1 compared to K of 0.563 ± 0.028 W m−1 K−1 of normal tissues. Further fisher's combined probability test and regression analysis asserted that these three parameters play a key role in identifying tumor tissues from BrC patients.
Palpreast is a wearable device that is specially designed to imitate the process of breast self-examination in women for the detection of cancer. The device is non-invasive and uses pressure-sensing-based textiles and further enhances confidence and self-awareness among women. The device is based on large- and medium-sized breast phantoms. It mimics the exact function of a human hand. The textile pressure sensor and the inflator system, as shown in Fig. 6, are embedded inside the smart top or fabrics used by women, and it protects the entire breast. To avoid any excessive expansion of the inflator, the smart wearable device consists of an internal pocket. The inflator system has a bulb pump with an air release valve, and a manometer to verify the pressure, along with properly designed T-valve structures to balance the air flux present in each compartment, as a result of which balloons inserted in each compartment layer inter-connected to the pumping system through rubber hoses are inflated.89
 | ||
| Fig. 6 Prototype design of the Palpreast device, including a smart wearable and inflator system. Reproduced from ref. 89 published under the terms and conditions of the Creative Commons Attribution (CC BY) license. | ||
2.7 Thermal-based technique (infrared thermography)
It is a non-invasive technique that creates a series of infrared scans of the breasts by mapping the temperature difference. The temperature of the tumor tissue is different from that of the non-cancerous tissue, and this difference is generated as a particular thermographic pattern, which can be analyzed for disease characterization. The physiologic image obtained from infrared imaging is in sync with the anatomic image created through mammography, with a very high sensitivity and a negative predictive value. The scans of IR thermography can be additionally used for ensuring the presence of a breast abnormality seen in mammograph.98 The images are further analyzed by computer algorithms to compare the infrared patterns. It can also assist in differentiating the benign or malignant nature of the lesion.99 Ekici and Jawzal100 developed an algorithm for early detection of BrC using image-processing techniques for analyzing thermal breast images. The proposed algorithm is based on convolutional neural networks optimized by Bayes algorithm with an accuracy rate of 98.95%. Likewise, Resmini et al.101 used genetic algorithms and support vector machine for early diagnosis of BrC using infrared thermography with 97.18% of accuracy and 94.79% area under the receiver operating characteristic curve.86 Another extensive work describes a system that produces thermographic images of breast and further characterizes them as cancerous and non-cancerous. The study suggests that a combination of gradient vector flow and convolutional neural network can detect BrC using the thermographic image characterization.1022.8 Imaging technique
It is a lesion detection technique based on functional abnormalities using near-infra-red light, evidenced to detect up to 85% lesions. The breast lesions are identified on the basis of increased hemoglobin concentration and reduced oxygen saturation, which are related to the increased vascularization of tumor cells. DOI techniques include Transillumination, which creates 2D images, and diffuse optical tomography (DOT), which generates 3D images.103 A digital breast tomosynthesis (DBT)/DOT fusion imaging for initial diagnosis of BrC on 28 women has been explored. The receiver operating characteristic curves showed significant improvement, and the technical success rate was 96.4%.104 Diffuse optical tomography breast imaging system (DOTBIS) non-invasively measures hemoglobin concentration, which is a potential biomarker for short-term response to neoadjuvant chemotherapy. The study conducted by McGuinness105 analyzed 7 postmenopausal women with stage I-III BrC enrolled in pre-surgical studies and performed DOTBIS pre- and post-therapy in the affected and unaffected breast. It was observed that DOTBIS-derived observations are modifiable with pre-surgical targeted therapies. The findings support DOTBIS as a potential imaging tool for assessing neoadjuvant targeted therapies in early-stage BrC. Further, to explore the clinical applicability of the diffuse optical inspection device, a compact multi-wavelength DOT system for breast imaging with a fiber-free parallel-plane structure is developed for acquiring the 3D optical properties of the breast.1062.9 Microwave-based technique
Microwave-based diagnostics for BrC offers a pain-free method with less scanning time and cost effectiveness.107 These can be classified into wearable, RF and imaging (radar and tomography). This technique is a contrast to the electrical method, wherein sensors sense the change in signal passing through waveguides, transmission lines lumped capacitors, etc., against the mass/temperature of the tissues.108A fully textile antenna-based detection system envisioned as “smart bra”, which can be worn on the breasts with dimensions 24 × 45 × 0.17 mm3, has been presented.107 An impedance bandwidth from 1.6 GHz up to 10 GHz at |S11| ≤ −6 dB (VSWR ≤ 3) and from 1.8 to 2.4 GHz and from 4 up to 10 GHz at |S11| ≤ −10 dB (VSWR ≤ 2) has been realized. The authors also used machine learning algorithms to classify different scanning states using recorded S parameters. In the imaging process, the antenna sends signals to the breasts and receives the backscattered signals.109 Islam et al.110 developed a small-side slotted antenna for the microwave imaging of the breast tumor. A heterogeneous breast phantom has been synthesized to simulate the human breast, and it contained the same dielectric properties as those of humans. Since the tumor cells have a higher water content than that of the healthy ones, the dielectric value of the tumor cells is also very high. This caused a change in the backscattered signals received from both tumor and healthy cells. The system consisted of a 1 × 9 antenna array with an RF switching system to control the receivers, signal processing and image reconstruction unit. The collected back scattered signals were processed using the iteratively corrected delay and sum imaging algorithm, which reconstructed the image of the breast interior for multiple tumor sensing.
A clinical prototype as a wearable device for breast health monitoring comprising 16 flexible monopole antennas embedded in a bra has been developed.111 The data received from this prototype were compared with the table-top version. It works on the principle of multistatic radar. A small pulse is generated, amplified and transmitted through a switching matrix to a transmitting antenna. A 16-element wideband antenna array surrounds the breast under test. The back scattered waves received from the breasts after the waves pass through the breasts are received at 15 receiving antennas. A picoscope was employed for collecting and storing data. The wearable prototype has reduced the uncertainties by improvement in the fixed positioning of breast and the collection of stronger signal levels, leading to a high signal-to-noise ratio. Since wearables can be directly worn, they reduce the chances of offset error, which is caused in the table-top version due to an additional medium required.
3. Commercially available solutions for cancer detection
3.1 Blue box device
The blue box112 is an electronic nose containing chemical sensors that detect BrC biomarkers present in the urine samples. It is a test at-home BrC detection device. Designed with an integrated artificial intelligence (AI) algorithm along with sophisticated technologies, it is used to trace late-stage BrC. There are in total 6 chemical sensors placed inside the box. Within 30 minutes of sample collection, one can know results on the user's phone through an app. In terms of accuracy, the device produces an excellent result of 95%. It renders non-irradiating, painless, and low-cost diagnosis along with a simple and in-home BrC screening. It offers a sensitivity of 88%, an accuracy of 83% and a specificity of 75%.3.2 Human BrC BRCA1 protein ELISA kit
MBS008497 from MyBioSource113 is a hassle-free ready-to-use strip plate with an ELISA kit to identify the presence of the BRCA1 target analytes in the biological samples. The kit concentration gradient and positive controls offer a detection range in biological samples containing BRCA1. The kit follows the quantitative sandwich-immunoassay procedure. The MBS008497 kit exploits the BRCA1 antibody-BRCA1 antigen interaction (immunosorbent) along with an HRP-conjugated colorimetric detection system to identify BRCA1. It has a detection range as 0.625–20 ng mL−1 with a sensitivity of 0.1 ng mL−1.3.3 Human cancer antigen CA15-3 ELISA kit
Human cancer antigen CA15-3 ELISA kit (ab108633_abcam)114 is another product intended to quantitatively measure the concentration of CA15-3 protein in the human serum sample. In patients with breast tumors, there is an elevated amount of CA15-3 (i.e. 25% more) concentrations being estimated (the normal concentration of CA15-3 usually ranges up to 30 units per mL). This protein is mostly shredded by the tumor cells into the bloodstream and acts as a disease-specific tumor marker. Using this kit, the minimum detectable concentration of CA15-3 is estimated to be 5 U mL−1.3.4 HercepTest kits
HercepTest is an FDA-approved semi-quantitative immunohistochemical assay for the recognition of HER2 proteins that are overexpressed in BrC-associated tissues.115 The kit comprises various reagents needed for the immunohistochemical staining. The HercepTest™ is interpreted as negative for Her2 protein at 0 and 1± score, equivocal at 2+ score, and positive for 3± score.3.5 Xpert® BrC STRAT4
The Xpert® BrC STRAT4116 test kit provides an accurate, standardized, and reliable biomarker for the qualitative estimation of ER, PR, HER2, and Ki-67 mRNA within 2 hours. This strip follows the semi-quantitative method for the detection of biomarkers in the case of invasive BrC. The method involves robust testing and workflow that does not need any PCR laboratory testing.4. Implantable devices for BrC detection
In recent times, medical devices in the form of in vivo sensors and therapeutics have played an important role in the early diagnosis and treatment. The traditional hospital-centered diagnostic process and monitoring is now moving towards patient-centered monitoring. Such type of remote healthcare monitoring needs reliable and affordable solutions. Thus, automated biomedical devices are being developed to address the needs of patients. In this context, implantable devices, which are electronic devices used in medical field, have played a key role. The research is, however, also shifting towards biodegradable implantable devices that degrade harmlessly in the human body and work in the human system without being rejected by the patient's immune response. This, further, rules out the dependency of battery and surgical removal after use which imposes a financial load and severe pain on patients. Triboelectric nanogenerators, which utilize mechanical energy and further transform it into electricity, are also new-age biodegradable implantable devices, showcasing massive potential in cancer therapeutics.117,118An implantable device capable of detecting a BrC biomarker (lactate) has been reported by Gil and his team.119 The device has demonstrated high sensitivity, a pH less than 0.1 (4.2 mV potential) and 1 mM lactate (70 nA current) level. It can operate at distances of 50 mm (10 μW) as tested inside an anatomical model of the human breast. Such safe and specific devices are highly beneficial for BrC screening and provide real-time access to intrabody tissue monitoring post-surgery. This simultaneously assists in assessing the healing or development of any infection in the tissue.
A microporous poly(ε-caprolactone) (PCL) scaffold has been developed as an implant that can identify metastatic BrC cells at early stage. The number of immune cells at the PCL scaffold, which had stabilized prior to tumor inoculation, altered after tumor inoculation. This scaffold can be used in combination with label-free imaging of early stage metastatic cells, or implanted in patients for monitoring and scheduling follow-up visits using the optical imaging technique.120
A multifunctional implantable anticancer device has been fabricated for postsurgical BrC treatment. It is a nanocomposite made of iron oxide nanoparticles, graphene oxide and doxorubicin in a nanofiber matrix that enables target-specific and sustained drug release over 60 days in vitro. It has shown significant results in eradicating the local regional recurrence of BrC. Additionally, it reportedly helps in breast reconstruction post-surgery via the adipogenic process. The implantable scaffold exhibits enhanced specificity with real-time monitoring and long-term anticancer efficacy.121
5. Future diagnostics and key challenges for BrC detection
The Internet of Things (IoT) has emerged as a novel and progressive way in the last decade, providing a way for real-time as well as past data analysis. With the use of artificial intelligence (AI), wireless sensor network (WSN), and machine learning (ML), it has advanced rapidly into medical diagnostics. AI and ML tools render enhanced computer-aided diagnosis, medical image processing, interpretation, retrieval, and analysis with add ons of predicting diseases, thus aiding to early diagnosis.122 Onasanya and Elshakankiri123 proposed an IoT-enabled system for enhanced detection, and monitoring of malignant cancer using cancer care services and business analytics/cloud services. Using these services, the availability of patient data can be streamlined for further treatment. The use of smart devices and WSN can improve cancer care services by seamless and secure integration of the equipment used in chemotherapy procedures. Moreover, follow-up and monitoring can be shifted into the home through remote monitoring, process automation, and alert communication. In addition to this, WSNs can be employed in achieving better planning of treatment and finalizing appropriate prescription doses. All the systems including pharmacy, medical and radiation oncology servers may allow access to a detailed patient chart that can be enabled from any smart device via a remote VPN access from anywhere.In another such study,124 an ML-based classifier support vector machine (SVM) has been demonstrated for classifying the malignant and benign BrC by using a recursive feature selection algorithm to include more information from the dataset. The results reveal that the proposed algorithm identifies and selects the best subsets, and the SVM classifier reported optimal classification performance with 99% classification accuracy and specificity and 98% sensitivity. The proposed system is suitable for early detection of BrC with more effective recovery and treatment.
Salvi and Kadam125 also presented an ML-based model, trained through CNN algorithm with 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 images. The study proposes that the raspberry pi-integrated thermal imaging sensor can be used by the patient to capture cancer cell images, which is further given as an input to the CNN trained model to identify the status of the image (benign or malignant). In addition, using the Wi-Fi and cloud technology, the captured images are wirelessly sent to the doctor for further examination.
000 images. The study proposes that the raspberry pi-integrated thermal imaging sensor can be used by the patient to capture cancer cell images, which is further given as an input to the CNN trained model to identify the status of the image (benign or malignant). In addition, using the Wi-Fi and cloud technology, the captured images are wirelessly sent to the doctor for further examination.
Deep learning systems (DLSs) have been used to predict the presence of ER, PR, and HER2 using the ‘patch level model’ and hematoxylin-and-eosin-stained images as input.126 The conventional and commercially existing methods employ costly preparation methods and produce inconstant scoring systems. Moreover, the contradiction in the histology and the expected biomarker results lead to repeated testing. In the study, computer software is trained to identify and classify the image features in order to find ER/PR/HER2 biomarkers in digitized images, instead of tissue stains. ML automatically predicts the status of the biomarkers in pathology images and further testing confirms the specific image features that assist in making the predictions. This method is cost-effective and less time consuming, and provides high-quality control in marker detection.
Despite the high advances made in various fields of cancer diagnostics, certain challenges need to be addressed. The most common of all issues is creating reproducible calibration and separation methods. Besides, identifying the appropriate technology while keeping the highest specificity and sensitivity is another challenge. Further, the device should offer the lowest detection time and detection limit with minimum consumption of energy and cost.127 Research is also directed on identifying novel biomarkers using a single gene/protein for precise detection. It also includes the use of nanomaterial-based substrates along with a combination of different mechanisms on a common platform, resulting in multiple outputs from a single sensor.
Nanomaterials have a large surface-to-volume ratio that assists in better functionalization of the substrate for the binding of biomolecules, and thus, specific detection of cancer cells. Nanomaterial-based point-of-care devices have shown exceptionally low limit of detection towards the BrC biomarker HER-2.128–130 Nanorobots are biocompatible, biodegradable, and biomimetic nanostructures that can detect and kill BrC cells. These can perform multiple tasks on a small scale, including targeted drug delivery and localized minimally invasive microsurgery. DNA nanorobots are specifically known to cause lysosomal degradation of BrC proteins, in different pH environments inside the cell and can also be used for imaging purposes through fluorescent tags.131 Similarly, nanomotors, nanobodies,132 nanoflowers,133 nanosubmarines,134 nanotrains,135 nanomachines, nanoballons,136 nanostars,137 nanocages,138etc., are all futuristic programmable DNA-based nanostructures that can be employed for safe imaging and therapeutic purposes. These nanostructures can autonomously navigate throughout the body and are able to find and kill cancerous cells.139 However, scalable fabrication or synthesis of nanomaterials is still a challenge. Moreover, the nanosafety concern must be developed to handle the unique and specific behavior of nanomaterials and further understand their potential toxicity.140
Most methods can identify one analyte at a time due to the limitation of a single biomarker. Therefore, for early diagnosis, simultaneous detection of multi-biomarkers is also necessary.141 In the case of IoT-based solutions and systems, the issues of confidentiality and privacy are a major concern. Thus, it is imperative to ensure the operational and security challenges, about patient-sensitive information to avoid the breach of patient data. For this, it is necessary to ensure that all the connected devices conform to industry standards and provide necessary information with minimal equipment failure and lesser human intervention. AI, though a boom for medical diagnostics, requires a large amount and span of data sets to train the models. Thus, it will take some time for AI-based techniques to come up to full use till the current devices fetch, store and keep the data for the AI models.
6. Conclusion
Despite the conventional techniques still being used today for BrC diagnosis, there is a need for the development of pain-free, low-LOD, higher sensitivity and less-time-consuming methods. This review embraces various techniques (optical, mechanical, electrical, electrochemical, imaging, microwave, color based) and commercial techniques for BrC detection. The commercial options available to a future vision including AI and IoT techniques as a part of overcoming challenges for next-generation diagnostics of BrC are also included in the review. This review has furnished the working principle of techniques and works recently cited in the literature relevant to the above-mentioned methods. It renders all important information for scientists working in the field of BrC diagnostics.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Author contributions
Parikshana Mathur: writing – original draft, review and editing, visualization. Saakshi Dhanekar: conceptualization, supervision, writing – review and editing. B. D. Malhotra: writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Shradha Suman Panda, Master's student in Medical Technologies Program in Smart Healthcare, IIT Jodhpur-AIIMS Jodhpur for helping in organizing a few topics for this manuscript.References
- L. Jing, C. Xie, Q. Li, M. Yang, S. Li and H. Li, et al., Electrochemical Biosensors for the Analysis of Breast Cancer Biomarkers: From Design to Application, Anal. Chem., 2022, 94(1), 269–296 CrossRef CAS PubMed , Available from: https://pubs.acs.org/doi/10.1021/acs.analchem.1c04475.
- Breast cancer [Internet], [cited 2024 Jul 15], Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- I. Abrao Nemeir, J. Saab, W. Hleihel, A. Errachid, N. Jafferzic-Renault and N. Zine, The Advent of Salivary Breast Cancer Biomarker Detection Using Affinity Sensors, Sensors, 2019, 19(10), 2373 CrossRef.
- S. Afzal, M. Hassan, S. Ullah, H. Abbas, F. Tawakkal and M. A. Khan, Breast Cancer; Discovery of Novel Diagnostic Biomarkers, Drug Resistance, and Therapeutic Implications, Front. Mol. Biosci., 2022, 9, 783450 CrossRef CAS PubMed.
- F. Aminolroayaei, S. Shahbazi-Gahrouei, A. Khorasani and D. Shahbazi-Gahrouei, A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis, Information, 2023, 15(1), 10 CrossRef.
- T. Mahmoudi, M. de la Guardia and B. Baradaran, Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends, TrAC, Trends Anal. Chem., 2020, 125, 115842 CrossRef CAS.
- Z. Yang, X. Mao, M. Zhu, S. Chen, Z. Gao and T. Jiang, et al., Chinese expert consensus on flow cytometric detection of hematological malignant cells in tissue samples, J. Natl. Cancer Cent., 2025, 5(1), 28–37 CrossRef.
- L. Vázquez-Iglesias, G. M. Stanfoca Casagrande, D. García-Lojo, L. Ferro Leal, T. A. Ngo and J. Pérez-Juste, et al., SERS sensing for cancer biomarker: Approaches and directions, Bioact. Mater., 2024, 34, 248–268 Search PubMed.
- M. Sharifi, A. Hasan, F. Attar, A. Taghizadeh and M. Falahati, Development of point-of-care nanobiosensors for breast cancers diagnosis, Talanta, 2020, 217, 121091 CrossRef CAS.
- A. Pulumati, A. Pulumati, B. S. Dwarakanath, A. Verma and R. V. L. Papineni, Technological advancements in cancer diagnostics: Improvements and limitations, Cancer Rep., 2023, 6(2), e1764 CrossRef , Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cnr2.1764.
- S. Mansouri, Y. Alharbi, F. Haddad, S. Chabcoub, A. Alshrouf and A. A. Abd-Elghany, Electrical Impedance Tomography – Recent Applications and Developments, J. Electr. Bioimpedance, 2021, 12(1), 50 CrossRef , Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8667811/.
- F. Achi, A. M. Attar and L. A. Ait, Electrochemical nanobiosensors for the detection of cancer biomarkers in real samples: Trends and challenges, TrAC, Trends Anal. Chem., 2024, 170, 117423 CrossRef CAS.
- A. Krilaviciute, J. A. Heiss, M. Leja, J. Kupcinskas, H. Haick and H. Brenner, et al., Detection of cancer through exhaled breath: a systematic review, Oncotarget, 2015, 6(36), 38643–38657 CrossRef PubMed , Available from: https://www.oncotarget.com/article/5938/text/.
- P. Praveen and S. Lakshmi, Cancer Detection Using MEMS Technology. 5th International Conference on Circuits, Control, Communication and Computing, I4C 2024, 2024, 546–553 Search PubMed.
- S. V. Joshi, S. Sadeghpour, N. Kuznetsova, C. Wang and M. Kraft, Flexible micromachined ultrasound transducers (MUTs) for biomedical applications, Microsyst. Nanoeng., 2025, 11(1), 1–21 CrossRef , Available from: https://www.nature.com/articles/s41378-024-00783-5.
- M. Aleixandre and M. C. Horrillo, Recent Advances in SAW Sensors for Detection of Cancer Biomarkers, Biosensors, 2025, 15(2), 88 CrossRef CAS PubMed , Available from: https://www.mdpi.com/2079-6374/15/2/88/htm.
- S. Akgönüllü, E. Özgür and A. Denizli, Quartz Crystal Microbalance-Based Aptasensors for Medical Diagnosis, Micromachines, 2022, 13(9), 1441 CrossRef , Available from: https://www.mdpi.com/2072-<?pdb_no 666X?>666X<?pdb END?>/13/9/1441/htm.
- P. Jaglan, R. Dass and M. Duhan, Breast Cancer Detection Techniques: Issues and Challenges, J. Inst. Eng. (India): B, 2019, 100(4), 379–386 Search PubMed , Available from: https://link.springer.com/article/10.1007/s40031-019-00391-2.
- K. Lee, Optical mammography: Diffuse optical imaging of breast cancer, World J. Clin. Oncol., 2011, 2(1), 64–72 CrossRef PubMed , Available from: http://www.ncbi.nlm.nih.gov/pubmed/21603315.
- A. A. Abdul Halim, A. M. Andrew, M. N. Mohd Yasin, M. A. Abd Rahman, M. Jusoh and V. Veeraperumal, et al., Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review, Appl. Sci., 2021, 11(22), 10753 CrossRef CAS , Available from: https://www.mdpi.com/2076-3417/11/22/10753/htm.
- K. M. Koczula and A. Gallotta, Lateral flow assays, Essays Biochem., 2016, 60(1), 111–120 CrossRef PubMed.
- C. Liu, L. Yang, W. Zhang, D. Li, L. Li and H. Wang, et al., A magnetic nanoparticle-based lateral flow immunochromatography assay for the rapid detection of fluoroquinolones in milk, Eur. Food Res. Technol., 2021, 247(10), 2645–2656 CrossRef CAS , Available from: https://link.springer.com/article/10.1007/s00217-021-03820-z.
- V. Shirshahi, S. N. Tabatabaei, S. Hatamie and R. Saber, Functionalized reduced graphene oxide as a lateral flow immuneassay label for one-step detection of Escherichia coli O157:H7, J. Pharm. Biomed. Anal., 2019, 164, 104–111 CrossRef CAS PubMed.
- L. Ye, X. Xu, A. Qu, L. Liu, C. Xu and H. Kuang, Quantitative assessment of the breast cancer marker HER2 using a gold nanoparticle-based lateral flow immunoassay, Nano Res., 2024, 17(6), 5452–5460 CrossRef CAS , Available from: https://link.springer.com/article/10.1007/s12274-024-6471-2.
- K. Deng, Z. L. Yu, X. Hu, J. Liu, X. Hong and G. G. L. Zi, et al., NIR-II fluorescent Ag2Se polystyrene beads in a lateral flow immunoassay to detect biomarkers for breast cancer, Microchim. Acta, 2023, 190(12), 1–9 CrossRef , Available from: https://link.springer.com/article/10.1007/s00604-023-06039-9.
- Y. Wang, M. A. Ali, E. K. C. Chow, L. Dong and M. Lu, An optofluidic metasurface for lateral flow-through detection of breast cancer biomarker, Biosens. Bioelectron., 2018, 107, 224–229 CrossRef CAS PubMed.
- J. P. Robinson, R. Ostafe, S. N. Iyengar, B. Rajwa and R. Fischer, Flow Cytometry: The Next Revolution, Cells, 2023, 12(14), 1875 CrossRef CAS PubMed.
- H. K. Mishra, Clinical Applications of Flow Cytometry in Cancer Immunotherapies: From Diagnosis to Treatments, in Signal Transduction Immunohistochemistry, ed. A. E. Kalyuzhny, Methods in Molecular Biology, Humana, New York, NY, 2023, vol. 2593, DOI:10.1007/978-1-0716-2811-9_6.
- K. Bhattacharyya, B. S. Goldschmidt and J. A. Viator, Detection and capture of breast cancer cells with photoacoustic flow cytometry, J. Biomed. Opt., 2016, 21(8), 087007 CrossRef , Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5005571/.
- W. Xu, Y. Zhang, D. Hou, J. Shen, J. Dong and Z. Gao, et al., Quantitative SERS detection of multiple breast cancer miRNAs based on duplex specific nuclease-mediated signal amplification, Anal. Methods, 2023, 15(24), 2915–2924 RSC , Available from: https://pubs.rsc.org/en/content/articlehtml/2023/ay/d3ay00583f.
- L. Vázquez-Iglesias, G. M. Stanfoca Casagrande, D. García-Lojo, L. Ferro Leal, T. A. Ngo and J. Pérez-Juste, et al., SERS sensing for cancer biomarker: Approaches and directions, Bioact. Mater., 2024, 34, 248–268 Search PubMed.
- V. Moisoiu, A. Stefancu, D. Gulei, R. Boitor, L. Magdo and L. Raduly, et al., SERS-based differential diagnosis between multiple solid malignancies: breast, colorectal, lung, ovarian and oral cancer, Int. J. Nanomed., 2019, 14, 6165–6178 CrossRef CAS.
- V. Moisoiu, A. Socaciu, A. Stefancu, S. Iancu, I. Boros and C. Alecsa, et al., Breast Cancer Diagnosis by Surface-Enhanced Raman Scattering (SERS) of Urine, Appl. Sci., 2019, 9(4), 806 CrossRef CAS.
- N. Gupta, S. Dhanekar, S. Chattopadhyay, S. S. Panda and H. Singh, An approach towards development of point of care diagnostics using ELISA, Proceedings of IEEE Sensors, 2021.
- M. C. C. G. Carneiro, L. R. Rodrigues, F. T. C. Moreira and M. G. F. Sales, Paper-based ELISA for fast CA 15–3 detection in point-of-care, Microchem. J., 2022, 181, 107756 CrossRef.
- J. W. Choi, B. I. Moon, J. W. Lee, H. J. Kim, Y. Jin and H. J. Kim, Use of CA15-3 for screening breast cancer: An antibody-lectin sandwich assay for detecting glycosylation of CA15-3 in sera, Oncol. Rep., 2018, 40(1), 145–154 CAS , Available from: https://pubmed.ncbi.nlm.nih.gov/29749490/.
- J. Lee, B. Kim, B. Park, Y. Won, S. Y. Kim and S. Lee, Real-time cancer diagnosis of breast cancer using fluorescence lifetime endoscopy based on the pH, Sci. Rep., 2021, 11(1), 16864 CrossRef CAS.
- B. Kim, B. Park, S. Lee and Y. Won, GPU accelerated real-time confocal fluorescence lifetime imaging microscopy (FLIM) based on the analog mean-delay (AMD) method, Biomed. Opt. Express, 2016, 7(12), 5055 CrossRef PubMed.
- W. T. Dou, L. F. Liu, J. Gao, Y. Zang, G. R. Chen and R. A. Field, et al., Fluorescence imaging of a potential diagnostic biomarker for breast cancer cells using a peptide-functionalized fluorogenic 2D material, Chem. Commun., 2019, 55(88), 13235–13238 RSC.
- J. Eisermann, A. Kerth and D. Hinderberger, Dynamic self-assembly of ions with variable size and charge in solution, RSC Adv., 2019, 9(32), 18627–18640 RSC.
- K. Glunde, Z. M. Bhujwalla and S. M. Ronen, Choline metabolism in malignant transformation, Nat. Rev. Cancer, 2011, 11(12), 835–848 CrossRef CAS PubMed.
- U. Sharma and N. R. Jagannathan, In vivo MR spectroscopy for breast cancer diagnosis, BJR Open, 2019, 1(1), 20180040 Search PubMed.
- A. G. V. Bitencourt, J. Goldberg, K. Pinker and S. B. Thakur, Clinical applications of breast cancer metabolomics using high-resolution magic angle spinning proton magnetic resonance spectroscopy (HRMAS 1H MRS): systematic scoping review, Metabolomics, 2019, 15(11), 148 CrossRef.
- A. Bitencourt, V. Sevilimedu, E. A. Morris, K. Pinker and S. B. Thakur, Fat Composition Measured by Proton Spectroscopy: A Breast Cancer Tumor Marker?, Diagnostics, 2021, 11(3), 564 CrossRef CAS PubMed.
- M. A. Bilal Ahmadani, S. Bhatty, Z. ul Abideen, M. S. Yaseen, T. Laique and J. Malik, Imaging in Breast Cancer: Use of Magnetic Resonance Spectroscopy, Cureus, 2020, 12(8), e9734 Search PubMed.
- J. C. Gómez-Cortés, J. J. Díaz-Carmona, J. A. Padilla-Medina, A. E. Calderon, A. I. B. Gutiérrez and M. Gutiérrez-López, et al., Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review, Micromachines, 2022, 13(4), 496 CrossRef.
- Q. Dong, Y. Zhang, Q. He, C. Xu and X. Pan, Image reconstruction method for electrical impedance tomography based on RBF and attention mechanism, Comput. Electr. Eng., 2023, 110, 108826 CrossRef.
- J. Lee, S. Gweon, K. Lee, S. Um, K. R. Lee and K. Kim, et al., A 9.6 mW/Ch 10 MHz Wide-bandwidth Electrical Impedance Tomography IC with Accurate Phase Compensation for Breast Cancer Detection, in 2020 IEEE Custom Integrated Circuits Conference (CICC), IEEE, 2020, pp. 1–4 Search PubMed.
- S. Hong, K. Lee, U. Ha, H. Kim, Y. Lee and Y. Kim, et al., A 4.9 mΩ-sensitivity mobile electrical impedance tomography IC for early breast-cancer detection system, IEEE J. Solid-State Circuits, 2015, 50(1), 245–257 Search PubMed.
- H. H. Wan, H. Zhu, C. C. Chiang, J. S. Li, F. Ren and C. T. Tsai, et al., High sensitivity saliva-based biosensor in detection of breast cancer biomarkers: HER2 and CA15-3, J. Vac. Sci. Technol., B:Nanotechnol. Microelectron.:Mater., Process., Meas., Phenom., 2024, 42(2), 023202 CAS.
- C. O'Brien, C. K. Khor, S. Ardalan and A. Ignaszak, Multiplex electrochemical sensing platforms for the detection of breast cancer biomarkers, Front. Med. Technol., 2024, 6, 1360510 CrossRef PubMed.
- A. A. Zare, H. Naderi-Manesh, S. M. Naghib, M. Shamsipur and F. Molaabasi, Label-free electrochemical cancer cell detection leveraging hemoglobin-encapsulated silver nanoclusters and Cu-MOF nanohybrids on a graphene-assisted dual-modal probe, Sci. Rep., 2023, 13(1), 21980 CrossRef CAS PubMed.
- C. Pothipor, J. Jakmunee, S. Bamrungsap and K. Ounnunkad, An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/graphene quantum dots/graphene oxide film, Analyst, 2021, 146(12), 4000–4009 RSC.
- P. Ranjan, M. Abubakar Sadique, S. Yadav and R. Khan, An Electrochemical Immunosensor Based on Gold-Graphene Oxide Nanocomposites with Ionic Liquid for Detecting the Breast Cancer CD44 Biomarker, ACS Appl. Mater. Interfaces, 2022, 14(18), 20802–20812 CrossRef CAS.
- H. Ilkhani, A. Ravalli and G. Marrazza, Design of an Affibody-Based Recognition Strategy for Human Epidermal Growth Factor Receptor 2 (HER2) Detection by Electrochemical Biosensors, Chemosensors, 2016, 4(4), 23 CrossRef.
- M. Emami, M. Shamsipur, R. Saber and R. Irajirad, An electrochemical immunosensor for detection of a breast cancer biomarker based on antiHER2–iron oxide nanoparticle bioconjugates, Analyst, 2014, 139(11), 2858–2866 RSC.
- Q. A. M. Al-Khafaji, M. Harris, S. Tombelli, S. Laschi, A. P. F. Turner and M. Mascini, et al., An Electrochemical Immunoassay for HER2 Detection, Electroanalysis, 2012, 24(4), 735–742 CrossRef CAS.
- J. X. Zhang, C. L. Lv, C. Tang, A. J. Wang, L. P. Mei and P. Song, et al., Sandwich-type ultrasensitive immunosensing of breast cancer biomarker based on core-shell Au@PdAg dog-bone-like nanostructures and Au@PtRh nanorods, Sens. Actuators, B, 2023, 382, 133497 CrossRef CAS.
- E. Arkan, R. Saber, Z. Karimi and M. Shamsipur, A novel antibody–antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode, Anal. Chim. Acta, 2015, 874, 66–74 CrossRef CAS PubMed.
- A. Ravalli, C. G. da Rocha, H. Yamanaka and G. Marrazza, A label-free electrochemical affisensor for cancer marker detection: The case of HER2, Bioelectrochemistry, 2015, 106, 268–275 CrossRef CAS PubMed.
- L. Chun, S. E. Kim, M. Cho and W. Choe, et al., Electrochemical detection of HER2 using single stranded DNA aptamer modified gold nanoparticles electrode, Sens. Actuators, B, 2013, 186, 446–450 CrossRef CAS.
- S. K. Arya, P. Zhurauski, P. Jolly, M. R. Batistuti, M. Mulato and P. Estrela, Capacitive aptasensor based on interdigitated electrode for breast cancer detection in undiluted human serum, Biosens. Bioelectron., 2018, 102, 106–112 CrossRef CAS.
- R. C. B. Marques, S. Viswanathan, H. P. A. Nouws, C. Delerue-Matos and M. B. González-García, Electrochemical immunosensor for the analysis of the breast cancer biomarker HER2 ECD, Talanta, 2014, 129, 594–599 CrossRef CAS.
- S. Patris, P. De Pauw, M. Vandeput, J. Huet, P. Van Antwerpen and S. Muyldermans, et al., Nanoimmunoassay onto a screen printed electrode for HER2 breast cancer biomarker determination, Talanta, 2014, 130, 164–170 CrossRef CAS.
- C. Li, X. Qiu, D. keqin and Z. Hou, Electrochemical co-reduction synthesis of Au/ferrocene–graphene nanocomposites and their application in an electrochemical immunosensor of a breast cancer biomarker. Anal, Methods, 2014, 6(22), 9078–9084 CAS.
- H. Li, J. He, S. Li and A. P. F. Turner, Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3, Biosens. Bioelectron., 2013, 43, 25–29 CrossRef CAS PubMed.
- S. Ge, X. Jiao and D. Chen, Ultrasensitive electrochemical immunosensor for CA 15-3 using thionine-nanoporous gold–graphene as a platform and horseradish peroxidase-encapsulated liposomes as signal amplification, Analyst, 2012, 137(19), 4440 RSC.
- W. Li, R. Yuan, Y. Chai and S. Chen, Reagentless amperometric cancer antigen 15-3 immunosensor based on enzyme-mediated direct electrochemistry, Biosens. Bioelectron., 2010, 25(11), 2548–2552 CrossRef CAS PubMed.
- C. Hong, R. Yuan, Y. Chai and Y. Zhuo, Ferrocenyl-doped silica nanoparticles as an immobilized affinity support for electrochemical immunoassay of cancer antigen 15-3, Anal. Chim. Acta, 2009, 633(2), 244–249 CrossRef CAS.
- Y. Yang, Z. Zhong, H. Liu, T. Zhu, J. Wu and M. Li, et al., Double-Layer Nanogold and Double-Strand DNA-Modified Electrode for Electrochemical Immunoassay of Cancer Antigen 15-3, Electroanalysis, 2008, 20(24), 2621–2628 CrossRef CAS.
- L. C. Ward and S. Brantlov, Bioimpedance basics and phase angle fundamentals, Rev. Endocr. Metab. Disord., 2023, 24(3), 381–391 CrossRef , Available from: https://pubmed.ncbi.nlm.nih.gov/36749540/.
- S. Mansouri, T. Alhadidi and M. B. Azouz, Breast Cancer Detection Using Low-Frequency Bioimpedance Device, Breast Cancer: Targets Ther., 2020, 12, 109–116 CAS , Available from: https://www.dovepress.com/breast-cancer-detection-using-low-frequency-bioimpedance-device-peer-reviewed-fulltext-article-BCTT.
- V. Cappelletti, E. Iorio, P. Miodini, M. Silvestri, M. Dugo and M. G. Daidone, Metabolic Footprints and Molecular Subtypes in Breast Cancer, Dis. Markers, 2017, 2017, 1–19 CrossRef.
- P. Divyashree, H. A. Quadri, P. Dwivedi and S. Dhanekar, Demonstration of Intelligent Sensing by Nanosensors and use of Classification and Regression Models for Electronic Nose Applications, IEEE Sens. J., 2024, 24(21), 35423–35428 Search PubMed.
- H. Y. Yang, Y. C. Wang, H. Y. Peng and C. H. Huang, Breath biopsy of breast cancer using sensor array signals and machine learning analysis, Sci. Rep., 2021, 11(1), 1–9 CrossRef , Available from: https://www.nature.com/articles/s41598-020-80570-0.
- J. Liu, H. Chen, Y. Li, Y. Fang, Y. Guo and S. Li, et al., A novel non-invasive exhaled breath biopsy for the diagnosis and screening of breast cancer, J. Hematol. Oncol., 2023, 16(1), 1–5 CrossRef , Available from: https://jhoonline.biomedcentral.com/articles/10.1186/s13045-023-01459-9.
- O. Barash, W. Zhang, J. M. Halpern, Q. L. Hua, Y. Y. Pan and H. Kayal, et al., Differentiation between genetic mutations of breast cancer by breath volatolomics, Onco Targets Ther, 2015, 6(42), 44864–44876 Search PubMed.
- L. Lavra, A. Catini, A. Ulivieri, R. Capuano, L. Baghernajad Salehi and S. Sciacchitano, et al., Investigation of VOCs associated with different characteristics of breast cancer cells, Sci. Rep., 2015, 5(1), 13246 CrossRef CAS.
- C. B. Ambrosone, Oxidants and Antioxidants in Breast Cancer, Antioxid. Redox Signaling, 2000, 2(4), 903–917 CrossRef CAS.
- O. Herman-Saffar, Z. Boger, S. Libson, D. Lieberman, R. Gonen and Y. Zeiri, Early non-invasive detection of breast cancer using exhaled breath and urine analysis, Comput. Biol. Med., 2018, 96, 227–232 CrossRef.
- L. D. De León-Martínez, M. Rodríguez-Aguilar, P. Gorocica-Rosete, C. A. Domínguez-Reyes, V. Martínez-Bustos and J. A. Tenorio-Torres, et al., Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: a case-control study, J. Breath Res., 2020, 14(4), 046009 CrossRef , Available from: https://iopscience.iop.org/article/10.1088/1752-7163/aba83f.
- M. J. Madou, Fundamentals of Microfabrication and Nanotechnology, Three-Volume Set, 2018, Available from: https://www.taylorfrancis.com/books/mono/10.1201/9781315274164/fundamentals-microfabrication-nanotechnology-three-volume-set-marc-madou Search PubMed.
- A. V. G. K., G. Gogoi, B. Behera, S. Rila, A. Rangarajan and H. J. Pandya, RapidET: a MEMS-based platform for label-free and rapid demarcation of tumors from normal breast biopsy tissues, Microsyst. Nanoeng., 2022, 8(1), 1 CrossRef CAS PubMed.
- I. Acerbi, L. Cassereau, I. Dean, Q. Shi, A. Au and C. Park, et al., Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration, Integr. Biol., 2015, 7(10), 1120–1134 CrossRef CAS PubMed.
- E. Leikina, M. V. Mertts, N. Kuznetsova and S. Leikin, Type I collagen is thermally unstable at body temperature, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(3), 1314–1318 CrossRef CAS.
- N. Ignatieva, O. Zakharkina, A. Dadasheva, A. Shekhter, A. Sviridov and V. Lunin, Transformation of the dermal collagen framework under laser heating, J. Biophotonics, 2019, 12(12), e201960024 CrossRef CAS PubMed.
- A. Khosla, MEMS Based Eit Technology for Non-Invasive Breast Cancer Diagsnostics, ECS Meeting Abstracts, 2014, 02(11), 690 CrossRef , Available from: https://iopscience.iop.org/article/10.1149/MA2014-02/11/690.
- A. V. Anil, G. Gogoi, B. Behera, S. Rila, A. Rangarajan and H. J. Pandya, RapidET: a MEMS-based platform for label-free and rapid demarcation of tumors from normal breast biopsy tissues, Microsyst. Nanoeng., 2022, 8(1), 1–16 CrossRef , Available from: https://www.nature.com/articles/s41378-021-00337-z.
- L. Arcarisi, L. Di Pietro, N. Carbonaro, A. Tognetti, A. Ahluwalia and C. De Maria, Palpreast—A New Wearable Device for Breast Self-Examination, Appl. Sci., 2019, 9(3), 381 CrossRef.
- J. Jung, W. Lee, W. Kang, E. Shin, J. Ryu and H. Choi, Review of piezoelectric micromachined ultrasonic transducers and their applications, J. Micromech. Microeng., 2017, 27(11), 113001 CrossRef.
- L. Li, J. Zheng, J. Chen, Z. Luo, Y. Su and W. Tang, et al., Flexible Pressure Sensors for Biomedical Applications: From Ex Vivo to In Vivo, Adv. Mater. Interfaces, 2020, 7(17), 2000743 CrossRef , Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/admi.202000743.
- C. Liu, C. Xue, B. Zhang, G. Zhang and C. He, The Application of an Ultrasound Tomography Algorithm in a Novel Ring 3D Ultrasound Imaging System, Sensors, 2018, 18(5), 1332 CrossRef PubMed.
- K. Chang, Y. Pi, W. Lu, F. Wang, F. Pan and F. Li, et al., Label-free and high-sensitive detection of human breast cancer cells by aptamer-based leaky surface acoustic wave biosensor array, Biosens. Bioelectron., 2014, 60, 318–324 CrossRef CAS PubMed.
- W. Geng, Y. Liu, N. Yu, X. Qiao, M. Ji and Y. Niu, et al., An ultra-compact acoustofluidic device based on the narrow-path travelling surface acoustic wave (np-TSAW) for label-free isolation of living circulating tumor cells, Anal. Chim. Acta, 2023, 1255, 341138 CrossRef CAS.
- A. Karczmarczyk, K. Haupt and K. H. Feller, Development of a QCM-D biosensor for Ochratoxin A detection in red wine, Talanta, 2017, 166, 193–197 CrossRef CAS.
- M. Bakhshpour, A. K. Piskin, H. Yavuz and A. Denizli, Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor, Talanta, 2019, 204, 840–845 CrossRef CAS PubMed.
- M. Yılmaz, M. Bakhshpour, I. Göktürk, A. K. Pişkin and A. Denizli, Quartz Crystal Microbalance (QCM) Based Biosensor Functionalized by HER2/neu Antibody for Breast Cancer Cell Detection, Chemosensors, 2021, 9(4), 80 CrossRef.
- N. Köü, A. Köü, M. Duran, S. Simavl and N. Turhan, Comparison of standard mammography with digital mammography and digital infrared thermal imaging for breast cancer screening Meme kanseri taramasnda standart mamografi ile dijital mamografi ve dijital infrared termal görüntülemenin karlatrlmas, J. Turk.-Ger. Gynecol. Assoc., 2010, 11, 152–159 CrossRef.
- Y. R. Parisky, A. Sardi, R. Hamm, K. Hughes, L. Esserman and S. Rust, et al., Efficacy of Computerized Infrared Imaging Analysis to Evaluate Mammographically Suspicious Lesions, AJR, Am. J. Roentgenol., 2003, 180(1), 263–269 CrossRef CAS PubMed.
- S. Ekici and H. Jawzal, Breast cancer diagnosis using thermography and convolutional neural networks, Med. Hypotheses, 2020, 137, 109542 CrossRef.
- R. Resmini, L. Silva, A. S. Araujo, P. Medeiros, D. Muchaluat-Saade and A. Conci, Combining Genetic Algorithms and SVM for Breast Cancer Diagnosis Using Infrared Thermography, Sensors, 2021, 21(14), 4802 CrossRef PubMed.
- S. Tello-Mijares, F. Woo and F. Flores, Breast Cancer Identification via Thermography Image Segmentation with a Gradient Vector Flow and a Convolutional Neural Network, J. Healthc. Eng., 2019, 2019, 1–13 CrossRef.
- L. V. Wang and H. I. Wu, Definitions of Optical Properties, Biomedical Optics: Principles and Imaging, 2007, pp. 343–346, Available from: https://www.wiley.com/en-us/Biomedical+Optics%3A+Principles+and+Imaging-p-9780471743040 Search PubMed.
- E. Y. Chae, H. H. Kim, S. Sabir, Y. Kim, H. Kim and S. Yoon, et al., Development of digital breast tomosynthesis and diffuse optical tomography fusion imaging for breast cancer detection, Sci. Rep., 2020, 10(1), 13127 CrossRef CAS PubMed.
- J. E. McGuinness, M. L. Altoe, A. Marone, L. E. Franks, S. M. Lee and H. K. Kim, et al., Diffuse optical tomography breast imaging measurements are modifiable with pre-surgical targeted and endocrine therapies among women with early stage breast cancer, Breast Cancer Res. Treat., 2021, 189(1), 297–304 CrossRef CAS , Available from: https://link.springer.com/article/10.1007/s10549-021-06320-6.
- Y. Wang, Y. Wang, S. Li, S. Li, Y. Wang and Q. Yan, et al., Compact fiber-free parallel-plane multi-wavelength diffuse optical tomography system for breast imaging, Opt. Express, 2022, 30(5), 6469–6486 CrossRef CAS PubMed , Available from: https://opg.optica.org/viewmedia.cfm?uri=oe-30-5-6469&seq=0&html=true.
- D. N. Elsheakh, R. A. Mohamed, O. M. Fahmy, K. Ezzat and A. R. Eldamak, Complete Breast Cancer Detection and Monitoring System by Using Microwave Textile Based Antenna Sensors, Biosensors, 2023, 13(1), 87 CrossRef CAS PubMed.
- E. C. Fear, P. M. Meaney and M. A. Stuchly, Microwaves for breast cancer detection? IEEE Potentials, 2003, 221, pp. 12–18 Search PubMed.
- L. Wang, Microwave Imaging and Sensing Techniques for Breast Cancer Detection, Micromachines, 2023, 14(7), 1462 CrossRef PubMed , Available from: https://www.mdpi.com/2072-<?pdb_no 666X?>666X<?pdb END?>/14/7/1462/htm.
- M. T. Islam, M. Z. Mahmud, M. T. Islam, S. Kibria and M. Samsuzzaman, A Low Cost and Portable Microwave Imaging System for Breast Tumor Detection Using UWB Directional Antenna array, Sci. Rep., 2019, 9(1), 15491 CrossRef CAS PubMed.
- IEEE Xplore, Full-Text PDF: [Internet], [cited 2024 May 16], Available from: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=7384493.
- Hello - The Blue Box Biomedical Solutions, S.L. [Internet], [cited 2024 May 17], Available from: https://thebluebox.ai/.
- BRCA1 elisa kit | Human Breast Cancer Susceptibility Protein 1 ELISA Kit-AAC37594.1.
- ab108633 Cancer Antigen CA15-3 Human ELISA Kit Instructions for Use.
- HercepTest Kits | Agilent [Internet], [cited 2024 May 17], Available from: https://www.agilent.com/en/product/pharmdx/herceptest-kits.
- Xpert® Breast Cancer STRAT4 [Internet], [cited 2024 May 17], Available from: https://www.cepheid.com/en-GB/tests/oncology-human-genetics/xpert-breast-cancer-strat4.html.
- G. Jian, S. Zhu, X. Yuan, S. Fu, N. Yang and C. Yan, et al., Biodegradable triboelectric nanogenerator as a implantable power source for embedded medicine devices, NPG Asia Mater., 2024, 16(1), 12 CrossRef CAS.
- S. Solanki, A. K. Gupta, U. Saha, A. V. Krasnoslobodtsev, R. K. Gupta and B. D. Malhotra, Triboelectric Nanogenerator-based smart biomedical sensors for healthcare, Sustain. Energy Technol. Assessments, 2023, 57, 103233 CrossRef.
- B. Gil, S. Anastasova and G. Z. Yang, Low-powered implantable devices activated by ultrasonic energy transfer for physiological monitoring in soft tissue via functionalized electrochemical electrodes, Biosens. Bioelectron., 2021, 182, 113175 CrossRef CAS PubMed.
- S. S. Rao, G. G. Bushnell, S. M. Azarin, G. Spicer, B. A. Aguado and J. R. Stoehr, et al., Enhanced Survival with Implantable Scaffolds That Capture Metastatic Breast Cancer Cells In Vivo, Cancer Res., 2016, 76(18), 5209–5218 CrossRef CAS PubMed.
- A. R. K. Sasikala, A. R. Unnithan, R. G. Thomas, S. W. Ko, Y. Y. Jeong and C. H. Park, et al., Multifaceted Implantable Anticancer Device for Potential Postsurgical Breast Cancer Treatment: A Single Platform for Synergistic Inhibition of Local Regional Breast Cancer Recurrence, Surveillance, and Healthy Breast Reconstruction, Adv. Funct. Mater., 2018, 28(8), 1704793 CrossRef.
- R. Jalloul, H. K. Chethan and R. Alkhatib, A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images, Diagnostics, 2023, 13(14), 2460 CrossRef PubMed.
- A. Onasanya and M. Elshakankiri, Smart integrated IoT healthcare system for cancer care, Wireless Networks, 2021, 27(6), 4297–4312 CrossRef.
- M. H. Memon, J. P. Li, A. U. Haq, M. H. Memon and W. Zhou, Breast Cancer Detection in the IOT Health Environment Using Modified Recursive Feature Selection, Wirel. Commun. Mob. Comput., 2019, 2019, 1–19 CrossRef.
- S. Salvi and A. Kadam, Breast Cancer Detection Using Deep learning and IoT Technologies, J. Phys.: Conf. Ser., 2021, 1831, 012030 CrossRef.
- P. Gamble, R. Jaroensri, H. Wang, F. Tan, M. Moran and T. Brown, et al., Determining breast cancer biomarker status and associated morphological features using deep learning, Commun. Med., 2021, 1(1), 14 CrossRef PubMed.
- M. Sadeghi, S. Sadeghi, S. M. Naghib and H. R. Garshasbi, A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer, Biosensors, 2023, 13(4), 481 CrossRef CAS.
- S. Augustine, P. Kumar and B. D. Malhotra, Amine-Functionalized MoO 3 @RGO Nanohybrid-Based Biosensor for Breast Cancer Detection, ACS Appl. Bio Mater., 2019, 2(12), 5366–5378 CrossRef CAS.
- S. Augustine, A. G. Joshi, B. K. Yadav, A. Mehta, P. Kumar and V. Renugopalakrishanan, et al., An emerging nanostructured molybdenum trioxide-based biocompatible sensor platform for breast cancer biomarker detection, MRS Commun., 2018, 8(3), 668–679 CrossRef CAS , Available from: https://link.springer.com/article/10.1557/mrc.2018.182.
- M. A. Ali, K. Mondal, C. Singh, B. Dhar Malhotra and A. Sharma, Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics, Nanoscale, 2015, 7(16), 7234–7245 RSC , Available from: https://pubs.rsc.org/en/content/articlehtml/2015/nr/c5nr00194c.
- R. Arvidsson and S. F. Hansen, Environmental and health risks of nanorobots: an early review, Environ. Sci.: Nano, 2020, 7(10), 2875–2886 RSC.
- H. Bakherad, F. Ghasemi, M. Hosseindokht and H. Zare, Nanobodies; new molecular instruments with special specifications for targeting, diagnosis and treatment of triple-negative breast cancer, Cancer Cell Int., 2022, 22(1), 245 CrossRef PubMed.
- H. Patel, K. Parekh, L. Fernel Gamarra, J. Bustamante Mamani, A. A. da Hora and A. M. Figueiredo Neto, In vitro evaluation of magnetic fluid hyperthermia therapy on breast cancer cells using monodispersed Mn0.5Zn0.5Fe2O4 nanoflowers, J. Magn. Magn. Mater., 2023, 587, 171275 CrossRef CAS.
- M. Pumera, Electrochemically powered self-propelled electrophoretic nanosubmarines, Nanoscale, 2010, 2(9), 1643 RSC.
- Z. Xu, R. Ni and Y. Chen, Targeting breast cancer stem cells by a self-assembled, aptamer-conjugated DNA nanotrain with preloading doxorubicin, Int. J. Nanomed., 2019, 14, 6831–6842 CrossRef CAS.
- B. Xie, X. Yang, R. Zhang, J. Guo, Z. Chen and Y. He, Hollow and Porous Fe3C-NC Nanoballoons Nanozymes for Cancer Cell H2O2 Detection, Sens. Actuators, B, 2021, 347, 130597 CrossRef CAS.
- M. K. Hameed, J. B. M. Parambath, M. T. Gul, A. A. Khan, Y. Park and C. Han, et al., Arylated gold nanostars aided SERS study of breast cancer cells, Appl. Surf. Sci., 2022, 583, 152504 CrossRef CAS.
- P. Ji, X. Wang, J. Yin, Y. Mou, H. Huang and Z. Ren, Selective delivery of curcumin to breast cancer cells by self-targeting apoferritin nanocages with pH-responsive and low toxicity, Drug Delivery, 2022, 29(1), 986–996 CrossRef CAS PubMed.
- A. N. Neagu, T. Jayaweera, K. Weraduwage and C. C. Darie, A Nanorobotics-Based Approach of Breast Cancer in the Nanotechnology Era, Int. J. Mol. Sci., 2024, 25(9), 4981 CrossRef CAS.
- F. Lebre, N. Chatterjee, S. Costa, E. Fernández-de-Gortari, C. Lopes and J. Meneses, et al., Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment, Nanomaterials, 2022, 12(11), 1810 CrossRef CAS.
- B. Kaur, S. Kumar and B. K. Kaushik, Recent advancements in optical biosensors for cancer detection, Biosens. Bioelectron., 2022, 197, 113805 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



