 Open Access Article
Open Access ArticleA novel two-photon fluorescent probe for non-destructive imaging of Hg2+ in fresh plant tissues†
Xiao
Liu‡
a,
Zheng
Zhu‡
b,
Ruitao
Sun
a,
Jun
Li
*a and
Shengzhen
Xu
 *a
*a
aCollege of Chemistry, Huazhong Agricultural University, Wuhan, Hubei 430070, China. E-mail: xusz@mail.hzau.edu.cn
bCollege of Life Science and Technology, Huazhong Agricultural University, Wuhan, Hubei 430070, China
First published on 29th April 2025
Abstract
In this work, we developed a small-molecule fluorescent probe (termed as LJTP3) for the specific detection of Hg2+ with high sensitivity in living plant tissues. LJTP3 can not only effectively indicate the spatiotemporal distribution of Hg2+ in the plant subcellular level, but also enable to realize 3D imaging of Hg2+ in plant roots.
Mercury, a highly toxic heavy metal, is widely distributed in the natural environment. However, with the increasing intensity of human activities such as industrial production, coal combustion, waste incineration and agricultural practices, mercury emissions have risen significantly, leading to serious environmental contamination.1,2 As a major form of Hg, Hg2+ exhibited a strong affinity for proteins with bioaccumulation properties.3 As a critical component of ecosystems, plants are particularly sensitive to mercury pollution.4 Studies have shown that Hg2+ tends to accumulate in plant roots and leaves, and elevated levels can cause visible damage to plant tissues and further affect plant growth and crop production.5,6 Therefore, developing an efficient tool for the detection of Hg2+ in plants has great significance for agricultural management.
During the past decades, various traditional methods for detecting Hg2+ have been developed, including but not limited to inductively coupled plasma atomic emission spectrometry (ICP-AES), inductively coupled plasma mass spectrometry (ICP-MS), atomic fluorescence spectrometry (AFS), atomic absorption spectrometry (AAS), and chemiluminescence methods.7–10 Compared to these techniques, fluorescence sensors displayed distinct advantages, such as high sensitivity, superior spatiotemporal resolution, and non-invasive in situ imaging capabilities.11–15 As a result, several fluorescent probes have been employed for the in vivo detection of Hg2+.16–21 However, only a few small-molecule organic fluorescent probes have been reported to achieve clear imaging at subcellular levels in plants.22–28 In particular, two-photon fluorescent probes possess NIR excitation wavelengths, enabling deeper penetration into plant tissues to achieve plant subcellular imaging with minimum interference of background. However, two-photon-based small-molecule probes for subcellular imaging in plants are still few, and the dynamic distribution of Hg2+ at subcellular lever still needs to be further investigated.29–32
In this study, a water-soluble fluorescent probe, LJTP3 was tailored for the detection and imaging of Hg2+ in plant tissues. It comprises a 2-(naphthalen-2-yl)benzo[d]oxazole-based fluorophore for signal output, and a hydrophilic tetrakis(N-2-hydroxyethyl)acetamide group as the Hg2+ specific binding component. LJTP3 exhibited not only excellent selectivity but also a low detection limit (LOD) of 0.08 μM for the early detection of Hg2+. Moreover, the fluorescence signals for Hg2+ detection were observed in the model plant Arabidopsis, allowing visualization of its localization at the subcellular level. More importantly, the temporal and spatiotemporal distribution of mercury (Hg) was clearly observed under two-photon microscopy and 3D reconstruction (Scheme 1).
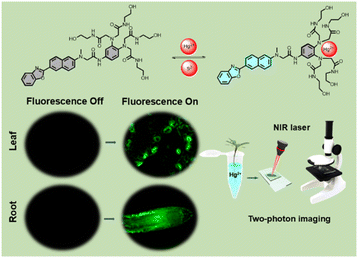 | ||
| Scheme 1 Illustration of a two-photon fluorescent probe (LJTP3) for the detection of Hg2+ in Arabidopsis thaliana. | ||
The synthetic procedures of LJTP3 are shown in Scheme S1 (ESI†) and the molecular characterization data are shown in Fig. S1–S17 (ESI†). The synthesis of LJTP3 was ultimately achieved through an 8-step process involving nucleophilic substitution, nitration, reduction, and condensation reactions to get the probe with moderate yields.
Following the successful synthesis of LJTP3, evaluation of its response to Hg2+ was then performed in HEPES solution. As shown in Fig. S18 (ESI†), the probe itself has an obvious UV absorption peak at 355 nm in HEPES solution, which was employed as the excitation wavelength of LJTP3. The fluorescence titration experiment of LJTP3 revealed that only Hg2+ induced significant fluorescence enhancement at the emission peak of 480 nm, while other metal ions including Ag+, Ba2+, Ca2+, Cr3+, Cd2+, Fe2+, Fe3+, Mg2+, Mn2+, Na+, Pd2+, and Zn2+ did not induce obvious fluorescence enhancement, indicating the good selectivity of LJTP3 (Fig. 1A). In addition, fluorescence interference tests for different ions were carried out in HEPES solution. As shown in Fig. 1B, the fluorescence of the probe LJTP3 shows minimal interference from other coexisting metal ions, demonstrating its strong anti-interference capability. This suggests that LJTP3 can be well-suited for the selective detection of Hg2+ in complex systems. The fluorescence spectra of the probe LJTP3 (1 μM) were measured at varying concentrations of Hg2+ (0–10 μM), as shown in Fig. 1C. The fluorescence intensity gradually increased with increasing Hg2+ concentrations, until reaching a plateau. During the titration experiments, a good linear relationship was observed between the fluorescence intensity and concentrations of Hg2+ in the range of 0–3 μM (Fig. 1D). The limit of detection (LOD) was determined to be 0.08 μM.
The binding mode of the probe LJTP3 for Hg2+ was hypothesized, as shown in the Fig. S19.† Upon coordination of the polyamide ligands with Hg2+, the PET effect was weakened, leading to enhance fluorescence intensity. To confirm this hypothesis, the detection mechanism of LJTP3 toward Hg2+ was thoroughly validated using ESI-MS. As shown in Fig. S20 (ESI†), the molecular ion peak (m/z: 1044.3536) was observed, which matches the calculated value (m/z: 1044.3538).
Additionally, a Job-plot experiment was conducted (Fig. 1F). The intersection of the curve at a ratio of 0.5 indicates a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio between LJTP3 and Hg2+. Due to its specific affinity for S2−, the Hg2+-enhanced fluorescence was restored to the level of the free probe. This rapid and reversible sensing behavior was repeated five times without significant signal attenuation (Fig. 1E), confirming the reversibility of the binding. Besides, LJTP3 exhibited high stability within the pH range of 6.5–8.0, making it suitable for Hg2+ sensing under physiological conditions (Fig. S21 ESI†).
1 binding ratio between LJTP3 and Hg2+. Due to its specific affinity for S2−, the Hg2+-enhanced fluorescence was restored to the level of the free probe. This rapid and reversible sensing behavior was repeated five times without significant signal attenuation (Fig. 1E), confirming the reversibility of the binding. Besides, LJTP3 exhibited high stability within the pH range of 6.5–8.0, making it suitable for Hg2+ sensing under physiological conditions (Fig. S21 ESI†).
To investigate the sensing mechanism (Fig. 2), density functional theory (DFT) calculations were performed using Gaussian 16 software.33 The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of LJTP3 were primarily localized on the fluorophore, although the HOMO also exhibited partial distribution in the recognition group. The energy gap between the HOMO and LUMO was calculated to be 3.66 eV, with photo-induced electron transfer (PET) occurring from the recognition group to the fluorophore, resulting in fluorescence quenching. Upon binding with Hg2+, the distribution of both the HOMO and LUMO shifted towards the fluorophore and recognition group, respectively, with a reduced energy gap of 3.45 eV, leading to the inhibition of PET and consequently, fluorescence restoration.
 | ||
| Fig. 2 Molecular orbitals and corresponding energy levels of LJTP3 and LJTP3 + Hg2+ in both the ground state and excitation state. | ||
Given the advantages of two-photon microscopy, we employed this technique to further verify the probe's efficiency in detecting Hg2+ at the tissue and cellular level. To evaluate the probe's specificity in vivo (Fig. 3A and B), the model plant Arabidopsis thaliana was treated with various metal ions including Cd2+, Mg2+, Zn2+, K+ and Hg2+ respectively and then imaged under two-photon microscopy (λex = 750 nm);34 only the Hg2+ treated group showed significant fluorescence signal output, indicating LJTP3 can be employed for Hg2+ specific imaging in plant tissues. As evidenced in Fig. S23,† two-photon comparative experiments were systematically conducted to examine the system before and after S2− introduction. The experimental data demonstrate near-complete fluorescence quenching upon S2− addition, strongly suggesting the reversible binding behavior between LJTP3 and Hg2+ in plant systems. In addition, the translocation of Hg2+ in plant tissues at the subcellular level, as well as the stress response of plant cells under Hg2+ exposure, were visualized in a real time manner (Fig. 3D and E). In the control group, where Hg2+ was absent, only a faint fluorescence signal was detected. However, after 1 hour of incubation with Hg2+, fluorescence corresponding to the probe's interaction with Hg2+ appeared on the epidermal cells of the root tip. After 3 hours, the fluorescence became more widespread, reflecting a significant uptake of the probe within the root tip cells. Moreover, after 5 hours, the fluorescence intensity increased markedly, indicating a strong and clear signal. Similar trends were observed in the Arabidopsis leaf epidermis (Fig. S21C and D ESI†).
To further investigate the fluorescence signal transmission in Arabidopsis under different Hg2+ concentrations, two-photon imaging was performed on root tips under varying Hg2+ stress levels (Fig. 3F and G). Only weak fluorescence signals were detected in the control group (no Hg2+ treatment). Under the stress of 10 μM Hg2+, fluorescence signals began to appear around the cells of Arabidopsis root tips. At 100 μM Hg2+, signals are present in most cells of the root tip. When the concentration was increased to 1 mM, signals appeared in all cells of the root tip and exhibited very high fluorescence intensity. In the leaf epidermis under the same treatment, the signal changed in a similar trend (Fig. S21A and B ESI†). The above results manifested that the dynamic distribution of Hg2+ can be visualized using LJTP3.
Under a single photon microscope with 3D imaging and reconstruction, LJPT3 can also directly visualize the spatial distribution of Hg2+ in plant organs (Fig. 3C). In summary, LJPT3 can realize the non-destructive detection of Hg2+ in plant organs and tissues in a very short time, with good selectivity and sensitivity, and can accurately indicate the location and content of Hg2+ in plants.
To evaluate the capability of LJPT3 in detecting Hg2+ distribution differences within plant tissue microstructures, fluorescence signals in Arabidopsis root tips and leaf epidermis were analyzed using two-photon microscopy. Under 10 μM Hg2+ treatment, the root epidermis exhibited stronger fluorescence than the stele, reflecting a defense strategy against mercury. Likewise, the leaf epidermis showed higher fluorescence than the root stele, indicating differential Hg2+ accumulation (Fig. 3H), consistent with previous reports.
This disparity is attributed to plant cell defense mechanisms against Hg2+, aligning with previous findings.35 In leaves, stomata exhibited stronger fluorescence than epidermal cells (Fig. 3I), as they serve as key sites for Hg2+ exchange between plants and the environment. Plants absorb elemental mercury via the stomata and convert accumulated mercury in leaves into elemental form for release.36
In conclusion, we have designed a highly efficient fluorescent probe (LJTP3) specifically to study Hg2+ stress in plant tissues. LJTP3 demonstrated excellent selectivity and sensitivity for the early detection of Hg2+ in aqueous solution, with a detection limit of 0.08 μM. Remarkably, LJTP3 exhibited outstanding selectivity for Hg2+ in both in vitro tests and plant imaging. Moreover, under two-photon imaging, the distribution of Hg2+, along with Hg2+-induced rupture of root tip cells and leaf stomata, was clearly observed. We believe that this study not only provides a novel imaging tool for investigating Hg2+-induced stress on plant cell structures but also contributes to the management of Hg pollution in agriculture.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
Shengzhen Xu and Jun Li conceived the basic idea and reviewed the manuscript. Xiao Liu designed and performed the experiment and drafted the manuscript. Zheng Zhu performed the experiments and imaging; Ruitao Sun provided suggestions on experiment. All authors read and approved the manuscript. Xiao Liu and Zheng Zhu contributed equally to this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Minhui Cao Studio of Huazhong Agricultural University.Notes and references
- C. T. Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob and N. Pirrone, Environ. Sci. Technol., 2013, 47, 4967–4983 CrossRef CAS PubMed.
- Y. S. Wu, A. I. Osman, M. Hosny, A. M. Elgarahy, A. S. Eltaweil, D. W. Rooney, Z. H. Chen, N. S. Rahim, M. Sekar, S. C. B. Gopinath, N. N. I. M. Rani, K. Batumalaie and P. S. Yap, ACS Omega, 2024, 9, 5100–5126 CrossRef CAS PubMed.
- P. A. Nogara, C. S. Oliveira, G. L. Schmitz, P. C. Piquini, M. Farina, M. Aschner and B. T. Rocha, Biochim. Biophys. Acta, Gen. Subj., 2019, 1863, 129–284 CrossRef.
- B. Gworek, W. Dmuchowski and A. H. Baczewska-Dąbrowsk, Environ. Sci. Eur., 2020, 32, 128 CrossRef CAS.
- J. Zhou, D. Obrist, A. Dastoor, M. Jiskra and A. Ryjkov, Nat. Rev. Earth Environ., 2021, 2, 269284 Search PubMed.
- W. Yuan, X. Wang, C. J. Lin, F. Wu, K. Luo, H. Zhang, Z. Y. Lu and X. B. Feng, Environ. Sci. Technol., 2022, 56, 14154–14165 CrossRef CAS.
- S. Li, C. C. Zhang, S. N. Wang, Q. Liu, H. H. Feng, X. Ma and J. H. Guo, Analyst, 2018, 143, 4230–4246 RSC.
- T. A. Saleh, G. Fadillah, E. Ciptawati and M. Khaled, TrAC, Trends Anal. Chem., 2020, 132, 116016 CrossRef CAS.
- K. Schlöglova, M. Wälle and C. A. Heinrich, J. Anal. At. Spectrom., 2017, 32, 1052–1063 RSC.
- K. Srinivasan, K. Subramanian, A. Rajasekar, K. Murugan, G. Benelli and K. Dinakaran, Bull. Mater. Sci., 2017, 40, 1455–1462 CrossRef CAS.
- M. C. Dai, Y. J. Yang, S. Sarkar and K. H. Ahn, Chem. Soc. Rev., 2023, 52, 6344–6358 RSC.
- X. L. Ding, Q. Wang, D. Chen, Y. L. Chen, W. W. Pan, Q. Sun, Q. Chen and X. Y. Han, Adv. Agrochem, 2023, 2, 364–370 CrossRef CAS.
- J. Yin, Y. Hu and J. Y. Yoon, Chem. Soc. Rev., 2015, 44, 4619–4644 RSC.
- Z. J. Zhang, B. R. Adhikari, P. Sen, L. Soleymani and Y. F. Li, Adv. Agrochem, 2023, 2, 246–257 CrossRef CAS.
- J. Li, D. Yim, W.-D. Jang and J. Yoon, Chem. Soc. Rev., 2017, 46, 2437–2458 RSC.
- L. H. Feng, Y. Deng, X. J. Wang and M. G. Liu, Sens. Actuators, B, 2017, 245, 441–447 CrossRef CAS.
- G. J. Li, J. L. Wang, D. Y. Li, S. H. Liu, J. Yin, Z. B. Lai and G. F. Yang, Chin. Chem. Lett., 2021, 32, 1527–1531 CrossRef CAS.
- J. H. Wang, Y. M. Liu, Z. M. Dong, J. B. Chao, H. Wang, Y. Wang and S. M. Shuang, J. Hazard. Mater., 2020, 382, 121056 CrossRef CAS.
- L. N. Neupane, J. Park, P. K. Mehta, E. T. Oh, H. J. Park and K. H. Lee, Chem. Commun., 2020, 56, 2941–2944 RSC.
- Z. G. Wang, Y. Zhang, J. Yin, Y. Q. Yang, H. Luo, J. Song, X. Xu and S. F. Wang, ACS Sustainable Chem. Eng., 2020, 33, 12348–12359 CrossRef.
- L. Wang, Y. Ma and W. Y. Lin, J. Hazard. Mater., 2024, 461, 132604 CrossRef CAS.
- Y. Y. Zhang, W. Shi, D. Feng, H. M. Ma, Y. Liang and J. R. Zuo, Sens. Actuators, B, 2011, 153, 261–265 CrossRef CAS.
- Y. Yang, R. Shen, Y. Z. Wang, F. Z. Qiu, Y. Feng, X. L. Tang, D. C. Bai, G. L. Zhang and W. S. Liu, Sens. Actuators, B, 2018, 255, 3479–3487 CrossRef CAS.
- K. Ramki, G. Thiruppathi, S. K. Ramasamy, P. Sundararaj and P. Sakthivel, Methods, 2024, 221, 1–11 CrossRef CAS.
- H. Y. Niu, T. Q. Ye, L. Y. Yao, Y. F. Lin, K. Chen, Y. B. Zeng, L. Li, L. H. Guo and J. B. Wang, J. Hazard. Mater., 2024, 475, 134914 CrossRef CAS.
- Y. An, B. Li, Y. Z. Yu, Y. C. Zhou, J. F. Yi, L. P. Li, Y. Q. Sun, Z. Z. Qiang, Y. Q. Liu and P. Wang, J. Hazard. Mater., 2024, 465, 133331 CrossRef CAS.
- C. Zhao, A. Aziz, W. J. Lu, H. M. Xu, M. Asif, S. M. Shuang and C. Dong, J. Hazard. Mater., 2024, 479, 135694 CrossRef CAS.
- J. H. Wang, Y. M. Liu, Z. M. Dong, J. B. Chao, H. Wang, Y. Wang and S. M. Shuang, J. Hazard. Mater., 2020, 382, 121056 CrossRef CAS PubMed.
- J. Z. Du, K. M. Chen, Z. Y. Yu, Y. H. Qiao, J. X. Liu, Q. Q. Zhai, Z. Hu, S. G. Yang, J. Li and H. L. Teng, Adv. Agrochem, 2022, 1, 162–173 CrossRef.
- V. Juvekar, S. J. Park, J. Y. Yoon and H. M. Kim, Coord. Chem. Rev., 2021, 427, 213574 CrossRef CAS.
- H. W. Lee, V. Juvekar, D. J. Lee and H. M. Kim, TrAC, Trends Anal. Chem., 2023, 165, 117128 CrossRef CAS.
- S. Asghar, Z. Yu, Z. Zhu, D. Zheng, Z. Zhao, Y. Xu, X. Liu, C. Yuan, Y. Li, W. Wang, J. F. Xu, H. L. Teng, J. Li, W. C. Yang and C. Chen, Research, 2025, 8, 0570 CrossRef CAS.
- G. J. Li, J. L. Wang, D. Y. Li, S. H. Liu, J. Yin, Z. B. Lai and G. F. Yan, Chin. Chem. Lett., 2021, 32, 1527–1531 CrossRef CAS.
- H. J. Kim, J. H. Han, M. K. Kim, C. S. Lim, H. M. Kim and B. R. Cho, Angew. Chem., 2010, 122, 6938–6941 CrossRef.
- J. J. Wang, Y. Y. Guo, D. L. Guo, S. L. Yin, D. L. Kong, Y. S. Liu and H. Zeng, Environ. Sci. Technol., 2012, 46, 769–777 CrossRef CAS PubMed.
- W. Yuan, X. Wang, C. J. Lin, F. Wu, K. Luo, H. Zhang, Z. Y. Lu and X. B. Feng, Environ. Sci. Technol., 2022, 56, 14154–14165 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00023h |
| ‡ X. Liu and Z. Zhu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |


