Open Access Article
Open Access ArticleTwo in one: a facile modular approach for the assembly of pnictogen-rich heteroleptic organometallic complexes
Bijan
Mondal
 *ab,
Christoph
Riesinger
a and
Manfred
Scheer
*ab,
Christoph
Riesinger
a and
Manfred
Scheer
 *a
*a
aInstitute of Inorganic Chemistry, University of Regensburg, Universitätsstr. 31, 93053 Regensburg, Germany. E-mail: bijan.mondal@chemie.uni-regensburg.de; manfred.scheer@chemie.uni-regensburg.de
bCorporate Research and Development Centre, Bharat Petroleum Corporation Limited, Plot 2A Udyog Kendra, Surajpur, Greater Noida, UP 201306, India. E-mail: bijanmondal@bharatpetroleum.in
First published on 5th November 2025
Abstract
The reactions between soluble 1D polymer [Ag{(η2:η1-A)}2]n[TEF]n, obtained from five-fold symmetric building blocks [Cp*Fe(η5-P5)] (A, Cp* = η5-C5Me5) and a weakly coordinating salt of Ag(I), and a series of ditopic–tetrahedral organometallic building blocks, [{CpMo(CO)2}2(η2-E2)] (E = P, As) or [{CpMo(CO)2}2(η2-PE)] (E = As, Sb), have been investigated. It is shown that, depending on the choice of the ditopic–tetrahedral complexes, a rational design of unprecedented heteroleptic 1D polymer or discrete molecular systems containing two different pnictogenyl building blocks is possible. Weak π–π interactions, adaptive coordination behavior of the Ag(I), argentophilic interactions and the fine-tuned electronic properties of the ditopic–tetrahedral complexes directed the product formation. The modular synthetic approach paves the way for hybrid systems containing heteroleptic pnictogen-rich supramolecular assemblies.
Introduction
Self-organization of discrete units to form supramolecular aggregates and networks has been an exciting multidisciplinary domain of contemporary chemical research that goes beyond conventional scientific boundaries.1 Supramolecules offer elegant molecular structures with intriguing physical and chemical properties,2 rich host–guest chemistries,3 and separation properties,4 and are used in catalysis.5 To access complex supramolecular architectures, coordination-driven self-assembly has emerged as a commonly adopted rational synthetic strategy.6 By utilizing the directional advantages of metal–ligand coordination, this technique provides better control over the design of various supramolecular products. Therefore, the choice of a connecting ligand and a metal-centered node is crucial for the construction of the desired molecular assemblies.7 In addition, the self-assembly process has an important feature known as “self-correction” which allows the system to thermodynamically control the formation of a specific architecture over other possible forms.8 However, in the absence of a clear thermodynamic preference several species may exist in solution, occasionally in an equilibrium.9 Nevertheless, in a dynamic coordination environment the modular synthetic approach stands out from the two extremes to prepare selective target molecules: nature's way of constructing biomolecules (amino acids are combined into proteins, nucleosides to DNA and RNA, and monosaccharides to carbohydrates) and host-directed receptor design with increasingly demanding synthetic efforts.10 Within the last category, the majority of formed supramolecules contain only one set of linker molecules. The creation of supramolecules containing two or more different linker molecules is a rather new and synthetically challenging direction of research.11 Moreover, to the best of our knowledge, no compound is known to date, with two or more different organometallic moieties as linkers.In our synthetic endeavors, we have shown the individual potential of the ditopic–tetrahedrane complex [{CpMo(CO)2}2(μ,η2-P2)] (D, Cp = C5H5) and the five-fold symmetric penta-phosphaferrocene [CpRFe(η5-P5)] (CpR = C5Me5 (Cp*, A), C5Me4Et, C5(CH2Ph)5, C5H3tBu2-1,3 (Cp′′, C), C5(4-nBuC6H4)5) as efficient building blocks together with Ag(I) or Cu(I) salts for the formation of oligomers and polymers.12 Flexible coordination modes of the five-fold symmetric building block [CpRFe(η5-P5)] allow access to a large library of nanobowls, nano-sized capsules and fullerene-like spherical supramolecular assemblies.13 Analyses of the molecular structure of these compounds revealed the crucial coordinating ability of the halogens in Cu(I) halides in determining the final structures resulting from reactions with [CpRFe(η5-P5)].
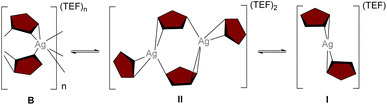
Polyphosphorus (Pn) ligand complexes exhibit dynamic behavior in solution when coinage metal salts of weakly coordinating anions (WCAs) are employed.14 For example, utilization of the Ag(I) salt of the weakly coordinating anion [TEF] ([TEF] = [Al{OC(CF3)3}4]) in the reaction with [Cp*Fe(η5-P5)] (A) yielded the 1D polymer [Ag{(η2:η1-A)}2]n[TEF]n (B).14 However, B dissolves (in CH2Cl2) by depolymerization to the mono-cation (I) and exists in dynamic equilibria with the di-cation (II) (Scheme 1), which is associated with a low enthalpy of dissociation of II to I (2 kJ mol−1 at 25 °C and 10 kJ mol−1 at −90 °C).15 The mono-cation I potentially creates a coordinatively unsaturated Ag(I) center which may be accessible by an additional organometallic ligand thereby making I a suitable precursor for expanded coordination networks.
Previously, we demonstrated the potential utilization of the dimeric aggregate, [Ag2{(μ,η2:2-(D)}2){μ,η2:η1:η1-(D)2}][TEF]2 (III)16 (D = [Cp2Mo2(CO)4(μ,η2-P2)]), with pyridine-based organic linkers, affording novel organometallic–organic hybrid polymers and molecular rectangles.17 However, in view of the so far unknown supramolecular aggregates with different organometallic linkers, the question arose if one could synthesise assemblies containing more than one type of organometallic Pn building block to form heteroleptic polymeric networks or discrete molecular aggregates by coordination to Lewis acidic metal centers. By taking advantage of the dynamic coordination abilities of A and D, we herein present a novel modular approach to design, for the first time, heteroleptic pnictogen rich organometallic hybrid materials composed of two different organometallic linker moieties.
Results and discussion
The competitive electronic properties of [Cp*Fe(η5-P5)] (A) and [Cp2Mo2(CO)4(μ,η2-P2)] (D) have been studied by employing DFT calculations (B3LYP/def2-TZVP) with respect to the ease of donating the lone pair electrons located on P atoms or the P–P bonds.18 The results show that the P-centred lone pairs in D are slightly lower in energy compared to those of A. Further, the σ(P–P) bond of D is located at high energy (energy gaps between the P-centred lone pair and σ(P–P) bond: 0.55 eV (D) and 0.23 eV (A), Table S2 and Fig. S7). These observations suggest D is a stronger ligand compared to A. Therefore, D was reacted with in situ prepared B in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CH2Cl2 under the exclusion of light which leads to the formation of 2, comprising the repeating units {Ag2(D)2(A)2} (Scheme 2 and Fig. 1a). Note that the addition of D in a higher ratio degrades the 1D polymer B and converts it into the homoleptic dimer III by replacing A. Moreover, compound 2 was unattainable if the dimer III was treated with an excess of A. Following the modular approach to 2, when D is treated with the homoleptic 1D polymer [Ag{(η2:η1-C)}2]n[TEF]n (1), obtained from [Cp′′Fe(η5-P5)] (Cp′′ = η5-C5H3tBu2-1,3) (C) and AgTEF in CH2Cl2 (Scheme S1, see the SI), it selectively produced another heteroleptic 1D polymer 3 consisting of the repeating unit {Ag2(D)2(C)} (Scheme 1 and Fig. 1b).
1 ratio in CH2Cl2 under the exclusion of light which leads to the formation of 2, comprising the repeating units {Ag2(D)2(A)2} (Scheme 2 and Fig. 1a). Note that the addition of D in a higher ratio degrades the 1D polymer B and converts it into the homoleptic dimer III by replacing A. Moreover, compound 2 was unattainable if the dimer III was treated with an excess of A. Following the modular approach to 2, when D is treated with the homoleptic 1D polymer [Ag{(η2:η1-C)}2]n[TEF]n (1), obtained from [Cp′′Fe(η5-P5)] (Cp′′ = η5-C5H3tBu2-1,3) (C) and AgTEF in CH2Cl2 (Scheme S1, see the SI), it selectively produced another heteroleptic 1D polymer 3 consisting of the repeating unit {Ag2(D)2(C)} (Scheme 1 and Fig. 1b).
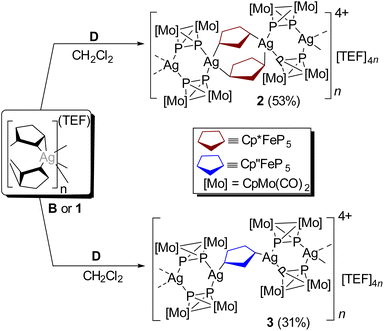 | ||
| Scheme 2 Synthesis of compounds [Ag2(D)2(A)2]n[TEF]2n (2) and [Ag2(D)2(C)]n[TEF]2n (3). Isolated yields are given in parentheses. B = [Ag{(η2:η1-A)}2]n[TEF]n and 1 = [Ag{(η2:η1-C)}2]n[TEF]n. | ||
Compounds 2 and 3 are obtained as red-orange crystalline plates suitable for single crystal X-ray structure analysis. The molecular structures of 2 and 3 unequivocally reveal the inclusion of both the organometallic complexes D and A or C, respectively, representing 2 and 3 as first examples of heteroleptic 1D polymers solely assembled from Ag(I) and two different polyphosphorus ligand complexes (Fig. 1a and b). The asymmetric unit of 2 consists of two Ag(I) ions bridged by two units of A in a 1,3-coordination mode resulting in a novel eight-membered macrocyclic {Ag2P6} ring. Each Ag(I) center is further connected to two units of D utilizing an η1-binding mode. The interplanar distance between two cyclo-P5 planes of A in 2 (distance between the cyclo-P5 ring centroids (4.311(10) Å) as well as the closest P⋯P distance (3.535(3) Å)) suggests a possible π–π stacking. The linear polycationic chain of 2 propagates in such a way that each Ag(I) centre adopts a distorted tetrahedral coordination sphere consisting of four P atoms. The solid-state structure of 3 is reminiscent to that of 2, the only difference being the {Ag2P4} nodes are now bridged by one cyclo-P5 unit of C, instead of two cyclo-P5 units, due to the steric influence of two tBu groups present in C. As a result, in 3 each Ag(I) centre possesses a distorted trigonal planar geometry coordinated by only three P atoms.
Interestingly, the six-membered {Ag2P4} ring motifs of III remain intact in the solid-state structures of 2 and 3 albeit they are more planar in 2 and slightly distorted in a kind of chair conformation in 3 (folding angle: 20.69(2)° in III, 4.52(12)° in 2, 15.6(2)° in 3).19 The six-membered {Ag2P4} and eight-membered {Ag2P6} motifs in 2 are arranged in alternate and virtually perpendicular positions along the chain propagation. The cationic 1D chain in 3 propagates in such a way that two {Ag2P4} nodes are bridged by a unit of C and the cyclo-P5 plane of C becomes coplanar to that of the {Ag2P4} plane (dihedral angle 3.37(7)°). Although the average P–P bond lengths of A and C in 2 (2.102(3)–2.130(3) Å) and 3 (2.072(4)–2.111(3) Å) are comparable to the uncoordinated ligands A (2.120(5) Å)20 and C (2.095(3)–2.117(3) Å),20b respectively, the P–P distances of D in 2 (2.099(3)–2.102(3) Å) and 3 (2.094(3)–2.090(4) Å) are slightly elongated compared to the free complex D (2.079(6) Å).21 The Ag(I)⋯Ag(I) separations of 4.7736(14) and 6.081(1) Å found in 2 and 4.344(3) Å observed in 3 certainly exclude any argentophilic interactions.
In the 31P{1H} NMR spectrum of 2 (Fig. S16) in CD2Cl2 at room temperature two sharp singlets (broadened at low temperature) at −98.1 and 152.4 ppm are detected, which are shifted markedly compared to the uncoordinated ligands D (δ = −43.7 ppm) and A (δ = 151 ppm). It is important to note for comparison that the 31P NMR chemical shifts of the homoleptic 1D polymer B and dimer III in CD2Cl2 are 154.2 and −96.1 ppm, respectively. Compound 3 shows a similar trend in 31P{1H} NMR chemical shifts (δ = −99.2 and 166.2 ppm, Fig. S23). The 1H, 13C{1H}, and 19F{1H} NMR spectra of 2 (Fig. S15, S18 and S19) and 3 (Fig. S22, S24 and S25) exhibit characteristic signals for the η5-coordinated Cp/Cp* ligands, as well as the counter-anion. The ESI mass spectrum of 2 in CH2Cl2 shows the mono-cation [Ag(A)(D)]+ as the base peak in the cation mode as well as peaks for smaller fragments (Fig. S21). Similarly, the mono-cationic fragment [Ag(C)(D)]+ was detected in the ESI-MS+ of 3 which was further confirmed by the calculated isotopic distribution pattern (Fig. S27). In the anionic mode, the peak with 100% intensity corresponds to the intact [TEF] anion. These results suggest that apparently dissociative dynamic equilibria may exist in solution between different monocationic species rendering the two/five-phosphorus nuclei equivalent on the NMR timescale (Fig. S17).
To test the generality of the above shown modular approach and enrich the library of the heteroleptic self-assembly of the organometallic complexes, other tetrahedral ligand complexes containing mixed pnictogens [{CpMo(CO)2}2(μ,η2-PE′)] (E′ = As (E), Sb (F)) and heavier pnictogens [{CpMo(CO)2}2(μ,η2-As2)] (G) were exploited. Incorporation of a heavier group 15 heteroatom as in E and F directed the P–E′ σ-bond to higher energy, which is even more pronounced in the heavier homo-diatomic congener G (Table S2 and Fig. S7). This indicates that σ(P–E′)/σ(As–As) allows an effective orbital overlap with the Ag(I) orbitals which have recently been recognized.22 Therefore, under identical conditions to the formation of 2, E was reacted with B to produce 4 exclusively (Scheme 3 and Fig. 2a). Compound 4 is the first example of a metallaparacyclophane composed solely of metal salt cations and two different types of organometallic polypnictogen ligand complexes.23 On the other hand, a molecular aggregate 5 containing an {Ag3} ring is obtained when F is reacted with B (Scheme 3 and Fig. 2b). In stark contrast upon moving to the heavier congener G under similar reaction conditions the formation of the 1D coordination polymer (6) is observed (Scheme 2). The structural modifications in the final assembled compounds can be attributed to the fine-tuned electronic properties of the tetrahedral complexes E–G that directed the interesting product formation.
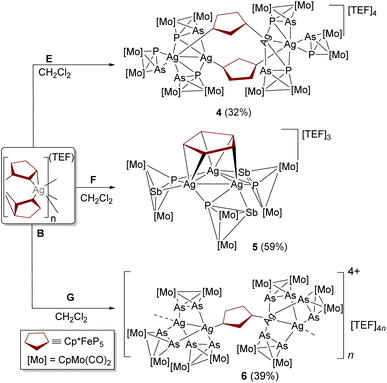 | ||
| Scheme 3 Synthesis of compounds [Ag4(A)2(E)6][TEF]4 (4), [Ag3(A)(F)3][TEF]3 (5) and [Ag4(A)(G)6]n[TEF]4n (6). Isolated yields are given in parentheses. | ||
 | ||
| Fig. 2 Molecular structure of (a) [Ag4(A)2(E)6][TEF]4 (4), (b) [(A)Ag3(F)3][TEF]3 (5) and (c) [(A)Ag2(G)3]n[TEF]2n (6). H atoms, anions, CO and Cp ligands attached to Mo are omitted for clarity. | ||
Compounds 4 and 5 crystallize in the triclinic space groups P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and P1, respectively. The X-ray structural analysis of 4 shows that each Ag atom is disordered in two positions and most of the positions of the P and As atoms of E show mixed occupancy. The asymmetric unit of 4 contains two independent cationic grown fragments (Fig. S4) having identical structural compositions [Ag4(A)2(E)6]. The side arms, [Ag2(E)3], are linked through two 1,3-coordinated molecules of A to construct the metallaparacyclophane core. The closest interplanar P–P distances (3.508(12)–3.562(16) Å), as well as the distance between the centroids (4.539(5)–4.586(5) Å) of the cyclo-P5 rings in 4, suggest intra-molecular π–π stacking between cyclo-P5 rings as found also in 2. The intermetallic Ag(I)⋯Ag(I) distances in 4 (2.954(2)–2.9893(15) Å) are significantly shorter than the sum of the van der Waals radii for two silver atoms (3.44 Å) suggesting the possible existence of argentophilic interactions.24 As a consequence, two different Ag(I) environments in 4 (penta- and hexa-coordinated) are observed which corroborated satisfactorily with the DFT computed (gas phase) optimized structure (see the SI).
and P1, respectively. The X-ray structural analysis of 4 shows that each Ag atom is disordered in two positions and most of the positions of the P and As atoms of E show mixed occupancy. The asymmetric unit of 4 contains two independent cationic grown fragments (Fig. S4) having identical structural compositions [Ag4(A)2(E)6]. The side arms, [Ag2(E)3], are linked through two 1,3-coordinated molecules of A to construct the metallaparacyclophane core. The closest interplanar P–P distances (3.508(12)–3.562(16) Å), as well as the distance between the centroids (4.539(5)–4.586(5) Å) of the cyclo-P5 rings in 4, suggest intra-molecular π–π stacking between cyclo-P5 rings as found also in 2. The intermetallic Ag(I)⋯Ag(I) distances in 4 (2.954(2)–2.9893(15) Å) are significantly shorter than the sum of the van der Waals radii for two silver atoms (3.44 Å) suggesting the possible existence of argentophilic interactions.24 As a consequence, two different Ag(I) environments in 4 (penta- and hexa-coordinated) are observed which corroborated satisfactorily with the DFT computed (gas phase) optimized structure (see the SI).
The triangular Ag3 core in 5 is stabilized by three F ligands, where each F ligand bridges (through its η2:η1-coordination mode) each side of the equilateral triangle (Ag⋯Ag distances (Å): 2.967(5), 2.958(4), 2.964(5); Ag–Ag–Ag angles (°): 60.04(10), 59.83(9), 60.13(11)). Although the observed Ag(I)⋯Ag(I) distances in 5 are similar to those of 4, these are slightly elongated compared to the homoleptic carbene-bridged species [(μ-NHC)3Ag3]3+ (Ag⋯Ag distances (Å): 2.7718(9), 2.7249(10), 2.7688(9) Å; Ag–Ag–Ag angles (°): 60.49(2), 60.60(2), 58.92(2)).25 The [Ag3(F)3] fragment is further anchored to A through novel 1,2,3,4,5-η2:η2:η1-coordination giving rise to a triple-decker sandwich complex, [(η5-C5Me5)Fe{μ,η5-P5}{η3-Ag3(F)3}]3+, where the cyclo-P5 ring is sandwiched between {Cp*Fe} and [Ag3(F)3] moieties. Such η5-coordination mode of the cyclo-P5 ring of A is only observed in the triple-decker sandwich complexes resulting from A.26
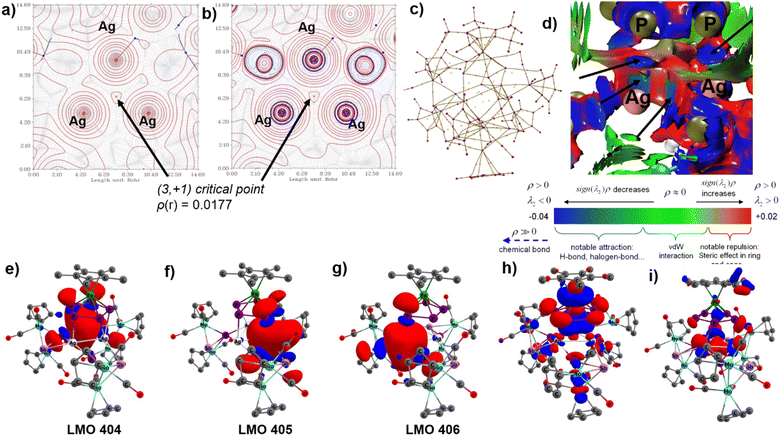
DFT calculations at the BP86(D3)/def2SVP level of theory disclosed that although the HOMO of 5 showed weaker antibonding interaction between the Ag3 and the P5 planes, the HOMO-62/HOMO-67 shows substantial Ag⋯Ag interactions (Fig. 3).18 The weak argentophilic interactions are further supported by the interaction region indicators surface plot as well as the extended-transition-state natural orbitals for chemical valence analysis (Fig. S8, interaction between the dZ2 orbitals of the Ag(I) ion with the hybrid orbitals of neighboring Ag(I) centers). Although the quantum theory of atoms in molecules analysis could not detect the bond critical points between two neighboring Ag(I) ions, the ring critical point at the center of the Ag3 ring was successfully located (Fig. 3a and b). The NICS(0) (nucleus independent chemical shift) value of −10 indicates a σ-aromaticity at the Ag3 plane (Fig. S9).
Compound 6 is isolated as a brown solid which crystallized in the monoclinic space group P21/n. According to the X-ray diffraction data (Fig. 2c), the dinuclear Ag(I) complex showed a paddle wheel arrangement with three bridging ligands of G around two Ag(I) centers. Two [Ag2(G)3] units are bridged by connector A. The Ag(I)–Ag(I) distance of 2.8922(9) Å is significantly shorter than those found in 4 and 5, respectively; however, it is comparable to those of compounds obtained only from G and Ag(I) ions.27
Consistent with the solid-state structure, the 31P{1H} NMR of 4 (Fig. S29) shows two resonances at 154.4 and −39.2 ppm corresponding to coordinated A and E units. Similar to 4, compound 5 also shows two signals at 155.6 and 35.1 ppm in the 31P{1H} NMR spectrum (Fig. S35). Note that distinct upfield 31P{1H} NMR chemical shifts related to E and F are present in 4 and 5, respectively, due to their coordination (uncoordinated ligands E (δ = 30.1) and F (δ = 90.7 ppm)).28,29 A low temperature 31P{1H} NMR spectrum of 5 at 193 K showed further splitting of the downfield signal (δ = 155.6 ppm) into three signals with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio corresponding to the cyclo-P5 ligand A (Fig. S36). The mass spectrum (ESI-MS+) in CH2Cl2 clearly shows intense peaks corresponding to [(A)2Ag], [(A)Ag(E)], and [(E)2Ag] fragments for 4 (Fig. S33) and [(A)2Ag], [(A)Ag(F)], and [(F)2Ag] fragments for 5 (Fig. S40). The 31P{1H} NMR spectrum of 6 (Fig. S42) displays a resonance at δ = 156.2 ppm corresponding to the coordinated ligand A, and the ESI mass spectrum supports the existence of [(G)2Ag] and [(A)Ag(G)] fragments in CH2Cl2 (Fig. S46). The negative mode mass spectrometry data as well as the 19F{1H} NMR displayed the existence of the [TEF]− anion in compounds 4–6.
1 ratio corresponding to the cyclo-P5 ligand A (Fig. S36). The mass spectrum (ESI-MS+) in CH2Cl2 clearly shows intense peaks corresponding to [(A)2Ag], [(A)Ag(E)], and [(E)2Ag] fragments for 4 (Fig. S33) and [(A)2Ag], [(A)Ag(F)], and [(F)2Ag] fragments for 5 (Fig. S40). The 31P{1H} NMR spectrum of 6 (Fig. S42) displays a resonance at δ = 156.2 ppm corresponding to the coordinated ligand A, and the ESI mass spectrum supports the existence of [(G)2Ag] and [(A)Ag(G)] fragments in CH2Cl2 (Fig. S46). The negative mode mass spectrometry data as well as the 19F{1H} NMR displayed the existence of the [TEF]− anion in compounds 4–6.
Conclusions
In conclusion, an innovative synthetic approach for the design of unique pnictogen-rich polymeric or discrete architectures has been explored. The results unveil the first example of heteroleptic self-assembly of organometallic complexes containing different bare pnictogen ligands connecting more than one Ag(I) ion. Fine-tuned electronic properties of the ditopic–tetrahedral complexes turned out to be crucial to generate different architectures, which were rationalised by DFT computations. Moreover, these aggregates also contain different pnictogen atoms within the connecting moieties, a unique feature of the products. Utilizing the solution phase labile behaviour of the assembled complexes, the results suggest new avenues to complex supramolecular systems/networks with fascinating architectures in the presence of other pnictogenyl compounds as well as diverse N donor organic linkers.Author contributions
B. M. conceived the idea for this research, designed and carried out the experiments as well as DFT calculations, interpreted the results, and wrote the manuscript. C. R. carried out the structural refinements. M. S. mentored the project, edited the manuscript and raised funding. All authors participated in and contributed to reviewing, editing, and revising the paper.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information: experimental details, single-crystal X-ray structural analyses, DFT calculations, NMR spectra and MS spectra. CCDC 2466232–2466237 contain the supplementary crystallographic data for this paper.30a–f See DOI: https://doi.org/10.1039/d5sc07723k.Acknowledgements
B. M. is grateful to the Alexander von Humboldt Foundation for a postdoctoral fellowship. Dr Michael Seidl is gratefully acknowledged for his initial crystallographic help. This work was supported by the Deutsche Forschungsgemeinschaft within the project Sche 384/44-1.Notes and references
- (a) P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS; (b) M. Fujita, Chem. Soc. Rev., 1998, 27, 417 RSC; (c) J. M. Lehn, Proc. Natl. Acad. Sci. U.S.A., 2002, 99, 4769 CrossRef PubMed; (d) B. H. Northrop, Y.-R. Zheng, K.-W. Chi and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554 CrossRef CAS PubMed.
- (a) G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240 CrossRef CAS PubMed; (b) J. G. Yu, L. Y. Sun, C. Wang, Y. Li and Y. F. Han, Chem.–Eur. J., 2021, 27, 1556 CrossRef CAS.
- (a) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418 CrossRef CAS PubMed; (b) D. Zhang, T. R. Ronson and J. R. Nitschke, Acc. Chem. Res., 2018, 51, 2423 CrossRef CAS PubMed; (c) S. Pullen, J. Tessarolo and G. H. Clever, Chem. Sci., 2021, 12, 7269 RSC; (d) J. E. M. Lewis, Chem. Commun., 2022, 58, 13873 RSC; (e) R. Banerjee, D. Chakraborty and P. S. Mukherjee, J. Am. Chem. Soc., 2023, 145, 7692 CrossRef CAS PubMed.
- (a) I. A. Riddell, M. M. J. Smulders, J. K. Clegg and J. R. Nitschke, Chem. Commun., 2011, 47, 457 RSC; (b) B. Zhang and J. N. H. Reek, Chem.–Eur. J., 2021, 16, 3851 CAS; (c) D. Chakraborty, R. Saha, J. K. Clegg and P. S. Mukherjee, Chem. Sci., 2022, 13, 11764 RSC.
- (a) J. Meeuwissen and J. N. H. Reek, Nat. Chem., 2010, 2, 615 CrossRef CAS PubMed; (b) B. Mitschke, M. Turberg and B. List, Chem, 2020, 6, 2515 CrossRef CAS; (c) M. Morimoto, S. M. Bierschenk, K. T. Xia, R. G. Bergman, K. N. Raymond and F. D. Toste, Nat. Catal., 2020, 3, 969 CrossRef CAS; (d) R. Saha, B. Mondal and P. S. Mukherjee, Chem. Rev., 2022, 122, 12244 CrossRef CAS PubMed; (e) D. Roy, S. Paul and J. Dasgupta, Angew. Chem., Int. Ed., 2023, 62, e202312500 CrossRef CAS PubMed.
- (a) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369 CrossRef CAS PubMed; (b) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728 RSC; (c) W. Wang, Y. X. Wang and H. B. Yang, Chem. Soc. Rev., 2016, 45, 2656 RSC; (d) C. Lescop, Acc. Chem. Res., 2017, 50, 885 CrossRef CAS PubMed; (e) W. X. Gao, H. J. Feng, B. B. Guo, Y. Lu and G. X. Jin, Chem. Rev., 2020, 120, 6288 CrossRef CAS PubMed.
- (a) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed; (b) D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563 CrossRef CAS PubMed; (c) A. Tarzia and K. E. Jelfs, Chem. Commun., 2022, 58, 3717 RSC; (d) T. K. Piskorz, V. Martí-Centelles, T. A. Young, P. J. Lusby and F. Duarte, ACS Catal., 2022, 12, 5806 CrossRef CAS PubMed; (e) A. J. McConnell, Chem. Soc. Rev., 2022, 51, 2957 RSC.
- (a) K. Acharyya, S. Mukherjee and P. S. Mukherjee, J. Am. Chem. Soc., 2013, 135, 554 CrossRef CAS; (b) N. Sinha, T. T. Y. Tan, E. Peris and F. E. Hahn, Angew. Chem., Int. Ed., 2017, 56, 7393 CrossRef CAS PubMed.
- (a) M. Ferrer, M. Mounir, O. Rossell, E. Ruiz and M. A. Maestro, Inorg. Chem., 2003, 42, 5890 CrossRef CAS PubMed; (b) S. Ghosh and P. S. Mukherjee, Inorg. Chem., 2009, 48, 2605 CrossRef CAS; (c) Y. Liu, F. Z. Liu and K. K. Yan, Angew. Chem., Int. Ed., 2022, 61, e202116980 CrossRef CAS; (d) K. Hema, A. B. Grommet, M. J. Białek, J. Wang, L. Schneider, C. Drechsler, O. Yanshyna, Y. Diskin-Posner, G. H. Clever and R. Klajn, J. Am. Chem. Soc., 2023, 145, 24755 CAS.
- I. Higler, P. Timmerman, W. Verboom and D. N. Reinhoudt, Eur. J. Org Chem., 1998, 2689 CrossRef CAS.
- (a) Y. Liang, Q. Jing, X. Li, L. Shi and K. Ding, J. Am. Chem. Soc., 2005, 127, 7694 CrossRef CAS PubMed; (b) M. Yoshizawa, J. Nakagawa, K. Kumazawa, M. Nagao, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2005, 44, 1810–1813 CrossRef CAS PubMed; (c) Z. He, W. Jiang and Ch. A. Schalley, Chem. Soc. Rev., 2015, 44, 779 RSC; (d) W. M. Bloch and G. H. Clever, Chem. Commun., 2017, 53, 8506 RSC; (e) J. E. M. Lewis and J. D. Crowley, ChemPlusChem, 2020, 85, 815 CrossRef CAS.
- (a) J. Bai, E. Leiner and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 783 CrossRef CAS; (b) L. J. Gregoriades, J. Bai, M. Sierka, G. Brunklaus, H. Eckert and M. Scheer, Chem.–Eur. J., 2005, 11, 2163 CrossRef PubMed; (c) M. E. Moussa, M. Fleischmann, E. Peresypkina, L. Dütsch, M. Seidl, G. Balázs and M. Scheer, Eur. J. Inorg. Chem., 2017, 3222 CrossRef PubMed.
- (a) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781 CrossRef CAS; (b) E. Peresypkina, C. Heindl, A. V. Virovets and M. Scheer, in Structure and Bonding, ed. S. Dehnen, Springer International Publishing, Switzerland, 2016, vol. 174, pp. 321–373 Search PubMed; (c) E. Peresypkina, A. V. Virovets and M. Scheer, Coord. Chem. Rev., 2021, 446, 213995 CrossRef CAS.
- (a) B. Mondal, C. Riesinger and M. Fleischmann, Eur. J. Inorg. Chem., 2024, 27, e202400213 CrossRef CAS; (b) I. Krossing, Chem.–Eur. J., 2001, 7, 490 CrossRef CAS.
- M. Scheer, L. J. Gregoriades, A. V. Virovets, W. Kunz, R. Neueder and I. Krossing, Angew. Chem., Int. Ed., 2006, 45, 5689 CrossRef CAS PubMed.
- M. Scheer, L. J. Gregoriades, M. Zabel, J. Bai, I. Krossing, G. Brunklaus and H. Eckert, Chem.–Eur. J., 2008, 14, 282 CrossRef CAS PubMed.
- B. Attenberger, S. Welsch, M. Zabel, E. Peresypkina and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 11516 CrossRef CAS.
- See the computational details in the SI.
- The folding angle is the dihedral angle between the planes containing P–Ag–P and the plane comprising the central four P atoms.
- (a) M. Scheer, L. J. Gregoriades, A. V. Virovets, W. Kunz, R. Neueder and I. Krossing, Angew. Chem., Int. Ed., 2006, 45, 5689 CrossRef CAS PubMed; (b) M. Detzel, G. Friedrich, O. J. Scherer and G. Wolmershäuser, Angew. Chem., Int. Ed., 1995, 34, 1321 CrossRef CAS.
- (a) M. Fleischmann, J. S. Jones, F. P. Gabbaï and M. Scheer, Chem. Sci., 2015, 6, 132 RSC; (b) O. J. Scherer, H. Sitzmann and G. Wolmershäuser, J. Organomet. Chem., 1984, 268, C9 CrossRef CAS.
- (a) M. E. Moussa, M. Seidl, G. Balázs, M. Hautmann and M. Scheer, Angew. Chem., Int. Ed., 2019, 58, 12903 CrossRef; (b) P. A. Shelyganov, M. E. Moussa, M. Seidl and M. Scheer, Angew. Chem., Int. Ed., 2023, 62, e202215650 CrossRef CAS PubMed.
- (a) C. M. Hartshorn and P. J. Steel, Inorg. Chem., 1996, 35, 6902 CrossRef CAS PubMed; (b) B. Nohra, S. Graule, C. Lescop and R. Réau, J. Am. Chem. Soc., 2006, 128, 3520 CrossRef CAS PubMed.
- (a) J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC; (b) H. Schmidbauer and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746 CrossRef.
- V. J. Catalano and M. A. Malwitz, Inorg. Chem., 2003, 42, 5483 CrossRef CAS PubMed.
- (a) B. Rink, O. J. Scherer, G. Heckmann and G. Wolmershäuser, Chem. Ber., 1992, 125, 1011 CrossRef CAS; (b) A. R. Kudinov, D. A. Loginov, Z. A. Starikova, P. V. Petrovskii, M. Corsini and P. Zanello, Eur. J. Inorg. Chem., 2002, 3018 CrossRef CAS; (c) C. Riesinger, D. Röhner, I. Krossing and M. Scheer, Chem. Commun., 2023, 59, 4495 RSC.
- M. E. Moussa, J. Schiller, E. Peresypkina, M. Seidl, G. Balázs, P. Shelyganov and M. Scheer, Chem.–Eur. J., 2020, 26, 14315 CrossRef CAS PubMed.
- J. E. Davies, L. C. Kerr, M. J. Mays, P. R. Raithby, P. K. Tompkin and A. D. Woods, Angew. Chem., Int. Ed., 1998, 37, 1428 CrossRef CAS.
- L. Dütsch, C. Riesinger, G. Balázs and M. Scheer, Chem.–Eur. J., 2021, 27, 8804 CrossRef PubMed.
- (a) CCDC 2466232: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ns9wn; (b) CCDC 2466233: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ns9xp; (c) CCDC 2466234: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ns9yq; (d) CCDC 2466235: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ns9zr; (e) CCDC 2466236: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nsb0t; (f) CCDC 2466237: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nsb1v.
| This journal is © The Royal Society of Chemistry 2025 |