Open Access Article
Open Access ArticleDMF/DMSO-catalyzed selective ring-opening polymerization of salicylate cyclic esters
Ge
Yao
a,
Jiyu
Liu
 a,
Hongjun
Fu
a,
Chunmei
Wang
a,
Guojie
Li
a,
Luya
Cao
b,
Xiaobo
Pan
a,
Hongjun
Fu
a,
Chunmei
Wang
a,
Guojie
Li
a,
Luya
Cao
b,
Xiaobo
Pan
 a and
Jincai
Wu
a and
Jincai
Wu
 *a
*a
aState Key Laboratory of Natural Product Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China. E-mail: wujc@lzu.edu.cn
bBaotou Research Institute of Rare Earths, China Northern Rare Earth (Group) High-tech Co., Ltd, Baotou 014030, People's Republic of China
First published on 27th October 2025
Abstract
Salicylate copolyesters are promising biomedical materials due to their biodegradability, biocompatibility, and bioactivity. Eliminating toxic catalyst/initiator residues in polymers is of great importance for their medical applications; however, achieving this remains a formidable challenge in controllable high-molecular-weight polyester synthesis in certain cases. In this work, dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) as weak hydrogen-bonding catalysts are found to efficiently catalyze the controllable ring-opening polymerization (ROP) of salicylate cyclic esters, where the phenolic ester bond in the monomer is selectively cleaved rather than that in the polymer chains. Therefore, high-molecular-weight salicylate copolyesters in a controlled manner (Mn, PSG = 177.5 kg mol−1 and Mn, PSMG = 530.0 kg mol−1) are successfully synthesized due to effective suppression of backbiting and intermolecular transesterification side reactions. DMF and DMSO are easily removed through devolatilization after polymerization, eliminating tedious purification steps and toxicity concerns for potential medical applications. In addition, the polymerization remains well-controlled even in the presence of water. Theoretical calculations indicate that DMF/DMSO catalyze the ROP through weak hydrogen interactions with the monomer and initiator/phenolic propagation species, which enables the selective ROP of salicylate cyclic esters.
Introduction
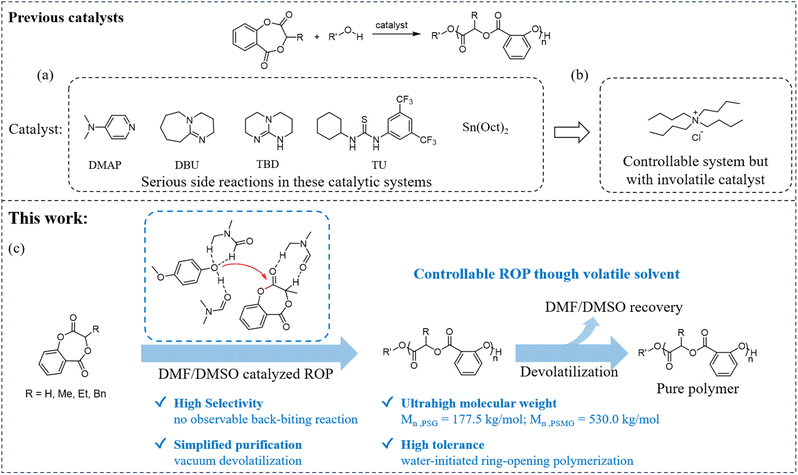
The production of biodegradable polymers reached 2.47 million tonnes in 2024 and will hit 5.73 million tonnes by 2029 (ref. 1) due to their potential to end plastic pollution.2,3 Among them, polyesters such as polylactide (PLA), polycaprolactone (PCL), poly(glycolic acid) (PGA), poly(hydroxy alkanoates), and poly(butylene adipate-co-terephthalate) are the primary ones.4–13 Some of them are widely applied in the drug, food, and healthcare industries due to their good biocompatibility. For medical applications, catalyst residues in polymers are subject to strict limits; for example, tin residues in medical-grade polylactide are required to be below 20 ppm by the U.S. Food and Drug Administration (FDA).14,15 Therefore, non-toxic metal and metal-free catalysts are widely explored.16–28 However, the toxicity of organic catalysts and most ligands of non-toxic metal complexes remains ambiguous.Salicylate-based polyesters are promising medical polymers that exhibit better biodegradability than polylactide.4,29,30 In addition, salicylate-based copolyesters can demonstrate good antimicrobial activity.31 However, the synthesis methodology for high-molecular-weight salicylate-based polyesters still requires further exploration because the highly active phenolic ester bonds in monomer and polymer chains lead to serious side reactions during the ROP of related cyclic esters, such as intermolecular transesterification and backbiting reactions when using tin(II) 2-ethylhexanoate (Sn(Oct)2)30 or 4-dimethylaminopyridine (DMAP)32 as a catalyst. Therefore, synthesizing high-molecular-weight salicylate-based polyesters is challenging. Recently, it was reported that tetrabutylammonium halides can selectively ring-open monomers while remaining inactive toward polymer chains in bulk polymerization, and high-molecular-weight lactic acid salicylate copolyester (PSMG with Mn = 361.8 kg mol−1 and narrow dispersity Đ = 1.25) was achieved.33 However, it is difficult to completely remove tetrabutylammonium chloride from the polymer (tetrabutylammonium chloride can decompose and volatilize above 170 °C).34 At such high temperatures, salicylate-based copolyesters (such as PSMG) tend to undergo a certain degree of degradation, which prevents the complete removal of tetrabutylammonium halides.33
In this work, DMF/DMSO was fortunately discovered as a green catalyst for the ROP of salicylate cyclic esters (Scheme 1). In this catalytic system, DMF/DMSO shows good selectivity toward the cleavage of the phenolic ester bond in the monomer rather than in polymer chains. Controllable high-molecular-weight copolyesters can also be achieved (PSMG with Mn = 198 kg mol−1 and Đ = 1.25 using DMF and Mn = 530.0 kg mol−1 and Đ = 1.28 using DMSO). In addition, traces of water do not inhibit the controllability of the ROP of salicylate cyclic esters; replacing phenolic initiators with water still enables controlled ROP. More importantly, DMF/DMSO can be easily removed via a simple devolatilization process at room temperature. In this system, DMF/DMSO acts as both solvent and the catalyst and can be recycled through devolatilization under vacuum.
Results and discussion
Catalytic ROP of SMG, SEG, SG, and SBG using DMF/DMSO as solvent and catalytic species
Based on previous controllable ROP of salicylate cyclic esters catalyzed by tetrabutylammonium halides via hydrogen bond interactions between the catalyst, monomer, and initiator, the weak activation of the monomer is necessary to avoid activation of the phenolic ester bond in polymer chains and to selectively cleave the phenolic ester bond in the monomer rather than in polymer chains. After careful literature research, it was found that DMF35,36 and DMSO37–39 can act as weak Lewis bases to stabilize cationic intermediates and catalyze some organic reactions, for example, by raising intramolecular hydroarylation of alkene with simple arene,35 by acting as Lewis base catalysts in the chlorination of (hetero)arenes,38,39 and sometimes by playing a synergistic role with a base in the initiation of the radical process of α-arylation of enolizable aryl ketones.36Thus, by employing DMF as both the solvent and catalyst, the ROP of lactic acid salicylate copolyester (PSMG) was conducted at 40 °C with p-methoxyphenol as the initiator, maintaining a ratio of [I]0/[PSMG]0 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and a monomer concentration in DMF of 1 M. Under these conditions, 90% of the monomer was converted after 1 hour (Table S1, entry 2). Both the NMR molecular weight (Fig. S1) and the gel permeation chromatography molecular weight were consistent with the theoretical value of 18.0 kg mol−1. When the temperature was decreased to 20 °C, the polymerization process became significantly slower (Table S1, entry 1), indicating that the energy barrier for this polymerization is not particularly low. Upon changing the solvent to a mixture of DMF and toluene, the polymerization was almost undetectable within 2.5 hours when the DMF content was below 20% (Table S2, entry 6). At a DMF proportion of 60 vol%, 56% of the monomer was converted under identical conditions, and the conversion gradually increased with higher DMF concentrations (Table S2, entries 1–5). All synthesized polymers exhibited Mn values that closely matched theoretical predictions and displayed narrow dispersity (Đ = 1.2–1.3). However, the Size-Exclusion Chromatography (SEC) traces revealed shoulder peaks when 60–80 vol% DMF was used as the solvent (Fig. S2c–e), which disappeared when the DMF concentration reached ≥90 vol%, resulting in monomodal distributions (Fig. S2a and b). Therefore, DMF concentrations below 90 vol% not only reduced catalytic efficiency but also raised side reactions, likely due to insufficient activation of both the initiator and the monomer. Polymerization nearly ceased below 20 vol% DMF, further emphasizing the essential role of high DMF content in maintaining polymerization control.
100 and a monomer concentration in DMF of 1 M. Under these conditions, 90% of the monomer was converted after 1 hour (Table S1, entry 2). Both the NMR molecular weight (Fig. S1) and the gel permeation chromatography molecular weight were consistent with the theoretical value of 18.0 kg mol−1. When the temperature was decreased to 20 °C, the polymerization process became significantly slower (Table S1, entry 1), indicating that the energy barrier for this polymerization is not particularly low. Upon changing the solvent to a mixture of DMF and toluene, the polymerization was almost undetectable within 2.5 hours when the DMF content was below 20% (Table S2, entry 6). At a DMF proportion of 60 vol%, 56% of the monomer was converted under identical conditions, and the conversion gradually increased with higher DMF concentrations (Table S2, entries 1–5). All synthesized polymers exhibited Mn values that closely matched theoretical predictions and displayed narrow dispersity (Đ = 1.2–1.3). However, the Size-Exclusion Chromatography (SEC) traces revealed shoulder peaks when 60–80 vol% DMF was used as the solvent (Fig. S2c–e), which disappeared when the DMF concentration reached ≥90 vol%, resulting in monomodal distributions (Fig. S2a and b). Therefore, DMF concentrations below 90 vol% not only reduced catalytic efficiency but also raised side reactions, likely due to insufficient activation of both the initiator and the monomer. Polymerization nearly ceased below 20 vol% DMF, further emphasizing the essential role of high DMF content in maintaining polymerization control.
With the optimized catalytic system, the polymerization of the SMG monomer was systematically investigated at a constant monomer concentration ([M]0 = 1 M) and varying [I]0/[M]0 ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 to 1
25 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (Table 1, entries 1–7). The experimental Mn values closely matched the theoretical predictions across the full [SMG]0/[I]0 range, exhibiting linear growth behavior (Fig. 1a). SEC analysis further confirmed narrow dispersity (Đ ≈ 1.25) for all synthesized polymers, indicating controlled polymerization. Under optimal conditions ([I]0/[M]0 = 1
1000 (Table 1, entries 1–7). The experimental Mn values closely matched the theoretical predictions across the full [SMG]0/[I]0 range, exhibiting linear growth behavior (Fig. 1a). SEC analysis further confirmed narrow dispersity (Đ ≈ 1.25) for all synthesized polymers, indicating controlled polymerization. Under optimal conditions ([I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), PSMG with Mn = 198.0 kg mol−1 was successfully synthesized (Table 1, entry 7 and Fig. S11). After completion of the polymerization, direct solvent recovery was performed. Initial vacuum treatment at room temperature yielded a polymer with approximately 3% residual solvent, as determined by 1H NMR analysis (Fig. S16). This corresponds to a solvent recovery efficiency of 97.8%. The polymer was heated to 130 °C under continued vacuum, after which no solvent signal was observed in the 1H NMR spectrum (Fig. S17) to remove residual solvent. This method successfully enabled direct solvent recovery and efficient solvent removal. At an initial [I]0/[M]0 ratio of 1
1000), PSMG with Mn = 198.0 kg mol−1 was successfully synthesized (Table 1, entry 7 and Fig. S11). After completion of the polymerization, direct solvent recovery was performed. Initial vacuum treatment at room temperature yielded a polymer with approximately 3% residual solvent, as determined by 1H NMR analysis (Fig. S16). This corresponds to a solvent recovery efficiency of 97.8%. The polymer was heated to 130 °C under continued vacuum, after which no solvent signal was observed in the 1H NMR spectrum (Fig. S17) to remove residual solvent. This method successfully enabled direct solvent recovery and efficient solvent removal. At an initial [I]0/[M]0 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis of the resulting polymer (Table 1, entry 1) displayed a series of well-defined peak clusters at m/z = 123 + n × 192 + 1 + 23 (Fig. 1b), corresponding to polymer chains composed of n repeating SMG units (Δm/z = 192 matching the monomer molecular weight). The terminated p-methoxyphenoxy end group, verified by both NMR and MALDI-TOF MS, confirmed the formation of linear polymers with controlled architecture. No cyclic polymers were detected in this MALDI-TOF MS, indicating that side reactions were minimal in this catalytic system. These findings indicated that DMF, serving as both solvent and the catalytic medium, can effectively and selectively activate phenolic ester groups in the monomer, enabling the controlled synthesis of high molecular weight polymers.
25, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis of the resulting polymer (Table 1, entry 1) displayed a series of well-defined peak clusters at m/z = 123 + n × 192 + 1 + 23 (Fig. 1b), corresponding to polymer chains composed of n repeating SMG units (Δm/z = 192 matching the monomer molecular weight). The terminated p-methoxyphenoxy end group, verified by both NMR and MALDI-TOF MS, confirmed the formation of linear polymers with controlled architecture. No cyclic polymers were detected in this MALDI-TOF MS, indicating that side reactions were minimal in this catalytic system. These findings indicated that DMF, serving as both solvent and the catalytic medium, can effectively and selectively activate phenolic ester groups in the monomer, enabling the controlled synthesis of high molecular weight polymers.
| Entry | [I]0/[M]0 | T/°C | t/h | Conv. b/% | M n,calcd (g mol−1) | M n,NMR (g mol−1) | M n,obsd (g mol−1) | Đ |
|---|---|---|---|---|---|---|---|---|
| a Conditions: under a dry N2 atmosphere, DMF, initiator = p-methoxyphenol, [M]0 = 1 M, monomer = SMG. b Determined using the 1H NMR spectrum. c Calculated from MSMG × [SMG]0/[I]0 × conversion yield + Mp-methoxyphenol. d Absolute values of Mn and Đ determined by SEC in THF. e SEG. | ||||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
40 | 0.5 | 97 | 4700 | 6700 | 6300 | 1.16 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
40 | 1 | 90 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.28 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
40 | 2 | 97 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.31 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
40 | 2.5 | 99 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.25 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
40 | 3 | 99 | 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.23 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
40 | 3.5 | 99 | 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 155![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.29 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 3.5 | 97 | 182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.25 |
| 8e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40 | 3 | 96 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.18 |
| 9e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 4 | 98 | 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.31 |
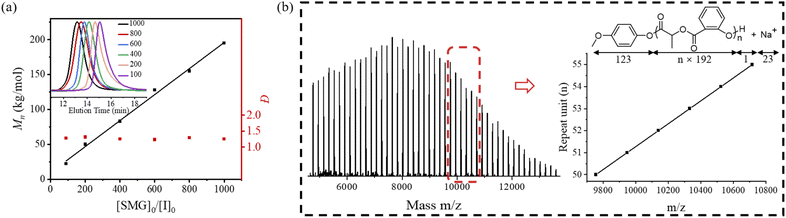 | ||
| Fig. 1 (a) Plots of the relationship between Mn and molecular weight distribution Đ of the polymer versus [SMG]0/[I]0 ratios, and representative SEC traces of PSMG (Table 1, entries 2–7); the black fitted line exhibits excellent correlation (R2 = 0.99). (b) MALDI-TOF MS of PSMG was obtained in THF using DMF as a catalyst (Table 1, entry 1). | ||
Methyl 2-((2-acetoxypropanoyl)oxy)benzoate (MAOB) was designed as a model compound representing the phenolic ester bond in polymer chains (Scheme S3, Fig. S3 and 4) to clarify the high selectivity of the DMF solvent system toward the cleavage of phenolic ester bonds in monomers rather than those within polymer chains. The chiral methine proton signal of MAOB at 5.37–5.41 ppm shifted to 5.22–5.26 ppm in the corresponding 4-methoxyphenyl-2-acetoxypropanoate (4-MAP) after the methyl salicylate group was substituted by the p-methoxyphenoxy group through a transesterification reaction between MAOB and p-methoxyphenol (Fig. 2 and S5–7). No reaction was observed between p-methoxyphenol and MAOB in DMF at 40 °C, even after 5 hours, as evidenced by the unchanged NMR spectrum of MAOB. This result indicates that DMF cannot catalyze the cleavage of phenolic ester bonds in linear polymer chains. In contrast, under identical conditions with DMAP as a catalyst, 23% of MAOB was converted to 4-MAP, indicating a pronounced transesterification side reaction. These results demonstrate that high concentrations of DMF selectively activate phenolic ester bonds in cyclic SMG monomers while preserving those within polymer chains. This high selectivity ensures chain-end stability during propagation and is critical for synthesizing high molecular weight PSMG with narrow dispersity.
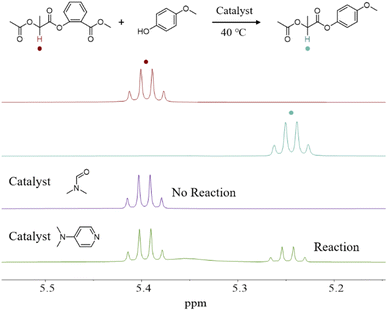 | ||
| Fig. 2 Methine proton signals in 1H NMR spectra for exploring the phenolic ester bond cleavage reaction of MAOB using DMF and DMAP as catalysts. | ||
Then the ROP behaviors of other similar cyclic monomers, including salicylic glycolide (SG) (Scheme S1 and Fig. S8), salicylic ethyl glycolide (SEG) (Scheme S2 and Fig. S12), and salicylic benzyl glycolide (SBG) (Scheme S2 and Fig. S14), were systematically evaluated. Using DMF as a catalyst, the ROP of SEG exhibited good controllability. When the initiator-to-monomer ratio ([I]0/[M]0) varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 to 1
500 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (Table 1, entries 8–9; Fig. S18a and b), the Mn values of poly(SEG) (PSEG) (Fig. S13) were consistent with theoretical predictions, achieving Mn = 217.0 kg mol−1 under 1
1000 (Table 1, entries 8–9; Fig. S18a and b), the Mn values of poly(SEG) (PSEG) (Fig. S13) were consistent with theoretical predictions, achieving Mn = 217.0 kg mol−1 under 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 conditions with narrow dispersity (Đ = 1.31). However, experimental observations revealed that the DMF/p-methoxyphenol initiating system failed to promote the ROP of SG (Table S3, entry 1). When DMF was replaced with DMSO, due to its higher catalytic activity, SG polymerization proceeded effectively at [I]0/[M]0 = 1
1000 conditions with narrow dispersity (Đ = 1.31). However, experimental observations revealed that the DMF/p-methoxyphenol initiating system failed to promote the ROP of SG (Table S3, entry 1). When DMF was replaced with DMSO, due to its higher catalytic activity, SG polymerization proceeded effectively at [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (Table 2, entry 1). DFT calculations (Fig. S40) showed that the transition state of the SG monomer in DMF (TSpath6) is approximately 2 kcal mol−1 higher than that in DMSO (TSpath7). Therefore, the lower energy barrier in DMSO facilitates more efficient ring-opening polymerization of SG. The carbonyl carbon in TSpath7 of the DMSO system carries a charge of +0.697, which is higher than that in TSpath6 of the DMF system (+0.681), indicating that the phenolic ester bond of SG is more activated in DMSO than in DMF. In addition, with DMSO as a solvent, PSG maintained homogeneous dissolution throughout the ROP process, which represents a significant advancement considering its insolubility in THF/toluene. Systematic evaluation of the polymerization with [M]0/[I]0 ratios ranging from 1
25 (Table 2, entry 1). DFT calculations (Fig. S40) showed that the transition state of the SG monomer in DMF (TSpath6) is approximately 2 kcal mol−1 higher than that in DMSO (TSpath7). Therefore, the lower energy barrier in DMSO facilitates more efficient ring-opening polymerization of SG. The carbonyl carbon in TSpath7 of the DMSO system carries a charge of +0.697, which is higher than that in TSpath6 of the DMF system (+0.681), indicating that the phenolic ester bond of SG is more activated in DMSO than in DMF. In addition, with DMSO as a solvent, PSG maintained homogeneous dissolution throughout the ROP process, which represents a significant advancement considering its insolubility in THF/toluene. Systematic evaluation of the polymerization with [M]0/[I]0 ratios ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 to 1
25 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in DMSO ([M]0 = 1 M, Table 2, entries 1–4) revealed linear molecular weight growth and narrow dispersity (Đ < 1.24). With [I]0/[M]0 at 1
1000 in DMSO ([M]0 = 1 M, Table 2, entries 1–4) revealed linear molecular weight growth and narrow dispersity (Đ < 1.24). With [I]0/[M]0 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (Table 2, entry 4 and Fig. S18c), the Mn of PSG (Fig. S9) achieved a record value of 177.5 kg mol−1, surpassing literature-reported values.32 Therefore, DMSO promotes polymerization due to its dual functionality as both a catalytic species and a solubilizing agent. Unlike SG, SMG, and SEG, the solubility of the SBG monomer in DMF is very low (monomer-insoluble/polymer-soluble), leading to anomalous SEC profiles (shoulder peaks, Table S3, entries 2 and 3, Fig. S19a and b) in the DMF catalytic system, possibly due to localized concentration gradients. In contrast, DMSO effectively resolved the solubility limitations of SBG (Table 2, entries 5 and 6), enabling the controlled synthesis of poly(SBG) (PSBG) (Fig. S15) with an Mn of up to 286.0 kg mol−1 (Đ = 1.33) (Table 2, entry 6 and Fig. S18d). Under optimized conditions ([I]0/[M]0 = 1
1000 (Table 2, entry 4 and Fig. S18c), the Mn of PSG (Fig. S9) achieved a record value of 177.5 kg mol−1, surpassing literature-reported values.32 Therefore, DMSO promotes polymerization due to its dual functionality as both a catalytic species and a solubilizing agent. Unlike SG, SMG, and SEG, the solubility of the SBG monomer in DMF is very low (monomer-insoluble/polymer-soluble), leading to anomalous SEC profiles (shoulder peaks, Table S3, entries 2 and 3, Fig. S19a and b) in the DMF catalytic system, possibly due to localized concentration gradients. In contrast, DMSO effectively resolved the solubility limitations of SBG (Table 2, entries 5 and 6), enabling the controlled synthesis of poly(SBG) (PSBG) (Fig. S15) with an Mn of up to 286.0 kg mol−1 (Đ = 1.33) (Table 2, entry 6 and Fig. S18d). Under optimized conditions ([I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) in the DMSO solvent catalytic system, the molecular weight of PSMG reached a high value of 530.0 kg mol−1 (Đ = 1.31) (Table 2, entry 8; Fig. S18f). However, the observed Mn,obs significantly exceeded the theoretical predictions for PSMG (Table 2, entries 7 and 8), which can be attributed to the high ring strain of the SMG monomer, endowing it with exceptionally high polymerization activity and enabling rapid chain propagation within a short timeframe. In addition, the dense solvation shell formed by highly polar DMSO molecules around the initiator could have caused the initiation rate to be slower than the propagation rate during ROP of SMG. Hence, the dependence of Mn on conversion was analyzed. Table S4 and Fig. S20 indicate that at low monomer conversion, the polymers exhibited relatively broad dispersities ranging from 1.7 to 1.9 (Table S4, entries 1 and 2, Fig. S21a and b). As conversion increased, dispersity gradually decreased to approximately 1.19 (Table S4, entry 4 and Fig. S21d). This observation indicates that during the early stage of polymerization, the initiator efficiency was relatively low, preventing complete initiation within a short time, while the high monomer reactivity led to rapid chain growth. Therefore, broad dispersity appeared in the SEC traces. As polymerization progressed and monomer conversion increased, the polymer chains reached higher molecular weights, and the non-uniform initiation in the early stage was masked by rapid chain growth and the formation of high molecular weight species, leading to narrow dispersity in the SEC traces. This set of experiments further confirmed that in DMSO, the initiator efficiency was suppressed, whereas high monomer reactivity drives rapid polymer chain growth, resulting in Mn,obsd values deviating from Mn,calcd values.
2000) in the DMSO solvent catalytic system, the molecular weight of PSMG reached a high value of 530.0 kg mol−1 (Đ = 1.31) (Table 2, entry 8; Fig. S18f). However, the observed Mn,obs significantly exceeded the theoretical predictions for PSMG (Table 2, entries 7 and 8), which can be attributed to the high ring strain of the SMG monomer, endowing it with exceptionally high polymerization activity and enabling rapid chain propagation within a short timeframe. In addition, the dense solvation shell formed by highly polar DMSO molecules around the initiator could have caused the initiation rate to be slower than the propagation rate during ROP of SMG. Hence, the dependence of Mn on conversion was analyzed. Table S4 and Fig. S20 indicate that at low monomer conversion, the polymers exhibited relatively broad dispersities ranging from 1.7 to 1.9 (Table S4, entries 1 and 2, Fig. S21a and b). As conversion increased, dispersity gradually decreased to approximately 1.19 (Table S4, entry 4 and Fig. S21d). This observation indicates that during the early stage of polymerization, the initiator efficiency was relatively low, preventing complete initiation within a short time, while the high monomer reactivity led to rapid chain growth. Therefore, broad dispersity appeared in the SEC traces. As polymerization progressed and monomer conversion increased, the polymer chains reached higher molecular weights, and the non-uniform initiation in the early stage was masked by rapid chain growth and the formation of high molecular weight species, leading to narrow dispersity in the SEC traces. This set of experiments further confirmed that in DMSO, the initiator efficiency was suppressed, whereas high monomer reactivity drives rapid polymer chain growth, resulting in Mn,obsd values deviating from Mn,calcd values.
| Entry | [I]0/[M]0 | T/°C | t/h | Conv. b/% | M n,calcd (g mol−1) | M n,NMR (g mol−1) | M n,obsd (g mol−1) | Đ |
|---|---|---|---|---|---|---|---|---|
| a Conditions: under a dry N2 atmosphere, DMSO, initiator = p-methoxyphenol, [M]0 = 1 M, monomer = SG. b Determined using the 1H NMR spectrum. c Calculated from MSMG × [SG]0/[I]0 × conversion yield + Mp-methoxyphenol. d Absolute values of Mn and Đ determined by SEC in mixed solvent CHCl3 +30% HFIP (SG) and in THF (SBG, SMG, and SEG). e SBG. f SMG. g SEG. | ||||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
40 | 0.5 | 95 | 4200 | 4500 | 4250 | 1.05 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
40 | 1 | 97 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.11 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40 | 3 | 97 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.15 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 5 | 99 | 176![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 220 |
— | 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.24 |
| 5e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40 | 1 | 94 | 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.20 |
| 6e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 1.5 | 98 | 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.33 |
| 7f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 1 | 93 | 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 371![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.25 |
| 8f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
40 | 4 | 75 | 288![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.28 |
| 9g | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 1.5 | 98 | 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 288![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.30 |
Melt polymerization represents a scalable production technology consistent with the principles of green chemistry and holds considerable industrial importance. DMSO was utilized to construct a solvation environment suitable for high-temperature reactions due to its high boiling point (189 °C) and low volatility to evaluate the applicability of solvent-catalyzed systems in melt polymerization. Controlled polymerization of SMG was achieved at an initiator-to-solvent ratio ([I]0/[DMSO]0) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 and [I]0/[M]0 = 1
140 and [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (a high monomer concentration [M]0 = 80 M), producing PSMG with Mn = 101.0 kg mol−1 and Đ = 1.14 (Table S5, entry 9). These findings confirm that even under molten-state high-temperature conditions, DMSO effectively stabilizes active species through its solvation effects, suppressing side reactions.
1000 (a high monomer concentration [M]0 = 80 M), producing PSMG with Mn = 101.0 kg mol−1 and Đ = 1.14 (Table S5, entry 9). These findings confirm that even under molten-state high-temperature conditions, DMSO effectively stabilizes active species through its solvation effects, suppressing side reactions.
Mechanistic studies
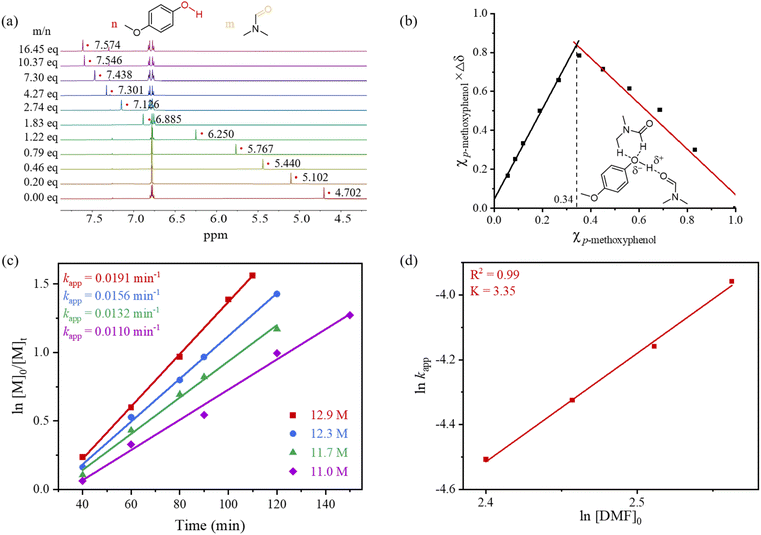
The interaction between DMF and the p-methoxyphenol initiator was examined using 1H NMR titration in chloroform-d (CDCl3) (Fig. 3a and Table S6) to gain further insight into the polymerization mechanism. The hydroxyl proton signal of p-methoxyphenol appeared as a single resonance, which exhibited significant downfield shifts upon incremental DMF addition (0.20–16.45 eq.). These shifts indicate dynamic hydrogen bonding between the phenolic OH and DMF, accompanied by rapid proton exchange on the 1H NMR timescale. Job plot40,41 analysis revealed a maximum change at a mole fraction value of approximately 0.34 (Fig. 3b), corresponding to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry between p-methoxyphenol and DMF. This observation demonstrates that two DMF molecules simultaneously coordinate with the initiator. Previous studies have shown that phenolic hydroxyl groups exhibit enhanced acidity in polar aprotic solvents.42–44 In such systems, solvent molecules form a solvation shell around the polarized hydroxyl group of p-methoxyphenol through hydrogen bonding interactions, attenuating the coulombic interaction between the oxygen and proton while stabilizing the ionized state (schematic structural representation in Fig. 3b). In the present case, dynamic coordination with DMF compensates for entropic penalties through a synergistic effect, necessitating excess DMF (≥10.37 eq.) for full initiator activation. This cooperative solvation mechanism accounts for the requirement of high DMF concentrations to ensure effective initiator activation, overcoming the weak hydrogen-bonding ability of individual DMF molecules and enhancing their collective catalytic performance. This mechanism closely parallels that observed in recent studies on hexafluoroisopropanol (HFIP)-catalyzed cationic polymerization of vinyl monomers.45 Similarly, 1H NMR titration in CDCl3 demonstrated that DMSO interacts with p-methoxyphenol in a manner analogous to that of DMF (Fig. S22 and Table S7). Job plot analysis revealed a maximum change at a mole fraction value of approximately 0.38 (Fig. S23), confirming a 1
2 binding stoichiometry between p-methoxyphenol and DMF. This observation demonstrates that two DMF molecules simultaneously coordinate with the initiator. Previous studies have shown that phenolic hydroxyl groups exhibit enhanced acidity in polar aprotic solvents.42–44 In such systems, solvent molecules form a solvation shell around the polarized hydroxyl group of p-methoxyphenol through hydrogen bonding interactions, attenuating the coulombic interaction between the oxygen and proton while stabilizing the ionized state (schematic structural representation in Fig. 3b). In the present case, dynamic coordination with DMF compensates for entropic penalties through a synergistic effect, necessitating excess DMF (≥10.37 eq.) for full initiator activation. This cooperative solvation mechanism accounts for the requirement of high DMF concentrations to ensure effective initiator activation, overcoming the weak hydrogen-bonding ability of individual DMF molecules and enhancing their collective catalytic performance. This mechanism closely parallels that observed in recent studies on hexafluoroisopropanol (HFIP)-catalyzed cationic polymerization of vinyl monomers.45 Similarly, 1H NMR titration in CDCl3 demonstrated that DMSO interacts with p-methoxyphenol in a manner analogous to that of DMF (Fig. S22 and Table S7). Job plot analysis revealed a maximum change at a mole fraction value of approximately 0.38 (Fig. S23), confirming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry between p-methoxyphenol and DMSO.
2 binding stoichiometry between p-methoxyphenol and DMSO.
The ROP of SMG was conducted with four different DMF concentrations (100, 95, 90, and 85 vol% DMF in toluene), corresponding to 12.9, 12.3, 11.7, and 11.0 M, respectively, while maintaining a DMF volume fraction >80 vol% to suppress side reactions to determine the kinetic order of DMF in the catalytic system. Real-time monitoring of the polymerization (Fig. 3c) enabled determination of apparent rate constants (kapp) through first-order kinetic fitting on the monomer. Linear regression of −ln(kapp) versus −ln([DMF]0) yielded a slope of 3.35 (R2 = 0.99; Fig. 3d), indicating a third-order dependence on the DMF concentration. Together with the previously established 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solvent–initiator binding stoichiometry, these results support a trimolecular cooperative activation model in which the third DMF molecule likely interacts with the monomer via a hydrogen-bonding interaction (see DFT calculations, vide infra).
2 solvent–initiator binding stoichiometry, these results support a trimolecular cooperative activation model in which the third DMF molecule likely interacts with the monomer via a hydrogen-bonding interaction (see DFT calculations, vide infra).
Fourier-transform infrared spectroscopy (FT-IR) was employed to probe dynamic interactions between DMF and the p-methoxyphenol initiator (Fig. S24) to substantiate the proposed mechanism. A gradient concentration protocol was applied. The O–H stretching vibration of p-methoxyphenol at 3367.53 cm−1 exhibited a pronounced red shift when the equivalents of DMF to p-methoxyphenol increased to 10.37 (Δν ≈ −120 cm−1, Fig. S24b). Simultaneously, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of neat DMF at 1670.16 cm−1 displayed an initial red shift (Δν ≈ −8 cm−1) at low DMF equivalents to p-methoxyphenol (0.203 eq.; Fig. S24c), followed by a gradual return to the initial wavenumber (1670.16 cm−1) as the DMF equivalents increased. Therefore, strong hydrogen bonding of O–H⋯O
O stretching vibration of neat DMF at 1670.16 cm−1 displayed an initial red shift (Δν ≈ −8 cm−1) at low DMF equivalents to p-methoxyphenol (0.203 eq.; Fig. S24c), followed by a gradual return to the initial wavenumber (1670.16 cm−1) as the DMF equivalents increased. Therefore, strong hydrogen bonding of O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C between the phenolic hydroxyl and the carbonyl group of DMF is evident. The C–O stretching vibration of p-methoxyphenol at 1231.06 cm−1 exhibited a distinct blue shift (Δν ≈ +4 cm−1, Fig. S24d), further indicating the DMF coordination effect. This shift plateaued between 1.83 and 2.74 DMF equivalents, corroborating the [I]0
C between the phenolic hydroxyl and the carbonyl group of DMF is evident. The C–O stretching vibration of p-methoxyphenol at 1231.06 cm−1 exhibited a distinct blue shift (Δν ≈ +4 cm−1, Fig. S24d), further indicating the DMF coordination effect. This shift plateaued between 1.83 and 2.74 DMF equivalents, corroborating the [I]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DMF]0 = 1
[DMF]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry determined by Job plot analysis through 1H NMR titration. This spectral evolution reflects a dynamic coordination equilibrium, wherein excess DMF molecules offset entropic penalties through transient interactions. Collectively, these findings demonstrate that DMF dynamically coordinates with p-methoxyphenol to enhance oxyanion electronegativity and nucleophilicity while maintaining rapid exchange.
2 stoichiometry determined by Job plot analysis through 1H NMR titration. This spectral evolution reflects a dynamic coordination equilibrium, wherein excess DMF molecules offset entropic penalties through transient interactions. Collectively, these findings demonstrate that DMF dynamically coordinates with p-methoxyphenol to enhance oxyanion electronegativity and nucleophilicity while maintaining rapid exchange.
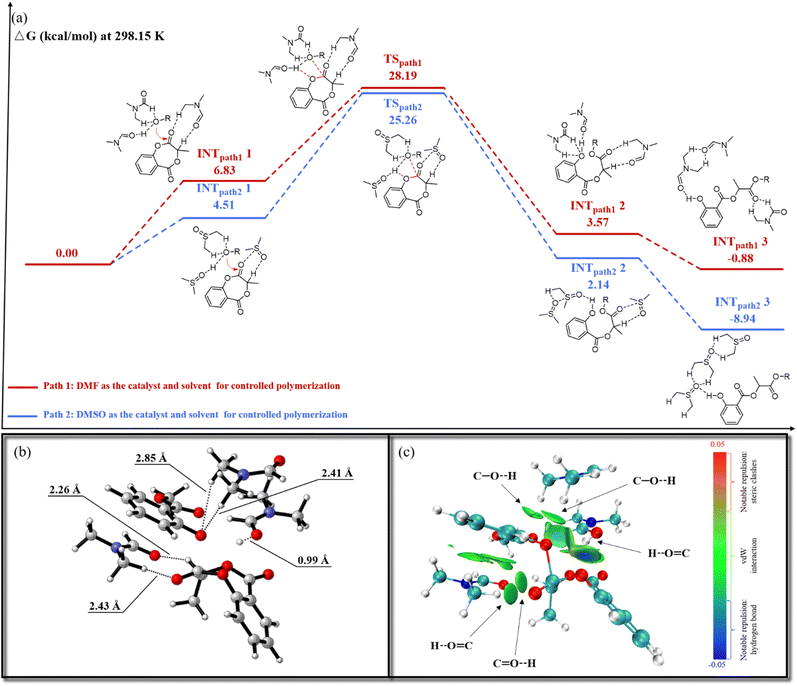
However, the interaction between the SMG monomer and DMF at a DMF/SMG ratio ranging from 0.2 to 10 cannot be detected using FT-IR and 13C NMR titration experiments, possibly because the interaction is very weak and the equilibrium constant is small for the complex formed between SMG and DMF. When large amounts of DMF were utilized, SMG became labile due to the ROP initiated by residual water (vide infra) with DMF acting as a catalyst. However, the third-order dependence on DMF indicates a potential interaction between SMG and DMF. Therefore, to clarify the catalytic role of DMF/DMSO in the ROP of the phenolic ester monomer, DFT calculations were conducted using methyl salicylate as a model initiator to simulate chain propagation conditions (Fig. 4). Initially, a trimolecular solvent-activation mechanism was proposed (Path 1 in Fig. 4a).
At the outset, two adducts were formed: INTpath11 (Fig. S29a), a complex consisting of two DMF molecules and one methyl salicylate molecule, and another complex composed of one DMF molecule and one SMG monomer. The catalytic mechanism proceeds through three coordinated steps: (1) initiator activation, one DMF molecule interacts with the initiator through pronounced hydrogen bonding (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O); (2) oxygen anion stabilization, the second DMF molecule stabilizes the emerging oxyanion through weaker hydrogen bonds, enhancing ionization efficiency and electronegativity; and (3) monomer activation, the third DMF molecule polarizes the carbonyl group of SMG through hydrogen bonding, thus lowering the activation barrier for the ring-opening of the monomer. The transition state TSpath1 (Fig. S29b) exhibited an activation barrier of 28.19 kcal mol−1, which is dramatically lower than that of the solvent-free TSpath3 (Fig. S25) barrier (42.26 kcal mol−1). The Independent Gradient Model based on Hirshfeld partition (IGMH)46–48 analysis revealed five characteristic hydrogen-bonding interactions (Fig. 4b and c). The synergistic effects of these interactions increased the electron density of the hydroxyl oxyanion, elevating its charge from −0.648e (INTpath11) to −0.731e (TSpath1). Simultaneously, monomer activation enhanced carbonyl polarization, increasing the ester carbon charge from +0.638e (INTpath11) to +0.649e (TSpath1). These findings confirm that DMF at a high concentration dynamically activates dormant species through weak non-covalent interactions. The trimolecular DMF coordination substantially reduces the activation energy barrier, facilitating efficient nucleophilic attack by the initiator on the SMG monomer. This process generates the active intermediate INTpath12 (Fig. S29c), which converts into the thermodynamically stabilized dormant species INTpath13 (Fig. S29d). The catalytic pathway involving two DMF molecules, without monomer activation, was also examined (Fig. S26); the energy barrier of TS′path1 at 35.71 kcal mol−1 was higher, indicating that during the catalytic process, the monomer is likely activated through the formation of a DMF-SMG adduct.
O⋯H–O); (2) oxygen anion stabilization, the second DMF molecule stabilizes the emerging oxyanion through weaker hydrogen bonds, enhancing ionization efficiency and electronegativity; and (3) monomer activation, the third DMF molecule polarizes the carbonyl group of SMG through hydrogen bonding, thus lowering the activation barrier for the ring-opening of the monomer. The transition state TSpath1 (Fig. S29b) exhibited an activation barrier of 28.19 kcal mol−1, which is dramatically lower than that of the solvent-free TSpath3 (Fig. S25) barrier (42.26 kcal mol−1). The Independent Gradient Model based on Hirshfeld partition (IGMH)46–48 analysis revealed five characteristic hydrogen-bonding interactions (Fig. 4b and c). The synergistic effects of these interactions increased the electron density of the hydroxyl oxyanion, elevating its charge from −0.648e (INTpath11) to −0.731e (TSpath1). Simultaneously, monomer activation enhanced carbonyl polarization, increasing the ester carbon charge from +0.638e (INTpath11) to +0.649e (TSpath1). These findings confirm that DMF at a high concentration dynamically activates dormant species through weak non-covalent interactions. The trimolecular DMF coordination substantially reduces the activation energy barrier, facilitating efficient nucleophilic attack by the initiator on the SMG monomer. This process generates the active intermediate INTpath12 (Fig. S29c), which converts into the thermodynamically stabilized dormant species INTpath13 (Fig. S29d). The catalytic pathway involving two DMF molecules, without monomer activation, was also examined (Fig. S26); the energy barrier of TS′path1 at 35.71 kcal mol−1 was higher, indicating that during the catalytic process, the monomer is likely activated through the formation of a DMF-SMG adduct.
The catalytic ROP employing DMSO as both solvent and the catalyst was also analyzed (Fig. 4a, Path 2). Initially, the adducts INTpath21 (Fig. S31a) were formed through the coordination of two DMSO molecules with methyl salicylate and one DMSO molecule with SMG. The hydrogen bonding formation energy barrier between DMSO and methyl salicylate was calculated to be 4.51 kcal mol−1, significantly lower than the 6.83 kcal mol−1 observed in the DMF system (INTpath11), indicating stronger thermodynamic stabilization of the DMSO-initiator adduct. Then, the transition state TSpath2 (Fig. S31b) exhibited an activation energy of 25.26 kcal mol−1, which is 2.93 kcal mol−1 lower than that of TSpath1 catalyzed by DMF. Similarly, for transition state TSpath2 (Fig. S27a and b), non-covalent interaction (NCI)49,50 analysis revealed four hydrogen bonds and a distinctive dipole–dipole interaction (2.87 Å). The sulfur atom in DMSO, bearing a substantial positive charge (+0.830e), enhanced dipole coupling with the carbonyl oxygen of the monomer. This interaction amplified carbonyl polarization, increasing the carbon charge to +0.701e, which is significantly higher than that in the DMF system (+0.649e). The reaction then proceeded to form the active intermediate INTpath22 (Fig. S31c), ultimately yielding the stabilized dormant species INTpath23 (Fig. S31d). Collectively, the DFT calculations validated the trimolecular solvent-cooperative activation mechanism. Both pathways exhibited significantly lower activation barriers than the solvent-free condition (TSpath3, 42.26 kcal mol−1), highlighting the synergistic role of high-polarity aprotic solvents (DMF/DMSO) at elevated concentrations in activating phenolic initiators and cyclic ester monomers through multi-molecular coordination. The DMSO system demonstrated consistently lower barriers than DMF for both intermediates and transition states, signifying stronger intermolecular interactions among DMSO, the initiator, and the monomer. These enhanced interactions, particularly the robust dipole–dipole coordination, substantially improved the thermodynamic favorability of the ROP process.
Catalyst water tolerance experiment
Given the advantages of weak interactions in adapting to environmental conditions, the water tolerance of the catalytic system was systematically examined. Interestingly, this solvent catalytic system exhibited remarkable resistance to moisture. In DMF solvent under conditions of [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, the addition of water at five equivalents (corresponding to 500 ppm in the solvent) relative to the initiator (Table S8, entry 4 and Fig. S33c) resulted in only a slight reduction in catalytic efficiency, with the monomer conversion decreasing by merely 16%. When water was considered an initiator, the experimental molecular weight closely matched the theoretical value. The SEC trace revealed a bimodal distribution (Đ = 1.57), indicating that water participated in the initiation process. However, the lower initiating efficiency of water compared to p-methoxyphenol likely caused the observed bimodality. Simultaneously, MALDI-TOF MS analysis (Fig. S34) revealed two distinct polymer populations initiated by p-methoxyphenol and water, respectively. At a lower water content of 100 ppm (Table S8, entry 2 and Fig. S33a), the SEC trace displayed a unimodal peak with a narrow dispersity (Đ < 1.20), and the conversion exceeded 95%, indicating well-controlled polymerization. These results indicate that water-initiated ROP follows a slow initiation but a rapid propagation mechanism. In addition, DFT calculations revealed that employing water as an initiator (Fig. S36) yielded the transition state TSpath4 (Fig. S37) with an activation barrier of 29.21 kcal mol−1, slightly higher than that of TSpath1 (28.19 kcal mol−1). This finding validates the lower initiation efficiency of water relative to phenol initiators. Within the water initiation system, weak hydrogen bond interactions with DMF facilitated its activation as an initiator, promoting the ROP of monomers. This observation motivated further investigation of water as an initiator for the controlled ROP of phenolic ester monomers. At [I]0/[M]0 = 1
600, the addition of water at five equivalents (corresponding to 500 ppm in the solvent) relative to the initiator (Table S8, entry 4 and Fig. S33c) resulted in only a slight reduction in catalytic efficiency, with the monomer conversion decreasing by merely 16%. When water was considered an initiator, the experimental molecular weight closely matched the theoretical value. The SEC trace revealed a bimodal distribution (Đ = 1.57), indicating that water participated in the initiation process. However, the lower initiating efficiency of water compared to p-methoxyphenol likely caused the observed bimodality. Simultaneously, MALDI-TOF MS analysis (Fig. S34) revealed two distinct polymer populations initiated by p-methoxyphenol and water, respectively. At a lower water content of 100 ppm (Table S8, entry 2 and Fig. S33a), the SEC trace displayed a unimodal peak with a narrow dispersity (Đ < 1.20), and the conversion exceeded 95%, indicating well-controlled polymerization. These results indicate that water-initiated ROP follows a slow initiation but a rapid propagation mechanism. In addition, DFT calculations revealed that employing water as an initiator (Fig. S36) yielded the transition state TSpath4 (Fig. S37) with an activation barrier of 29.21 kcal mol−1, slightly higher than that of TSpath1 (28.19 kcal mol−1). This finding validates the lower initiation efficiency of water relative to phenol initiators. Within the water initiation system, weak hydrogen bond interactions with DMF facilitated its activation as an initiator, promoting the ROP of monomers. This observation motivated further investigation of water as an initiator for the controlled ROP of phenolic ester monomers. At [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (Table 3, entry 1 and Fig. S33d), the experimental molecular weight closely approached the theoretical value, although the SEC trace exhibited a relatively broad distribution (Đ = 1.43), again reflecting the slow initiation and fast propagation nature of water in DMF. Increasing the feed ratio to [I]0/[M]0 = 1
200 (Table 3, entry 1 and Fig. S33d), the experimental molecular weight closely approached the theoretical value, although the SEC trace exhibited a relatively broad distribution (Đ = 1.43), again reflecting the slow initiation and fast propagation nature of water in DMF. Increasing the feed ratio to [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (Table 3, entry 5) significantly improved polymerization control, producing a polymer with a molecular weight of 142.0 kg mol−1 and a narrow dispersity (Đ < 1.20). The molecular weight increased linearly with the monomer-to-initiator ratio, confirming the controllability of the solvent system. The presence of water did not disrupt the dynamic hydrogen bonding network in DMF. Instead, water molecules were activated by DMF through hydrogen bonding and directly participated in ROP. The strong moisture tolerance of the solvent system was further verified in DMSO. At [I]0/[M]0 = 1
1000 (Table 3, entry 5) significantly improved polymerization control, producing a polymer with a molecular weight of 142.0 kg mol−1 and a narrow dispersity (Đ < 1.20). The molecular weight increased linearly with the monomer-to-initiator ratio, confirming the controllability of the solvent system. The presence of water did not disrupt the dynamic hydrogen bonding network in DMF. Instead, water molecules were activated by DMF through hydrogen bonding and directly participated in ROP. The strong moisture tolerance of the solvent system was further verified in DMSO. At [I]0/[M]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, the addition of up to three equivalents of water (300 ppm) caused only a minor decrease in molecular weight (>300.0 kg mol−1) without significantly affecting catalytic efficiency (Table S9, entries 2–4, Fig. S32g and h). Even at 500 ppm water (Table S9, entry 5 and Fig. S33i), the conversion decreased to 69%, but the polymerization remained well-controlled, yielding a high-molecular-weight polymer (282.0 kg mol−1) with a narrow dispersity (Đ = 1.16). MALDI-TOF MS analysis detected polymer chains initiated solely by p-methoxyphenol, with no signals arising from water initiation (Fig. S35). DFT calculations demonstrated that the energy barrier for the process initiated by water was 3.22 kcal mol−1 higher (TSpath5 = 28.48 kcal mol−1vs.TSpath2 = 25.26 kcal mol−1, Fig. S38) than that for the reaction initiated by p-methoxyphenol in DMSO. Therefore, ROP initiated by water was suppressed by p-methoxyphenol. This DMF/DMSO solvent catalytic system exhibits outstanding water tolerance. Significantly, in DMF solvent, water can be effectively utilized as an initiator to achieve controlled ROP. This strategy not only addresses the challenge of catalyst residues but also eliminates initiator residue issues, greatly enhancing the biocompatibility and safety of the resulting polymers—an essential advantage for medical polymer development.
1000, the addition of up to three equivalents of water (300 ppm) caused only a minor decrease in molecular weight (>300.0 kg mol−1) without significantly affecting catalytic efficiency (Table S9, entries 2–4, Fig. S32g and h). Even at 500 ppm water (Table S9, entry 5 and Fig. S33i), the conversion decreased to 69%, but the polymerization remained well-controlled, yielding a high-molecular-weight polymer (282.0 kg mol−1) with a narrow dispersity (Đ = 1.16). MALDI-TOF MS analysis detected polymer chains initiated solely by p-methoxyphenol, with no signals arising from water initiation (Fig. S35). DFT calculations demonstrated that the energy barrier for the process initiated by water was 3.22 kcal mol−1 higher (TSpath5 = 28.48 kcal mol−1vs.TSpath2 = 25.26 kcal mol−1, Fig. S38) than that for the reaction initiated by p-methoxyphenol in DMSO. Therefore, ROP initiated by water was suppressed by p-methoxyphenol. This DMF/DMSO solvent catalytic system exhibits outstanding water tolerance. Significantly, in DMF solvent, water can be effectively utilized as an initiator to achieve controlled ROP. This strategy not only addresses the challenge of catalyst residues but also eliminates initiator residue issues, greatly enhancing the biocompatibility and safety of the resulting polymers—an essential advantage for medical polymer development.
| Entry | [H2O]0/[M]0 | T/°C | t/h | Conv. b% | M n,calcd (g mol−1) | M n,obsd (g mol−1) | Đ |
|---|---|---|---|---|---|---|---|
| a Conditions: solvent = DMF, initiator = H2O, [M]0 = 1 M, monomer = SMG. b Determined using the 1H NMR spectrum. c Calculated from MSMG × [SMG]0/[I]0 × conversion yield + Mp-methoxyphenol. d Absolute values of Mn and Đ determined by SEC in THF. | |||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
40 | 2.5 | 91 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.43 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
40 | 3.5 | 93 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.24 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
40 | 3.5 | 90 | 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.21 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
40 | 4 | 94 | 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.16 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
40 | 5 | 90 | 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.19 |
Conclusion
This study establishes a green and controllable polymerization system catalyzed by DMF and DMSO, enabling efficient ROP of cyclic phenolic ester monomers such as SG and SMG. In this system, solvent molecules simultaneously act as both solvent and the catalyst, challenging conventional paradigms of catalyst design. Mechanistic investigations reveal a “solvent cooperative activation” mechanism: (1) the first solvent molecule activates the phenol initiator through a strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O hydrogen bond; (2) the second solvent molecule stabilizes the phenoxide anion intermediate via a weak C–H⋯O interaction; and (3) the third solvent molecule activates the ester bond in the monomer, reducing the nucleophilic attack barrier. The system demonstrates good moisture tolerance. Even in the presence of five equivalents of water relative to the initiator, effective polymerization control is maintained. Controlled ROP is successfully achieved using water as the initiator in DMF, yielding PSMG with a molecular weight of up to 142 kg mol−1 and a narrow dispersity (Đ < 1.20). This approach fundamentally addresses the problems associated with catalyst and initiator residues, enhancing the biocompatibility and safety of the resulting polymers, simplifying post-processing, and reducing production costs. This solvent-catalyzed strategy presents a new perspective for ROP and broadens the application potential of bio-based phenolic ester polymers.
O⋯H–O hydrogen bond; (2) the second solvent molecule stabilizes the phenoxide anion intermediate via a weak C–H⋯O interaction; and (3) the third solvent molecule activates the ester bond in the monomer, reducing the nucleophilic attack barrier. The system demonstrates good moisture tolerance. Even in the presence of five equivalents of water relative to the initiator, effective polymerization control is maintained. Controlled ROP is successfully achieved using water as the initiator in DMF, yielding PSMG with a molecular weight of up to 142 kg mol−1 and a narrow dispersity (Đ < 1.20). This approach fundamentally addresses the problems associated with catalyst and initiator residues, enhancing the biocompatibility and safety of the resulting polymers, simplifying post-processing, and reducing production costs. This solvent-catalyzed strategy presents a new perspective for ROP and broadens the application potential of bio-based phenolic ester polymers.
Author contributions
Jincai Wu supervised the project. Ge Yao carried out most of the experiments and wrote the initial manuscript draft. Jincai Wu and Ge Yao revised the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Additional data supporting this article were provided in supplementary information (SI). Supplementary information: experimental details, the spectra of compouds and polymers, and theoretical calculation details. See DOI: https://doi.org/10.1039/d5sc06353a.Acknowledgements
Financial support from the National Key R&D Program of China (2023YFA1506804) and the National Natural Science Foundation of China (nos. 22471110, 22131007, and 22171111) is gratefully acknowledged. We thank Dr Qingfeng Wu at the Institute of Modern Physics, Chinese Academy of Sciences for the MALDI-TOF mass measurement. We thank Prof. Shaoyu Lü and Dr Haofei Qie at the Lanzhou University College of Chemistry and Chemical Engineering for the FT-IR test.Notes and references
- “Bioplastic market development update 2024”(European Bioplastics, 2024), European Bioplastics, https://www.european-bioplastics.org/bioplastics-market-development-update-2024/ Search PubMed.
- United Nations Environment Programme, “Zero Draft of the plastics treaty (UNEP/PP/INC.3/4)” (UNEP, 2023), https://wedocs.unep.org/bitstream/handle/20.500.11822/43239/ZERODRAFT.pdf Search PubMed.
- B. A. Abel and G. W. Coates, Introduction: The Future of Plastics Sustainability, Chem. Rev., 2025, 125, 1255–1256 CrossRef CAS PubMed.
- M. S. Kim, H. Chang, L. Zheng, Q. Yan, B. F. Pfleger, J. Klier, K. Nelson, E. L. W. Majumder and G. W. Huber, A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs, Chem. Rev., 2023, 123, 9915–9939 CrossRef CAS PubMed.
- X. L. Li, R. W. Clarke, H. Y. An, R. R. Gowda, J. Y. Jiang, T. Q. Xu and E. Y. X. Chen, Dual Recycling of Depolymerization Catalyst and Biodegradable Polyester that Markedly Outperforms Polyolefins, Angew. Chem., Int. Ed., 2023, 62, e202303791 CrossRef CAS PubMed.
- Z. Zhang, E. C. Quinn, J. L. Olmedo-Martinez, M. R. Caputo, K. A. Franklin, A. J. Muller and E. Y. Chen, Toughening Brittle Bio-P3HB with Synthetic P3HB of Engineered Stereomicrostructures, Angew. Chem., Int. Ed., 2023, 62, e202311264 CrossRef CAS PubMed.
- L. Zhou, Z. Zhang, C. Shi, M. Scoti, D. K. Barange, R. R. Gowda and E. Y.-X. Chen, Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates, Science, 2023, 380, 64–69 CrossRef CAS PubMed.
- Q. Cao, Y. M. Tu, H. Z. Fan, S. Y. Shan, Z. Cai and J. B. Zhu, Torsional Strain Enabled Ring-Opening Polymerization towards Axially Chiral Semiaromatic Polyesters with Chemical Recyclability, Angew. Chem., Int. Ed., 2024, 63, e202400196 CrossRef CAS PubMed.
- H. Z. Fan, X. Yang, J. H. Chen, Y. M. Tu, Z. Cai and J. B. Zhu, Advancing the Development of Recyclable Aromatic Polyesters by Functionalization and Stereocomplexation, Angew. Chem., Int. Ed., 2022, 61, e202117639 CrossRef CAS PubMed.
- C. Li, L. Wang, Q. Yan, F. Liu, Y. Shen and Z. Li, Rapid and Controlled Polymerization of Bio-sourced δ-Caprolactone toward Fully Recyclable Polyesters and Thermoplastic Elastomers, Angew. Chem., Int. Ed., 2022, 61, e202201407 CrossRef CAS PubMed.
- X. T. Wu, C. Yang, J. S. Xi, C. Shi, F. S. Du and Z. C. Li, Enabling Closed-Loop Circularity of “Non-Polymerizable” α, β-Conjugated Lactone Towards High-Performance Polyester with the Assistance of Cyclopentadiene, Angew. Chem., Int. Ed., 2024, 63, e202404179 CrossRef CAS PubMed.
- W. Zhao, Z. Guo, J. He and Y. Zhang, Solvent-Free Chemical Recycling of Polyesters and Polycarbonates by Magnesium-Based Lewis Acid Catalyst, Angew. Chem., Int. Ed., 2025, 64, e202420688 CrossRef CAS PubMed.
- J. Xian, H. Chen, G. Yao, F. Chen, Z. Chen, H. Cao, L. Cao, X. Pan, Y. Tang and J. Wu, Enantiomorphic Site-Assisted Chain End Control Stereospecific Alternating Copolymerization of Chiral Cyclic Diesters, Angew. Chem., Int. Ed., 2025, 64, e202420316 CrossRef CAS PubMed.
- A. Gadomska-Gajadhur and P. Ruśkowski, Biocompatible Catalysts for Lactide Polymerization—Catalyst Activity, Racemization Effect, and Optimization of the Polymerization Based On Design of Experiments, Org. Process Res. Dev., 2020, 24, 1435–1442 CrossRef CAS.
- S. C. Yoon, S. Y. Park, D. Y. Sim and I. S. Lee, Polylactide Resin Compositions with Good Mechanical Properties and Heat Resistance, US Pat., 20120289675A1, 2012 Search PubMed.
- X. Geng, X. Liu, Q. Yu, C. Zhang and X. Zhang, Advancing H-Bonding Organocatalysis for Ring-Opening Polymerization: Intramolecular Activation of Initiator/Chain End, J. Am. Chem. Soc., 2024, 146, 25852–25859 CrossRef CAS PubMed.
- M. S. Young, A. M. LaPointe, S. N. MacMillan and G. W. Coates, Highly Enantioselective Polymerization of β-Butyrolactone by a Bimetallic Magnesium Catalyst: An Interdependent Relationship Between Favored and Unfavored Enantiomers, J. Am. Chem. Soc., 2024, 146, 18032–18040 CrossRef CAS PubMed.
- R. Morodo, D. M. Dumas, J. Zhang, K. H. Lui, P. J. Hurst, R. Bosio, L. M. Campos, N. H. Park, R. M. Waymouth and J. L. Hedrick, Ring-Opening Polymerization of Cyclic Esters and Carbonates with (Thio)urea/Cyclopropenimine Organocatalytic Systems, ACS Macro Lett., 2024, 13, 181–188 CrossRef CAS PubMed.
- Y. Xia, P. Yuan, Y. Zhang, Y. Sun and M. Hong, Converting Non-strained γ-Valerolactone and Derivatives into Sustainable Polythioesters via Isomerization-driven Cationic Ring-Opening Polymerization of Thionolactone Intermediate, Angew. Chem., Int. Ed., 2023, 62, e202217812 CrossRef CAS PubMed.
- J. Zhang, K. H. Lui, R. Zunino, Y. Jia, R. Morodo, N. Warlin, J. L. Hedrick, G. Talarico and R. M. Waymouth, Highly Selective O-Phenylene Bisurea Catalysts for ROP: Stabilization of Oxyanion Transition State by a Semiflexible Hydrogen Bond Pocket, J. Am. Chem. Soc., 2024, 146, 22295–22305 CrossRef CAS PubMed.
- J.-B. Zhu and E. Y. X. Chen, From meso-Lactide to Isotactic Polylactide: Epimerization by B/N Lewis Pairs and Kinetic Resolution by Organic Catalysts, J. Am. Chem. Soc., 2015, 137, 12506–12509 CrossRef CAS PubMed.
- A. Sanchez-Sanchez, I. Rivilla, M. Agirre, A. Basterretxea, A. Etxeberria, A. Veloso, H. Sardon, D. Mecerreyes and F. P. Cossío, Enantioselective Ring-Opening Polymerization of rac-Lactide Dictated by Densely Substituted Amino Acids, J. Am. Chem. Soc., 2017, 139, 4805–4814 CrossRef CAS PubMed.
- P. Lu, V. K. Kensy, R. L. Tritt, D. T. Seidenkranz and A. J. Boydston, Metal-Free Ring-Opening Metathesis Polymerization: From Concept to Creation, Acc. Chem. Res., 2020, 53, 2325–2335 CrossRef CAS PubMed.
- H.-Y. Huang, W. Xiong, Y.-T. Huang, K. Li, Z. Cai and J.-B. Zhu, Spiro-salen catalysts enable the chemical synthesis of stereoregular polyhydroxyalkanoates, Nat. Catal., 2023, 6, 720–728 CrossRef CAS.
- R. Xie, Y. Y. Zhang, G. W. Yang, X. F. Zhu, B. Li and G. P. Wu, Record Productivity and Unprecedented Molecular Weight for Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides Enabled by Organoboron Catalysts, Angew. Chem., Int. Ed., 2021, 60, 19253–19261 CrossRef CAS PubMed.
- C. K. Xu, G. W. Yang, C. Lu and G. P. Wu, A Binary Silicon-Centered Organoboron Catalyst with Superior Performance to That of Its Bifunctional Analogue, Angew. Chem., Int. Ed., 2023, 62, e202312376 CrossRef CAS PubMed.
- Q. Yan, J. Ma, W. Pei, Y. Zhang, R. Zhong, S. Liu, Y. Shen and Z. Li, Chemoselective Ring-Opening Polymerization of α-Methylene-δ-valerolactone Catalyzed by a Simple Organoaluminum Complex to Prepare Closed-Loop Recyclable Functional Polyester, Angew. Chem., Int. Ed., 2024, 64, e202418488 CrossRef PubMed.
- Y. Zhu and Y. Tao, Stereoselective Ring-opening Polymerization of S-Carboxyanhydrides Using Salen Aluminum Catalysts: A Route to High-Isotactic Functionalized Polythioesters, Angew. Chem., Int. Ed., 2024, 63, e202317305 CrossRef CAS PubMed.
- A.-C. Albertsson and M. Hakkarainen, Designed to degrade, Science, 2017, 358(6365), 872–873 CrossRef CAS PubMed.
- H. J. Kim, M. A. Hillmyer and C. J. Ellison, Enhanced Polyester Degradation through Transesterification with Salicylates, J. Am. Chem. Soc., 2021, 143, 15784–15790 CrossRef CAS PubMed.
- P. Salas-Ambrosio, S. Vexler, R. P Sivasankaran, N. Vlahakis, R. S. Lai, C. Johnson, S. I. Baas-Maynard, D. S. Min, H. Lower, A. G. Doyle, Y. Tang, J. A. Rodriguez, I. A. Chen, J. Read de Alaniz and H. D. Maynard, Biosourced Functional Hydroxybenzoate-co-Lactide Polymers with Antimicrobial Activity, J. Am. Chem. Soc., 2025, 147, 19230–19238 CrossRef CAS PubMed.
- H. J. Kim, Y. Reddi, C. J. Cramer, M. A. Hillmyer and C. J. Ellison, Readily Degradable Aromatic Polyesters from Salicylic Acid, ACS Macro Lett., 2020, 9, 96–102 CrossRef CAS PubMed.
- F. Ren, J. Xian, Z. Jia, Z. Chen, H. Fu, R. Wang, W. D. Chu, X. Pan and J. Wu, Tetrabutylammonium Halides as Selectively Bifunctional Catalysts Enabling the Syntheses of Recyclable High Molecular Weight Salicylic Acid-Based Copolyesters, Angew. Chem., Int. Ed., 2023, 62, e202306759 CrossRef CAS PubMed.
- W. Dołębska and T. Jaroń, Structural and thermal study of solvent-free tetrabutylammonium chloride and its novel solvates, J. Mol. Struct., 2020, 1206, 127748 CrossRef.
- K. Lu, X.-W. Han, W.-W. Yao, Y.-X. Luan, Y.-X. Wang, H. Chen, X.-T. Xu, K. Zhang and M. Ye, DMF-Promoted Redox-Neutral Ni-Catalyzed Intramolecular Hydroarylation of Alkene with Simple Arene, ACS Catal., 2018, 8, 3913–3917 CrossRef CAS.
- M. Pichette Drapeau, I. Fabre, L. Grimaud, I. Ciofini, T. Ollevier and M. Taillefer, Transition-Metal-Free α-Arylation of Enolizable Aryl Ketones and Mechanistic Evidence for a Radical Process, Angew. Chem., Int. Ed., 2015, 54, 10587–10591 CrossRef CAS PubMed.
- Y. Ashikari, A. Shimizu, T. Nokami and J.-I. Yoshida, Halogen and Chalcogen Cation Pools Stabilized by DMSO. Versatile Reagents for Alkene Difunctionalization, J. Am. Chem. Soc., 2013, 135, 16070–16073 CrossRef CAS PubMed.
- S. Song, X. Li, J. Wei, W. Wang, Y. Zhang, L. Ai, Y. Zhu, X. Shi, X. Zhang and N. Jiao, DMSO-catalysed late-stage chlorination of (hetero)arenes, Nat. Catal., 2019, 3, 107–115 CrossRef.
- H.-Q. Yue, D.-W. Shi, P. Zhang, B. Xiao, L.-T. Jia, R. Li, S.-N. Zhao, S.-D. Yang and B. Yang, DMSO-Catalyzed Double P–O Bond or Double P–S Bond Formations of Phosphinic Acids, Org. Lett., 2024, 26, 8939–8944 CrossRef CAS PubMed.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- W. Fang, Y. D. Meng, S. Y. Ding, J. Y. Wang, Z. H. Pei, M. L. Shen, C. Z. Yao, Q. Li, Z. Gu, J. Yu and H. J. Jiang, Asymmetric S-Arylation of Sulfenamides to Access Axially Chiral Sulfilimines Enabled by Anionic Stereogenic-at-Cobalt(III) Complexes, Angew. Chem., Int. Ed., 2025, 64, e202419596 CrossRef CAS PubMed.
- Z. Pawelka and T. Zeegers-Huyskens, Mid- and near-infrared study of hydrogen bond complexes between phenols and N,N-dimethylformamide, Vib. Spectrosc., 1998, 18, 41–49 CrossRef CAS.
- M. G. Siskos, V. G. Kontogianni, C. G. Tsiafoulis, A. G. Tzakos and I. P. Gerothanassis, Investigation of solute–solvent interactions in phenol compounds: accurate ab initio calculations of solvent effects on 1H NMR chemical shifts, Org. Biomol. Chem., 2013, 11, 7400–7411 RSC.
- F. G. Bordwell, R. J. McCallum and W. N. Olmstead, Acidities and Hydrogen Bonding of Phenols in Dimethyl Sulfoxide, J. Org. Chem., 1984, 49, 1424–1427 CrossRef CAS.
- M. Li, X. Ma and Y. Tao, Unlocking Hexafluoroisopropanol as a Practical Anion-Binding Catalyst for Living Cationic Polymerization, Angew. Chem., Int. Ed., 2025, 64, e202425178 CrossRef CAS PubMed.
- T. Lu and Q. Chen, Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu, A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn, J. Chem. Phys., 2024, 161, 082503 CrossRef CAS PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, Revealing Noncovalent Interactions, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graph., 1996, 14, 33–38 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |