Open Access Article
Open Access ArticlePrecision pore engineering via fit-topology assembly in a Zn-porphyrin MOF for selective C2H2 capture
Zhenliang
Zhu
a,
Jianfei
Xiao
a,
Min
Zhang
a,
Yaoqi
Huang
*ab and
Shaojun
Yuan
 *a
*a
aLow-carbon Technology & Chemical Reaction Engineering Lab, School of Chemical Engineering, Sichuan University, Chengdu 610065, China. E-mail: ysj@scu.edu.cn; Fax: +86-028-85405201; Tel: +86-028-85405201
bSchool of Engineering and Applied Sciences, Harvard University, MA 02138, USA. E-mail: yhua@seas.harvard.edu
First published on 22nd October 2025
Abstract
The topology-guided design of porphyrin-based metal–organic frameworks (PMOFs) with tailored ultramicroporosity (<7 Å) remains a formidable challenge, as conventional strategies often fail to balance structural rigidity with precise pore confinement. To address this limitation, we propose a dual-ligand coordination approach, integrating Zn2+-porphyrin chelation and triazole-mediated SBU assembly, to construct Zn-TCPP-dmtrz—a novel PMOF featuring a unique fit topology for C2H2/CO2 separation. Unlike traditional PMOFs with oversized pores (e.g., Al/Y-TCPP), this strategy exploits the synergistic coordination of tetrakis(4-carboxyphenyl)porphyrin (TCPP) and 3,5-dimethyl-1,2,4-triazole (dmtrz) to compress pore apertures to 6.3 × 6.8 Å, closely matching the molecular dimensions of the C2H2 molecule. The resulting ultramicroporous channels, reinforced by staggered porphyrin planes and hydrophobic methyl groups, exhibit high C2H2 uptake (4.30 mmol g−1) and a separation potential (ΔQ = 1.62 mmol g−1) under ambient conditions, outperforming existing PMOFs. Crucially, the framework retains structural integrity under high humidity (90% RH) and cyclic adsorption–desorption, with a low regeneration energy barrier (Qst = 28.5 kJ mol−1). Computational studies attribute the selectivity to confinement-enhanced van der Waals interactions and electrostatic alignment at the porphyrin-Zn interface and methyl-decorated channels. This work establishes a topology-driven pore engineering strategy for PMOFs, advancing the design of next-generation adsorbents for challenging gas separations.
1. Introduction
Metal–organic frameworks (MOFs) are a versatile class of crystalline porous materials assembled from metal ions or clusters and multitopic organic ligands into periodic architectures.1–3 Their modularity not only affords ultrahigh surface areas and permanent porosity but also enables precise control over pore structures and functionalities.4–6 These attributes have made MOFs attractive candidates for applications in gas storage and separation,7–9 heterogeneous catalysis,10,11 and chemical sensing.12 One of the fundamental challenges in this field is the translation of structural diversity into rationally designed pore systems capable of delivering predictable adsorption properties and effective separation of small molecules with closely similar physicochemical characteristics.The network topology of a MOF provides a critical link between local coordination environments and global material properties.13,14 While metal–ligand coordination defines primary connectivity, the resulting topology dictates the dimensionality of channels, the continuity of pore windows, and the overall packing density of the framework.15 These features directly impact how guest molecules are confined and interact within the pore space.16–18 Even subtle variations in connectivity or linker orientation can reshape pore apertures, reorganize channels, and ultimately transform adsorption behavior.19,20 Accordingly, rational topology engineering, guided by reticular chemistry principles, has become an effective strategy for constructing MOFs with precisely tuned pore systems.21
Such considerations are particularly important for the separation of small gas molecules with similar physicochemical properties. Acetylene (C2H2) and carbon dioxide (CO2), for example, both have kinetic diameters of ∼3.3 Å and similar polarizabilities, making size-based discrimination extremely challenging.22,23 Their quadrupole moments, however, differ substantially (C2H2: −29 × 10−40 C m2; CO2: −14 × 10−40 C m2), offering an opportunity for frameworks with confined pores and well-distributed adsorption sites to selectively recognize one molecule over the other.24–26 Topology-directed pore confinement thus provides a powerful means to amplify such differences, enabling MOFs to achieve separation performance that cannot be obtained through simple surface functionalization.27,28
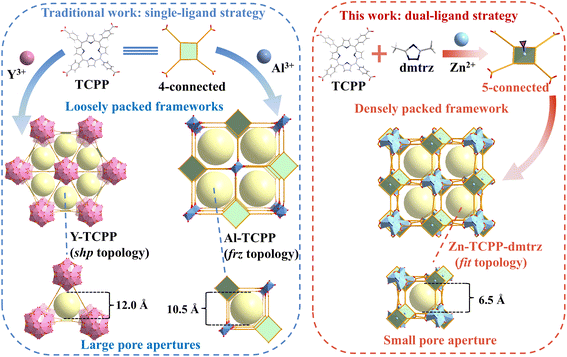
Porphyrin-based MOFs (PMOFs) represent a promising but underexplored platform for implementing this concept. The rigid, square-planar geometry of porphyrin ligands promotes ordered frameworks, while their π-conjugated cores impart stability and tunability.29,30 Among them, tetrakis(4-carboxyphenyl)porphyrin (TCPP) has been most widely employed, as its four carboxylate groups readily coordinate with high-valent metals.31,32 However, when combined with metals such as Al3+ or Y3+, TCPP typically forms frameworks with large one-dimensional channels (>10 Å).33,34 These structures are chemically stable but suffer from loose packing and oversized pore apertures, thereby limiting their ability to discriminate between small molecules. Attempts with transition metals such as Zn2+ or Cu2+ can, in principle, yield more compact frameworks since these metals can also coordinate to the porphyrin core.35,36 Nevertheless, most reported transition-metal PMOFs crystallize as layered structures or interpenetrated networks, leading to limited porosity and reduced tunability.37–39 Traditional single-ligand PMOFs (e.g., Al- and Y-TCPP) therefore produce loosely packed frameworks with large pore apertures, whereas the dual-ligand approach, by introducing an auxiliary triazole ligand, converts TCPP into a 5-connected node and directs the assembly of densely packed frameworks with compressed pore windows (Scheme 1).
Building on this rationale, short N-donor linkers such as imidazoles and triazoles provide an effective means of stabilizing high-connectivity secondary building units (SBUs), suppressing interpenetration, and guiding frameworks toward compact topologies.40–42 Integrating such linkers with porphyrins offers a powerful means to merge the rigidity of porphyrins with tunable pore confinement, yet this dual-ligand approach has rarely been applied in porphyrinic systems. In this work, we report a new PMOF, Zn-TCPP-dmtrz, synthesized from Zn2+, TCPP, and 3,5-dimethyl-1,2,4-triazole (dmtrz). The introduction of dmtrz converts TCPP into a 5-connected node, which, together with Zn SBUs, directs the formation of a distinctive (5,10)-connected fit topology. The resulting framework is densely packed and features one-dimensional rectangular channels with ultramicroporous apertures of 6.3 × 6.8 Å. Compared with conventional Al- and Y-TCPP analogues, this design compresses pore size and enhances confinement, enabling more effective recognition of C2H2 relative to CO2. Static adsorption isotherms at 298 K and 1 bar reveal preferential C2H2 uptake (4.30 mmol g−1) over CO2 (2.61 mmol g−1), with a separation potential (ΔQ) of 1.6 mmol g−1 for equimolar C2H2/CO2 mixtures—substantially higher than conventional TCPP-based MOFs. Dynamic breakthrough experiments demonstrate stable C2H2/CO2 separation over 5 cycles, while static adsorption–desorption tests confirm full retention of capacity after 10 cycles, underscoring the framework's robust recyclability. Even under high humidity (90% RH), Zn-TCPP-dmtrz preserves its separation performance without noticeable degradation. The relatively low initial isosteric heat of adsorption (28.5 kJ mol−1 for C2H2) suggests facile regeneration, a critical advantage for industrial applications. Moreover, Grand Canonical Monte Carlo (GCMC) simulations and density functional theory (DFT) calculations reveal that C2H2 molecules preferentially adsorb at the porphyrin-Zn interface, where confinement-enhanced van der Waals interactions dominate. This work pioneers a topology-guided design strategy for PMOFs, demonstrating how coordination-driven pore compression can unlock their potential for precision gas separations.
2. Results and discussion
Zn-TCPP-dmtrz was synthesized through a solvothermal reaction of Zn(NO3)2·6H2O, TCPP, and dmtrz in a mixed solution of N,N-dimethylformamide (DMF), water, and a minimal amount of HNO3 at 140 °C for 72 hours, yielding black-purple block-shaped crystals. Single-crystal X-ray diffraction (SCXRD) studies reveal that the structure of Zn-TCPP-dmtrz belongs to the monoclinic crystal system and C2 space group (Table S1). Structural analysis identifies three distinct Zn2+ coordination environments (Fig. 1a and S1): the first Zn center adopts a five-coordinate geometry, ligated by three oxygen atoms from two carboxylate groups of adjacent TCPP ligands, one nitrogen atom from a dmtrz ligand, and one oxygen atom from a μ3-OH group; the second Zn center exhibits a six-coordinate octahedral geometry, coordinated to four carboxylate oxygen atoms from four distinct TCPP ligands and two μ3-OH oxygen atoms; and the third Zn center is chelated within the porphyrin macrocycle, coordinating to four nitrogen atoms of the porphyrin core and axially binding to a nitrogen atom from a dmtrz ligand, forming a five-coordinate square pyramidal geometry. This unusual axial coordination mode at the porphyrin center arises directly from the dense packing of the framework. The assembly of a pentanuclear Zn cluster—comprising four five-coordinate Zn ions and one six-coordinate Zn ion (Fig. 1a)—shortens the interlayer distance between adjacent porphyrin planes, sterically hindering ligand binding at the opposite axial site (Fig. 1b). Consequently, the porphyrin-chelated Zn center adopts an asymmetric five-coordinate geometry, bonding exclusively to a single dmtrz ligand. This multimodal coordination strategy drives the formation of two distinct secondary building units (SBUs) (Fig. 1a and b): a pentanuclear Zn cluster acting as a 10-connected node through linkages to eight TCPP carboxylates and two dmtrz ligands, and a 5-connected TCPP unit formed by the porphyrin-chelated Zn center, which bridges four carboxylate-linked Zn clusters and one axial dmtrz ligand. The interconnection of these SBUs, mediated by tritopic dmtrz spacers, generates a three-dimensional framework with a novel (5,10)-connected fit topology in porous coordination polymers (Fig. 1c), marking the first example of this topology in metal–organic frameworks. The framework features unidirectional rectangular channels along the c-axis (Fig. 1d and e), with pore dimensions of 6.3 × 6.8 Å, as determined by Connolly surface analysis (Fig. 1f). Notably, the staggered arrangement of porphyrin planes and Zn clusters at all four channel corners (Fig. 1e) creates a confined ultramicroporous environment, distinct from conventional PMOFs, where partial corner occupation by elongated metal-carboxylate chains or vertically aligned porphyrins leads to larger apertures. This asymmetric distribution of SBUs compresses the pore aperture into the ultramicroporous regime while introducing a diverse chemical environment: the π-conjugated porphyrin walls enhance the van der Waals interactions with C2H2, facilitating its selective adsorption in C2H2/CO2 mixtures, while the hydrophobic methyl groups of dmtrz ligands project into the channels, minimizing competitive H2O adsorption that could otherwise interfere with the C2H2 recognition.The phase purity and structural integrity of Zn-TCPP-dmtrz are confirmed by powder X-ray diffraction (PXRD, Fig. S2). The experimental patterns of both the as-synthesized and activated samples closely match the simulated pattern derived from single-crystal data. Scanning electron microscopy (SEM) images, presented in Fig. S3a, reveal uniform block-shaped particles with sizes ranging from 50 to 100 μm. Elemental mapping via SEM-EDX (Fig. S3b) shows a homogeneous distribution of C, N, O, and Zn across the framework, which is further confirmed by X-ray photoelectron spectroscopy (XPS) data (Fig. S4). According to the single-crystal X-ray diffraction results (CCDC 2410049) and the XPS elemental composition, the molecular formula of Zn-TCPP-dmtrz is determined to be Zn7(μ3-OH)2(TCPP)2(dmtrz)2(DMF)2. The XPS analysis confirms the presence of Zn2+, with a binding energy of 1021.9 eV for the Zn 2p3/2 peak, and provides information about the coordination environment of nitrogen species, including triazole N at 399.8 eV and pyrrolic N at 401.7 eV (Fig. S5).43 Fourier-transform infrared spectroscopy (FT-IR, Fig. S6) reveals characteristic peaks at 1119.5 and 1347.0 cm−1 corresponding to C–N stretching vibrations of the porphyrin ring, at 989.2 cm−1 for N–N stretching in dmtrz ligands, and at 531.4 and 429.7 cm−1 for Zn–O and Zn–N bonds, respectively.44 The N2 adsorption isotherm at 77 K (Fig. S7) exhibits a type I adsorption curve, characteristic of microporous materials, with a calculated Brunauer–Emmett–Teller (BET) surface area of 829 m2 g−1 and a pore volume of 0.34 cm3 g−1. Non-local density functional theory (NLDFT) calculations show a narrow pore size distribution centered at 0.5–0.8 nm, which aligns well with the crystallographic pore dimensions. Thermogravimetric analysis (TGA, Fig. S8) shows that the framework remains stable up to 380 °C before decomposition begins, demonstrating its excellent thermal stability. Overall, these comprehensive characterization studies confirm the successful synthesis of Zn-TCPP-dmtrz as a highly crystalline, thermally stable, and ultramicroporous material, making it suitable for gas separation applications.
Inspired by its ultramicroporous characteristics, the adsorption performance and separation potential of Zn-TCPP-dmtrz for C2H2 and CO2 are systematically examined. As shown in Fig. 2a, single-component adsorption isotherms for both gases are measured at various temperatures using a BSD-660M sorption analyzer (BSD Instruments). The results clearly indicate a stronger affinity for C2H2 than for CO2 under identical conditions. Specifically, at 298 K and 1 bar, Zn-TCPP-dmtrz exhibits a C2H2 adsorption capacity of 4.30 mmol g−1, outperforming several well-established porous materials, such as SOFOUR-TEPE-Zn (3.98 mmol g−1),45 ZJU-74 (3.83 mmol g−1),46 JNU-1 (3.35 mmol g−1),47 Zn-bpy-DLmal (3.10 mmol g−1),48 and NKMOF-1-Ni (2.74 mmol g−1)49 (Table S4). In contrast, the CO2 uptake is considerably lower, with Zn-TCPP-dmtrz adsorbing only 2.61 mmol g−1 under the same conditions. Furthermore, when the temperature is adjusted to 283 K or 313 K, the C2H2 uptake remains significantly higher than that of CO2 at both temperatures, reaffirming the stronger interaction between C2H2 and the framework. To better understand the differences in C2H2 and CO2 adsorption behavior across TCPP-based adsorbents, we synthesize seven different TCPP-based PMOFs incorporating various metals, comparing their C2H2 and CO2 adsorption performance with that of Zn-TCPP-dmtrz (Fig. S9–S17). Among these materials, Al-TCPP shows the highest C2H2 uptake (3.89 mmol g−1), but it is still lower than that of Zn-TCPP-dmtrz, while most of the other PMOFs, such as Y-TCPP, demonstrate much lower capacities due to the larger pore size weakening the interactions with C2H2. Interestingly, Al-TCPP and In-TCPP also exhibit relatively strong CO2 adsorption capacities, further distinguishing Zn-TCPP-dmtrz for its superior C2H2/CO2 adsorption capacity difference, highlighting its significant potential for C2H2/CO2 separation.
The stability and regeneration potential of Zn-TCPP-dmtrz are evaluated through 10 adsorption–desorption cycles for both C2H2 and CO2 (Fig. 2c and S18). The results indicate that the adsorption capacities are well-maintained, confirming excellent cycling stability. To further assess its separation capability, we employ the Ideal Adsorbed Solution Theory (IAST) to calculate the selectivity for C2H2/CO2 (50/50). The dual-site Langmuir (DSL) model fits the adsorption isotherms with high precision (Fig. S11–S17, S19 and Table S2), and the IAST selectivity for C2H2/CO2 at 298 K and 1 bar is 2.63 for Zn-TCPP-dmtrz, outperforming other TCPP-based PMOFs such as In-TCPP (2.39), Y-TCPP (1.77), and Al-TCPP (1.07) (Fig. S20). Furthermore, the maximum recoverable C2H2 from a C2H2/CO2 mixture, represented by the separation potential (ΔQ), is calculated to be 1.62 mmol g−1 for Zn-TCPP-dmtrz, which is significantly higher than other PMOFs, including In-TCPP (1.10 mmol g−1) (Fig. 2d). This highlights the exceptional potential of Zn-TCPP-dmtrz for efficient C2H2 selectivity and purification. Additionally, the adsorption heat (Qst), indicative of the binding affinity between the framework and the guest molecules, is determined using adsorption isotherms at different temperatures and fitted with the Virial equation (Fig. S11–S17, S21, and Table S3). The Qst for C2H2 is 28.5 kJ mol−1 at low loading, demonstrating that Zn-TCPP-dmtrz allows for energy-efficient regeneration (<30 kJ mol−1), whereas the Qst for CO2 is 25.0 kJ mol−1. The noticeable difference in Qst supports the preferential adsorption of C2H2, providing moderate C2H2/CO2 selectivity. Despite other PMOFs having similarly low binding energies for C2H2, their low adsorption capacities limit their performance in C2H2/CO2 separation. In contrast, Zn-TCPP-dmtrz, as a representative of a fit-topology PMOF, combines high C2H2 adsorption capacity and excellent regeneration potential, making it a promising candidate for C2H2 purification with lower energy consumption compared to other PMOFs and conventional adsorbents (Fig. 2f).
To evaluate the practical C2H2/CO2 separation performance of Zn-TCPP-dmtrz, dynamic breakthrough measurements are carried out at 298 K on a fixed-bed column packed with the activated PMOF and fed with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 C2H2/CO2 mixture at 2 mL min−1 (Fig. 3a). CO2 elutes first at 12.3 min g−1, affording 9.08 cm3 g−1 of 99.95% pure CO2, whereas C2H2 remains on the column until 23.5 min g−1, yielding a dynamic uptake of 41.22 cm3 g−1 (61.1% of the 298 K isotherm capacity). In contrast, Y-TCPP demonstrates weak interactions with C2H2, resulting in a shorter breakthrough time for C2H2 (9.1 min g−1), and although Al-TCPP delays C2H2 breakthrough to 16.4 min g−1, its CO2 elution at 19.8 min g−1 does not precede C2H2, precluding direct recovery of high-purity CO2. Since C2H2 is recovered during desorption, the regeneration step is assessed by purging the saturated column with He (10 mL min−1) at 333 K (Fig. 3b, S22 and S23). Zn-TCPP-dmtrz releases CO2 completely within 4 min g−1, enabling collection of C2H2 at >99.5% purity with a yield of 29.80 cm3 g−1 (72.3% of the dynamic uptake), whereas Y-TCPP desorbs both gases rapidly—resulting in only 5.00 cm3 g−1 C2H2—and Al-TCPP shows no selective C2H2 recovery (Fig. 3c). To probe operational feasibility, breakthrough tests under varied flow rates (3 and 5 mL min−1; Fig. 3d) and temperatures (283 and 313 K; Fig. S24) maintain one-step high-purity CO2 elution. Finally, Zn-TCPP-dmtrz retains its separation performance over five consecutive cycles without detectable change (Fig. 3e), and exhibits outstanding moisture resilience: after six months of air exposure, 48 h at 90% RH, or 24 h water immersion, PXRD patterns and 77 K N2 isotherms show unchanged crystallinity and BET surface area (Fig. S25), and humid-condition breakthrough (Fig. 3f) yields identical elution profiles—attributable to the hydrophobic methylated triazole groups—underscoring its recyclability and stability under realistic conditions.
50 C2H2/CO2 mixture at 2 mL min−1 (Fig. 3a). CO2 elutes first at 12.3 min g−1, affording 9.08 cm3 g−1 of 99.95% pure CO2, whereas C2H2 remains on the column until 23.5 min g−1, yielding a dynamic uptake of 41.22 cm3 g−1 (61.1% of the 298 K isotherm capacity). In contrast, Y-TCPP demonstrates weak interactions with C2H2, resulting in a shorter breakthrough time for C2H2 (9.1 min g−1), and although Al-TCPP delays C2H2 breakthrough to 16.4 min g−1, its CO2 elution at 19.8 min g−1 does not precede C2H2, precluding direct recovery of high-purity CO2. Since C2H2 is recovered during desorption, the regeneration step is assessed by purging the saturated column with He (10 mL min−1) at 333 K (Fig. 3b, S22 and S23). Zn-TCPP-dmtrz releases CO2 completely within 4 min g−1, enabling collection of C2H2 at >99.5% purity with a yield of 29.80 cm3 g−1 (72.3% of the dynamic uptake), whereas Y-TCPP desorbs both gases rapidly—resulting in only 5.00 cm3 g−1 C2H2—and Al-TCPP shows no selective C2H2 recovery (Fig. 3c). To probe operational feasibility, breakthrough tests under varied flow rates (3 and 5 mL min−1; Fig. 3d) and temperatures (283 and 313 K; Fig. S24) maintain one-step high-purity CO2 elution. Finally, Zn-TCPP-dmtrz retains its separation performance over five consecutive cycles without detectable change (Fig. 3e), and exhibits outstanding moisture resilience: after six months of air exposure, 48 h at 90% RH, or 24 h water immersion, PXRD patterns and 77 K N2 isotherms show unchanged crystallinity and BET surface area (Fig. S25), and humid-condition breakthrough (Fig. 3f) yields identical elution profiles—attributable to the hydrophobic methylated triazole groups—underscoring its recyclability and stability under realistic conditions.
To gain deeper insights into the underlying mechanism of C2H2/CO2 separation in Zn-TCPP-dmtrz, grand canonical Monte Carlo (GCMC) simulations and dispersion-corrected density functional theory (DFT-D) calculations are employed to probe the binding sites and interaction strengths of both gases within the framework. GCMC results reveal distinct density distributions for C2H2 and CO2 within the confined pore architecture (Fig. 4a and d). Both gases localize near the porphyrin walls, aromatic rings, and alkyl-functionalized channels, but C2H2 exhibits significantly higher adsorption density, consistent with experimental uptake trends. Two primary adsorption sites for C2H2 are identified: Site I is situated near symmetrically arranged SBUs formed by TCPP porphyrin rings and dmtrz ligands, where C2H2 engages in multiple interactions, including hydrogen bonding between the H atoms of C2H2 and both the carboxylate O atoms of TCPP and the methyl H atoms of dmtrz (H⋯O and H⋯H distances ∼4.0 Å) (Fig. 4b). Simultaneously, the C atoms of C2H2 form electrostatic interactions (Cδ−⋯Hδ+) with aromatic H atoms (3.79–4.07 Å). Site II, located near the bilayered porphyrin region adjacent to the 10-connected Zn5 clusters, allows C2H2 to form multiple Cδ−⋯Hδ+ interactions with pyrrole moieties (3.21–3.98 Å) and engage in H⋯π interactions with the benzene rings (3.68 Å), as shown in Fig. 4c. In comparison, CO2 occupies analogous regions (Fig. 4e and f) but interacts via weaker and fewer non-directional van der Waals forces. At Site I, CO2 forms contacts (3.42–4.08 Å) between its O atoms and aromatic/pyrrolic H atoms, while at Site II, interactions are limited to distances of 3.57–3.99 Å. DFT-D calculations corroborate these observations: the binding energies for C2H2 at Sites I and II are 30.5 and 28.0 kJ mol−1, respectively, significantly exceeding those for CO2 (26.6 and 26.2 kJ mol−1). These findings underscore the critical role of the densely packed π-conjugated porphyrin arrays and alkyl moieties within the ultramicroporous channels in Zn-TCPP-dmtrz, which enable enhanced C2H2-framework recognition through the synergistic interplay of hydrogen bonding, electrostatic complementarity, and confinement-enhanced van der Waals interactions, thereby boosting its separation performance. The results highlight the topology-guided structural design principles for developing PMOF-based materials for challenging gas separations.
3. Conclusions
In summary, this study presents a strategic advancement in PMOF design by integrating Zn2+ coordination and dual-ligand assembly to construct an ultramicroporous architecture (6.3 × 6.8 Å) with tailored pore confinement for efficient C2H2/CO2 separation. Distinct from conventional PMOFs (e.g., Al- and Y-TCPP) with enlarged apertures, the Zn-TCPP-dmtrz framework adopts a novel fit topology—enabled by porphyrin chelation and triazole-mediated SBU densification—which achieves pore channels optimized for C2H2 accommodation. This structural design enhances host–guest interactions while maintaining framework stability, resulting in superior C2H2 uptake (4.30 mmol g−1) and separation potential (ΔQ = 1.62 mmol g−1) at 298 K and 1 bar, outperforming existing PMOFs in both selectivity (2.63) and regenerability (Qst = 28.5 kJ mol−1). Computational analyses reveal that staggered porphyrin planes and methyl-functionalized channels synergistically amplify C2H2 recognition through van der Waals and electrostatic interactions—mechanisms unattainable in PMOFs with less confined geometries. Remarkable moisture resistance and cycling stability further validate its practical feasibility. By demonstrating the effectiveness of topology-driven pore engineering in PMOFs, this work expands the scope of precision adsorbent design for gas mixtures with near–identical properties, offering a blueprint for future studies to extend this strategy to other industrially relevant separations.Author contributions
Zhenliang Zhu performed the methodology, data curation, and writing – original draft; Jianfei Xiao contributed to methodology and formal analysis; Min Zhang was involved in formal analysis; Yaoqi Huang contributed to methodology and writing – review & editing; Shaojun Yuan supervised the project, acquired funding, and contributed to project administration, resources, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary Information is available. See DOI: https://doi.org/10.1039/d5sc07319g.Acknowledgements
The authors would like to acknowledge the financial support provided by the National Natural Science Foundation of China (22578284) and Beijing Welltrailing Science and Technology Co., Ltd (contact no. 2025510005000242) to provide financial support for this study. They also express their gratitude to Dr Ji Li and Mr Pan Wu from the Engineering Teaching Center, School of Chemical Engineering, Sichuan University, for their support in conducting the measurements using the BSD-MAB Analyzer. Furthermore, the authors would like to thank Dr Yingming Zhu from the Institute of New Energy and Low Carbon Technology, Sichuan University, for assistance with the XRD characterization. Additionally, the authors extend their gratitude to Mr Sheng Liu from Scientific Compass (https://www.shiyanjia.com/) for providing invaluable assistance with the single crystal structure determination.References
- H. W. H. Lai, F. M. Benedetti, J. M. Ahn, A. M. Robinson, Y. Wang, I. Pinnau, Z. P. Smith and Y. Xia, Hydrocarbon ladder polymers with ultrahigh permselectivity for membrane gas separations, Science, 2022, 375, 1390–1392 CrossRef CAS PubMed.
- K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Synergistic sorbent separation for one-step ethylene purification from a four-component mixture, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- X. Jiang, Y. Wang, H. Wang, L. Cheng, J.-W. Cao, J.-B. Wang, R. Yang, D.-H. Zhang, R.-Y. Zhang, X.-B. Yang, S.-H. Wang, Q.-Y. Zhang and K.-J. Chen, Integration of ordered porous materials for targeted three-component gas separation, Nat. Commun., 2025, 16, 694 CrossRef CAS.
- S. Capelo-Avilés, M. de Fez-Febré, S. R. G. Balestra, J. Cabezas-Giménez, R. Tomazini de Oliveira, I. I. Gallo Stampino, A. Vidal-Ferran, J. González-Cobos, V. Lillo, O. Fabelo, E. C. Escudero-Adán, L. R. Falvello, J. B. Parra, P. Rumori, G. Turnes Palomino, C. Palomino Cabello, S. Giancola, S. Calero and J. R. Galán-Mascarós, Selective adsorption of CO2 in TAMOF-1 for the separation of CO2/CH4 gas mixtures, Nat. Commun., 2025, 16, 3243 CrossRef.
- X. Zhang, M. Li, Y.-l. Zhao, X.-Y. Li, Y. Fang, L.-H. Xie and J.-R. Li, Simultaneous Capture of N2O and CO2 from a N2O/N2/CO2/O2 Mixture with a Ni(II)-Pyrazolecarboxylate Framework, J. Am. Chem. Soc., 2025, 147(20), 17042–17048 CrossRef CAS PubMed.
- M. Yi, S. Wang, S. Li, S. Zhang, Y. Liu, L. Zhang, Z. You, X. Liu, L. Li, J. Wang, H. Wang, Q. Zhao, B. Li and X.-H. Bu, Superhydrophobic Molecular Selector for Efficient Separation of Ethane over Ethylene under Dry and Humid Conditions, J. Am. Chem. Soc., 2025, 147, 13592–13600 CrossRef CAS PubMed.
- T. Li, P. Cui and D. Sun, Uncoordinated Hexafluorosilicates in a Microporous Metal–Organic Framework Enabled C2H2/CO2 Separation, Inorg. Chem., 2022, 61, 4251–4256 CrossRef CAS PubMed.
- S.-Q. Yang, R. Krishna, H. Chen, L. Li, L. Zhou, Y.-F. An, F.-Y. Zhang, Q. Zhang, Y.-H. Zhang, W. Li and T.-L. Hu, Immobilization of the polar group into an ultramicroporous metal–organic framework enabling benchmark inverse selective CO2/C2H2 separation with record C2H2 production, J. Am. Chem. Soc., 2023, 145, 13901–13911 CrossRef CAS PubMed.
- S.-Q. Yang, B. Xing, L.-L. Wang, L. Zhou, F.-Y. Zhang, Y.-L. Li and T.-L. Hu, Boosting Acetylene Packing Density within an Isoreticular Metal–Organic Framework for Efficient C2H2/CO2 Separation, Chem. Biol. Eng., 2024, 1, 245–251 CrossRef CAS.
- W. Chen, P. Cai, H. C. Zhou and S. T. Madrahimov, Bridging Homogeneous and Heterogeneous Catalysis: Phosphine-Functionalized Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2024, 63, e202315075 CrossRef CAS PubMed.
- R. R. Liang, Z. Liu, Z. Han, Y. Yang, J. Rushlow and H. C. Zhou, Anchoring Catalytic Metal Nodes within a Single-Crystalline Pyrazolate Metal-Organic Framework for Efficient Heterogeneous Catalysis, Angew. Chem., Int. Ed., 2025, 64, e202414271 CrossRef CAS PubMed.
- J. He, G. Wen, Q. Peng and X. Hou, The design, synthesis and application of metal–organic framework-based fluorescence sensors, Chem. Commun., 2024, 60, 11237–11252 RSC.
- S.-M. Wang, L. Xu, L.-P. Zhang, Y.-T. Li, T. Wang and Q.-Y. Yang, Rational Design of a π-Electron Rich Co-MOF Enabling Benchmark C2H6/CH4 Selectivity in Natural Gas Purification, Adv. Funct. Mater., 2025, 2504251 CrossRef CAS.
- H. Kim, Y. Seo, J. Park, E. Lee and H. Oh, A Gate-Opening Control Strategy via Nitrate–Chloride Anion Exchange for Enhanced Hydrogen Isotope Separation in Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2025, 64, e202421756 CrossRef CAS PubMed.
- G. Lee, D. Choi and M. Oh, Activating the Gate-Opening of a Metal–Organic Framework and Maximizing Its Adsorption Capacity, J. Am. Chem. Soc., 2025, 147, 12811–12820 CrossRef CAS PubMed.
- Y.-Z. Hao, K. Shao, X. Zhang, Y.-H. Yu, D. Liu, H.-M. Wen, Y. Cui, B. Li, B. Chen and G. Qian, Pore Space Partition Enabled by Lithium(I) Chelation of a Metal–Organic Framework for Benchmark C2H2/CO2 Separation, J. Am. Chem. Soc., 2025, 147, 11257–11266 CrossRef CAS.
- Z. Zhu, J. Xiao, M. Zhang, Y. Wang, K. Xin Yao and S. Yuan, Nonpolar microporous Metal-Organic framework decorated with multiple functional sites for efficient Ethane/Ethylene separation, Sep. Purif. Technol., 2025, 354, 128696 CrossRef CAS.
- J. Xiao, Z. Zhu, M. Zhang, Y. Huang, T. C. Zhang and S. Yuan, Efficient One-Step Purification of Methanol-to-Olefin Products Using a Porphyrinyl MOF to Achieve Record C2H4 and C3H6 Productivity, ACS Appl. Mater. Interfaces, 2025, 17, 21630–21642 CrossRef CAS PubMed.
- M. Jung, J. Park, R. Muhammad, T. Park, S.-Y. Jung, J. Yi, C. Jung, J. Ollivier, A. J. Ramirez-Cuesta, J. T. Park, J. Kim, M. Russina and H. Oh, Lattice-driven gating in a Cu-based zeolitic imidazolate framework for efficient high-temperature hydrogen isotope separation, Nat. Commun., 2025, 16, 2032 CrossRef CAS PubMed.
- B. Pramanik, R. Sahoo, R. Krishna and M. C. Das, A Chemically Robust Microporous Zn-MOF for C2H2 Separation from CO2 and Industrially Relevant Four Component Gas Mixtures, Small, 2025, 21, 2411456 CrossRef CAS PubMed.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n), Science, 1999, 283, 1148–1150 CrossRef CAS.
- J.-S. Zou, Z.-P. Wang, Y. H. Andaloussi, J. Xue, W. Zhang, B. E. G. Lucier, Z. Zhang, Y. Jia, X.-C. Wu, J. Li, Y. Huang, M. J. Zaworotko, G. Chen, S. Chen and Y.-L. Peng, Benchmarking selective capture of trace CO2 from C2H2 using an amine-functionalized adsorbent, Nat. Commun., 2025, 16, 2598 CrossRef CAS PubMed.
- Y. Zhang, Y. Han, B. Luan, L. Wang, W. Yang, Y. Jiang, T. Ben, Y. He and B. Chen, Metal–Organic Framework with Space-Partition Pores by Fluorinated Anions for Benchmark C2H2/CO2 Separation, J. Am. Chem. Soc., 2024, 146, 17220–17229 CrossRef CAS PubMed.
- Y.-L. Zhao, Q. Chen, X. Zhang and J.-R. Li, Enabling C2H2/CO2 Separation Under Humid Conditions with a Methylated Copper MOF, Adv. Sci., 2024, 11, 2310025 CrossRef CAS PubMed.
- L. Zhang, T. Xiao, X. Zeng, J. You, Z. He, C.-X. Chen, Q. Wang, A. Nafady, A. M. Al-Enizi and S. Ma, Isoreticular Contraction of Cage-like Metal–Organic Frameworks with Optimized Pore Space for Enhanced C2H2/CO2 and C2H2/C2H4 Separations, J. Am. Chem. Soc., 2024, 146, 7341–7351 CrossRef CAS PubMed.
- D. Song, S. Zou, Z. Ji, Y. Li, H. Li, Y. Zhou, C. Chen, Q. Chen and M. Wu, One-Step Ethylene Purification from Ternary Mixture through Adaptive Recognition Sites, Angew. Chem., Int. Ed., 2025, 64, e202423496 CrossRef CAS PubMed.
- F. Yuan, Y. Li, Z. Yuan, L. Li, C. Chen, L. He, H. Lin, X. Fan, B. Chen, S. Xiang and Z. Zhang, A Grafting Hydrogen-bonded Organic Framework for Benchmark Selectivity of C2H2/CO2 Separation under Ambient Conditions, Angew. Chem., Int. Ed., 2025, 64, e202414215 CrossRef CAS PubMed.
- Y.-Z. Hao, H.-M. Wen, Y.-H. Yu, X. Zhang, Y. Cui, B. Chen, B. Li and G. Qian, Anchoring Highly Unsaturated Nickel(II) Sites into a Metal–Organic Framework for Simultaneous High C2H2 Adsorption and Separation, Angew. Chem., Int. Ed., 2025, e202506055 CAS.
- P. Zhang, Y. Zhong, Y. Zhang, Z. Zhu, Y. Liu, Y. Su, J. Chen, S. Chen, Z. Zeng and H. Xing, Synergistic binding sites in a hybrid ultramicroporous material for one-step ethylene purification from ternary C2 hydrocarbon mixtures, Sci. Adv., 2022, 8, eabn9231 CrossRef CAS.
- Y. Wang, C. Hao, W. Fan, M. Fu, X. Wang, Z. Wang, L. Zhu, Y. Li, X. Lu, F. Dai, Z. Kang, R. Wang, W. Guo, S. Hu and D. Sun, One-step Ethylene Purification from an Acetylene/Ethylene/Ethane Ternary Mixture by Cyclopentadiene Cobalt-Functionalized Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CrossRef CAS PubMed.
- M. Sun, H. Liu, X. Wang, X. Yang, F. Gao, D. Xie, W. Fan, Y. Han, B. Xu and D. Sun, Metal-ion-tuned metal-organic frameworks for C2H2/CO2 separation, Chin. J. Struct. Chem., 2023, 42, 100146 CAS.
- L. Xu, M.-K. Zhai, F. Wang, L. Sun and H.-B. Du, A series of robust metal–porphyrinic frameworks based on rare earth clusters and their application in N–H carbene insertion, Dalton Trans., 2016, 45, 17108–17112 RSC.
- X. He, S. Gao, R. Peng, D. Zhu and F. Yu, A novel topological indium–organic framework for reversible ammonia uptake under mild conditions and catalysis, J. Mater. Chem. A, 2024, 12, 14501–14507 RSC.
- M. V. Nguyen, H. C. Dong, V. T. N. Truong, H. N. Nguyen, L. C. Luu, N. N. Dang and T. A. T. Nguyen, A new porphyrinic vanadium-based MOF constructed from infinite V(OH)O4 chains: syntheses, characterization and photoabsorption properties, New J. Chem., 2022, 46, 632–641 RSC.
- R. L. Mander, A. Schmidt, M. Ruf and M. D. Korzyński, Design and synthesis of pillared metal–organic frameworks featuring olefinic fragments, Dalton Trans., 2024, 53, 18873–18879 RSC.
- E.-Y. Choi, C. A. Wray, C. Hu and W. Choe, Highly tunable metal–organic frameworks with open metal centers, CrystEngComm, 2009, 11, 553–555 RSC.
- Z. Zhu, J. Xiao, M. Zhang, Y. Huang and S. Yuan, Two isostructural pillar-layered metal-organic frameworks with hydrophobic properties for efficient C2H6/C2H4 separation under humid conditions, Sep. Purif. Technol., 2025, 361, 131343 CrossRef CAS.
- Z.-H. Guo, X.-Q. Wu, Y.-P. Wu, D.-S. Li, G.-P. Yang and Y.-Y. Wang, A Scalable Pore-space-partitioned Metal-organic Framework Powered by Polycatenation Strategy for Efficient Acetylene Purification, Angew. Chem., Int. Ed., 2025, 64, e202421992 CrossRef CAS PubMed.
- J.-W. Cao, T. Zhang, Y.-Q. Liu, Y. Wang, F.-P. Pan, J. Chen and K.-J. Chen, Precise C2H2 Adsorption Affinity Modulation by Nitrogen Functionalization in Isostructural Coordination Networks, Small, 2025, 21, 2501924 CrossRef CAS.
- A. Fateeva, J. Clarisse, G. Pilet, J.-M. Grenèche, F. Nouar, B. K. Abeykoon, F. Guegan, C. Goutaudier, D. Luneau, J. E. Warren, M. J. Rosseinsky and T. Devic, Iron and Porphyrin Metal–Organic Frameworks: Insight into Structural Diversity, Stability, and Porosity, Cryst. Growth Des., 2015, 15, 1819–1826 CrossRef CAS.
- X.-N. Wang, J.-L. Li, Y.-M. Zhao, J. Pang, B. Li, T.-L. Zhang and H.-C. Zhou, Structural tuning of zinc–porphyrin frameworks via auxiliary nitrogen-containing ligands towards selective adsorption of cationic dyes, Chem. Commun., 2019, 55, 6527–6530 RSC.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, One-Step Ethylene Purification from Ternary Mixtures in a Metal–Organic Framework with Customized Pore Chemistry and Shape, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- D. Jia, Z. Shen, W. Zhou, Y. Li, J. He, L. Jiang, Y. Wei and X. He, Ultrahigh N-doped carbon with hierarchical porous structure derived from metal-organic framework for high-performance zinc ion hybrid capacitors, Chem. Eng. J., 2024, 485, 149820 CrossRef CAS.
- J. Liu, H. Xiong, H. Shuai, X. Liu, Y. Peng, L. Wang, P. Wang, Z. Zhao, Z. Deng, Z. Zhou, J. Chen, S. Chen, Z. Zeng, S. Deng and J. Wang, Molecular sieving of iso-butene from C4 olefins with simultaneous high 1,3-butadiene and n-butene uptakes, Nat. Commun., 2024, 15, 2222 CrossRef CAS PubMed.
- X. Liu, P. Zhang, H. Xiong, Y. Zhang, K. Wu, J. Liu, R. Krishna, J. Chen, S. Chen, Z. Zeng, S. Deng and J. Wang, Engineering Pore Environments of Sulfate-Pillared Metal-Organic Framework for Efficient C2H2/CO2 Separation with Record Selectivity, Adv. Mater., 2023, 35, 2210415 CrossRef CAS.
- J. Pei, K. Shao, J. X. Wang, H. M. Wen, Y. Yang, Y. Cui, R. Krishna, B. Li and G. Qian, A Chemically Stable Hofmann-Type Metal–Organic Framework with Sandwich-Like Binding Sites for Benchmark Acetylene Capture, Adv. Mater., 2020, 32, 1908275 CrossRef CAS PubMed.
- H. Zeng, M. Xie, Y. L. Huang, Y. Zhao, X. J. Xie, J. P. Bai, M. Y. Wan, R. Krishna, W. Lu and D. Li, Induced fit of C2H2 in a flexible MOF through cooperative action of open metal sites, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS PubMed.
- S. Shang, Z. Zhou, H. Wang, Y. Wang, X. Liu, Z. Zhu, Y. Zeng, C. Liu, H. Xiong, H. Liu, F. Zhao, J. Chen, S. Chen, Z. Zhou and J. Wang, A Rigid, Stable, and Scalable Aliphatic MOF Adsorbent for Efficient C2H2/CO2 Separation with Record Acetylene Packing Density, Angew. Chem., Int. Ed., 2025, e202503317 CAS.
- Y. L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K. J. Chen, K. A. Forrest, B. Space, P. Cheng and M. J. Zaworotko, Robust ultramicroporous metal–organic frameworks with benchmark affinity for acetylene, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |