Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photogenerated-radical cyclopropylation of in situ generated iminiums mediated by NaI/PPh3: direct access to α-cyclopropyl tertiary alkylamines
Ying
Zhou†
a,
Shan
Wang†
 b,
Yi-Chuan
Liu
a,
Yan
Liu
c,
Fei
Tan
b,
Yi-Chuan
Liu
a,
Yan
Liu
c,
Fei
Tan
 a,
Hongbo
Dong
a and
Jian
Wang
a,
Hongbo
Dong
a and
Jian
Wang
 *a
*a
aAntibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106, P. R. China. E-mail: wangjianchem@cdu.edu.cn
bZhejiang Key Laboratory of Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, China
cSchool of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China
First published on 21st October 2025
Abstract
Using cyclopropyl radicals to install cyclopropanes has been a fast-growing research field in recent years. Meanwhile, direct radical carbonyl alkylative amination has emerged as an ideal protocol for constructing α-branched tertiary amines. Based on the strategy of direct addition of cyclopropyl radicals to in situ generated iminium ions, we disclose a method for preparing diverse α-cyclopropyl tertiary alkylamines by photogenerated-radical cyclopropylation mediated by NaI/PPh3 using abundant feedstocks (aldehydes and amines) and easily procured cyclopropyl active esters. Importantly, NaI/PPh3 works as both the photoinitiator and sacrificial reductant in this reaction and hence gives an economical variant of carbonyl alkylative amination under mild reaction conditions. In addition, the electrochemical variant of this photogenerated-radical cyclopropylation was also investigated with the preliminary results.
Introduction
The cyclopropyl motif can be frequently found in natural products and pharmaceuticals, and it usually possesses unique physicochemical and pharmacokinetic properties.1 As the smallest carbocycles, cyclopropanes have also been widely studied and applied in organic synthesis due to their high ring strain and unique bonding properties.2 Generally, two types of strategies for acquisition of the cyclopropyl unit have been summarized, namely de novo construction of cyclopropyl rings and installation of an existing cyclopropane (Scheme 1a).3De novo cyclopropane syntheses have made great achievements such as classical Simmons–Smith cyclopropanation, the Kulinkovich reaction, Corey–Chaykovsky cyclopropanation, and so on. Driven by the recent renaissance in photochemistry, a variety of new methods based on carbene transfer or radical reactions have been developed to construct cyclopropanes using the de novo strategy under relatively mild conditions.4 On the other hand, traditionally, the direct installation of an existing cyclopropane into target molecules has been focused on transition-metal-catalyzed cross-coupling reactions of cyclopropyl-containing substrates, especially the polar reactions of cyclopropyl nucleophiles.3a,f It's known that two-electron chemistry at cyclopropyl electrophiles can easily lead to cyclopropyl ring opening. In contrast, cyclopropyl-radical-involved processes usually retain the cyclopropane ring.5 Accordingly, using cyclopropyl radicals to install cyclopropanes has been intensively investigated in recent years.6α-Cyclopropyl tertiary alkylamines have appeared in many biologically active compounds and pharmaceuticals (Scheme 1b). But tertiary alkylamines possessing geminal alkyl and cyclopropyl groups at the α-positions of nitrogen are scarce due to the shortage of straightforward methods for their construction. Like other α-branched aliphatic amines, preparing branched α-cyclopropyl tertiary alkylamines via conventional two-component synthesis methods, such as carbonyl reductive amination,7N-alkylation of amines,8 transition metal-catalyzed C–N cross-coupling,9 imine addition,10 and hydroamination of alkenes,11 is often problematic. They may suffer from poor steric hindrance tolerance, overalkylation, high cost and chemical waste from metal catalysts, unstable organometallic reagents, requiring special auxiliary groups, and narrow availability of the starting materials. To address these challenges, some multicomponent reactions based on the strategy of the direct addition of carbon nucleophiles to in situ generated imines or iminium ions were developed, such as Strecker reactions,12 Mannich reactions,13 Petasis boron–Mannich reactions and aza–Morita–Baylis–Hillman reactions.14 However, these aforementioned multicomponent reactions usually require special nucleophiles with attenuated basicity, and a general method for the direct addition of alkyl groups to in situ generated imines and iminium ions, which could rapidly synthesize complex α-branched aliphatic amines, is highly desirable.15
In 2020, Gaunt and co-workers reported a radical carbonyl alkylative amination system for constructing structurally diverse α-branched tertiary amines from commercially available feedstocks under irradiation with blue light-emitting diodes (LEDs) (Scheme 1c).16 They used alkyl iodide to generate a neutral carbon-centred radical through a halogen atom transfer (XAT) step between a silyl radical and alkyl halide. Then, the alkyl radical added to the iminium intermediates in situ generated from secondary amines and aldehydes to form the aminium radical cation, which was rapidly terminated by the silane reagent through hydrogen-atom transfer (HAT). Most importantly, during this reaction, the novel elementary mechanistic step, adding an alkyl radical to positively charged iminium ions, provided great opportunities for the development of new alkyl amine synthetic methods.17 Following this precedent, the addition of carbamoyl and fuoromethyl radicals to iminium ions is successfully realized to prepare corresponding valuable amines.18 Meanwhile, different starting materials working as alkyl iminium ions or neutral alkyl imine sources, such as the combinations of primary amines with α-ketoesters or aldehydes and even secondary amides, were also reported to engage similar radical carbonyl alkylative amination.19 Furthermore, the Gaunt group recently disclosed another two new “higher-order” variants of carbonyl reductive amination, which involved the 2e− addition process to iminium ions using alkyl zinc and 2-azinyl indium species, namely zinc-mediated carbonyl alkylative amination and carbonyl azinylative amination.20 Inspired by the fast-growing higher-order carbonyl reductive amination, we envisioned that cyclopropyl radicals could participate in the addition to iminium ions (Scheme 1d). This assumption enables us to efficiently prepare α-cyclopropyl tertiary alkylamines in a streamlined synthesis method. For this desired reaction manifold, the key points are how to generate the initial radical and how to terminate the aminium radical cation derived from the addition of the radical to iminium ion.
Results and discussion
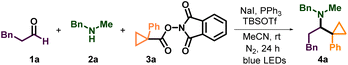
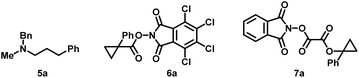
In our initial investigations, the easily procured cyclopropyl N-hydroxyphthalimide (NHPI) ester (3a) was first chosen as the cyclopropyl radical precursor, and N-methylbenzylamine (2a) and hydrocinnamaldehyde (1a) worked as the iminium ion sources. In 2019, the Fu group showed that the combination of sodium iodide and triphenylphosphine (NaI/PPh3) under blue light irradiation could reduce redox-active NHPI esters to generate alkyl radicals.21 Since then, numerous iodide/phosphine-mediated photoredox radical transformations have been reported, which have the advantage of having a more accessible and cost-effective catalyst system.22 Hence, we tried the first condition of the model reaction by using NaI/PPh3 as a photocatalyst and using tris(trimethylsilyl)silane ((Me3Si)3Si–H) as a reductant under blue light irradiation (Table 1, entry 1). The desired amine (4a) was obtained with a 45% isolated yield, and the reductive amination byproduct (5a) was also isolated in 12% yield. Surprisingly, without (Me3Si)3Si–H, in the presence of NaI/PPh3 and tert-butyldimethylsilyl trifluoromethanesulfonate (TBSOTf), the model reaction could also produce 4a and no 5a was detected. During this transformation, triphenylphosphine oxide (TPPO) was generated as the most conspicuous byproduct. Therefore, we conjectured that PPh3 worked not only as a photoinitiator but also as the terminal reductant. Crucially, as there is no reductive amination happening here, we inferred that it would be easy to get a high yield of this reaction through modification of the conditions. After screening of the reaction parameters, the optimized reaction conditions with a yield of 87% were identified to be using blue LED light to irradiate the stirred reaction mixture of 1a (0.4 mmol), 2a (0.4 mmol), 3a (0.2 mmol), NaI (0.4 mmol), PPh3 (0.4 mmol) and TBSOTf (0.4 mmol) in acetonitrile (1 mL) with cooling using a fan for 24 hours (Table 1, entry 2). The use of an excess amount of amine and aldehyde is necessary to achieve high yields, but unreactive amine and aldehyde can be efficiently recovered. Importantly, the one-pot strategy by directly using carboxylic acids as raw materials by their in situ activation without further purification and presynthesis of NHPI esters is also successful (Table 1, entry 3). Control experiments validated that light, NaI and PPh3 are indispensable to conduct this transformation successfully (entry 4–7). Reducing the amount of NaI or PPh3 would make the yield significantly decrease (entry 8 and 9). Other solvents such as ethyl acetate and dichloromethane resulted in lower yields (entry 10 and 11). When TBSOTf was replaced by acetic acid or trimethylsilyl trifluoromethanesulfonate (TMSOTf), the reaction worked but with lower yields (entry 12 and 13). Maintaining a high concentration of the reactive alkyl iminium ion, which captures the alkyl radical, is crucial to obtaining high yield. As such, reactions that used excess amounts of 3a over 1a and 2a gave lower yield than the standard conditions (entry 14). Except for 3a, two other cyclopropyl radical precursors N-hydroxytetrachlorophthalimide (TCNHPI) esters 6a and N-hydroxyphthalimide oxalate 7a were also examined under similar conditions (entry 14 and 15). More easily reduced redox active ester 6a (6a: Ep = −1.213 V vs. Fc+/Fc, 3a: Ep = −1.690 V vs. Fc+/Fc)23 generated the product 4a in a slightly higher yield (entry 15), while 7a could not produce the product at all (entry 16).24| Entry | Deviation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.4 mmol, 2.0 equiv.), 2a (0.4 mmol, 2.0 equiv.), 3a (0.2 mmol, 1.0 equiv.), NaI (0.4 mmol, 2.0 equiv.), PPh3 (0.4 mmol, 2.0 equiv.), TBSOTf (0.4 mmol, 2.0 equiv.), MeCN (1 mL), rt, N2, 24 h, blue LEDs. b Isolated yield. c Yield of 5a is given in parentheses. d (i) 1-Phenyl-1-cyclopropane-carboxylic acid (0.2 mmol), NHPI (0.22 mmol), 4-dimethylaminopyridine (5%), N,N-dicyclohexyl-carbodiimide (0.22 mmol), MeCN, 0 °C to rt, 2 h. (ii) Standard conditions without 3a. e 1a (0.2 mmol, 1.0 equiv.). | ||
| 1c | With 2.0 equiv. (TMS)3SiH as a reductant | 48 (12) |
| 2 | None | 87 |
| 3d | One-pot method using carboxylic acid | 67 |
| 4 | In the dark | 0 |
| 5 | Without NaI | 0 |
| 6 | Without PPh3 | 0 |
| 7 | In the dark, at 50 or 80 °C | 0 |
| 8 | NaI (0.25 equiv.) | 45 |
| 9 | PPh3 (0.25 equiv.) | 14 |
| 10 | In EA | 48 |
| 11 | In DCM | 31 |
| 12 | CH3COOH instead of TBSOTf | 30 |
| 13 | TMSOTf instead of TBSOTf | 51 |
| 14e |
1a/2a/3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
55 |
| 15 | 6a instead of 3a | 90 |
| 16 | 7a instead of 3a | 0 |
|
||
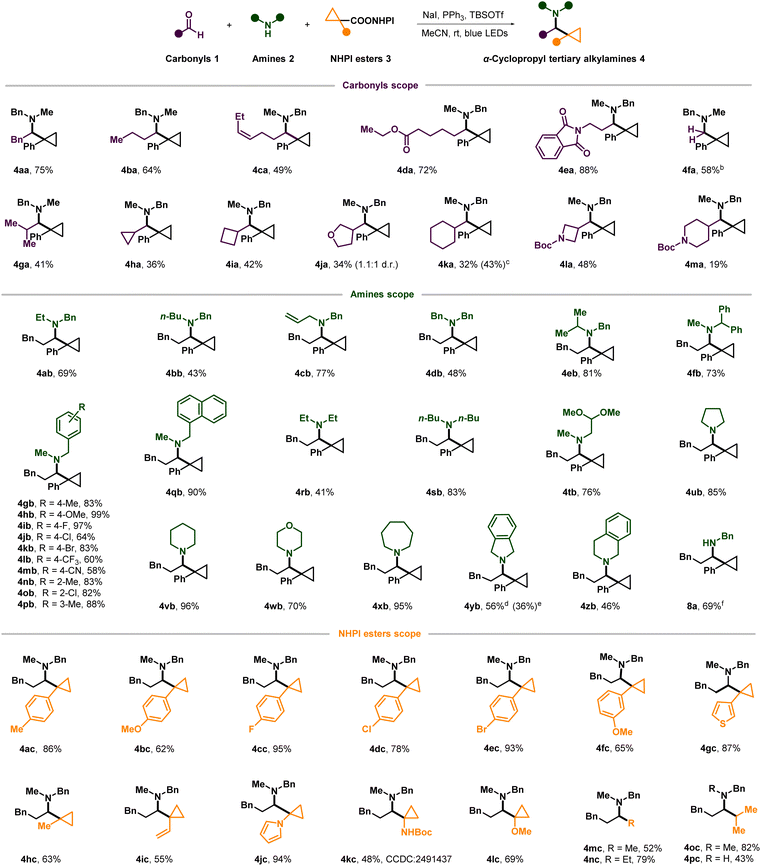
After optimization, we first explored the substrate scope of aldehydes in the benchmark reaction (Scheme 2). Various linear aldehydes, including those bearing carboxylic ester, phthalimide, alkenyl and aryl groups, reacted efficiently to give the desired amines in moderate to high yields (4aa–4ea). Formaldehyde also reacted with 2a and 3a to afford the unbranched amine 4fa using paraformaldehyde as a coupling partner. Branched aldehydes, including strained or unstrained saturated cyclic and heterocyclic rings, resulted in moderate to low yields, presumably due to the corresponding high steric hindrance in condensation or cyclopropyl radical addition and the lower reactivity of less electrophilic iminium ions (4ga–4ma).25 Unfortunately, other more sterically hindered carbonyls such as α-tertiary aldehydes, ketones and α-ketoesters were all totally unreactive in our reaction.
Subsequently, we turned to explore the scope of amines by varying the amine component while retaining hydrocinnam-aldehyde 1a and the redox-active NHPI ester 3a (Scheme 2). Secondary benzylamine derivatives containing the allyl, benzyl, linear or branched alkyl on nitrogen were all amenable to this transformation (4ab–4fb). However, when more sterically hindered tertiary-butyl is connected to the nitrogen center of benzylamine, corresponding photogenerated-radical cyclopropylation didn't work at all. N-methylbenzylamines bearing various substituents attached to the aromatic ring were also widely investigated, and all of them delivered the corresponding products in high yield, except for those which bear a strong electron-withdrawing group at the para position of arene (4gb–4pb). The N-methyl-1-naphthalenyl methanamine reacted smoothly and afforded the desired product with a yield of 90% (4qb). Besides benzylamine derivatives, dialkylated secondary amines also acted as good reaction partners (4rb–4xb), especially for the cyclic amine derivatives of varying ring sizes including pyrrolidine (4ub), piperidine (4vb), morpholine (4wb), and azepane (4xb). In contrast to the aforementioned cyclic amines, benzo-fused cyclic amines such as isoindoline (4yb) and 1,2,3,4-tetrahydroisoquinoline (4zb) showed relatively low activities and resulted in moderate yields (4yb and 4zb). It is needless to mention that amine hydrochloride salt can be used directly in this reaction but with a lower yield (36% of 4yb) compared to the approach where the amine hydrochloride salt was freed beforehand by stirring it with an equal equivalent of triethylamine (Et3N) (56% of yield). Last but not least, after a little modification of the standard reaction conditions by using ethyl acetate as solvent, primary benzylamines were also applicable to this multicomponent reaction to access complex secondary amines directly (8a).
Then, reactions with a variety of cyclopropyl redox-active NHPI esters coupled with compounds 1a and 2a were conducted to assess the applicability of this method (Scheme 2). Various functional groups on the benzene ring directly connected to the cyclopropyl were tolerated, and the yields of cyclopropyl alkylamines were generally high (4ac–4fc).
Noteworthily, although it was not easy to get high yields for tertiary alkylating agents during previously reported carbonyl alkylative amination, a-aryl cyclopropyl NHPI esters can react efficiently in our reaction to furnish α-tertiary cyclopropyl alkylamines in good yields. In addition, heteroaromatic variants (4gc and 4jc) also reacted smoothly during this photocatalyzed process in very high yields (87–94%). Apart from aryl, other groups including methyl and vinyl and even heteroatom groups at the a-position of carboxylic acid NHPI esters on the cyclopropane ring were also tolerated in moderate to high yields, which gave diverse cyclopropanes that enable subsequent elaboration (4hc–4lc). At last, the more common alkyl carboxylic acid NHPI esters could also provide desired tertiary or secondary amines (4mc-4pc) in good yield.
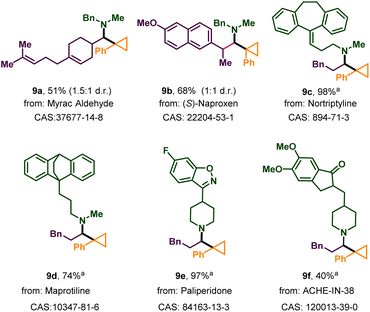
Next, the robustness of the protocol was demonstrated on complex natural products, drugs, or their derivatives (Scheme 3). Myracaldehyde and the aldehyde derived from naproxen reacted with 2a and 3a to afford the corresponding cyclopropylation products 9a and 9b in 51% and 68% yield respectively. Meanwhile, as a less sterically hindered secondary amine, the antidepressant nortriptyline gave the desired product 9c in almost quantitative yields (98%). Maprotiline with a similar structure also reacted efficiently (9d). The substructure of paliperidone (9e), which contains isoxazole, was also tolerated in our reaction in very high yield (97%). ACHE–IN–38, which can alleviate memory deficits in patients with Alzheimer's disease, can be transferred into complex α-cyclopropyl tertiary alkylamines (9f) by this method. In conclusion, all of these results showed the strong practicality of this protocol.
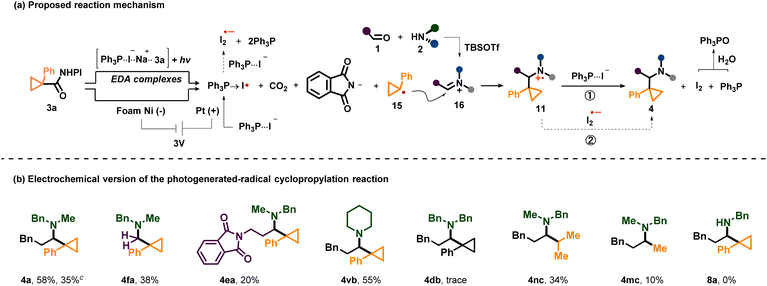
To gain more information about the mechanism of the three-component photogenerated-radical cyclopropylation reaction, we carried out a series of mechanistic experiments. First, we used one prepared iminium salt (IM-1) to replace the corresponding amine and aldehyde to engage in this reaction (Scheme 4a). The product 4fa was isolated in 28% yield, and when TBSOTf was omitted, the yield of 4fa could raise to 62%. These results indicated the in situ formation of the iminium ion and its involvement in the subsequent reactions. It also indicated that TBSOTf might be important during the formation of the iminium ion from 1a and 2a but unnecessary in other fundamental reaction steps. Then, a radical scavenger experiment was carried out, and only 11% yield of 4a was obtained when one equivalent of (2,2,6,6 tetramethylpiperidin-1-yl)oxyl (TEMPO) was added into the model reaction under standard reaction conditions (Scheme 4b). More equivalents of TEMPO could totally inhibit the reaction (see the SI). Meanwhile, the TEMPO adduct of the cyclopropyl radical was detected using a high resolution mass spectrometer (HRMS). Next, we used 1,1-diphenylethylene to successfully couple with the cyclopropyl radical and generate 10a in 24% yield (Scheme 4c). In this system, the model reaction was not thoroughly suppressed and could also proceed in 23% yield. Generally, the results of these two radical capturing experiments indicated that carbon-centered radical generation from NHPI esters was highly possible, which was in accord with previous reports.21 To validate the existence of the aminium radical cation species during the reaction pathway, cyclopropylamine was used as the amine substrate under standard conditions (Scheme 4d). The reaction ultimately yielded only 8a, which could be explained by the fast ring opening of the cyclopropane structure of the aminium radical cation intermediates, proving that the step in which the radical was added to the iminium ion to form the aminium radical cation was feasible.
We then performed UV-visible (UV-vis) spectroscopic absorption experiments on various combinations of reaction components to inspect their possible interactions. An observable redshift of absorption onset occurred when NaI was mixed with redox-active ester (3a) (Fig. 1a-1). Meanwhile, NaI also induced the mixture of aldehyde (1a), amine (2a) and TBSOTf to present one comparatively larger redshift of absorption onset (Fig. 1a and 2). Without TBSOTf, the mixture of NaI, 1a and 2a still gave an obvious redshift of absorption onset compared with the mixture of 1a and 2a (Fig. S2-1). For all three groups of UV-vis experiments, addition of PPh3 induced a slight blueshift of absorption onset compared with the respective redshift system, but only PPh3 cannot have an effect on 3a, or the mixture of 1a and 2a. Consequently, the combination of NaI and PPh3 may activate both the redox-active ester and the mixture of amine and aldehyde under blue LED irradiation. In order to learn more about the effect of TBSOTf on our reaction, we also tested the UV-vis absorption of 3a in the presence of TBSOTf (Fig. S2-2). We found that TBSOTf would inhibit the redshift of 3a caused by NaI/PPh3, which meant that the photoactivation of 3a by NaI/PPh3 might be weakened by TBSOTf. This result may explain why the excess amount of TBSOTf to 1a and 2a will lead to a low yield (Table S1, entry 16). At last, the mixture of 1a, 2a, 3a and TBSOTf didn't show any interaction between them according to the UV-vis spectrum (Fig. S2-4).
Iodide has been reported to be capable of direct single-electron transfer (SET) reduction of carbocation, diazonium, and 1,2-diketones,26 and NaI/PPh3 may have an effect on the iminium ion during our reaction, but the process in which NaI/PPh3 reduces the iminium ion to an a-amino radical followed by final product formation through radical–radical cross-coupling (a-amino radical and cyclopropyl radical) is less possible because α-amino radicals from trialkylamines are readily oxidized (E1/2 = −1.03 V vs. SCE) and unstable under acidic conditions.27 The interaction between NaI and the mixture of 1a and 2a, leading to a decrease in the concentration of iminium ions, may be the reason why this reaction requires an excess amount of NaI, 1a and 2a to achieve high yield. In addition, the light on/off experiments showed that continuous irradiation was necessary for this transformation, and the radical chain mechanism is unlikely (Fig. 1b). From all these results, the two sequential steps of the mechanism, NaI/PPh3 initiating the radical generation from 3a under light irradiation and the generated cyclopropyl radical adding to the in situ generated iminium ions, are clear and proved now.
Next, cyclic voltammetry (CV) studies were performed on NaI (1 mM), PPh3 (1 mM), and their mixture (1 mM NaI and 1 mM PPh3) in acetonitrile (Fig. 1c-1). There was a noticeable increase in the current density and cathodic shift of the onset potential for the mixture of NaI and PPh3, which suggested that complexation of NaI and PPh3 in acetonitrile would form one more easily oxidizable species that should be PPh3⋯I− (12) (the complexation of NaI and PPh3 is exergonic by 4.6 kcal mol−1 in acetonitrile).21 Therefore, even though PPh3 has not shown an obvious effect on 3a in UV-vis experiments, PPh3 still should engage in the initial reduction of 3a. Similarly, 12 may also reduce aminium radical cations 11a to yield the final product 4ad and PPh3 → I˙ (13) radical, which can be terminated by another 13, and these are supported by our computational studies (Fig. 1d-1, and see the SI). Additionally, the CV shows that the first oxidation wave of 4a can be suppressed by the mixture of NaI and PPh3, which means that NaI/PPh3 should be able to reduce 11a (Fig. 1c-2). On the other hand, CV studies revealed that the product 4a exhibited one irreversible oxidation wave with half-wave potentials Eox1/2 = +0.84 V (versus Ag/AgCl; Fig. 1c and 3), and iodine (I2) had the first reduction waves at Ered1/2 = +0.74 V (versus Ag/AgCl; Fig. 1c and 3). These results indicated that aminium radical cations (11) were also able to be reduced by the anionic diiodide radical (I2˙−), which was in accord with the Maulide group's report.28 Meanwhile, the computational studies revealed that the single electron transfer from I2˙− to aminium radical cations was a highly exergonic process (ΔG298(11a → 4ad) = −15.3 kcal mol−1; (Fig. 1d and 2). In our system, I2˙− (14) may come from the reaction between 12 and 13 (see the SI). However, due to the large amounts of NaI and PPh3, we prefer that PPh3⋯I− works as an electron donor for 11 in the secondary SET process of this transformation.
Taking into account all the results obtained from the aforementioned mechanistic studies and previous literature,16,21,28 we propose the mechanism outlined in Scheme 5a. Initially, visible-light irradiation of the transiently formed EDA complexes leads to electron transfer from iodide to the N-phthalimide moiety of 3a to further generate a cyclopropyl radical (15) through extrusion of carbon dioxide and the phthalimide anion. Then, the resulting 15 adds to the positively charged alkyl iminium ions (16) to give the amine radical cation 11, which soon undergoes a SET reduction with two possible electron donors including PPh3⋯I− (12) and I2˙− (14) to deliver the final product 4.
Due to its inherent tunability and scalability, we envisioned that electrochemistry could be applied to our reaction successfully. Based on the proposed reaction mechanism of this photochemical reaction, a large number of electrolysis conditions were screened (see the SI). Finally, 4 could be obtained in moderate yields under electrolysis conditions (6 examples, 10–58% yields) (Scheme 5b). Because NaI, PPh3 and TBSOTf were still indispensable to get products in the electrochemical reaction (electrolyte was not indispensable), we suggested that it had a similar mechanism to the photochemical reaction except that the active ester 3 was reduced at the cathode and PPh3⋯I− (12) was oxidized to PPh3 → I˙ (13) at the anode.
Conclusions
In summary, we have developed a general photochemical method of preparing diverse α-cyclopropyl tertiary alkylamines by radical cyclopropylation using abundant feedstocks (aldehydes and amines) and easily procured cyclopropyl active esters under mild and metal-free reaction conditions. Notably, this methodology used NaI/PPh3 as both the photoinitiator and electron donor, and no other traditional dye- or metal complex-based photoredox catalyst and expensive sacrificial reductant was needed. Meanwhile, a preliminary electrochemical version of this photogenerated-radical cyclopropylation was also developed. Mechanistic studies indicate that the reaction proceeds via the addition of the cyclopropyl radical, which is generated by visible-light-induced SET during the formation of EDA complexes upon aggregation of NaI/PPh3 and NHPI ester, to the in situ generated iminium ions from amines and aldehydes to obtain the aminium radical cations, which are finally terminated by reactive intermediates derived from NaI/PPh3 through the secondary SET.Author contributions
The manuscript was written through the contributions of all authors and all authors have given approval to the final version.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2491437 contains the supplementary crystallographic data for this paper.29All data associated with this work can be found in the supplementary information (SI). Supplementary information: experimental procedures and compound characterization. See DOI: https://doi.org/10.1039/d5sc06039g.
Acknowledgements
We gratefully acknowledge financial support from the Fund of Chengdu University (No. T202315), Sichuan Science and Technology Program (NO. 2024NSFSC1126) and National Natural Science Foundation of China (82003619).Notes and references
- (a) T. T. Talele, J. Med. Chem., 2016, 59, 8712 CrossRef CAS PubMed; (b) S. S. Uthumange, A. J. H. Liew, X. W. Chee and K. Y. Yeong, Bioorg. Med. Chem., 2024, 116, 117980 CrossRef CAS.
- A. de Meijere, Angew. Chem., Int. Ed., 1979, 18, 809 CrossRef.
- (a) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (b) C. Ebner and E. M. Carreira, Chem. Rev., 2017, 117, 11651 CrossRef CAS PubMed; (c) J. Wenbing, Y. Hua and T. Gongli, Chin. J. Org. Chem., 2018, 38, 2324 CrossRef; (d) W. Wu, Z. Lin and H. Jiang, Org. Biomol. Chem., 2018, 16, 7315 RSC; (e) Z. L. Chen, Y. Xie and J. Xuan, Eur. J. Org Chem., 2022, e202201066 CrossRef CAS; (f) A. L. Gabbey, K. Scotchburn and S. A. L. Rousseaux, Nat. Rev. Chem., 2023, 7, 548 CrossRef CAS PubMed; (g) C. B. Kelly, L. Thai-Savard, J. Hu, T. B. Marder, G. A. Molander and A. B. Charette, ChemCatChem, 2024, 16, e202400110 CrossRef CAS; (h) M. Liu and C. Uyeda, Angew. Chem., Int. Ed., 2024, 63, e202406218 CrossRef CAS; (i) X. Han, N. Zhang, Q. Li, Y. Zhang and S. Das, Chem. Sci., 2024, 15, 13576 RSC; (j) P. I. C. Godinho, R. G. Soengas and A. M. S. Silva, Synthesis, 2025, 57, 1769 CrossRef CAS; (k) N. Wang, J.-X. Zhao and J.-M. Yue, Org. Chem. Front., 2025, 12, 2439 RSC.
- Recent examples of visible light-mediated cyclopropanation using the de novo strategy: (a) B. T. Boyle, N. W. Dow, C. B. Kelly, M. C. Bryan and D. W. C. MacMillan, Nature, 2024, 631, 789 CrossRef CAS PubMed; (b) D. P. Poudel, A. Pokhrel, R. K. Tak, M. Shankar and R. Giri, Science, 2023, 381, 545 CrossRef CAS PubMed; (c) D. M. Fischer, H. Lindner, W. M. Amberg and E. M. Carreira, J. Am. Chem. Soc., 2023, 145, 774 CrossRef CAS PubMed; (d) J. H. G. Teye-Kau, M. J. Ayodele and S. P. Pitre, Angew. Chem., Int. Ed., 2024, 63, e202316064 CrossRef CAS PubMed; (e) P. Pérez-Ramos, P. I. C. Godinho, R. G. Soengas and H. Rodríguez-Solla, RSC Adv., 2025, 15, 15155 RSC; (f) Y. Zhang, J. Wang, X. He, S. Peng, L. Yuan, G. Huang, Y. Guo and X. Lu, Adv. Sci., 2025, 12, e2411788 CrossRef PubMed; (g) Z. Wang, Y. Chen, J. Li and C. Zhu, Sci. China:Chem., 2024, 68, 241 CrossRef; (h) L.-X. Li, J. Zhao, C.-R. Li, G. Sun, X. Guo, H.-W. Li, B. Qiao, Z. Li, K. Hu and Z. Zhang, Org. Chem. Front., 2024, 11, 1062 RSC; (i) Y. Tian, Y. Zhang, J. Niu and C. Zhang, ChemCatChem, 2024, 16, e202400972 CrossRef CAS; (j) S. Y. Wen, J. J. Chen, Y. Zheng, J. X. Han and H. M. Huang, Angew. Chem., Int. Ed., 2025, 64, e202415495 CrossRef CAS PubMed; (k) D. D. Snabilie, R. Ham, J. N. H. Reek and B. de Bruin, Organometallics, 2024, 43, 1299 CrossRef CAS PubMed; (l) Z. Mu, H. Xie, S. Gan, H. Li, Y. Hou, M. Qian, S. Zhao and X. Chen, Tetrahedron, 2024, 167, 134296 CrossRef CAS; (m) R. R. Indurmuddam, P. C. Huang, B. C. Hong and S. Y. Chien, Org. Lett., 2024, 26, 5752 CrossRef CAS PubMed; (n) J. Aragon, S. Sun, S. Fernandez and J. Lloret-Fillol, Angew. Chem., Int. Ed., 2024, 63, e202405580 CrossRef CAS PubMed; (o) J. P. Milton, A. Milanowski, M. Andersson and D. Gryko, Chem Commun, 2024, 60, 4483 RSC; (p) L. Huai, L. Zhang, Z. Wang, H. Wub and Y. Fang, Org. Chem. Front., 2023, 10, 1245 RSC; (q) D. Qi, J. Bai, Y. Yao and C. Liu, Org. Biomol. Chem., 2025, 23, 2823 RSC; (r) C. Zhu, S. Das, A. Guin, C. K. De and B. List, Nat. Catal., 2025, 8, 487 CrossRef CAS; (s) S. Mondal, S. Pramanik and S. Maity, Chem Commun, 2025, 61, 7999 RSC.
- L. R. Mills, J. J. Monteith, G. dos Passos Gomes, A. Aspuru-Guzik and S. A. L. Rousseaux, J. Am. Chem. Soc., 2020, 142, 13246 CrossRef CAS PubMed.
- Recent examples of using cyclopropyl radicals to install cyclopropanes: (a) R. Giri, P. K. Peng, A. J. Fernandes, S. Yu, J. G. West and D. Katayev, Angew. Chem., Int. Ed., 2025, 64, e202508377 CrossRef CAS PubMed; (b) S. Timmann, M. T. H. Dilchert, J. Dietzel, V. S. Poltl, M. R. Wennekamp, C. Golz and M. Alcarazo, ACS Catal., 2025, 15, 7232 CrossRef CAS PubMed; (c) Z. Gao, L. Liu, J.-R. Liu, W. Wang, N.-Y. Yang, L. Tao, Z.-L. Li, Q.-S. Gu and X.-Y. Liu, Nat. Synth., 2024, 4, 84 CrossRef; (d) J. J. Monteith, J. W. Pearson and S. A. L. Rousseaux, Angew. Chem., Int. Ed., 2024, 63, e202402912 CrossRef CAS PubMed; (e) X. K. He, L. Q. Lu, B. R. Yuan, J. L. Luo, Y. Cheng and W. J. Xiao, J. Am. Chem. Soc., 2024, 146, 18892 CrossRef CAS PubMed; (f) J. Hu, T. Xia, X. Wu, H. Feng, J. Qu and Y. Chen, Org. Chem. Front., 2024, 11, 6311 RSC; (g) J. Pan, H. Qu, Y. Li, X. Bu, H. Deng, H. Gong, M. Ma, L. Xu and F. Xue, J. Org. Chem., 2024, 89, 16929 CrossRef CAS PubMed; (h) Z. Feng, L. Riemann, Z. Guo, D. Herrero, M. Simon, C. Golz, R. A. Mata and M. Alcarazo, Angew. Chem., Int. Ed., 2023, 62, e202306764 CrossRef CAS PubMed; (i) C. Liu, T. Feng, X. Wu and C. Zhu, ACS Catal., 2023, 13, 8394 CrossRef CAS; (j) S. Biswas, P. Chandu, S. Garai and D. Sureshkumar, Org. Lett., 2023, 25, 7863 CrossRef CAS PubMed; (k) B. Li, J. L. Shi and Y. Xia, Org. Lett., 2023, 25, 2674 CrossRef CAS PubMed.
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov and D. Chusov, Chem. Rev., 2019, 119, 11857 CrossRef CAS PubMed.
- D. G. Brown and J. Böstrom, J. Med. Chem., 2016, 59, 4443 CrossRef CAS PubMed.
- A. Trowbridge, S. M. Walton and M. J. Gaunt, Chem. Rev., 2020, 120, 2613 CrossRef CAS PubMed.
- R. Bloch, Chem. Rev., 1998, 98, 1407 CrossRef CAS PubMed.
- T. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed.
- V. V. Kouznetsov and C. E. P. Galvis, Tetrahedron, 2018, 74, 773 CrossRef CAS.
- J. F. A. Filho, B. C. Lemos, A. S. de Souza, S. Pinheiro and S. J. Greco, Tetrahedron, 2017, 73, 6977 CrossRef.
- (a) P. Wu, M. Givskov and T. E. Nielsen, Chem. Rev., 2019, 119, 11245 CrossRef CAS PubMed; (b) V. Declerck, J. Martinez and F. Lamaty, Chem. Rev., 2009, 109, 1 CrossRef CAS PubMed.
- (a) C. Hu, J. Tsien, S.-J. Chen, M. Kong, R. R. Merchant, Y. Kanda and T. Qin, J. Am. Chem. Soc., 2024, 146, 21769 CrossRef PubMed; (b) L. H. Choudhury and T. Parvin, Tetrahedron, 2011, 67, 8213 CrossRef CAS PubMed.
- R. Kumar, N. J. Floden, W. G. Whitehurst and M. J. Gaunt, Nature, 2020, 581, 415 CrossRef CAS PubMed.
- J. Tauber, D. Imbri and T. Opatz, Molecules, 2014, 19, 16190 CrossRef PubMed.
- (a) J. Liu and M. J. Gaunt, J. Am. Chem. Soc., 2024, 146, 24699 CrossRef CAS PubMed; (b) P. J. Deneny, R. Kumar and M. J. Gaunt, Chem. Sci., 2021, 12, 12812 RSC.
- (a) J. H. Blackwell, R. Kumar and M. J. Gaunt, J. Am. Chem. Soc., 2021, 143, 1598 CrossRef CAS PubMed; (b) M. A. Smith, R. J. D. Kang, R. Kumar, B. Roy and M. J. Gaunt, Chem. Sci., 2024, 15, 14888 RSC; (c) A. Pulcinella, S. Bonciolini, R. Stuhr, D. Diprima, M. T. Tran, M. Johansson, A. J. von Wangelin and T. Noël, Nat. Commun., 2025, 16, 948 CrossRef CAS PubMed.
- (a) J. M. Phelps, R. Kumar, J. D. Robinson, J. C. K. Chu, N. J. Flodén, S. Beaton and M. J. Gaunt, J. Am. Chem. Soc., 2024, 146, 9045 CrossRef CAS PubMed; (b) A. A. Rafaniello, R. Kumar, R. C. Phillips and M. J. Gaunt, Angew. Chem., Int. Ed., 2024, 63, e202408287 CAS.
- M. C. Fu, R. Shang, B. Zhao, B. Wang and Y. Fu, Science, 2019, 363, 1429 CrossRef CAS PubMed.
- (a) T. Liu, Y. Zhou, J. Tang and C. Wang, Beilstein J. Org. Chem., 2023, 19, 1785 CrossRef CAS PubMed; (b) L. Yang, Y. Y. Tang, S. Y. Gao and P. L. Zhang, Chem Commun, 2025, 61, 10558 RSC; (c) D. X. Jiang, Z. Chen, Q. Shu, Y. Tan, J. Chen, X. Zhang, Z. Lei, Z. Wang and Y. F. Zeng, Org. Biomol. Chem., 2025, 23, 9094 RSC; (d) J. L. Sui, X. Q. Liu, S. D. Li, P. F. Huang, Y. Liu and J. H. Li, Chin. J. Chem., 2024, 42, 3373 CrossRef CAS; (e) Y. Wang, Z. Wang, H. Luo, G. Wang, Z. Shi, Y. Ma, J. Wu, D. Li and J. Yang, Org. Chem. Front., 2024, 11, 5524 RSC; (f) Q. Li, Z.-Q. Zhu, W.-Y. Zhang, Z.-G. Le and Z.-B. Xie, Org. Biomol. Chem., 2024, 22, 965 RSC; (g) J. X. Wang, M. C. Fu, L. Y. Yan, X. Lu and Y. Fu, Adv. Sci., 2024, 11, 2307241 CrossRef CAS PubMed; (h) M. Moczulski, A. Artelska, Ł. Albrecht and A. Albrecht, Eur. J. Org Chem., 2022, e202200630 CrossRef CAS; (i) R.-H. Li, Y.-L. Zhao, Q.-K. Shang, Y. Geng, X.-L. Wang, Z.-M. Su, G.-F. Li and W. Guan, ACS Catal., 2021, 11, 6633 CrossRef CAS; (j) X. J. Liu, S. Y. Zhou, Y. Xiao, Q. Sun, X. Lu, Y. Li and J. H. Li, Org. Lett., 2021, 23, 7839 CrossRef CAS PubMed; (k) Z. Qu, X. Chen, S. Zhong, G.-J. Deng and H. Huang, Org. Lett., 2021, 23, 5349 CrossRef CAS PubMed; (l) Y.-T. Wang, M.-C. Fu, B. Zhao, R. Shang and Y. Fu, Chem Commun, 2020, 56, 2495 RSC; (m) K. Wadekar, S. Aswale and V. R. Yatham, RSC Adv., 2020, 10, 16510 RSC; (n) H. Y. Wang, L. J. Zhong, G. F. Lv, Y. Li and J. H. Li, Org. Biomol. Chem., 2020, 18, 5589 RSC.
- D. C. Salgueiro, B. K. Chi, I. A. Guzei, P. Garcia-Reynaga and D. J. Weix, Angew. Chem., Int. Ed., 2022, 61, e202205673 CrossRef CAS PubMed.
- L. R. Mills, J. J. Monteith, G. dos Passos Gomes, A. Aspuru-Guzik and S. A. L. Rousseaux, J. Am. Chem. Soc., 2020, 142, 13246 CrossRef CAS PubMed.
- For example, for 4ka, yield can be increased by about 10% by using more equivalents of amines and aldehydes.
- J. A. Luján-Montelongo, J. B. Mateus-Ruiz and R. M. Valdez-García, Eur. J. Org Chem., 2023, 26, e202201156 CrossRef.
- K. Nakajima, Y. Miyake and Y. Nishibayashi, Acc. Chem. Res., 2016, 49, 1946 CrossRef CAS PubMed.
- M. Lemmerer, V. Tona, D. Just, M. Vavrík, B. Maryasin, G. D. Mauro, A. B. ȥur Bonsen, D. Kaiser and N. Maulide, Angew. Chem., Int. Ed., 2025, 64, e202409688 CrossRef CAS PubMed.
- CCDC 2491437: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmjyt.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |