Open Access Article
Open Access ArticleSelf-sorting systems of photoresponsive polymer micelles: isomerization drives reversible and switchable self-assembly
Rikuto
Kanno
 ,
Makoto
Ouchi
,
Makoto
Ouchi
 and
Takaya
Terashima
and
Takaya
Terashima
 *
*
Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: terashima.takaya.2e@kyoto-u.ac.jp
First published on 16th October 2025
Abstract
Herein, we report self-sorting systems of photoresponsive polymer micelles obtained from amphiphilic statistical copolymers bearing hydrophilic poly(ethylene glycol) (PEG) chains and hydrophobic/photoresponsive azobenzene (Azo) groups. The PEG/Azo copolymers self-folded or intermolecularly self-assembled via the association of the azobenzene pendants to form photoresponsive unimer or multichain micelles in water, where the core-forming Azo groups show cis/trans isomerization in response to UV/vis irradiation. The PEG/Azo copolymer micelles not only induced self-sorting in the presence of other PEG copolymer micelles with different compositions and/or pendants but also exhibited photoresponsive and reversible self-sorting/co-self-assembly with an anionic copolymer micelle via the cis/trans isomerization of the Azo units. We successfully developed self-sorting and co-self-assembly systems of binary polymer mixtures that can be dually controlled by molecular design and UV/vis light without using externally added reagents.
Introduction
Light is a ubiquitous, non-destructive, and permeable energy source and thereby effective as an external trigger to modulate the structures (e.g., stereoisomerism), motion, and association properties of molecules for desired functions. The importance of light is highlighted in photoresponsive self-assembly systems in nature: for instance, rhodopsin, a light-sensitive receptor protein, converts light signals into visual information through the photoisomerization of its retinal chromophore.1 Such examples have inspired chemists to create photoresponsive amphiphilic self-assemblies2 and photoresponsive supramolecular polymers,3,4 among others.5,6An even more attractive possibility is to use light for controlling self-sorting phenomena, in which molecules in complex media containing multiple components selectively recognize and organize themselves through dynamic interactions among each other.7–9 Analogous to precision self-assembly of proteins and DNA in vivo,10–12 self-sorting systems based on supramolecular compounds13–22 and synthetic polymers23–30 have been created. For instance, low-molecular-weight supramolecular compounds can recognize themselves from non-selves based on structural factors including size,13,14 shape,15–17 and chirality18 through noncovalent interaction such as hydrogen bonding. Self-sorting on macromolecules can also be realized by tuning primary structures: molecular weight,23,30 tacticity,24,25 monomer unit structures,26 and composition.27–29 Introducing stimuli-responsive properties to such self-sorting systems would allow reversible switching of the association modes of macromolecules in response to external stimuli including light, whereas such switchable self-sorting systems are rare among previous achievements.22
Our group has developed self-assembly and self-sorting systems of amphiphilic random copolymer micelles in water.27–32 Random copolymers bearing hydrophilic poly(ethylene glycol) (PEG) and hydrophobic alkyl groups as side chains induce chain-folding via the association of the hydrophobic segments, like proteins, in water to form small micelles. The size of the micelles is predominantly determined by the hydrophilic/hydrophobic balance, i.e., composition or side chain structures. Thus, binary PEG random copolymers with different compositions and/or alkyl groups self-sort to simultaneously form discrete micelles.27–30 In contrast, such a PEG random copolymer co-self-assembles with an ionic random copolymer into a PEG/ion-fused micelle in pure water, and the fused micelle is separated into discrete PEG or ionic micelles in water containing a salt.31,32 These results imply that folding polymer micelles containing light-sensitive units open a new possibility to create self-sorting systems controlled by not only molecular structures (e.g., molecular weight, composition, and side chain functional groups) as encoded information but also light as an external trigger.
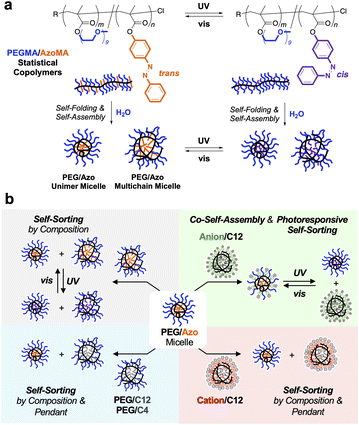
Herein, we report self-sorting systems of photoresponsive polymer micelles in water (Fig. 1a). For this, we designed photoresponsive amphiphilic statistical copolymers bearing hydrophilic PEG and hydrophobic azobenzene (Azo) units as side chains (Fig. 1a), focusing on the fact that Azo units are reversibly switchable between a thermodynamically stable trans-state and a metastable cis-state upon visible and UV light irradiation, respectively.33–37 The PEG/Azo statistical copolymers induce self-folding or intermolecular self-assembly via the association of the hydrophobic Azo pendants and form unimer or multichain micelles, depending on the content of the Azo units. The Azo units within the micelle cores allow reversible isomerization between trans and cis states in water by irradiating visible (470 nm) and UV (365 nm) light, respectively. Importantly, the PEG/Azo copolymer micelles not only induced self-sorting, depending on the difference of the composition and pendant structures, but also afforded photoresponsive self-sorting/co-self-assembly with an anionic copolymer micelle via the reversible isomerization of the Azo units (Fig. 1b). To the best of our knowledge, this is the first example of self-sorting/co-self-assembly systems of polymers reversibly controlled by light in complex aqueous media.
Results and discussion
Design and synthesis of PEG/Azo copolymers
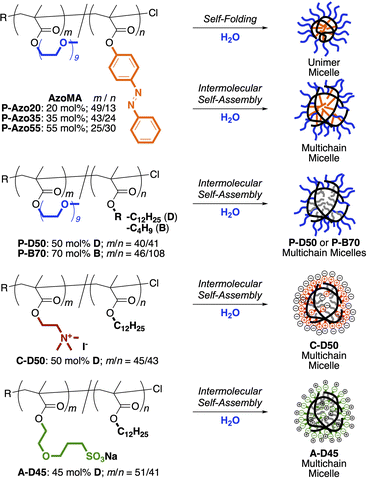
The PEG/Azo statistical copolymers with different compositions (Azo = 20, 35, 55 mol%) were synthesized by ruthenium-catalyzed living radical copolymerization of PEG methyl ether methacrylate (PEGMA, Mn = 500 g mol−1, average oxyethylene units = 9) and (E)-4-(phenyldiazenyl)phenyl methacrylate (AzoMA)37 with ethyl 2-chloro-2-phenylacetate (ECPA) as a chloride initiator (Scheme S1). According to the AzoMA content, the PEG/Azo copolymers were named P-Azo20, P-Azo35, and P-Azo55. The feed ratio of PEGMA and AzoMA in the copolymerization was set to 85/15, 70/30, 55/45 to modulate the copolymer composition.In all the cases, the two monomers were smoothly and simultaneously consumed to give PEGMA/AzoMA statistical copolymers with a controlled molecular weight and narrow molecular weight distribution (Mn = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200–27
200–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, Mw/Mn < 1.3, by size-exclusion chromatography (SEC) in N,N-dimethylformamide (DMF) with poly(methyl methacrylate) standard calibration, Fig. S1). Although AzoMA was consumed faster than PEGMA, the averaged AzoMA content estimated from the conversion in the copolymerization was almost constant along a polymer chain without biassed sequence distribution such as gradient copolymers. The degree of polymerization of PEGMA and AzoMA units in the copolymers (m/n) was calculated from the initial monomer feed ratio and the monomer conversion. The m/n values agreed well with the monomer unit composition estimated from the area ratio of the monomer units in 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S2): m/n = 49/13 (P-Azo20), 43/24 (P-Azo35), 25/30 (P-Azo55) (Fig. 2 and Table 1).
200 g mol−1, Mw/Mn < 1.3, by size-exclusion chromatography (SEC) in N,N-dimethylformamide (DMF) with poly(methyl methacrylate) standard calibration, Fig. S1). Although AzoMA was consumed faster than PEGMA, the averaged AzoMA content estimated from the conversion in the copolymerization was almost constant along a polymer chain without biassed sequence distribution such as gradient copolymers. The degree of polymerization of PEGMA and AzoMA units in the copolymers (m/n) was calculated from the initial monomer feed ratio and the monomer conversion. The m/n values agreed well with the monomer unit composition estimated from the area ratio of the monomer units in 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S2): m/n = 49/13 (P-Azo20), 43/24 (P-Azo35), 25/30 (P-Azo55) (Fig. 2 and Table 1).
 | ||
| Fig. 2 Self-folding or intermolecular self-assembly of amphiphilic statistical or random copolymers into unimer or multichain micelles in water. | ||
| Polymera | AzoMAb (mol%) NMR | m/nc calcd | M n (g mol−1) SEC | M w/Mnd SEC | M n (g mol−1) calcd | M w,DMF (g mol−1) MALLS |
|---|---|---|---|---|---|---|
| a P-Azo20, P-Azo35, and P-Azo55 were synthesized by living radical copolymerization of poly(ethylene glycol) methyl ether methacrylate (PEGMA) and (E)-4-(phenyldiazenyl)phenyl methacrylate (AzoMA). b AzoMA content (mol%) determined by 1H NMR. c Degrees of polymerization of PEGMA (m) and AzoMA (n) were calculated from the initial monomer feed ratio and the final conversion of each monomer species. The calculated composition agreed well with the values (mol%, NMR) estimated from the area ratio of their pendant units in the 1H NMR spectra of the purified polymers. d Number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of P-Azo20, P-Azo35 and P-Azo55 determined by SEC in DMF containing 10 mM LiBr with PMMA standard calibration. e M n of the copolymers calculated from the initial monomer feed ratio, the final conversion of each monomer species, and the molecular weight of the monomers. f Absolute weight-average molecular weight of the polymers (Mw) determined by SEC-MALLS in DMF (10 mM LiBr). | ||||||
| P-Azo20 | 20 | 49/13 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.28 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| P-Azo35 | 35 | 43/24 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.29 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| P-Azo55 | 55 | 25/30 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.29 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Besides, other amphiphilic random copolymers carrying different side chains were prepared (Table S1 and Fig. 2): P-D50 or B70 bearing PEG and alkyl groups (P-D50; 50 mol% dodecyl units, or P-B70; 70 mol% butyl units),29,32A-D45 bearing a sulfonate anion and a dodecyl group (45 mol%),32 and C-D50 bearing a quaternary ammonium cation and a dodecyl group (50 mol%).31
Self-assembly of PEG/Azo copolymers into micelles in water
Micellization of PEG/Azo statistical copolymers in water was evaluated using SEC equipped with a multi-angle laser light scattering detector (SEC-MALLS) (Fig. 3a–c and Table 2). The bulk polymers were dissolved in water containing 100 mM NaCl. The aqueous solution was sonicated for 10 minutes at 25 °C and kept for 1 h at 25 °C. After filtering with a membrane filter (pore size = 0.45 μm), the aqueous solutions of the polymer micelles were injected into SEC-MALLS in water containing 100 mM NaCl as an eluent to determine the absolute weight-average molecular weight (Mw,H2O) and aggregation number (Nagg) (for details of the sample preparation see the SI). Note that all the injected samples were prepared in water containing 100 mM salts whose concentration and species were identical to those included in eluents for the aqueous SEC.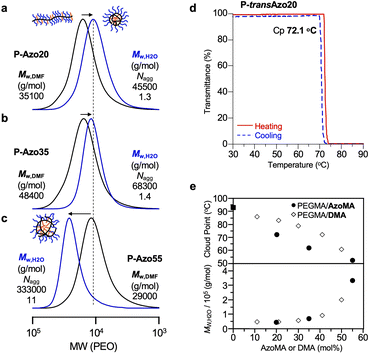 | ||
| Fig. 3 Self-assembly of PEG/azo statistical copolymers in water. (a–c) SEC curves of (a) P-Azo20, (b) P-Azo35, and (c) P-Azo55 in DMF (black lines) and their micelles in H2O containing 100 mM NaCl (blue lines): [polymer] = 10 mg mL−1 in DMF or 1 mg mL−1 in H2O with 100 mM NaCl. (d) Thermoresponsive solubility of P-transAzo20 micelles in water. Transmittance of the aqueous solutions of the polymer micelles was monitored at 670 nm upon heating at 1 °C min−1 from 30 to 90 °C (red lines) and subsequent cooling at −1 °C min−1 from 90 to 30 °C (blue dashed lines): [polymer] = 4.0 mg mL−1. (e) Mw,H2O and cloud point (Cp) temperature of PEGMA/AzoMA statistical copolymer micelles and PEGMA/DMA (dodecyl methacrylate) random copolymers/micelles in water. Mw,H2O and Cp were determined by SEC-MALLS and transmittance measurements upon heating. Mw,H2O and Cp values of the PEGMA/DMA copolymers in water are cited from ref. 40. | ||
| Polymera | R h (nm) | M w,H2O (g mol−1) | N agg | C p (°C) | CMCe (mg mL−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| trans | cis | trans | cis | trans | cis | trans | cis | ||
| a Hydrodynamic radius (Rh) of the polymer micelles was determined by DLS in H2O containing 100 mM NaCl at 25 °C: [polymer] = 1.0 mg mL−1. b Absolute weight-average molecular weight of the polymer micelles (Mw,H2O) was determined by SEC-MALLS in H2O containing 100 mM NaCl as an eluent. c Aggregation number (Nagg) of the polymer micelles was estimated as follows: Nagg = Mw,H2O (MALLS)/Mw,DMF (MALLS). d Cloud point temperature (Cp) was determined by monitoring the transmittance of the aqueous solutions of their polymer micelles ([polymer] = 4.0 mg mL−1) at 670 nm upon heating from 25 °C to 90 °C. Cp was defined as the temperature at which the transmittance became 90%. e Critical micellization concentration (CMC) of the copolymers in water was determined by using Nile Red. | |||||||||
| P-Azo20 | 5.0 | 5.5 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 | 1.3 | 72 | 72 | 0.010 |
| P-Azo35 | 4.4 | 4.9 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.4 | 1.3 | 62 | 62 | 0.005 |
| P-Azo55 | 6.6 | 6.7 | 333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
297![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11 | 10 | 52 | 51 | 0.005 |
P-Azo20, P-Azo35, and P-Azo55 self-assembled into uniform micelles in water, whereas the Mw,H2O, hydrodynamic radius (Rh), and Nagg depended on the composition. Mw,H2O of their micelles increased from 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 to 333
500 to 333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 on increasing the Azo content from 20 to 55 mol%. P-Azo20 and P-Azo35 mostly formed unimer micelles (Nagg ∼ 1), while a more hydrophobic P-Azo55 formed a multichain micelle (Nagg ∼ 11). As analyzed by dynamic light scattering (DLS), Rh for the P-Azo55 micelle (6.6 nm) was larger than that for the P-Azo35 micelle (4.4 nm) (Fig. S3).
000 on increasing the Azo content from 20 to 55 mol%. P-Azo20 and P-Azo35 mostly formed unimer micelles (Nagg ∼ 1), while a more hydrophobic P-Azo55 formed a multichain micelle (Nagg ∼ 11). As analyzed by dynamic light scattering (DLS), Rh for the P-Azo55 micelle (6.6 nm) was larger than that for the P-Azo35 micelle (4.4 nm) (Fig. S3).
PEG/Azo copolymer micelles further showed lower critical solution temperature (LCST)-type thermoresponsive solubility in water (Fig. 3d). The cloud point temperatures (Cp) of the polymer micelles decreased with increasing Azo units (Fig. 3e and S4). The size (Mw,H2O, Rh) of the PEG/Azo statistical copolymer micelles was close to that of corresponding PEG/dodecyl random copolymer micelles (Fig. 3e).40 In contrast, Cp for PEG/Azo copolymer micelles was lower than that for PEG/dodecyl counterparts. This implies that the Azo units promote intermolecular aggregation of their micelles upon heating. Critical micellization concentration (CMC) of the copolymers was determined to be approximately 0.005–0.01 mg mL−1 by using the Nile Red chromophore (Fig. S5).
Photoresponsive isomerization of PEG/Azo copolymers
Photoresponsive isomerization of the PEG/Azo copolymers was evaluated by UV/vis spectroscopy. An as-prepared aqueous solution of a P-Azo20 micelle showed a UV-vis spectrum with a strong band around 320 nm and a weak one around 430 nm, which correspond to the arrowed π–π* transition and the forbidden n–π* transition, respectively, characteristic of trans-azobenzene (Fig. 4a, black line). After UV (λ = 365 nm) irradiation for 600 seconds, the π–π* transition band decreased and the n–π* transition band in turn slightly increased, indicative of the isomerization of the trans-Azo units into the cis-state within the micelle core (Fig. 4a). The irradiation time was then varied from 5 seconds to 600 seconds to evaluate the isomerization behavior of the Azo units. The isomerization quickly proceeded to be saturated just after 60 seconds of irradiation to reach the photostationary state (Fig. 4f and S6). By irradiating visible light (λ = 470 nm), the PEG/cis-Azo micelle was again transformed to its initial trans-state (Fig. 4a, red line).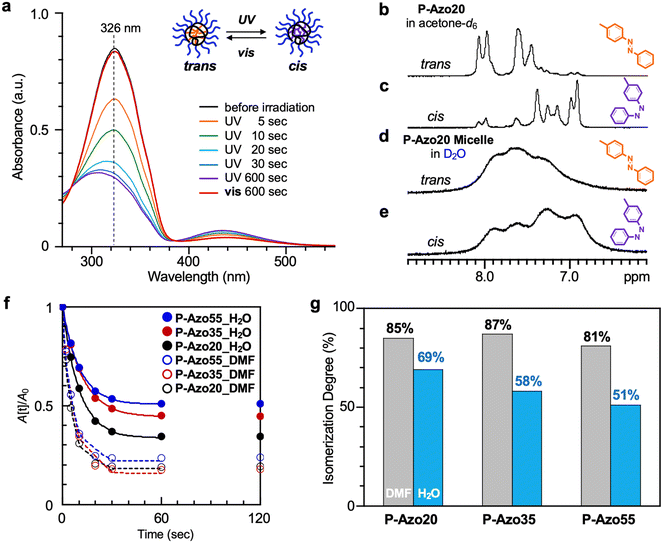 | ||
| Fig. 4 Photo-induced isomerization of PEG/azo statistical copolymers in water. (a) UV-vis spectra of the P-Azo20 micelle in water before (black line) and after being irradiated with UV light (λ = 365 nm) for 5, 10, 20, 30, and 600 seconds, and then visible light (λ = 470 nm) for 600 seconds: [polymer] = 0.1 mg mL. (b–e) 1H NMR spectra of P-Azo20 in acetone-d6 and P-Azo20 micelles in D2O before (trans) and after (cis) being irradiated with UV light (λ = 365 nm) for 600 seconds. (f) Plots of A(t)/A0 of the aqueous solutions of P-Azo20 (black circle), P-Azo35 (red circle), and P-Azo55 (blue circle) micelles, and the DMF solutions of P-Azo20 (black hollow circle), P-Azo35 (red hollow circle), and P-Azo55 (blue hollow circle) before and after UV irradiation (λ = 365 nm). The plots were obtained from the spectra in Fig. 4a, S6, and S7, and fitted using eqn (S1): [P-Azo20] = 0.10 mg mL−1, [P-Azo35] = 0.050 mg mL−1, [P-Azo55] = 0.025 mg mL−1. (g) The isomerization degree of P-Azo20, P-Azo35, and P-Azo55 in DMF and their micelles in H2O after being irradiated with UV light for 600 seconds. | ||
The effects of composition and solvents (water or DMF) on the photoisomerization of P-Azo20, P-Azo35, and P-Azo55 were examined. The isomerization degree of the Azo units at the photostationary state was calculated from eqn (1):38,39
| Isomerization degree (%) = 100 × 1.055 × (1 − Aafter/A0) | (1) |
In all the copolymers, the isomerization degree of the Azo pendants in DMF (∼85%) was higher than that in water. The isomerization degree in water decreased from 69% to 51% on increasing the Azo content from 20 to 55 mol%, though the isomerization degree in DMF was independent of the composition (Fig. 4g). This is probably because, in water, multiple Azo pendants are accumulated within a hydrophobic core to sterically hinder the trans-Azo groups from transforming into the cis-state.39 The isomerization rate was hardly affected by either Azo content (hydrophobicity) of the copolymers or solvents (Fig. 4f, S6, S7, and Table S2).
The in-core cis-Azo groups showed gradual relaxation into trans-Azo ones in water at 30 °C even under dark conditions (e.g., P-Azo20: from cis 68% to 44% in 24 h). However, the process was much slower than that by irradiating visible light (Fig. S8); the cis-Azo state is virtually kept at 30 °C at least for 1 h. The isomerization degree from the trans to cis-state for P-Azo20 in acetone-d6 was estimated to be 81% from 1H NMR (Fig. 4b–e). This was close to that (82%) determined by UV-vis spectroscopy in DMF. The Azo units within the micelle cores in D2O showed peaks broader than those in acetone-d6 (Fig. 4d and e), indicative of the reduced mobility.
The size (Mw,H2O, Rh), Cp values, and Nagg of PEG/cis-Azo micelles were further examined after UV irradiation (Fig. S3, S4, S9, and Table 2), and the values were almost identical to those for PEG/trans-Azo micelles. These results indicate that the isomerization of the in-core Azo units hardly affects the associated structures of the micelles.
Self-sorting or co-self-assembly in binary mixtures of copolymer micelles
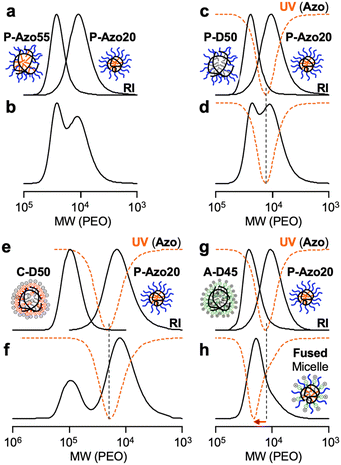
We then investigated self-sorting and co-self-assembly of PEG/Azo statistical copolymers in the presence of other amphiphilic copolymers with different compositions and/or side chains. The aqueous solution of a P-Azo20 micelle was mixed with the aqueous solutions of P-Azo55, P-D50, P-B70, C-D50, and A-D45 micelles. The aqueous micelle solutions were prepared with 100 mM NaCl (PEG and/or anion micelle mixtures) or NaNO3 (PEG/cation micelle mixtures). The micelle mixtures were then heated at 50 °C for 24 hours. After cooling to 25 °C, the mixtures were injected into SEC in water containing 100 mM NaCl or NaNO3 as an eluent; the SEC system was equipped with a refractive index (RI) detector and a UV-vis detector whose absorbance was set at 320 nm for the selective detection of the Azo unit.The binary mixtures consisting of a P-Azo20 micelle and P-Azo55, P-D50, P-B70, or C-D50 micelles (1/1 wt ratio) showed bimodal SEC curves on the RI detector, whose peak tops were at the same retention time as their corresponding micelles before mixing (Fig. 5a–f and S10a, b). The UV-vis SEC curves originating from P-Azo20 were intact before and after mixing. These results indicate that a P-Azo20 unimer micelle (Nagg = 1.3) induced self-sorting in the presence of a P-Azo55 multichain micelle (Nagg = 11, Table 2) or other PEG or cation/alkyl random copolymer multichain micelles (Nagg > 5, Table S1) in water. Such a self-sorting was achieved not only in the binary mixture of P-cisAzo20 micelles and P-cisAzo55 micelles but also by the direct dissolution of a bulk blend of P-Azo20 and P-Azo55 in water (Fig. S10c–h). It should be noted that, in the direct dissolution method, their discrete micelles are gradually formed via dynamic chain exchange during the solubilization process of the polymers. It means that the self-sorting is the thermodynamically stable state independent of the isomerization or the mixing procedures. Self-sorting against the C-D50 micelle was also realized with the P-Azo35 micelle and P-Azo55 micelles (Fig. S11). This is in clear contrast to our previous report: the mixture of a PEG/alkyl copolymer and a cation/alkyl copolymer induced co-self-assembly to yield a PEG/cation fused micelle.31 This importantly indicates that the structural difference between dodecyl groups of C-D50 and azobenzene pendants of PEG/Azo copolymers is a driving force for the self-sorting (immiscible) behavior, similar to the self-sorting of PEG/dodecyl micelles and PEG/butyl ones.29
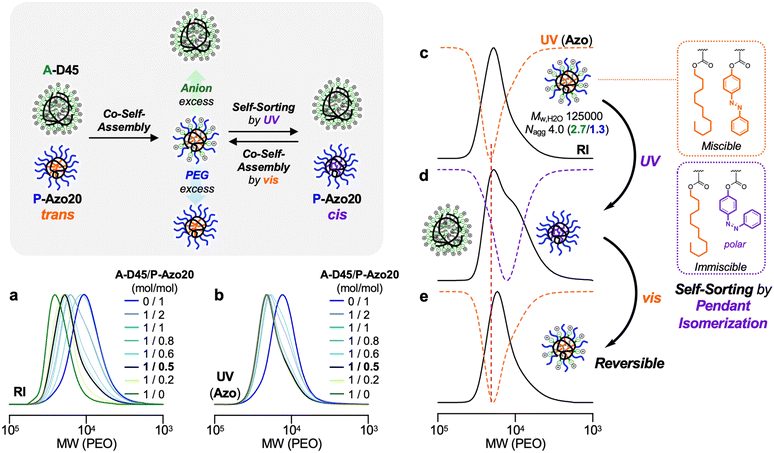
In contrast, the mixture of P-transAzo20 and A-D45 showed a unimodal RI SEC curve, whose peak top was at the middle between single micelles of the two copolymers (Fig. 5g and h). The UV-vis curve of the mixture was shifted to a higher molecular weight region compared to that of an original P-transAzo20 unimer micelle. This indicates that P-transAzo20 co-self-assembled with A-D45 to form an A-D45/P-transAzo20-fused micelle in water.
We evaluated effects of the mixing molar ratio of an A-D45 micelle and a P-Azo20 micelle (1/0.2 to 1/2) on their co-self-assembly behavior in water. In both SEC curves using RI and UV-vis detectors, the shoulder peak originating from a P-Azo20 micelle turned smaller as the molar ratio of P-Azo20 decreased, and the SEC curve became almost unimodal at the mixing ratio of 1/0.5 (Fig. 6a and b). Importantly, the UV-vis SEC curves for 1/0.5 and 1/0.2 mixtures were almost identical. This supports the observation that the yield of the A-D45/P-Azo20-fused micelle is the highest at the 1/0.5 ratio while there are excess A-D45 micelles in addition to the fused micelles at the 1/0.2 ratio. We thus concluded that the 1/0.5 mixing ratio almost quantitatively yielded the A-D45/P-Azo20-fused micelle. Mw,H2O and Nagg of the fused micelle was determined to be 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 4.0 (A-D45/P-Azo20 = 2.7/1.3) by SEC-MALLS (Fig. 6c), and Rh of the fused micelle by DLS was 5.6 nm (Fig. S12). The zeta potential of the fused micelle in water was −35 mV, whose value was between −11 mV (P-Azo20 micelle) and −42 mV (A-D45 micelle) (Fig. S13).
000 g mol−1 and 4.0 (A-D45/P-Azo20 = 2.7/1.3) by SEC-MALLS (Fig. 6c), and Rh of the fused micelle by DLS was 5.6 nm (Fig. S12). The zeta potential of the fused micelle in water was −35 mV, whose value was between −11 mV (P-Azo20 micelle) and −42 mV (A-D45 micelle) (Fig. S13).
These results indicate the following co-self-assembly process and mechanism: by mixing a P-Azo20 unimer micelle (Nagg = 1.3) and an A-D45 multichain micelle (Nagg = 5.5) at 50 °C, a P-Azo20 unimer micelle collides with the A-D45 micelle to associate with two or three A-D45 chains into the A-D45/P-Azo20-fused micelle. Nagg of P-Azo20 in the fused micelle is almost the same as that of the original unimer micelle, whereas Nagg of A-D45 decreased from 5.5 to 2.7. This is consistent with the fact that the zeta potential value of the fused micelle is ranged in the middle between that of the P-Azo20 micelle and that of the A-D45 micelle. The co-self-assembly is thus driven by the reduction of electrostatic repulsion among anionic pendants of A-D45via the co-self-assembly with a neutral P-Azo20. A similar trend was observed previously in the formation of the fused micelles of an anion/dodecyl random copolymer and a PEG/dodecyl counterpart.32
While the contrasting outcomes observed for the two systems—self-sorting of the C-D50/P-Azo20 mixture or co-self-assembly into A-D45/P-Azo20 fused micelles—may appear contradictory, they suggest that differences in hydrophilic pendants can have a significant impact on the self-assembly of binary mixtures of random copolymer micelles. In fact, the absolute value of zeta potential of the A-D45 micelle (−42 mV) was larger than that of the C-D50 micelle (23 mV). This means that the former A-D45 micelle has larger electrostatic repulsion in water and thus co-self-assembles with P-Azo20 to minimize the repulsion even though the core-forming hydrophobic groups are different. Namely, the strength of electrostatic repulsion among ionic pendants may play an important role in determining whether co-self-assembly or self-sorting occurs.
Reversible co-self-assembly and self-sorting via isomerization
The aqueous solution of the A-D45/P-Azo20-fused micelle was irradiated with UV light (365 nm) at 50 °C for 24 hours to induce isomerization of the trans-Azo units into the cis-state (Fig. 6c, d, and S14). Interestingly, the irradiated sample showed a shoulder peak at a lower molecular weight region in the RI SEC curve. Moreover, the UV-vis SEC curve turned to be almost identical to that of an original P-Azo20 micelle. This means that a P-Azo20 unimer micelle carrying cis-Azo groups was released from the fused micelle via self-sorting in water. Such self-sorting was also confirmed by mixing an A-D45 micelle and a P-Azo20 micelle containing cis-Azo groups (irradiated with UV) in water (Fig. S15). By irradiating visible light, the discrete A-D45 or P-Azo20 micelles were once again co-self-assembled into an A-D45/P-Azo20-fused micelle (Fig. 6e). Self-sorting is probably due to the change of polarity: cis-Azo units are more polar than trans-ones. This results in less miscibility of the cis-Azo units against apolar dodecyl groups within the micelle core, leading to the separation of the cis-state P-Azo20 from A-D45. To wrap up these results, co-self-assembly/self-sorting can reversibly be switched by UV/vis light irradiation without using externally added reagents.32Conclusions
In conclusion, we developed self-sorting systems of photoresponsive polymer micelles formed by the self-assembly of amphiphilic statistical copolymers bearing PEG and Azo pendants in water. The PEG/Azo statistical copolymer micelles induced not only self-sorting in the presence of other PEG micelles with different compositions or alkyl groups but also reversible co-self-assembly and self-sorting with an anionic random copolymer micelle via photoresponsive trans–cis isomerization of the Azo units. The reversible self-sorting system can be controlled by photo-irradiation without any chemical additives, in sharp contrast to previous counterparts driven by the external addition of a salt. This study offers a new strategy to create self-assembled and self-sorting polymer materials with not only photoresponsive properties and functions but also association properties dynamically controlled by light as desired in complex aqueous media.Author contributions
Takaya Terashima and Rikuto Kanno suggested the project and conceptualized the research. Rikuto Kanno conducted all experimental studies under the supervision of Takaya Terashima and Makoto Ouchi. All authors contributed to the writing and editing of the manuscript and agreed on the final version.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this study have been included as part of the supplementary information (SI). Supplementary information: experimental details of the synthesis and characterization of polymers and SEC, NMR, DLS, thermoresponsive solubility, and UV-vis spectroscopy. See DOI: https://doi.org/10.1039/d5sc06852e.Acknowledgements
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants (JP19K22218, JP20H02787, JP20H05219, JP22H04539, JP23H02008, JP23K26701, JP23KJ135, and JP25H00425), JST PRESTO Grant Number JPMJPR24M6, the Ogasawara Foundation for the Promotion of Science and Engineering, the Noguchi Institute, the Iketani Science and Technology Foundation, and the Kurita Water and Environment Foundation.References
- O. P. Ernst, D. T. Lodowski, M. Elstner, P. Hegemann, L. S. Brown and H. Kandori, Chem. Rev., 2014, 114, 126–163 CrossRef CAS PubMed
.
- S. Chen, R. Costil, F. K.-C. Leung and B. L. Feringa, Angew. Chem., Int. Ed., 2021, 60, 11604–11627 CrossRef CAS
.
- S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520–1529 RSC
.
- F. Xu and B. L. Feringa, Adv. Mater., 2023, 35, e2204413 CrossRef PubMed
.
- R. Göstl, A. Senf and S. Hecht, Chem. Soc. Rev., 2014, 43, 1982–1996 RSC
.
- E. Blasco, M. Wegener and C. Barner-Kowollik, Adv. Mater., 2017, 29, 1604005 CrossRef PubMed
.
- J.-M. Lehn, Science, 2002, 295, 2400–2403 CrossRef CAS PubMed
.
- A. Wu and L. Isaacs, J. Am. Chem. Soc., 2003, 125, 4831–4835 CrossRef CAS PubMed
.
- M. M. Safont-Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784–5814 CrossRef CAS PubMed
.
- A. C. Mendes, E. T. Baran, R. L. Reis and H. S. Azevedo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 582–612 CAS
.
- B. J. G. E. Pieters, M. B. van Eldijk, R. J. M. Nolte and J. Mecinović, Chem. Soc. Rev., 2016, 45, 24–39 RSC
.
- J. D. Watson and F. H. Crick, Nature, 1953, 171, 737–738 CrossRef CAS
.
- A. Pal, S. Karthikeyan and R. P. Sijbesma, J. Am. Chem. Soc., 2010, 132, 7842–7843 CrossRef CAS PubMed
.
- M. Obana, T. Fukino, T. Hikima and T. Aida, J. Am. Chem. Soc., 2016, 138, 9246–9250 CrossRef CAS PubMed
.
- N. Tomimasu, A. Kanaya, Y. Takashima, H. Yamaguchi and A. Harada, J. Am. Chem. Soc., 2009, 131, 12339–12343 CrossRef CAS PubMed
.
- S. Takahashi and S. Yagai, J. Am. Chem. Soc., 2022, 144, 13374–13383 CrossRef CAS PubMed
.
- K. Matsumoto, N. Bäumer, S. Ogi and S. Yamaguchi, Angew. Chem., Int. Ed., 2024, e202416361 Search PubMed
.
- F. Helmich, M. M. J. Smulders, C. C. Lee, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 12238–12246 CrossRef CAS PubMed
.
- S. Onogi, H. Shigemitsu, T. Yoshii, T. Tanida, M. Ikeda, R. Kubota and I. Hamachi, Nat. Chem., 2016, 8, 743–752 CrossRef CAS
.
- Q. Xiao, J. D. Rubien, Z. Wang, E. H. Reed, D. A. Hammer, D. Sahoo, P. A. Heiney, S. S. Yadavalli, M. Goulian, S. E. Wilner, T. Baumgart, S. A. Vinogradov, M. L. Klein and V. Percec, J. Am. Chem. Soc., 2016, 138, 12655–12663 CrossRef CAS PubMed
.
- A. Sarkar, R. Sasmal, C. Empereur-Mot, D. Bochicchio, S. V. K. Kompella, K. Sharma, S. Dhiman, B. Sundaram, S. S. Agasti, G. M. Pavan and S. J. George, J. Am. Chem. Soc., 2020, 142, 7606–7617 CrossRef CAS
.
- Y.-Y. Zhan, T. Kojima, K. Ishii, S. Takahashi, Y. Haketa, H. Maeda, S. Uchiyama and S. Hiraoka, Nat. Commun., 2019, 10, 1440 CrossRef PubMed
.
- A. Harada and K. Kataoka, Science, 1999, 283, 65–67 CrossRef CAS PubMed
.
- J. Kumaki, T. Kawauchi, K. Ute, T. Kitayama and E. Yashima, J. Am. Chem. Soc., 2008, 130, 6373–6380 CrossRef CAS PubMed
.
- J. M. Ren, J. Lawrence, A. S. Knight, A. Abdilla, R. B. Zerdan, A. E. Levi, B. Oschmann, W. R. Gutekunst, S.-H. Lee, Y. Li, A. J. McGrath, C. M. Bates, G. G. Qiao and C. J. Hawker, J. Am. Chem. Soc., 2018, 140, 1945–1951 CrossRef CAS PubMed
.
- B. Bilgiçer, X. Xing and K. Kmar, J. Am. Chem. Soc., 2001, 123, 11815–11816 CrossRef
.
- Y. Hirai, T. Terashima, M. Takenaka and M. Sawamoto, Macromolecules, 2016, 49, 5084–5091 CrossRef CAS
.
- S. Imai, Y. Hirai, C. Nagao, M. Sawamoto and T. Terashima, Macromolecules, 2018, 51, 398–409 CrossRef CAS
.
- S. Imai, M. Takenaka, M. Sawamoto and T. Terashima, J. Am. Chem. Soc., 2019, 141, 511–519 CrossRef CAS PubMed
.
- Y. Kimura, M. Takenaka, M. Ouchi and T. Terashima, Macromolecules, 2020, 53, 4942–4951 CrossRef CAS
.
- R. Kanno, K. Tanaka, T. Ikami, M. Ouchi and T. Terashima, Macromolecules, 2022, 55, 5213–5221 CrossRef CAS
.
- R. Kanno, H. Kono, A. Ryoki, M. Ouchi and T. Terashima, J. Am. Chem. Soc., 2024, 146, 30848–39859 CrossRef CAS PubMed
.
- F. D. Jochum and P. Theato, Chem. Commun., 2010, 46, 6717–6719 RSC
.
- Y. Huang, R. Dong, X. Zhu and D. Yan, Soft Matter, 2014, 10, 6121–6138 RSC
.
- E. Blasco, B. V. K. J. Schmidt, C. Barner-Kowollik, M. Piñol and L. Oriol, Macromolecules, 2014, 47, 3693–3700 CrossRef CAS
.
- M. Younis, S. Ahmad, A. Atiq, M. Amjad Farooq, M.-H. Huang and M. Abbas, Chem. Rec., 2023, 23, e202300126 CrossRef CAS PubMed
.
- M. Moniruzzaman, C. J. Sabey and G. F. Fernando, Macromolecules, 2004, 37, 2572–2577 CrossRef CAS
.
- J. G. Victor and J. M. Torkelson, Macromolecules, 1987, 20, 2241–2250 CrossRef CAS
.
- Y. Li, Y. Deng and X. Wang, Macromolecules, 2006, 39, 1108–1115 CrossRef CAS
.
- M. Hishida, R. Kanno and T. Terashima, Macromolecules, 2023, 56, 7587–7596 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |