Open Access Article
Open Access ArticleExciplex spin-flip acceleration enables high-performance narrowband electroluminescence
Mengcheng
Wang†
a,
Zhanxiang
Chen†
*a,
Manli
Huang
b,
Dengke
Wang
c,
Cheng
Zhong
d,
Zheng-Hong
Lu
 ce and
Chuluo
Yang
ce and
Chuluo
Yang
 *a
*a
aShenzhen Key Laboratory of New Display and Storage Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, P. R. China. E-mail: zxchen@szu.edu.cn; clyang@whu.edu.cn
bInstitute of Technology for Future Industry, School of Science and Technology Instrument Application Engineering, Shenzhen Institute of Information Technology, Shenzhen 518172, P. R. China
cDepartment of Physics, Center for Optoelectronics Engineering Research, Yunnan University, Kunming 650500, P. R. China
dHubei Key Lab on Organic and Polymeric Optoelectronic Materials, Department of Chemistry, Wuhan University, Wuhan, 430072, P. R. China
eDepartment of Materials Science and Engineering, University of Toronto, Toronto, ON M5S 3E4, Canada
First published on 23rd October 2025
Abstract
Exciplex-forming systems that harvest triplet excitons via a triplet-to-singlet spin flip (reverse intersystem crossing, RISC) enable thermally activated delayed fluorescence, providing a route to boost light emission in organic light-emitting diodes. Here, we report heavy-atom-incorporated exciplexes in which the triplet state is predominantly localized on the heavy-atom fragment, resulting in large spin–orbit coupling. Through positional isomer optimization, the RISC rate constant reaches 4.9 × 106 s−1, approximately an order of magnitude higher than in typical exciplexes. Organic light-emitting diodes based on the optimized exciplex host achieve a maximum external quantum efficiency (EQE) exceeding 40% and exhibit low efficiency roll-off (EQE > 33% at 1000 cd m−2).
Introduction
The spin-triplet excitons are key determinants of efficiency and stability in organic optoelectronic devices.1–4 For organic emitters, their triplet excitons are primarily harnessed by two pathways: phosphorescence through radiative triplet decay,5–7 or thermally activated delayed fluorescence (TADF) via reverse intersystem crossing (RISC) from spin-forbidden triplets to spin-allowed singlets.8–10 Recent developments have shown that TADF can be realized in materials featuring twisted donor (D)–acceptor (A) configurations,11–15 or multiple-resonance (MR) nitrogen-boron fused polycyclic frameworks.16–20 In both cases, the electron densities of the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO) can be spatially separated by precise molecular design, thereby facilitating spin-flip of triplet excitons for efficient utilization.21,22Another class of TADF materials, known as exciplexes, is formed in donor/acceptor (D/A) blends and offers greater design flexibility, attracting considerable industrial interest.23,24 Upon photo- or electrical excitation, D and A form an excited-state complex in which the HOMO resides mainly on D and the LUMO on A, enabling through-space charge transfer and triplet-to-singlet spin flip.25,26 In such exciplex systems, however, the lowest singlet (S1) and triplet (T1) states typically have dominant charge-transfer (CT) character, resulting in weak spin–orbit coupling (SOC) and consequently slow RISC;27,28 reported exciplex TADF systems usually exhibit RISC rates (kRISC) below 106 s−1.29–32 Slow RISC prolongs triplet lifetimes and promotes bimolecular exciton annihilation events, leading to severe efficiency roll-off in exciplex-based OLEDs.33
In this context, we are driven to accelerate the triplet-to-singlet spin flip in exciplex systems, achieving kRISC of approximately 4.9 × 106 s−1, about an order of magnitude higher than those typically other reported exciplexes. This RISC enhancement enables excellent performance in exciplex-based OLEDs, particularly at high brightness, surpassing state-of-the-art TADF, phosphorescent, TADF-sensitized hyperfluorescent and phosphorescence-sensitized hyperfluorescent OLEDs based on exciplex-forming co-hosts. Theoretical calculations and photophysical studies confirm that forming localized triplet states on heavy-atom-containing fragments, together with inducing additional orbital angular momentum through positional isomer optimization, drives the enhanced kRISC, so as to reduce the triplet exciton concentration and minimize triplet-involved annihilations in the emission layer, and ultimately overcome the efficiency roll-off.
Results and discussion
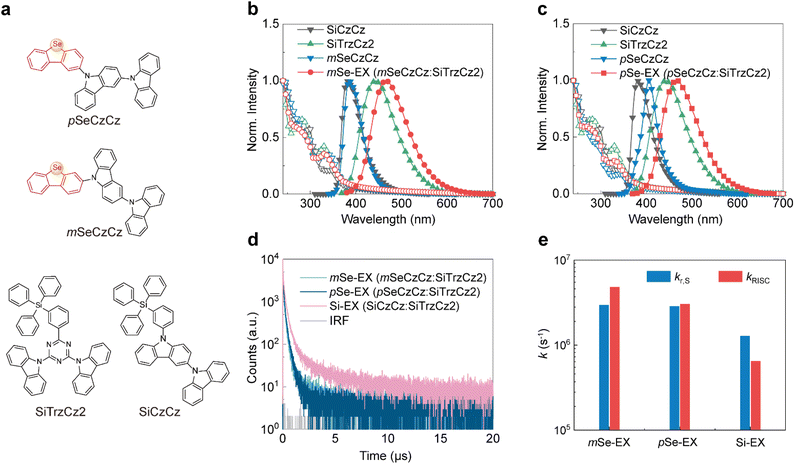
Given that exciplex-forming co-hosts are based on bimolecular donor–acceptor systems,27,34 and recognizing the relatively high electronegativity of selenium,35–37 we strategically incorporated a planar five-membered selenium-containing heterocycle (dibenzo[b,d]selenophene) into the donor moiety. To minimize the formation of labile bonds,38 we specifically compared meta- and para-conjugation linkages (mSeCzCzvs.pSeCzCz, Fig. 1a) for integrating selenium atom within the electron-donating framework. Density functional theory calculations (Fig. S1) indicated that mSeCzCz and pSeCzCz have similar dihedral angles between the dibenzo[b,d]selenophene and bicarbazole moieties (55.1° for mSeCzCz and 56.1° for pSeCzCz).The selenium-containing donors (mSeCzCz and pSeCzCz) were synthesized via a multi-step procedure (Scheme S1). The synthetic route commenced with a lithium–halogen exchange reaction, followed by hydrolysis to yield aromatic boric acids. Chlorinated dibenzo[b,d]selenophenes, serving as key intermediates, were prepared by trimethylsilyl cyanide-catalyzed intramolecular cyclization. The final products were obtained through the Buchwald–Hartwig coupling reaction. The chemical structures were fully characterized by 1H NMR, 13C NMR, and high-resolution mass spectrometry (Fig. S2–S11). Furthermore, the single-crystal structure of mSeCzCz (Fig. S12) revealed a twisted geometry between the peripheral dibenzo[b,d]selenophene group and the bicarbazole core. Notably, the crystal architecture displayed no significant π–π interactions. However, intermolecular C–H⋯Se interactions between the phenyl rings of bicarbazole and a neighboring molecule's selenium atom, along with multiple C–H⋯π interactions between neighboring dibenzo[b,d]selenophene and carbazole rings, were observed. Additionally, thermogravimetric analysis (Fig. S13) demonstrated their excellent thermal stability, with high decomposition temperatures (5% weight loss) of 420 °C and 408 °C for mSeCzCz and pSeCzCz, respectively, making them suitable for forming uniform films by thermal evaporation.
To construct blue-light-emitting exciplex systems, SiTrzCz2 (9,9′-(6-(3-(triphenylsilyl)phenyl)-1,3,5-triazine-2,4-diyl)bis(9H-carbazole)) with high triplet energy (T1 = 2.89 eV) was selected as an electron-withdrawing component and blended with selenium-containing donors of mSeCzCz and pSeCzCz, respectively (Fig. 1a). The steady-state photophysical properties of mSeCzCz, pSeCzCz, SiTrzCz2, and their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar blends (mSeCzCz:SiTrzCz2 and pSeCzCz:SiTrzCz2, abbreviated as mSe-EX and pSe-EX) were investigated in solid films. As shown in Fig. 1b and c, both mSeCzCz and pSeCzCz exhibited absorption bands below 350 nm, closely matching the absorption of the reference molecule SiCzCz (9-(3-(triphenylsilyl)phenyl)-9H-3,9′-bicarbazole). The photoluminescence (PL) spectra of mSeCzCz and pSeCzCz films displayed emission maxima at 386 nm and 406 nm, respectively, with the latter showing a distinct red shift relative to SiCzCz. Interestingly, their 1
1 molar blends (mSeCzCz:SiTrzCz2 and pSeCzCz:SiTrzCz2, abbreviated as mSe-EX and pSe-EX) were investigated in solid films. As shown in Fig. 1b and c, both mSeCzCz and pSeCzCz exhibited absorption bands below 350 nm, closely matching the absorption of the reference molecule SiCzCz (9-(3-(triphenylsilyl)phenyl)-9H-3,9′-bicarbazole). The photoluminescence (PL) spectra of mSeCzCz and pSeCzCz films displayed emission maxima at 386 nm and 406 nm, respectively, with the latter showing a distinct red shift relative to SiCzCz. Interestingly, their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar blends with SiTrzCz2 (mSe-EX and pSe-EX) exhibited identical broad emission bands centered at 467 nm, slightly blue-shifted compared to the control exciplex (SiCzCz:SiTrzCz2, termed Si-EX). Phosphorescence spectra measured at 77 K (Fig. S14) revealed T1 energies of 2.77 eV for mSeCzCz and 2.84 eV for pSeCzCz, determined from the onsets of these spectra. The broad phosphorescence emission band around 500 nm arises from the cumulative contribution of multiple vibronic bands associated with LE-state emission. As anticipated, mSe-EX and pSe-EX films clearly exhibited prompt and delayed fluorescence components (Fig. 1d), confirming efficient RISC within these exciplexes. The corresponding prompt (τPF) and delayed (τDF) lifetimes of mSe-EX (58.5 ns/0.3 μs) and pSe-EX (72.0 ns/0.4 μs) were significantly shorter than those of Si-EX (186.8 ns/1.9 μs), despite similar PL quantum yields (31%, 29%, and 30% for mSe-EX, pSe-EX, and Si-EX, respectively). Key kinetic constants, including singlet radiative decay rate (kr,S), singlet nonradiative decay rate (knr,S), intersystem crossing rate (kISC) and RISC rate (kRISC), were extrapolated and are summarized in Fig. 1e and Table 1 (see SI for detailed calculation methods).39,40 Notably, mSe-EX and pSe-EX showed significantly enhanced kr,S values (3.0 × 106 s−1 and 2.9 × 106 s−1, respectively) relative to Si-EX (1.3 × 106 s−1). Selenium incorporation also accelerated singlet nonradiative contributions, including knr,S (5.4 × 106 s−1 for mSe-EX, 6.8 × 106 s−1 for pSe-EX and 2.9 × 106 s−1 for Si-EX) and kISC (6.1 × 106 s−1 for mSe-EX, 3.3 × 106 s−1 for pSe-EX and 1.0 × 106 s−1 for Si-EX), resulting in similar PL quantum yields across these exciplex systems. More importantly, mSe-EX and pSe-EX demonstrated substantially increased kRISC values (4.9 × 106 s−1 and 3.1 × 106 s−1, respectively) compared to Si-EX (6.9 × 105 s−1), indicating that selenium integration in exciplex systems promotes faster consumption of triplet excitons. This trend is consistent with other selenium-containing TADF emitters, and the kRISC values of mSe-EX and pSe-EX surpass those of most previously reported selenium-containing TADF molecules (typically in the order of 105–106 s−1).41–45
1 molar blends with SiTrzCz2 (mSe-EX and pSe-EX) exhibited identical broad emission bands centered at 467 nm, slightly blue-shifted compared to the control exciplex (SiCzCz:SiTrzCz2, termed Si-EX). Phosphorescence spectra measured at 77 K (Fig. S14) revealed T1 energies of 2.77 eV for mSeCzCz and 2.84 eV for pSeCzCz, determined from the onsets of these spectra. The broad phosphorescence emission band around 500 nm arises from the cumulative contribution of multiple vibronic bands associated with LE-state emission. As anticipated, mSe-EX and pSe-EX films clearly exhibited prompt and delayed fluorescence components (Fig. 1d), confirming efficient RISC within these exciplexes. The corresponding prompt (τPF) and delayed (τDF) lifetimes of mSe-EX (58.5 ns/0.3 μs) and pSe-EX (72.0 ns/0.4 μs) were significantly shorter than those of Si-EX (186.8 ns/1.9 μs), despite similar PL quantum yields (31%, 29%, and 30% for mSe-EX, pSe-EX, and Si-EX, respectively). Key kinetic constants, including singlet radiative decay rate (kr,S), singlet nonradiative decay rate (knr,S), intersystem crossing rate (kISC) and RISC rate (kRISC), were extrapolated and are summarized in Fig. 1e and Table 1 (see SI for detailed calculation methods).39,40 Notably, mSe-EX and pSe-EX showed significantly enhanced kr,S values (3.0 × 106 s−1 and 2.9 × 106 s−1, respectively) relative to Si-EX (1.3 × 106 s−1). Selenium incorporation also accelerated singlet nonradiative contributions, including knr,S (5.4 × 106 s−1 for mSe-EX, 6.8 × 106 s−1 for pSe-EX and 2.9 × 106 s−1 for Si-EX) and kISC (6.1 × 106 s−1 for mSe-EX, 3.3 × 106 s−1 for pSe-EX and 1.0 × 106 s−1 for Si-EX), resulting in similar PL quantum yields across these exciplex systems. More importantly, mSe-EX and pSe-EX demonstrated substantially increased kRISC values (4.9 × 106 s−1 and 3.1 × 106 s−1, respectively) compared to Si-EX (6.9 × 105 s−1), indicating that selenium integration in exciplex systems promotes faster consumption of triplet excitons. This trend is consistent with other selenium-containing TADF emitters, and the kRISC values of mSe-EX and pSe-EX surpass those of most previously reported selenium-containing TADF molecules (typically in the order of 105–106 s−1).41–45
| Film | λ PL [nm] | Φ PL [%] | τ p [ns] | τ d [μs] | k r,S [s−1] | k nr,S [s−1] | k ISC [s−1] | k RISC [s−1] |
|---|---|---|---|---|---|---|---|---|
| a Peak wavelength of the photoluminescence spectra in the film. b Absolute photoluminescence quantum yield measured with an integration-sphere system under an argon atmosphere. c Lifetime of the prompt component determined from the transient photoluminescence decay curve at 298 K. d Lifetime of the delayed component determined from the transient photoluminescence decay curve at 298 K. e Rate constant of singlet radiative decay. f Rate constant of singlet nonradiative decay. g Rate constant of intersystem crossing. h Rate constant of reverse intersystem crossing. | ||||||||
| mSe-EX | 467 | 31 | 58.5 | 0.3 | 3.0 × 106 | 5.4 × 106 | 6.1 × 106 | 4.9 × 106 |
| pSe-EX | 467 | 29 | 72.0 | 0.4 | 2.9 × 106 | 6.8 × 106 | 3.3 × 106 | 3.1 × 106 |
| Si-EX | 470 | 30 | 186.8 | 1.9 | 1.3 × 106 | 2.9 × 106 | 1.0 × 106 | 6.9 × 105 |
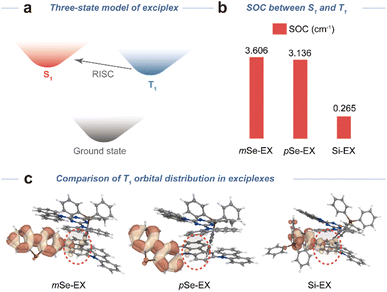
To clarify the enhanced kRISC in mSe-EX relative to pSe-EX, we performed time-dependent density functional theory calculations to analyse SOC matrix elements and natural transition orbital (NTO) distributions, with Si-EX as the reference. The SOC matrix elements for the T1 → S1 transition (Fig. 2a) in mSe-EX (3.606 cm−1) and pSe-EX (3.136 cm−1) were markedly higher than that of Si-EX (0.265 cm−1), corresponding to 13.6- and 11.8-fold enhancements, respectively (Fig. 2b). NTO analysis indicated charge-transfer characteristics in the S1 states of all exciplexes (Fig. S15), while their T1 states exhibited localized excitations (Fig. 2c). In Si-EX, the T1 state primarily localized on the benzene unit of tetraphenylsilane and the meta-connected carbazole groups. Conversely, the T1 state in pSe-EX was mainly localized on the dibenzo[b,d]selenophene fragment, and the selenium incorporation enhanced the SOC between T1 and S1 states. Notably, for mSe-EX, the T1 state extended from the dibenzo[b,d]selenophene fragment to the meta-connected carbazole group via two-lobed p-orbitals. The rotational overlap of adjacent p-orbitals generated additional orbital angular momentum, amplifying SOC and thereby enhancing kRISC in mSe-EX.46
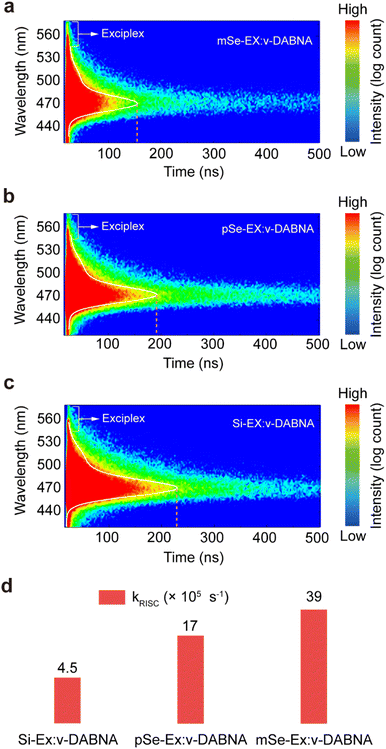
To further investigate the exciplex hosts with fast kRISC on MR-TADF emitters, we incorporated v-DABNA as the emitter into mSe-EX, pSe-EX, and Si-EX host matrices. PL spectra of the doped films (Fig. S16) showed a narrow emission peak at 470 nm with a FWHM of 17 nm, confirming efficient Förster resonance energy transfer (FRET) from the exciplex host to the MR-TADF emitter. Time-resolved emission spectra (Fig. 3a–c) revealed shorter delayed fluorescence lifetimes for mSe-EX:v-DABNA (2.8 μs) and pSe-EX:v-DABNA (3.0 μs) compared to Si-EX:v-DABNA (4.0 μs). Additionally, the delayed component contribution was highest for mSe-EX:v-DABNA (91%), followed by pSe-EX:v-DABNA (80%) and Si-EX:v-DABNA (45%). All doped films exhibited near-unity PL quantum yields of 99% (mSe-EX:v-DABNA), 97% (pSe-EX:v-DABNA), and 99% (Si-EX:v-DABNA). Consequently, the calculated kRISC values (Fig. 3d) were progressively increased from 4.5 × 105 s−1 (Si-EX:v-DABNA) to 1.7 × 106 s−1 (pSe-EX:v-DABNA) and 3.9 × 106 s−1 (mSe-EX:v-DABNA). These results align closely with theoretical predictions, clearly demonstrating that exciplex hosts with fast kRISC substantially accelerate the RISC process in MR-TADF emitters.
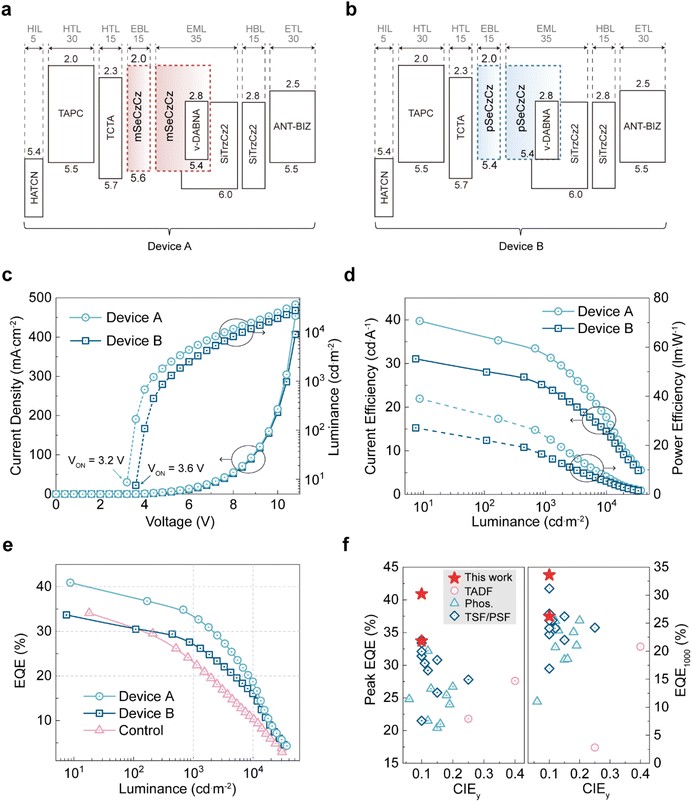
Encouraged by the near-unity PL quantum yield and accelerated RISC process of v-DABNA in the selenium-based exciplex hosts, we fabricated corresponding OLED devices with the following architecture: indium tin oxide (50 nm)/1,4,5,8,9,11-hexaazatriphenylenehexacarbonitrile (HATCN, 5 nm)/4,4′-cyclohexylidenebis[N,N-bis(4-methylphenyl)benzenamine] (TAPC, 30 nm)/tris(4-carbazoyl-9-ylphenyl)amine (TCTA, 15 nm)/Se donor (15 nm)/Se donor:SiTrzCz2:v-DABNA (35 nm, 49.5 wt%:49.5 wt%:1 wt%)/SiTrzCz2 (15 nm)/1-(4-(10-([1,1′-biphenyl]-4-yl)anthracen-9-yl)phenyl)-2-ethyl-1H-benzo[d]-imidazole (ANT-BIZ, 30 nm)/lithium quinolin-8-olate (Liq, 2 nm)/aluminium (Al, 100 nm). Specifically, for the emitting layers, v-DABNA was co-evaporated with the mSe-EX (mSeCzCz:SiTrzCz2) host for device A, and the pSe-EX (pSeCzCz:SiTrzCz2) host for device B. For comparison, a control device based on the Si-EX (SiCzCz:SiTrzCz2) host was also prepared. In this device architecture, HATCN, TAPC and TCTA served as hole-injection and hole-transport layers, while ANT-BIZ and Liq functioned as the electron-transport and electron-injection layers, respectively. Additionally, Se-containing donors (mSeCzCz or pSeCzCz) and SiTrzCz2 acted as electron-blocking and hole-blocking layers, respectively. The device structure and corresponding energy level diagram are illustrated in Fig. 4a and b, and chemical structures of the organic materials are provided in Fig. S17. The electroluminescence properties are summarized in Table 2.
| Device | λ PL [nm] | L max [cd m−2] | EQEc [%] | CEd [cd A−1] | PEe [lm W−1] | FWHMf [nm] | CIE(x,y)g | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | 1000 cd m−2 | Roll-off | Max | 1000 cd m−2 | Max | 1000 cd m−2 | |||||
| a Electroluminescence peak wavelength recorded at 1000 cd m−2. b Maximum luminance. c Maximum external quantum efficiency, and value at 1000 cd m−2. d Maximum current efficiency, and value at 1000 cd m−2. e Maximum power efficiency, and value at 1000 cd m−2. f Full width at half-maximum. g CIE coordinates. | |||||||||||
| Device A | 470 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
40.9 | 33.6 | 17.8 | 39.7 | 32.2 | 39.0 | 23.9 | 17 | (0.13, 0.12) |
| Device B | 470 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 204 |
33.7 | 26.2 | 22.3 | 31.1 | 23.9 | 27.1 | 14.4 | 17 | (0.12, 0.12) |
| Control | 470 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
34.1 | 22.4 | 34.3 | 35.8 | 23.1 | 40.2 | 16.5 | 17 | (0.13, 0.12) |
All fabricated devices exhibited nearly identical blue emission profiles (Fig. S18) peaking at 470 nm, with a narrow FWHM of 17 nm, closely matching the corresponding PL spectra obtained from thin films. This indicates complete FRET from the exciplex hosts to the v-DABNA emitter. According to the current density–voltage–luminance (J–V–L) curves (Fig. 4c), device A exhibited a turn-on voltage of approximately 3.2 V, an operating voltage of 4.2 V at a luminance of 1000 cd m−2, and a maximum luminance of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 cd m−2. In comparison, device B showed a higher turn-on voltage of approximately 3.6 V, an operating voltage of 5.0 V at 1000 cd m−2, and a lower maximum luminance of 33
603 cd m−2. In comparison, device B showed a higher turn-on voltage of approximately 3.6 V, an operating voltage of 5.0 V at 1000 cd m−2, and a lower maximum luminance of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 cd m−2. The EL spectra remained stable under high-brightness (∼10
204 cd m−2. The EL spectra remained stable under high-brightness (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2) or high-voltage (∼8 V) for all devices, indicating that the exciton recombination zone was essentially unchanged under these operating conditions (Fig. S19). Device A also achieved higher maximum current efficiency (39.7 cd A−1) and power efficiency (39.0 lm W−1) compared with device B, which displayed maximum current and power efficiencies of 31.1 cd A−1 and 27.1 lm W−1, respectively (Fig. 4d).
000 cd m−2) or high-voltage (∼8 V) for all devices, indicating that the exciton recombination zone was essentially unchanged under these operating conditions (Fig. S19). Device A also achieved higher maximum current efficiency (39.7 cd A−1) and power efficiency (39.0 lm W−1) compared with device B, which displayed maximum current and power efficiencies of 31.1 cd A−1 and 27.1 lm W−1, respectively (Fig. 4d).
As shown in Fig. 4e, device A achieved a maximum EQE of 40.9% (average: 40.45 ± 0.29%, based on the measurements of 11 independent devices; Fig. S20), substantially exceeding those of device B (33.7%) and the control device (34.1%). This exceptional performance stemmed from the near-unity PL quantum yield of the emissive layer and favorable horizontal orientation of the transition dipole moment of v-DABNA doped in the mSe-EX host (transition dipole moment vector S = −0.46, Fig. S21).47 Compared to the control device, devices A and B demonstrated significantly reduced efficiency roll-off, resulting from enhanced SOC values between the singlet and triplet manifolds provided by the mSe-EX and pSe-EX hosts, which effectively suppresses triplet-involved annihilation processes at high brightness levels. Notably, owing to the stronger SOC provided by the mSe-EX host, device A exhibited considerably lower efficiency roll-off compared to recent blue OLEDs utilizing exciplex-forming co-host systems. Remarkably, device A maintained an EQE of 33.6% at a practical luminance of 1000 cd m−2, surpassing those of state-of-the-art TADF, phosphorescent, TADF-sensitized hyperfluorescent and phosphorescence-sensitized hyperfluorescent OLEDs based on exciplex-forming co-hosts (Fig. 4f and Table S1). These results clearly demonstrate that the exciplex hosts with fast RISC can effectively accelerate the RISC process in MR-TADF emitters, thereby substantially enhancing device performance under realistic display operating conditions.
Conclusions
In summary, we have developed a heavy-atom-incorporated exciplex with kRISC as far as 4.9 × 106 s−1, an order of magnitude higher than the typical values for exciplexes. The heavy atom participates directly in the triplet-state orbital distribution, resulting in strong spin–orbit coupling; combined with positional isomer optimization, this accelerates the spin-flip process. Notably, employing this exciplex with fast RISC as a host also enhances the kRISC of the MR-TADF emitter to the 106 s−1 range. Devices employing the selenium-containing mSe-EX host exhibited exceptional electroluminescence performance, achieving a high EQEmax of 40.9% and minimal efficiency roll-off (EQE = 33.6% at 1000 cd m−2). We believe that the simple design and in-depth structure–property relationship established here would aid in the development of exciplex systems with efficient spin-flip transition and benefit the electroluminescence efficiency in narrowband OLEDs.Author contributions
Z. Chen and C. Yang conceived and supervised the project. M. Wang synthesized and characterized the hosts, and performed photophysical measurements under the supervision of Z. Chen. Z. Chen and M. Huang fabricated the OLEDs and characterized the performance of the devices. Z. Chen performed theoretical calculations. D. Wang and Z.-H. Lu contributed to the UPS results. Z. Chen, M. Huang, C. Zhong, and C. Yang participated in discussions. All authors engaged in result analysis and provided feedback on the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the SI. Supplementary information: all synthetic procedures, characterization data, spectroscopic data, supplementary figures and tables, and detailed information. See DOI: https://doi.org/10.1039/d5sc06200d.CCDC 2408523 (mSeCzCz) contains the supplementary crystallographic data for this paper.48
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (52130308 and 52403237), GuangDong Basic and Applied Basic Research Foundation (2022A1515110445), the Shenzhen Technology and Innovation Commission (RCBS20231211090518026, ZDSYS20210623091813040), Research Team Cultivation Program of Shenzhen University (2023DFT004), and Scientific Foundation for Youth Scholars of Shenzhen University (868-000001033371). We also thank the Instrumental Analysis Center of Shenzhen University for the analytical support.References
- Z. Tang, F. Lyu, J. Gu, H. Guo, W. Yu, Y. Zou, L. Gong, R. Tang, B. Qu, X. Guo, Y. Chen, Y. Deng, M. Bian, Y. Li, D. Zhang, M. Wei, S. M. Park, P. Xia, Y. Lv, Q. Gong, S. Wang, Z. Chen and L. Xiao, Sub-Second Long Lifetime Triplet Exciton Reservoir for Highly Efficient and Stable Organic Light-Emitting Diode, Adv. Mater., 2024, 36, 2313746 CrossRef CAS PubMed.
- Q. Meng, R. Wang, Y. Wang, X. Guo, Y. Liu, X. Wen, C. Yao and J. Qiao, Longevity gene responsible for robust blue organic materials employing thermally activated delayed fluorescence, Nat. Commun., 2023, 14, 3927 CrossRef CAS PubMed.
- Y. H. Jung, G. S. Lee, S. Muruganantham, H. R. Kim, J. H. Oh, J. H. Ham, S. B. Yadav, J. H. Lee, M. Y. Chae, Y. H. Kim and J. H. Kwon, Modified t-butyl in tetradentate platinum (II) complexes enables exceptional lifetime for blue-phosphorescent organic light-emitting diodes, Nat. Commun., 2024, 15, 2977 CrossRef CAS PubMed.
- H. H. Cho, D. G. Congrave, A. J. Gillett, S. Montanaro, H. E. Francis, V. Riesgo-Gonzalez, J. Ye, R. Chowdury, W. Zeng, M. K. Etherington, J. Royakkers, O. Millington, A. D. Bond, F. Plasser, J. M. Frost, C. P. Grey, A. Rao, R. H. Friend, N. C. Greenham and H. Bronstein, Suppression of Dexter transfer by covalent encapsulation for efficient matrix-free narrowband deep blue hyperfluorescent OLEDs, Nat. Mater., 2024, 23, 519–526 CrossRef CAS PubMed.
- H. Zhao, C. E. Arneson, D. Fan and S. R. Forrest, Stable blue phosphorescent organic LEDs that use polariton-enhanced Purcell effects, Nature, 2024, 626, 300–305 CrossRef CAS PubMed.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices, Nature, 1998, 395, 151–154 CrossRef CAS.
- Y. Ma, H. Zhang, J. Shen and C. Che, Electroluminescence from triplet metal-ligand charge-transfer excited state of transition metal complexes, Synth. Met., 1998, 94, 245–248 CrossRef CAS.
- Z. Chen, F. Ni, Z. Wu, Y. Hou, C. Zhong, M. Huang, G. Xie, D. Ma and C. Yang, Enhancing Spin-Orbit Coupling by Introducing a Lone Pair Electron with p Orbital Character in a Thermally Activated Delayed Fluorescence Emitter: Photophysics and Devices, J. Phys. Chem. Lett., 2019, 10, 2669–2675 CrossRef CAS PubMed.
- Z. Chen, Z. Wu, F. Ni, C. Zhong, W. Zeng, D. Wei, K. An, D. Ma and C. Yang, Emitters with a pyridine-3,5-dicarbonitrile core and short delayed fluorescence lifetimes of about 1.5 μs: orange-red TADF-based OLEDs with very slow efficiency roll-offs at high luminance, J. Mater. Chem. C, 2018, 6, 6543–6548 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- T. Huang, Q. Wang, H. Zhang, Y. Xin, Y. Zhang, X. Chen, D. Zhang and L. Duan, Delocalizing electron distribution in thermally activated delayed fluorophors for high-efficiency and long-lifetime blue electroluminescence, Nat. Mater., 2024, 23, 1523–1530 CrossRef CAS PubMed.
- T. Huang, Q. Wang, H. Zhang, Y. Zhang, G. Zhan, D. Zhang and L. Duan, Enhancing the efficiency and stability of blue thermally activated delayed fluorescence emitters by perdeuteration, Nat. Photonics, 2024, 18, 516–523 CrossRef CAS.
- Y. Wada, H. Nakagawa, S. Matsumoto, Y. Wakisaka and H. Kaji, Organic light emitters exhibiting very fast reverse intersystem crossing, Nat. Photonics, 2020, 14, 643–649 CrossRef CAS.
- M. Y. Wong and E. Zysman-Colman, Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed.
- S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Highly efficient blue electroluminescence based on thermally activated delayed fluorescence, Nat. Mater., 2015, 14, 330–336 CrossRef CAS PubMed.
- T. Hua, X. Cao, J. Miao, X. Yin, Z. Chen, Z. Huang and C. Yang, Deep-blue organic light-emitting diodes for ultrahigh-definition displays, Nat. Photonics, 2024, 18, 1161–1169 CrossRef CAS.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- M. Yang, I. S. Park and T. Yasuda, Full-Color, Narrowband, and High-Efficiency Electroluminescence from Boron and Carbazole Embedded Polycyclic Heteroaromatics, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- J. M. Dos Santos, D. Hall, B. Basumatary, M. Bryden, D. Chen, P. Choudhary, T. Comerford, E. Crovini, A. Danos, J. De, S. Diesing, M. Fatahi, M. Griffin, A. K. Gupta, H. Hafeez, L. Hämmerling, E. Hanover, J. Haug, T. Heil, D. Karthik, S. Kumar, O. Lee, H. Li, F. Lucas, C. F. R. Mackenzie, A. Mariko, T. Matulaitis, F. Millward, Y. Olivier, Q. Qi, I. D. W. Samuel, N. Sharma, C. Si, L. Spierling, P. Sudhakar, D. Sun, E. Tankelevičiūtė, M. Duarte Tonet, J. Wang, T. Wang, S. Wu, Y. Xu, L. Zhang and E. Zysman-Colman, The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation, Chem. Rev., 2024, 124, 13736–14110 CrossRef CAS PubMed.
- X. Fan, K. Wang, Y. Shi, Y. Cheng, Y. Lee, J. Yu, X. Chen, C. Adachi and X. Zhang, Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design, Nat. Photonics, 2023, 17, 280–285 CrossRef CAS.
- T. Wu, M. Huang, C. Lin, P. Huang, T. Chou, R. Chen-Cheng, H. Lin, R. Liu and C. Cheng, Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off, Nat. Photonics, 2018, 12, 235–240 CrossRef CAS.
- X. Fan, X. Tang, T. Zhang, S. Kohata, J. Yu, X. Chen, K. Wang, T. Hatakeyama, C. Adachi and X. Zhang, Stable narrowband blue OLEDs by modulating frontier molecular orbital levels, Nat. Commun., 2025, 16, 4936 CrossRef CAS PubMed.
- H. Shin, J. H. Lee, C. K. Moon, J. S. Huh, B. Sim and J. J. Kim, Sky-Blue Phosphorescent OLEDs with 34.1% External Quantum Efficiency Using a Low Refractive Index Electron Transporting Layer, Adv. Mater., 2016, 28, 4920–4925 CrossRef CAS PubMed.
- H. G. Kim, K. H. Kim and J. J. Kim, Highly Efficient, Conventional, Fluorescent Organic Light-Emitting Diodes with Extended Lifetime, Adv. Mater., 2017, 29, 1702159 CrossRef PubMed.
- C. K. Moon, K. Suzuki, K. Shizu, C. Adachi, H. Kaji and J. J. Kim, Combined Inter- and Intramolecular Charge-Transfer Processes for Highly Efficient Fluorescent Organic Light-Emitting Diodes with Reduced Triplet Exciton Quenching, Adv. Mater., 2017, 29, 1606448 CrossRef PubMed.
- X. Tang, L. Cui, H. Li, A. J. Gillett, F. Auras, Y. Qu, C. Zhong, S. T. E. Jones, Z. Jiang, R. H. Friend and L.-S. Liao, Highly efficient luminescence from space-confined charge-transfer emitters, Nat. Mater., 2020, 19, 1332–1338 CrossRef CAS PubMed.
- C. K. Moon, J. S. Huh, J. M. Kim and J. J. Kim, Electronic Structure and Emission Process of Excited Charge Transfer States in Solids, Chem. Mater., 2018, 30, 5648–5654 CrossRef CAS.
- K. H. Kim, S. J. Yoo and J. J. Kim, Boosting Triplet Harvest by Reducing Nonradiative Transition of Exciplex toward Fluorescent Organic Light-Emitting Diodes with 100% Internal Quantum Efficiency, Chem. Mater., 2016, 28, 1936–1941 CrossRef CAS.
- K. Goushi, K. Yoshida, K. Sato and C. Adachi, Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion, Nat. Photonics, 2012, 6, 253–258 CrossRef CAS.
- Z. Chen, C. Zhong, J. Han, J. Miao, Y. Qi, Y. Zou, G. Xie, S. Gong and C. Yang, High-Performance Circularly Polarized Electroluminescence with Simultaneous Narrowband Emission, High Efficiency, and Large Dissymmetry Factor, Adv. Mater., 2022, 34, 2109147 CrossRef CAS PubMed.
- J. Sun, H. Ahn, S. Kang, S. Ko, D. Song, H. A. Um, S. Kim, Y. Lee, P. Jeon, S. Hwang, Y. You, C. Chu and S. Kim, Exceptionally stable blue phosphorescent organic light-emitting diodes, Nat. Photonics, 2022, 16, 212–218 CrossRef CAS.
- C. Duan, C. Han, J. Zhang, X. Zhang, C. Fan and H. Xu, Manipulating Charge-Transfer Excitons by Exciplex Matrix: Toward Thermally Activated Delayed Fluorescence Diodes with Power Efficiency beyond 110 lm W-1, Adv. Funct. Mater., 2021, 31, 2102739 CrossRef CAS.
- S. Diesing, L. Zhang, E. Zysman-Colman and I. D. W. Samuel, A figure of merit for efficiency roll-off in TADF-based organic LEDs, Nature, 2024, 627, 747–753 CrossRef CAS PubMed.
- H. A. Al Attar and A. P. Monkman, Electric Field Induce Blue Shift and Intensity Enhancement in 2D Exciplex Organic Light Emitting Diodes; Controlling Electron-Hole Separation, Adv. Mater., 2016, 28, 8014–8020 CrossRef CAS PubMed.
- G. L. Gibson, T. M. McCormick and D. S. Seferos, Atomistic Band Gap Engineering in Donor-Acceptor Polymers, J. Am. Chem. Soc., 2012, 134, 539–547 CrossRef CAS PubMed.
- Z. Chen, X. Li, J. Zhao, S. Zhang, J. Wang, H. Zhang, J. Zhang, Q. Dong, W. Zhang, W. Hu and X. Han, Stabilizing Pt Single Atoms through Pt-Se Electron Bridges on Vacancy-enriched Nickel Selenide for Efficient Electrocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2023, 62, e202308686 CrossRef CAS PubMed.
- W. Jiang, S. Wu, D. Xu, L. Tu, Y. Xie, P. Pasqués-Gramage, P. G. Boj, M. A. Díaz-García, F. Li, J. Wu and Z. Li, Stable Xanthene Radicals and Their Heavy Chalcogen Analogues Showing Tunable Doublet Emission from Green to Near-infrared, Angew. Chem., Int. Ed., 2025, 64, e202418762 CrossRef CAS PubMed.
- R. Wang, Y. Wang, N. Lin, R. Zhang, L. Duan and J. Qiao, Effects of Ortho-Linkages on the Molecular Stability of Organic Light-Emitting Diode Materials, Chem. Mater., 2018, 30, 8771–8781 CrossRef CAS.
- M. Huang, Z. Chen, J. Miao, S. He, W. Yang, Z. Huang, Y. Zou, S. Gong, Y. Tan and C. Yang, Harmonization of rapid triplet up-conversion and singlet radiation enables efficient and stable white OLEDs, Nat. Commun., 2024, 15, 8048 CrossRef CAS PubMed.
- Z. Chen, M. Huang, C. Zhong, X. Cao, G. Xie, S. Gong and C. Yang, Cascade Chirality Transfer through Diastereomeric Interaction Enables Efficient Circularly Polarized Electroluminescence, Adv. Funct. Mater., 2023, 33, 2215179 CrossRef CAS.
- B. H. Drummond, G. C. Hoover, A. J. Gillett, N. Aizawa, W. K. Myers, B. T. McAllister, S. T. E. Jones, Y. Pu, D. Credgington and D. S. Seferos, Selenium Substitution Enhances Reverse Intersystem Crossing in a Delayed Fluorescence Emitter, J. Phys. Chem. C, 2020, 124, 6364–6370 CrossRef CAS.
- Y. Hu, J. Miao, T. Hua, Z. Huang, Y. Qi, Y. Zou, Y. Qiu, H. Xia, H. Liu, X. Cao and C. Yang, Efficient selenium-integrated TADF OLEDs with reduced roll-off, Nat. Photonics, 2022, 16, 803–810 CrossRef CAS.
- Y. Zou, M. Yu, Y. Xu, Z. Xiao, X. Song, Y. Hu, Z. Xu, C. Zhong, J. He, X. Cao, K. Li, J. Miao and C. Yang, Acceleration of reverse intersystem crossing in multi-resonance TADF emitter, Chem, 2024, 10, 1485–1501 CAS.
- J. Hu, J. Liang, Z. Yan, H. Ni, X. Liao and Y. Zheng, One-shot synthesis of heavy-atom-modified carbazole-fused multi-resonance thermally activated delayed fluorescence materials, Sci. China Mater., 2024, 67, 2789–2795 CrossRef CAS.
- Q. Zheng, Y. Qu, P. Zuo, H. Yuan, Y. Yang, Y. Qiu, L. Liao, D. Zhou and Z. Jiang, Enhancing multi-resonance thermally activated delayed fluorescence emission via through-space heavy-atom effect, Chem, 2025, 11, 102353 CAS.
- H. Ma, Q. Peng, Z. An, W. Huang and Z. Shuai, Efficient and Long-Lived Room-Temperature Organic Phosphorescence: Theoretical Descriptors for Molecular Designs, J. Am. Chem. Soc., 2019, 141, 1010–1015 CrossRef CAS PubMed.
- C. Chan, M. Tanaka, Y. Lee, Y. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission, Nat. Photonics, 2021, 15, 203–207 CrossRef CAS.
- CCDC 2408523: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lv891.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |