Open Access Article
Open Access ArticleSingle atom-bridged Au nanozymes boost glucose oxidase-like activity in acidic media
Xin
Luo†
a,
Feilong
Tan†
a,
Zhenglong
Mao
a,
Yan
Zhang
a,
Yinjun
Tang
b,
Canglong
Wang
 e,
Wenling
Gu
b,
Cao
Li
a,
Juewen
Liu
e,
Wenling
Gu
b,
Cao
Li
a,
Juewen
Liu
 *c and
Chengzhou
Zhu
*c and
Chengzhou
Zhu
 *bd
*bd
aNational “111” Center for Cellular Regulation and Molecular Pharmaceutics, Key Laboratory of Fermentation Engineering (Ministry of Education), Cooperative Innovation Center of Industrial Fermentation (Ministry of Education & Hubei Province), School of Life and Health Sciences, Hubei University of Technology, Wuhan 430068, P. R. China
bState Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensing Technology and Health, College of Chemistry, Central China Normal University, Wuhan 430079, P. R. China. E-mail: czzhu@ccnu.edu.cn
cDepartment of Chemistry, Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, Canada. E-mail: liujw@uwaterloo.ca
dCollege of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou 311121, P. R. China
eInstitute of Modern Physics, Chinese Academy of Science, Lanzhou 730000, P. R. China
First published on 13th October 2025
Abstract
Although Au nanozymes hold great promise as glucose oxidase (GOx) mimics, their catalytic activity and pH dependence remain significant challenges. Herein, we synthesize Fe single atom bridged Au nanozymes (AuNPs/FeNC) with dual catalytic sites, achieving a 3.7-fold enhancement in GOx-like activity under acidic media compared to AuNPs/NC. Experimental and theoretical analyses reveal that charge transfer from Au to Fe single atom facilitates O2 adsorption at Fe sites, synergistically boosting glucose oxidation. Unlike previously reported Au–H intermediates formed under alkaline conditions, in situ monitoring identifies the formation of Au–Fe–OO intermediates in AuNPs/FeNC, which facilitate the dehydrogenation of glucose and enhance the catalytic efficiency in acidic environments. Benefiting from optimal GOx- and peroxidase-like activities at pH 4.0, an AuNPs/FeNC-based glucose cascade system is constructed with exceptional properties. As a proof of concept, this system is integrated into a portable, gel-based sensor for real-time and visual determination of organophosphorus pesticides. This study provides valuable insights into the rational design of high-performance nanozymes featuring dual catalytic sites for advanced sensing applications.
Introduction
Glucose oxidase (GOx), with its exceptional catalytic efficiency and substrate specificity, plays a pivotal role in bioassays and biomedicine.1–3 However, its practical applications are hindered by intrinsic drawbacks such as poor stability, high production costs, and short shelf life.4–6 Nanozymes, artificial enzymes with intrinsic enzyme-catalytic activities, have emerged as promising alternatives owing to their structural stability, cost-effectiveness, and adjustable activity.7–11 Among them, Au nanoparticles (NPs) have emerged as particularly promising GOx mimics, catalyzing glucose oxidation to generate H2O2, a critical molecule in biosensing and disease diagnosis.12–15 However, the GOx-like activity of currently reported Au nanozymes remains unsatisfactory owing to their insufficient intrinsic activity.16,17 Moreover, their underlying mechanisms are not yet fully elucidated.18,19 Therefore, the development of novel Au nanozymes with enhanced catalytic performance is highly desirable and remains a priority for advancing practical applications.Recently, extensive efforts have been devoted to optimizing the catalytic performance of Au nanozymes.20,21 Carbon-based materials, for instance, have been widely explored as catalyst supports for Au NPs due to their ability to establish strong metal–support interactions, facilitate mass/charge transfer, and improve catalytic efficiency.22–24 Additionally, doping Au with other metals has emerged as an effective strategy to tune its electronic configuration and enhance GOx-like activity.25,26 Despite these advances, the GOx-like activity of Au nanozymes remains predominantly restricted to alkaline media, with limited catalytic efficiency and mechanistic understanding in acidic or neutral conditions.27–29 In natural GOx, a histidine (His) residue serves as a Brønsted base, initially abstracting the C1 hydroxyl proton from glucose, thereby facilitating glucose oxidation (Fig. 1a).30,31 Similarly, mechanistic investigations suggest that traditional Au nanozymes follow a comparable reaction pathway, with OH− ions acting as the Brønsted base and O2 serving as the terminal electron acceptor (Fig. 1b).32–34 Consequently, the overall reaction rate is governed by the dehydrogenation of glucose. However, the continuous generation of gluconic acid progressively depletes OH− ions, leading to catalytic slowdown. To this end, the rational design of catalytic centers to boost glucose oxidation while overcoming pH constraints is of paramount importance.
In this work, we design Fe single atom (SA)-bridged Au nanozymes (AuNPs/FeNC) with dual catalytic sites to overcome the pH-dependence limitations of GOx-like activity (Fig. 1c), which exhibit a 3.7-fold enhancement in acidic media in comparison to AuNPs/NC. Fe SAs on the N-doped carbon serve as anchoring sites for Au NPs, establishing strong interactions that provide an Au–Fe charge transfer pathway and facilitate O2 adsorption. Notably, in situ experiments provide the first direct visualization of Au–Fe–OO intermediates, rather than conventional Au–H intermediates, during glucose oxidation. Based on these insights, a plausible mechanism for glucose oxidation is proposed in which the reaction bypasses OH−-dependence pathways, allowing for efficient catalysis in acidic media. Leveraging the aligned optimal conditions at pH 4.0 for both GOx- and peroxidase (POD)-like activity, AuNPs/FeNC enable an efficient glucose cascade catalysis system, further applied in a portable, gel-based biosensor for real-time, visual determination of organophosphorus pesticides (OPs) in the range of 10–1000 ng mL−1, with a low limit of detection (LOD) of 1.9 ng mL−1.
Results and discussion
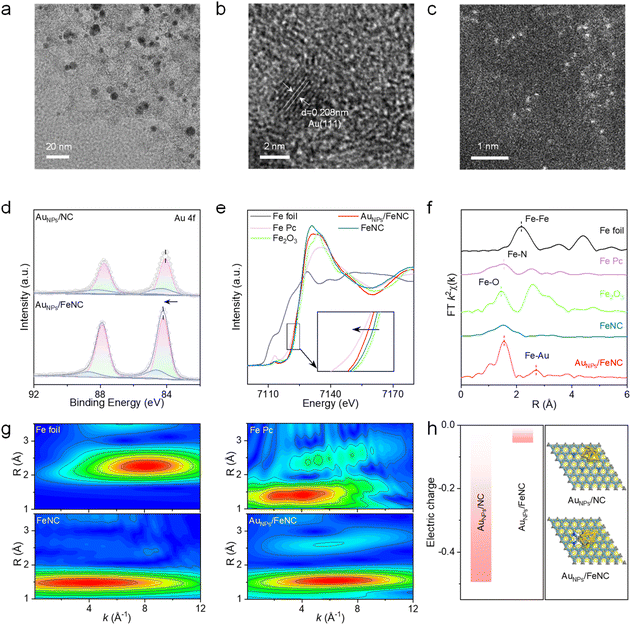
FeNC was synthesized via pyrolysis of a mixture of glucosamine and FeCl3 in the presence of dual templates of SiO2 and ZnCl2. Subsequently, HAuCl4 served as the Au source for the in situ growth of Au to obtain AuNPs/FeNC. For comparison, AuNPs & FeNC were obtained by physically mixing FeNC with AuNPs. Fig. 2a reveals that distinct black particles are uniformly distributed on the carbon support. Energy-dispersive spectroscopy (EDS) mapping images further demonstrate that Au is primarily localized within the particles, whereas Fe is randomly dispersed across the carbon support (Fig. S1). High-resolution TEM (HRTEM) image (Fig. 2b) shows a lattice spacing of 0.208 nm, corresponding to the (111) plane of Au. Furthermore, the aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HADDF-STEM) image (Fig. 2c) reveals the abundant Fe SAs on the support of AuNPs/FeNC. The crystalline phases of the samples analyzed through X-ray diffraction (XRD) patterns reveal no characteristic metal peaks for FeNC (Fig. S2), while AuNPs/FeNC displays distinct peaks matching the standard peaks of Au (JCPDS-04-0784). N2 adsorption–desorption isotherm analysis indicates a surface area of 199 m2 g−1 and abundant mesopores in AuNPs/FeNC (Fig. S3), which facilitate substrate and product diffusion as well as efficient electron transport during catalytic reactions. X-ray photoelectron spectroscopy (XPS) was utilized to investigate chemical composition. The N 1s spectrum of AuNPs/FeNC is deconvoluted into pyridinic N, Fe–N, pyrrolic N, and graphite N components (Fig. S4).35 Notably, the Au 4f peak in AuNPs/FeNC shifts slightly toward higher binding energy compared to AuNPs/NC (Fig. 2d), suggesting that the introduction of Fe SAs enables electron transfer from AuNPs to the support. To further elucidate the electronic structure of AuNPs/FeNC, X-ray absorption near-edge structure (XANES) and X-ray absorption fine structure (EXAFS) analyses were employed. In the Fe K-edge spectra, the absorption edges of Fe K-edge XANES curves in AuNPs/FeNC and FeNC fall between those of iron phthalocyanine (Fe Pc) and Fe2O3. Additionally, the absorption edge of AuNPs/FeNC exhibits a negative shift relative to FeNC, suggesting electron transfer from AuNPs to Fe sites (Fig. 2e). Fourier transformed (FT) k3-weighted EXAFS spectra of AuNPs/FeNC display a primary peak at 1.63 Å, attributed to the Fe–N bond, without evidence of Fe–Fe bond at 2.18 Å (Fig. 2f). Additionally, a peak at around 2.7 Å is observed in AuNPs/FeNC but not in FeNC, illustrating the formation of the Fe–Au bond in AuNPs/FeNC. EXAFS fitting results (Fig. S5 and Table S1) indicate a Fe–N coordination number of 3.95 with a bond length of 2.01 Å, suggesting a Fe–N4 configuration in AuNPs/FeNC. In addition, wavelet transform (WT) analysis further confirms the coordination of the Fe–N coordination structure of AuNPs/FeNC (Fig. 2g). Additionally, the Au EXAFS curve of AuNPs/FeNC in Fig. S6 exhibits the main peak at around 1.95 and 2.42 Å, associated with the Au–Fe and Au–Au interactions, respectively.36 To explore the electronic properties, density functional theory (DFT) calculations were conducted. As shown in Fig. 2h, the electric charge at Au sites in AuNPs/FeNC (−0.055e) is higher than that in AuNPs/NC (−0.494e), indicating that the introduction of Fe SAs facilitates electron transfer from AuNPs to the substrate, leading to a redistribution of electrons at the catalytic centers, consistent with the experimental results. Ultraviolet photoelectron spectroscopy (UPS) was employed to better understand the band structure information of nanozymes (Fig. S7). Notably, the cutoff energy of AuNPs/FeNC and AuNPs/NC is 16.76 and 16.62 eV, respectively. The calculated work functions are 4.46 eV for AuNPs/FeNC and 4.60 eV for AuNPs/NC, indicating that AuNPs/FeNC more readily donate electrons to reaction intermediates.37Fig. 3a presents a schematic illustration of the reaction catalyzed by AuNPs/FeNC, where O2 adsorption and activation realize the glucose cascade reaction. To explore the ability to mimic GOx, the glucose-nanozyme supernatant was assessed using a horseradish peroxidase (HRP)-based colorimetric system with 3,3,5,5-tetramethyl-benzidine (TMB) as the chromogenic substrate.38 As shown in Fig. 3b, the glucose-like activity of AuNPs/FeNC is 3.7- and 2.9-fold higher than that of AuNPs/NC and AuNPs & FeNC. To further explore the GOx-like property, the production of glucose acid was evaluated by adding hydroxylamine and Fe3+ to the reaction supernatant.39 As displayed in Fig. 3c, the absorbance band in both AuNPs/FeNC and GOx confirms the presence of gluconic acid, demonstrating the GOx-like behavior of AuNPs/FeNC. Additionally, AuNPs/FeNC exhibit 2.2-fold higher intensity than AuNPs/NC, suggesting that the introduction of Fe SAs facilitates the generation of gluconic acid. To further investigate the specificity mechanism, the activation energy (Ea) of glucose catalysis was calculated according to the Arrhenius equation. In a chemical reaction, Ea represents the minimum energy required for reactants to reach the activated state and is closely related to the reaction rate.40 The Ea value for AuNPs/FeNC is 1.15 kJ mol−1, lower than that of AuNPs/NC (6.46 kJ mol−1) and AuNPs & FeNC (8.33 kJ mol−1), indicating its superior glucose catalytic efficiency (Fig. 3d). Similar to natural GOx, the GOx-like activity of AuNPs/FeNC is pH-dependent. As revealed in Fig. 3e, its optimal GOx-like activity is observed at pH 4.0, aligning with its optimal POD-like activity. Consequently, the AuNPs/FeNC cascade system shows superior catalytic activity under the same acidic conditions (Fig. S8a). Additionally, the GOx-like activity of AuNPs increased with increasing pH, implying that the introduction of Fe SAs regulates the catalytic centers (Fig. S8b). Regarding POD-like activity, AuNPs/FeNC, AuNPs & FeNC, and FeNC display significantly higher POD-like activity than AuNPs/NC (Fig. S9a), highlighting Fe SAs as the primary active sites for H2O2 activation. Notably, the introduction of AuNPs slightly enhances the POD-like activity of AuNPs/FeNC, which is 8.7 times greater than its oxidase-like activity (Fig. S9b), indicating a remarkable preference for POD-like activity. To validate the cascade catalytic activity, a colorimetric assay was conducted (Fig. 3f). The absorbance at 652 nm for the AuNPs/FeNC cascade system is 3.8-fold higher than that of AuNPs/NC, indicating that the introduction of Fe SAs significantly enhances the catalytic efficiency. Interestingly, the cascade catalytic activity of AuNPs/FeNC is 3.3-fold higher than that of AuNPs & FeNC, whereas its POD-like activity is slightly higher than that of AuNPs & FeNC. This result suggests that Fe SA-bridged Au NPs significantly optimize the activation efficiency of the glucose cascade system. When glucose is the only substrate, the GOx–HRP system produces oxTMB (Fig. S10), whereas neither the GOx nor HRP system alone does, suggesting that AuNPs/FeNC possess both GOx- and HRP-like catalytic activities. To quantitatively evaluate the catalytic performance of the biomimetic cascade system, a typical Michaelis–Menten kinetic analysis was conducted. As shown in Fig. 3g and Table S2, the maximum reaction velocity (Vmax) of AuNPs/FeNC for the glucose cascade system is calculated to be 75.6 × 10−8 M s−1, which is 2.9-fold and 6.9-fold higher than that of AuNPs/NC and AuNPs & FeNC. Moreover, AuNPs/FeNC possess the smallest Michaelis–Menten constants (Km) of 0.28 mM, indicating their superior affinity for glucose. Benefiting from the highly compatible pH conditions of AuNPs/FeNC with both GOx- and POD-like activities, the engineered AuNPs/FeNC-based cascade catalysis system was applied for the sensitive and selective colorimetric detection of glucose. As shown in Fig. 3h, the absorbance of oxTMB at 652 nm increases progressively with glucose concentration, exhibiting a good linear correlation in the range of 0.01–2 mM with a LOD of 2.9 μM. Furthermore, AuNPs/FeNC show outstanding recyclability (Fig. S11), maintaining nearly constant glucose oxidation activity after five catalytic cycles. In addition, the biomimetic AuNPs/FeNC system displays satisfactory selectivity for glucose over common interfering substances, including sucrose, fructose, L-cysteine, galactose, lactose, dopamine, ascorbic acid, maltose, and uric acid (Fig. 3i).
To investigate the catalytic mechanism of glucose oxidation, DFT calculations were performed to study glucose adsorption on the nanozymes. Fig. S12a reveals that the adsorption energy of glucose on the AuNPs/FeNC model (−2.06 eV) is lower than that on the AuNPs/NC model (−1.90 eV), indicating that the introduction of Fe SAs is favorable for glucose adsorption. In the AuNPs/FeNC model, the adsorption energy of O2 at the Fe sites (−0.94 eV) is lower than that at the Au sites (−0.39 eV), suggesting that Fe sites are more favorable for O2 adsorption (Fig. 4a). The projected state density (PDOS) analysis for AuNPs/FeNC and AuNPs/NC (Fig. 4b) reveals enhanced orbital hybridizations between Fe and C in AuNPs/FeNC, consistent with its stronger glucose adsorption capacity. Mulliken charge analysis (Fig. S12b) indicates that the charge transfer from glucose to AuNPs/FeNC is 0.123e, higher than that to AuNPs/NC (0.104e), verifying that the introduction of Fe SAs optimizes the interfacial charge transfer process. To elucidate reaction pathways and identify intermediates, in situ attenuated total reflection-FTIR (ATR-FTIR) experiments were conducted for AuNPs/NC and AuNPs/FeNC under reaction conditions (Fig. 4c and d). Upon glucose introduction, a distinct signal peak at 1573 cm−1, corresponding to O–C–O bending, confirms the formation of gluconate.41 The intensity of this peak is significantly higher than for AuNPs/NC, suggesting enhanced glucose oxidation. A peak at 1639 cm−1 corresponds to the H–O–H bending, while the peak at 1279 cm−1 is attributed to the O–O stretching mode of adsorbed OOH (*OOH).42,43 In addition, the band at 1410 cm−1 is assigned to adsorbed O2, observed exclusively in AuNPs/FeNC, indicating that the introduction of Fe SAs facilitates O2 absorption on the surface of AuNPs/FeNC.44 Notably, Fe–O and *OOH species, represented by peaks at 653 and 930 cm−1, are unique to AuNPs/FeNC. Furthermore, the *OO species absorption band at 1018 cm−1 shifts to a lower frequency in AuNPs/FeNC compared to AuNPs/NC, indicating a stronger Fe–O interaction that enhances O–O bond polarization.45 To monitor real-time catalytic processes, in situ electron paramagnetic resonance (EPR) measurement was conducted. As displayed in Fig. 4e, in AuNPs, an initial ˙H signal at 20 s transitions into a ˙OOH signal at 100 s, with increasing intensity facilitating H2O2 formation. In contrast, AuNPs/FeNC follow a distinct radical pathway, transitioning from O2˙− to ˙OOH. To further verify these findings, 2,2,6,6-tetramethylpiperidinooxy (TEMPO) was employed as a hydrogen-extracting reagent.46 The catalytic activity of AuNPs decreases by 89% in the presence of TEMPO, whereas AuNPs/FeNC exhibits no significant decrease (Fig. S13), demonstrating that the crucial role of surface Au–H species in glucose oxidation by AuNPs and suggesting an alternative catalytic pathway in AuNPs/FeNC. Based on these findings, a plausible reaction mechanism is proposed (Fig. 4f). In AuNPs, OH− acts as a Brønsted base, initially abstracting H+ from the C1 hydroxyl group of glucose. Subsequently, certain Au atoms extract H from the glucose C–H bond, forming Au–H intermediates. Then, O2 accepts electrons, becoming activated and integrating into Au–H to form Au–OOH species, which ultimately dissociate to generate H2O2. In contrast, under a proton-rich acidic environment, glucose first adsorbs onto the Au sites of AuNPs/FeNC. Subsequently, the electron-rich Fe sites adsorb O2 to form *OO intermediates. The Au–Fe–OO intermediates then combine with H+ derived from glucose, forming Au–Fe–OOH species. The *OOH at the Fe sites further extracts H from the C–H bond of glucose to generate *H2O2, thereby completing the catalytic cycle and enabling efficient glucose oxidation in acidic conditions.
Leveraging the exceptional glucose cascade system of AuNPs/FeNC, a colorimetric assay is developed as a proof-of-concept application for AChE and OP detection. AChE catalyzes the hydrolysis of acetylthiocholine (ATCh) into thiocholine (TCh), which is essential for regulating the neurotransmitter acetylcholine levels.47 TCh, a sulfhydryl molecule, binds the active sites of AuNPs/FeNC, thereby inhibiting the biomimetic cascade reaction. Au anchors mercapto molecules via the Au–S bond. To confirm this inhibitory effect, cysteine (Cys) and glutathione (GSH) were introduced into the FeNC–H2O2 system, resulting in a noticeable decrease in absorbance (Fig. S14), suggesting that the active sites of FeNC were effectively blocked by the mercapto molecules.48 Leveraging the enzyme-like inhibitory mechanism of active sites, an AuNPs/FeNC-based biosensor was constructed to further evaluate its sensitivity to AChE activity. As shown in Fig. S15a, the catalytic activity of AuNPs/FeNC gradually decreases with increasing AChE concentrations in the presence of ATCh (5 mM). The AuNPs/FeNC-based biosensor displays a good linear relationship between the absorbance of oxTMB and AChE concentrations in the range of 0.5 to 50 mU mL−1 with an LOD of 0.16 mU mL−1 (Fig. S15b). Notably, the AuNPs/FeNC-based biosensor displays a broader detection range compared to the AuNPs/NC-based biosensor, suggesting that the introduction of Fe SAs enhances the sensitivity and detection performance of biosensors.
OPs can rapidly inhibit the AChE activity, leading to nervous system dysfunction. In this study, fenthion was selected as a model OP due to its inhibition effect on AChE activity, consistent with observations of the AuNPs/FeNC system (Fig. S16). Compared with AuNPs/NC-based biosensor, AuNPs/FeNC-based biosensor exhibits a wider detection range for OP detection (Fig. S17), indicating the main role of Fe single atoms. To realize visual, rapid, and real-time detection, a portable, smartphone-integrated gel biosensor was developed for the quantitative OP analysis. As illustrated in Fig. 5a, agarose hydrogels were formed on the snap caps of EP tubes by immersing them in a solution containing AuNPs/FeNC and TMB. When OPs were introduced into the EP tube lumen along with AChE for 5 min, the tubes were inverted, causing a color shift from colorless to blue. The color intensity was captured using the smartphone app ColorDesk, which quantified the results obtained by converting images into RGB values. As shown in Fig. 5b, the biosensor exhibits a progressive blue shift with increasing OP concentrations, detectable by the naked eye. Linear regression reveals a strong correlation between the RGB values and OP concentrations within the range of 10 to 1000 ng mL−1, with a calculated LOD of 1.9 ng mL−1. Compared to other OP sensing platforms, the AuNPs/FeNC-based gel biosensor kit exhibits competitive sensitivity (Table S3). To confirm the potential for practical application, the anti-interference capabilities of the biosensor were tested. As shown in Fig. 5c, several biomolecules show no significant impact on the performance of the biosensor, validating its reliability for OP detection. Remarkably, its stability is confirmed with RGB values showing minimal decline after 15 days (Fig. 5d). Furthermore, the applicability of the biosensor for OP detection in real samples was evaluated. As shown in Table S4, the recovery rates of OPs range from 102.0% to 107.6%, demonstrating the high potential of this sensing platform for real-world OP detection.
Conclusions
In summary, this work achieves highly efficient glucose oxidation in acidic media by constructing a dual-site nanozyme composed of Fe single atom-bridged Au NPs, which serve as the binding sites for O2 and glucose. The formation of metallic-bonded Fe–Au pairs enhances electron transfer and optimizes the electronic structure of active sites. Notably, in situ experiments and theoretical calculations reveal the presence of Au–Fe–OO intermediates during the reaction, which modifies the catalytic pathway, eliminates OH− dependence, and enables efficient glucose activation under acid conditions. Benefiting from the aligned optimal conditions for both GOx- and POD-like activities at pH 4.0, an AuNPs/FeNC-based glucose cascade system is successfully constructed. As a proof-of-concept, a portable biosensor kit based on this system is developed for real-time, ultrasensitive determination of OPs. This work advances the design of highly efficient nanozymes to meet the growing demands of practical applications.Author contributions
Investigation, writing—original draft, X. L., and F. T.; providing help in measurements, Z. M., Y. Z. and Y. T.; investigation, C. W. and W. G.; funding acquisition, X. L., C. Z., and C. L.; supervision, writing—review & editing, J. L. and C. Z.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the supplementary information (SI) of this article. Supplementary information: experimental details, additional characterizations, supplementary data, and supporting tables. See DOI: https://doi.org/10.1039/d5sc05430c.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (no. 22204045), the Fundamental Research Funds for Hubei University of Technology (no. XBTK-2024007 and GCC2024013), the Fundamental Research Funds for the Central Universities (no. CCNU24JCPT032) and the Open Research Fund of the Key Laboratory of Ministry of Education, Hangzhou Normal University (KFJJ2023009).Notes and references
- W. Xu, L. Jiao, Y. Wu, L. Hu, W. Gu and C. Zhu, Adv. Mater., 2021, 33, 2005172 CrossRef CAS PubMed.
- X. Zhang, G. Li, G. Chen, D. Wu, Y. Wu and T. D. James, Adv. Funct. Mater., 2021, 31, 2106139 CrossRef CAS.
- D. M. Vriezema, P. M. L. Garcia, N. Sancho Oltra, N. S. Hatzakis, S. M. Kuiper, R. J. M. Nolte, A. E. Rowan and J. C. M. van Hest, Angew. Chem., Int. Ed., 2007, 46, 7378 CrossRef CAS PubMed.
- S. Liu and Y. Sun, Angew. Chem., Int. Ed., 2023, 62, e202308562 CrossRef CAS PubMed.
- Y. Li, R. Fu, Z. Duan, C. Zhu and D. Fan, Small, 2022, 18, 2200165 CrossRef CAS PubMed.
- X. Jia, L. Jiao, R. Li, C. Chen, X. Li, L. Hu, Y. Zhai, C. Zhu and X. Lu, Adv. Funct. Mater., 2024, 34, 2406380 CrossRef CAS.
- L. Gao, H. Wei, S. Dong and X. Yan, Adv. Mater., 2024, 36, 2305249 CrossRef CAS PubMed.
- M. Zandieh and J. Liu, Adv. Mater., 2024, 36, 2211041 CrossRef CAS PubMed.
- S. He, L. Ma, Q. Zheng, Z. Wang, W. Chen, Z. Yu, X. Yan and K. Fan, Bioact. Mater., 2024, 42, 284 CAS.
- Y. Zhou, W. Wei, F. Cui, Z. Yan, Y. Sun, J. Ren and X. Qu, Chem. Sci., 2020, 11, 11344 RSC.
- R. Zhang, B. Jiang, K. Fan, L. Gao and X. Yan, Nat. Rev. Bioeng., 2024, 2, 849 CrossRef CAS.
- J. Chen, Q. Ma, M. Li, D. Chao, L. Huang, W. Wu, Y. Fang and S. Dong, Nat. Commun., 2021, 12, 3375 CrossRef CAS PubMed.
- W. Luo, C. Zhu, S. Su, D. Li, Y. He, Q. Huang and C. Fan, ACS Nano, 2010, 4, 7451 CrossRef CAS PubMed.
- Z. Wang, R. Chen, W. Zhang, P. Sun, N. Zhang and Y. Zhao, Adv. Funct. Mater., 2024, 35, 2412767 CrossRef.
- Y. Huang, M. Zhao, S. Han, Z. Lai, J. Yang, C. Tan, Q. Ma, Q. Lu, J. Chen, X. Zhang, Z. Zhang, B. Li, B. Chen, Y. Zong and H. Zhang, Adv. Mater., 2017, 29, 1700102 CrossRef PubMed.
- Y. Wu, W. Xu, L. Jiao, W. Gu, D. Du, L. Hu, Y. Lin and C. Zhu, Chem. Soc. Rev., 2022, 51, 6948 RSC.
- X. Cai, L. Jiao, H. Yan, Y. Wu, W. Gu, D. Du, Y. Lin and C. Zhu, Mater. Today, 2021, 44, 211 CrossRef CAS.
- L.-H. Fu, C. Qi, J. Lin and P. Huang, Chem. Soc. Rev., 2018, 47, 6454 RSC.
- Q. Wang, K. Chen, H. Jiang, C. Chen, C. Xiong, M. Chen, J. Xu, X. Gao, S. Xu, H. Zhou and Y. Wu, Nat. Commun., 2023, 14, 5338 CrossRef CAS PubMed.
- J. Chen, X. Liu, G. Zheng, W. Feng, P. Wang, J. Gao, J. Liu, M. Wang and Q. Wang, Small, 2023, 19, e2205924 CrossRef PubMed.
- Y. Wang, T. Chu, T. Jin, S. Xu, C. Zheng, J. Huang, S. Li, L. Wu, J. Shen, X. Cai and H. Deng, Adv. Sci., 2024, 11, 2308587 CrossRef CAS PubMed.
- B. Wu, H. Yang, L. Li, X. Tang, Y. Wu, B. Huang, D. Lutzenkirchen-Hecht, M. Qiu, K. Yuan and Y. Chen, Adv. Mater., 2025, 37, 2500096 CrossRef CAS PubMed.
- J. Zhang, Y. Yuan, L. Gao, G. Zeng, M. Li and H. Huang, Adv. Mater., 2021, 33, 2006494 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhao, B. Danil, W. Xiao and X. Yang, Adv. Mater., 2024, 36, 2400198 CrossRef CAS PubMed.
- C. Wang, L. Wang, V. Nallathambi, Y. Liu, J. Kresse, R. Hübner, S. Reichenberger, B. Gault, J. Zhan, A. Eychmüller and B. Cai, Adv. Mater., 2024, 36, 2405200 CrossRef CAS PubMed.
- H. Shi, T. Wang, Z. Lin, S. Liu, X. Liu, R. Zhou, Z. Cai, Y. Huang and Q. Li, Angew. Chem., Int. Ed., 2025, 64, e202424476 CrossRef CAS PubMed.
- D. Jana, D. Wang, A. K. Bindra, Y. Guo, J. Liu and Y. Zhao, ACS Nano, 2021, 15, 7774 CrossRef CAS PubMed.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2019, 31, 1805368 CrossRef CAS PubMed.
- H. Shen, S. Chen, S.-C. Mo, H. Huang, H. Liang, J. Zhang, Z.-L. Xu, W. Liu and Y. Liu, Adv. Funct. Mater., 2024, 35, 2418360 CrossRef.
- X. Wang, Q.-Z. Li, J.-J. Zheng and X. Gao, ACS Catal., 2024, 14, 13040 CrossRef CAS.
- M. Comotti, C. Della Pina, E. Falletta and M. Rossi, Adv. Synth. Catal., 2006, 348, 313 CrossRef CAS.
- Y. Tang, X. Liu, P. Qi, W. Xu, Y. Wu, Y. Cai, W. Gu, H. Sun, C. Wang and C. Zhu, Nano Lett., 2024, 24, 9974 CrossRef CAS PubMed.
- X. Shen, W. Liu, X. Gao, Z. Lu, X. Wu and X. Gao, J. Am. Chem. Soc., 2015, 137, 15882 CrossRef CAS PubMed.
- K. Wang, Q. Hong, C. Zhu, Y. Xu, W. Li, Y. Wang, W. Chen, X. Gu, X. Chen, Y. Fang, Y. Shen, S. Liu and Y. Zhang, Nat. Commun., 2024, 15, 5705 CrossRef CAS PubMed.
- X. Luo, Z. Luo, S. Li, Q. Fang, W. Xu, H. Wang, Y. Wang, G. M. Bao, W. Gu and C. Zhu, Anal. Chem., 2023, 95, 12306 CrossRef CAS PubMed.
- Y. Yang, Y. Xiao, L. Jiang, J. Li, J. Li, J. Jia, C. T. Yavuz, F. Cui, X. Jing and G. Zhu, Adv. Mater., 2024, 36, 2404791 CrossRef CAS PubMed.
- X. Wei, S. Song, W. Cai, X. Luo, L. Jiao, Q. Fang, X. Wang, N. Wu, Z. Luo, H. Wang, Z. Zhu, J. Li, L. Zheng, W. Gu, W. Song, S. Guo and C. Zhu, Chem, 2023, 9, 181 CAS.
- Q. Li, S. Wu, B. Li, P. Zhou, H. Wang, X. Zhang, Q. Meng, X. Li, H. Chen, Y. Pang and R. Chen, Small, 2024, 20, 2405321 CrossRef CAS PubMed.
- J. Yu, T. Chen, X. Wen, H. Shi, L. Wang and Y. Xu, Biosens. Bioelectron., 2024, 253, 116169 CrossRef CAS PubMed.
- Y. Tang, Y. Chen, Y. Wu, W. Xu, Z. Luo, H.-R. Ye, W. Gu, W. Song, S. Guo and C. Zhu, Nano Lett., 2023, 23, 267 CrossRef CAS PubMed.
- E. G. Mahoney, W. Sheng, M. Cheng, K. X. Lee, Y. Yan and J. G. Chen, J. Power Sources, 2016, 305, 89 CrossRef CAS.
- C. Feng, Z. Zhang, D. Wang, Y. Kong, J. Wei, R. Wang, P. Ma, H. Li, Z. Geng, M. Zuo, J. Bao, S. Zhou and J. Zeng, J. Am. Chem. Soc., 2022, 144, 9271 CrossRef CAS PubMed.
- K. Kunimatsu, T. Yoda, D. A. Tryk, H. Uchida and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 12, 621 RSC.
- Y. Zhou, Y. Wu, Z. Luo, L. Ling, M. Xi, J. Li, L. Hu, C. Wang, W. Gu and C. Zhu, J. Am. Chem. Soc., 2024, 146, 12197 CrossRef CAS PubMed.
- W. Xu, Y. Wu, X. Wang, Y. Qin, H. Wang, Z. Luo, J. Wen, L. Hu, W. Gu and C. Zhu, Angew. Chem., Int. Ed., 2023, 62, e202304625 CrossRef CAS PubMed.
- X. Li, W. Tan, J. Fan and K. Li, ACS Nano, 2024, 18, 24162 CrossRef CAS PubMed.
- M. Lei, X. Ding, J. Liu, Y. Tang, H. Chen, Y. Zhou, C. Zhu and H. Yan, Anal. Chem., 2024, 96, 6072 CrossRef CAS PubMed.
- Y. Wu, L. Jiao, X. Luo, W. Xu, X. Wei, H. Wang, H. Yan, W. Gu, B. Z. Xu, D. Du, Y. Lin and C. Zhu, Small, 2019, 15, e1903108 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |