 Open Access Article
Open Access ArticleNitrogen fixation using metallic lithium nanoparticles formed by electrospray deposition†
Dylan T.
Holden
 a,
Myles Quinn
Edwards
a,
Myles Quinn
Edwards
 a,
Zhongxia
Shang
a,
Zhongxia
Shang
 b and
R. Graham
Cooks
b and
R. Graham
Cooks
 *a
*a
aDepartment of Chemistry, Purdue University, 560 Oval Dr, West Lafayette, IN 47907, USA. E-mail: cooks@purdue.edu
bBirck Nanotechnology Center, Purdue University, 1205 W State St, West Lafayette, IN 47907, USA
First published on 17th June 2025
Abstract
Multiphase reduction of ambient dinitrogen (N2) was observed during the deposition of charged or uncharged aqueous microdroplets containing a lithium salt pre-catalyst onto conducting or inert surfaces to yield mixed lithium nanoparticles. We propose that this method leverages the high electric fields and partial solvation of ions at the gas–water–solid interface to enhance reaction rates and perform normally moisture-sensitive chemistry in aqueous droplets. Ammonia (NH3) is formed and Li+ is regenerated from the hydrolysis of transient lithium nitride Li3N, constituting a complete catalytic cycle. The ammonia is captured in situ using formaldehyde to yield hexamethylenetetramine, a solid nitrogenous fuel. By measuring product formation with mass spectrometry, it was determined that 2.97 ± 0.36 μg h−1 of NH3 was produced by a single spray source when using pure N2 sheath gas and an applied potential, though microdroplets alone appear a sufficient source of electrons in cases where an external potential is not applied; air is also an adequate source of N2 to produce NH3. The mixed lithium metal nanoparticles were characterized with (S)TEM, EDS, and EELS. This ammonia synthesis has implications for the formation of nitrogenous compounds in environmental, prebiotic, and traditional synthetic contexts.
Introduction
Ammonia (NH3) is among the most important of all commodity chemicals due to its use as fertilizer. It is primarily synthesized via the energy intensive and environmentally harmful Haber–Bosch (H–B) process where molecular nitrogen (N2) and petroleum-derived hydrogen react under extreme temperatures (400–550 C) and pressures (150–300 atm) using fused-iron catalysts. This process consumes approximately 2% of the world's yearly fossil fuel energy output and generates over half a billion metric tons of carbon dioxide (CO2) as a byproduct.1–4 Despite nearly a century of research into scalable chemical and biological nitrogen fixation, Haber–Bosch still dominates industrial ammonia production, signifying a clear need for efficient and sustainable alternatives.4–17While numerous methods exist for cleaving the highly stable N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond (BDE 941 kJ mol−1),18 these typically require the use of toxic and/or costly transition metal catalysts.19 Electrochemical strategies have been heralded as green alternatives to the H–B process due to the availability of less-toxic electrocatalysts, as well as the relative ease of scaling up such reactions.20 Practical implementation of this approach has been limited due to competition with hydrogen evolution reactions and water incompatibility. Several publications describe the use of ‘physical catalysts' such as high local electric fields and the resulting ion chemistry that activates N2 prior to reduction,21 often utilizing water or H2 as a proton source. Related examples include spontaneous aqueous electrochemical reduction at N-doped carbon nanospikes,22 use of metal plasmas,23,24 corona discharges in humid N2-rich atmospheres,25 contact electrification at hydrophobic interfaces,26 as well as reactions at the charged gas–water–solid interfaces of aqueous microdroplets and iron catalysts.27
N triple bond (BDE 941 kJ mol−1),18 these typically require the use of toxic and/or costly transition metal catalysts.19 Electrochemical strategies have been heralded as green alternatives to the H–B process due to the availability of less-toxic electrocatalysts, as well as the relative ease of scaling up such reactions.20 Practical implementation of this approach has been limited due to competition with hydrogen evolution reactions and water incompatibility. Several publications describe the use of ‘physical catalysts' such as high local electric fields and the resulting ion chemistry that activates N2 prior to reduction,21 often utilizing water or H2 as a proton source. Related examples include spontaneous aqueous electrochemical reduction at N-doped carbon nanospikes,22 use of metal plasmas,23,24 corona discharges in humid N2-rich atmospheres,25 contact electrification at hydrophobic interfaces,26 as well as reactions at the charged gas–water–solid interfaces of aqueous microdroplets and iron catalysts.27
Recently, lithium-metal-based nitrogen fixation approaches have garnered attention due to the high speed and efficiency with which the corresponding nitride Li3N can be formed under ambient conditions and subsequently reacted with water to yield NH3 and regenerate Li+ ions.28,29 By spatially or temporally separating the processes of Li+ reduction, Li metal nitridation, and Li3N hydrolysis, it is possible to achieve efficient NH3 production while retaining water as a proton source and potentially limiting unwanted H2 production.30 Further, lithium may also serve an alternative role in direct NH3 production by enhancing electric field effects at sharp tips.22
Inspired by the discovery that microdroplets accelerate chemical reactions, largely via generation of reactive species and partial solvation of reagents at the spontaneously charged gas–liquid interface,31–33 we explored the possibility that lithium-containing water microdroplets might be a viable strategy for rapid and sustainable small-scale NH3 production. Taking advantage of the high concentration34 and partial solvation of Li+ ions at the (relatively) water-deficient surface35 of the microdroplet, we deposited droplets formed with or without an applied external potential to a grounded graphite counter electrode or a non-conductive surface to facilitate Li+ reduction to Li0. Simultaneous formation of mixed Li nanoparticles (Li NPs) on the deposition surfaces was observed. Electrospray deposition (ESD) is a widely utilized technique in industrial and academic contexts for preparing NPs and other nanostructured materials from polymers or metal salts, and their presence can increase the rates of heterogeneous reactions.36–42 By carefully controlling the sheath gas and liquid flow rates, spray voltage, as well as the spray distance, it was possible to form Li NPs or Li dendrites at the cathode surface followed by reaction of N2 (or compressed air) sheath gas with ambient water provided by the nearly de-solvated microdroplets to yield NH3. Formaldehyde, added to the Li+ solution, enabled in situ derivatization of produced ammonia via hexamethylenetetramine (hexamine) synthesis, a solid adamantine nitrogenous fuel of considerable industrial,43–46 synthetic,47 and prebiotic48–53 relevance.
Results and discussion
Mechanistic investigation of ammonia synthesis via electrospray deposition
To examine the feasibility of reducing Li+ in an aqueous ESD system, nano-electrospray (nESI) analysis was performed following cathodic ESD of a solution of 100 mM LiBr 3.7% formaldehyde (v/v) with N2 sheath gas, revealing a strong signal at m/z 141 attributed to protonated hexamine. Fig. 1A details the (electro)spray deposition apparatus and conditions utilized to perform the electrospray deposition and NH3 trapping experiments. To verify the identity of the presumptive product (Fig. 2A), high-resolution mass spectrometry (HRMS) and tandem mass spectrometry (MS/MS) were performed on the surface wash solution following ESD. The elemental composition of the m/z 141 peak (Fig. S1†) is C6H13N4+, corresponding to protonated hexamine. Analysis of the product ion spectrum of hexamine reveals a series of neutral loss species with alternating mass differences of 13 and 14 Da (Fig. 2B). From precursor ion m/z 141 [hexamine + H]+, the first neutral loss corresponds to methanimine (CH2NH), giving a product ion at m/z 112. The remaining product ion peaks reveal neutral losses of C2H5N (m/z 98), C2H4N2 (m/z 85), C3H6N2 (m/z 71), and C3H5N3 (m/z 58), corresponding to fragmentation events along the C–N backbone of hexamine. The identity of the product at m/z 141 formed following ESD was confirmed with a hexamine standard (Fig. S2†) and with previously reported hexamine MS/MS spectra.48Lithium has been shown to assist in the synthesis of NH3 from N2 through at least two mechanisms: via a Li3N intermediate or through enhancement of local electric fields, the latter allowing for activation and direct electrolysis of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in aqueous systems.22,28 Previous work has described a cation size effect on the rate of aqueous NH3 production at N-doped carbon nanospikes (Li+ > Na+ > K+), where the smaller Li+ ions might provide a greater charge density at the nanospike tip while also limiting access of water to the Stern layer where N2 reduction occurs.22 Given that microdroplets inherently possess large interfacial electric fields,54,55 even in the absence of an externally applied potential, various alkali bromide salts were screened to examine their effect on NH3 production via local field enhancement. NaBr and KBr were added to formaldehyde solutions and, despite extensive effort at optimizing the ESD conditions, the hexamine product was undetectable by MS in all cases (Table S3†). This suggests that NH3 is formed via the hydrolysis of transient Li3N rather than through direct N2 activation by a cation-augmented electric field (Fig. 1B). Further description of the electrochemical half-cell reactions is detailed in Scheme S1.† While alignment and activation of N2 might still occur at the surface of the microdroplet's air–water interface, this alone cannot explain the observed ammonia production.
N triple bond in aqueous systems.22,28 Previous work has described a cation size effect on the rate of aqueous NH3 production at N-doped carbon nanospikes (Li+ > Na+ > K+), where the smaller Li+ ions might provide a greater charge density at the nanospike tip while also limiting access of water to the Stern layer where N2 reduction occurs.22 Given that microdroplets inherently possess large interfacial electric fields,54,55 even in the absence of an externally applied potential, various alkali bromide salts were screened to examine their effect on NH3 production via local field enhancement. NaBr and KBr were added to formaldehyde solutions and, despite extensive effort at optimizing the ESD conditions, the hexamine product was undetectable by MS in all cases (Table S3†). This suggests that NH3 is formed via the hydrolysis of transient Li3N rather than through direct N2 activation by a cation-augmented electric field (Fig. 1B). Further description of the electrochemical half-cell reactions is detailed in Scheme S1.† While alignment and activation of N2 might still occur at the surface of the microdroplet's air–water interface, this alone cannot explain the observed ammonia production.
To probe Li3N involvement, various lithium salts were screened for their ability to produce NH3. Of the three salts examined, LiBr and LiClO4 produced similar amounts of hexamine after 20 minutes of deposition, with a thin and evenly distributed white coating of LiBr and polymerized formaldehyde (paraformaldehyde), as well as faint some faint brown-orange material (Li3N) on the graphite surface (Fig. S3A†). However, ESD of a 100 mM LiOH and formaldehyde solution resulted in the formation of macroscopic tower-like structures (Fig. S3B†) that, when dissolved in water, yielded a detectable but significantly lower intensity hexamine signal. This observation is presumably due to rapid base-catalyzed polymerization of formaldehyde to paraformaldehyde,56 which formed in the LiBr and LiClO4 solutions, but is of considerably lower concentration due to neutralization of any LiOH by strong conjugate acids HBr and HClO4, respectively. The observed selectivity of hexamine formation with inclusion of only Li-containing salts implicates the involvement of Li0 and Li3N as reactive intermediates in this method. Note that it might also be possible for formaldehyde to react directly with Li3N to form hexamine, though investigation of this secondary mechanism is beyond the scope of this study.57
To control for environmental NH3 contamination and demonstrate N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage, the N2 sheath gas stream was diluted 10% with compressed 15N-labeled N2 and the ESD experiment was performed as previously described. Peaks corresponding to multiple 15N incorporation events are observed in the high-resolution full scan mass spectrum, largely favoring the incorporation of 1–2 15N atoms, supporting the argument that this method primarily utilizes N2 as the nitrogen feedstock (Table S1, Fig. S4A and B†). While instrument contamination reduced the quality of the high-resolution MS/MS spectrum of m/z 145 (Fig. S4C†), the low-resolution mass spectrometer did not show signs of contamination (Table S2†) and the tetrasubstituted product at m/z 145 could be isolated and subject to MS/MS (Fig. 2C); the resulting product ions correspond well with the mass shifts expected when compared to those of unlabeled hexamine at m/z 141 (cf.Fig. 2B).
N bond cleavage, the N2 sheath gas stream was diluted 10% with compressed 15N-labeled N2 and the ESD experiment was performed as previously described. Peaks corresponding to multiple 15N incorporation events are observed in the high-resolution full scan mass spectrum, largely favoring the incorporation of 1–2 15N atoms, supporting the argument that this method primarily utilizes N2 as the nitrogen feedstock (Table S1, Fig. S4A and B†). While instrument contamination reduced the quality of the high-resolution MS/MS spectrum of m/z 145 (Fig. S4C†), the low-resolution mass spectrometer did not show signs of contamination (Table S2†) and the tetrasubstituted product at m/z 145 could be isolated and subject to MS/MS (Fig. 2C); the resulting product ions correspond well with the mass shifts expected when compared to those of unlabeled hexamine at m/z 141 (cf.Fig. 2B).
Quantitation of NH3 production in charged and uncharged droplet systems
While the inherent electric field at the gas–water interface does not appear to be sufficient for direct electrolysis of N2 (at least in quantities capable of determination by MS on this limited timescale), the surface charge layer might still contribute to the reduction of surface Li+ in instances when an external potential is not applied (and when a nonconductive surface is used). To investigate this possibility, a calibration curve was constructed to quantify the amount of hexamine produced under various deposition conditions (Fig. 3A). Standard ESD conditions using LiBr as the electrolyte with a positive charge applied to the metal syringe needle and a grounded graphite surface resulted in an average rate of 2.97 ± 0.36 μg NH3 per hour per sprayer with an RSD of 8.7% as determined by the original peak ratios and hexamine concentration (Fig. 3A, blue data point), as determined by the observed hexamine yield (see ESI,† Methods and materials). This value of 2.97 ± 0.36 μg NH3 per hour per sprayer is approximately 4 times greater than previously reported microdroplet or electrospray-related ammonia formation methodologies.25,27 Changing the electrolyte from LiBr to LiClO4 returned a similar rate of ammonia production (Table S2†), demonstrating no significant counterion effect. Note that all reported values are likely underestimates given that MS detection is dependent on capture of NH3 by formaldehyde, an undoubtedly inefficient process given the volatility of both the formaldehyde and the NH3 in conjunction with the pneumatic force of the surrounding sheath gas.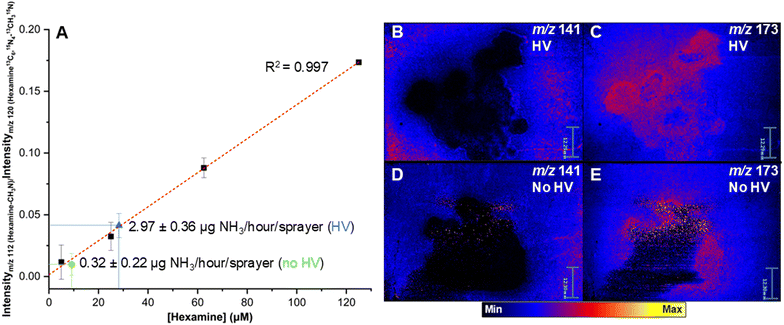 | ||
| Fig. 3 (A) Calibration curve constructed to determine the approximate rate of NH3 production across various experimental conditions. The blue extrapolated point refers to the average hexamine concentration found after washing a graphite surface following electrospray deposition of the LiBr and formaldehyde solution used in Fig. 2, whereas the green point corresponds to the same experiment albeit without an applied potential. (B–E) DESI-MS ion images of two separate graphite surfaces following 20-minute deposition experiments. (B) and (C) Show the distribution of protonated hexamine (m/z 141) and the lithium adduct of a paraformaldehyde unit (m/z 173) with a potential applied to the spray apparatus and a grounded graphite surface. (D) and (E) Show the distribution of the same two species without an externally applied potential. The signals for each pair of images (B–E) are normalized to the total ion current from each round of imaging and the scale bar is 12.30 mm. | ||
When the external spray potential was removed, the rate of NH3 production decreased to approximately 320 ± 220 ng per hour per sprayer (Fig. 3A, green data point). This value was remarkably constant across trials where a potential was not applied to graphite and in those where a non-conductive surface such as Parafilm was used (Fig. S5†). The RSD for this point falls between the limit of quantitation and the limit of detection so, while this value is consistent across trials and conditions, one cannot compare it directly to the high voltage NH3 yield. This suggests that while it is certainly more efficient for Li+ to be reduced at a negatively biased conductive surface, it is still possible for transient Li metal to form from spray deposition alone. Surface effects in aqueous microdroplets, such as partial solvation, can produce strong reducing agents58–60 (e.g. hydrated electrons61–64) and enable redox chemistry normally prohibited in bulk solution. We propose that electrons with a sufficiently high electrochemical potential can be primed and transferred to the deposited Li+ ion from impinging charged droplets to perform single-electron reduction and form highly reactive Li metal (Fig. 1C).
Additional considerations
A peak at m/z 141 is often observed even in cases where formaldehyde is not included (Fig. S6A†). This species, identified as lithium-adducted diethylene glycol monoethyl ether (DEGMEE), has previously been reported as a ubiquitous contaminant in ambient ionization experiments.65 Given that this species can be differentiated from protonated hexamine (also at m/z 141) in unit-resolution MS experiments via MS/MS (Fig. S6B†), simultaneous quantitation of hexamine (and thus NH3) yields and contaminant abundance were performed by employing a wide-isolation window MS/MS method (see ESI,† Methods and materials for more information on this strategy of quantitation).Paraformaldehyde formation is present in all ESD experiments which include formaldehyde, as evidenced by a polymeric distribution of peaks from ca. m/z 150–400 in the corresponding mass spectra (Fig. 2A; not observed with direct nESI of the reaction solution (Fig. S7†)). Assuming Li+ reduction primarily occurs at the electrode surface (though not exclusively, as demonstrated previously) and that the resulting Li0 must contact N2 gas to form the corresponding nitride, the continuous vertical deposition of hydrated paraformaldehyde inhibits both of these processes through blocking of the surface as well as any transiently formed Li3N, deceasing hexamine yields over time.
The reduced hexamine yields on the deposition surface can be visualized by desorption electrospray ionization mass spectrometry (DESI-MS) imaging of the graphite surface before (Fig. S8C†) and after (electro)spray deposition (Fig. 3B–E). The ion [hexamine + H]+ gives strong signals along the periphery of the deposition area. The distribution of paraformaldehyde (selected peak at m/z 173, [C5H10O6 + Li]+) is effectively the opposite of that observed for hexamine. The highest intensity signals for paraformaldehyde occur in the middle of the deposition surface, centered on the most direct point of contact of the spray plume. When the spray potential is removed, the overall hexamine abundance is decreased relative to the total ion current for each set of DESI-MS images.
Thin films, contaminant control, and NH3 production from air
Li metal is highly reactive both with water and O2, thus necessitating the use of spray methods and a protective sheath gas to place reagents at the ‘dry’ air–water interface35 and to prevent significant accumulation of water on the deposition surface, respectively. To verify the role of microdroplets in this reduction, solutions of pure water, aqueous formaldehyde, aqueous LiBr, and the formaldehyde/LiBr reaction mixture were directed onto a grounded graphite surface using the standard ESD parameters albeit with a significantly increased solution flow rate (250 μL min−1, an order of magnitude faster than in the standard ESD experiments to minimize microdroplet formation) in the absence of a sheath gas. Solutions were then spread radially across the surface to produce thin films and an N2 gas flow was directed from above to ensure contact with the thin films. After 3 hours, the dried surfaces were washed and analyzed by nESI-MS. Hexamine was not detected from any of the solutions (Fig. S9A–D†). Thin films have been shown to serve as high surface-to-volume ratio systems that retain some aspects of the increased reactivity observed for microdroplets, thus this result highlights the importance of spray deposition and droplet evaporation in performing moisture-sensitive chemistry from an aqueous parent solution.Given that indoor NH3 concentrations can reach upwards of 1 part per million66,67 and that NH3 has been reported as a contaminant in some compressed gas cylinders,68 a more detailed inquiry into the origin of the nitrogen fixed during the spray deposition experiments beyond the already mentioned isotope labeling experiment was performed. As a precaution, all spray deposition experiments were performed inside of a fume hood to minimize the potential impact of ambient contaminants. To determine whether the compressed N2 or Ar tanks contained any trace NH3, the gases were bubbled through a solution of formaldehyde and LiBr, similar to that used in the spray deposition experiments. After two hours of bubbling with either gas, the reaction mixtures were directly analyzed by MS and no signals which might correspond to protonated hexamine were observed (Fig. S10A and B†); this further reinforces the null results found for the thin film control experiments (Fig. S9A–D†). Ar gas was used when depositing standard solutions used in constructing the calibration curve shown previously (Fig. 3A). The procedure was repeated with compressed laboratory air from outside the fume hood which resulted in a low intensity signal matching that of hexamine (Fig. S10D†), suggesting that ambient laboratory NH3 could be a source of error if introduced into the fume hood. However, bubbling compressed laboratory air from inside of the fume hood did not yield any hexamine product (Fig. S10C†), likely due to limited introduction and efficient ventilation of NH3. Neither contaminant NH3 in the gas cylinders nor ambient NH3 from outside the fume hood were indicated to play a significant role in the reported nitrogen fixation.
Knowing that compressed laboratory air from inside the fume hood is not a significant source of NH3, we investigated whether air could be used as a convenient source of N2 for Li-mediated NH3 production. After 60 minutes of ESD using compressed laboratory fume hood air as the sheath gas, a detectable hexamine peak was observed in the corresponding mass spectrum (Fig. S11†). It should be noted that the significant decrease in the hexamine peak intensity relative to the paraformaldehyde peaks compared to that in Fig. 2A (pure N2) can be explained both by the ca. 20% decrease in total N2 in ambient air in addition to the consumption of Li metal and Li3N via reaction with O2 and H2O.
Li nanoparticle synthesis and characterization
Heterogeneous reactions are generally accelerated as catalytic particle size decreases due to the increased availability of active surface sites relative to the total amount of catalyst.69 Given that ESD of metal salts often results in formation of metal nanoparticles,40 we investigated whether Li NPs (e.g. Li0, Li3N, Li2O) form during our ESD experiments which might further accelerate surface chemistry, outcompeting hydrolysis.Transmission electron microscopy (TEM) analysis was performed following nano-electrospray ESD of the formaldehyde/LiBr solution onto a grounded Cu TEM grid. As evident in Fig. 4A and B, spheroidal NPs formed with an average diameter of 8.44 ± 2.24 nm and a polydispersity index of 0.07, indicating a highly monodispersed population.70 An additional population of larger cubic particles was also observed (Fig. 4A, inset top). When the spray voltage was increased to 3 kV, dendritic species were formed upon deposition rather than NPs (Fig. 4A, inset bottom). Energy-dispersive X-ray spectroscopy (EDS) of an area similar to Fig. 4A (though also including cubic particles) revealed unique atomic signatures corresponding to Br and N (Fig. 3C), which were not observed for the blank TEM grid (Fig. S12†). Scanning transmission electron microscropy (STEM) combined with EDS showed that the cubic structures primarily consist of Br and O (Fig. S13D and E†). STEM-EDS imaging revealed the increased presence of nitrogen in areas the smaller spheroidal NPs reside (Fig. S13C†). This, in conjunction with the overall average increase in N-related EDS signals with both LiBr and LiBr/HCHO compared to a blank TEM grid (Fig. S12†), suggests that Li3N is formed during this experiment and is present in the smaller spheroidal NPs. Li metal is likely present in the area analyzed, though traditional EDS is insensitive to the low-energy X-rays characteristic of Li.71
To directly confirm the presence of metallic Li in the observed NPs, electron energy loss spectroscopy (EELS) was performed given its sensitivity to light elements.72 As shown in Fig. 4D, the K-edge for metallic Li can be directly observed with a peak at a threshold energy loss value of ca. 54.8 eV, in agreement with previously reported values.73 An additional set of peaks can be observed at 59.0 eV and 60.4 eV. While interpretation of the fine structure of these peaks is difficult due to the intensity of the plasmon peak spanning 25–200 eV, the threshold values and peak shapes resemble to those previously found for LiOH, Li halides (LiBr in this case), Li2Ox, and LiOH.73–75 Elemental mapping of the EELS spectra to an STEM image reveals that metallic Li and oxygen are primarily localized in the cubic NPs, while lower densities are observed in the surrounding paraformaldehyde films and smaller spheroidal NPs (Fig. S14A–D†). Similarly, EELS confirmed the dendritic species shown in the bottom inset panel of Fig. 4A primarily consist of metallic Li and Li2Ox (Fig. S15†). These dendrites, the formation of which is of major concern in Li-based batteries,76 might prove to be advantageous in terms of heterogeneous catalysis (as described in this manuscript) due to the greater surface area compared to spheroidal or cubic NPs.
The presence of lithium oxides is further substantiated by nESI-MS analysis of the deposited LiBr/HCHO solution. Compared to the original bulk LiBr solution (Fig. S16A†), this sample revealed increased formation of small LiBr/Li2Ox/LiOH clusters (Fig. S16B†) and is likely representative of initial clustering events leading to the cubic NPs. No pure or mixed metallic Li clusters were observed by MS, likely due to the reconstitution of the NPs in water prior to nESI analysis. Together, these data suggest that the cubic NPs likely consist of both LiBr, Li2O, Li2O2, and LiOH.
The formation of Li NPs with large surface area likely facilitates the observed NH3 production via accelerated Li3N formation, outcompeting reactions with water from the spray. Paraformaldehyde formed in situ may also contribute to Li NP stability by providing a water deficient protective coating. To this point, the abundance of NPs observed with the inclusion of HCHO generally appeared to be significantly greater than without. To the authors' knowledge this is the first example of mixed Li/LiBr/Li2Ox/LiOH/Li3N NP synthesis via ESD. Li NPs have garnered attention for their distinctive qualities as electrodes and additives for lithium-ion and lithium–air batteries and hence this approach might also represent a feasible strategy for novel materials preparation.77,78
Conclusions
With atmospheric CO2 levels at climate-altering levels and a lack of widespread sustainable energy and NH3 sources, deployment of environmentally sustainable strategies to the Haber–Bosch process are imperative, even if initially made on a small scale; they include the catalytic lithium-mediated NH3 cycle shown here. The ambient water microdroplet serves as a reaction vessel that contains the proton source, the electron source (in the case of deposition onto non-conducting surfaces), and it provides a means for generating metal nanoparticles upon surface collisions. The multifunctionality of the demonstrated microdroplet chemistry has potential to lessen the environmental and economic burden that the use of conventional solvents, extreme reaction environments, and the generation of H2 gas from fossil fuels normally entail. We also demonstrate rapid in situ capture of transient NH3 with simple and inexpensive reagents in the spray solution to form hexamine without need to access high temperatures or pressures.These results add to growing evidence that the air–water interface of microdroplets is effectively water-deficient and may serve as a means of performing water-sensitive reactions in aqueous systems. The successful generation of Li metal NPs in an aqueous system shown here is largely made possible through the combination of primed reagents at the microdroplet interface reacting with gas–solid nanoparticle surfaces. We envision that similar reaction formats will enable a new branch of green synthesis for condensation reactions, organometallic reactions, electrochemical processes, and might also serve as laboratory models for multiphase prebiotic chemistry. Furthermore, the role of charged microdroplet collisions with solid surfaces is demonstrated by the generation of NH3 without the need for application of an external potential or a conductive deposition surface, a seldom explored possibility in previous electrospray deposition studies. Similar chemistry has recently been highlighted by Pradeep and coworkers as a potentially major source of spontaneous mineral weathering in nature.79
This method's relatively rapid rate of NH3 production compared to other reported microdroplet syntheses can be attributed to both the general speed of Li3N formation and hydrolysis, as well as the presence of Li NPs formed during the spray deposition process. The inclusion of protecting ligands in the Li salt solution might increase the lifetime of the Li NPs by shielding them from water, allowing for additional NH3-capture reactions to be implemented. Methods of multiplexing sprayers, solvent recycling,80 continuous surface de-fouling, improved reactor design, as well as direct ammonia condensation could also be employed to increase yields and further assist scaling up of the microdroplet deposition process. With these modifications, this system has the potential to be a valuable method of generating preparative amounts of NH3 from pure N2 or air for use in as a bulk chemical or in organic synthesis, utilizing minimal resources in the process.
Data availability
All data on which this publication is based appears either in the main text or in the ESI.† The underlying raw data is available on request to the authors.Author contributions
All authors contributed to experimental design and data interpretation. DTH and MQE performed TEM and MS experiments. ZS performed all STEM, EDS, and EELS experiments with contributions by DTH and MQE. DTH, MQE, and RGC wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Dr Nicolás M. Morato for his generous contributions in performing DESI-MS imaging experiments. We thank the Purdue University Department of Chemistry Research Instrumentation Center for access to a high-resolution mass spectrometer. This work was supported in part by the Multi-University Research Initiative (MURI) of the Air Force Office of Scientific Research (FA9550-21-1-0170) via Stanford University (sub-award 62741613-204669). D. T. H. acknowledges support from the National Science Foundation Graduate Research Fellowship (DGE-1842166).Notes and references
- L. Wang, M. Xia, H. Wang, K. Huang, C. Qian, C. T. Maravelias and G. A. Ozin, Joule, 2018, 2, 1055–1074 CrossRef CAS.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 RSC.
- S. Ghavam, M. Vahdati, I. A. G. Wilson and P. Styring, Front. Energy Res., 2021, 9, 1–19 Search PubMed.
- M. A. Nawaz, R. Blay-Roger, M. Saif, F. Meng, J. González-Arias, B. Miao, L. F. Bobadilla, T. Ramirez-Reina and J. A. Odriozola, ACS Catal., 2023, 13, 14415–14453 CrossRef CAS.
- D. Chanda, R. Xing, T. Xu, Q. Liu, Y. Luo, S. Liu, R. A. Tufa, T. H. Dolla, T. Montini and X. Sun, Chem. Commun., 2021, 57, 7335–7349 RSC.
- A. J. Martín, T. Shinagawa and J. Pérez-Ramírez, Chem, 2019, 5, 263–283 Search PubMed.
- S. L. Foster, S. I. P. Bakovic, R. D. Duda, S. Maheshwari, R. D. Milton, S. D. Minteer, M. J. Janik, J. N. Renner and L. F. Greenlee, Nat. Catal., 2018, 1, 490–500 CrossRef.
- Y. Abghoui, S. B. Sigtryggsson and E. Skúlason, ChemSusChem, 2019, 12, 4265–4273 CrossRef CAS PubMed.
- B. M. Hoffman, D. Lukoyanov, Z. Y. Yang, D. R. Dean and L. C. Seefeldt, Chem. Rev., 2014, 114, 4041–4062 CrossRef CAS PubMed.
- H. Himmel and M. Reiher, Angew. Chem., Int. Ed., 2006, 45, 6264–6288 CrossRef CAS PubMed.
- S. Reichle, M. Felderhoff and F. Schüth, Angew. Chem., Int. Ed., 2021, 60, 26385–26389 CrossRef CAS PubMed.
- D. Ziegenbalg, J. Zander and R. Marschall, ChemPhotoChem, 2021, 5, 792–807 CrossRef CAS.
- T. Dai, T. Wang, Z. Wen and Q. Jiang, Adv. Funct. Mater., 2024, 2400773 CrossRef CAS.
- S. Bennaamane, B. Rialland, L. Khrouz, M. Fustier-Boutignon, C. Bucher, E. Clot and N. Mézailles, Angew. Chem., Int. Ed., 2023, 62, e202209102 CrossRef CAS PubMed.
- T. Wang and F. Abild-Pedersen, Proc. Natl. Acad. Sci. U.S.A., 2021, 118, 1–5 Search PubMed.
- W. Qiu, X. Xie, J. Qiu, W. Fang, R. Liang, X. Ren, X. Ji, G. Cui, A. M. Asiri, G. Cui, B. Tang and X. Sun, Nat. Commun., 2018,(9), 3485 CrossRef PubMed.
- E. Spatolisano and L. A. Pellegrini, Chem. Eng. Res. Des., 2023, 195, 651–661 CrossRef CAS.
- P. Wang, S. Gong, Y. Li and Y. Mo, J. Chem. Phys., 2024, 160, 014304 CrossRef CAS PubMed.
- R. Akter, S. S. Shah, M. A. Ehsan, M. N. Shaikh, M. H. Zahir, M. A. Aziz and A. J. S. Ahammad, Chem.–Asian J., 2024, 19, e202300797 CrossRef CAS PubMed.
- S. C. Jesudass, S. Surendran, J. Y. Kim, T. Y. An, G. Janani, T. H. Kim, J. K. Kim and U. Sim, Electrochem. Energy Rev., 2023, 6, 27 CrossRef CAS.
- A. I. Gerasimchuk and D. A. Zhogolev, Theor. Exp. Chem., 1977, 13, 5–9 CrossRef.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, 1–8 Search PubMed.
- C. Geng, J. Li, T. Weiske and H. Schwarz, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 21416–21420 CrossRef CAS PubMed.
- C. Geng, J. Li, T. Weiske and H. Schwarz, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11680–11687 CrossRef CAS PubMed.
- X. Gong, L. Feng, S. Tan, S. Cheng, R. Zhai, T. Peng, Y. Jiang, X. Dai, Y. Pan and X. Fang, ACS Energy Lett., 2024, 9, 2153–2161 CrossRef CAS.
- J. Li, Y. Xia, X. Song, B. Chen and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(4), e2318408121 CrossRef CAS PubMed.
- X. Song, C. Basheer and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2301206120 CrossRef CAS PubMed.
- N. Lazouski, Z. J. Schiffer, K. Williams and K. Manthiram, Joule, 2019, 3, 1127–1139 CrossRef CAS.
- N. Futamura, T. Ichikawa, N. Imanishi, Y. Takeda and O. Yamamoto, ECS Meet. Abstr., 2012, 1137 CrossRef.
- J. M. McEnaney, A. R. Singh, J. A. Schwalbe, J. Kibsgaard, J. C. Lin, M. Cargnello, T. F. Jaramillo and J. K. Nørskov, Energy Environ. Sci., 2017, 10, 1621–1630 RSC.
- X. Yan, R. M. Bain and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 12960–12972 CrossRef CAS PubMed.
- Z. Wei, Y. Li, R. G. Cooks and X. Yan, Annu. Rev. Phys. Chem., 2020, 71, 31–51 CrossRef CAS PubMed.
- T. Majumder, D. B. Eremin, B. Delibas, A. Sarkar, V. Fokin and J. M. Dawlaty, Angew. Chem., Int. Ed., 2024, e202414746 Search PubMed.
- V. Kwan and S. Consta, J. Am. Soc. Mass Spectrom., 2021, 32, 33–45 CrossRef CAS PubMed.
- D. T. Holden, N. M. Morato and R. G. Cooks, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, 1–8 CrossRef PubMed.
- J. K. Lee, D. Samanta, H. G. Nam and R. N. Zare, Nat. Commun., 2018, 9, 1562 CrossRef PubMed.
- S. Zhang and K. Kawakami, Int. J. Pharm., 2010, 397, 211–217 CrossRef CAS PubMed.
- A. Jana, S. K. Jana, D. Sarkar, T. Ahuja, P. Basuri, B. Monday, S. Bose, J. Ghosh and T. Pradeep, J. Mater. Chem. A, 2019, 7, 6387–6394 RSC.
- T. Ahuja, A. Ghosh, S. Mondal, P. Basuri, S. K. Jenifer, P. Srikrishnarka, J. S. Mohanty, S. Bose and T. Pradeep, Analyst, 2019, 144, 7412–7420 RSC.
- J. Ghosh and R. G. Cooks, ChemPlusChem, 2022, 87, e202200252 CrossRef CAS PubMed.
- J. Ghosh and R. G. Cooks, Trends Anal. Chem., 2023, 161, 117010 CrossRef CAS.
- A. Li, Q. Luo, S. J. Park and R. G. Cooks, Angew. Chem., Int. Ed., 2014, 53, 3147–3150 CrossRef CAS PubMed.
- F. Meissner, E. Schwiedessen and D. F. Othmer, Ind. Eng. Chem., 1954, 46, 724–727 CrossRef CAS.
- T. S. Lo, K. D. P. Hammer, M. Zegarra and W. C. Cho, Expert Rev. Anti-Infect. Ther., 2014, 12, 549–554 CrossRef CAS PubMed.
- P. Roose, K. Eller, E. Henkes, R. Rossbacher and H. Höke, in Ullmann's Encyclopedia of Industrial Chemistry, 2015, pp. 1–55 Search PubMed.
- A. Alamdari and F. Tabkhi, Chem. Eng. Process., 2004, 43, 803–810 CrossRef CAS.
- N. Blažević, D. Kolbah, B. Belin, V. Šunjić and F. Kajfež, Synthesis, 1979, 3, 161–176 CrossRef.
- Y. Oba, Y. Takano, H. Naraoka, Y. Furukawa, D. P. Glavin, J. P. Dworkin and S. Tachibana, Nat. Commun., 2020, 11, 1–8 CrossRef PubMed.
- V. Vinogradoff, S. Bernard, C. Le Guillou and L. Remusat, Icarus, 2018, 305, 358–370 CrossRef CAS.
- C. He, G. Lin and M. A. Smith, Icarus, 2012, 220, 627–634 CrossRef CAS.
- A. Zeffiro, S. Lazzaroni, D. Merli, A. Profumo, A. Buttafava, N. Serpone and D. Dondi, Origins Life Evol. Biospheres, 2016, 46, 223–231 CrossRef CAS PubMed.
- H. Cottin, S. Bachir, F. Raulin and M. C. Gazeau, Adv. Space Res., 2002, 30, 1481–1488 CrossRef.
- D. P. Glavin, J. P. Dworkin, M. O. Alexander, J. C. Aponte, A. A. Baczynski, J. J. Barnes, H. A. Bechtel, E. L. Berger, A. A. Burton, P. Caselli, A. H. Chung, S. J. Clemett, G. D. Cody, G. Dominguez, J. E. Elsila, K. K. Farnsworth, D. I. Foustoukos, K. H. Freeman, Y. Furukawa, Z. Gainsforth, H. V. Graham, T. Grassi, B. M. Giuliano, V. E. Hamilton, P. Haenecour, P. R. Heck, A. E. Hofmann, C. H. House, Y. Huang, H. H. Kaplan, L. P. Keller, B. Kim, T. Koga, M. Liss, H. L. McLain, M. A. Marcus, M. Matney, T. J. McCoy, O. M. McIntosh, A. Mojarro, H. Naraoka, A. N. Nguyen, M. Nuevo, J. A. Nuth III, Y. Oba, E. T. Parker, T. S. Peretyazhko, S. A. Sandford, E. Santos, P. Schmitt-Kopplin, F. Seguin, D. N. Simkus, A. Shahid, Y. Takano, K. L. Thomas-Keprta, H. Tripathi, G. Weiss, Y. Zheng, N. G. Lunning, K. Righter, H. C. Connolly Jr and D. S. Lauretta, Nat. Astron., 2025, 9, 199–210 CrossRef PubMed.
- H. Hao, I. Leven and T. Head-Gordon, Nat. Commun., 2022, 13, 1–8 Search PubMed.
- H. Xiong, J. K. Lee, R. N. Zare and W. Min, J. Phys. Chem. Lett., 2020, 11, 7423–7428 CrossRef CAS PubMed.
- N. Brown, J. Macromol. Sci., Part A: Pure Appl. Chem., 1967, 1, 209–230 CrossRef.
- J. M. Morris, R. B. Dunmire, P. E. Koenig and G. R. Newkome, J. Org. Chem., 1972, 37, 1244–1248 CrossRef CAS.
- Q. Liang, C. Zhu and J. Yang, J. Am. Chem. Soc., 2023, 145, 10159–10166 CrossRef CAS PubMed.
- L. Qiu and R. G. Cooks, Angew. Chem., Int. Ed., 2024, 63(17), e202400118 CrossRef CAS PubMed.
- M. Mofidfar, M. A. Mehrgardi and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2315940121 CrossRef CAS PubMed.
- M. Armbruster, H. Haberland and H.-G. Schindler, Phys. Rev. Lett., 1981, 47, 323–326 CrossRef CAS.
- F. Arnold, Nature, 1981, 294, 732–733 CrossRef CAS.
- W. A. Maza, V. M. Breslin and J. C. Owrutsky, Curr. Opin. Chem. Eng., 2024, 44, 101015 CrossRef.
- J. K. Lee, D. Samanta, H. G. Nam and R. N. Zare, J. Am. Chem. Soc., 2019, 141, 10585–10589 CrossRef CAS PubMed.
- S. R. Kumbhani, L. M. Wingen, V. Perraud and B. J. Finlayson-Pitts, Rapid Commun. Mass Spectrom., 2017, 31, 1659–1668 CrossRef CAS PubMed.
- M. Li, C. J. Weschler, G. Bekö, P. Wargocki, G. Lucic and J. Williams, Environ. Sci. Technol., 2020, 54, 5419–5428 CrossRef CAS PubMed.
- L. Ampollini, E. F. Katz, S. Bourne, Y. Tian, A. Novoselac, A. H. Goldstein, G. Lucic, M. S. Waring and P. F. Decarlo, Environ. Sci. Technol., 2019, 53, 8591–8598 CrossRef CAS PubMed.
- R. Dabundo, M. F. Lehmann, L. Treibergs, C. R. Tobias, M. A. Altabet, P. H. Moisander and J. Granger, PLoS One, 2014, 9(10), e110335 CrossRef PubMed.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J. M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- N. Raval, R. Maheshwari, D. Kalyane, S. R. Youngren-Ortiz, M. B. Chougule and R. K. Tekade, in Basic Fundamentals of Drug Delivery, Elsevier Inc., 2019, pp. 369–400 Search PubMed.
- J. A. Österreicher, C. Simson, A. Großalber, S. Frank and S. Gneiger, Scr. Mater., 2021, 194, 0–3 CrossRef.
- L. Yu, M. Li, J. Wen, K. Amine and J. Lu, Mater. Chem. Front., 2021, 5, 5186–5193 RSC.
- F. Wang, J. Graetz, M. S. Moreno, C. Ma, L. Wu, V. Volkov and Y. Zhu, ACS Nano, 2011, 5, 1190–1197 CrossRef CAS PubMed.
- P. Zhang, B. Han, X. Yang, Y. Zou, X. Lu, X. Liu, Y. Zhu, D. Wu, S. Shen, L. Li, Y. Zhao, J. S. Francisco and M. Gu, J. Am. Chem. Soc., 2022, 144, 2129–2136 CrossRef CAS PubMed.
- K. Nishikawa and K. Shinoda, J. Phys. Chem. Lett., 2021, 12, 3922–3927 CrossRef CAS PubMed.
- J. Lang, L. Qi, Y. Luo and H. Wu, Energy Storage Mater., 2017, 7, 115–129 CrossRef.
- J. R. Rodriguez, M. Flores, B. Trujillo, J. N. Díaz de León, S. B. Aguirre, R. D. Cadena-Nava, R. M. Félix, D. Sauceda and S. Camacho, J. Colloid Interface Sci., 2024, 657, 953–959 CrossRef CAS PubMed.
- A. Kondori, M. Esmaeilirad, A. M. Harzandi, R. Amine, M. T. Saray, L. Yu, T. Liu, J. Wen, N. Shan, H. H. Wang, A. T. Ngo, P. C. Redfern, C. S. Johnson, K. Amine, R. Shahbazian-Yassar, L. A. Curtiss and M. Asadi, Science, 2023, 379, 499–505 CrossRef CAS PubMed.
- B. K. Spoorthi, K. Debnath, P. Basuri, A. Nagar, U. V. Waghmare and T. Pradeep, Science, 2024, 384, 1012–1017 CrossRef CAS PubMed.
- H. Nie, Z. Wei, L. Qiu, X. Chen, D. T. Holden and R. G. Cooks, Chem. Sci., 2020, 11, 2356–2361 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02558c |
| This journal is © The Royal Society of Chemistry 2025 |



