 Open Access Article
Open Access ArticleFrom the synthesis of wearable polymer sensors to their potential for reuse and ultimate fate
Arya
Ajeev
a,
Theodore
Warfle
 ab,
Sara
Maslaczynska-Salome
a,
Saeideh
Alipoori
ac,
Colton
Duprey
*ab and
Evan K.
Wujcik
ab,
Sara
Maslaczynska-Salome
a,
Saeideh
Alipoori
ac,
Colton
Duprey
*ab and
Evan K.
Wujcik
 *ab
*ab
aMaterials Engineering And Nanosensor [MEAN] Laboratory, Department of Chemical and Biomedical Engineering, The University of Maine, Orono, Maine, USA. E-mail: Evan.Wujcik@maine.edu; Fax: +1 207-581-2323; Tel: +1 207-581-2742
bAdvanced Structures and Composites Center [ASCC], The University of Maine, Orono, Maine, USA. E-mail: Evan.Wujcik@maine.edu; Fax: +1 207-581-2323; Tel: +1 207-581-2742
cUNAM Institute of Materials Science and Nanotechnology, Bilkent University, Ankara 06800, Turkey. E-mail: saeideh@unam.bilkent.edu.tr
First published on 1st May 2025
Abstract
The objective of this perspective is to review the high-interest field of wearable polymer-based sensors—from synthesis to use and detection mechanisms—with a focus on their transient nature, potential for reuse, and ultimate fate. While many bulk polymers have long been mass-produced, the materials needed to create polymer-based sensors—often with unique properties (e.g., being electronically conductive)—are still highly active areas of research. Polymer-based materials and composites, when investigated as wearable sensors, have a wide range of applications with most falling under the umbrellas of biochemical and environmental sensing (i.e., chemical reactivity-based detection) or physical sensing (e.g., piezoresistive response). Since the long-term viability of these sensors is a function of not just their initial syntheses but also their ability to be durable, recyclable, or otherwise renewable, a discussion of both the technical and societal aspects of the reuse and ultimate fate of these materials will be covered. This discussion will focus on topics such as environmental impact, sterilization, and other methods for ensuring continued biocompatibility, as well as methods for the transformation, reclamation, or re-implementation of the sensor devices—a major issue the polymer community is facing.
1 The importance of advancing wearable polymer-based sensors
Wearable polymer-based sensors have emerged as indispensable tools in healthcare,1,2 environmental monitoring,3,4 and fitness5,6 due to their inherent flexibility, stretchability, sensitivity, durability, and biocompatibility.7 Because of their excellent properties and seamless integration with the human body, they improve the daily life of humans by reducing manual effort and human error, and thus provide reliable real-time data.8 The transition from rigid metal and metal oxide-based sensors to polymer-based sensors provides a high degree of flexibility and compliance to adapt to natural skin movements, including bending, stretching, twisting, and folding.9 In short, these sensors should behave like human skin to minimize discomfort when worn directly on human skin or attached to clothes or garments. In this perspective, we will broadly categorize these into two types depending on their mode of operation: chemical reactivity-based sensors10,11 and physical interaction-based sensors,12,13 which are both utilized depending on what the targeted application specifies.Research on wearable sensors or polymer-based wearable sensors has spiked significantly in the last decade.8,14 Many researchers are interested in this area due to the impact these studies can have on the daily activities of humans.15 The global wearable sensors market is projected to reach USD 1.516 billion in 2025 and is forecast to reach around USD 5.485 billion in 2034, reflecting a compound annual growth rate (CAGR) of 15.40%. However, while wearable polymer sensors are invaluable for both the economy and human health monitoring, it is equally important to address and ensure how waste materials generated are treated and managed. Most of the wearable sensors are based on synthetic polymers (petroleum-based),16,17 and thus their end-of-life on earth is longer due to a lack of biodegradability. The accumulation of these sensors in landfills can cause environmental pollution and problems for the ecosystem. Moreover, the one-time use and shorter lifespans of many wearable devices contribute significantly to waste generation.
The environmental impact of wearable sensors due to improper waste disposal and synthetic raw materials necessitates the need for sustainable materials and proper degradation mechanisms. The usage of naturally derived polymers, many of which are biodegradable18 and bio-based materials,19,20 helps to reduce this issue to an extent.21 Additionally, exploring the degradation or recycling mechanism for the developed sensor can minimize the environmental impact, particularly when its end-of-life can be planned. Self-healing materials also provide great help in reducing the environmental footprint by repairing themselves, allowing for increased longevity and usage before reaching their end of life.22–24 Understanding the ultimate fate of wearable polymer sensors, whether through recycling, proper degradation, or controlled disposal, is crucial to the sustainable development of this technology.
This review examines the synthesis, applications, and degradation mechanism of physical and chemical sensors. The degradation mechanism for both sensors is explained thoroughly to encourage researchers to extend their focus to recyclability or degradability governing material breakdown. In the later sections, we cover the reuse and ultimate fate of wearable polymer-based sensors, discussing the transient nature of these materials, the need for recycling, and strategies for extending their lifespan through sterilization and reusability. In the last part, the future prospects of wearable polymer-based sensors are summarized, emphasizing the need for innovative polymeric solutions that align with environmental and technological advancements.
2 Defining sustainability metrics
The increasing demand for wearable polymer-based sensors necessitates a clear understanding of degradability to effectively assess their environmental impact. Most of the sensors are made of non-biodegradable materials and are non-processible in nature, which can lead to accumulation in the environment. It is commendable that researchers are increasingly focusing on degradable materials in the development of sensor technologies. The degradation pathways can be classified as biodegradable, compostable, recyclable, and transient.Biodegradable materials are substances that can be consumed or broken down by microorganisms, including bacteria, fungi, or enzymes into H2O, CO2, and methane. This biodegradation process depends heavily on the structure, size, morphology, and chemical modifications of the polymers. In addition, the environmental conditions such as temperature, humidity, and presence of oxygen also play a vital role in determining the rate of degradation.25 Hence, the degradation time is challenging to determine, as it can range from days to months or even years based on the material's composition and environmental conditions. In implantable biomedical applications, biodegradable materials are designed to degrade or resorbed into the body by eliminating device retraction procedures.26 On the other hand, compostable materials are those that can be broken down naturally to form nutrient-rich compost. In industrial composting facilities, this can happen under controlled aerobic conditions, especially for bio-plastics. All compostable materials are biodegradable in nature, but not all biodegradable materials are compostable.27
In contrast, recyclable materials are not designed to degrade, whereas they can be reprocessed into new products by physical or chemical means.28 Recycling helps reduce waste accumulation in landfills thereby minimizing environmental concerns. Recycling in wearable electronics can be challenging because of the two or more kinds of functional materials and various solvents used for the fabrication.29 However, with proper reprocessing techniques, these materials can be effectively recycled. A solvent-based separation or dissolution of functional materials or the complete shredding, followed by reprocessing by hot pressing, are the main ways in which recycling is done. Interestingly, transient materials represent an emerging field, and they can disappear into the surrounding environment without leaving a trace. These types of material are widely used in wearable electronics to reduce electronic waste.30 Different types of solutions, light, temperature, pH or enzymatic activity can be used to trigger the degradation process. Chemical degradation is the process by which a material is broken down by chemical agents such as acids, bases, oxidants, or solvents. Most chemical degradation studies are performed in a controlled laboratory setting. Although it is useful for evaluating short-term degradation behavior, chemical degradation does not necessarily indicate environmental compatibility unless followed by toxicity and residue assessments.
3 Key applications of wearable polymer-based sensors
Physical sensors are capable of detecting and quantifying changes in physical stimuli, such as pressure, strain, and motion. To quantify these physical stimuli, these sensors rely on changes in resistance and capacitance across the material. Most of the physical sensors have applications in human-motion monitoring, prosthetics, soft robotics, and electronic skin.31 Environmental sensors that monitor the changes in temperature and humidity also fall into the physical sensing category.4,32,33 On the other hand, chemical-type sensors utilize molecular interactions to detect and quantify specific chemical or biological analytes. They monitor the changes in metrics such as glucose, pH, or gas levels for early disease detection and personalized medicine by chemically targeting specific analytes.34,353.1 Physical sensing mechanisms
A plethora of polymers are used in the design and fabrication of wearable physical sensors. Such materials are chosen because of their flexibility, sensitivity, and skin-like conformability, as these properties are indispensable for the materials to seamlessly integrate with the human body, ensuring efficient and accurate sensing. The synthesis mechanisms of different polymers used in physical sensing and their applications are described in detail below.3.1.1.1 Conductive polymers as active sensing materials. Conducting polymers (CP) are materials that combine the electrical properties of metals with inexpensive synthesis, high sensitivity, and excellent flexibility while maintaining the processability of conventional polymers.36 Various types of conducting polymers are polyaniline (PANI), polypyrrole (PPY), polythiophene, polycarbazoles, and polyaminonaphthalenes. Their ability to detect and respond to physical stimuli with high sensitivity and their tunable properties solidify their role in physical sensing applications for wearable technology. Despite their advantages, these CPs are synthetically derived and generally nonbiodegradable, causing environmental pollution. More importantly, most sensor materials, particularly chemical-type sensors, can be used only once, creating a huge environmental impact. This emphasizes the need to develop wearable sensors that are biodegradable and recyclable. PANI is an intrinsically conductive polymer that exhibits high electrical conductivity due to its π-conjugated structure, tunable oxidation states, and environmental stability. Moreover, its flexibility and compatibility with various substrates make PANI an attractive choice for flexible electronics and wearable devices. PANI is synthesized by electrochemical and chemical polymerization methods.37 Chemical polymerization is the most widely used technique for synthesis from an aniline monomer, typically employing various oxidants to initiate the process, with ammonium persulfate (APS) being the most commonly employed. PANI is not inherently biodegradable, but researchers have found ways to make it degradable by combining it with other polymers. Lu et al.38 developed a flexible, self-healable and electrically conductive wearable strain sensor using PANI, phytic acid, and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAAMPSA) for applications in human motion detection. This sensor is completely soluble in water and can be effortlessly recycled to create a new batch, making it an environmentally sustainable option.
The use of paper-based substrates has increased in sensor development due to their unique properties, including flexibility, abundance, low cost, and biodegradability.10 Zheng and co-workers39 developed an innovative dual-mode sensor with pressure and ammonia detection capabilities using a paper substrate. The paper was dip-coated in the graphene oxide solution followed by thermal reduction and in situ polymerization of aniline to achieve a PANI/rGO/paper substrate. The degradation performance of the composite was analyzed by immersing it in a NaOH solution and a 74.3% mass loss was observed after 60 days. In another device, triple sensing of pressure, strain, and temperature was made possible by the combination of Bi2S3 nanosticks, PVDF (Polyvinylidene fluoride), and polypyrrole. The hydrothermally synthesized Bi2S3 nanosticks were added into PVDF/PPy solution and electrospun to achieve the nanomembrane.40 This sensor is completely dissolved in an organic solvent, acetone, after 5 days, confirming the chemical degradation of the sensor (Table 1).
| Active materials | Stretchability | Sensitivity | Application | Ref. |
|---|---|---|---|---|
| BC/ImClO4 | — | 4 mV kPa−1 | Piezoelectric | 41 |
| Poly(vinyl alcohol) (PVA)/SA/BC/Modified carbon nanotube and carbon black | 200% | 5.01 kPa−1 | Strain/pressure sensing | 42 |
| MXene/tissue paper/PLA | — | 3.81 kPa−1 | Pressure sensor | 43 |
| MXene/cotton fiber-based piezoresistive textile | — | 17.73 kPa−1 | Wearable bio-monitoring | 44 |
| BC/PPy/EF | 90% | 4.86 | Human-body motion monitoring | 45 |
| BPU/CNT | 250% | 2468 | Human-body motion monitoring | 46 |
| Paper/MWCNT/PDMS | — | 263.34 | Underwater vibration monitoring | 47 |
| MXene/PLA | — | 5.37 kPa−1 | Wearable biomonitoring | 48 |
| SPI/HBT/GL | 100% | — | Human-body motion monitoring | 7 |
| Origami paper | — | — | Humidity sensing | 3 |
| PVA/LMPs | — | 0.828 kPa−1 | Epidermal sensing | 49 |
| MXene/tissue paper | — | 344.0 kPa−1 | Pressure sensing | 50 |
| PLGA/PCL | — | 0.863 ± 0.025 kPa−1 | Pressure sensing | 51 |
| Graphene nanoplatelets/cellulose/SA | 25% | 32.62 | Pressure and human-body motion monitoring | 52 |
| Paper/AgNWs/nanocellulose | — | 1.5 kPa−1 | Pressure sensing | 53 |
| PPy/PVDF/Bi2S3 | — | 1.51 kPa−1 | Pressure, strain, and temperature sensing | 40 |
| SnS/cellulose paper | — | 14.8 kPa−1 | Pressure, strain, and pressure sensing | 54 |
| PANI/rGO/paper | — | 143.41 kPa−1 | Pressure and ammonia sensing | 39 |
| PANI/PAAMPSA/PA | 1935% | 14.52 | Human-body motion monitoring | 38 |
| β-Glycine/chitosan | — | 2.82 ± 0.2 mV kPa−1 | Piezoelectric pressure sensor | 55 |
| Ellipsoidal CNT/polyvinylpyrrolidone/CA | — | 3.38 kPa−1 | Human-body motion monitoring | 56 |
| MXene/methyl cellulose | — | 19.41 kPa−1 | Human-body motion monitoring and pressure sensing | 57 |
3.1.1.2 Composite materials. Another way of manufacturing wearable sensors is through the creation of composites, where the properties of dissimilar materials are combined to improve both the electrical and mechanical properties of the composite material. Usually, conducting fillers like graphene,34,58 carbon nanotubes,59 MXenes,57,60 and metallic nanoparticles35 are utilized to increase the performance of a polymeric matrix. Solution blending, in situ growth, and layer-by-layer assembly56,57 are the synthesis approaches utilized for the incorporation of additives into a polymer in order to create composite wearable sensors.61–63 MXenes belong to the category of two-dimensional transition metal carbides and nitrides and the attention they have received in the field of wearable sensors is huge.64 Regardless of their potential for wearable technology, the impact of these on the environment remains a complex issue. However, to address this, researchers often combine MXene with other materials through a solution-blending process, which has been widely investigated. The drip coating method was adopted by Pan et al.44 for the preparation of a cotton fiber-based piezoresistive film with MXene flakes for pressure sensing, as seen in Fig. 1a. This sensor exhibited a high sensitivity of 17.73 kPa−1 within the pressure range of 100 Pa to 30 kPa due to the large number of active sites provided by cellulose to bind with MXene. After 20 days of preservation in 2 M H2SO4 solution, the film faded to a white color and shrunk, indicating the chemical degradability. At the same time, decomposition in a real-world setting is still a question. Similarly, Hung et al.48 developed a completely degradable MXene/PLA composite by immersing electrospun PLA fibers into the MXene solution and drying (Fig. 1b). The degradation performance is evaluated by treating the sensor with 1 wt% sodium carbonate solution. After 120 hours, the complete degradation of the sensor is achieved. A MXene/PVA/bacterial cellulose (BC) based epidermal sensor was developed by Sun et al.60 through a repeated freeze-thaw process. The degradability of the sensor was tested by submerging it in hydrogen peroxide (H2O2) and phosphate-buffered saline solutions. Immersing the sensor in H2O2 resulted in film degradation in 53 minutes, while the other method took 56 days. A similar trend is observed in Du et al.’s57 MXene/methylcellulose-based sensor for applications in human motion detection and pressure sensing. This sensor required 48 hours to completely degrade in H2O2 solution. Although these studies offer approaches to enhance the environmental friendliness of MXene-based sensors, natural degradation mechanisms like enzymatic or microbial breakdown are still unexplored. In another composite system, by a simple mixing of starch and CaCl2, followed by heating the mixture at 80 °C for 5 min, Liu et al.65 developed a disposable electrode for health monitoring. These electrodes are easily degradable and can be consumed safely by plankton or aquatic plants. The degradation here is not biodegradation, hence the material is not broken down by microorganisms. However, aquatic biodegradability and products can be safely consumed by plankton or aquatic plants, indicating potential environmental compatibility.
 | ||
| Fig. 1 (a) Schematic of the fabrication process for conductive MXene/cellulose textiles. Reprinted with permission from ref. 44. Copyright 2023 Elseiver. (b) Synthesis procedure and composition design of degradable MXene-PLA textiles. Reprinted with permission from ref. 48. Copyright 2023 American Chemical Society. (c) SEM image of the degradation performance of different molecular weights of BPU, weight loss rate and gel permeation chromatography curves after 0, 1, 3, and 5 weeks of degradation under the action of PBS buffer. Schematic diagram of degradation of the ester and carbamate bond. Reprinted with permission from ref. 46 Copyright 2022 American Chemical Society. (d) Schematics of the processes to biodegrade and recycle the ImClO4/BC sensor; optical and SEM images illustrating the degradation process of the recycled BC membrane. Reprinted with permission from ref. 41. Copyright 2022 American Chemical Society. | ||
The fabrication of sensors by incorporating carbon-based materials into a matrix is a method commonly employed by many researchers. CNTs are such functional fillers. They are not biodegradable in nature, and studies have shown that the toxicity of CNTs can cause adverse effects on the kidneys, heart, and eyes of humans.66 The highly stable nature of CNT can resist the natural degradation process, and this can cause toxicity to the soil ecosystem and aquatic organisms. Researchers have proposed several strategies to reduce the adverse effect of CNTs, including the combination of CNT with biodegradable materials. Such a method was used by Liu et al.46 to develop a strain sensor by incorporating carbon nanotubes (CNTs) into biodegradable polyurethane (BPU). The composite showed degradability in a phosphate buffered saline solution (PBS) with 19.45% weight loss after 42 days (Fig. 1c). However, the degradation pathway of this strain sensor is long and incomplete, raising environmental concerns, as it is not fully addressing the electronic waste accumulation problem. In another work, a biodegradable silk fibroin hydrogel was combined with CNTs to develop strain sensors.67 The incorporation of CNTs in the hydrogel may reduce the risk of toxicity by limiting the release of CNT during degradation, but this strategy does not degrade CNTs completely. CNTs are widely used as various types of implants in the human body. This includes dental, orthopedic, cardiovascular, and neural implants, etc. Despite the excellent application potential of CNTs, their direct exposure to the human body can be harmful. Modifying the surface of CNTs with biocompatible materials or polymer coatings can reduce the potential toxicity by avoiding direct contact. Magnesium-doped CNTs are widely utilized in implants due to their improved degradation, biocompatibility, and reduced toxicity.68,69 As the magnesium-doped CNTs undergo degradation, Mg2+ ions will be released, which are biocompatible and can also stimulate bone regeneration, cell growth and reduce inflammatory responses.70 Francis et al.71 developed a surgical implant containing CNT, magnesium and chitosan. The chitosan coating here also acts as a protective coating to improve the biocompatibility, biodegradability and antibacterial activity.
Similarly, PVDF is the other composite material that is widely used for wearable and implantable applications due to its piezoelectricity, thermal stability, chemical resistance, and dielectric and mechanical properties.72,73 However, the highly stable nature of the flourinated backbone makes it resistant to degradation under environmental or biological conditions. A PVDF/PPy/gelatin based multifunctional device for UV photodetection, tactile and strain sensing applications was developed by Veeralingam et al.74 This study utilized a biodegradable material-gelatin, which reduces the total amount of PVDF used. But the leaching of toxic flourinated by-products (hydrogen fluoride and perfluorinated compounds) when exposed to high temperatures and UV makes this material an environmental threat. Similarly, a biodegradable poly(butylene succinate) based PVDF/organic montmorillonite implant in the gastrointestinal tract was developed by Kuo et al.75 PVDF is used in this work to impart mechanical stability in harsh gastrointestinal environments. Although PVDF-based nanocomposites demonstrate excellent mechanical stability and short-term biocompatibility for gastrointestinal implants, the long-term retention of non-biodegradable PVDF in the body remains a concern. Future research should prioritize on the biodegrdable piezoelectric alternatives, PVDF-based copolymers with degradation properties, or transient encapsulation strategies to address the challenges and risks associated with PVDF in degradable sensor platforms In contrast, Lu et al.41 developed a piezoelectric sensor by embedding imidazolium perchlorate (ImClO4), a molecular ferroelectric, into a BC hydrogel matrix. By dissolving ImClO4/BC into pure water, complete dissolution of ImClO4 occurs, leaving a solution of imidazolium and perchlorate ions that can be recycled and reused. BC film is completely decomposed into glucose and oligosaccharides by immersing in a 5 mg mL−1 concentration cellulase solution (Fig. 1d). Lu et al.41 achieved both recyclability and complete degradation of the sensor, representing a valuable step forward in the development of eco-friendly sensors.
Guo et al.43 and Yang et al.50 developed a pressure sensor based on MXene and tissue paper. Guo et al. sandwiched a PLA thin sheet and an interdigitated electrode-coated PLA thin sheet into the MXene incorporated tissue paper as seen in Fig. 2a. The sensor could detect a sugar granule of 2.3 mg by loading it onto the sensor's surface. Additionally, low-frequency changes in the range of 4–6 Hz can also be detected using the sensor, which would be beneficial for early neurodegenerative disease detection (Fig. 4c). Moreover, the sensor is attached to a robot femorotibial joint and can detect the hand touching of the sensor to the corresponding arm uplift cycles by wireless connection. Degradation studies are conducted by placing the sensor in 0.5 M NaOH and PBS solution for 14 days, and mass loss of 23% and 68% was observed, indicating a partial mass loss, not complete degradation. Yang et al.50 also used a layer-by-layer method for the fabrication of the sensor. They coated the tissue paper with MXene and sandwiched it between a polyimide encapsulation layer and printing paper with interdigital electrodes. The sensor is utilized for the early detection of opioids by integrating the sensor with signal processing and a wireless communication module on a face mask. This sensor can detect changes in the flow of breath as the resistance changes. More importantly, the incineration of the sensor for 20 seconds generates ash of paper with the fragments of a silver interdigitated electrode. The remaining fragment of the electrode is treated with ethanol and crushed using a cell crusher for 60 min at a power of 300 W after drying to generate the silver powder for reuse (Fig. 2b). The incineration of paper-based sensors is a rapid disposal method but might lead to the emission of harmful gases as byproducts, which can affect both the environment and all the living organisms in an adverse manner. Future research in this field should focus on environmental fate beyond the laboratory setup by developing materials and methods that offer complete biodegradation.
 | ||
| Fig. 2 (a) Schematic illustration of the fabrication procedure of flexible wearable transient pressure sensors with MXene nanosheets/tissue paper/PLA. Reprinted with permission from ref. 43. Copyright 2019 American Chemical Society. (b) Recycling procedure for the Ag interdigital electrode by burning and collecting the fragmented silver interdigital electrode with ethanol, followed by sonication and drying. Reprinted with permission from ref. 50. Copyright 2021 American Chemical Society. (c) Disposal of the paper-based piezoresistive pressure sensor by incineration. Reprinted with permission from ref. 53. Copyright 2019 American Chemical Society. (d) Fabrication process of PVA/SA/BC/MCC hydrogels. Reprinted with permission from ref. 42. (e) Schematic diagram showing the synthesis of soy protein isolate/glycerin/surface-hydroxylated BaTiO3. Reprinted with permission from ref. 61. Copyright 2021 American Chemical Society. | ||
Barreto's Mars fold structure was utilized by Chen et al.3 to develop an origami based humidity sensor. It is fabricated by transferring the polyester conductive tape to the paper substrate using sensitive adhesive tape and then folding. This sensor is used to monitor nasal and oral breathing by securing the sensor onto a facial mask. In addition to this, they also used the sensor for diaper-wetting monitoring by stitching the sensor to the bottom of the diaper through capacitance changes. Even though the paper-based substrate does not cause environmental issues, the biodegradation potential of this sensor has not been fully explored and remains an area for further investigation. Similarly, another humidity sensor using carbonized fabric (CF) and oxidized carbonized fabric (OCF) was developed by Yi et al.4 (Fig. 4d). The cotton woven fabric used in this work is generally biodegradable in nature, but the high-temperature carbonization may alter the chemical structure and lead to a slower degradation than the untreated ones. Significantly, another pressure sensor is fabricated by stacking the tissue paper coated with silver nanowires and nanocellulose paper printed with the silver interdigitated electrode. A thermal degradation method-incineration was carried out to analyze the degradation of the sensor (Fig. 2c).53 When organic materials like tissue papers are incinerated, they mostly convert to CO2 and water. However, silver nanowires and printed silver electrodes may produce metallic residues, nanoparticles, or toxic fumes that may affect the environment. Incineration may reduce the waste; emission control and residue handling are essential to minimize environmental impact.
Huang et al.76 utilized environment-friendly polyvinyl alcohol (PVA), sodium alginate (SA), bacterial cellulose (BC), carboxylic multiwall carbon nanotubes (c-MWCNTs) and carbon black (CB) to develop the piezoresistive strain and capacitive pressure sensor. The dual-functioning hydrogel was fabricated through the freezing-thawing process and the Ca2+ crosslinking method (Fig. 2d). Wei et al.7 used soy protein isolate for the preparation of conductive film by incorporating surface-hydroxylated BaTiO3 (0.5 wt%), glycerin (0.5 wt%), and PEG-200 (Fig. 2d). This film exhibited a tensile strength of 21.63 MPa and a toughness of 17.70 MJ m−3 along with a conductivity of 0.912 S m−1. The high repeatability of the film over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles made the sensor suitable for application in human-motion monitoring. The degradability of the sensor is analyzed in two ways: first, the film is dissolved in a NaOH solution for 1 hour at 100 °C. The centrifugation process is carried out to separate the precipitated milk white protein solution and BaTiO3 nanoparticles. The second method is the in vitro degradation test by placing the sample in a PBS buffer solution and then placed on a shaker with a water bath at a 60 rpm shaking speed and 37 °C. The film completely degraded after 4 days.
000 cycles made the sensor suitable for application in human-motion monitoring. The degradability of the sensor is analyzed in two ways: first, the film is dissolved in a NaOH solution for 1 hour at 100 °C. The centrifugation process is carried out to separate the precipitated milk white protein solution and BaTiO3 nanoparticles. The second method is the in vitro degradation test by placing the sample in a PBS buffer solution and then placed on a shaker with a water bath at a 60 rpm shaking speed and 37 °C. The film completely degraded after 4 days.
3.1.1.3 Elastomeric platforms for stretchable sensor applications. Elastomers like ecoflex, PDMS, natural rubber, dextrin, and polyurethanes that provide excellent compatibility to the human skin have been widely explored in wearable applications. The skin can deform around 20% to 30% and these substrates provide stretchability more than that.77 Gao et al.45 reported a piezoresistive strain sensor prepared by the in situ fermentation of polypyrrole in BC and encapsulated by the ecoflex. This sensor is used to detect voluntary and involuntary functions associated with the human body, as the sensor can detect subtle movements or vibrations as a function of relative change in resistance. More interestingly, they embedded five sensors into a textile-based smart glove for the wireless real-time sensing of gesture and machine learning with an accuracy of 99.2% (Fig. 4a). The exciting part of this work is that they conducted degradation studies in the natural soil environment, and the sensor took approximately 60–90 days for complete degradation, leaving one piece of residue, and the rest is completely transformed into soil. This sensor exhibited fast biodegradation in the soil compared to 10–20 years of degradation time for plastics.78
A paper-based strain sensor was prepared by Liu et al.47 It was inspired by the ultrasensitive vibration sensing capacity of the scorpion and the superhydrophobic properties of a lotus leaf. It was fabricated on photo paper (high gloss on one side and the other side rough) by making grooves using a mechanical cutter plotter on the rough side of the paper, and silver nanoparticles were sputter-coated to enhance conductivity. A superhydrophobic coating was prepared by first making a base solution by mixing hexamethyldisilazane and n-butyl acetate and then adding MWCNTs and SiO2 into it. PDMS is also mixed with the previous solution to improve the elasticity and hydrophobic properties of the sensor (Fig. 3a). Apart from using the sensor for real-time human motion detection, they also used the paper-based sensor for underwater detection of vibration waves of the water droplet, where most of the paper-based sensors fail (Fig. 4b). Although the hydrophobic coating prohibits the paper from degrading in water, the removal of this hydrophobic coating would lend the sensor to being degradable. An excellent degradable, reprocessable, self-healing PDMS/CNT nanocomposite elastomer was developed by Lv et al.79 The reprocessability was examined by hot pressing the cut pieces of nanocomposite at 120 °C for 30 minutes. Even after 4 cycles of cutting and recycling process they were able to maintain the original mechanical properties. Additionally, the degradation studies were carried out by soaking the elastomer in trifluoroacetic acid (TFA), O-ethylhydroxylamine (EHA), and benzaldehyde (BA). The films took just 3 minutes to completely degrade in TFA and 24 hours for EHA and BA solutions. The degradation product-CNT can be recovered and reused. However, what happens to the solvents with dissolved PDMS remains unanswered.
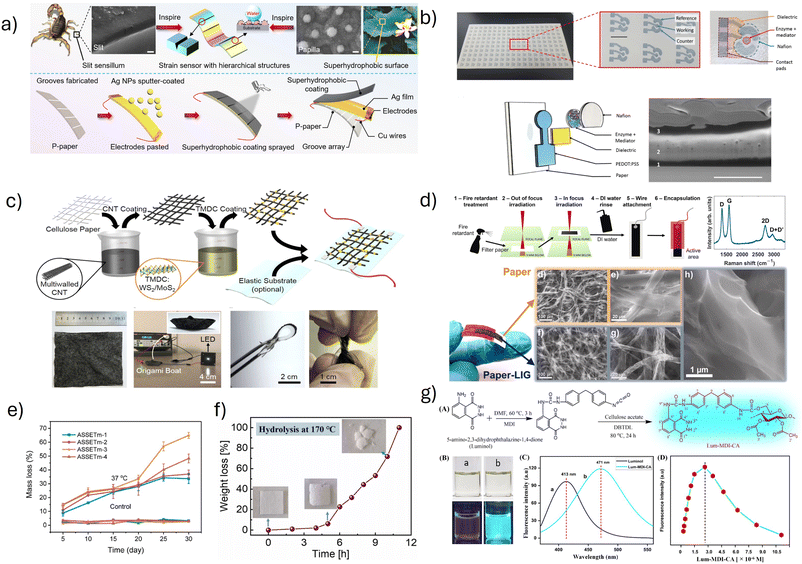 | ||
| Fig. 3 (a) The design concept and fabrication process of the bioinspired strain sensor based on the strategy of coupling bionics. SEM images of the slit unit geometry of a scorpion's ultrasensitive vibration-sensing organ and the surface morphology of the lotus leaf. Reprinted with permission from ref. 47. Copyright 2021 American Chemical Society. (b) Photograph of a glucose biosensor inkjet printed on paper. Schematic of the working electrode with separately printed layers, namely the electrode (PEDOT:PSS), the dielectric (SunTronic EMD6415), the biological coating containing the enzyme and the mediator (glucose oxidase and ferrocene), and the encapsulation layer (Nafion). Cross sectional SEM image of the working electrode taken using a focused ion beam. Reprinted with permission from ref. 80. (c) Schematic of the preparation process of the CNT–TMDC-integrated cellulose paper for the NO2 sensor. Photographs of sensor, bending and twisting demonstrating its deformability. Reprinted with permission from ref. 81. Copyright 2019 American Chemical Society. (d) Schematic illustration of the preparation of the paper-LIG electrodes. Schematic illustration of the preparation of the paper-laser induced graphene electrodes. Raman spectra and SEM micrographs of the unmodified paper, laser irradiated paper and magnified view of the electrode. Reprinted with permission from ref. 11. Copyright 2022 Elsevier. (e) Mass loss with enzymatic degradation at 37 °C of the advanced scalable supersoft elastic transparent material (ASSETm). Reprinted with permission from ref. 82. Copyright 2022 American Chemical Society. (f) Biodegradability of PLA films by hydrothermal reaction with a temperature of 170 °C. Reprinted with permission from ref. 83. Copyright 2022 Elsevier. (g) Preparation method of luminol/MDI/CA for pH sensing. Photographs of luminol and luminol-MDI-CA in DMSO in visible light (top) and at 365 nm (bottom). Fluorescence intensity of luminol and Lum-MDI-CA excited at 365 nm. Effect of the concentration of Lum-MDI-CA/DMSO solution on fluorescence intensity excited at 365 nm. Reprinted with permission from ref. 84. Copyright 2022 American Chemical Society. | ||
 | ||
| Fig. 4 (a) Application of the BC/PPy/Ecoflex sensor in human body motion monitoring. Reprinted with permission from ref. 45. Copyright 2023 Elsevier. (b) Application of the paper-based strain sensor in real-time detection under humid/underwater harsh conditions. Reprinted with permission from ref. 47. Copyright 2021 American Chemical Society. (c) Photograph of the E-skin assembled from the MXene/tissue-paper-based sensors with a size of 4 pixels × 4 pixels for pressure sensing applications and the corresponding pressure distribution mapping from the sensing responses for two-finger and three-finger sensing. Reprinted with permission from ref. 43. Copyright 2019 American Chemical Society. (d) Atmospheric humidity detection and moisture monitoring of a plant by carbonized fabric and oxidized carbonized fabric-based sensors. Reprinted with permission from ref. 4. Copyright 2022 Elsevier. (e) Applications of a luminol/cellulose acetate/4,4′-diphenylmethane diisocyanate sensor for pH sensing, fluorescent films, and security purposes. Reprinted with permission from ref. 84. Copyright 2023 American Chemical Society. (f) Glucose sensing application of PLA/overoxidized polypyrrole/gold nanoparticles/glucose oxidase/Nafion. Reprinted with permission from ref. 85. Copyright 2020 Elsevier. (g) schematic illustration for the detection of NO2 molecules by an MWCNTs/CeO2/jelly substrate. Reprinted with permission from ref. 86. Copyright 2022 Elsevier. (h) Real-time monitoring of sweat by a subject wearing a smart sweat headband device during stationary cycling and a flexible printed circuit board communicating with a personal mobile application. Reprinted with permission from ref. 87. Copyright 2019 American Chemical Society. | ||
Natural rubber is a biodegradable material and can be broken down by microorganisms over a long period of time. Zhen et al.88 and Ajeev et al.89 used naturally derived rubber as the substrate to develop strain sensors owing to the reduction of e-waste. Zhen and coworkers used milk protein fabric (MPF), natural rubber, tannic acid and vitamin C to make the strain sensor. Initially, graphene oxide is mixed with epoxidized natural rubber latex, tannic acid, and vitamin C, and the suspension obtained is coated on the MPF which is pretreated with Ca2+. This sensor is rich in biodegradable materials and the environmental aspects of this work are excellent. On the other hand, Ajeev et al. developed a conducting latex rubber band by soaking it in a conducting ink composed of toluene, Pluronic F127, sodium dodecyl sulfate (SDS) and carbon nanoparticles. The soaking of latex rubber in toluene leads to the swelling of rubber and drying at 40 °C for 12 hours which results in recycling of the rubber for another use. This is an innovative way for the reuse of latex rubber, but the hazardous effects of toluene can introduce health and environmental risks. Exploring more green solvents, which offer lower toxicity and environmental impact, will be a more sustainable approach. Another stretchable elastomer, dextrin, was utilized by Lan et al.90 for touch-sensing applications. For degradability studies, they treated the film with a PBS buffer solution containing 5 U mL−1 lipases for 30 days. The film remained in their original shape but a mass loss of 65% was obtained. A bio-based strain sensor was developed by Zhang and coworkers91 using candle soot particles, chitosan, potato starch and PVA. The degradation analysis was carried out by immersing the solution in 2 wt% CH3COOH at room temperature and at 90 °C. The film took 10 minutes to completely degrade at 90 °C and 120 minutes at room temperature. This confirms a safe disposal of the sensor in mild, nontoxic acetic acid rather than harsh chemicals. Lignin-based polyurethane elastomer was utilized to develop a strain sensor for the detection of human motions, including finger, elbow, wrist, knee bending, and swallowing. The degradability test was carried out in two different solutions including 1 mol L−1 NaOH water/ethanol and 1 mol per L NaOH aqueous solution. The sensor is completely degraded in 1 mol per L NaOH water/ethanol solution within 2 hours. And the sensor remained unchanged after immersing in 1 mol per L NaOH aqueous solution, indicating no degradability. The 4-aminophenyl disulfide (APDS) using as a chain extender in this work is insoluble in water and many organic solvents, which describes the non-solubility in 1 mol per L NaOH aqueous solution. However, the ethanol in the other solution helps penetrate through the elastomer to make it swell to achieve complete degradation. It is also important to note that they reprocessed the waste elastomer by hot-pressing at 140 °C and 20 MPa for 30 min, to obtain a new sensor.92 Another interesting polyurethane based strain sensor was developed by Zhu et al.93 They incorporated 1,4-cyclohexanedimethanol and 4,4′-diaminodiphenyl sulfide into the polyurethane via in situ bulk polymerization, followed by spin-coating with PEDOT:PSS conducting ink. The biodegradation study was conducted on the films by placing it in the PBS buffer solution containing proteinase XIV and incubated in a constant temperature shaker at 37 °C. The film was monitored from week 0 to week 8 and the remaining mass was found to be 76.04%. This indicates partial biodegradability of the elastomer with stable components present in the backbone of the elastomer, that resists the enzymatic attack. A tactile sensor based on poly(caproactone-urethane) elastomer was designed by Reddy et al.94 The degradability test was conducted by immersing the elastomer in lipase solution for 60 days and degradation of 53% was reported. A 53% degradation rate over 60 days indicates only a partial degradation and does not guarantee complete biodegradability under environmental or physiological conditions.
3.2 Chemical reactivity-based sensing for biochemical and environmental applications
Chemical sensors are devices or instruments that are capable of converting the presence, concentration, or quantity of an analyte into a measurable signal. It contains three major components, including a receptor, a transducer, and a signal processor.34,35 These chemical sensors can be used in various applications, including healthcare, environmental monitoring, food, and industrial safety. Based on the type of sensing material present, chemical sensors can be classified into conducting polymer-based, carbon-based, metal-based, and biopolymer-based.Paper is the most attractive and widely used substrate for chemical sensing applications due to its biodegradability and recyclability. Paper based chemical sensors developed by various research groups showcase different fabrication methods and applications.95,96 Glucose sensors based on paper substrates are designed by utilizing different conducting polymers like PEDOT:PSS and PANI by Bihar et al.80 and Das et al.97 Bihar and coworkers developed the glucose sensor by an inkjet printing method using PEDOT:PSS, glucose oxidase, and ferrocene as enzyme and electron mediator (Fig. 3b). However, Das incorporated PANI/graphite into cellulose paper for the glucose sensing applications. Interestingly, an acetone sensor was fabricated by Davis et al.98 by coating conductive carbon ink and acetone sensitive PANI-ZnO ink, using a blade coating method on the paper substrate.
While conducting polymers offer tunable conductivity and redox activity for chemical sensing, carbon based nanomaterials are also widely explored for chemical sensing. Their high surface area, conductivity, stability, and tunable surface chemistry make them ideal for detecting gases or biomolecules. A disposable paper-based sensor for sulfamethoxazole detection is prepared by depositing a conducting ink made of shellac, graphite, and solvent over the paper and drying.99 Interestingly, a NO2 sensor, composed of cellulose paper, CNT, and transition metal dichalcogenides (TMDCs) is synthesized by Lee et al.81 The sensor is fabricated by dip coating the cellulose paper in CNT for 5 s and drying, then dipping in TMDCs and drying (Fig. 3c). Kulyk et al.11 developed another paper-based, biodegradable sensor for the non-enzymatic electrochemical sensing of uric acid in human urine. The paper substrate is treated with fire retardant initially and irradiated twice. The first irradiation is out of focus, which increases the conversion of cellulose into char, and the second one is in focus irradiation, which graphitizes it into laser-induced graphene (Fig. 3d). Paper-based sensors are a promising step forward for the development of environmentally friendly wearable devices. However, it is important to examine the complete lifecycle and degradation pathways as these sensors often combine with additional polymers, nanomaterials, metals, and solvents. Relying completely on the biodegradability of paper alone might mask the potential environmental impact of other materials in these devices. A glucose sensor is fabricated by using the biodegradable substrates PLA and PEG and then screen printing carbon electrodes on it. This device is completely made by polymer based biodegradable materials possessing nontoxicity and biodegradability.100
Metal-based chemical sensors typically utilize metal oxides or metal nanoparticles as sensing materials for analyte detection. Molina et al. developed a NO2 gas detection sensor using a jelly-based substrate86 (Fig. 4g) and algae-based substrate.101 Both the substrates are environmentally friendly, but they are also depositing Yb doped nickel oxides, MWCNTs, and CeO2 into it, which questions the complete degradability of the material. Another NO2 sensor was fabricated by Ko et al.102 using a single-crystal silicon nanomembrane. The device is fabricated by thinning down the silicon layer to about 100 nm and doping it with phosphorus. The silicon membrane is then patterned using plasma etching and magnesium electrodes were deposited on it. Finally, the entire device was printed onto a biodegradable polycaprolactone (PCL) substrate. The ultra-thin silicon structures exhibit higher degradation potential than the bulk silicon, while PCL further accelerates the degradation. A lignin containing cellulose nanofiber/ZnO based ammonia sensing device was developed by Li et al.103 by hydrothermal reaction followed by freeze-drying. The degradability of the sensor was examined by burying the film in soil and the substrate completely degraded within three weeks, indicating excellent biodegradability.
An interesting sensor for the detection of temperature, sweat pH, and UV was developed by Liu et al.104 Thermochromic, methyl red, and photochromic microcapsules were added into the gelatinized starch solution to obtain the sensing ink and then microprinted into a starch support bath for fabrication. The highlight of this work is that they carried out the degradation studies in tap water at 37 °C, and complete degradation was observed after 10 days. The mass loss of different molecular weight ASSETm upon degradation is given in Fig. 3e. Compared with the enzymatic or alkali treatments required by most of the sensors, the degradation pathway of this sensor is more sustainable. A self-healable sweat sensor (Fig. 4h) was developed by Yoon et al.103 by integrating carbon fiber thread into a poly(1,4-cyclohexanedimethanol succinate-co-citrate) (PCSC) matrix. Similarly, a PLA microneedle-based glucose sensor was developed by Zhang et al.,85 by coating it with gold nanaoparticles, and the sensing graph is shown in Fig. 4f. Through the chemical modification of cellulose acetate (CA) by 5-amino-2,3-dihydrophthalazine-1,4-dione (luminol), using 4,4′-diphenylmethane diisocyanate (MDI) as a cross-linking agent, Nawaz et al.91 (Fig. 3g) developed a pH sensor (Fig. 4e) that can identify pH 1–2 and pH 12–14 by significant change in fluorescent color. Although cellulose acetate is derived from cellulose, the acetylation process disrupts the hydrogen bonding, which can actually affect biodegradability. A fully degradable pH sensor was developed by Sakabe et al.105 to detect the soil acidity. This wireless sensor consists of patterned octacalcium phosphate coated with magnesium on a poly-lactic acid sheet. The fully degradable nature of this sensor allows it to be safely left in soil, where it will naturally degrade by environmental processes (Table 2).
| Active materials | Response time | Sensitivity | Detection limit | Degradation mechanism | Application | Ref. |
|---|---|---|---|---|---|---|
| BC/PANI/PAAMPSA/SSA | 4.1 s | 4.1 s | 10 ppb | — | Wearable ammonia sensor | 10 |
| Paper/graphene | — | 0.363 μA cm−2 μM−1 | 3.97 μM | Biodegradation of cellulose | Uric acid sensing | 11 |
| CNT/TMDCs/cellulose paper | — | 4.57% ppm−1 | — | Biodegradation of cellulose | NO2 sensor | 81 |
| MWCNTs/CeO2/jelly substrate | 22.9 s | 0.42 | — | Biodegradability of a jelly substrate | NO2 sensor | 86 |
| Paper/shellac/graphite | — | 0.09 μA μ mol−1 L | 0.4 μmol−1 L | Biodegradation of cellulose | Sulfamethoxazole detection | 99 |
| PLA/PPy/AuNPs/glucose oxidase/Nafion | 28 s | 8.09 μA mM−1 | 40 μM | Biodegradability of PLA | Glucose sensing | 85 |
| Paper/PANI/graphite | — | 18.78 μA (mg dL−1) | 1.6 mg dL−1 | Biodegradation of cellulose | Glucose sensing | 97 |
| Whatman Paper/CuO/PEDOT:PSS | — | 9.1279 μA nM−1 | 0.42 nM | Degradation of paper | Paraoxon-ethyl sensing | 96 |
4 Reuse and ultimate fate of wearable polymer-based sensors
4.1 The transient nature of wearables
Transient wearables are pioneering innovations in wearable technology. Unlike conventional wearable sensors, transient wearables are designed for temporary use. Due to their biodegradable nature, this provides significant opportunities for their application across healthcare, defense, and environmental sciences by eliminating long-term waste, removing the need for sensor retrieval, and ensuring sensitive data protection.106 As the demand for sustainable technology grows, the global market for biodegradable electronic components and devices is expected to grow at a CAGR of 12.23%, reaching $1.08 billion by 2027.106Based on their functions and composition, transient wearables can be split into two categories: biodegradable polymers and biodegradable metals. Polymers are primarily used as substrates, encapsulation layers, and dielectric layers due to their mechanical robustness. This makes them ideal components for implants that may be used in drug delivery or tissue regeneration, among a variety of other applications.106 Some polymers used in biodegradable substrates may be derived from nature, such as collagen, silk, gelatin, cellulose, alginate, chitosan and natural waxes.106 They are particularly beneficial due to their global availability, cost-effectiveness, and biocompatibility. Sedghi et al.107 developed a biodegradable composite conductive hydrogel via a “thiol–ene” click reaction between the amphiphilic groups of modified chitosan and thiol-functionalized graphene oxide. Later on, these biocompatible chitosan-based hydrogel wearable sensors were tested for human activity monitoring and achieved full degradation within 21 days. Zeng et al. synthesized a wood-based hydrogel by cross-linking cellulose fibers, PVA, and lignin via the Hoffmeister effect. The hydrogel showed high mechanical strength, notable flexibility, electrical conductivity, and excellent degradation.108 With their promising results, such hydrogels hold great potential for green, next-generation, flexible bioelectronics.107 Qian et al. grafted a conductive PPy onto gelatin molecular chains, resulting in a highly water-soluble conductive hydrogel. Due to its biocompatibility, congruent electrical conductivity to the heart, and easy insertion, the hydrogel was successfully injected on the surface of the damaged heart for myocardial repair.107
Other commonly used polymers, such as poly(lactic acid) (PLA), poly(octanediol-co-citrate) (POC), poly(glycerol sebacate) (PGS), PVA, and poly(caprolactone) (PCL), are acquired synthetically, although with some exceptions.106 PLA can be derived from plant materials, such as corn or sugarcane. On the other hand, PVA and PCL are obtained from fossil fuels. Though these polymers have proven their biocompatibility, they are oftentimes combined with other natural ingredients to enhance their mechanical properties and biodegradation. Ma et al.83 proposed a flexible and disposable sensor based on a PLA piezoelectric film (DS-PLA) that could be used for bio-motion monitoring. As shown in Fig. 3f, the film successfully degraded within 11 hours in deionized water with a temperature of 170 °C.
Boutry et al.109 presented a biodegradable strain sensor with potential use in real-time monitoring of tendon healing. The polymer matrix, composed of PGS, PLA, and poly(octamethylene maleate anhydride citrate) (POMaC), demonstrated a linear degradation rate of 11% to 14% per week. Silicone-based substrates are equally as prevalent and implemented in biodegradable sensors due to their flexibility, conformability, and biocompatibility. Commonly used forms include monocrystalline micromembranes, nanomembranes, and porous structures. Their degradation occurs through hydrolysis, with the process typically completing within days to weeks, depending on the thickness of the SI film. Hwang et al.110 presented the degradation of monocrystalline SI nanomembranes at a rate of 4.5 nm per day under physiological conditions.
On the molecular level, polymer degradation may occur through hydrolytic, enzymatic, and/or oxidative routes—offering different degradation rates. First, the polymer is broken down into short-chain molecules through the abiotic processes mentioned previously. Subsequently, the short chains are further broken down into smaller molecular weight compounds by microorganisms found in the media.107 The rate of degradation will vary depending on the degree of polymerization, molecular weight, intramolecular interactions, and aggregation rate. Typically, polymers with higher molecular weights and complex structures exhibit slower degradation rates. In terms of structure, polymers with active bonds such as hydrolyzable and oxidizable bonds are more prone to degradation by natural environmental factors and microbial activity.107 Unlike crystalline polymers, amorphous polymers are more susceptible to degradation due to fewer intramolecular interactions and larger spaces between polymer chains. By tuning some microstructured properties of the polymer matrix, such as grain size, texture, defects, and porosity, the rate of degradation can be managed based on the preferred outcome and application.
On a larger scale, polymer degradation may occur in two ways: bulk degradation or surface erosion. In bulk degradation, the polymer matrix breaks down uniformly, which reduces the polymer's mechanical strength and molecular weight. Over time, it may cause disintegration and debris formation. In contrast, surface erosion occurs only on the surface of an implant. Though the size and mass of the matrix gradually decreases, its shape, mechanical strength, and molecular weight remain stable for a longer period. This form of degradation is more predictable, provides greater control over the ongoing process, and offers significant protection to the core of the polymer matrix.106
Though options are fairly limited, some biodegradable metals, such as magnesium (Mg), iron (Fe), zinc (Zn), and their alloys, have excellent biocompatibility, mechanical properties and adjustable degradation rates, making them great candidates in the field of orthopedics and cardiology.106 Mg-based metals have been shown to be particularly advantageous for biomedical applications, as their elastic modulus (45 GPa) closely matches that of human bone (40–57 GPa), and their density (1.7 g cm−3) aligns with the density of human calvarium bone (1.75 g cm−3).111 Mg-based metals also exhibit faster degradation and bulk conductivity compared to Fe-based and Zn-based metals.106 As this Mg-based sensor degrades, body fluids gradually infiltrate the packaging layer, which leads to corrosion. As a result, magnesium oxides are formed. Ultimately, these highly soluble salts are naturally expelled from the body, removing the reminder of an implant entirely from the system. This makes Mg-based metals highly suitable for short-term applications.109 Although less commonly used, Zn has been implemented into ink formulations by Feng et al.112 as a filler, and has been used in different printing approaches to fabricate biodegradable conductors. Among all biodegradable metals, Fe has shown a slower and less predictable degradation rate. Therefore, it is uncommon in emerging biodegradable sensors. However, iron-based particles have been introduced as catalysts in implantable biosensors.106
Meant for temporary use, transient wearables are often used for medical monitoring, environmental sensors, and data security, where short-term functionality is needed. They serve as a great medium for temporary medical implants that dissolve after fulfilling their purpose and avoid the risks associated with removal surgery. Due to their biodegradability, bio-friendliness, and nontoxic nature, these materials have been extensively used in medical applications such as sutures, wound dressings, controlled drug delivery, blood flow monitoring, oxygen content monitoring, and tissue regeneration inter alia.106,113–115 Kang et al.116 successfully developed an implantable sensor intended for intracranial pressure monitoring that was entirely biodegradable. The sensor was designed to operate under human body conditions for a certain period of time, and then fully degrade via hydrolysis and metabolism without any health complications. Boutry et al.113 designed a flexible and fully biodegradable arterial-pulse sensor meant for wireless monitoring of the patient's blood flow. This sensor would be particularly valuable in post-surgery follow up care after reconstructive surgeries resulting from trauma or cancer. Xu and Yadavalli114 proposed a flexible and fully biodegradable sensor meant for vascular endothelial growth factor (VEGF) biomarker detection in urine samples and human serum. The biosensor consisted of a biocompatible ink from mixing a conducting polymer, PEDOT:PSS, sericin photoresist (SPP), and anti-VEGF16. The sensor showed high sensitivity to VEGF across all the samples, yielding promising results and the potential for detecting cancer at an early stage.
4.2 Moving towards recyclability
As wearable technologies evolve, it is vital to redesign these devices from a sustainability perspective, moving toward materials that promote recycling and reuse.117–119 The use of specialized polymers with functional properties120 offers superior performance compared to traditional plastics for intended applications. Although, these materials enable high-performance sensing, their specialized compositions consisting of conjugated backbones complicate the recycling approach. Traditional recycling methods, often designed for polymers such as polyethylene and polypropylene, are not compatible with the unique polymers used in sensors. Thus, a key challenge is to modify these recycling methods or develop a new process that can effectively handle the unique chemical and structural properties of sensor materials.29,121,122A promising approach for recycling wearable sensors is the development of biodegradable polymers designed to decompose after a specific lifespan as mentioned in the previous sections. Biodegradable polymers have shown promise in a variety of applications but still require enhancements to meet the functional demands of sensors. For example, biodegradable polymers generally lack the electronic properties essential for sensing and monitoring applications.123,124 Current research is focused on functionalizing these materials with conductive 0D, 1D, or 2D additives or coatings, such as graphene, CNTs, or metallic nanoparticles, to enable their use in wearable sensors. This strategy could lead to a new generation of wearables that can perform effectively during their operational lifespan and then degrade naturally, significantly reducing environmental impact.125–127
Durability and reusability are key considerations in the sustainability of wearable sensors. Many wearable sensors are designed for single-use or have limited lifespan due to concerns about contamination, particularly in biomedical applications. Therefore, reusability has not been a priority in wearable sensor design.124 However, recent advancements in sterilization and surface treatment methods are opening up opportunities for developing sensors that can be reused safely.128 Techniques such as ultraviolet (UV) sterilization, autoclaving, and chemical disinfectants have been successfully applied to a range of polymers, allowing reliable decontamination without compromising the functional performance of the sensor performance.129,130 Additionally, the incorporation of biocompatible coatings that resist bacterial or viral contamination can further enhance the reuse potential of wearables, especially in medical or fitness applications where direct skin contact is common. These coatings not only protect the sensor's integrity but also extend its operational lifespan, reducing the need for frequent disposal or replacement.124,131
To fully support recycling, new design philosophies must be adopted that consider the entire lifecycle of wearable sensors. For instance, wearable sensors could be engineered with modular components that can be easily separated, enabling the selective recycling of specific parts.128 Conductive inks, adhesives, and encapsulants that are soluble or removable through environmentally friendly processes could enable the recovery of electronic components without extensive chemical treatment. For polymer substrates, this strategy might involve the use of reversible cross-linking agents or bonds that break under specific conditions, simplifying the separation of sensing elements from the substrate when the device reaches the end of its lifecycle.132–134
Furthermore, exploring bio-based polymers offers a promising route for creating sustainable wearables. For example, cellulose nanofibrils can be engineered to exhibit mechanical properties suitable for wearable devices while maintaining biodegradability.14,135 These bio-based materials offer a viable alternative to petroleum-derived polymers and can be processed into forms compatible with wearable applications. Beyond their sustainability, bio-based polymers may provide unique benefits, such as natural antimicrobial properties, that enhance both the functionality and hygiene of sensors. By integrating natural polymers into wearable sensor design, it may be possible to develop devices that not only perform their intended functions but also biodegrade naturally after use, reducing long-term environmental impact.136–138
Chemical recycling techniques also offer a promising solution for the recycling and reprocessing of polymer-based sensors.139 In contrast to mechanical recycling, which typically involves physical processes, chemical recycling breaks down polymers into their monomers or other basic chemicals for reuse. This process enables the restoration of polymers to their molecular precursors, enabling the creation of new materials with properties identical to the original. For example, depolymerizing materials like PET or polycarbonate can yield monomers that can be repolymerized into high-quality materials, effectively closing the loop on plastic waste.140–142 While chemical recycling is traditionally energy-intensive, recent developments in catalysis and process optimization have significantly reduced its energy consumption, making it a more viable and sustainable approach. This option is particularly beneficial for wearable sensors made from polymers unsuitable for conventional mechanical recycling, offering a sustainable route for disposing of and reusing high-performance materials.28,143
Finally, societal and economic factors play a crucial role in advancing sustainable wearable sensors. The growing demand for wearables is driving the need for faster production cycles, cost-effective manufacturing, and single-use devices, creating tension between affordability and environmental impact.144,145 To address this, consumer education about the benefits of reusable or recyclable wearables is crucial, along with the establishment of industry standards and regulatory measures that promote sustainable alternatives. Furthermore, collaboration between researchers, manufacturers, and policymakers can establish closed-loop systems where wearable sensors are returned, reprocessed, and reintroduced into the market.130,146 Extended producer responsibility (EPR) programs, where manufacturers take on the responsibility for the end-of-life management of their products, can further enhance the recycling infrastructure needed for wearable sensors.145,147,148
4.3 Can we reuse them? A look at sterilization/lifetime
As wearable sensors emerge in industrial and consumer devices, research on lifespan and sterilization is needed to further study life-cycle analysis and compliance with safety regulations. To ensure the safety of polymer-based sensors in applications such as healthcare, where a sensor may contact multiple patients, effective sterilization is required between uses. The FDA recognizes three classes of medical devices for regulatory control; however, most polymer-based sensors developed fall into Class I and Class II devices, those developed for low-risk application and typically only make dermal contact.149,150 The FDA divides sterilization of such medical devices into established methods and novel methods. Established methods of sterilization include dry heat, ethylene oxide, steam (autoclave), radiation, and hydrogen peroxide treatment.151 Steam and dry heat methods of sterilization use high-temperature convection to sterilize medical devices, which may exceed the degradation temperatures of sensors. Ethylene oxide may be used for sterilization at ambient temperatures and pressures for heat-sensitive equipment due to its highly reactive alkylation sterilization mechanism, though sterilant residual analysis is required to avoid contact with ethylene oxide during normal use.152 Ultraviolet (UV) light is recognized as a novel sterilization method by the FDA and offers another low-temperature method for heat-sensitive sensors. UV sterilization offers an advantage over other techniques as it avoids direct contact with high temperatures or solvents. UV light may induce photo-oxidation in polymers and composite fillers, leading to premature degradation, though the incorporation of stabilizing agents can minimize UV degradation. Low band-gap filler materials that promote high UV absorption have been shown to preserve the properties of polymers and elastomers in repeated UV sterilization. A study by Kuo et al.153 found the addition of graphene nanosheets at 1 wt% offered protection of polyurethane for up to 30 hours (150 cycles) of UV-C sterilization with significant improvement in Young's modulus compared to untreated polyurethane.Mechanical and environmental degradation testing can estimate how sensors perform and change in real-world conditions. Fatigue testing, in which a sensor is continuously engaged while performance is monitored, is one method of understanding how a sensor changes with repeated use. Although many publications containing a wide range of physical and chemical properties have reported some degree of fatigue testing, these studies more commonly report on high-amplitude (maximum elongation) and low-cycle testing while not reporting on the number of uses to failure.154 High-cycle testing in the typical domain of a sensor's sensing mechanism conditions, along with the sensor's performance, provides needed insight into the useful lifespan of sensors. A broad analysis of the effect of cyclic loading on nanomaterial-based elastomers by Boland155 found a consistent power-law scaling of relative resistance with fatigue cycles, likely due to the Mullins effect: stress softening in composites with repeated mechanical loading. Testing, however, is not limited to mechanical fatigue. Reporting of degradation from high temperatures, such as thermo-gravimetric analysis, or exposure to chemical sterilization agents such as ethylene oxide over time in future literature may be useful in determining which sterilization method is appropriate for a sensor and how sensors may behave in the long term in clinical settings.
5 Future outlook of wearable polymer-based sensors
Wearable polymer-based sensors have emerged as a transformative technology with a broad range of applications, from healthcare monitoring to environmental sensing and interactive devices. Their significance in modern society continues to grow as they play a central role in advancing wearable technology. However, as the use of these sensors increases, it becomes increasingly important to address their long-term sustainability, particularly with reuse, recycling, and disposal at the end of their life cycle. The polymers used in these sensors are often engineered with unique properties such as conductivity, flexibility, and responsiveness to various stimuli, but these advanced materials pose significant challenges in terms of recycling and have potential environmental impact.As the scientific community continues to innovate and advance these materials, the need for sustainability becomes increasingly crucial. In this regard, evaluating the potential of transient and biodegradable polymers is urgent, as they could enable the development of wearables that are not only high-performing and efficient but also environmentally responsible. The goal of this perspective is to offer insights into how the polymer science community, in collaboration with researchers, manufacturers, and policymakers, can solve these challenges. The rising demand for wearable sensors calls for a shift toward sustainable materials and processes that support the continued growth of the field while safeguarding the health of the planet.
5.1 The need for sustainable wearables
The rapid progression of wearable technology has resulted in remarkable advancements in health monitoring, sports science, immersive gaming, environmental sensing, and various other fields. Wearable polymer sensors are specifically designed to be sensitive to a broad range of physical, chemical, and biological stimuli, making them essential elements in devices for fitness tracking, medical diagnostics, environmental monitoring, and even smart textiles. These sensors can detect changes in temperature, pressure, humidity, gases, chemicals, and electrical signals from the human body, offering real-time data for immediate feedback or continuous monitoring over extended periods.The growing market for wearable sensors presents a significant environmental challenge. Many of the polymers used in these sensors are sourced from petrochemicals and possess properties that make them difficult to recycle through traditional methods. These polymers are engineered to be flexible, stretchable, and durable, allowing them to endure continuous use and exposure to various environmental conditions. However, their durability often hinders their decomposition and reuse once the sensor reaches the end of its life cycle. The difficulty in recycling or disposing of these materials in an environmentally responsible manner poses a considerable concern as the wearable technology market continues to grow. Life cycle assessment (LCA) is a tool for evaluating the environmental impacts from raw material extraction to use, and disposal of a product or process.156 This approach helps to identify the possible environmental impacts that can occur during each stage of the product. In most cases, the lack of awareness is the root cause, and tools like LCA can provide an excellent opportunity for the researchers and manufactures to address the sustainability issues.
In light of this challenge, the demand for sustainable polymer materials that are recyclable, reusable, or biodegradable has become increasingly urgent. Recently, there has been a growing emphasis on creating polymers that not only provide the essential functional properties for wearables but also contribute to environmental sustainability objectives. The development of such materials offers the polymer community a valuable opportunity to address critical global issues, including plastic pollution and the depletion of natural resources.
5.2 Transient polymers: a new era of sustainability
A promising solution to the sustainability challenge of wearable polymer sensors lies in the use of transient polymers. These materials are specifically designed to degrade or break down after serving their intended purpose. For wearable sensors, transient polymers could be engineered to decompose under specific environmental conditions, such as exposure to moisture, temperature, or UV radiation. This controlled degradation process would ensure that the sensor devices have a defined lifespan, after which they would naturally decompose, resulting in minimal environmental impact.The development of transient polymers for wearable sensors presents a promising alternative to traditional plastics, which are non-biodegradable and can persist in the environment for decades.157 By incorporating transient polymers, the reliance on conventional waste disposal methods like incineration or landfilling could be significantly reduced. Moreover, these polymers can be engineered to degrade in a controlled manner, ensuring that the sensor devices break down without releasing harmful chemicals into the environment. However, a key challenge lies in maintaining the right balance between the functional properties required for wearable sensors—such as conductivity, flexibility, and strength—and the degradation characteristics needed for transient polymers. While transient polymers have been explored in other industries, such as packaging and medical devices, their use in wearable sensors is still in the early stages. Continued research is essential to advance these materials, ensuring they meet the demanding performance standards of wearables without sacrificing their functionality.
5.3 The role of recycling in wearable sensors
While transient polymers offer an exciting future for wearables, there is still significant work to be done in improving the recyclability of existing polymer materials. As wearable sensors become more complicated, the incorporation of integrated electronics and functional coatings for these materials causes the recycling processes to become increasingly complex. Many of the polymers used in wearable sensors, including conductive polymers, thermoplastics, and elastomers, require advanced recycling methods that extend beyond traditional mechanical recycling techniques.Chemical recycling offers a promising approach to recycling the complex polymer materials used in wearable sensors. Unlike mechanical recycling, which breaks down polymers into smaller pieces for reprocessing, chemical recycling decomposes the polymers into their original monomers, allowing them to be re-polymerized and processed into new materials. This method offers the potential to produce high-quality materials that retain the original properties of the polymers, enabling wearable sensors to be recycled into new devices without compromising their performance. However, chemical recycling comes with its own set of challenges, as it typically requires high temperatures and significant energy inputs, which can reduce its environmental benefits. Recent advancements in catalytic processes and the development of more energy-efficient techniques are helping to address these issues. For instance, researchers are investigating innovative catalysts that lower the energy demands of chemical recycling, improving its cost-effectiveness and sustainability.
Furthermore, the development of new polymers tailored for easier recycling could play a vital role in the future of wearable sensors. For example, polymers with reversible chemical bonds can be engineered to break down under specific conditions, such as heat or light, enabling the efficient separation of materials and recovery of valuable components. This approach would not only facilitate the recycling process for wearable sensors but also help reduce the overall waste generated by the wearable technology industry.
5.4 Global scientific community collaboration
The increasing demand for sustainable wearable polymer sensors requires a unified effort from the global scientific community. Collaboration among researchers in polymer science, materials engineering, environmental science, and other related fields is essential to solve the challenges of reuse, recycling, and end-of-life management of wearable sensors. This collective effort is key to developing new materials, processes, and technologies that enhance the sustainability of wearable sensors. A crucial aspect of this collaboration is the establishment of standardized testing and regulations to ensure the recyclability and sustainability of these devices. Governments, industries, and research institutions must join forces to create guidelines and standards for the design, production, and disposal of wearable sensors. By creating a regulatory framework that encourages the use of recyclable and biodegradable materials, the global scientific community can help guide the wearable technology industry toward more sustainable practices.Education and awareness will also play a key role in encouraging the adoption of sustainable wearable sensors. Researchers and manufacturers must focus on educating consumers about the importance of recycling and reusing wearable sensors, as well as the environmental benefits of using biodegradable or transient materials. Recycling programs or incentives for returning used devices could help foster a more sustainable ecosystem for wearable sensors. Additionally, partnerships between academic researchers, private industry, and government organizations are essential for accelerating the commercialization of sustainable wearable technologies. By combining resources and expertise, these stakeholders can drive innovation in materials and processes that facilitate not only the reuse and recycling of wearable sensors but also their re-purposing.
Re-purposing refers to the reuse of materials or entire devices for alternative applications beyond their original intended function. As most of the wearable sensors are not fully degradable and others only partially, they still pose environmental concerns after their functional lifespan. In such scenarios, exploring secondary uses for these devices provide a valuable opportunity to minimize waste and extend material utility. Alongside the primary research objectives, researchers should also consider the potential for repurposing at the initial stages of design and development if the material cannot be degraded. However, practical implementation of repurposing has its own challenges. Especially wearable devices are frequently exposed to sweat, mechanical strain and other contaminants. However, repurposing provides an intermediate strategy between single-use disposal and full degradation or recycling from a sustainability standpoint. The ultimate goal should be to establish a circular economy, where wearable sensors are not discarded after use but instead returned to the production cycle, reprocessed, or repurposed into new devices.
List of abbreviations
| APDS | 4-Aminophenyl Disulfide |
| APS | Ammonium Persulfate |
| ASSETm | Advanced Scalable Super Soft Elastic Transparent Material |
| BA | Benzaldehyde |
| BC | Bacterial cellulose |
| BPU | Biodegradable polyurethane |
| CA | Cellulose acetate |
| CAGR | Compound annual Growth Rate |
| CB | Carbon Black |
| CF | Carbonized Fabric |
| CNT | Carbon Nanotube |
| CP | Conducting Polymer |
| CS | Candle Soot |
| c-MWCNT | Carboxylic Multi-walled Carbon Nanotube |
| EHA | O-Ethylhydroxylamine |
| EPR | Extended Producer Responsibility |
| FDA | Food and Drug Administration |
| LCA | Life Cycle Assessment |
| MCC | Modified Carbon Nanotube |
| MDI | 4,4-Diphenylmethane diisocyanate |
| MCNT | Multi-walled Carbon Nanotube |
| MPF | Milk Protein Fabric |
| OCF | Oxidated Carbonized Fabric |
| PAAMPSA | Poly(2-Acrylamido-2-Methyl-1-Propanesulfonic Acid) |
| PANI | Polyaniline |
| PBS | Phosphate Buffered Solution |
| PCCU | Poly(Carbonate-Chair Cyclohexane-Urethane) |
| PCL | Polycaprolactone |
| PCL | Poly(Caprolactone) |
| PDMS | Polydimethyl siloxane |
| PEDOT:PSS | Poly(3,4-Ethylenedioxythiophene)Polystyrene Sulfonate |
| PGS | Poly(Glycerol Sebacate) |
| PLA | Polylactic Acid |
| POC | Poly(Octandefiol-co-Citrate) |
| POMaC | Poly(Octanemethylene Maleate Anhydride Citrate) |
| PPy | Polypyrrole |
| PS | Potato Starch |
| PVDF | Poly(Vinylidene Fluoride) |
| PVA | Poly(Vinyl Alcohol) |
| rGO | Reduced Graphene Oxide |
| SA | Sodium Alginate |
| SDS | Sodium Dodecyl Sulfate |
| SEM | Scanning Electron Microscopy |
| SPP | Sericin Photoresist |
| TMDC | Transition Metal Dichalcogenide |
| TFA | Trifluoroacetic Acid |
| UV | Ultraviolet |
| VEGF | Vascular Endothelial Growth Factor |
Data availability
All data in this article were taken from published articles online and are publicly available for reading and download.Author contributions
Conceptualization was carried out by E. K. W. Writing of the original draft was conducted by A. A., T. W, S. M.-S., C. D., S. A., and E. K. W. Manuscript review & editing were conducted by A. A., T. W., S. M.-S., C. D., S. A., and E. K. W. Visualization was done by A. A., T. W, S. M.-S., C. D., S. A., and E. K. W. Supervision, project administration, and funding acquisition were conducted by E. K. W.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
E. Wujcik and A. Ajeev would like to acknowledge support from a National Science Foundation (NSF) CAREER Award from the Electronic/Photonic Materials program (NSF/MPS/DMR/EPM; award: 2305282). Partial support for A. Ajeev was provided by The University of Maine, Maine College of Engineering and Computing (MCEC). C. Duprey would like to acknowledge support from the Advanced Structures and Composites Center (ASCC) at the University of Maine's general effort and fundamental research funds. E. K. W. also thanks Dr Amanda Koh for helpful discussions related to the article. Any opinions, findings and conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the ASCC or The University of Maine.Notes and references
- N. J. Blasdel, E. K. Wujcik, J. E. Carletta, K. S. Lee and C. N. Monty, IEEE Sens. J., 2015, 15, 300–306 CAS.
- J. Horne, L. McLoughlin, E. Bury, A. S. Koh and E. K. Wujcik, Adv. Mater. Interfaces, 2020, 7, 1901851 CrossRef.
- X. Chen, Y. Li, X. Wang and H. Yu, ACS Appl. Mater. Interfaces, 2022, 14, 36227–36237 CrossRef CAS PubMed.
- Y. Yi, C. Yu, H. Zhai, L. Jin, D. Cheng, Y. Lu, Z. Chen, L. Xu, J. Li, Q. Song, P. Yue, Z. Liu and Y. Li, Nano Energy, 2022, 103, 107780 CrossRef CAS.
- E. K. Wujcik, N. J. Blasdel, D. Trowbridge and C. N. Monty, IEEE Sens. J., 2013, 13, 3430–3436 CAS.
- C. Harito, L. Utari, B. R. Putra, B. Yuliarto, S. Purwanto, S. Z. Zaidi, D. V. Bavykin, F. Marken and F. C. Walsh, J. Electrochem. Soc., 2020, 167, 037566 CrossRef CAS.
- Y. Wei, S. Jiang, X. Li, J. Li, Y. Dong, S. Q. Shi, J. Li and Z. Fang, ACS Appl. Mater. Interfaces, 2021, 13, 37617–37627 CrossRef CAS.
- Y. Li, W. Chen and L. Lu, ACS Appl. Bio Mater., 2020, 4, 122–139 CrossRef.
- Y. Lu, M. C. Biswas, Z. Guo, J.-W. Jeon and E. K. Wujcik, Biosens. Bioelectron., 2019, 123, 167–177 CrossRef CAS PubMed.
- L. Yang, X. Xu, M. Liu, C. Chen, J. Cui, X. Chen, K. Wu and D. Sun, Sens. Actuators, B, 2021, 334, 129647 CrossRef CAS.
- B. Kulyk, S. O. Pereira, A. J. Fernandes, E. Fortunato, F. M. Costa and N. F. Santos, Carbon, 2022, 197, 253–263 CrossRef CAS.
- X. Luo, H. Wu, C. Wang, Q. Jin, C. Luo, G. Ma, W. Guo and Y. Long, Chem. Eng. J., 2024, 483, 149330 CrossRef CAS.
- C. Wei, H. Zhou, Z. Wang, B. Zheng, H. Zheng, X. Jin, A. Ma, W. Chen and H. Liu, ACS Appl. Polym. Mater., 2024, 6, 4014–4024 CrossRef CAS.
- S. N. Banitaba, S. Khademolqorani, V. V. Jadhav, E. Chamanehpour, Y. K. Mishra, E. Mostafavi and A. Kaushik, Mater. Today Electron., 2023, 5, 100055 CrossRef.
- J. Horne, L. McLoughlin, B. Bridgers and E. K. Wujcik, Sens. Acutators Rep., 2020, 2, 100005 Search PubMed.
- W. Li, S. Zhao, N. Wu, J. Zhong, B. Wang, S. Lin, S. Chen, F. Yuan, H. Jiang and Y. Xiao, et al. , ACS Appl. Mater. Interfaces, 2017, 9, 23716–23722 CrossRef CAS.
- A. Giuliani, M. Placidi, F. Di Francesco and A. Pucci, React. Funct. Polym., 2014, 76, 57–62 CrossRef CAS.
- A. M. Soomro, F. Jabbar, M. Ali, J.-W. Lee, S. W. Mun and K. H. Choi, J. Mater. Sci.: Mater. Electron., 2019, 30, 9455–9465 CrossRef CAS.
- X. Hu, X.-X. Xia, S.-C. Huang and Z.-G. Qian, Biomacromolecules, 2019, 20, 3283–3293 CrossRef CAS PubMed.
- Z. Wang, N. Yi, Z. Zheng, J. Zhou, P. Zhou, C. Zheng, H. Chen, G. Shen and M. Weng, Chem. Eng. J., 2024, 497, 154443 CrossRef CAS.
- Z. Yang, T. Huang, P. Cao, Y. Cui, J. Nie, T. Chen, H. Yang, F. Wang and L. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 18110–18119 CrossRef CAS PubMed.
- Y. Lu, Z. Liu, H. Yan, Q. Peng, R. Wang, M. E. Barkey, J. W. Jeon and E. K. Wujcik, ACS Appl. Mater. Interfaces, 2019, 11, 20453–20464 CrossRef CAS.
- J.-W. J. Yang Lu and E. K. Wujcik, Piezoelectric ultra-stretchable strain sensor with excellent linearity and unique self-healing ability, IEEE Xplore, 2019 Search PubMed.
- E. K. Wujcik, J.-W. Jeon and Y. Lu, Self-healing and Stretchable Polymeric Compositions, US Pat., 11915838, 2021 Search PubMed.
- A. Samir, F. H. Ashour, A. A. Hakim and M. Bassyouni, npj Mater. Degrad., 2022, 6, 68 CrossRef CAS.
- C. Li, C. Guo, V. Fitzpatrick, A. Ibrahim, M. J. Zwierstra, P. Hanna, A. Lechtig, A. Nazarian, S. J. Lin and D. L. Kaplan, Nat. Rev. Mater., 2020, 5, 61–81 CrossRef.
- E. Rudnik, Compostable polymer materials, Newnes, 2019 Search PubMed.
- A. Rahimi and J. M. García, Nat. Rev. Chem, 2017, 1, 0046 CrossRef.
- X. Tao, S. Liao and Y. Wang, EcoMat, 2021, 3, e12083 CrossRef CAS.
- K. K. Fu, Z. Wang, J. Dai, M. Carter and L. Hu, Chem. Mater., 2016, 28, 3527–3539 CrossRef CAS.
- J. C. Yeo and C. T. Lim, et al. , Microsyst. Nanoeng., 2016, 2, 1–19 Search PubMed.
- Y. Guo, S. Chen, L. Sun, L. Yang, L. Zhang, J. Lou and Z. You, Adv. Funct. Mater., 2021, 31, 2009799 CrossRef CAS.
- C. N. Monty, E. K. Wujcik and N. J. Blasdel, Flexible Electrode for Detecting Changes in Temperature, Humidity, and Sodium Ion Concentration in Sweat, US Pat., US9603560B2, 2017 Search PubMed.
- S. S. Hassan, A. H. Kamel and M. A. Fathy, Talanta, 2023, 253, 123907 CrossRef CAS.
- A. Santhosh, S. Sandeep, D. J. Bound, S. Nandini, S. Nalini, G. Suresh, N. K. Swamy, J. R. Rajabathar and A. Selvaraj, Surf. Interfaces, 2021, 26, 101377 CrossRef CAS.
- C. X. Zhan, G. Q. Yu, Y. Lu, L. Y. Wang, E. Wujcik and S. Y. Wei, Polym. Sci., Ser. C, 2017, 5, 1569–1585 CAS.
- Z. A. Boeva and V. G. Sergeyev, J. Mater. Chem. C, 2014, 56, 144–153 CAS.
- Y. Lu, Z. Liu, H. Yan, Q. Peng, R. Wang, M. E. Barkey, J.-W. Jeon and E. K. Wujcik, ACS Appl. Mater. Interfaces, 2019, 11, 20453–20464 CrossRef CAS.
- S. Zheng, R. Du, N. Wang, M. Cao, Y. Zhang, Y. Jiang, Z. Liu, W. Yang, M. Yang and X. Xia, Composites, Part A, 2021, 151, 106649 CrossRef CAS.
- S. Veeralingam and S. Badhulika, ACS Appl. Bio Mater., 2021, 4, 14–23 CrossRef CAS PubMed.
- J. Lu, S. Hu, W. Li, X. Wang, X. Mo, X. Gong, H. Liu, W. Luo, W. Dong, C. Sima, Y. Wang, G. Yang, J.-T. Luo, S. Jiang, Z. Shi and G. Zhang, ACS Nano, 2022, 16, 3744–3755 CrossRef CAS PubMed.
- J. Huang, M. Zhao, Y. Cai, M. Zimniewska, D. Li and Q. Wei, Adv. Electron. Mater., 2020, 6, 1900934 CrossRef CAS.
- Y. Guo, M. Zhong, Z. Fang, P. Wan and G. Yu, Nano Lett., 2019, 19, 1143–1150 CrossRef CAS PubMed.
- H. Pan, G. Chen, Y. Chen, A. Di Carlo, M. A. Mayer, S. Shen, C. Chen, W. Li, S. Subramaniam and H. Huang, et al. , Biosens. Bioelectron., 2023, 222, 114999 CrossRef CAS.
- C. Gao, Y. Liu, F. Gu, Z. Chen, Z. Su, H. Du, D. Xu, K. Liu and W. Xu, Chem. Eng. J., 2023, 460, 141769 CrossRef CAS.
- Z. Liu, C. Li, X. Zhang, B. Zhou, S. Wen, Y. Zhou, S. Chen, L. Jiang, S. Jerrams and F. Zhou, ACS Sustainable Chem. Eng., 2022, 10, 8788–8798 CrossRef CAS.
- L. Liu, Z. Jiao, J. Zhang, Y. Wang, C. Zhang, X. Meng, X. Jiang, S. Niu, Z. Han and L. Ren, ACS Appl. Mater. Interfaces, 2021, 13, 1967–1978 CrossRef CAS.
- J. Huang, G. Xie, Q. Wei, Y. Su, X. Xu and Y. Jiang, ACS Appl. Mater. Interfaces, 2023, 15, 5600–5607 CrossRef CAS.
- M. Liao, H. Liao, J. Ye, P. Wan and L. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 47358–47364 CrossRef CAS.
- L. Yang, H. Wang, W. Yuan, Y. Li, P. Gao, N. Tiwari, X. Chen, Z. Wang, G. Niu and H. Cheng, ACS Appl. Mater. Interfaces, 2021, 13, 60531–60543 CrossRef CAS PubMed.
- M. A. U. Khalid, M. Ali, A. M. Soomro, S. W. Kim, H. B. Kim, B.-G. Lee and K. H. Choi, Sens. Actuators, A, 2019, 294, 140–147 CrossRef.
- L. Sun, F. Wang, J. Jiang, H. Liu, B. Du, M. Li, Y. Liu and M. Li, Cellulose, 2020, 27, 8923–8935 CrossRef CAS.
- L. Gao, C. Zhu, L. Li, C. Zhang, J. Liu, H.-D. Yu and W. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 25034–25042 CrossRef CAS PubMed.
- N. Sakhuja, R. Kumar, P. Katare and N. Bhat, ACS Sustainable Chem. Eng., 2022, 10, 9697–9706 CrossRef CAS.
- E. S. Hosseini, L. Manjakkal, D. Shakthivel and R. Dahiya, ACS Appl. Mater. Interfaces, 2020, 12, 9008–9016 CrossRef CAS.
- J. Huang, G. Xie, X. Xu, Z. Geng and Y. Su, ACS Appl. Mater. Interfaces, 2024, 16, 58838–58847 CrossRef CAS PubMed.
- C. Du, H. Zhang, X. Liu, S. Zhou, Y. Ma, S. Li and Y. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 12996–13005 CrossRef CAS.
- R. Scaffaro, A. Maio, G. L. Re, A. Parisi and A. Busacca, Compos. Sci. Technol., 2018, 156, 166–176 CrossRef CAS.
- V. Sencadas, C. Tawk and G. Alici, ACS Appl. Mater. Interfaces, 2020, 12, 8761–8772 CrossRef CAS.
- S. Sun, R. Yuan, S. Ling, T. Zhou, Z. Wu, M. Fu, H. He, X. Li and C. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 7826–7837 CrossRef CAS PubMed.
- H. Wei, D. Kong, T. Li, Q. Xue, S. Wang, D. Cui, Y. Huang, L. Wang, S. Hu and T. Wan, et al. , ACS Sens., 2021, 6, 2938–2951 CrossRef CAS PubMed.
- H. Kim, A. Shaqeel, S. Han, J. Kang, J. Yun, M. Lee, S. Lee, J. Kim, S. Noh and M. Choi, et al. , ACS Appl. Mater. Interfaces, 2021, 13, 39868–39879 CrossRef CAS.
- H. Zhang, D. Zhang, B. Zhang, D. Wang and M. Tang, ACS Appl. Mater. Interfaces, 2022, 14, 48907–48916 CrossRef CAS.
- V. Kedambaimoole, K. Harsh, K. Rajanna, P. Sen, M. Nayak and S. Kumar, Mater. Adv., 2022, 3, 3784–3808 RSC.
- P. Liu, C. Ma, Y. Li, L. Wang, L. Wei, Y. Yan and F. Xie, ACS Sustainable Chem. Eng., 2020, 8, 19117–19128 CrossRef CAS.
- S. Awasthi, A. Srivastava, D. Kumar, S. K. Pandey, N. M. Mubarak, M. H. Dehghani and K. Ansari, Environ. Adv., 2024, 100601 CrossRef CAS.
- S. Zhang, Z. Zhou, J. Zhong, Z. Shi, Y. Mao and T. H. Tao, Adv. Sci., 2020, 7, 1903802 CrossRef CAS.
- J. Zhao, M. Haowei, A. Saberi, Z. Heydari and M. S. Baltatu, Coatings, 2022, 12, 1589 CrossRef CAS.
- M. Zhao, R. Su, L. Ji, Y. Zhang, H. Wu, Z. Wen and C. Dai, J. Nanomater., 2023, 2023, 5012576 CrossRef.
- S. Abazari, A. Shamsipur, H. Bakhsheshi-Rad, M. Keshavarz, M. Kehtari, S. Ramakrishna and F. Berto, J. Mater. Res. Technol., 2022, 20, 976–990 CrossRef CAS.
- A. Francis, S. Abdel-Gawad and M. Shoeib, J. Coat. Technol. Res., 2021, 18, 971–988 CrossRef CAS.
- G. Laroche, Y. Marois, R. Guidoin, M. W. King, L. Martin, T. How and Y. Douville, J. Biomed. Mater. Res., 1995, 29, 1525–1536 CrossRef CAS PubMed.
- Y. Yu, H. Sun, H. Orbay, F. Chen, C. G. England, W. Cai and X. Wang, Nano Energy, 2016, 27, 275–281 CrossRef CAS.
- S. Veeralingam and S. Badhulika, Mater. Res. Bull., 2022, 150, 111779 CrossRef CAS.
- C.-F. J. Kuo, H.-Y. Wang, A. Prasannan, J.-Y. Lai, J.-S. Wang, H.-M. Chang and H.-C. Tsai, Polym. Degrad. Stab., 2020, 172, 109058 CrossRef CAS.
- J. Huang, M. Zhao, Y. Cai, M. Zimniewska, D. Li and Q. Wei, Adv. Electron. Mater., 2020, 6, 1900934 CrossRef CAS.
- Y. Li, A. Veronica, J. Ma and H. Y. Y. Nyein, Adv. Mater., 2024, 2408456 CrossRef.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustain. Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- C. Lv, J. Wang, Z. Li, K. Zhao and J. Zheng, Composites, Part B, 2019, 177, 107270 CrossRef CAS.
- E. Bihar, S. Wustoni, A. M. Pappa, K. N. Salama, D. Baran and S. Inal, npj Flexible Electron., 2018, 2, 30 CrossRef.
- W. S. Lee and J. Choi, ACS Appl. Mater. Interfaces, 2019, 11, 19363–19371 CrossRef CAS PubMed.
- X. Lan, W. Li, C. Ye, L. Boetje, T. Pelras, F. Silvianti, Q. Chen, Y. Pei and K. Loos, ACS Appl. Mater. Interfaces, 2022, 15, 4398–4407 CrossRef.
- X. Ma, Q. Hu, Y. Dai, P. He and X. Zhang, Sens. Actuators, A, 2022, 346, 113834 CrossRef CAS.
- H. Nawaz, S. Chen, X. Zhang, X. Li, T. You, J. Zhang and F. Xu, ACS Nano, 2023, 17, 3996–4008 CrossRef CAS.
- B. L. Zhang, Y. Yang, Z. Q. Zhao and X. D. Guo, Electrochim. Acta, 2020, 358, 136917 CrossRef CAS.
- A. Molina, M. Al-Sardar, V. Rodriguez-Gonzalez, V. Escobar-Barrios, A. Zakhidov, A. Mtz-Enriquez, A. Encinas and J. Oliva, Synth. Met., 2022, 287, 117091 CrossRef CAS.
- J. H. Yoon, S.-M. Kim, Y. Eom, J. M. Koo, H.-W. Cho, T. J. Lee, K. G. Lee, H. J. Park, Y. K. Kim and H.-J. Yoo, et al. , ACS Appl. Mater. Interfaces, 2019, 11, 46165–46175 CrossRef CAS.
- Z. Lv, J. Liu, X. Yang, D. Fan, J. Cao, Y. Luo and X. Zhang, ACS Appl. Mater. Interfaces, 2020, 12(19), 22163–22169 CrossRef CAS PubMed.
- A. Ajeev, B. H. Javaregowda, A. Ali, M. Modak, S. Patil, S. Khatua, M. Ramadoss, P. A. Kothavade and A. K. Arulraj, Adv. Mater. Technol., 2020, 5, 2000690 CrossRef.
- X. Lan, W. Li, C. Ye, L. Boetje, T. Pelras, F. Silvianti, Q. Chen, Y. Pei and K. Loos, ACS Appl. Mater. Interfaces, 2023, 15, 4398–4407 CrossRef CAS.
- S. Zhang, H. Li, Z. Yang, B. Chen, K. Li, X. Lai and X. Zeng, J. Colloid Interface Sci., 2022, 626, 554–563 CrossRef CAS PubMed.
- Z. Yang, S. Zhang, Z. Chen, X. Lai, H. Li and X. Zeng, ACS Appl. Polym. Mater., 2023, 6, 905–914 CrossRef.
- Y. Zhu, Y. He, W. Lu, H. Tian, F. Fei, P. Zhou and J. Wang, J. Mater. Chem. A, 2024, 12, 28716–28730 RSC.
- P. G. Reddy, V. Sharma, V. S. Parihar, I. Haider, A. Barua, A. Koivikko, K. Yiannacou, H. Jongprasitkul, M. Kellomäki and V. Sariola, Adv. Eng. Mater., 2024, 26, 2401704 CrossRef CAS.
- V. Gupta, R. Malik and L. Kumar, Mater. Chem. Phys., 2023, 310, 128388 CrossRef CAS.
- S. Paneru, Sweety and D. Kumar, J. Appl. Electrochem., 2023, 53(11), 2229–2238 CrossRef CAS.
- D. Das, J. Das, A. Debnath, S. Chakraborty and B. Saha, Cellulose, 2023, 30, 6423–6433 CrossRef CAS.
- D. Davis, S. K. Narayanan, A. Ajeev, J. Nair, J. Jeeji, A. Vijayan, M. Viyyur Kuttyadi, A. Nelliparambil Sathian and A. K. Arulraj, ACS Appl. Mater. Interfaces, 2023, 15, 25734–25743 CrossRef CAS PubMed.
- J. M. Henrique, J. R. Camargo, G. G. de Oliveira, J. S. Stefano and B. C. Janegitz, Microchem. J., 2021, 170, 106701 CrossRef.
- N. O. Gomes, R. T. Paschoalin, S. Bilatto, A. R. Sorigotti, C. S. Farinas, L. H. C. Mattoso, S. A. Machado, O. N. Oliveira Jr and P. A. Raymundo-Pereira, ACS Sustain. Chem. Eng., 2023, 11, 2209–2218 CrossRef CAS.
- A. Molina, A. Oliva, A. Zakhidov, E. Valadez-Renteria, V. Rodriguez-Gonzalez, A. Encinas and J. Oliva, J. Alloys Compd., 2022, 903, 163896 CrossRef CAS.
- G.-J. Ko, S. D. Han, J.-K. Kim, J. Zhu, W. B. Han, J. Chung, S. M. Yang, H. Cheng, D.-H. Kim and C.-Y. Kang, et al. , NPG Asia Mater., 2020, 12, 71 CrossRef CAS.
- S. Li, W. Chen, Y. Kou, S. Wang, D. Hu, X. Ji and Z. Lu, Cellulose, 2025, 1–21 Search PubMed.
- Y. Liu, H. Li, Q. Feng, H. Su, D. Li, Y. Shang, H. Chen, B. Li and H. Dong, ACS Omega, 2022, 7, 9834–9845 CrossRef CAS PubMed.
- K. Sakabe, T. Kan and H. Onoe, Adv. Mater. Technol., 2024, 9, 2400038 CrossRef CAS.
- Z. Janićijević, T. Huang, D. I. S. Bojórquez, T. H. Tonmoy, S. Pané, D. Makarov and L. Baraban, Adv. Sci., 2024, 11(20), 2307232 CrossRef.
- M. Nezafati, N. Salimiyan, S. Salighehdar, R. Sedghi, A. Dolatshahi-Pirouz and Y. Mao, Chem. Eng. J., 2025, 509, 161112 CrossRef CAS.
- Y. Guihua, H. Shuaiming, C. Gaofeng, M. Sen, Z. Anqi, C. Binglin, Y. Shuliang, T. Xing, S. Yong, X. Feng, L. Lin and Z. Xianhai, Nano-Micro Lett., 2022, 14(1), 84 CrossRef.
- C. M. Boutry, Y. Kaizawa, B. C. Schroeder, A. Chortos, A. Legrand, Z. Wang, J. Chang, P. Fox and Z. Bao, Nat. Electron., 2018, 1, 314–321 CrossRef.
- S.-W. Hwang, H. Tao, D.-H. Kim, H. Cheng, J.-K. Song, E. Rill, M. A. Brenckle, B. Panilaitis, S. M. Won, Y.-S. Kim, Y. M. Song, K. J. Yu, A. Ameen, R. Li, Y. Su, M. Yang, D. L. Kaplan, M. R. Zakin, M. J. Slepian, Y. Huang, F. G. Omenetto and J. A. Rogers, Science, 2012, 337, 1640–1644 CrossRef CAS PubMed.
- R. Zeng, W. Dietzel, F. Witte, N. Hort and C. Blawert, Adv. Eng. Mater., 2008, 10(8), B3–B14 CrossRef CAS.
- S. Feng, Z. Tian, J. Wang, S. Cao and D. Kong, Adv. Electron. Mater., 2019, 5(3), 1800693 CrossRef.
- C. M. Boutry, L. Beker, Y. Kaizawa, C. Vassos, H. Tran, A. C. Hinckley, R. Pfattner, S. Niu, J. Li, J. Claverie, Z. Wang, J. Chang, P. M. Fox and Z. Bao, Nat. Biomed. Eng., 2019, 3, 47–57 CrossRef CAS PubMed.
- X. Meng and V. K. Yadavalli, Flexible biosensors for the impedimetric detection of protein targets using silk-conductive polymer biocomposites, ACS Sens., 2019, 4(4), 1040–1047 CrossRef.
- X. Peng, K. Dong, Z. Wu, J. Wang and Z. L. Wang, J. Mater. Sci., 2021, 56, 16765–16789 CrossRef CAS.
- S.-K. Kang, R. K. J. Murphy, S.-W. Hwang, S. M. Lee, D. V. Harburg, N. A. Krueger, J. Shin, P. Gamble, H. Cheng, S. Yu, Z. Liu, J. G. McCall, M. Stephen, H. Ying, J. Kim, G. Park, R. C. Webb, C. H. Lee, S. Chung, D. S. Wie, A. D. Gujar, B. Vemulapalli, A. H. Kim, K.-M. Lee, J. Cheng, Y. Huang, S. H. Lee, P. V. Braun, W. Z. Ray and J. A. Rogers, Nature, 2016, 530, 71–76 CrossRef CAS.
- A. Nag, S. Nuthalapati and S. C. Mukhopadhyay, IEEE Sens. J., 2022, 22, 10235–10245 CAS.
- H. Kudo, T. Sawada, E. Kazawa, H. Yoshida, Y. Iwasaki and K. Mitsubayashi, Biosens. Bioelectron., 2006, 22, 558–562 CrossRef CAS.
- C. Pang, C. Lee and K.-Y. Suh, J. Appl. Polym. Sci., 2013, 130, 1429–1441 CrossRef CAS.
- Y. Wang, A. Liu, Y. Han and T. Li, Polym. Int., 2020, 69, 7–17 CrossRef CAS.
- M. Dulal, S. Afroj, J. Ahn, Y. Cho, C. Carr, I.-D. Kim and N. Karim, ACS Nano, 2022, 16, 19755–19788 CrossRef CAS PubMed.
- H. Park, S. Kim, J. Lee, I. Lee, S. Bontapalle, Y. Na and K. Sim, Nat. Electron., 2024, 7, 39–50 CrossRef CAS.
- L. M. Koh and S. M. Khor, Anal. Chim. Acta, 2022, 1217, 339989 CrossRef PubMed.
- J. A. Chiong, H. Tran, Y. Lin, Y. Zheng and Z. Bao, Adv. Sci., 2021, 8, 2101233 CrossRef CAS PubMed.
- T. A. Baldo, L. F. de Lima, L. F. Mendes, W. R. de Araujo, T. R. Paixao and W. K. Coltro, ACS Appl. Electron. Mater., 2020, 3, 68–100 CrossRef.
- B. Piro, H. V. Tran and V. T. Thu, Sensors, 2020, 20, 5898 CrossRef CAS PubMed.
- J. Min, Y. Jung, J. Ahn, J. G. Lee, J. Lee and S. H. Ko, Adv. Mater., 2023, 35, 2211273 CrossRef CAS.
- O. Y. Kweon, S. J. Lee and J. H. Oh, NPG Asia Mater., 2018, 10, 540–551 CrossRef CAS.
- A. B. Singh, C. Khandelwal and G. S. Dangayach, Polymer-Plastics Technology and Materials, 2024, pp. 1–37 Search PubMed.
- T. SINGH and T. MOHAPATRA, Role of Emerging Technologies in Social Science, 2024, p. 156 Search PubMed.
- J. M. García, Sensory Polymers, 2024, pp. 803–828 Search PubMed.
- S. Xu and W. Wu, Adv. Intell. Syst., 2020, 2, 2000117 CrossRef.
- J. R. Camargo, L. O. Orzari, D. A. G. Araujo, P. R. de Oliveira, C. Kalinke, D. P. Rocha, A. L. dos Santos, R. M. Takeuchi, R. A. A. Munoz and J. A. Bonacin, et al. , Microchem. J., 2021, 164, 105998 CrossRef CAS.
- Y. Htwe and M. Mariatti, J. Sci.:Adv. Mater. Devices, 2022, 7, 100435 CAS.
- C. Cui, Q. Fu, L. Meng, S. Hao, R. Dai and J. Yang, ACS Appl. Bio Mater., 2020, 4, 85–121 CrossRef.
- M. Sikka, J. Bioact. Compat Polym., 2024, 08839115241279867 Search PubMed.
- Y. Long, M. Bai, X. Liu, W. Lu, C. Zhong, S. Tian, S. Xu, Y. Ma, Y. Tian and H. Zhang, et al. , Carbohydr. Polym., 2022, 297, 119974 CrossRef CAS.
- Z. Wang, Z. Ma, S. Wang, M. Pi, X. Wang, M. Li, H. Lu, W. Cui and R. Ran, Carbohydr. Polym., 2022, 298, 120128 CrossRef CAS PubMed.
- R. Prajapati, K. Kohli, S. K. Maity and B. K. Sharma, Recent Developments in Plastic Recycling, 2021, pp. 15–41 Search PubMed.
- S. Lanzalaco, G. Fabregat, H. Muñoz-Galan, J. Cabrera, X. Munoz-Pascual, J. Llorca and C. Aleman, ACS Sustain. Chem. Eng., 2020, 8, 12554–12560 CrossRef CAS.
- D. J. da Silva and H. Wiebeck, Prog. Rubber Plast. Recycl. Technol., 2020, 36, 284–303 CrossRef.
- K. Makenji, Management, recycling and reuse of waste composites, Elsevier, 2010, pp. 217–252 Search PubMed.
- A. R. Prado, A. G. Leal-Junior, C. Marques, S. Leite, G. L. De Sena, L. C. Machado, A. Frizera, M. R. Ribeiro and M. J. Pontes, Opt. Express, 2017, 25, 30051–30060 CrossRef CAS PubMed.
- J. Lee, D. Kim, H.-Y. Ryoo and B.-S. Shin, Sustainability, 2016, 8, 466 CrossRef.
- V. Chaudhary, P. Gaur and S. Rustagi, Sustainable Mater. Technol., 2024, e00952 CrossRef.
- A. Habibipour, A. M. Padyab and A. Ståhlbröst, AMCIS 2019, Twenty-fifth Americas Conference on Information Systems, Cancun, México, Augusti 15-17, 2019, 2019 Search PubMed.
- F. Gu, J. Guo, P. Hall and X. Gu, Int. J. Prod. Res., 2019, 57, 1458–1477 CrossRef.
- S. H. Leclerc, PhD thesis, McGill University, Canada, 2024.
- C. for Devices and R. Health, Classify your medical device, 2020, https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device.
- N. P. Tipnis and D. J. Burgess, Int. J. Pharm., 2018, 544, 455–460 CrossRef CAS.
- U. Food and D. Administration, 2020, https://www.fda.gov/media/74445/download.
- G. C. Mendes, T. R. Brandão and C. L. Silva, Am. J. Infect. Control, 2007, 35, 574–581 CrossRef PubMed.
- L. Kuo, B. J. Luijten, S. Li, A. C. M. de Moraes, A. J. Silvaroli, S. G. Wallace, J. Hui, J. R. Downing, K. R. Shull and M. C. Hersam, ACS Appl. Mater. Interfaces, 2022, 14, 53241–53249 CrossRef CAS PubMed.
- A. K. Persons, J. E. Ball, C. Freeman, D. M. Macias, C. L. Simpson, B. K. Smith and R. F. Burch V, Materials, 2021, 14, 4070 CrossRef CAS.
- C. S. Boland, ACS Appl. Polym. Mater., 2020, 2, 3474–3480 CrossRef CAS.
- S. Das, C. Liang and J. B. Dunn, Circular Economy of Polymers: Topics in Recycling Technologies, ACS Publications, 2021, pp. 143–170 Search PubMed.
- A. O. Chinomso Iroegbu and S. S. Ray, ACS Omega, 2022, 7, 10854–10863 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


