Open Access Article
Open Access ArticleTandem reaction-powered near-infrared fluorescent molecular reporter for real-time imaging of lung diseases†
Yan
Hu‡
a,
Hongshuai
Zhang‡
a,
Yiteng
Ding‡
a,
Weirui
Chen
a,
Changqie
Pan
*e,
Longwei
He
 *bc,
Dan
Cheng
*bc,
Dan
Cheng
 *ac and
Lin
Yuan
*ac and
Lin
Yuan
 d
d
aDepartment of Gastroenterology, The Affiliated Nanhua Hospital, Hengyang Medical School, University of South China, Hengyang 421002, Hunan, China. E-mail: flychand0110@163.com
bMOE Key Lab of Rare Pediatric Diseases, Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study, Department of Pharmacy and Pharmacology, Hengyang Medical School, University of South China, Hengyang, China. E-mail: helongwei0110@163.com
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, P. R. China
dState Key Laboratory of Chemo and Biosensing, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China
eDepartment of Thoracic Oncology, Hunan Cancer Hospital/The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, 410013, China. E-mail: panchangqie@hnca.org.cn
First published on 24th April 2025
Abstract
Diabetes and its complications have drawn growing research attention due to their detrimental effects on human health. Although optical probes have been used to help understand many aspects of diabetes, the lung diseases caused by diabetes remain unclear and have rarely been explored. Herein, a tandem-reaction (TR) strategy is proposed based on the adjacent diol esterification-crosslinking reaction and the nicotinamide reduction reaction of nicotinamide adenine dinucleotide (NADH) to design a lung-targeting near-infrared (NIR) small molecule probe (NBON) for accurate imaging of diabetic lung diseases. NBON was designed by coupling a phenylboronic acid analog that can form borate ester bonds by reversibly binding with NADH via an esterification-crosslinking reaction. Streptozotocin (STZ)-induced diabetic mice and metformin (MET)/epalrestat (EPS)-repaired model studies demonstrated that NBON allowed the sensitive imaging of NADH for lung disease diagnosis and therapeutic monitoring. The proposed antioxidant mechanism by which EPS alleviates diabetic lung disease was studied for the first time in living cells and in vivo. Furthermore, NBON was successfully applied in the detection of NADH in tumors and lung metastases. Overall, this work provides a general platform for a NIR NADH probe design, and advances the development of NADH probes for mechanistic studies in lung diseases.
Introduction
Diabetes mellitus is a metabolic disorder that is characterized by chronic hyperglycemia and which leads to multiple systemic diseases, rendering it one of the main causes of human death.1,2 Indeed, the probabilities of premature death and functional disability are known to be higher in diabetic patients than in healthy individuals. The pathophysiological process of diabetes involves multiple organs, such as the liver and the kidneys.3 In the 1970s, Schuyler et al. first proposed that the lungs may be one of the target organs of diabetic injury.4 Since patients with diabetes are often in a state of hyperglycemia for long periods of time, a series of changes can be induced in the lungs, causing various lung diseases, such as pneumonia, pulmonary interstitial fibrosis, and lung cancer, among others.5,6 Thus, the growing incidence rates of diabetes in recent decades poses a significant threat to human health.Diabetes originates from glucose metabolism disorders and is characterized by sustained high blood sugar levels, which can lead to glucose toxicity. Nicotinamide adenine dinucleotide (NADH) is the most important coenzyme in cells, and is involved in substance and energy metabolism.7 Because glucose is one of the main sources of NADH, its accumulation often leads to the excessive production of NADH, resulting in an intracellular NAD+/NADH redox imbalance. During the entry of upregulated NADH electrons into the electron transfer chain, electrons may leak and bind to oxygen, producing a large amount of reactive oxygen species (ROS), thereby inducing oxidative stress, and ultimately leading to cell death.8 Despite this knowledge, the specific regulatory mechanism of NADH in diabetes-induced lung diseases and the relationship between NADH levels and lung injury remain controversial. One of the main obstacles hindering the study of these molecular events is a lack of precise methods for the non-invasive detection of NADH concentration changes in cells, tissues, and in vivo. It is therefore desirable to construct a highly sensitive and selective fluorescent reporter to monitor NADH levels in diabetes-induced lung disease in vivo.
In recent years, with the rapid development of molecular imaging technologies, optical probes have attracted increasing attention owing to their high sensitivities, high spatial resolutions, rapid analysis, and noninvasive nature.9–24 Consequently, they have become a powerful tool for the real-time monitoring and imaging of diabetes and lung diseases. To date, a number of NADH probes have been reported.25–31 For example, Chang et al. developed a highly sensitive NADH probe inspired by enzyme-catalyzed reactions to detect NADH in living cells.25 In addition, Kim et al. selected a rhodamine dye as the fluorescent group and constructed an NADH probe using the reducing ability of NAD(P)H to detect NAD(P)H during cellular hypoxia.28 Furthermore, Yin et al. designed an NADH fluorescent probe using rhodamine dye to distinguish p53-abnormal tumors from normal tumors.30 Although there has been some progress in the detection of NADH in cells using existing molecular fluorescent probes, the majority of advances have been achieved in situations where the concentration of NADH changes dramatically owing to human stimulation, rather than reflecting true fluctuations during pathological processes in living cells. As a result, these developed probes cannot provide accurate information regarding intracellular molecular events. In addition, the imaging wavelengths of most probes are relatively short, and significant improvements are required in terms of their sensitivities. Furthermore, considering that the lungs are located deep within the human body, issues related to probe targeting, spontaneous fluorescence interference and the imaging signal-to-noise (S/N) ratio must be addressed to successfully conduct noninvasive optical imaging in vivo. Owing to the ability of near-infrared (NIR) fluorescence imaging to detect the light emission of fluorescent dyes in the range of 650–900 nm, the spontaneous fluorescence of biological tissues in this range is significantly reduced, resulting in reduced light scattering.32–47 Therefore, the development of NIR NADH fluorescence probes would be expected to permit the non-invasive in vivo imaging and detection of diabetic lung diseases with a high S/N ratio and a high sensitivity, thereby enhancing the accuracy and reliability of analysis and detection.
Herein, a tandem-reaction (TR) strategy is developed based on the adjacent diol esterification-crosslinking reaction and nicotinamide reduction reaction of NADH by introducing both an NADH recognition group and an NADH crosslinking group on the probe. This method is aimed at improving the probe sensitivity, whilst also promoting its specific targeting and accumulation in the lungs. A NIR semi-cyanine dye derivative is selected as the fluorescent reporting group, and hydroxyl protection and deprotection strategies are employed on the probe to achieve fluorescence regulation with the aim of constructing a highly sensitive lung-targeted NIR NADH probe (NBON). Subsequently, the developed probe is applied in the detection of endogenous NADH changes in cells, and to visualize trace fluctuations of the NADH levels in cells under high glucose stimulation conditions. The ability of the probe to visualize changes in the NADH levels in streptozotocin-induced diabetic lung diseases and in a metformin (MET)/epalrestat (EPS) repair model in vivo is also evaluated. Furthermore, antioxidant mechanism by which EPS alleviates diabetic lung disease is examined in vivo. Moreover, the developed probe is employed to sensitively detect changes in the NADH levels in lung tumors and metastases.
Results and discussion
Design and synthesis of NBON probe
NADH is a reducing agent composed of adenine, sugars (ortho-dihydroxyl compounds), phosphate linkers, and niacinamide (Fig. S1†). Its reduction mainly originates from the niacinamide site, which is often employed as the reactive attack site for NADH probes. However, the sensitivities of traditional NADH probes are often limited (Scheme 1A), and so an alternative approach should be considered. In this context, NADH contains an adjacent diol structure which is prone to reactions with phenylboronic acids. Inspired by this reaction, a TR strategy was considered for the design of a novel NADH probe (Scheme 1B). More specifically, a group containing a phenylboronic acid analog was initially attached onto the probe molecule to promote an esterification-crosslinking reaction with adjacent diols on NADH (Reaction 1). Subsequently, the recognition group on the probe molecule was modified for reduction by NADH, rendering it highly reactive (Reaction 2). Based on the above design idea, a highly stable semi-cyanine NIR dye derivative with a tunable hydroxy group was selected as the fluorescence reporting unit (CS), which exhibits a high fluorescence quantum yield and long absorption and emission wavelengths in the NIR region (λabs/λem = 700/721 nm).48 Consequently, changes in the fluorescence signal can be regulated by protection and deprotection of the hydroxyl group. For this molecular structure, a long-chain phenylboronic acid binding group was introduced (NBCS) to impart the compound with a good hydrophilicity and promote effective circulation in the blood and accumulation in lungs.49 Furthermore, compared to commercial MitoTracker Deep Red (Fig. S2 and S3†), NBCS exhibits a good photostability in solution and in cells, which is beneficial for biological imaging of the lung in vivo. For the NADH recognition group, a quinoline salt derivative was selected, and the target NBON was prepared (Scheme 1C). A control compound without the modified boronic acid binding group was also synthesized (NCCN), as outlined in Schemes 1C and S1.† Compounds were characterized by nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry and high-performance liquid chromatography (HPLC), as detailed in the ESI.†Optical properties of fluorescent probes toward NADH
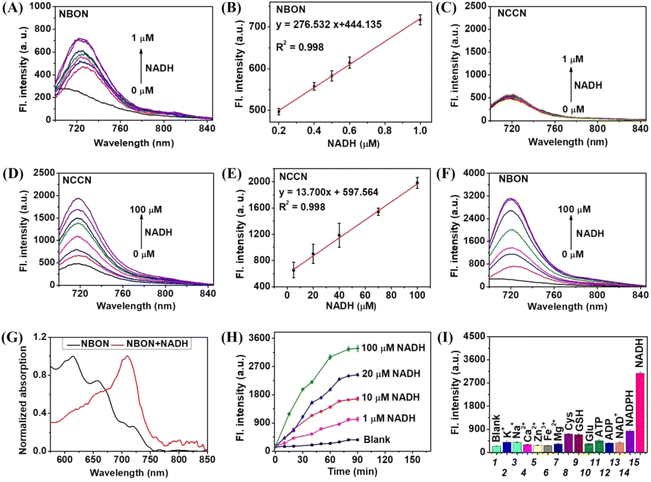
Initially, the feasibility of NBON to detect NADH in a buffer solution (phosphate buffered saline (PBS)/ethanol (EtOH) = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, pH 7.4) was investigated. As shown in Fig. 1A, the fluorescence spectrum indicated that NBON exhibited a sensitive response toward NADH (0–1 μM), with a 45 nM detection limit (Fig. 1B). In contrast, the control compound NCCN, which did not contain the long-chain phenylboronic acid binding group, showed no obvious signal changes in the presence of NADH (0–1 μM) under comparable conditions (Fig. 1C). It was speculated that this may have been caused by the low sensitivity of NCCN toward NADH. Indeed, in the presence of a higher NADH concentration (0–100 μM) (Fig. 1D), a relatively poor fluorescence enhancement was observed (Fig. S4†), and the detection limit was determined to be 2.5 μM (Fig. 1E), confirming the low sensitivity of this system. Thus, the detection of NADH by NBON was further evaluated upon increasing the NADH concentration to 100 μM. Notably, an apparent enhanced NIR signal (λem = 721 nm) was captured with a 12-fold increase in fluorescence intensity (Fig. 1F), while the absorption peak at 615/657 nm was red-shifted to 669/709 nm in response to NADH (Fig. 1G). These differences were attributed to the superior sensitivity of the NBON probe toward NADH (Table S1†), which in turn was caused by the presence of a phenylboronic acid binding group enabling a rapid response.
3, pH 7.4) was investigated. As shown in Fig. 1A, the fluorescence spectrum indicated that NBON exhibited a sensitive response toward NADH (0–1 μM), with a 45 nM detection limit (Fig. 1B). In contrast, the control compound NCCN, which did not contain the long-chain phenylboronic acid binding group, showed no obvious signal changes in the presence of NADH (0–1 μM) under comparable conditions (Fig. 1C). It was speculated that this may have been caused by the low sensitivity of NCCN toward NADH. Indeed, in the presence of a higher NADH concentration (0–100 μM) (Fig. 1D), a relatively poor fluorescence enhancement was observed (Fig. S4†), and the detection limit was determined to be 2.5 μM (Fig. 1E), confirming the low sensitivity of this system. Thus, the detection of NADH by NBON was further evaluated upon increasing the NADH concentration to 100 μM. Notably, an apparent enhanced NIR signal (λem = 721 nm) was captured with a 12-fold increase in fluorescence intensity (Fig. 1F), while the absorption peak at 615/657 nm was red-shifted to 669/709 nm in response to NADH (Fig. 1G). These differences were attributed to the superior sensitivity of the NBON probe toward NADH (Table S1†), which in turn was caused by the presence of a phenylboronic acid binding group enabling a rapid response.
To further verify the reaction mechanism between NBON and NADH, high-performance liquid chromatography (HPLC)-mass spectrometry (MS) and mass spectrometry were performed. As shown in the HPLC-MS results presented in Fig. S5,†NBON (m/z 924.4, [M]+) exhibited a single sharp characteristic peak with a retention time of 8.60 min. However, upon the addition of NADH, a new peak appeared with a retention time of 10.41 min, which was confirmed to originate from the expected product NBCS (m/z 767.3, [M−H]−) = 767.3. A compound I (m/z 1572.45, [M]+) was also detected in the reaction mixture by mass spectrometry, which likely originated from the NADH response intermediate (Fig. S6†). The absorption spectra further supported the above results (Fig. S7†), thereby confirming the complete conversion of NBON to NBCS upon the reduction of NADH. This is consistent with the proposed reaction mechanism, and supports the design idea that NBON efficiently reacts with NADH via a TR strategy (Scheme S2†).
Subsequently, the fluorescence kinetic curves were recorded for NBON upon incubation with NADH (0–100 μM) for different incubation times (0–90 min). No apparent signal changes were observed in the absence of NADH (Fig. 1H); however, the fluorescence intensity of NBON increased rapidly after incubation with different NADH concentrations, and reached a plateau after 60 min, thereby suggesting that NBON can sensitively and rapidly detect changes in the NADH concentration. Furthermore, the selectivity of NBON toward NADH was investigated in the presence of various ions (Ca2+, K+, Na+, Zn2+, Fe2+, and Mg2+), reactive species (Cysteine (Cys) and glutathione (GSH)), and biomolecules (Glucose (Glu), adenosine triphosphate (ATP), adenosine diphosphate (ADP), nicotinamide adenine dinucleotide (NAD+), and nicotinamide adenine dinucleotide phosphate (NADPH)). As shown in Fig. 1I, none of the other species responded to NBON, indicating the excellent selectivity of NBON toward NADH. In addition, it possessed a good photostability (Fig. S8†), and it was observed that NBON could successfully monitor NADH between pH 6.0 and 8.0 (Fig. S9†) upon variation in the solution pH, thereby confirming that NBON is a suitable agent for recognizing NADH in complex systems.
Fluorescence imaging of NADH in living cells using the NBON probe
Inspired by the above experiments performed using a buffer solution, the ability of NBON to detect NADH was evaluated in human lung cancer A549 cells overexpressing NADH. Initially, a MTT assay was conducted for 24 h using different NBON concentrations to confirm the low cytotoxicity of NBON toward the living cells (Fig. S10†). As shown in Fig. 2A, after incubation of NBON with the A549 cells for 60 min, the fluorescence signal gradually increased in intensity, reaching a maximum value at ∼40 min. This bright fluorescence signal demonstrated that NBON was activated by the overexpression of NADH in the A549 cells. Images were also obtained to detect exogenous NADH (0–3 mM) (Fig. 2B and C), further confirming that NBON can be sensitively activated by exogenous NADH during live-cell imaging.Subsequently, the cellular distribution of NBON was investigated in A549 cells using the commercial agents MitoTracker Green and LysoTracker Green (Fig. 2D). Co-localization imaging results revealed enhanced NBON localization in the mitochondria (Pearson correlation coefficient = 0.94) compared to in the lysosomes (Pearson correlation coefficient = 0.78), likely due to the electrostatic interactions between the positively charged NBON and the negative potential of the inner mitochondrial membrane. These results therefore suggest that NBON was mainly located in mitochondria.
It is well known that the pyruvate/lactate imbalance greatly influences NADH levels in living cells,50 wherein lactate dehydrogenase is able to efficiently catalyze the oxidation of NADH by transporting two electrons from NADH to pyruvate for the generation of lactate. Therefore, changes in NADH levels were investigated during pyruvate/lactate by using NBON. As shown in Fig. 3A and B, a considerably increased fluorescence signal (∼1.4-fold) was observed upon the incubation of A549 cells with exogenous lactate. In contrast, pyruvate-treated cells exhibited a large fluorescence reduction (Fig. 3B and S11†), suggesting that NBON can be used to detect changes in the endogenous NADH levels caused by the pyruvate/lactate imbalance in living cells.
Changes in NADH levels were subsequently evaluated in the respiratory cascade, considering the important role of NADH in mitochondrial oxidative phosphorylation during rotenone stimulation (a known respiratory complex I inhibitor). Notably, an increased fluorescence was achieved with different concentrations of rotenone (Fig. S12†), indicating that the NADH levels increased during the oxidation of complex I in the mitochondrial respiratory chain. The experiment was also performed using carbonyl cyanide chlorophenylhydrazone (CCCP)-treated cells. The uncoupler, CCCP, affects ATP synthesis by increasing the proton permeability across the inner mitochondrial membrane. The fluorescence images show an obvious decrease in the fluorescence intensity of NBON in the CCCP dose-dependent experiment (Fig. S13†), likely due to the reduction of NADH under these conditions. Moreover, the existence of crosstalk was evaluated between the redox regulators GSH and NADH.51 Consequently, a reduced fluorescence intensity was observed for NBON in the presence of high GSH concentrations (Fig. S14†), and this was attributed to the decrease in endogenous NADH levels, ultimately suggesting a close connection between the redox balance and NADH.
Fluorescence imaging of NADH in the glucose-stimulated cells
The above observations inspired the use of NBON to detect NADH in a diabetic disease environment. For this purpose, high glucose levels were used to stimulate cells to mimic the diabetic environment. As shown in Fig. 3C, compared to the control group, the cells pretreated with increasing doses of glucose (5, 20, and 30 mM) exhibited significantly enhanced fluorescence signals (1.1-, 1.2-, and 1.8-fold) (Fig. 3D). A similar phenomenon was observed in normal fibroblasts (WI-38) upon incubation with glucose (Fig. 3C and E). This may be due to the excessive formation of NADH during the electrochemical reduction of NAD+ to NADH, indicating that the NBON probe could sensitively monitor NADH fluctuations in cells incubated with high glucose concentrations.Under high-glucose conditions, glucose is converted into fructose through the aldose reductase (AR, a rate-limiting enzyme in the polyol pathway) and sorbitol dehydrogenase (SD, involved in oxidative stress) pathways, which can disturb the redox balance between NAD+/NADH (Fig. 4A).52–54 EPS, which is an AR inhibitor, can reduce the intracellular sorbitol content under high-glucose conditions, and has also been implicated in the pathogenesis of diabetic complications.55 It was therefore speculated that EPS could alleviate or repair diabetes by inhibiting the AR enzyme and enhancing the antioxidant capacity to regulate the redox balance in living cells. Thus, experiments were conducted using living A549 and WI-38 cells. As shown in Fig. 4B, when the cells were pre-treated with different concentrations of EPS (0–50 μM) during incubation with glucose, the resulting NAD+/NADH imbalance led to significant reductions in the NBON fluorescence intensity, with 6.1- and 3.3-fold reductions being observed for the A549 and WI-38 cells (c.f., the strong signal obtained in the presence of glucose alone) (Fig. 4C and D). These results were attributed to the downregulation of NADH under the experimental conditions. Flow cytometry was then performed (Fig. 4E), and commercial NAD+/NADH and GSH/GSSG assay kits were employed to detect the contents of NADH and GSH/GSSG in the EPS-treated cells. It was found that the level of NADH was reduced 1.4-fold (Fig. 4F), while the GSH/GSSG level was increased 1.8-fold compared to their corresponding levels in the glucose-treated cells (Fig. 4G), thereby confirming the downregulation of NADH levels and the upregulation of GSH levels. A total antioxidant capacity assay kit based on the ferric reducing ability of plasma (FRAP) method (i.e., a T-AOC Assay Kit) was also used to evaluate the redox state of the system, demonstrating an enhanced antioxidant capacity for the cells treated with EPS (Fig. 4H). Importantly, for further evaluation of the ROS and GSH levels in cells under EPS treatment conditions, experiments were also performed using a commercial ROS assay kit56 and a GSH probe57 that was previously reported by our group. It was found that the ROS levels decreased following EPS stimulation (Fig. 4I and J), while the GSH levels increased (Fig. S15†), thereby confirming the above findings and suggesting that EPS has the potential to relieve high glucose-induced diabetes by regulating the redox balance. Moreover, these results demonstrate that the small molecule NBON probe is suitable for monitoring NADH levels in living cells under these conditions.
In vivo imaging of NADH in a diabetic lung disease model
Given its impressive in vitro performance, the ability of NBON to detect NADH in a mouse model of diabetic lung disease was evaluated. Initially, the biosafety of NBON was assessed in healthy Kunming mice to ensure a low systemic toxicity during in vivo treatment. The major organs (i.e., heart, liver, spleen, lungs, and kidneys) of the mice were collected for hematoxylin and eosin (H&E) staining 24 h after the intravenous injection of NBON. Importantly, no obvious histopathological or physiological diseases were observed in the mice treated with NBON (c.f., the saline treatment group) (Fig. S16†). In addition, a hemolysis test was performed using NBON, indicating a slight change in hemolysis (Fig. S17†), and suggesting that the test dose of NBON exhibited a small degree of toxicity during in vivo imaging.Subsequently, the biodistribution of NBON was examined after its intravenous administration in mice, and as shown in Fig. S18,†NBON was preferentially accumulated in the livers within 2 hours, and also enriched in lungs after 4.5 hours injection. The biodistribution of the NBCS product (i.e., from the reaction between NBON and NADH) was also tracked, and it was found that NBCS was mainly localized in the lungs (Fig. 5A), while CS was mainly distributed in the liver (Fig. 5B). These results indicate that the introduction of the phenylboronic acid analog into the molecular skeleton of the CS dye promotes its accumulation in the lungs, likely due to the change in its hydrophilic/hydrophobic properties. These results indicate that the NBON reporter is suitable for imaging diabetic lung disease in vivo.
The response of NBON to NADH was then evaluated in mice. As shown in Fig. S19,† the signal recorded for the right thigh, which was stimulated with exogenous NADH, clearly increased compared to that observed for the left thigh, which was treated only with NBON. This result reveals the potential of NBON for detecting NADH in vivo. Subsequently, the ability of NBON to visualize changes in the NADH level was evaluated in a mouse diabetic lung disease model (Fig. 5C). To the best of our knowledge, direct evidence for NADH generation in this context is lacking. Thus, a diabetic lung disease model was constructed using streptozotocin (STZ)58 to image endogenous NADH generation. Metformin (MET), a biguanide derivative,58 was selected as the traditional medication for diabetes, and EPS was also used to evaluate its therapeutic effects in diabetes based on its excellent performance in living cells. The experiments were divided into four groups, namely the control (PBS treatment only), STZ, STZ/MET, and STZ/EPS groups. As shown in Fig. 5D, the mice in the control group showed no fluorescence signal. For the STZ group, the mice from were intraperitoneally injected with STZ over 7 days following the intravenous injection of NBON (100 μL, 200 μM). Consequently, the fluorescence signals in the mice lungs gradually increased (1.8-, 1.9-, and 2.0-fold, respectively) at time intervals (30, 90, and 150 min) compared with the mice of the control group (Fig. 5E). This result was mainly attributed to the NAD+/NADH imbalance in the STZ-stimulated mice, which resulted in excessive NADH formation, as demonstrated using the commercial NAD+/NADH kit (Fig. 5F). In living systems, a NAD+/NADH imbalance is known to affect the antioxidant capacity of the organism. It was therefore speculated that compared to the control group, the antioxidant capacities of the tissues would be significantly reduced under STZ stimulation, as demonstrated using the T-AOC assay kit (Fig. 5G). Notably, when mice from the STZ/MET and STZ/EPS groups were treated with MET for 28 d and EPS for 49 d, respectively, maximum reductions of 2.0- and 2.1- fold were observed for the fluorescence signals of the lungs in the STZ/MET and STZ/EPS groups. These results were attributed to recovery of the NAD+/NADH balance in the lung after MET and EPS treatment for diabetic lung disease, resulting in lower NADH levels (Fig. 5F) and an enhanced antioxidant capacity (Fig. 5G). Moreover, H&E staining revealed different pathological characteristics before and after treatment of the lung injury (Fig. 5H), which were supported by an ex vivo organ experiment performed in the presence of NBON (Fig. 5I and S20†). Taken together, these results imply that EPS has the potential to treat diabetic lung disease based on its regulation of the system redox state, thereby demonstrating that the probe NBON is applicable for the high-fidelity imaging of lung disease and repair in vivo.
In vivo imaging of NADH for lung metastases and tumors
The worst-case outcome of lung disease is the development of lung tumors, which primarily include metastatic and primary tumors. Based on the favorable properties of NBON identified above, investigations were carried out to assess its performance in the imaging of lung metastases and tumors in mice. For this purpose, the mice were divided into two groups, namely normal mice and mice with lung metastases. More specifically, BALB/c mice with lung metastases were established by the intravenous injection of 4T1 cells for three weeks (Fig. 6A). Subsequently, these mice were intravenously injected with NBON (100 μL, 200 μM), and as shown in Fig. 6B, apparent signal enhancements were observed in the metastasis group over time (i.e., at 25 and 120 min), whereas a negligible signal change was observed for the normal mice. After sacrifice, lung samples were collected from the mice (Fig. 6B(c) and 6B(g)), and the ex vivo fluorescence images also showed a large difference in the signal intensity between the two groups (Fig. 6B(d) and 6B(h)). More specifically, cancer cell metastases were only observed on the lung tissue surfaces of the mice with lung metastasis; no changes were observed for the normal lungs. These results were further verified by H&E staining (Fig. 6C), which indicated that NBON can track metastatic lung tumor lesions and image NADH levels during lung metastasis in vivo. Finally, an additional experiment was conducted using NBON to image NADH and guide the resection of lung tumors. The tumor model was established by implanting A549 cells into BALB/c mice for 20 d. PBS and NBON (30 μL, 100 μM) were then simply sprayed onto the tumor (Fig. S21†). Notably, the tumor was accurately lit up in situ within 25 min, while no fluorescence signal was obtained after spraying with PBS alone (Fig. 6D). Consequently, it was possible to completely remove the tumor under the fluorescence guidance provided by NBON (Fig. 6D). Additionally, in vitro lung tumor and para-carcinoma tissues could also be effectively distinguished via the in situ spraying of NBON at a tumor/normal tissue ratio of 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6E and F). These results confirm the potential of NBON for NADH detection/imaging in lung tumors, and its role in aiding in the accurate resection of lung tumors.
1 (Fig. 6E and F). These results confirm the potential of NBON for NADH detection/imaging in lung tumors, and its role in aiding in the accurate resection of lung tumors.
Conclusions
In summary, a lung-targeted nicotinamide adenine dinucleotide (NADH)-sensitive long-wavelength near-infrared (NIR) fluorescent reporter (NBON) was designed and developed using a tandem reaction (TR) strategy based on the dual reactions of NADH. More specifically, a group containing a phenylboronic acid analog was initially attached onto a probe molecule to promote an esterification-crosslinking reaction with the adjacent diols present in the NADH structure. Subsequently, the recognition group on the probe molecule was modified for reduction by NADH, rendering it highly reactive and leading to a strong fluorescence signal in the presence of NADH. This approach generated a sensitive probe with a low NADH detection limit (45 nM), and which also selectively accumulated in the lung. Importantly, these characteristics indicated the potential of the NBON for detecting the NADH generated in diabetic lung disease. Owing to the high sensitivity and selectivity observed for NBONin vitro, it was subsequently confirmed that this probe could detect small fluctuations in the NADH levels in living cells during high-glucose stimulations and epalrestat (EPS) repair. In addition, NBON was above to track changes in the NADH levels in streptozotocin-induced diabetic lung injury and MET/EPS repair models in mice. More importantly, the proposed antioxidant mechanism by which EPS alleviates diabetic lung disease was confirmed for the first time in living cells and in vivo. Finally, the endogenous NADH present in lung tumors and metastases was successfully monitored using NBON, and its role in aiding in the accurate resection of lung tumors was demonstrated. It is expected that this novel TR strategy will be applicable to the development other activatable probes. Moreover, this work should promote further research into the design of NADH probes for biological applications, including disease diagnosis and therapy.Data availability
The data supporting this article have been included in the ESI.† Additional data are available upon request from the corresponding author. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved (approval number: No. SYXK (Xiang) 2020-0002) by University of South China.Author contributions
Y. Hu, H. Zhang and Y. Ding: data curation, methodology, investigation, and visualization. W. Chen: investigation, data curation, methodology, and visualization. C. Pan: resources. L. He: conceptualization, writing – review and editing, resources and funding acquisition. D. Cheng and L. Yuan: conceptualization, writing – review and editing, resources and funding acquisition. All authors discussed the results and assisted during manuscript preparation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22074036), the Science and Technology Project of Hunan Province (2021RC3108), the Hunan Provincial Natural Science Foundation of China (2022JJ10042, 2022JJ30484, 2023JJ40412), the Special Funding for the Construction of Innovative Provinces in Hunan (2021SK4031).Notes and references
- M. Brownlee, Nature, 2001, 414, 813–820 CrossRef CAS PubMed.
- S. Amanat, S. Ghahri, A. Dianatinasab, M. Fararouei and M. Dianatinasab, Adv. Exp. Med. Biol., 2020, 1228, 91–105 CrossRef CAS PubMed.
- N. H. Cho, J. E. Shaw, S. Karuranga, Y. Huang, J. D. da Rocha Fernandes, A. W. Ohlrogge and B. Malanda, Diabetes Res. Clin. Pract., 2018, 138, 271–281 CrossRef CAS PubMed.
- M. R. Schuyler, D. E. Niewoehner, S. R. Inkley and R. Kohn, Am. Rev. Respir. Dis., 1976, 113, 37–41 CAS.
- D. Pitocco, L. Fuso, E. G. Conte, F. Zaccardi, C. Condoluci, G. Scavone, R. A. Incalzi and G. Ghirlanda, Rev. Diabet. Stud., 2012, 9, 23–35 CrossRef PubMed.
- S. F. Ehrlich, C. P. Quesenberry, S. K. Van Den Eeden, J. Shan and A. Ferrara, Diabetes Care., 2010, 33, 55–60 CrossRef PubMed.
- S. S. Garg and J. Gupta, Pharmacol. Res., 2022, 182, 106326 CrossRef CAS PubMed.
- J. Wu, Z. Jin and L. J. Yan, Redox Biol., 2017, 11, 51–59 CrossRef CAS PubMed.
- D. Cheng, J. Peng, Y. Lv, D. Su, D. Liu, M. Chen, L. Yuan and X. Zhang, J. Am. Chem. Soc., 2019, 141, 6352–6361 CrossRef CAS PubMed.
- H. Chang, S. Clemens, P. Gao, Q. Li, H. Zhao, L. Wang, J. Zhang, P. Zhou, K. Johnsson and L. Wang, J. Am. Chem. Soc., 2024, 146, 20569–20576 CrossRef CAS PubMed.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Nat. Commun., 2022, 13, 2009 CrossRef CAS PubMed.
- Z. Wang, W. Wang, P. Wang, X. Song, Z. Mao and Z. Liu, Anal. Chem., 2021, 93, 3035–3041 CrossRef CAS PubMed.
- X. Wu, W. Shi, X. Li and H. Ma, Acc. Chem. Res., 2019, 52, 1892–1904 CrossRef CAS PubMed.
- Z. Yuwen, Q. Zeng, Q. Ye, Y. Zhao, J. Zhu, K. Chen, H. Liu and R. Yang, Angew. Chem., Int. Ed., 2023, 135, e202302957 CrossRef.
- X. Zhang, F. Yang, T. Ren, Y. Zheng, X. Zhang and L. Yuan, Chin. Chem. Lett., 2023, 34, 107835 CrossRef CAS.
- Q. Liu, J. Yuan, R. Jiang, L. He, X. Yang, L. Yuan and D. Cheng, Anal. Chem., 2023, 95, 2062–2070 CrossRef CAS PubMed.
- B. Hu, Q. Liu, Y. Jiang, Y. Huang, H. Ji, J. Zhang, X. Wang, X.-C. Shen and H. Chen, Angew. Chem., Int. Ed., 2025, 64, e202418378 CrossRef CAS PubMed.
- X. Wang, S. S. Liew, J. Huang, Y. Hu, X. Wei and K. Pu, J. Am. Chem. Soc., 2024, 146, 22689–22698 CrossRef CAS PubMed.
- M. Vendrell, D. Zhai, J. C. Er and Y. T. Chang, Chem. Rev., 2012, 112, 4391–4420 CrossRef CAS PubMed.
- D. Cheng, W. Xu, X. Gong, L. Yuan and X. B. Zhang, Acc. Chem. Res., 2021, 54, 403–415 CrossRef CAS PubMed.
- Z. Mao, J. Xiong, P. Wang, J. An, F. Zhang, Z. Liu and J. S. Kim, Coord. Chem. Rev., 2022, 454, 214356 CrossRef CAS.
- R. Wu, Z. Chen, H. Huo, L. Chen, L. Su, X. Zhang, Y. Wu, Z. Yao, S. Xiao, W. Du and J. Song, Anal. Chem., 2022, 94, 10797–10804 CrossRef CAS PubMed.
- Y. Xu, W. Tuo, L. Yang, Y. Sun, C. Li, X. Chen, W. Yang, G. Yang, P. J. Stang and Y. Sun, Angew. Chem., Int. Ed., 2022, 61, e202110048 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- L. Wang, J. Zhang, B. Kim, J. Peng, S. N. Berry, Y. Ni, D. Su, J. Lee, L. Yuan and Y. T. Chang, J. Am. Chem. Soc., 2016, 138, 10394–10397 CrossRef CAS PubMed.
- Y. Zhao, K. Wei, F. Kong, X. Gao, K. Xu and B. Tang, Anal. Chem., 2019, 91, 1368–1374 CrossRef CAS PubMed.
- X. Pan, Y. Zhao, T. Cheng, A. Zheng, A. Ge, L. Zang, K. Xu and B. Tang, Chem. Sci., 2019, 10, 8179–8186 RSC.
- A. Podder, S. Koo, J. Lee, S. Mun, S. Khatun, H. G. Kang, S. Bhuniya and J. S. Kim, Chem. Commun., 2019, 55, 537–540 RSC.
- R. Freeman, R. Gill, I. Shweky, M. Kotler, U. Banin and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 309–313 CrossRef CAS PubMed.
- K. Ma, H. Yang, X. Wu, F. Huo, F. Cheng and C. Yin, Angew. Chem., Int. Ed., 2023, 62, e202301518 CrossRef CAS PubMed.
- J. Joo, D. Youn, S. Park, D. Shin and M. Lee, Dyes Pigm., 2019, 170, 107561 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- H. Li, H. Kim, F. Xu, J. Han, Q. Yao, J. Wang, K. Pu, X. Peng and J. Yoon, Chem. Soc. Rev., 2022, 51, 1795–1835 RSC.
- E. A. Owens, M. Henary, G. El Fakhri and H. S. Choi, Acc. Chem. Res., 2016, 49, 1731–1740 CrossRef CAS PubMed.
- H. Li, Y. Kim, H. Jung, J. Y. Hyun and I. Shin, Chem. Soc. Rev., 2022, 51, 8957–9008 RSC.
- C. Li, Y. Pang, Y. Xu, M. Lu, L. Tu, Q. Li, A. Sharma, Z. Guo, X. Li and Y. Sun, Chem. Soc. Rev., 2023, 52, 4392–4442 RSC.
- J. Weng, Z. Huang, Y. Liu, X. Wen, Y. Miao, J. J. Xu and D. Ye, J. Am. Chem. Soc., 2024, 146, 13163–13175 CrossRef CAS PubMed.
- J. Liu, W. Zhang, C. Zhou, M. Li, X. Wang, W. Zhang, Z. Liu, L. Wu, T. D. James, P. Li and B. Tang, J. Am. Chem. Soc., 2022, 144, 13586–13599 CrossRef CAS PubMed.
- S. S. Liew, Z. Zeng, P. Cheng, S. He, C. Zhang and K. Pu, J. Am. Chem. Soc., 2021, 143, 18827–18831 CrossRef CAS PubMed.
- Y. Ding, R. Zhong, R. Jiang, X. Yang, L. He, L. Yuan and D. Cheng, ACS Sens., 2023, 8, 914–922 CrossRef CAS PubMed.
- W. Zhang, T. Chen, L. Su, X. Ge, X. Chen, J. Song and H. Yang, Anal. Chem., 2020, 92, 6094–6102 CrossRef CAS PubMed.
- K. Li, Y. Lyu, Y. Huang, S. Xu, H. W. Liu, L. Chen, T. B. Ren, M. Xiong, S. Huan, L. Yuan, X. B. Zhang and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2018033118 CrossRef CAS PubMed.
- R. Zhong, R. Jiang, J. Zeng, X. Gong, X. Yang, L. He, L. Yuan and D. Cheng, Anal. Chem., 2023, 95, 2428–2435 CrossRef CAS PubMed.
- J. Liu, W. Zhang, C. Zhou, M. Li, X. Wang, W. Zhang, Z. Liu, L. Wu, T. D. James, P. Li and B. Tang, J. Am. Chem. Soc., 2022, 144, 13586–13599 CrossRef CAS PubMed.
- S. Zhang, H. Chen, L. Wang, X. Qin, B. P. Jiang, S. C. Ji, X. C. Shen and H. Liang, Angew. Chem., Int. Ed., 2022, 61, e202107076 CrossRef CAS PubMed.
- D. Cheng, Y. Pan, L. Wang, Z. Zeng, L. Yuan, X. Zhang and Y. T. Chang, J. Am. Chem. Soc., 2017, 139, 285–292 CrossRef CAS PubMed.
- H. Bian, D. Ma, F. Pan, X. Zhang, K. Xin, X. Zhang, Y. Yang, X. Peng and Y. Xiao, J. Am. Chem. Soc., 2022, 144, 22562–22573 CrossRef CAS PubMed.
- Y. Wei, D. Cheng, T. Ren, Y. Li, Z. Zeng and L. Yuan, Anal. Chem., 2016, 88, 1842–1849 CrossRef CAS PubMed.
- J. S. Brody, C. A. Vaccaro, N. S. Hill and S. Rounds, Circ. Res., 1984, 55, 155–167 CrossRef CAS PubMed.
- Y. P. Hung and G. Yellen, Cell Metab., 2014, 1071, 83–95 CAS.
- C. Busu, V. Atanasiu, G. Caldito and T. Y. Aw, J. Med. Life, 2014, 7, 611–618 CAS.
- T. F. Ng, F. K. Lee, Z. T. Song, N. A. Calcutt, A. Y. Lee, S. S. Chung and S. K. Chung, Diabetes, 1998, 47, 961–966 CrossRef CAS PubMed.
- A. Y. Lee and S. S. Chung, FASEB J., 1999, 13, 23–30 CrossRef CAS PubMed.
- J. Wu, Z. Jin, H. Zheng and L. J. Yan, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2016, 9, 145–153 CAS.
- J. W. Steele, D. Faulds and K. L. Goa, Drugs Aging, 1993, 3, 532–555 CrossRef CAS PubMed.
- H. Hao, L. Cao, C. Jiang, Y. Che, S. Zhang, S. Takahashi, G. Wang and F. J. Gonzalez, Cell Metab., 2017, 25, 856–867 CrossRef CAS PubMed.
- R. Jiang, H. Zhang, Q. Liu, X. Yang, L. He, L. Yuan and D. Cheng, Adv. Healthcare Mater., 2024, 13, 2302466 CrossRef CAS PubMed.
- S. Li, K. Yang, Y. Liu, P. Wang, D. Cheng and L. He, Sens. Actuators, B, 2023, 379, 133253 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01488c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |