Open Access Article
Open Access ArticleThe role of structural defects in the fluoride-mediated synthesis of aluminosilicate zeolites†
Kingsley Christian
Kemp
a,
Ömer F.
Altundal
 b,
Donghui
Jo
c,
Weidong
Huang
de,
Qiang
Wang
de,
Feng
Deng
b,
Donghui
Jo
c,
Weidong
Huang
de,
Qiang
Wang
de,
Feng
Deng
 de,
German
Sastre
de,
German
Sastre
 *b and
Suk Bong
Hong
*b and
Suk Bong
Hong
 *a
*a
aCenter for Ordered Nanoporous Materials Synthesis, Division of Environmental Science and Engineering, POSTECH, Pohang 37673, South Korea. E-mail: sbhong@postech.ac.kr
bInstituto de Tecnologia Quimica (UPV-CSIC), Universidad Politécnica de Valencia, Avenida Naranjos s/n, Valencia 46022, Spain. E-mail: gsastre@itq.upv.es
cLow-Carbon Petrochemical Research Center, Korea Research Institute of Chemical Technology, Daejeon 34114, South Korea
dState Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan 430071, P. R. China
eUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 25th March 2025
Abstract
The existence of framework defects in zeolites, an important class of industrial catalysts and adsorbents, has long been recognized, but little is known about their exact role in zeolite crystallization. Here we show that despite their relatively high framework Al content (Si/Al = 11.5–13.8), as-synthesized PWO, PWW and RTH zeolites, obtained using various trimethylpyridinium cation isomers as organic structure-directing agents (OSDAs) in concentrated fluoride media, contain unexpectedly large amounts of SiO−···HOSi defects which counterbalance the charge of 11–39% of the total OSDA cations occluded per unit cell, but have only a negligible amount (<0.1 ions per unit cell) of fluoride anions. The results suggest that the phase selectivity of the crystallization in the presence of fluoride ions may be determined by a combination of Al incorporation into the silicate framework, the type of OSDAs used and the microstructure and concentration of SiO−···HOSi defects formed. This study provides a new basis for better understanding the fundamental aspects of zeolite crystallization mechanisms.
Introduction
Zeolites, both natural and synthetic, are microporous crystalline materials that are widely used as commercial catalysts and adsorbents, largely because of their uniformity in pore shape and size. Yet despite this success, there is a need for zeolites with desired pore architectures and physicochemical properties, for example, to develop green technologies for carbon capture and energy conversion.1,2 However, the current understanding of zeolite crystallization mechanisms is poor so that the rational synthesis of novel zeolite structures and/or compositions remains highly challenging.None of the crystalline solids can be completely free of internal structural defects, which is also the case for zeolites and zeolite-like materials. In particular, many important properties of high-silica zeolites like their ion exchange capacity, catalytic activity and (hydro)thermal stability have long been recognized to differ according to the concentration and nature of their defects.3–8 Intrazeolitic defect sites can be broadly categorized as siloxy (SiO−) and silanol (SiOH) groups: the former can balance the positive charge of inorganic structure-directing agents (ISDAs) and organic structure-directing agents (OSDAs) or be involved in intermolecular hydrogen bonding to the SiOH group, whereas the latter is generated by hydrolysis of Si–O–Si linkages, by missing tetrahedral atoms (T-atoms; T = Si, Al, B, etc.), or by stacking disorder.9–12 On the other hand, the formation of discrete nuclei with the structural identity of the crystallizing phase is generally believed to trigger zeolite crystallization.13 Apparently, zeolite nuclei should be substantially more defective than well-grown zeolite crystals because of their embryonic nature. This led us to consider the possibility that when they are stable enough to survive in the crystallization medium, the microstructure and concentration of their defects, apart from or together with those of ISDAs and/or OSDAs in the synthesis mixture, could be determinant of the phase selectivity during zeolite crystallization. In fact, structural defects have long been reported to be present at early stages of zeolite synthesis.9 However, little attention has been paid to whether and how they could play a structure-directing role in zeolite nucleation and crystal growth, especially in fluoride media.14–16
PST-21 (framework type PWO) and PST-22 (PWW) are two high-silica (Si/Al ∼ 10) zeolites that were first synthesized via the so-called excess fluoride approach (HF/OSDAq+ ≥ 2q) using 1,2,3-trimethylimidazolium (123TMI) and 1,3,4-trimethylimidazolium (134TMI) or 1,2,3,4-tetramethylimidazolium (1234TMI) cations as OSDAs, respectively.17,18 While the PWO structure consists of two intersecting 9-ring (4.2 × 4.4 Å) channels, the PWW one has intersecting 10-ring (5.2 × 6.0 Å) and 8-ring (3.3 × 3.6 Å) channels. In the present study, we report the fluoride-mediated synthesis of aluminosilicate (Si/Al = 11.5–13.8) zeolites with PWO, PWW and RTH topologies in the presence of various trimethylpyridinium (TMP) cation isomers as OSDAs. RTH is a small-pore zeolite containing 22-hedral ([46586484]) t-rth cages, unlike PWO and PWW.19 Up to now, many alkylpyridinium-based SDAs have been used in the synthesis of a number of zeolites with different framework structures (e.g. ZSM-22 (TON), ferrierite (FER), ITQ-12 (ITW), RUB-13 (RTH), SCM-14 (SOR), ZSM-5 (MFI), etc.) and compositions (aluminosilicate, germanosilicate and pure-silica) in hydroxide or fluoride media.20–29 However, none of them were reported to direct the synthesis of PWO and PWW. More interestingly, our experimental results demonstrate that considerable (11–39% of the total OSDA cations occluded per unit cell) SiO−···HOSi defects exist in as-synthesized aluminosilicate PWO, PWW and RTH zeolites, unlike in as-synthesized pure-silica ITQ-12 (ITW) that was crystallized in the presence of any of the TMP cation isomers studied here. To elucidate the origin of this anomaly, we have carried out synthesis energy calculations for various TMP cations in a series of zeolites with different framework structures and anion compositions.30–32
Experimental section
Zeolite synthesis
In a typical preparation of a series of TMP cation isomers in their hydroxide form used as OSDAs in zeolite synthesis, a mixture of 0.10 mol of an appropriate dimethylpyridine and 0.3 mol of iodomethane (98%, Kanto) was stirred in 200 mL of acetone at 25 °C for 3 days. Further details can be found in the ESI.† The other reagents included tetraethylorthosilicate (98%, Aldrich), aluminum hydroxide (Al(OH)3, Aldrich), hydrofluoric acid (48%, J.T. Baker) and deionized water. The composition of the final zeolite synthesis mixture was 0.5R·xHF·yAl2O3·1.0SiO2·5.0H2O, where R is OSDA prepared here, x is varied between 0.5 < x < 1.5 and y is 0 or 0.05. The final synthesis mixture was transferred into a 23 mL Teflon-lined autoclave and heated under rotation (60 rpm) at 160 °C for 14–21 days. Further details can be found in the ESI.† When necessary, as-synthesized zeolites were calcined in air at 600–750 °C for 8 h, depending on their structure type, to remove the occluded organic SDA.Analytical methods
Powder X-ray diffraction (PXRD), elemental and thermal analyses, scanning electron microscopy (SEM), and 1H–13C cross polarization (CP), 1H, 19F, 27Al and 29Si Magic Angle Spinning (MAS) Nuclear Magnetic Resonance (NMR) measurements were performed as described in our previous papers.17,33 Synchrotron PXRD data for as-synthesized PWO and PWW zeolites were collected on the 2D and 5A beamlines equipped with a ceramic furnace at the Pohang Accelerator Laboratory (PAL; Pohang, Korea) using monochromated X-rays (λ = 0.7000 and 0.6926 Å, respectively). The Rietveld refinements on the occluded OSDAs was performed using the rigid-body method.34 A summary of experimental and crystallographic data for 134TMP-PWO and 135TMP-PWW are shown in Table S1,† and their atomic coordinates, thermal parameters and selected bond lengths and angles are given in Tables S2–S5.†1H double quantum–single quantum (DQ–SQ) MAS and triple quantum–single quantum (TQ–SQ) MAS NMR spectra were recorded on a Bruker Avance III 500 spectrometer and 1.9 mm triple resonance probe. Prior to the NMR experiments, the sample was dried at 200 °C under dynamic vacuum. All experiments were carried out at a spinning frequency of 25 kHz and a π/2 pulse length of 1.9 μs, using the R1225 symmetry-based recoupling scheme35 (τre ≈ 210 μs) for DQ and the SR30410 recoupling scheme36 (τre ≈ 632 μs) for TQ. A total of 512 scans were collected for each of the 64 rotor-synchronized t1 increments with a recycle delay of 2 s. The spectra are referenced with respect to adamantane at 1.78 ppm.
Synthesis energy calculations
The zeoTsda software37 contains a suite of programs with the General Utility Lattice Program (GULP)38 at the heart, whose Monte Carlo and lattice energy minimization algorithms are used to, respectively, include OSDA molecules until the optimum loading is found and optimise their location in the zeolite micropores. The total energies (Ezeolite–OSDAF) of these structures, for pure-silica composition, were calculated through a single point energy calculation and the so-called synthesis energy (Esyn) values obtained using eqn (1), as previously described:30–32 | (1) |
For defect-free aluminosilicate zeolite calculations the starting point was the unit cells for the optimally loaded OSDA pure-silica ones. Here the Si/Al ratio was chosen to either (i) compensate for the entire OSDA charge or (ii) to represent the experimentally obtained one. The zeoTAl software was used to generate random Al distributions for each zeolite–OSDA pair. Of these pairs the lowest energy selection following Lowenstein's rule was optimized and its Esyn value was calculated using eqn (2):
 | (2) |
The calculation was then expanded to accommodate for aluminosilicate zeolites containing 1SiOH–1SiO− clusters (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects) using the same work flow and the Esyn calculated using eqn (3a):
1 connectivity defects) using the same work flow and the Esyn calculated using eqn (3a):
 | (3a) |
When the defect type in the structure changes to the 3SiOH–1SiO− cluster model (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect or 3
1 connectivity defect or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defect), Esyn is defined by the following equation:
1 vacancy defect), Esyn is defined by the following equation:
 | (3b) |
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect, 2
1 connectivity defect, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect and 3
1 connectivity defect and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defect models were employed to generate the initial geometries of defects in as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH according to the 1H MAS NMR results (Fig. 3, S7 and S8†). Structures of 134TMP-PWO, 135TMP-PWW and 123TMP-RTH with various defect types were subsequently optimized using DFT calculations to acquire an accurate geometry of defects and O⋯O distances within these defects.
1 vacancy defect models were employed to generate the initial geometries of defects in as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH according to the 1H MAS NMR results (Fig. 3, S7 and S8†). Structures of 134TMP-PWO, 135TMP-PWW and 123TMP-RTH with various defect types were subsequently optimized using DFT calculations to acquire an accurate geometry of defects and O⋯O distances within these defects.
Details regarding the force field, Lennard-Jones, three-body, Morse and Buckingham potential parameters are given in the ESI and Tables S15–S19.† Additionally, force field potential parameters for species unused in this work are presented in Table S20.† This will make it easier for the users to get all parameters for this general and transferable force field.
Results and discussion
Zeolite synthesis
Table 1 lists the representative products from pure-silica (Si/Al = ∞) and aluminosilicate (Si/Al = 10) zeolite syntheses at different HF/OSDA ratios (1.0–3.0) using 1,2,3-trimethylpyridinium (123TMP), 1,2,4-trimethylpyridinium (124TMP), 1,2,5-trimethylpyridinium (125TMP), 1,2,6-trimethylpyridinium (126TMP), 1,3,4-trimethylpyridinium (134TMP), or 1,3,5-trimethylpyridinium (135TMP) ions under rotation (60 rpm) at 160 °C for 14 days. Here we selected these six TMP isomers as OSDAs because their positive charge is localized on the N atom, unlike in the TMI cations where the charge is spread over both N atoms. However, ITW was the only zeolite phase synthesized at HF/OSDA = 1.0 and 2.0 in pure-silica composition, regardless the type of TMP isomer used. Since the same result has been previously observed for the synthesis with 123TMI and 134TMI,17 differences in the charge distribution between TMP and TMI ions may be not large enough to induce changes in the pure-silica phase selectivity.| Si/Al | HF/OSDA | ||||||
|---|---|---|---|---|---|---|---|
| 123TMP | 124TMP | 125TMP | 126TMP | 134TMP | 135TMP | ||
| Productb | |||||||
| a The composition of the synthesis mixture is 0.5R·xHF·yAl2O3·1.0SiO2·5.0H2O, where R is the OSDA, x is varied between 0.5 ≤ x ≤ 1.5 and y is 0 or 0.05. All syntheses were performed under rotation (60 rpm) at 160 °C for 14 days, unless otherwise stated. OSDA abbreviations: 123TMP, 1,2,3-trimethylpyridinium; 124TMP, 1,2,4-trimethylpyridinium; 125TMP, 1,2,5-trimethylpyridinium; 126TMP, 1,2,6-trimethylpyridinium; 134TMP, 1,3,4-trimethylpyridinium; and 135TMP, 1,3,5-trimethylpyridinium. b The product appearing first is the major phase. c Product obtained after 21 days. | |||||||
| ∞ | 1.0 | ITW | ITW | ITW | ITW | ITW | ITW |
| 2.0 | ITW | ITW | ITW | ITW | ITW | ITW | |
| 3.0 | ITW | Amorphousc | Dense | Dense | ITW | Amorphousc | |
| 10.0 | 1.0 | RTH | PWO + RTH | PWO + RTHc | PWW + RTH | FER + RTH | PWW |
| 2.0 | RTH | Amorphousc | Amorphousc | Amorphousc | PWO + amorphousc | PWW | |
| 3.0 | Amorphousc | Amorphousc | Amorphousc | Amorphousc | PWOc | Amorphousc | |
On the other hand, when the Si/Al ratio in the synthesis mixture was fixed at 10.0, PWO and PWW were the products obtained using 134TMP and 135TMP as OSDAs at HF/OSDA = 3.0 and 2.0, respectively. Given that 135TMP directed the synthesis of PWW even at HF/OSDA = 1.0 (Table 1), this largest TMP isomer appears to have a stronger structure-directing ability for PWW formation than the imidazolium-based OSDAs such as 134TMI.17 We were also able to crystallize RTH from synthesis mixtures with HF/OSDA = 1.0 or 2.0 using 123TMP. These results strongly suggest that Al substitution in zeolites is the key to controlling the selectivity of crystallization in the presence of various TMP cation isomers and F− anions. Indeed, when HF/OSDA and Si/Al were set at 0.0 and 10.0, respectively, 134TMP and 135TMP gave amorphous material even after 21 days of heating, showing the need for F− ions during PWO and PWW crystallization.17 On the other hand, we obtained RTH using 123TMP in hydroxide media after 14 days. However, this is not so surprising because RTH has already been reported to crystallize in the presence of many different OSDA cations under F−-free conditions.39
Characterization
The PXRD patterns of the representative zeolites synthesized in this study (i.e. 135TMP-ITW, 134TMP-PWO, 135TMP-PWW and 123TMP-RTH, all of which, except 134TMP-PWO, were obtained at HF/OSDA = 1.0) show that each zeolite is highly crystalline and there are no reflections other than those from the corresponding structures (Fig. S1†).191H–13C CP MAS NMR spectroscopy reveals that the OSDA cations employed remain intact upon their occlusion into zeolite pores (Fig. S2†). While all Al atoms in as-synthesized zeolites occupy tetrahedral framework positions, calcination at high temperatures (550–750 °C; Fig. S3†) in air to remove the occluded OSDAs resulted in the formation of a non-negligible amount of extra-framework Al species, as evidenced by 27Al MAS NMR (Fig. S4†). No significant differences in the crystal morphology were observed when TMP and TMI cations directed the synthesis of zeolites with the same framework topology. However, in the case of 135TMP-PWW and 1234TMI-PWW, the crystal width and thickness were significantly larger for the former zeolite, leading to a considerable difference (ca. 740 vs. 590 °C) in the maximum temperature of exothermic weight losses due to OSDA combustion (Fig. S3†).Fig. 1 shows the locations and orientations of OSDA cations in as-synthesized 134TMP-PWO and 135TMP-PWW determined by the Rietveld analysis of synchrotron PXRD data (Tables S1–S5 and Fig. S5 and S6†). We did not determine the structures of as-synthesized 135TMP-ITW and 123TMP-RTH, because the location of original OSDAs in these two zeolites have already been reported.40–42 Both 134TMP and 135TMP ions were found to reside in the intersections (i.e. the 18-hedral t-pwo ([465894]) and t-pww ([465882102]) cavities, respectively) of two 9-ring channels in PWO and of 10- and 8-ring channels in PWW, with minimum distances between the TMP C and framework O atoms of 3.37 and 3.18 Å, respectively. However, the number (7 vs. 4) of C–O distances shorter than 3.5 Å is larger in 135TMP-PWW than in 134TMP-PWO. This can be explained by the bell shape of the t-pww cavity, giving a better fit to 135TMP. No signs of the presence of F− ions in 135TMP-PWW and 134TMP-PWO were observed, despite their formation in fluoride media, in good agreement with the 19F MAS NMR data in Fig. 2.
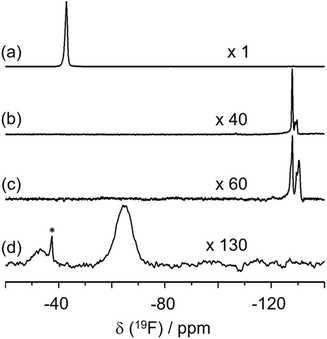 | ||
| Fig. 2 19F MAS NMR spectra of as-synthesized (a) 135TMP-ITW, (b) 134TMP-PWO, (c) 135TMP-PWW and (d) 123TMP-RTH. The asterisk indicates the spinning sideband. | ||
Fig. 2 shows the 19F MAS NMR spectra of as-synthesized 135TMP-ITW, 134TMP-PWO, 135TMP-PWW and 123TMP-RTH. As previously reported,43 135TMP-ITW exhibits a sharp resonance at 40 ppm due to the F− ions within the double 4-ring (d4r; 6-hedral ([46]) t-cub) cages, which was also observed for the other ITW zeolites synthesized here. Of particular interest is that the overall intensity of 19F resonances is exceedingly weaker in the spectra of the latter three aluminosilicate zeolites compared to pure-silica 135TMP-ITW, revealing the practical absence of F− ions, even in their small cages like 10-hedral ([445462]) t-tte, 6-hedral ([4254]) t-bru and 8-hedral ([4454]) t-cle cages: very weak resonances in the range −128 to −133 ppm can be attributed to the free F− and/or SiF62− species within the 9- and 10-ring channels in PWO and PWW, respectively.44–46 This indicates that in the fluoride-mediated synthesis of aluminosilicate zeolites under highly concentrated conditions, the counterbalance of OSDA cations by single framework negative charges (in the form of [AlO4/2]− tetrahedra) created by Al substitution is more favorable than that by F− ions encapsulated within small cages, probably because of the shorter cation–anion distance. Fig. 2 also shows that a resonance around 67 ppm observed for 123TMP-RTH is considerably broad compared to other zeolites, suggesting the location of F− ions at different positions within the large t-rth cages.
Elemental analysis shows that as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH have slightly higher bulk Si/Al ratios (11.5–13.8) than the ratio (10.0) of their synthesis mixtures (Tables 1 and 2). It should be noted that they possess 1.6, 2.7 and 2.2 Al atoms per unit cell that are smaller by 0.2, 1.5 and 1.6 than the numbers (1.8, 4.2 and 3.8) of OSDA cations, respectively, because all of their Al atoms are in framework positions (Fig. S4†). Therefore, charge balance requires that the positive charges of 0.2, 1.5 and 1.5 OSDAs per unit cell of 134TMP-PWO, 135TMP-PWW and 123TMP-RTH, when considering the presence of 0.1 F− ions per unit cell in the latter zeolite, should be counterbalanced by additional negative charge centers (i.e. internal SiO− groups), respectively. On the other hand, the SiO− groups are stabilized by hydrogen bonding with water molecules and/or vicinal SiOH groups47 to form defect sites. The chemical composition data in Table 2 reveal that as-synthesized 135TMP-PWW and 123TMP-RTH possess 0.7 and 1.3 water molecules per unit cell, which are smaller than those (1.5 both) of additional negative charge centers. Thus, we speculate that if all of their water molecules form hydrogen bonds with SiO− groups, they should then be immobilized, giving potentially intense spinning sidebands in the 1H MAS NMR spectra because of the intramolecular dipole interactions between the protons.9 However, no such sidebands were observed in the 1H MAS NMR spectra of not only 134TMP-PWO but also 135TMP-PWW and 123TMP-RTH, like the spectrum of pure-silica 135TMP-ITW (Fig. S7†). This led us to conclude, as expected, that considerable amounts of OSDA cations in our aluminosilicate zeolites must be balanced by SiO−···HOSi defects (Table 2).9,16
| Sample ID | HF/OSDAa | Unit cell compositionb | Si/Al | OSDAD/OSDATc | Crystal shape and average sized (μm) | Micropore volumee (cm−3 g) |
|---|---|---|---|---|---|---|
| a HF/OSDA ratio in the synthesis mixture that crystallized each product. b Determined from a combination of elemental and thermal analyses, and 19F MAS NMR measurements. The water content was calculated from the endothermic weight loss by TGA/DTA up to 400 °C and OH− (defect; see ESI eqn (4a) and (4b)) has been introduced to the zeolite framework to make the as-synthesized zeolites electrically neutral. c Ratio of OSDA cations compensated by SiO−···HOSi defects to the total OSDA cations in each zeolite. d Determined by SEM. e Calculated from N2 adsorption data. | ||||||
| 135TMP-ITW | 1.0 | |(135TMP)2.0F2.0(H2O)0.6|[Si24O48] | ∞ | 0.00 | Rhombic dodecahedra, 3 × 2 | 0.17 |
| 134TMP-PWO | 3.0 | |(134TMP)1.8(H2O)0.8|[Si18.4Al1.6O40(OH)0.2] | 11.5 | 0.11 | Overlapped plates, 1.0 × 0.1 | 0.12 |
| 135TMP-PWW | 1.0 | |(135TMP)4.2(H2O)0.7|[Si37.3Al2.7O80(OH)1.5] | 13.8 | 0.36 | Overlapped plates, 5.0 × 1.0 | 0.14 |
| 123TMP-RTH | 1.0 | |(123TMP)3.8F0.1(H2O)1.3|[Si29.8Al2.2O64(OH)1.5] | 13.6 | 0.39 | Rods, 15 × 2 | 0.24 |
We applied 1H DQ and TQ MAS NMR spectroscopy to ascertain the presence of SiO−···HOSi hydrogen bonds in as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH. Although multiple quantum transitions are quantum-mechanically forbidden for direct observation, they can be visible in a two-dimensional (2D) experiment where single quantum (SQ) chemical shifts are observable in one dimension and DQ or TQ chemical shifts in the other.16 As shown in Fig. 3, the shoulder at a SQ chemical shift of around 9 ppm shows a 1H DQ–SQ autocorrelation peak at 18.4 ppm (2 × 9.2 ppm), corresponding to the hydrogen-bonded SiOH protons at 9.2 ppm with an O⋯O distance of 2.74 Å between SiO− and SiOH groups,48 is resolved for the former two zeolites. However, since no TQ signal at 27.6 ppm (3 × 9.2 ppm) was found (Fig. S8†), it is clear that 134TMP-PWO and 135TMP-PWW have two clustered SiOH groups which are hydrogen-bonded to one SiO− group. Another interesting result is that 123TMP-RTH shows no DQ signal at 18.4 ppm, despite its higher defect concentrations (Table 2), revealing the presence of only one SiO−···HOSi hydrogen bond in defect sites. The existence of SiO−···HOSi defects in as-synthesized aluminosilicate zeolites obtained in hydroxide media has been previously observed.9,49 To our knowledge, however, this has never been reported for any of the aluminosilicate zeolites synthesized in fluoride media. The 1H DQ–SQ MAS NMR data in Fig. 3 also suggest that while the microstructure type of defects can differ according to the zeolite framework topology, they may not be primarily created by a T-atom vacancy with one SiO− and three SiOH groups in a SiOH nest.16 This is because the concentration of three clustered SiOH groups forming hydrogen bonds to SiO− was below the detection limit of our NMR experiments. It is remarkable here that all of 134TMP-PWO, 135TMP-PWW and 123TMP-RTH show two pairs of DQ peaks at (9.2, 13.7) and (4.5, 13.7) ppm and (9.2, 12.0) and (2.8, 12.0) ppm, suggesting close proximities between SiOH protons hydrogen-bonded to SiO− and the N- and C-methyl protons in TMP.
Synthesis energy calculations
To understand the origin and role of defect clusters as another important structure-directing factor in zeolite synthesis from a thermodynamic point of view, we first optimized the corresponding geometry (zeolite–OSDA) of each zeolite with various embedded TMP isomers using force fields and then calculated the Esyn using eqn (3a) and (3b) in the Experimental section. The lowest Esyn value, which corresponds to the theoretical prediction for the most stable zeolite–OSDA, has been shown to be a successful strategy,30–32 because it can be used for any zeolite composition, even in the presence of F− ions. For the pure-silica system, the charge of OSDA cations was counterbalanced by F− ions within the small cavities in zeolites in order to calculate the Esyn values for the optimized structures (Table S6†). The results showed that ITW was the most stable pure-silica zeolite for all OSDAs, which is in excellent agreement with the experimental results (Table 1). This, once again, proves the accuracy of the synthesis energy approach in predicting the phase selectivity of pure-silica zeolite crystallization.When zeolites have aluminosilicate composition, the characterization results revealed that, although the synthesis was performed in fluoride media, F− ions did not get occluded in small zeolite cages (Table 2 and Fig. 1 and 2). Therefore, we first calculated their Esyn values in the absence of F− ions, as well as of the experimentally observed SiO−···HOSi defects, so that the positive charge of the OSDAs was only counterbalanced by [AlO4/2]− tetrahedra (Table S7†). RTH was calculated to have the most favorable Esyn with all OSDAs when the OSDA charge was compensated only by [AlO4/2]− tetrahedra. It is worth noting that the Si/Al ratio (7.0) of all these OSDA–RTH structures used in the calculations is lower than not only the ratio (10.0) of the synthesis mixture but also that (13.6) of the experimentally crystallized product. Nevertheless, the Esyn calculation results for aluminosilicate zeolites support the experimental observation that the charge of OSDA cations occluded is balanced by both SiO−···HOSi defects and [AlO4/2]− tetrahedra (Table S8†).
To include the effect of SiO−···HOSi defects in our Esyn calculations, we derived new equations (i.e.eqn (3a) and (3b) in the Experimental section) and applied them to the calculations for the 135TMP and 123TMP cations embedded in FER and ITE structures, as well as in ITW, PWO, PWW and RTH with defects containing 1SiO− and 1SiOH which will be designated as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect. The other structure types of defect clusters considered in this work will be explained below. The first two structures (FER and ITE) were selected because they contain the same pore system (intersecting 10- and 8-ring channels) and composite building unit (t-cle cage) as those of PWW and RTH, respectively.19 The Si/Al ratio of these six zeolite structures was set to 12.3 or higher so that their Al content was always lower than that of 134TMP-PWO with the lowest Si/Al ratio (11.5) among the aluminosilicate zeolites synthesized in this study (Table 2). When the F− ions were not included in the calculations, PWO and PWW, which give the lowest Si/Al ratio, were determined to be the most favorable structures in the presence of 135TMP with Esyn values of −0.499 and −0.472 eV per T-atom, respectively (Table S8†). This implies that they are capable of incorporating more Al atoms, in good agreement with our previous work showing that the zeolite structure with a higher Al content (or a lower Si/Al ratio) is characterized by a more favorable Esyn value.30,31 However, although PWO was not experimentally synthesized with 135TMP (Table 1), the Esyn value of the observed PWW phase is higher by only 0.027 eV per T-atom than that of PWO. On the other hand, Esyn calculations for 123TMP were found to favor the formation of PWW and PWO again, unlike the experimental result giving RTH. However, since 123TMP-RTH contains only 0.1 F− ions per unit cell, we calculated the Esyn value of 123TMP-RTH with Si/Al ∼ 14, while considering a larger number of anionic combinations to counterbalance the OSDAs (Table 2 and S9†). We note here that the addition of 2.0 F− ions and 2.0 [AlO4/2]− tetrahedra to the RTH unit cell gives a Esyn value of −0.627 eV per T-atom for the RTH formation (Table S9†), which is lower than that in their absence (−0.505 eV per T-atom; Table S8†) and where PWW is favored. As the amount of F− ions occluded in 123TMP-RTH is negligible (Table 2), we speculate that RTH nuclei containing F− ions may ‘catalyse’ the formation of the rest of the crystal without need of further F− encapsulation.
1 connectivity defect. The other structure types of defect clusters considered in this work will be explained below. The first two structures (FER and ITE) were selected because they contain the same pore system (intersecting 10- and 8-ring channels) and composite building unit (t-cle cage) as those of PWW and RTH, respectively.19 The Si/Al ratio of these six zeolite structures was set to 12.3 or higher so that their Al content was always lower than that of 134TMP-PWO with the lowest Si/Al ratio (11.5) among the aluminosilicate zeolites synthesized in this study (Table 2). When the F− ions were not included in the calculations, PWO and PWW, which give the lowest Si/Al ratio, were determined to be the most favorable structures in the presence of 135TMP with Esyn values of −0.499 and −0.472 eV per T-atom, respectively (Table S8†). This implies that they are capable of incorporating more Al atoms, in good agreement with our previous work showing that the zeolite structure with a higher Al content (or a lower Si/Al ratio) is characterized by a more favorable Esyn value.30,31 However, although PWO was not experimentally synthesized with 135TMP (Table 1), the Esyn value of the observed PWW phase is higher by only 0.027 eV per T-atom than that of PWO. On the other hand, Esyn calculations for 123TMP were found to favor the formation of PWW and PWO again, unlike the experimental result giving RTH. However, since 123TMP-RTH contains only 0.1 F− ions per unit cell, we calculated the Esyn value of 123TMP-RTH with Si/Al ∼ 14, while considering a larger number of anionic combinations to counterbalance the OSDAs (Table 2 and S9†). We note here that the addition of 2.0 F− ions and 2.0 [AlO4/2]− tetrahedra to the RTH unit cell gives a Esyn value of −0.627 eV per T-atom for the RTH formation (Table S9†), which is lower than that in their absence (−0.505 eV per T-atom; Table S8†) and where PWW is favored. As the amount of F− ions occluded in 123TMP-RTH is negligible (Table 2), we speculate that RTH nuclei containing F− ions may ‘catalyse’ the formation of the rest of the crystal without need of further F− encapsulation.
Competition between Al substitution, F− encapsulation and defect formation
Fig. 4 shows an overall view of the Esyn calculation results of 123TMP-RTH, 134TMP-PWO and 135TMP-PWW, using different combinations of framework Al, F− ions and/or SiO−⋯HOSi defects for the anionic species compensating the OSDA positive charge. The Esyn calculation results predict that Al substitution is more favorable than F− encapsulation. For RTH, the Esyn value (−1.043 eV per T-atom) of 123TMP-RTH with 4.0 Al atoms is far more favorable than that (−0.627 eV per T-atom) with 2.0 Al and 2.0 F− ions (Table S9†). The other zeolite–OSDA pairs (i.e. 134TMP-PWO and 135TMP-PWW) in Fig. 4 are all showing a similar trend where Al is more readily incorporated into their structures than F− ions. This supports the previous suggestion that [AlO4/2]− tetrahedra may have stronger interactions with OSDA cations than F− ions.50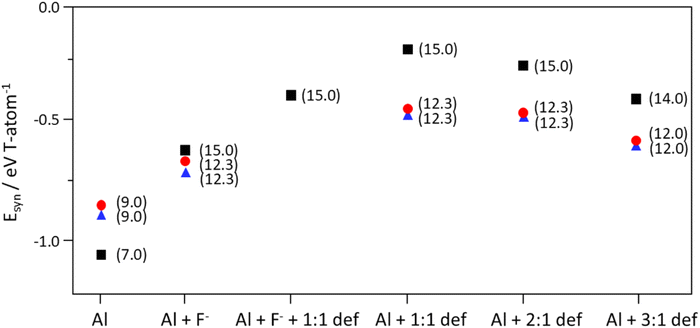 | ||
Fig. 4
E
syn values of zeolite–OSDA pairs with different framework Si/Al ratios and different combinations of anionic species compensating the OSDA positive charge: Al (framework Al atoms); Al + F− (Al atoms + F− ions); Al + F− + def (Al atoms + F− ions + SiO−⋯HOSi defects); Al + def (Al atoms + SiO−⋯HOSi defects). Three different types of defect clusters were considered: (i) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects (1 1 connectivity defects (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def); (ii) 2 1 def); (ii) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects (2 1 connectivity defects (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def); and (iii) 3 1 def); and (iii) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defects (3 1 vacancy defects (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def). The pairs studied are 123TMP-RTH (black squares; Table S9†), 134TMP-PWO (blue triangles; Table S11†) and 135TMP-PWW (red circles; Table S12†). The values in the parenthesis of pair identifications are the zeolite Si/Al ratios. 1 def). The pairs studied are 123TMP-RTH (black squares; Table S9†), 134TMP-PWO (blue triangles; Table S11†) and 135TMP-PWW (red circles; Table S12†). The values in the parenthesis of pair identifications are the zeolite Si/Al ratios. | ||
When 123TMP-RTH has a constant number of framework Al atoms (i.e. 2.0 per unit cell), Esyn becomes more favorable by F− encapsulation than by any type of SiO−⋯HOSi defect formation (Fig. 4 and Table S9†). Therefore, the existence of large amounts of framework defects in aluminosilicate zeolites synthesized here (Table 2) can not be explained by our energetic calculations and suggests the kinetic nature of zeolite crystallization.13 Although thermodynamically less favorable not only than Al substitution but also F− cage encapsulation, SiO−⋯HOSi defects are formed in the presence of Al and fluoride. We suggest that the incorporation of Al and fluoride may be limited by their restricted mobility in the gel and the stringent requirements of transport needed so that they migrate to the specific framework location in which they are close to the compensating positive charge of the OSDA. Opposite to this, SiO−⋯HOSi defects can be formed anywhere since they only require breaking a SiOSi linkage, without mobility constraints. We also believe that if the concentration of defects in zeolite nuclei reaches a significant level, it could then determine phase selectivity, when properly combined with the structure-directing effects of Al substitution and OSDA cations. This may be more likely in the hydroxide-mediated synthesis than in the fluoride-mediated one, given the more defective nature of the former route.14,50
Suggested role of fluoride in nucleation
As mentioned above, when 2.0 F− ions per unit cell are incorporated into the RTH structure, the Esyn value of RTH (−0.627 per T-atom; Table S9†) becomes lower than the values of PWW and PWO (−0.503 and −0.505 eV per T-atom, respectively; Table S8†). Since we were not able to crystallize any aluminosilicate zeolite, as well as pure-silica ITW, in the absence of F− ions, one possible hypothesis is that the nucleation of 123TMP-RTH, 134TMP-PWO and 135TMP-PWW in the aluminosilicate (Si/Al = 10) synthesis mixture, as well as their crystal growth, may begin around the F− ions, but without significant further incorporation of these anions. Thus, if the F− ions play a catalytic role in 123TMP-RTH synthesis, this would explain why the F− content in 134TMP-PWO and 135TMP-PWW is negligible as well (Table 2). In fact, we previously showed that all three levyne (LEV) zeolites with Si/Al = 10.7–11.0 synthesized using the same OSDA cation (N,N-dimethylpiperidinium) but at different HF/OSDA ratios (1.0–3.0) were nearly free of F− ions.33On the other hand, the Esyn difference (−0.689 eV per T-atom) between pure-silica 135TMP-ITW with 2.0 F− ions or 2.0 defects per unit cell is significantly larger than that (−0.412 eV per T-atom) between aluminosilicate 123TMP-RTH with 2.0 framework Al atoms and 2.0 F− ions and the same zeolite but with 2.0 Al atoms and 2.0 defects, highlighting the favorable interactions between fluoride anions and d4r units, absent in RTH,19 of the ITW structure. It thus appears, based on our experimental and theoretical results, that once F− ions are encapsulated within certain small cages of the growing zeolite with aluminosilicate composition, their mobility must be restricted so that further crystal growth mainly occurs around the F− ion-containing small cages without additional F− encapsulation.
Defects in zeolites
We calculated the Esyn values for 135TMP-ITW with various F− and SiO−⋯HOSi combinations (Table S10†). As expected, the Esyn value becomes less favorable when replacing F− ions by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects. This is in line with the well-known fact that the fluoride-mediated zeolite synthesis leads to the formation of far fewer internal defects than the hydroxide-mediated one, which validates our theoretical approach.14
1 connectivity defects. This is in line with the well-known fact that the fluoride-mediated zeolite synthesis leads to the formation of far fewer internal defects than the hydroxide-mediated one, which validates our theoretical approach.14
Three types of defects were considered in Fig. 4, labelled as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def, 2
1 def, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def, and 3
1 def, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def, depending on the respective number (1, 2 or 3) of SiOH groups that make a hydrogen bond with the siloxy (SiO−) group. Defects 1
1 def, depending on the respective number (1, 2 or 3) of SiOH groups that make a hydrogen bond with the siloxy (SiO−) group. Defects 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 belong to the class of ‘connectivity defects’, generated by breaking SiOSi linkages whilst ‘3
1 belong to the class of ‘connectivity defects’, generated by breaking SiOSi linkages whilst ‘3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def’ belongs to the class of ‘vacancy defects’ in which a Si is missing from the zeolite framework. For details on how these defects were generated, see ESI.†
1 def’ belongs to the class of ‘vacancy defects’ in which a Si is missing from the zeolite framework. For details on how these defects were generated, see ESI.†
The structural analysis of the calculated defects shows that in ‘2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def’ the O⋯H distances between two of the SiOH groups and the SiO− group were found to range from 1.43 to 1.52 Å, while the O⋯H distance for the third SiOH group exceeded 2.6 Å, indicating that only two of the SiOH groups form hydrogen bonds with the SiO− group. Since the 1H DQ–SQ MAS NMR data have shown two hydrogen-bonded SiOH per SiO− in defect sites of 134TMP-PWO and 135TMP-PWW, it is clear that experimentally detected defects correspond to the ‘2
1 def’ the O⋯H distances between two of the SiOH groups and the SiO− group were found to range from 1.43 to 1.52 Å, while the O⋯H distance for the third SiOH group exceeded 2.6 Å, indicating that only two of the SiOH groups form hydrogen bonds with the SiO− group. Since the 1H DQ–SQ MAS NMR data have shown two hydrogen-bonded SiOH per SiO− in defect sites of 134TMP-PWO and 135TMP-PWW, it is clear that experimentally detected defects correspond to the ‘2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def’. For our calculated ‘3
1 def’. For our calculated ‘3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 def’, the O⋯H distances between all three SiOH groups and the SiO− group ranged from 1.47 to 1.62 Å, revealing that all three SiOH groups are hydrogen-bonded to the SiO− group at the defect site.
1 def’, the O⋯H distances between all three SiOH groups and the SiO− group ranged from 1.47 to 1.62 Å, revealing that all three SiOH groups are hydrogen-bonded to the SiO− group at the defect site.
For the defects, the Esyn calculation results in Fig. 4 suggest that the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity and 3
1 connectivity and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defects is more favorable than that of 1
1 vacancy defects is more favorable than that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 defects. This is in good agreement with the 1H TQ–SQ MAS NMR results of aluminosilicate PWO and PWW zeolites with a considerable amount of 2
1 defects. This is in good agreement with the 1H TQ–SQ MAS NMR results of aluminosilicate PWO and PWW zeolites with a considerable amount of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects (Fig. 3 and S8†). To further investigate the major defect type present in the PWO and PWW structures, we calculated the average O⋯O distances between SiO− and SiOH groups in as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH zeolites, optimized by density functional theory (DFT) calculations (Table S14†). For all three zeolites, the O⋯O distances were calculated to range between 2.43 and 2.44 Å for 1
1 connectivity defects (Fig. 3 and S8†). To further investigate the major defect type present in the PWO and PWW structures, we calculated the average O⋯O distances between SiO− and SiOH groups in as-synthesized 134TMP-PWO, 135TMP-PWW and 123TMP-RTH zeolites, optimized by density functional theory (DFT) calculations (Table S14†). For all three zeolites, the O⋯O distances were calculated to range between 2.43 and 2.44 Å for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects. In contrast, the 2
1 connectivity defects. In contrast, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect models exhibited slightly longer O⋯O distances, ranging from 2.46 to 2.52 Å. These values are closer to the experimentally determined distance of 2.74 Å, derived from the 1H chemical shift of 9.2 ppm (Fig. 3) using the equation by Eckert et al.,48 supporting the formation of 2
1 connectivity defect models exhibited slightly longer O⋯O distances, ranging from 2.46 to 2.52 Å. These values are closer to the experimentally determined distance of 2.74 Å, derived from the 1H chemical shift of 9.2 ppm (Fig. 3) using the equation by Eckert et al.,48 supporting the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects in PWO and PWW structures.
1 connectivity defects in PWO and PWW structures.
However, the Esyn calculation results in Fig. 4 predict that 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defects are energetically more favorable than the experimentally observed 2
1 vacancy defects are energetically more favorable than the experimentally observed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defects. This suggests that the formation of SiO−⋯HOSi defects and their microstructure in aluminosilicate zeolites during crystallization, at least in fluoride media, may be kinetically rather than thermodynamically controlled, which deserves further study.
1 connectivity defects. This suggests that the formation of SiO−⋯HOSi defects and their microstructure in aluminosilicate zeolites during crystallization, at least in fluoride media, may be kinetically rather than thermodynamically controlled, which deserves further study.
Conclusions
In summary, we found that considerable amounts (11–39%) of OSDA cations in PWO, PWW and RTH zeolites with framework Si/Al ratios of 11.5–13.8 synthesized using different TMP cation isomers in concentrated fluoride media are counterbalanced by internal SiO−···HOSi defects, unlike observed for pure-silica ITW zeolite whose OSDA charge is fully compensated by the fluoride anions occluded in double 4-ring units. The practical absence of F− ions in these aluminosilicate zeolites suggests their role as a ‘catalyst’ in both nucleation and crystal growth processes rather than as a structure-directing agent. Experimental and theoretical analysis shows that zeolite phase selectivity for a given TMP cation in concentrated fluoride media is altered by the type and concentration of negative charge centers, most notably of framework [AlO4/2]− tetrahedra and SiO−···HOSi defects, although Esyn calculations predict that the energetic preference for zeolite–OSDA pairs studied is in the order of defect formation < F− encapsulation < Al substitution. The Esyn calculation results suggest that the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vacancy defect is more stable than the 2
1 vacancy defect is more stable than the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity one. However, the experimental 1H MAS NMR data indicate that the latter defect is predominant in PWO and PWW structures. At least, the Esyn calculation results agree with the experimental results in identifying the 1
1 connectivity one. However, the experimental 1H MAS NMR data indicate that the latter defect is predominant in PWO and PWW structures. At least, the Esyn calculation results agree with the experimental results in identifying the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connectivity defect as the least stable, consistent with their absence in these zeolites. According to these findings, pure-silica zeolites cannot be obtained from aluminosilicate mixtures, which aligns with the well-known fact that natural zeolites are aluminosilicates but not silicates. The results also suggest that defect formation may be entropically favored due to the limited mobility of framework Al atoms and encapsulated F− ions, a hypothesis that warrants further experimental and theoretical investigations. We anticipate that our work will serve as a stimulus to elucidate the exact role of structural defects and the mechanism by which they affect the structure of the crystallizing aluminosilicate zeolite.
1 connectivity defect as the least stable, consistent with their absence in these zeolites. According to these findings, pure-silica zeolites cannot be obtained from aluminosilicate mixtures, which aligns with the well-known fact that natural zeolites are aluminosilicates but not silicates. The results also suggest that defect formation may be entropically favored due to the limited mobility of framework Al atoms and encapsulated F− ions, a hypothesis that warrants further experimental and theoretical investigations. We anticipate that our work will serve as a stimulus to elucidate the exact role of structural defects and the mechanism by which they affect the structure of the crystallizing aluminosilicate zeolite.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 134TMP-PWO and 135TMP-PWW has also been deposited at the CCDC under 2366700 and 2366701.Author contributions
Kingsley Christian Kemp, Ömer F. Altundal, Donghui Jo and Weidong Huang: formal analysis, investigation, methodology. Qiang Wang and Feng Deng: methodology, funding acquisition. German Sastre: conceptualization, methodology, funding acquisition. Suk Bong Hong: conceptualization, methodology, project administration, funding acquisition. All authors co-wrote the paper, which involved writing the original draft as well as review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Creative Research Initiative Program (2021R1A3A-3088711) through the National Research Foundation of Korea, EcoPro HN, the KRICT Institutional Research Program (KK2412-10), GVA (PROMETEO/2021/077), MCIN (Severo Ochoa), ASIC-UPV and SGAI-CSIC for the use of computational facilities, the International Partnership Program of the Chinese Academy of Sciences (314GJH2022022FN) and Hubei International Scientific and Technological Cooperation Program (2024EHA043) and Base (SH2303). We thank H. Koller (University of Münster) and M. A. Camblor (ICMM) for valuable discussion and PAL (Pohang, Korea) for beam time at beamline 2D (D. H. Moon) and 5A (H. H. Lee). PAL is supported by MSIP and POSTECH.Notes and references
- D. Fu and M. E. Davis, Carbon Dioxide Capture with Zeotype Materials, Chem. Soc. Rev., 2022, 51, 9340–9370 RSC.
- E. Pérez-Botella, S. Valencia and F. Rey, Zeolites in Adsorption Processes: State of the Art and Future Prospects, Chem. Rev., 2022, 122, 17647–17695 CrossRef PubMed.
- A. W. Chester, Y. F. Chu, R. M. Dessau, G. T. Kerr and C. T. Kresge, Aluminium-Independent Cation Exchange of Internal Siloxy Groups in ZSM-5 and ZSM-11, J. Chem. Soc., Chem. Commun., 1985, 289–290 CAS.
- W. R. Moser, R. W. Thompson, C.-C. Chiang and H. Tong, Silicon-Rich H-ZSM-5 Catalyzed Conversion of Aqueous Ethanol to Ethylene, J. Catal., 1989, 117, 19–32 CAS.
- F. Vaudry, F. Di Renzo, F. Fajula and P. Schulz, Origin of the Optimum in Catalytic Activity of Zeolite Beta, J. Chem. Soc., Faraday Trans., 1998, 94, 617–621 CAS.
- H. Ichihashi, Vapor Phase Beckmann Rearrangement over a High Silica MFI Zeolite, Stud. Surf. Sci. Catal., 2003, 145, 73–78 CAS.
- K. Barbera, F. Bonino, S. Bordiga, T. V. W. Janssens and P. Beato, Structure–Deactivation Relationship for ZSM-5 Catalysts Governed by Framework Defects, J. Catal., 2011, 280, 196–205 CAS.
- S. Prodinger, H. Shi, S. Eckstein, J. Z. Hu, M. V. Olarte, D. M. Camaioni, M. A. Derewinski and J. A. Lercher, Stability of Zeolites in Aqueous Phase Reactions, Chem. Mater., 2017, 29, 7255–7262 CAS.
- H. Koller, R. F. Lobo, S. L. Burkett and M. E. Davis, SiO−⋯HOSi Hydrogen Bonds in As-Synthesized High-silica Zeolites, J. Phys. Chem., 1995, 99, 12588–12596 CAS.
- I. C. Medeiros-Costa, E. Dib, N. Nesterenko, J.-P. Dath, J.-P. Gilson and S. Mintova, Silanol Defect Engineering and Healing in Zeolites: Opportunities to Fine-Tune Their Properties and Performances, Chem. Soc. Rev., 2021, 50, 11156–11179 CAS.
- E. Dib, J. Grand, S. Mintova and C. Fernandez, Structure-Directing Agent Governs the Location of Silanol Defects in Zeolites, Chem. Mater., 2015, 27, 7577–7579 CAS.
- E. Dib, J. Grand, A. Gedeon, S. Mintova and C. Fernandez, Control the position of framework defects in zeolites by changing the symmetry of organic structure directing agents, Microporous Mesoporous Mater., 2021, 315, 110899 CAS.
- C. S. Cundy and P. A. Cox, The Hydrothermal Synthesis of Zeolites: Precursors, Intermediates and Reaction Mechanism, Microporous Mesoporous Mater., 2005, 82, 1–78 CrossRef CAS.
- M. A. Camblor, L. Villaescusa and M. J. Díaz-Cabañas, Synthesis of All-Silica and High-Silica Molecular Sieves in Fluoride Media, Top. Catal., 1999, 9, 59–76 CrossRef CAS.
- A. W. Burton, S. I. Zones and S. Elomari, The Chemistry of Phase Selectivity in the Synthesis of High-Silica Zeolites, Curr. Opin. Colloid Interface Sci., 2005, 10, 211–219 CrossRef CAS.
- G. Brunklaus, H. Koller and S. I. Zones, Defect Models of As-Made High-Silica Zeolites: Clusters of Hydrogen-Bonds and Their Interaction with the Organic Structure-Directing Agents Determined from 1H Double and Triple Quantum NMR Spectroscopy, Angew. Chem., Int. Ed., 2016, 55, 14459–14463 CrossRef CAS PubMed.
- D. Jo, G. T. Park, J. Shin and S. B. Hong, A Zeolite Family Nonjointly Built from the 1,3-Stellated Cubic Building Unit, Angew. Chem., Int. Ed., 2018, 57, 2199–2203 CrossRef CAS PubMed.
- J. Shin, D. Jo and S. B. Hong, Rediscovery of the Importance of Inorganic Synthesis Parameters in the Search for New Zeolites, Acc. Chem. Res., 2019, 52, 1419–1427 CrossRef CAS PubMed.
- Database of Zeolite Structures, https://www.iza-structure.org/databases/, accessed February 2025 Search PubMed.
- E. W. Valyocsik, Synthesis of Zeolite ZSM-22 with a Heterocyclic Organic Compound, US Pat., 4481177A, 1984 Search PubMed.
- W. J. Smith, J. Dewing and J. Dwyer, Zeolite Synthesis in the SiO2-Al2O3-Na2O-Pyridine-H2O System, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 3623–3628 RSC.
- S. J. Weigel, J.-C. Gabriel, E. G. Puebla, A. M. Bravo, N. J. Henson, L. M. Bull and A. K. Cheetham, Structure-Directing Effects in Zeolite Synthesis: A Single-Crystal X-Ray Diffraction, 29Si MAS NMR, and Computational Study of the Competitive Formation of Siliceous Ferrierite and Dodecasil-3C (ZSM-39), J. Am. Chem. Soc., 1996, 118, 2427–2435 CAS.
- G. Cao, M. J. Shah and S. C. Reyes, Synthesis of ITQ-12, US Pat., 7837979B2, 2010 Search PubMed.
- L. Zhu, L. Ren, S. Zeng, C. Yang, H. Zhang, X. Meng, M. Rigutto, A. van der Made and F.-S. Xiao, High Temperature Synthesis of High Silica Zeolite Y with Good Crystallinity in the Presence of N-Methylpyridinium Iodide, Chem. Commun., 2013, 49, 10495–10497 CAS.
- S. Cadars, M. Allix, D. H. Brouwer, R. Shayib, M. Suchomel, M. N. Garaga, A. Rakhmatullin, A. W. Burton, S. I. Zones, D. Massiot and B. F. Chmelka, Long- and Short-Range Constraints for the Structure Determination of Layered Silicates with Stacking Disorder, Chem. Mater., 2014, 26, 6994–7008 CAS.
- H. Xu, Q. Wu, Y. Chu, J. Jiang, L. Zhang, S. Pan, C. Zhang, L. Zhu, F. Deng, X. Meng, S. Maurer, R. McGuire, A.-N. Parvulescu, U. Müller and F.-S. Xiao, Efficient Synthesis of Aluminosilicate RTH Zeolite with Good Catalytic Performances in NH3-SCR and MTO Reactions, J. Mater. Chem. A, 2018, 6, 8705–8711 RSC.
- Y. Luo, S. Smeets, F. Peng, A. S. Etman, Z. Wang, J. Sun and W. Yang, Synthesis and Structure Determination of Large-Pore Zeolite SCM-14, Chem.–Eur. J., 2017, 23, 16829–16834 CrossRef CAS PubMed.
- Y. Luo, S. Smeets, Z. Wang, J. Sun and W. Yang, Synthesis and Structure Determination of SCM-15: A 3D Large Pore Zeolite with Interconnected Straight 12×12×10-Ring Channels, Chem.–Eur. J., 2019, 25, 2184–2188 CrossRef CAS PubMed.
- Y. Ma, X. Tang, J. Hu, Y. Ma, W. Chen, Z. Liu, S. Han, C. Xu, Q. Wu, A. Zheng, L. Zhu, X. Meng and F.-S. Xiao, Design of a Small Organic Template for the Synthesis of Self-Pillared Pentasil Zeolite Nanosheets, J. Am. Chem. Soc., 2022, 144, 6270–6277 CrossRef CAS PubMed.
- Ö. F. Altundal, S. Leon and G. Sastre, Different Zeolite Phases Obtained with the Same Organic Structure Directing Agent in the Presence and Absence of Aluminum: The Directing Role of Aluminum in the Synthesis of Zeolites, J. Phys. Chem. C, 2023, 127, 10797–10805 CrossRef.
- Ö. F. Altundal and G. Sastre, The Directing Role of Aluminum in the Synthesis of PST-21 (PWO), PST-22 (PWW), and ERS-7 (ESV) Zeolites, J. Phys. Chem. C, 2023, 127, 15648–15656 Search PubMed.
- J. Zeng, G. Sastre and S. B. Hong, Capturing Different Intermediate Phases During Zeolite Synthesis, J. Am. Chem. Soc., 2024, 146, 27468–27474 CAS.
- J. Bae and S. B. Hong, Zeolite Synthesis by the Excess Fluoride Approach in the Presence of Piperidinium-based Structure-Directing Agents, Microporous Mesoporous Mater., 2021, 327, 11142 Search PubMed.
- H. M. Rietveld, A Profile Refinement Method for Nuclear and Magnetic Structures, J. Appl. Crystallogr., 1969, 2, 65–71 CAS.
- M. Carravetta, M. Edén, X. Zhao, A. Brinkmann and M. H. Levitt, Symmetry Principles for the Design of Radiofrequency Pulse Sequences in the Nuclear Magnetic Resonance of Rotating Solids, Chem. Phys. Lett., 2000, 321, 205–215 CAS.
- Y. Nishiyama, V. Agarwal and R. Zhang, t1-Noise Suppression by γ-Free Recoupling Sequences in Solid-State NMR for Structural Characterization of Fully Protonated Molecules at Fast MAS, J. Phys. Chem. C, 2020, 124, 26332–26343 CAS.
- M. Gálvez-Llompart, A. Cantín, F. Rey and G. Sastre, Computational Screening of Structure Directing Agents for the Synthesis of Zeolites. A Simplified Model, Z. Kristallogr., 2019, 234, 451–460 Search PubMed.
- J. D. Gale, GULP: A Computer Program for the Symmetry-Adapted Simulation of Solids, J. Chem. Soc., Faraday Trans., 1997, 93, 629–637 CAS.
- J. E. Schmidt, M. A. Deimund, D. Xie and M. E. Davis, Synthesis of RTH-Type Zeolites Using a Diverse Library of Imidazolium Cations, Chem. Mater., 2015, 27, 3756–3762 CrossRef CAS.
- D. Jo, J. B. Lim, T. Ryu, I.-S. Nam, M. A. Camblor and S. B. Hong, Unseeded Hydroxide-Mediated Synthesis and CO2 Adsorption Properties of an Aluminosilicate Zeolite with the RTH Topology, J. Mater. Chem. A, 2015, 3, 19322–19329 RSC.
- X. B. Yang, M. A. Camblor, Y. Lee, H. M. Liu and D. H. Olson, Synthesis and Crystal Structure of As-Synthesized and Calcined Pure Silica Zeolite ITQ-12, J. Am. Chem. Soc., 2004, 126, 10403–10409 CrossRef CAS PubMed.
- B. Marler, U. Werthmann and H. Gies, Synthesis and Structure of Pure Silica-RUB-10 (Structure Type: RUT) Obtained with Pyrrolidine as the Structure Directing Agent, Microporous Mesoporous Mater., 2001, 43, 329–340 CrossRef CAS.
- P. A. Barrett, T. Boix, M. Puche, D. H. Olson, E. Jordan, H. Koller and M. A. Camblor, ITQ-12: A New Microporous Silica Polymorph and its Potential for Light Hydrocarbon Separations, Chem. Commun., 2003, 2114–2115 RSC.
- H. Koller, A. Wölker, L. A. Villaescusa, M. J. Díaz-Cabañas, S. Valencia and M. A. Camblor, Five-coordinate silicon in high-silica zeolites, J. Am. Chem. Soc., 1999, 121, 3368–3376 CrossRef CAS.
- S. I. Zones, R. J. Darton, R. Morris and S.-J. Hwang, Studies on the Role of Fluoride Ion vs. Reaction Concentration in Zeolite Synthesis, J. Phys. Chem. B, 2005, 109, 652–661 CrossRef CAS PubMed.
- R. M. Shayib, N. C. George, R. Seshadri, A. W. Burton, S. I. Zones and B. F. Chmelka, Structure-Directing Roles and Interactions of Fluoride and Organocations with Siliceous Zeolite Frameworks, J. Am. Chem. Soc., 2011, 133, 18728–18741 CrossRef CAS PubMed.
- M. Wiebcke, Structural Links between Zeolite-Type and Clathrate Hydrate-Type Materials, J. Chem. Soc., Chem. Commun., 1991, 2, 1507–1508 Search PubMed.
- H. Eckert, J. P. Yesinowski, L. A. Silver and E. M. Stolper, Water in Silicate Glasses: Quantitation and Structural Studies by Proton Solid Echo and Magic Angle Spinning NMR Methods, J. Phys. Chem., 1988, 92, 2055–2064 CAS.
- C. Schroeder, S. I. Zones, M. R. Hansen and H. Koller, Hydrogen Bonds Dominate Brønsted Acid Sites in Zeolite SSZ-42: A Classification of Their Diversity, Angew. Chem., Int. Ed., 2022, 61, e202109313 CrossRef CAS PubMed.
- M. A. Camblor, A. Corma and S. Valencia, Synthesis in Fluoride Media and Characterisation of Aluminosilicate Zeolite Beta, J. Mater. Chem., 1998, 8, 2137–2145 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: OSDA and zeolite preparation, analytical and computational methods, and characterization results. CIF files of all optimized geometries of the zeolite–OSDA systems in Tables S6–S13. CCDC 2366700 (134TMP-PWO) and 2366701 (135TMP-PWW). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00899a |
| This journal is © The Royal Society of Chemistry 2025 |