Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isothiourea-catalysed enantioselective synthesis of phosphonate-functionalised β-lactones†
Ffion M.
Platt‡
 a,
Yihong
Wang‡
a,
David B.
Cordes
a,
Yihong
Wang‡
a,
David B.
Cordes
 a,
Aidan P.
McKay
a,
Alexandra M. Z.
Slawin
a,
Aidan P.
McKay
a,
Alexandra M. Z.
Slawin
 a,
Heena
Panchal
b and
Andrew D.
Smith
a,
Heena
Panchal
b and
Andrew D.
Smith
 *a
*a
aEaStCHEM, School of Chemistry, University of St Andrews, St Andrews, Fife KY16 9ST, UK. E-mail: ads10@st-andrews.ac.uk
bChemical Development, PT&D, AstraZeneca, Etherow Building, Silk Road Business Park, Charter Way, Macclesfield, Cheshire SK10 2NA, UK
First published on 6th March 2025
Abstract
Despite growing interest in the reactivity and biological activity of phosphonate-containing molecules, the application of α-ketophosphonates in enantioselective formal [2 + 2] cycloadditions to generate β-lactones bearing a pendant phosphonate group remains unreported. In this manuscript, a highly diastereo- and enantioselective isothiourea-catalysed formal [2 + 2] cycloaddition of both alkyl- and aryl substituted C(1)-ammonium enolates and α-ketophosphonates is established. This strategy allows a mild, practical and scalable approach to highly enantioenriched C(3)-unsubstituted and C(3)-alkyl β-lactones bearing a phosphonate motif from their corresponding α-silyl acids, via a desilylative pathway (30 examples, up to 98%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Alternatively, the use of (hetero)arylacetic acids allows the preparation of C(3)-(hetero)aryl β-lactones to be accessed in high yields and stereocontrol (19 examples, up to 98%, >95
1 er). Alternatively, the use of (hetero)arylacetic acids allows the preparation of C(3)-(hetero)aryl β-lactones to be accessed in high yields and stereocontrol (19 examples, up to 98%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, 99
5 dr, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er).
1 er).
Introduction
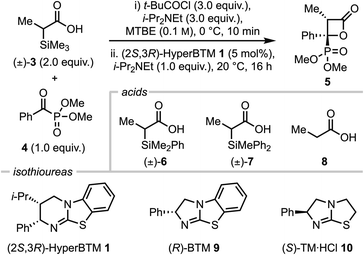
Over the last two decades, heterocyclic molecules containing a phosphonic acid or related organophosphorus derivative have drawn broad interest due to their versatile properties and widespread biological activities, finding use as medicines, herbicides, optoelectronic materials and flame retardants.1–4 Representative examples include the broad-spectrum antibiotic fosfomycin, the herbicide glyphosate and optoelectronic material benzo[b]phospholoxide (Fig. 1a).1–3 Given the significant interest in these functionalities, a number of catalytic, enantioselective processes have been developed to generate stereodefined products bearing the phosphonate functionality via the use of α-ketophosphonates.5–11 In the same vein, β-lactones are versatile motifs which are prevalent in medicinal chemistry both as synthetic building blocks and as warheads in several biologically active compounds (Fig. 1a).12 Consequently, many enantioselective protocols exist for their synthesis,13–16 among which the use of C(1)-ammonium enolates generated from chiral tertiary amines (including the use of cinchona alkaloids, planar chiral DMAP derivatives and isothioureas) have been widely reported.17C(1)-ammonium enolates are powerful reactive intermediates that have been harnessed for enantioselective C–C and C–X bond formation with a broad range of electrophiles. Classically, catalyst turnover occurred through intramolecular cyclisation to afford lactone or lactam products, however intermolecular turnover can be provided using electron-poor phenoxides, which has significantly broadened the scope of electrophiles compatible with this approach.18–20 Despite being widely applied, until recently intermolecular reactions with electrophiles involving C(1)-ammonium enolate formation from carboxylic acid starting materials using isothiourea catalysts (such as HyperBTM 1) was limited to (hetero)aryl-acetic acid derivatives. This presumably reflects the acidity of the α-C–H bond within an intermediate acyl ammonium ion pair for deprotonation to occur (Fig. 1b). To broaden the utility of C(1)-ammonium enolate reactivity to alkyl-substituted C(1)-ammonium enolates, we recently harnessed a desilylative approach from α-silyl carboxylic acids, expanding the scope of substitution within C(1)-ammonium enolates to include benzyl, alkyl and even acetic acid-derivatives (Fig. 1b).21
In previous work, Chi et al. demonstrated the enantioselective, NHC-catalysed formal [4 + 2] cycloaddition of α,β-unsaturated aldehydes with α-ketophosphonates in the presence of a stoichiometric oxidant to give 2-pyranylphosphonates (Fig. 1c).22 To the best of our knowledge enantioselective formal [2 + 2] cycloadditions using α-ketophosphonates to access β-lactones with a pendant phosphonate group remain unexplored to date. Building on these precedents, we describe a mild and highly enantioselective organocatalytic formal [2 + 2] cycloaddition of both alkyl C(1)-ammonium enolates (formed via a desilylative pathway) and (hetero)aryl C(1)-ammonium enolates (formed by a deprotonation pathway) with a range of α-ketophosphonates using the isothiourea (2S,3R)-HyperBTM 1 (Fig. 1d). This methodology gives access to a range of stereodefined β-lactones bearing two stereogenic centres, one of which bears a pendant phosphonate group, in excellent stereoselectivity.
Results and discussion
Investigation of optimal reaction conditions: reactivity of alkyl substituted C(1)-ammonium enolates by desilylation
Using α-trimethylsilyl propionic acid 3 and dimethyl benzoylphosphonate 4 as model substrates, several parameters were screened to find optimal conditions, including acid, catalyst, temperature and solvent (Table 1). Initially, using racemic acid 3 (2 equiv.), t-BuCOCl (3 equiv.) and i-Pr2NEt (3 equiv.) in MTBE to form the mixed anhydride in situ, followed by i-Pr2NEt (1 equiv.), (2S,3R)-HyperBTM 1 (5 mol%) and α-ketophosphonate 4 (1 equiv.) at room temperature gave the desired β-lactone 5 in 80% yield with exceptional diastereo- and enantioselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er) (entry 1). The use of alternative racemic acid 6 led to reduced yield, albeit with retention of high diastereo- and enantioselectivity (72%, >95
1 er) (entry 1). The use of alternative racemic acid 6 led to reduced yield, albeit with retention of high diastereo- and enantioselectivity (72%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er, entry 2), however further variation of the silyl group using racemic acid 7 led to significantly reduced yield (<5%, entry 3). Control reactions showed that the α-silyl-substituent is necessary for reactivity in this process, as the use of propionic acid 8 (for subsequent mixed anhydride formation, entry 4) or propionic anhydride as starting material (entry 5) led to no formation of β-lactone 5. Changing to alternative isothiourea catalysts 9 and 10 led to no reaction (entries 6 and 7) and reducing the stoichiometry of acid 3 led to a reduction in yield, although maintained high stereoselectivity (35%, >95
1 er, entry 2), however further variation of the silyl group using racemic acid 7 led to significantly reduced yield (<5%, entry 3). Control reactions showed that the α-silyl-substituent is necessary for reactivity in this process, as the use of propionic acid 8 (for subsequent mixed anhydride formation, entry 4) or propionic anhydride as starting material (entry 5) led to no formation of β-lactone 5. Changing to alternative isothiourea catalysts 9 and 10 led to no reaction (entries 6 and 7) and reducing the stoichiometry of acid 3 led to a reduction in yield, although maintained high stereoselectivity (35%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, entry 8). When the temperature was increased to 25 °C (entry 9) β-lactone 5 was obtained in 98% yield, with exceptional stereocontrol (>95
5 dr, entry 8). When the temperature was increased to 25 °C (entry 9) β-lactone 5 was obtained in 98% yield, with exceptional stereocontrol (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Variation of the solvent at 25 °C saw no improvement upon these conditions (entries 10–13). Hence, entry 9 was chosen as the optimal reaction conditions.
1 er). Variation of the solvent at 25 °C saw no improvement upon these conditions (entries 10–13). Hence, entry 9 was chosen as the optimal reaction conditions.
| Entry | Variation from standard | Yieldb/% | drc | erd |
|---|---|---|---|---|
| a t-BuCOCl (1.2 mmol), i-Pr2NEt (1.2 mmol) and acid 3 (0.8 mmol) in MTBE (4 mL, 0.1 M) was stirred at 0 °C for 10 min before the addition of i-Pr2NEt (0.4 mmol), α-ketophosphonate 4 (0.4 mmol) and (2S,3R)-HyperBTM 1 (5 mol%) at 20 °C for 16 h. Temperatures of 25 °C were maintained using an oil bath. MTBE = methyl tert-butyl ether. BTM = benzotetramisole. TM = tetramisole. b Isolated yield. c Determined by 1H NMR of crude reaction mixture. d Determined by HPLC analysis on a chiral stationary phase. e propionic anhydride (1.0 mmol), i-Pr2NEt (0.5 mmol), α-ketophosphonate 4 (0.4 mmol) and (2S,3R)-HyperBTM 1 (5 mol%) in MTBE (4 mL) at rt for 16 h. | ||||
| 1 | None | 80 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Acid 6 | 72 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Acid 7 | — | — | — |
| 4 | Acid 8 | — | — | — |
| 5 | Propionic anhydridee | — | — | — |
| 6 | (R)-BTM 9 | — | — | — |
| 7 | (S)-TM·HCl 10 | — | — | — |
| 8 | Acid 3 (1 equiv.) | 35 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 25°C | 98 |
>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
| 10 | CH2Cl2, 25 °C | 75 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 11 | CH3CN, 25 °C | 78 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | Toluene, 25 °C | 80 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | THF, 25 °C | 25 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
Scope and limitations
With optimised conditions in hand, the scope and limitations of the developed process were investigated through variation of the phosphonate alkyl group under the standard reaction conditions (Fig. 2). Increasing substitution of the phosphonate group from methyl to ethyl and iso-propyl gave β-lactones 11 and 12 in reduced yield (80% and 50% respectively) compared to 5 but with exceptional stereocontrol (both >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Conversely, taking unsubstituted α-silyl acetic acid with α-ketophosphonates of increasing substitution gave C(3)-unsubstituted β-lactones 13–15 in high yields (86–99%) but reduced enantioselectivity (87
1 er). Conversely, taking unsubstituted α-silyl acetic acid with α-ketophosphonates of increasing substitution gave C(3)-unsubstituted β-lactones 13–15 in high yields (86–99%) but reduced enantioselectivity (87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13–89
13–89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er).
11 er).
Subsequent work varied the steric and electronic nature of the aryl substituent within the benzoylphosphonate. The incorporation of electron-withdrawing groups in the 4-position were tolerated well, with compounds 16–19 (4-F3CC6H4-, 4-CO2MeC6H4, 4-ClC6H4 and 4-FC6H4) prepared in good to high yields (72–90%) with exceptional stereoselectivity (all >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Other aryl C(4)-substituents within the benzoylphosphonate were also well-tolerated, giving 4-Me β-lactone 20 and 4-Ph β-lactone 21 in good yields and stereoselectivities (67–78%, both >95
1 er). Other aryl C(4)-substituents within the benzoylphosphonate were also well-tolerated, giving 4-Me β-lactone 20 and 4-Ph β-lactone 21 in good yields and stereoselectivities (67–78%, both >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Further substitution patterns and groups were also compatible, with 3-MeOC6H4, 3-BrC6H4, 3-MeC6H4 and 3,5-(MeO)2C6H3 all providing efficient access to the corresponding β-lactones 22–25 with high diastereo- and enantioselectivity (62–90%, all >95
1 er). Further substitution patterns and groups were also compatible, with 3-MeOC6H4, 3-BrC6H4, 3-MeC6H4 and 3,5-(MeO)2C6H3 all providing efficient access to the corresponding β-lactones 22–25 with high diastereo- and enantioselectivity (62–90%, all >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er).
1 er).
Reduced acid stoichiometry (1.6 equiv.) was used with a 2-naphthoylphosphonate, facilitating access to the corresponding β-lactone 26 in 81% yield with exceptional stereoselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). In addition to substituted benzoylphosphonates, heterocyclic α-ketophosphonates were also well-tolerated, providing C(4)-3-furyl and 3-thiophenyl β-lactones 27 and 28 in high yields and stereoselectivities (68–82%, both >95
1 er). In addition to substituted benzoylphosphonates, heterocyclic α-ketophosphonates were also well-tolerated, providing C(4)-3-furyl and 3-thiophenyl β-lactones 27 and 28 in high yields and stereoselectivities (68–82%, both >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Single crystal X-ray diffraction of both 18 and 26 allowed confirmation of their relative and absolute configuration, which is consistent with the stereochemical rationale previously identified within the group for other formal [2 + 2]-cycloadditions using C(1)-ammonium enolates.23 Pleasingly, when dimethyl acetylphosphonate was employed, β-lactone 29 bearing a C(4)-methyl substituent was formed in 61% yield, with >95
1 er). Single crystal X-ray diffraction of both 18 and 26 allowed confirmation of their relative and absolute configuration, which is consistent with the stereochemical rationale previously identified within the group for other formal [2 + 2]-cycloadditions using C(1)-ammonium enolates.23 Pleasingly, when dimethyl acetylphosphonate was employed, β-lactone 29 bearing a C(4)-methyl substituent was formed in 61% yield, with >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr and >99
5 dr and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er.
1 er.
The reaction was scaled up, and β-lactone 30 was formed on gram scale with no erosion of stereoselectivity (76%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Furthermore, treatment of 30 with excess morpholine under ambient conditions provided ring-opened α-hydroxyl-β-amidylphosphonate 31 bearing two contiguous stereocentres in high yield, with no detriment to the diastereo- or enantioselectivity (94%, >95
1 er). Furthermore, treatment of 30 with excess morpholine under ambient conditions provided ring-opened α-hydroxyl-β-amidylphosphonate 31 bearing two contiguous stereocentres in high yield, with no detriment to the diastereo- or enantioselectivity (94%, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). The limitations of this protocol were discovered throughout the course of these investigations. Strongly electron-donating groups on the aryl phosphonate component were not well-tolerated, with for example 4-MeOC6H4 substituted α-ketophosphonate 32 leading to less than 5% conversion to the corresponding β-lactone. Furthermore, 2-methylbenzoyl phosphonate 33 was significantly less reactive than its 4-methyl counterpart, leading to <5% conversion of the starting material. While use of 3-furoylphosphonate gave β-lactone 27 in good yield and the product was bench stable, the 2-furoylphosphonate 34 gave no β-lactone product, but instead alkene 35 was obtained in 58% yield, which presumably arises from decarboxylation of the initially formed β-lactone product.24
1 er). The limitations of this protocol were discovered throughout the course of these investigations. Strongly electron-donating groups on the aryl phosphonate component were not well-tolerated, with for example 4-MeOC6H4 substituted α-ketophosphonate 32 leading to less than 5% conversion to the corresponding β-lactone. Furthermore, 2-methylbenzoyl phosphonate 33 was significantly less reactive than its 4-methyl counterpart, leading to <5% conversion of the starting material. While use of 3-furoylphosphonate gave β-lactone 27 in good yield and the product was bench stable, the 2-furoylphosphonate 34 gave no β-lactone product, but instead alkene 35 was obtained in 58% yield, which presumably arises from decarboxylation of the initially formed β-lactone product.24
Further investigations focused on the scope of the α-silyl carboxylic acid. A variety of alkyl (Me, Et), as well as allyl and propargyl groups were well-tolerated, giving the corresponding β-lactones 36–39 in high yields (77–98%) and exceptional diastereo- and enantioselectivity (all >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Benzyl-derived acids, including those bearing 4-MeC6H4 and 4-FC6H4 substituents, also showed excellent reactivity, affording β-lactones 40–42 in good yields (76–82%) with consistently high stereocontrol (all >95
1 er). Benzyl-derived acids, including those bearing 4-MeC6H4 and 4-FC6H4 substituents, also showed excellent reactivity, affording β-lactones 40–42 in good yields (76–82%) with consistently high stereocontrol (all >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Furthermore, 3-(naphthalen-2-yl)propanoic acid delivered the corresponding β-lactone 43 in 80% yield, with >95
1 er). Furthermore, 3-(naphthalen-2-yl)propanoic acid delivered the corresponding β-lactone 43 in 80% yield, with >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr and >99
5 dr and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. The reaction of α-trimethylsilyl phenylacetic acid was also investigated, which provided β-lactone 44 in only a moderate yield and with a noticeable decrease in diastereoselectivity, although high enantioselectivity was maintained (40%, 90
1 er. The reaction of α-trimethylsilyl phenylacetic acid was also investigated, which provided β-lactone 44 in only a moderate yield and with a noticeable decrease in diastereoselectivity, although high enantioselectivity was maintained (40%, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr, 99
10 dr, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Disappointingly, branched iso-propyl α-silyl acid 45 showed no reactivity under the reaction conditions.
1 er). Disappointingly, branched iso-propyl α-silyl acid 45 showed no reactivity under the reaction conditions.
Probing double stereodifferentiation: matched and mismatched reaction pairings with enantioenriched anhydride
As a control, rather than (±)-α-alkyl-α-silyl mixed anhydride, the use of enantioenriched α-silyl acid 6 (89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er) was investigated upon reaction with the enantiomers of HyperBTM 1 (Fig. 3). Using (2S,3R)-1 gave full conversion to (2R,3R)-30 within 10 hours in 72% yield and excellent stereocontrol (>95
11 er) was investigated upon reaction with the enantiomers of HyperBTM 1 (Fig. 3). Using (2S,3R)-1 gave full conversion to (2R,3R)-30 within 10 hours in 72% yield and excellent stereocontrol (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). However, the use of enantiomeric (2R,3S)-1 gave (2S,3S)-30 with identical stereocontrol (>95
1 er). However, the use of enantiomeric (2R,3S)-1 gave (2S,3S)-30 with identical stereocontrol (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, >99
5 dr, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er) but took 20 hours to reach completion. This is consistent with the previously observed kinetic resolution of the α-silyl anhydride taking place upon acylation in this protocol.21
1 er) but took 20 hours to reach completion. This is consistent with the previously observed kinetic resolution of the α-silyl anhydride taking place upon acylation in this protocol.21
 | ||
| Fig. 3 Probing double stereodifferentiation. All yields are isolated yields, dr determined by 1H NMR of crude reaction mixture, er determined by HPLC analysis on a chiral stationary phase. | ||
Re-optimisation of reaction conditions: (hetero)aryl substituted C(1)-ammonium enolates by deprotonation
Since the synthesis of β-lactone 44 from an aryl substituted-silyl acid had proceeded with reduced yield and diastereoselectivity compared to C(3)-alkyl or benzyl substituents, further work considered the propensity for formation of β-lactones bearing a C(3)-aryl substituent via a deprotonation route (Table 2). Building upon previous work that demonstrated the ability of phenylacetic anhydrides (either isolated or prepared in situ) to act as ammonium enolate precursors,23 the corresponding pivalic anhydride was prepared in situ from phenylacetic acid 47, with addition of α-ketophosphonate 4 leading to β-lactone 44 in 39% yield, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr and 98
10 dr and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er (entry 1). Reducing the equivalents of base in the generation of the mixed pivalic anhydride led to a modest increase in yield, whilst maintaining high stereoselectivity (entry 2). Further variation in stoichiometry (see Tables S7 to S10†) led to an optimal ratio of 3
2 er (entry 1). Reducing the equivalents of base in the generation of the mixed pivalic anhydride led to a modest increase in yield, whilst maintaining high stereoselectivity (entry 2). Further variation in stoichiometry (see Tables S7 to S10†) led to an optimal ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 of acid 47
3 of acid 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t-BuCOCl
t-BuCOCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2NEt. Further optimisation showed that varying the rate of acid had a significant impact on the reaction yield (entries 3–6), with addition of the acid over 5 min optimal, giving β-lactone 44 in an isolated yield of 74% with 90
i-Pr2NEt. Further optimisation showed that varying the rate of acid had a significant impact on the reaction yield (entries 3–6), with addition of the acid over 5 min optimal, giving β-lactone 44 in an isolated yield of 74% with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr and 96
10 dr and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er.
4 er.
| Entry | Variation | Yieldd/% | dre | erf |
|---|---|---|---|---|
| a t-BuCOCl (0.6 mmol), i-Pr2NEt (0.6 mmol) and acid 47 (0.4 mmol) in MTBE (2 mL, 0.1 M) was stirred at 0 °C for 10 min, then i-Pr2NEt (0.2 mmol), α-ketophosphonate 4 (0.2 mmol) and (2S,3R)-HyperBTM 1 (5 mol%) at 25 °C for 16 h. b 1.6 mL MTBE used in (i), then 0.4 mL added in (ii) to ensure all components rinsed into flask. c Rate of addition controlled using syringe pump, 0.32 mL min−1 for 5 min or 0.11 mL min−1 for 15 min. d Isolated yield. e Determined by 1H NMR of crude reaction mixture. f Determined by HPLC analysis on a chiral stationary phase. | ||||
| 1 | None | 39 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | 2 equiv. of i-Pr2NEt in (i) | 55 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3b | 3 equiv. 47, added over 1 min | 72 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 4b , | 3 equiv. 47, added over 5 min | 74 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 4
|
| 5b,c | 3 equiv. 47, added over 15 min | 56 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6 | (2S,3R)-HyperBTM (2.5 mol%) | 38 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
Probing in situ epimerisation of C(3)-aryl β-lactones under reaction conditions
During the optimisation of this reaction, significant variation in product diastereoselectivity was observed and it was considered that this may be due to epimerisation of the β-lactone product. To evaluate the potential epimerisation of β-lactone 44 under the reaction conditions, isolated diastereomerically enriched samples of (±)-anti- and (±)-syn-44 (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 10
6 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 dranti:syn respectively) were exposed to i-Pr2NEt (20.0 equiv.) in MTBE, at 25 °C for 2 h (Fig. 4). After this time the diastereomeric ratio of anti-44 was reduced from 94
90 dranti:syn respectively) were exposed to i-Pr2NEt (20.0 equiv.) in MTBE, at 25 °C for 2 h (Fig. 4). After this time the diastereomeric ratio of anti-44 was reduced from 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 82
6 to 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 dranti:syn, while anti-44 (68
18 dranti:syn, while anti-44 (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 dranti:syn) was also favoured starting from syn-β-lactone 44 (90
32 dranti:syn) was also favoured starting from syn-β-lactone 44 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 drsyn:anti). Consistent with previous observations, it is likely that the observed epimerisation occurs through base-mediated enolisation at the β-lactone C(3)-position, then reprotonation.25–28
10 drsyn:anti). Consistent with previous observations, it is likely that the observed epimerisation occurs through base-mediated enolisation at the β-lactone C(3)-position, then reprotonation.25–28
Scope and limitations
With optimised conditions in hand, the generality of this methodology was investigated through variation of the arylacetic acid component (Fig. 5). Electron-donating groups in the aryl C(4)-position were well tolerated, giving β-lactones 48 and 49 in good yields, with high diastereo- and enantioselectivity (70–74%, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–>95
9–>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, both 95
5 dr, both 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). Halogens were also well-tolerated in this position (61–84%, 94
5 er). Halogens were also well-tolerated in this position (61–84%, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–95
6–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er), although β-lactones 50 and 51 were isolated with reduced diastereoselectivity (82
5 er), although β-lactones 50 and 51 were isolated with reduced diastereoselectivity (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18–84
18–84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 dr), which may be due to the increased susceptibility towards epimerisation at this stereocentre under the reaction conditions. Further variation around the aryl ring was also successful, with 3-OMe, 3,5-OMe and even 3-CF3 β-lactones 52–54 formed in synthetically useful yields, with good diastereo- and enantioselectivities (41–73%, 86
16 dr), which may be due to the increased susceptibility towards epimerisation at this stereocentre under the reaction conditions. Further variation around the aryl ring was also successful, with 3-OMe, 3,5-OMe and even 3-CF3 β-lactones 52–54 formed in synthetically useful yields, with good diastereo- and enantioselectivities (41–73%, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14–89
14–89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr, 90
11 dr, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–95
10–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). Pleasingly, heteroaryl substituents were also well-tolerated, with β-lactones 55–57 prepared in good to high yields with high diastereo- and enantioselectivity (69–90%, 86
5 er). Pleasingly, heteroaryl substituents were also well-tolerated, with β-lactones 55–57 prepared in good to high yields with high diastereo- and enantioselectivity (69–90%, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14–89
14–89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr, 90
11 dr, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–98
10–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er). Single crystal X-ray analysis of 55 confirmed the relative and absolute configuration, which aligns with that within the C(3)-alkyl scope. Subsequent investigations focused on the scope of the α-ketophosphonate. Incorporation of the electron-withdrawing 4-F3CC6H4 substituents gave a disappointing 48% yield of β-lactone 58, although good diastereoselectivity and excellent enantioselectivity were observed (89
2 er). Single crystal X-ray analysis of 55 confirmed the relative and absolute configuration, which aligns with that within the C(3)-alkyl scope. Subsequent investigations focused on the scope of the α-ketophosphonate. Incorporation of the electron-withdrawing 4-F3CC6H4 substituents gave a disappointing 48% yield of β-lactone 58, although good diastereoselectivity and excellent enantioselectivity were observed (89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr, 99
11 dr, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Pleasingly, the reaction of both 4-tolylacetic acid and 3-thiophenylacetic acid with 4-ClC6H4– and 4-FC6H5-substituted benzoylphosphonates worked well, giving β-lactones 59–62 in high yields, with high diastereoselectivity and excellent enantioselectivity (77–98%, 83
1 er). Pleasingly, the reaction of both 4-tolylacetic acid and 3-thiophenylacetic acid with 4-ClC6H4– and 4-FC6H5-substituted benzoylphosphonates worked well, giving β-lactones 59–62 in high yields, with high diastereoselectivity and excellent enantioselectivity (77–98%, 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17–>95
17–>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, 95
5 dr, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–98
5–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er).
2 er).
 | ||
| Fig. 5 Scope and limitations of formal [2 + 2] cycloaddition of (hetero)arylacetic acids with α-ketophosphonates. All yields are isolated yields, dr determined by 1H NMR of crude reaction mixture, er determined by HPLC analysis on a chiral stationary phase. *α-ketophosphonate starting material showed dimerization over time (see ESI†). | ||
Electron-withdrawing groups in other positions were also well-tolerated in this methodology with β-lactones 63–65 prepared in good yields with good diastereoselectivity and excellent enantioselectivity throughout (57–91%, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14–89
14–89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr, 95
11 dr, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–99
5–99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Interestingly, although aryl C(2)-substituents had not been tolerated in the α-ketophosphonate scope in the reaction with α-silyl acids (Fig. 2) or in the arylacetic acid scope (Fig. 5), 2-FC6H5 β-lactone 66 was prepared in a synthetically useful 34% yield, with modest diastereoselectivity (82
1 er). Interestingly, although aryl C(2)-substituents had not been tolerated in the α-ketophosphonate scope in the reaction with α-silyl acids (Fig. 2) or in the arylacetic acid scope (Fig. 5), 2-FC6H5 β-lactone 66 was prepared in a synthetically useful 34% yield, with modest diastereoselectivity (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 dr) but excellent enantioselectivity (96
18 dr) but excellent enantioselectivity (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). Unfortunately, strongly Hammett electron-withdrawing 4-F3CC6H4-phenylacetic acid 67 showed low reactivity under the reaction conditions, which may be due to reduced nucleophilicity of the intermediate C(1)-ammonium enolate species. Sterically hindered acids 68 and 69 were also not tolerated. Disappointingly, the incorporation of an electron-donating methyl group in either the 4- or 2-position of the aryl-substituent of the phosphonate (70 and 32) showed no conversion to the desired β-lactone products, which presumably reflects the reduced electrophilicity of the carbonyl carbon, and/or steric hindrance in the case of the 2-MeC6H5 substituent.
4 er). Unfortunately, strongly Hammett electron-withdrawing 4-F3CC6H4-phenylacetic acid 67 showed low reactivity under the reaction conditions, which may be due to reduced nucleophilicity of the intermediate C(1)-ammonium enolate species. Sterically hindered acids 68 and 69 were also not tolerated. Disappointingly, the incorporation of an electron-donating methyl group in either the 4- or 2-position of the aryl-substituent of the phosphonate (70 and 32) showed no conversion to the desired β-lactone products, which presumably reflects the reduced electrophilicity of the carbonyl carbon, and/or steric hindrance in the case of the 2-MeC6H5 substituent.
Proposed mechanism
Based upon these observations and following established precedent, a catalytic cycle for the enantioselective formal [2 + 2] cycloaddition of C(1)-ammonium enolates and α-ketophosphonates is presented (Fig. 6a) with the method of C(1)-ammonium enolate generation dependent upon the starting material. When (hetero)arylacetic mixed anhydrides 71 are used (X = H), N-acylation of (2S,3R)-HyperBTM 1 occurs reversibly to generate acyl ammonium ion pair 73, with deprotonation at the α-position generating the corresponding (Z)-C(1)-ammonium enolate 75. When an excess of (±)-α-alkyl-α-silyl mixed anhydride 72 is used, following our previous work preferential acylation of the (R)-enantiomer of 72 in a kinetic resolution process is assumed to lead to acyl ammonium ion pair 74 and enantioenriched anhydride 72. Desilylation to generate the corresponding C(1)-ammonium enolate 75 occurs, which may be promoted by the pivalate counterion or an alternative nucleophile such as a chloride anion, or through a Brook-type C- to O-silyl rearrangement followed by O-desilylation.21 In both cases, key to the observed stereochemical outcome is formation of the (Z)-ammonium enolate and a stabilizing 1,5-O⋯S chalcogen bonding interaction which ensures coplanarity between the 1,5-O and S-atoms and provides a conformational bias.29–50 The relative and absolute configuration observed within the major diastereoisomer of the β-lactone products is consistent with that previously observed in related formal [2 + 2] cycloadditions of C(1)-ammonium enolates with trifluoromethylketones,23 isatins51 and pyrazolones52 and so by analogy a similar concerted asynchronous formal [2 + 2] cycloaddition pathway via transition state assembly 78 to give 77 is proposed. Following precedent,21,51–53 we speculate that this transition state assembly can be stabilised a non-classical CH⋯O interaction between the acidic α-C–H of the substrate-bound catalyst and the carbonyl C
which ensures coplanarity between the 1,5-O and S-atoms and provides a conformational bias.29–50 The relative and absolute configuration observed within the major diastereoisomer of the β-lactone products is consistent with that previously observed in related formal [2 + 2] cycloadditions of C(1)-ammonium enolates with trifluoromethylketones,23 isatins51 and pyrazolones52 and so by analogy a similar concerted asynchronous formal [2 + 2] cycloaddition pathway via transition state assembly 78 to give 77 is proposed. Following precedent,21,51–53 we speculate that this transition state assembly can be stabilised a non-classical CH⋯O interaction between the acidic α-C–H of the substrate-bound catalyst and the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 6b).54 Subsequent catalyst release via collapse of the spirocyclic tetrahedral intermediate 76 generates the β-lactone 77 in high diastereo- and enantioselectivity.
O (Fig. 6b).54 Subsequent catalyst release via collapse of the spirocyclic tetrahedral intermediate 76 generates the β-lactone 77 in high diastereo- and enantioselectivity.
Conclusion
This manuscript reports the development of a highly diastereo- and enantioselective formal [2 + 2] cycloaddition of α-unsubstituted, α-alkyl and α-(hetero)aryl-substituted C(1)-ammonium enolates with α-ketophosphonates using the isothiourea catalyst (2S,3R)-HyperBTM 1. The scope and limitations of this process have been explored, with good yields and high stereoselectivity observed across a broad range of substrate derivatives incorporating varied substitution at the C(3)- and C(4)-positions as well as the phosphonate group. This cycloaddition procedure is also amenable to scale up using bench grade solvents and reagents, with effective conversion on a 1 g scale demonstrated. The further application of this methodology towards unsymmetrical phosphonates is currently underway within our laboratory.Data availability
All data (experimental procedures and characterization data) that support the findings of this study are available within the article and its ESI.† Crystallographic data for compounds (2R,3R)-18, (2R,3R)-26 and (2R,3R)-55 have been deposited with the Cambridge Crystallographic Data Centre under deposition numbers 2395570, 2395571 and 2395572, respectively. The research data supporting this publication can be accessed at: DOI: https://doi.org/10.17630/a6b1715c-641b-47c2-8a28-d493726f8e5f, 2024, data underpinning: “Isothiourea-Catalysed Enantioselective Synthesis of Phosphonate-Functionalised β-lactones”, University of St Andrews Research Portal.Author contributions
ADS conceived the project. FMP and YW carried out all experimental studies in consultation with HP and ADS. FMP, YW and ADS wrote the manuscript. DBC, APM and AMZS carried out single crystal X-ray analysis. All authors agreed on the finalised version of the manuscript. FMP and YW contributed equally to this paper and both have the right to put their name first.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The research leading to these results has received funding from AstraZeneca (FMP), the EaSI-CAT Centre for Doctoral Training (FMP), the CSC-St Andrews PhD Scholarship Scheme (YW) and the EPRSC (CMY, EP/S019359/1). ADS thanks the EPRSC Programme Grant “Boron: Beyond the Reagent” (EP/W007517) for support. The authors thank Dr Tengfei Kang (St Andrews) for valuable discussions and suggestions. To meet institutional and research funder open access requirements, any accepted manuscript arising shall be open access under a Creative Commons Attribution (CC BY) reuse licence with zero embargo.Notes and references
- J. B. Rodriguez and C. Gallo-Rodriguez, ChemMedChem, 2019, 14, 190–216 CrossRef CAS PubMed.
- G. M. Dill, R. D. Sammons, P. C. C. Feng, F. Kohn, K. Kretzmer, A. Mehrsheikh, M. Bleeke, J. L. Honegger, D. Farmer, D. Wright and E. A. Haupfear, in Glyphosate Resistance in Crops and Weeds, ed. V. K. Nandula, Wiley, 1st edn, 2010, pp. 1–33 Search PubMed.
- N. Popovics-Tóth and E. Bálint, Acta Chim. Slov., 2022, 69, 735–755 CrossRef PubMed.
- D. Lanzinger, S. Salzinger, B. S. Soller and B. Rieger, Ind. Eng. Chem. Res., 2015, 54, 1703–1712 CrossRef CAS.
- S. Samanta and C.-G. Zhao, J. Am. Chem. Soc., 2006, 128, 7442–7443 CrossRef CAS PubMed.
- T. Mandal, S. Samanta and C.-G. Zhao, Org. Lett., 2007, 9, 943–945 CrossRef CAS PubMed.
- X. Chen, J. Wang, Y. Zhu, D. Shang, B. Gao, X. Liu, X. Feng, Z. Su and C. Hu, Chem.–Eur. J., 2008, 14, 10896–10899 CrossRef CAS.
- S. Kayal and S. Mukherjee, Org. Lett., 2015, 17, 5508–5511 CrossRef CAS.
- J. Yu, X. Zhao, Z. Miao and R. Chen, Org. Biomol. Chem., 2011, 9, 6721 RSC.
- V. B. Gondi, K. Hagihara and V. H. Rawal, Angew. Chem., Int. Ed., 2009, 48, 776–779 CrossRef CAS PubMed.
- M. Frings, I. Thomé, I. Schiffers, F. Pan and C. Bolm, Chem.–Eur. J., 2014, 20, 1691–1700 CrossRef CAS.
- S. L. Robinson, J. K. Christenson and L. P. Wackett, Nat. Prod. Rep., 2019, 36, 458–475 RSC.
- H. W. Yang and D. Romo, Tetrahedron, 1999, 55, 6403–6434 CrossRef CAS.
- A. D. Smith, J. Douglas, L. C. Morrill and E. Richmond, in Methods and Applications of Cycloaddition Reactions in Organic Syntheses, John Wiley & Sons, Ltd, 2014, pp. 89–114 Search PubMed.
- K. N. Van, L. C. Morrill, A. D. Smith and D. Romo, in Lewis Base Catalysis in Organic Synthesis, John Wiley & Sons, Ltd, 2016, pp. 527–654 Search PubMed.
- S. Mukherjee and A. T. Biju, Chem.–Asian J., 2018, 13, 2333–2349 CrossRef CAS.
- L. C. Morrill and A. D. Smith, Chem. Soc. Rev., 2014, 43, 6214–6226 RSC.
- C. McLaughlin, A. M. Z. Slawin and A. D. Smith, Angew. Chem., Int. Ed., 2019, 58, 15111–15119 CrossRef CAS.
- K. J. Schwarz, J. L. Amos, J. C. Klein, D. T. Do and T. N. Snaddon, J. Am. Chem. Soc., 2016, 138, 5214–5217 CrossRef CAS PubMed.
- S. Y. Lee, S. Neufeind and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 8899–8902 CrossRef CAS PubMed.
- Y. Wang, C. M. Young, H. Liu, W. C. Hartley, M. Wienhold, D. B. Cordes, A. M. Z. Slawin and A. D. Smith, Angew. Chem., Int. Ed., 2022, 61, e202208800 CrossRef CAS PubMed.
- J. Sun, F. He, Z. Wang, D. Pan, P. Zheng, C. Mou, Z. Jin and Y. R. Chi, Chem. Commun., 2018, 54, 6040–6043 RSC.
- D.-J. Barrios Antúnez, M. D. Greenhalgh, A. C. Brueckner, D. M. Walden, P. Elías-Rodríguez, P. Roberts, B. G. Young, T. H. West, A. M. Z. Slawin, P. Ha-Yeon Cheong and A. D. Smith, Chem. Sci., 2019, 10, 6162–6173 RSC.
- D. S. Noyce and E. H. Banitt, J. Org. Chem., 1966, 31, 4043–4047 CrossRef CAS.
- A. Matviitsuk, M. D. Greenhalgh, J. E. Taylor, X. B. Nguyen, D. B. Cordes, A. M. Z. Slawin, D. W. Lupton and A. D. Smith, Org. Lett., 2020, 22, 335–339 CrossRef CAS PubMed.
- G. Kang, M. Yamagami, S. Vellalath and D. Romo, Angew. Chem., Int. Ed., 2018, 57, 6527–6531 CrossRef CAS PubMed.
- S. R. Smith, J. Douglas, H. Prevet, P. Shapland, A. M. Z. Slawin and A. D. Smith, J. Org. Chem., 2014, 79, 1626–1639 CrossRef CAS PubMed.
- D.-S. Ji, H. Liang, K.-X. Yang, Z.-T. Feng, Y.-C. Luo, G.-Q. Xu, Y. Gu and P.-F. Xu, Chem. Sci., 2022, 13, 1801–1807 RSC.
- V. B. Birman, X. Li and Z. Han, Org. Lett., 2007, 9, 37–40 CrossRef CAS PubMed.
- P. Liu, X. Yang, V. B. Birman and K. N. Houk, Org. Lett., 2012, 14, 3288–3291 CrossRef CAS.
- M. E. Abbasov, B. M. Hudson, D. J. Tantillo and D. Romo, J. Am. Chem. Soc., 2014, 136, 4492–4495 CrossRef CAS PubMed.
- E. R. T. Robinson, D. M. Walden, C. Fallan, M. D. Greenhalgh, P. H.-Y. Cheong and A. D. Smith, Chem. Sci., 2016, 7, 6919–6927 RSC.
- M. D. Greenhalgh, S. M. Smith, D. M. Walden, J. E. Taylor, Z. Brice, E. R. T. Robinson, C. Fallan, D. B. Cordes, A. M. Z. Slawin, H. C. Richardson, M. A. Grove, P. H. Cheong and A. D. Smith, Angew. Chem., Int. Ed., 2018, 57, 3200–3206 CrossRef CAS PubMed.
- C. M. Young, A. Elmi, D. J. Pascoe, R. K. Morris, C. McLaughlin, A. M. Woods, A. B. Frost, A. de la Houpliere, K. B. Ling, T. K. Smith, A. M. Z. Slawin, P. H. Willoughby, S. L. Cockroft and A. D. Smith, Angew. Chem., Int. Ed., 2020, 59, 3705–3710 CrossRef CAS PubMed.
- Y. Nagao, S. Miyamoto, M. Miyamoto, H. Takeshige, K. Hayashi, S. Sano, M. Shiro, K. Yamaguchi and Y. Sei, J. Am. Chem. Soc., 2006, 128, 9722–9729 CrossRef CAS PubMed.
- B. R. Beno, K.-S. Yeung, M. D. Bartberger, L. D. Pennington and N. A. Meanwell, J. Med. Chem., 2015, 58, 4383–4438 CrossRef CAS PubMed.
- D. J. Pascoe, K. B. Ling and S. L. Cockroft, J. Am. Chem. Soc., 2017, 139, 15160–15167 CrossRef CAS PubMed.
- M. Breugst and J. J. Koenig, Eur. J. Org Chem., 2020, 2020, 5473–5487 CrossRef CAS.
- A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357–4416 CrossRef CAS.
- K. Fujita, M. Iwaoka and S. Tomoda, Chem. Lett., 1994, 23, 923–926 CrossRef.
- K. Fujita, K. Murata, M. Iwaoka and S. Tomoda, J. Chem. Soc., Chem. Commun., 1995, 1641 RSC.
- K. Fujita, K. Murata, M. Iwaoka and S. Tomoda, Tetrahedron Lett., 1995, 36, 5219–5222 CAS.
- T. Wirth, Angew. Chem., Int. Ed., 1995, 34, 1726–1728 CrossRef CAS.
- C. Bleiholder, R. Gleiter, D. B. Werz and H. Köppel, Inorg. Chem., 2007, 46, 2249–2260 CrossRef CAS PubMed.
- S. Kolb, G. A. Oliver and D. B. Werz, Angew. Chem., Int. Ed., 2020, 59, 22306–22310 CrossRef CAS PubMed.
- S. Benz, J. López-Andarias, J. Mareda, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2017, 56, 812–815 CrossRef CAS PubMed.
- P. Wonner, L. Vogel, M. Düser, L. Gomes, F. Kniep, B. Mallick, D. B. Werz and S. M. Huber, Angew. Chem., Int. Ed., 2017, 56, 12009–12012 CrossRef CAS PubMed.
- W. Wang, H. Zhu, S. Liu, Z. Zhao, L. Zhang, J. Hao and Y. Wang, J. Am. Chem. Soc., 2019, 141, 9175–9179 CrossRef CAS PubMed.
- J. Merad, J. Pons, O. Chuzel and C. Bressy, Eur. J. Org Chem., 2016, 2016, 5589–5610 CrossRef CAS.
- V. B. Birman, Aldrichimica Acta, 2016, 49, 23–33 CAS.
- Y. Abdelhamid, K. Kasten, J. Dunne, W. C. Hartley, C. M. Young, D. B. Cordes, A. M. Z. Slawin, S. Ng and A. D. Smith, Org. Lett., 2022, 24, 5444–5449 CrossRef CAS PubMed.
- A. Conboy, A. S. Goodfellow, K. Kasten, J. Dunne, D. B. Cordes, M. Bühl and A. D. Smith, Chem. Sci., 2024, 15, 8896–8904 RSC.
- J. Wu, C. M. Young, A. A. Watts, A. M. Z. Slawin, G. R. Boyce, M. Bühl and A. D. Smith, Org. Lett., 2022, 24, 4040–4045 CrossRef CAS PubMed.
- S. K. Agrawal, P. K. Majhi, A. S. Goodfellow, R. K. Tak, D. B. Cordes, A. P. McKay, K. Kasten, M. Bühl and A. D. Smith, Angew. Chem., Int. Ed., 2024, 63, e202402909 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2395570–2395572. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00322a |
| ‡ F. M. Platt and Y. Wang contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2025 |