Open Access Article
Open Access ArticleSequential metabolic probes illuminate nuclear DNA for discrimination of cancerous and normal cells†
Caiqi
Liu
a,
Sirui
Lu
a,
Chenxu
Yan
 *a,
Xingyuan
Zhao
a,
Jing
Yang
a,
Weixu
Zhang
a,
Xiuyan
Zhao
a,
Yao
Ge
a,
Xiaofan
You
a and
Zhiqian
Guo
*a,
Xingyuan
Zhao
a,
Jing
Yang
a,
Weixu
Zhang
a,
Xiuyan
Zhao
a,
Yao
Ge
a,
Xiaofan
You
a and
Zhiqian
Guo
 *ab
*ab
aKey Laboratory for Advanced Materials, Institute of Fine Chemicals, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: chenxuyan@ecust.edu.cn; guozq@ecust.edu.cn
bState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China
First published on 11th March 2025
Abstract
Elucidating the timing and spatial distribution of DNA synthesis within cancer cells is vital for cancer diagnosis and targeted therapy. However, current probes for staining nucleic acids rely on electrostatic interactions and hydrogen bonds with the nucleic acid, resulting in “static” DNA staining and the inability to distinguish cell types. Here, we present a proof-of-concept study of sequential metabolic probes, for the first time allowing for cancer-cell-specific illumination of DNA. This breakthrough is achieved by the combination of a “dual-locked” nucleoside analog VdU-Lys, and a new tetrazine-based bioorthogonal probe. Specifically, 5-vinyl-2′-deoxyuridine (VdU) release is only conducted in programmatically triggered histone deacetylases (HDACs) and cathepsin L (CTSL) as “sequential keys”, enabling the modification of vinyl groups into the nuclear DNA of cancerous cells rather than normal cells. Subsequently, tetrazine-based Et-PT-Tz could in situ light-up DNA containing VdUs with significant fluorescence illumination (120-fold enhancement) through rapid bioorthogonal reaction. We demonstrated the compatibility of our probe in cancer-specific sensing of DNA with a high signal-to-noise ratio ranging from in vitro multiple cell lines to whole-organism scale. This approach would serve as a benchmark for the development of cell-specific metabolic reporters for DNA labelling, to characterize DNA metabolism in various types of cell lines.
Introduction
DNA plays a central role in biological processes and understanding its regulation is critical for distinguishing normal and disease cell physiology.1,2 Specifically, labelling the nuclear DNA of cancer cells can help researchers comprehend the behavior of these cells, such as proliferation, metastasis, and apoptosis, to ultimately enhance the effectiveness of anti-tumor therapies.3–10 Towards this, DNA labelling with fluorescent probes11–17 is an essential tool.18,19 Currently available probes20–26 for DNA fluorescence labelling include DNA minor groove binders DAPI and Hoechst, which are established as fundamental imaging tools and are widely used in biological and biomedical fields.27,28 However, these fluorescent dyes stain nuclear acid through electrostatic interactions and hydrogen bonds with the nucleic acid,29,30 resulting in “static” DNA staining and the inability to distinguish cell types. Thus, there is an urgent need to develop fluorescence probes for elucidating the timing and spatial distribution of DNA synthesis in specific cell types, especially for cancer cells.Metabolic labelling31–33 of DNA, as an emerging chemical biology approach, provides an effective method for “dynamically” monitoring DNA.34–37 In this strategy, artificially modified nucleoside analogs were employed for DNA metabolic labelling through cellular DNA biosynthesis, enabling introduce chemical reporter groups (e.g. additional azide,38 alkyne,39 or vinyl group40) to be introduced into DNA. These reporter groups can then bioorthogonally react with additional chemical probes to fluorescently label the modified DNA.41–43 However, two major limitations exist in this metabolic-based approach: the highly conserved DNA metabolic pathway means nucleoside analogs can join the DNA synthesis process in almost all cell types,44 making it unable to cell-specifically label DNA. In addition, most of the current bioorthogonal dyes are in “always-on” mode, resulting in a low signal-to-noise ratio in fluorescence imaging. To address these issues, with the final goal of achieving cancer-cell-specific lighting-up of DNA, we envision a strategy of Boolean logic operation-based metabolic labelling: (i) chemically modifying nucleoside analogs by cell-specific and sequentially triggered masking group to engage DNA metabolic pathways only in cancer cells; (ii) designing a bioorthogonal probe that is capable of turn-on fluorescence detection with high signal-to-noise ratio and fast labelling kinetics.
Herein, we present a proof-of-concept study of sequential metabolic fluorescent probes, which have for the first time achieved cell-specific illumination of nuclear DNA within cancer cells (Fig. 1a). This breakthrough is facilitated by the de novo design of a sequentially responsive nucleoside analog VdU-Lys, and a tetrazine-based probe Et-PT-Tz (Fig. S1 and S2†). We integrated an ε-acetylated lysine residue that was incorporated into 5-vinyl-2′-deoxyuridine (VdU), serving as a “dual-lock” protective group. Utilizing histone deacetylases (HDACs) and cathepsin L (CTSL) as the “sequential keys” (enzymes known for their increased activity in cancer cells45–47), VdU-Lys could programmatically release VdU through Boolean logic operation-based sequential digestion (Fig. 1b). This process ensures highly selective activation to release VdU only in cancerous cells rather than normal cells, and enables metabolic modification of DNA with vinyl groups. Subsequently, the tetrazine group of Et-PT-Tz, functioning as both a bioorthogonal partner and a quencher, engages in an inverse electron-demand Diels–Alder (IEDDA) reaction48–51 with DNA containing VdUs, resulting in a 120-fold fluorescence enhancement for DNA detection. The specificity of our approach has been validated both in situ and in vivo, representing a reliable metabolic labelling method for targeted illumination of DNA within cancer cells. We believe this approach will serve as a benchmark for the development of cell-specific metabolic reporters for DNA labelling and open new avenues for using chemical and fluorescence techniques to characterize DNA metabolism in cancer cells.
Results and discussion
Uncaging “dual-locked” nucleoside analog to liberate VdU with HDAC and CTSL enzyme as the “sequential keys”
As established, HDAC and CTSL are known for their increased activity in cancer cells and have been extensively demonstrated across various cancer cell lines (including HeLa, HepG2, HCT116, A549, and HCA-7).52 To validate the sequential digestion reaction of the “dual-locked” nucleoside analog VdU-Lys (Fig. 2a), we conducted high-performance liquid chromatography (HPLC) and mass spectrometry (MS) analyses. We began with HDAC1 (a broad-spectrum histone deacetylase) to digest VdU-Lys (the first uncaging step). In HPLC analysis, the retention times of VdU-Lys and VdU are 7.5 and 5.5 min, respectively (Fig. 2b). After reaction with HDAC1, the deacetylated product VdU-(-Ac)Lys exhibited a new peak with a retention time of 6.5 min. As shown in Fig. 2c and S3,† MS analysis revealed the deacetylated product at m/z 631.3135. These results suggested a full conversion (triggered by HDAC) of VdU-Lys to deacetylated products VdU-(-Ac)Lys, with minimal further degradation to produce VdU.Next, we performed sequential digestion using HDAC1 and CTSL, which uncaged the protecting group and released VdU (the second uncaging step). The HPLC results indicated that the cleavage product VdU displayed an obvious peak at 5.5 min (Fig. 2d), which was further corroborated by MS analysis (Fig. 2e and S3†). Importantly, using CTSL alone did not digest VdU-Lys into VdU, indicating that deacetylation of VdU-Lys is a prerequisite for CTSL-mediated cleavage of the C1-amide bond. Taken together, these HPLC and MS results suggest that VdU-Lys can be deacetylated by HDAC, followed by CTSL cleavage of the amide bond, and self-cleavage of (4-aminophenyl)methanol and carbonate ester to release the metabolically active VdU. This demonstrated that “dual-locked” VdU-Lys could serve as a substrate for sequential activation by HDAC and CTSL enzymes (both are over-expressed in cancer cells), potentially enabling cancer-cell-specific metabolic labelling of DNA with vinyl groups.
Bioorthogonal reaction with significant lighting-up response fluorescence (120-fold enhancement) and fast kinetics
Subsequently, we explored the kinetics of the inverse electron-demand Diels–Alder (IEDDA) reaction of VdU with our tetrazine group-modified probe Et-PT-Tz (Fig. 3a). Initially, we examined the absorption spectra of Et-PT-Tz in various solutions, finding that the absorption wavelengths were primarily within the 425–450 nm range (Fig. 3b). Upon mixing Et-PT-Tz and VdU in a PBS buffer, a significantly fluorogenic reaction was observed (Fig. 3c). The fluorescence spectrum showed a sharp increase in fluorescence intensity (120-fold enhancement) after the reaction, with the peak emission at 550 nm and a large Stokes shift of around 100 nm (Fig. 3c). Notably, activated Et-PT-Tz exhibits a broad emission spectrum extending to the far-red region, with a tail peak reaching 700 nm, indicating the potential for in vivo imaging applications. To further assess the reaction kinetics, we conducted the reaction under pseudo-first-order conditions, tracking the changes in fluorescence intensity at 550 nm. The determined apparent rate constant for this reaction was 3.38 × 10−2 M−1 s−1 (Fig. 3d, e and S4†), around 30-fold faster than that of commonly used strain-promoted azide–alkyne cycloaddition “SPAAC” reactions (k ≈ 10−3 M−1 s−1)53 for cellular labelling. Therefore, the bioorthogonal reaction of Et-PT-Tz with VdU shows fast kinetics with significant fluorescence enhancement (120-fold).Cancer-cell-specific lighting-up of nuclear DNA in situ and in vivo
To evaluate our strategy for cancer-cell-specific metabolic labelling of nuclear DNA, we treated cancerous cells (HepG2, A549, HCT116, and HeLa) and noncancerous cells (HK2, 293T, HaCaT, and SV-Huc-1) with VdU (without “dual-lock”) or VdU-Lys (with “dual-lock”) (Fig. 4). We then stained these cells with Et-PT-Tz to illuminate the vinyl-modified DNA. MTT assays clearly showed that the probes were non-toxic to both cancerous and noncancerous cells (Fig. S5†). In VdU groups, fluorescence imaging revealed strong nuclear fluorescence signals in all cancerous and noncancerous cell lines (Fig. 4a and c), along with excellent colocalization between Et-PT-Tz and the commercial DNA binder, including Hoechst 33342 (Pr = 0.93, Fig. S6†) and PI (Pr = 0.94, Fig. S7†). Moreover, quantitative fluorescence intensity profiling confirmed that Et-PT-Tz stained nearly the same nuclear region as the commercial DNA binder (Fig. S6 and S7†), indicating its strong nuclear targeting ability. Remarkably, the nuclear imaging signal-to-noise ratio of Et-PT-Tz was 3.8 times higher than that of Hoechst due to the off-on fluorescent response of Et-PT-Tz with DNA containing vinyl groups (Fig. S8†). In addition, SIM images demonstrated that Et-PT-Tz effectively visualized the super-resolution nuclear structure at different depths (Fig. S9†). These results indicated the successful metabolic incorporation of VdU into the nuclear DNA, and the activation of Et-PT-Tz fluorescence through the bioorthogonal reaction.In contrast, for the cell lines treated with VdU-Lys, only cancerous cells exhibited bright nuclear fluorescence signals, while noncancerous cells displayed almost no fluorescence (Fig. 4b, d and S10†). Further, there was little difference in the nuclear fluorescence signal of cancerous cells treated with VdU-Lys compared to those treated with VdU (Fig. S11†). These findings demonstrated that, with the combination of the sequentially triggered VdU-Lys and probe Et-PT-Tz, we have successfully developed an effective method for cancer-cell-specific lighting-up of DNA and super-resolution of nuclear structures with a high signal-to-noise ratio.
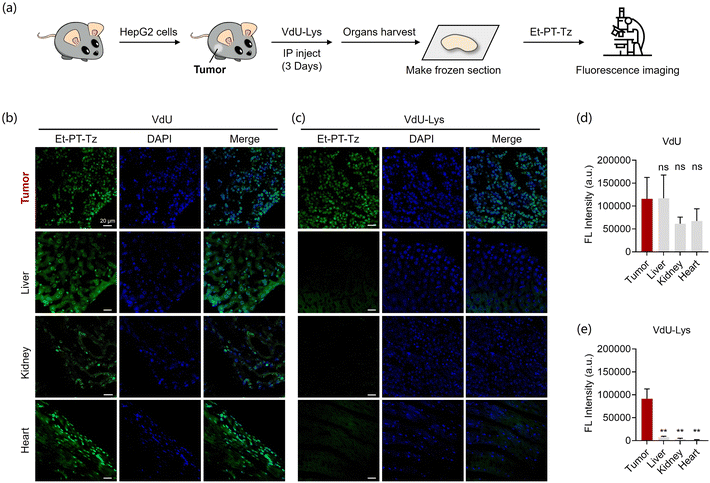
To evaluate the feasibility of our sequential metabolic probes in vivo, we established an in vivo xenograft tumor model (Fig. 5). After three weeks, we administered intraperitoneal injections of either VdU or VdU-Lys. We first evaluated the ability of our strategy for tumor-specific fluorescent labelling by in vivo imaging of mice, indicating that it could achieve tumor targeting at the whole-animal scale (Fig. S12†). We then harvested the organs and tumors to create frozen sections, which we stained with Et-PT-Tz for imaging (Fig. 5a). The fluorescent imaging results showed that mice injected with VdU displayed strong fluorescence in the tumor and various organs (Fig. 5b and d). In contrast, bright nuclear fluorescence was exclusively only in the tumors of mice injected with VdU-Lys (Fig. 5c, e and S13†), with no nuclear fluorescence observed in other organs (including the liver, kidney, and heart). All these results confirmed that our strategy of using sequential metabolic probes can selectively mark tumors in vivo by cancer-cell-selective metabolic lighting-up of DNA.
Conclusions
We developed an innovative sequence-activated metabolic labelling approach, for the first time allowing for cancer-cell-specific lighting-up of DNA. This strategy ingeniously uses two enzymes to regulate metabolic labelling, thereby achieving remarkable cell selectivity. It capitalizes on the unique activities of HDAC and CTSL in cancer cells (as “sequential keys”), to sequentially uncage “dual-locked” nucleoside analog VdU-Lys and release VdU for vinyl-modified DNA labelling. We also introduced a novel tetrazine-based bioorthogonal probe Et-PT-Tz, in which the tetrazine moiety serves dual functions: a fluorescence quenching group and a bioorthogonal functional unit. Et-PT-Tz only fluoresces once it reacts with vinyl-modified DNA via an IEDDA reaction with fast kinetics, resulting in a significant fluorescence enhancement and facilitating high-signal-to-noise cancer cell DNA sensing. Notably, we demonstrated the compatibility and cancer-specificity of our strategy in applications ranging from in vitro multiple cell lines to whole-organism scale, achieving real-time, subcellular resolution imaging/identifying cancerous cells. We believe this strategy could generate a series of imaging toolboxes, by designing other nucleoside analogs for selective metabolic labelling of DNA in other diseases. This would be possible if endogenous disease-specific triggers, such as oxidants, reductases, matrix metalloproteinases, and various cathepsins, could be identified and utilized for profiling DNA molecules from specified cells.Ethical statement
All animal care and experimental procedures were reviewed and approved by the East China University of Science and Technology Animal Studies Committee (ECUST-2021-07001).Data availability
Supporting data have been included in the article's ESI.† All data generated and analyzed during the study are available from the corresponding authors on reasonable request.Author contributions
All the experiments were conducted by C. L., S. L., X. Z., Y. G., and X. Y., with the supervision of C. Y. and Z. G. J. Y., W. Z., and X. Z. gave technical support in cell culturing. All the authors analyzed the data and contributed to the manuscript writing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by National Key Research and Development Program (2023YFA1802000), NSFC/China (22225805, 32121005, 32394001, T2488302, and 22378122), Shanghai Science and Technology Innovation Action Plan (No. 23J21901600), Innovation Program of Shanghai Municipal Education Commission, Shanghai Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism (Shanghai Municipal Education Commission, grant 2021 Sci & Tech 03-28), Science and Technology Commission of Shanghai Municipality (24DX1400200), and the Fundamental Research Funds for the Central Universities.Notes and references
- T. Misteli, Beyond the sequence: cellular organization of genome function, Cell, 2007, 128, 787–800 CrossRef CAS PubMed.
- Y. L. Ying, Z. L. Hu, S. Zhang, Y. Qing, A. Fragasso, G. Maglia, A. Meller, H. Bayley, C. Dekker and Y. T. Long, Nanopore-based technologies beyond DNA sequencing, Nat. Nanotechnol., 2022, 17, 1136–1146 CrossRef CAS PubMed.
- L. Yang, Q. Liu, X. Zhang, X. Liu, B. Zhou, J. Chen, D. Huang, J. Li, H. Li, F. Chen, J. Liu, Y. Xing, X. Chen, S. Su and E. Song, DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25, Nature, 2020, 583, 133–138 CrossRef CAS PubMed.
- S. Zeng, Y. Wang, C. Chen, H. Kim, X. Liu, M. Jiang, Y. Yu, Y. S. Kafuti, Q. Chen, J. Wang, X. Peng, H. Li and J. Yoon, An ER-targeted, viscosity-sensitive hemicyanine dye for the diagnosis of nonalcoholic fatty liver and photodynamic cancer therapy by activating pyroptosis pathway, Angew. Chem., Int. Ed., 2024, 63, e202316487 CrossRef CAS PubMed.
- K. X. Teng, L. Y. Niu and Q. Z. Yang, Supramolecular photosensitizer enables oxygen-independent generation of hydroxyl radicals for photodynamic therapy, J. Am. Chem. Soc., 2023, 145, 4081–4087 CrossRef CAS PubMed.
- L. Yu, Y. Xu, Z. Pu, H. Kang, M. Li, J. L. Sessler and J. S. Kim, Photocatalytic superoxide radical generator that induces pyroptosis in cancer cells, J. Am. Chem. Soc., 2022, 144, 11326–11337 CrossRef CAS PubMed.
- W. P. Roos, A. D. Thomas and B. Kaina, DNA damage and the balance between survival and death in cancer biology, Nat. Rev. Cancer, 2016, 16, 20–33 CrossRef CAS PubMed.
- W. Zhang, F. Huo, F. Cheng and C. Yin, Employing an ICT-FRET integration platform for the real-time tracking of SO2 metabolism in cancer cells and tumor models, J. Am. Chem. Soc., 2020, 142, 6324–6331 CrossRef CAS PubMed.
- J. Li, N. Niu, D. Wang, X. Liu, Y. Qin, L. Wang, B. Z. Tang and D. Wang, Acceptor-engineering tailored type-I photosensitizer with aggregation-induced NIR-II emission for cancer multimodal phototheranostics, Sci. China:Chem., 2024, 67, 2647–2660 CrossRef CAS.
- A. Wang, J. Fang, Y. Feng, Y. Zhang, Y. Zhao, J. Li, C. Cui, Y. Hou, H. Shi and M. Gao, MMP-2 and upconverted UV dual-mediated drug sequential delivery and on-site immobilization for enhanced multidrug-resistant cancer therapy, Sci. China:Chem., 2023, 66, 2317–2328 CrossRef CAS.
- J. Ma, F. Luo, C. H. Hsiung, J. Dai, Z. Tan, S. Ye, L. Ding, B. Shen and X. Zhang, Chemical control of fluorescence lifetime towards multiplexing imaging, Angew. Chem., Int. Ed., 2024, 63, e202403029 CrossRef CAS PubMed.
- J. Ouyang, L. Sun, Z. Zeng, C. Zeng, F. Zeng and S. Wu, Nanoaggregate probe for breast cancer metastasis through multispectral optoacoustic tomography and aggregation-induced NIR-I/II fluorescence imaging, Angew. Chem., Int. Ed., 2020, 59, 10111–10121 CrossRef CAS PubMed.
- C. Wang, Q. Qiao, W. Chi, J. Chen, W. Liu, D. Tan, S. McKechnie, D. Lyu, X. F. Jiang, W. Zhou, N. Xu, Q. Zhang, Z. Xu and X. Liu, Quantitative design of bright fluorophores and aiegens by the accurate prediction of twisted intramolecular charge transfer (TICT), Angew. Chem., Int. Ed., 2020, 59, 10160–10172 CrossRef CAS PubMed.
- Y. Liu, L. Teng, Y. Lyu, G. Song, X. B. Zhang and W. Tan, Ratiometric afterglow luminescent nanoplatform enables reliable quantification and molecular imaging, Nat. Commun., 2022, 13, 2216 CrossRef CAS PubMed.
- Y. Wu, S. Huang, J. Wang, L. Sun, F. Zeng and S. Wu, Activatable probes for diagnosing and positioning liver injury and metastatic tumors by multispectral optoacoustic tomography, Nat. Commun., 2018, 9, 3983 CrossRef PubMed.
- Y. Xu, C. V. Chau, J. Lee, A. C. Sedgwick, L. Yu, M. Li, X. Peng, J. S. Kim and J. L. Sessler, Lutetium texaphyrin: A photocatalyst that triggers pyroptosis via biomolecular photoredox catalysis, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2314620121 CrossRef CAS PubMed.
- S. Ye, H. Zhang, J. Fei, C. H. Wolstenholme and X. Zhang, A general strategy to control viscosity sensitivity of molecular rotor-based fluorophores, Angew. Chem., Int. Ed., 2021, 60, 1339–1346 CrossRef CAS PubMed.
- X. Peng, T. Wu, J. Fan, J. Wang, S. Zhang, F. Song and S. Sun, An effective minor groove binder as a red fluorescent marker for live-cell DNA imaging and quantification, Angew. Chem., Int. Ed., 2011, 50, 4180–4183 CrossRef CAS PubMed.
- M. Tian, J. Sun, B. Dong and W. Lin, Dynamically monitoring cell viability in a dual-color mode: Construction of an aggregation/monomer-based probe capable of reversible mitochondria-nucleus migration, Angew. Chem., Int. Ed., 2018, 57, 16506–16510 CrossRef CAS PubMed.
- N. Ruan, Q. Qiu, X. Wei, J. Liu, L. Wu, N. Jia, C. Huang and T. D. James, De novo green fluorescent protein chromophore-based probes for capturing latent fingerprints using a portable system, J. Am. Chem. Soc., 2024, 146, 2072–2079 CrossRef CAS PubMed.
- F. Xiao, H. Gao, Y. Lei, W. Dai, M. Liu, X. Zheng, Z. Cai, X. Huang, H. Wu and D. Ding, Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging, Nat. Commun., 2022, 13, 186 CrossRef CAS PubMed.
- A. F. Henwood, N. Curtin, S. Estalayo-Adrián, A. J. Savyasachi, T. A. Gudmundsson, J. I. Lovitt, L. C. Sigurvinsson, H. L. Dalton, C. S. Hawes, D. Jacquemin, D. F. O'Shea and T. Gunnlaugsson, Time-resolved fluorescence imaging with color-changing, “turn-on/turn-on” AIE nanoparticles, Chem, 2024, 10, 578–599 CAS.
- Y. Wang, H. Niu, K. Wang, L. Yang, G. Wang, T. D. James, J. Fan and H. Zhang, Fluorescence-plane polarization for the real-time monitoring of transferase migration in living cells, Chem. Sci., 2024, 15, 16291–16299 RSC.
- J. Li, N. Zhao, W. Zhang, P. Li, X. Yin, W. Zhang, H. Wang and B. Tang, Assessing the progression of early atherosclerosis mice using a fluorescence nanosensor for the simultaneous detection and imaging of pH and phosphorylation, Angew. Chem., Int. Ed., 2023, 62, e202215178 CrossRef CAS PubMed.
- Y. Lu, Y. Zhang, X. Wu, R. Pu, C. Yan, W. Liu, X. Liu, Z. Guo and W. H. Zhu, A de novo zwitterionic strategy of ultra-stable chemiluminescent probes: highly selective sensing of singlet oxygen in FDA-approved phototherapy, Chem. Sci., 2024, 15, 12431–12441 RSC.
- L. Wu, J. Huang, K. Pu and T. D. James, Dual-locked spectroscopic probes for sensing and therapy, Nat. Rev. Chem, 2021, 5, 406–421 CrossRef CAS PubMed.
- J. Kapuscinski, DAPI: a DNA-specific fluorescent probe, Biotech. Histochem., 1995, 70, 220–233 CrossRef CAS PubMed.
- G. Lukinavicius, C. Blaukopf, E. Pershagen, A. Schena, L. Reymond, E. Derivery, M. Gonzalez-Gaitan, E. D'Este, S. W. Hell, D. Wolfram Gerlich and K. Johnsson, SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy, Nat. Commun., 2015, 6, 8497 CrossRef CAS PubMed.
- N. P. Bazhulina, A. M. Nikitin, S. A. Rodin, A. N. Surovaya, Y. V. Kravatsky, V. F. Pismensky, V. S. Archipova, R. Martin and G. V. Gursky, Binding of Hoechst 33258 and its derivatives to DNA, J. Biomol. Struct. Dyn., 2009, 26, 701–718 CrossRef CAS PubMed.
- C. Y. Y. Yu, W. Zhang, R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, A photostable AIEgen for nucleolus and mitochondria imaging with organelle-specific emission, J. Mater. Chem. B, 2016, 4, 2614–2619 RSC.
- Q. Qiao, A. Song, K. An, N. Xu, W. Jia, Y. Ruan, P. Bao, Y. Tao, Y. Zhang, X. Wang and Z. Xu, Spontaneously blinkogenic probe for wash-free single-molecule localization-based super-resolution imaging in living cells, Angew. Chem., Int. Ed., 2024, e202417469 Search PubMed.
- L. Hu, W. Cao, Y. Jiang, W. Cai, X. Lou and T. Liu, Designing artificial fluorescent proteins and biosensors by genetically encoding molecular rotor-based amino acids, Nat. Chem., 2024, 16, 1960–1971 Search PubMed.
- S. Hong, D. W. Zheng, Q. L. Zhang, W. W. Deng, W. F. Song, S. X. Cheng, Z. J. Sun and X. Z. Zhang, An RGB-emitting molecular cocktail for the detection of bacterial fingerprints, Chem. Sci., 2020, 11, 4403–4409 RSC.
- M. Kuba, P. Khoroshyy, M. Lepsik, E. Kuzmova, D. Kodr, T. Kraus and M. Hocek, Real-time imaging of nascent DNA in live cells by monitoring the fluorescence lifetime of DNA-incorporated thiazole orange-modified nucleotides, Angew. Chem., Int. Ed., 2023, 62, e202307548 CrossRef CAS PubMed.
- D. E. Wagner, I. E. Wang and P. W. Reddien, Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration, Science, 2011, 332, 811–816 Search PubMed.
- J. Bargstedt, M. Reinschmidt, L. Tydecks, T. Kolmar, C. M. Hendrich and A. Jaschke, Photochromic nucleosides and oligonucleotides, Angew. Chem., Int. Ed., 2024, 63, e202310797 CrossRef CAS PubMed.
- N. Klocker, F. P. Weissenboeck and A. Rentmeister, Covalent labeling of nucleic acids, Chem. Soc. Rev., 2020, 49, 8749–8773 RSC.
- A. B. Neef and N. W. Luedtke, An azide-modified nucleoside for metabolic labeling of DNA, Chembiochem, 2014, 15, 789–793 CrossRef CAS PubMed.
- A. B. Neef and N. W. Luedtke, Dynamic metabolic labeling of DNA in vivo with arabinosyl nucleosides, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20404–20409 CrossRef CAS PubMed.
- U. Rieder and N. W. Luedtke, Alkene-tetrazine ligation for imaging cellular DNA, Angew. Chem., Int. Ed., 2014, 53, 9168–9172 CrossRef CAS PubMed.
- Y. Deng, T. Shen, X. Yu, J. Li, P. Zou, Q. Gong, Y. Zheng, H. Sun, X. Liu and H. Wu, Tetrazine-isonitrile bioorthogonal fluorogenic reactions enable multiplex labeling and wash-free bioimaging of live cells, Angew. Chem., Int. Ed., 2024, 63, e202319853 CrossRef CAS PubMed.
- A. Salic and T. J. Mitchison, A chemical method for fast and sensitive detection of DNA synthesis in vivo, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2415–2420 CrossRef CAS PubMed.
- I. H. Wang, M. Suomalainen, V. Andriasyan, S. Kilcher, J. Mercer, A. Neef, N. W. Luedtke and U. F. Greber, Tracking viral genomes in host cells at single-molecule resolution, Cell Host Microbe, 2013, 14, 468–480 CrossRef CAS PubMed.
- J. Cheng, N. Li, X. Wang, J. Hu, Y. Zhai and N. Gao, Structural insight into the assembly and conformational activation of human origin recognition complex, Cell Discovery, 2020, 6, 88 CrossRef CAS PubMed.
- J. H. Jang, H. Lee, A. Sharma, S. M. Lee, T. H. Lee, C. Kang and J. S. Kim, Indomethacin-guided cancer selective prodrug conjugate activated by histone deacetylase and tumour-associated protease, Chem. Commun., 2016, 52, 9965–9968 RSC.
- H. Wang, R. Wang, K. Cai, H. He, Y. Liu, J. Yen, Z. Wang, M. Xu, Y. Sun, X. Zhou, Q. Yin, L. Tang, I. T. Dobrucki, L. W. Dobrucki, E. J. Chaney, S. A. Boppart, T. M. Fan, S. Lezmi, X. Chen, L. Yin and J. Cheng, Selective in vivo metabolic cell-labeling-mediated cancer targeting, Nat. Chem. Biol., 2017, 13, 415–424 Search PubMed.
- N. Ueki, S. Lee, N. S. Sampson and M. J. Hayman, Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease, Nat. Commun., 2013, 4, 2735 CrossRef PubMed.
- W. Mao, W. Chi, X. He, C. Wang, X. Wang, H. Yang, X. Liu and H. Wu, Overcoming spectral dependence: A general strategy for developing far-red and near-infrared ultra-fluorogenic tetrazine bioorthogonal probes, Angew. Chem., Int. Ed., 2022, 61, e202117386 CrossRef CAS PubMed.
- S. Zheng, N. Dadina, D. Mozumdar, L. Lesiak, K. N. Martinez, E. W. Miller and A. Schepartz, Long-term super-resolution inner mitochondrial membrane imaging with a lipid probe, Nat. Chem. Biol., 2024, 20, 83–92 Search PubMed.
- A. Vázquez, R. Dzijak, M. Dračínský, R. Rampmaier, S. J. Siegl and M. Vrabel, Mechanism-based fluorogenic trans-cyclooctene-tetrazine cycloaddition, Angew. Chem., Int. Ed., 2017, 129, 1354–1357 CrossRef.
- W. Mao, J. Tang, L. Dai, X. He, J. Li, L. Cai, P. Liao, R. Jiang, J. Zhou and H. Wu, A general strategy to design highly fluorogenic far-red and near-infrared tetrazine bioorthogonal probes, Angew. Chem., Int. Ed., 2021, 60, 2393–2397 CrossRef CAS PubMed.
- O. C. Olson and J. A. Joyce, Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response, Nat. Rev. Cancer, 2015, 15, 712–729 CrossRef CAS PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Copper-free click chemistry for dynamic in vivo imaging, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00360a |
| This journal is © The Royal Society of Chemistry 2025 |