Open Access Article
Open Access ArticlePolyoxometalate condensation and transformation mediated by adaptive coordination-assembled molecular flasks†
Li-Xuan
Cai
 ac,
Yu-Hang
Hu
ab,
Li-Peng
Zhou
ac,
Yu-Hang
Hu
ab,
Li-Peng
Zhou
 ac,
Pei-Ming
Cheng
a,
Xiao-Qing
Guo
a,
Yi-Tsu
Chan
ac,
Pei-Ming
Cheng
a,
Xiao-Qing
Guo
a,
Yi-Tsu
Chan
 d and
Qing-Fu
Sun
d and
Qing-Fu
Sun
 *ac
*ac
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: qfsun@fjirsm.ac.cn
bCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
dDepartment of Chemistry, National Taiwan University, Taipei 10617, Taiwan
First published on 10th March 2025
Abstract
Here we report polyoxometalate (POM) condensation or transformation reactions mediated by adaptive coordination-assembled molecular flasks. Addition of Na2SiO3 to the (Mo6O19)2⊂1·(NO3)8 complex containing Lindquist-type clusters as guests leads to the formation of a new (SiMo12O40)⊂2·(NO3)8 host–guest complex, where the in situ generated Keggin-type cluster served as a trigger for the host transformation from cage 1 to isomeric bowl 2. Conversion from 1 to 2 driven by the in situ condensation was found to be 27.5-fold faster than the direct templation with independently prepared SiMo12O404−. As a comparison, cage 1 was noticed to bind only one W6O192− cluster in its cavity, and the formation of (W10O32)⊂2·(NO3)8 as the main product and (SiW12O40)⊂2·(NO3)8 as the minor host–guest complex was observed when it was used for the above condensation reaction, highlighting the crucial role of encapsulation in cavity-confined POM transformations. The reaction processes and the final structure of all the new host–guest complexes have been investigated by NMR, ESI-TOF-MS and SCXRD. Our findings not only showcase a unique example of inorganic-reaction-driven responsive supramolecular system, but also provide a new approach for the preparation of functional POMs⊂cage composite materials.
Introduction
Enzymes are biological catalysts with substrate-binding cavities that exhibit conformational dynamics and rich active sites, essential for efficient catalysis.1–3 Inspired by these natural systems, scientists have developed coordination molecular containers based on supramolecular interactions to mimic enzyme functionality. The confined spaces of coordination-assembled cages, often referred to as ‘molecular flasks’, provide unique local microenvironments that allow for the encapsulation of guest molecules and endow them with reactivity distinctly different from the bulk solvent.4–12 The tailored pockets of molecular flasks play a vital role in cage-promoted reactions through three major factors: preorganization and proximity of substrates to yield an unusual regioselectivity,13,14 local concentration enrichment to accelerate reactions,15,16 and reaction intermediate stabilization to lower the energy and enthalpy barrier.17–19 Despite extensive research on constructing stimuli-responsive supramolecular hosts with adaptive confined cavities,20–34 examples of using such adaptive hosts as molecular flasks for inorganic chemical transformations remain scarce.Polyoxometalates (POMs) are a well-known class of inorganic compounds that have shown wide-spread applications in diverse fields.35–39 In nature, polyoxomolybdates can be stored in a cage-like Mo-storage protein (MoSto) from Azotobacter vinelandii.40–42 Biological protein pockets are able to engineer condensation processes and stabilize the resulting fragile POM species by shielding against hydrolysis.43 In laboratory, POMs are usually generated using bottom-up synthetic approaches, through acidic condensation of tetraoxometalates MO42− (M = Mo, W etc.), the outcome of which is very sensitive to synthetic variables, such as the pH, ionic strength, concentration etc.44,45 POM transformation can happen by adjusting the solution conditions or by modifying the structural “vacancy” sites of lacunary POMs.46 Although an artificial encapsulation approach has been demonstrated by installing POM anions into host systems via non-covalent and specific interactions,47–59 the condensation or transformation of POM guests inside discrete adaptive coordination cages has been seldom targeted so far.
In our previous reports,34,60,61 a mitosis-like host transformation from Pd4L2 cage 1 to a unique conjoined Pd6L3 twin-cage 3 has been observed (Scheme 1A), driven by an organic self-coupling dimerization reaction of ortho-quinone methide precursors. Here we present the cavity-confined syntheses of Keggin-type POM clusters (SiM12O404−, M = Mo) from Lindquist-type Mo6O192− precursors and SiO32−, which exert the induced-fit power to force the structural conversion from cage 1 to isomeric bowl 2 (Scheme 1B). The same conversion also proceeds, but at a much slower rate, via the direct templating of independently made SiMo12O404−. As a comparison, the reaction of the other Lindqvist-type W6O192− cluster with SiO32− mediated by cage 1 led to not only Keggin-type SiW12O404− but also W10O324−, both also accompanied by the induced-fit cage-to-bowl transformation. These POM synthesis reactions mediated by adaptive cages have been thoroughly investigated by IR, UV-vis, NMR, ESI-TOF-MS, and SCXRD.
 | ||
| Scheme 1 (A) Organic self-coupling dimerization reaction triggered cage to twin-cage transformation. (B) Inorganic POM condensation reaction induced cage to bowl transformations. | ||
Results and discussion
Cage 1·(NO3)12 and (Mo6O19)2⊂1·(NO3)8 were synthesized according to our previous work (Fig. S1–S4†).60,61 The optimized structure of (Mo6O19)2⊂1·(NO3)8 was simulated using molecular mechanical modeling (Fig. S5†). Surprisingly, when 1 equiv. of Na2SiO3 was added into the aqueous solution of (Mo6O19)2⊂1·(NO3)8, a dramatic 1H NMR change was observed after heating at 70 °C for 12 h, accompanied by the darkening of the pale-yellow solution (Fig. 1, S6 and S7†). Two sets of protons signals consisting of 12 aromatic signals were observed after the reaction. The resonances of He in the CH2 groups of the ligand (L) split into two doublets, along with a new set of splitting signals of Hd and Hc in the pyridinium group, all of which experienced two different magnetic environments. The DOSY spectrum shows that all the new signals have the same diffusion coefficient (D = 1.75 × 10−10 m2 s−1, Fig. 1D and S8†), different from that of (Mo6O19)2⊂1·(NO3)8 (D = 2.43 × 10−10 m2 s−1, Fig. S3†). The host–guest complex was characterized by high-resolution ESI-TOF-MS (Fig. 1E, S9 and S10†). Highly resolved +8 peaks observed at m/z = 520.9201 could be assigned to [Pd4L2(SiMo12O40)]8+. It is noted that the 1H NMR of the inclusion complex (Mo6O19)2⊂1·(NO3)8 had almost no change even after heating at 70 °C for three days (Fig. S11†). Based on the above results, we inferred that a new POM species, SiMo12O404−, was formed in the system by the condensation of Mo6O192− and SiO32−, accompanied by the structural transformation of cage 1.Yellow block crystals suitable for SCXRD were obtained by slow evaporation of an aqueous solution of the host–guest complex (Fig. S46†). The inclusion complex crystallized in a hexagonal crystal system with the P63 space group. Remarkably, the crystal structure reveals that the Pd4L2-type cage 1 transformed into a structural isomer known as bowl 2, benefited by the semirigid feature of the ligand with flexible p-xylene linkers. A single Keggin-type α-SiMo12O404− anion is tightly accommodated in the cavity of bowl-shaped host structure 2 (Fig. 2A). Multiple hydrogen bonding interactions between the p-xylene group and SiMo12O404− (C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo), with distances ranging from 2.3778(1) to 2.9933(1) Å, were observed in the structure (Fig. S50†). Independent gradient model (IGM) analysis provides a visual depiction of the noncovalent bonding interactions (Fig. 2B and S48†), from which we inferred that the SiMo12O404− anion is stabilized in the cavity by electrostatic and anion–π interactions as well as multiple intermolecular hydrogen bonding interactions. Additionally, in the crystal packing, three host–guest complexes aggregate into a trimer via π–π interactions between p-xylene and TPT panels (center-to-center distances: 3.6822(3)–3.8283(2) Å) (Fig. S50†).
Mo), with distances ranging from 2.3778(1) to 2.9933(1) Å, were observed in the structure (Fig. S50†). Independent gradient model (IGM) analysis provides a visual depiction of the noncovalent bonding interactions (Fig. 2B and S48†), from which we inferred that the SiMo12O404− anion is stabilized in the cavity by electrostatic and anion–π interactions as well as multiple intermolecular hydrogen bonding interactions. Additionally, in the crystal packing, three host–guest complexes aggregate into a trimer via π–π interactions between p-xylene and TPT panels (center-to-center distances: 3.6822(3)–3.8283(2) Å) (Fig. S50†).
The bowl 2 skeleton adopts the C2v molecular symmetry (Fig. 2C), in contrast to the D2d-symmetry of cage 1, which aligns well with the NMR analysis. The cavity volume of bowl 2 was calculated to be ca. 963 Å3 using MoloVol calculations based on the crystal structure (Fig. 2C and S52†),62 which is larger than that of cage 1 (ca. 914 Å3). This difference explains why the large-sized SiMo12O404− anion is encapsulated by bowl 2 rather than cage 1. According to Rebek's “55% rule”,63 optimal binding between the host and guest can be expected when the occupancy factor falls within the range 0.55 ± 0.09. The SiMo12O404− anion has a maximum size of approximately 10.4 Å and a molecular volume of ca. 658 Å3 (Fig. S53†). The occupancy factor of the bowl-cavity space by SiMo12O404− is ca. 68%, smaller than that (72%) in the cage-cavity, which makes it more suitable for the optimal binding than 1. Therefore, shape complementarity between Keggin-type SiMo12O404− and the bowl complex 2 drives the supramolecular structural transformation.
To shed light on the POM condensation induced-fit cage-to-bowl transformation mechanism, independently prepared (TBA)4SiMo12O40 (TBA = [(n-C4H9)4N]+) was treated with 1·(NO3)12 in D2O solution. As observed in the 1H NMR spectra, cage 1 cannot encapsulate the Keggin-type SiMo12O404− in the cavity. Time-dependent 1H NMR spectra with heating were collected to monitor the solution of (Mo6O19)2⊂1·(NO3)8 after adding 1 equiv. of Na2SiO3, as well as the solution containing SiMo12O404− and cage 1 (Fig. 3 and S12–S15†). Upon heating at 70 °C, signals assignable to the host–guest complex (SiMo12O40)⊂2·(NO3)8 gradually evolved, implying the simultaneous POM condensation and induced-fit cage transformation. For the in situ POM condensation driven cage-to-bowl conversion, over 92% transformation from (Mo6O19)2⊂1·(NO3)8 to (SiMo12O40)⊂2·(NO3)8 was observed within 12 h. In sharp contrast, only 61% conversion for SiMo12O404−-induced structural transformation was observed even after heating at 70 °C for 8 d. Based on pseudo-first order reaction kinetics, a 27.5-fold higher rate constant of in situ-POM-condensation driven cage-to-bowl transformation was estimated, compared to that driven by SiMo12O404− (Fig. 3D).
We also examined a similar condensation process with another Lindqvist W6O192− cluster. Host–guest NMR titration experiments and ESI-TOF-MS revealed that only one W6O192− cluster could be trapped in the cavity of 1 (Fig. S16–S20†). Then we wondered whether the reaction of (TBA)2W6O19 and Na2SiO3 in the presence of 1·(NO3)12 could also produce a similar Keggin-type product. The solution of 1 equiv. of Na2SiO3 and 2 equiv. of W6O192− with 1·(NO3)12 was heated for 15 h, resulting in a complicated 1H NMR spectrum (Fig. S21†). The formation of the main product (W10O32)⊂2·(NO3)8, as well as the minor product (SiW12O40)⊂2·(NO3)8 was confirmed by ESI-TOF-MS (Fig. S22†). Observation of +4 and +3 charged species assigned to (W10O32)⊂2·(NO3)8 and (SiW12O40)⊂2·(NO3)8 provides the direct evidence. In addition, other signals attributable to [Pd4L2(NO3)3(W6O19)(HW2O7)(H2O)3]6+ and [Pd4L2(NO3)5(W6O19)(HW2O7)(H2O)3]4+ were observed in the mass spectrum. The cage-to-bowl transformation was then also checked with independently made SiW12O404−. Time-dependent 1H NMR spectra of 1·(NO3)12 and 1 equiv. of SiW12O404− with heating at 70 °C exhibit 27% yield of clean (SiW12O40)⊂2·(NO3)8 (Fig. S23†) without the formation of other POM species. ESI-TOF-MS analysis of (SiW12O40)⊂2·(BF4)8 revealed a clear series of multivalent signals assignable to [Pd4L2(BF4)nSiW12O40]8−n (n = 0–4) (Fig. S24†). These results indicate that the full-encapsulation of the small POM precursors inside the cage cavity is crucial for efficient condensation reactions.
Previous reports have shown that the rapid transformation of W6O192− to W10O324− occurs when water is added to a methanolic solution containing W6O192−.64,65 Thus the transformation of W6O192− without SiO32− was also examined in our system (Fig. 4A). When 2 equiv. of the W6O192− anion was added to the H2O solution of 1·(NO3)12 and heated at 70 °C, 1H NMR spectra show the disappearance of the starting material along with the coinstantaneous evolvement of two sets of new species (Fig. 4B and C). ESI-TOF-MS reveals the formation of new host–guest complexes assignable to (W10O32)⊂Pd4L2 (Fig. 4D, S27 and S28†). 1H NMR spectra of 1·(NO3)12 with the addition of independently prepared (TBA)2W10O32 showed almost identical NMR spectra (Fig. S34†). Based on the NMR of 1·(NO3)12 and (TBA)4W10O32 (Fig. S29–S32†), along with the symmetry of the cage and bowl, one set of signals is assigned to (W10O32)⊂1·(NO3)8 and the other to (W10O32)⊂2·(NO3)8. This is further supported by the ESI-TOF-MS analyses (Fig. S36†).
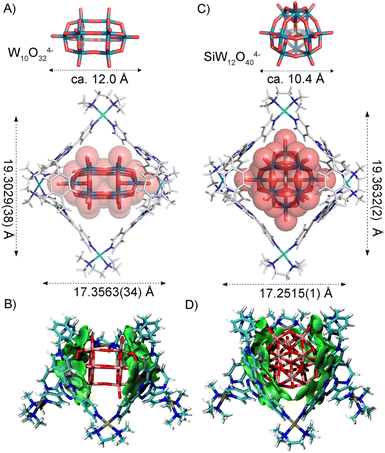
SCXRD provided direct proof of the structural transformation. After heating W6O192− in the aqueous solution of 1·(NO3)12 at 70 °C for 2 days, single crystals of (W10O32)⊂2·(NO3)8 were obtained by slow vapor diffusion of THF into the system. X-ray structural analysis reveals a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex, where a W10O324− anion was encapsulated into the cavity of bowl 2 (Fig. 5A). The average diagonal Pd–Pd distances in the structure of (W10O32)⊂2·(NO3)8 are 17.3563(34) Å and 19.3029(38) Å. IGM analysis revealed that electrostatic, anion–π interactions and multiple hydrogen bonds work together to stabilize this host–guest complex (Fig. 5B and S49†). As the maximum size and the volume of W10O324− are ca. 12.0 Å and 558 Å3, respectively, the occupancy factor of W10O324− in the bowl-cavity of 2 is ca. 58%, compared to 61% in the cage cavity of 1 (Table S1†). W10O324− also can be shape-matched well with the bowl-shape space of 2 and multiple non-covalent interactions drive the guest-reaction induced-fit structural transformation.
1 host–guest complex, where a W10O324− anion was encapsulated into the cavity of bowl 2 (Fig. 5A). The average diagonal Pd–Pd distances in the structure of (W10O32)⊂2·(NO3)8 are 17.3563(34) Å and 19.3029(38) Å. IGM analysis revealed that electrostatic, anion–π interactions and multiple hydrogen bonds work together to stabilize this host–guest complex (Fig. 5B and S49†). As the maximum size and the volume of W10O324− are ca. 12.0 Å and 558 Å3, respectively, the occupancy factor of W10O324− in the bowl-cavity of 2 is ca. 58%, compared to 61% in the cage cavity of 1 (Table S1†). W10O324− also can be shape-matched well with the bowl-shape space of 2 and multiple non-covalent interactions drive the guest-reaction induced-fit structural transformation.
Crystallization of the (SiW12O40)⊂2·(NO3)8 complex was also successful. In the crystal structure, the complex crystallized in the monoclinic crystal system with the P21/n space group. One SiW12O404− anion sits inside the bowl-shaped cage 2, similar to SiMo12O404− (Fig. 5C and D). IGM analysis confirmed similar non-covalent interactions in stabilizing this iso-structural host–guest complex as observed with (SiMo12O40)⊂2·(NO3)8.
UV-vis absorption spectra also supported the POM synthesis driven supramolecular transformation processes (Fig. S37 and S38†). After heating the solution of the inclusion complex (Mo6O19)2⊂1·(NO3)8 at 70 °C with NaSiO3 in H2O, the structural conversion to (SiMo12O40)⊂2·(NO3)8 was indicated by the appearance of a new absorption band tailing to the region until ca. 400 nm. In the IR spectra (Fig. S39†), the characteristic peaks of ν(Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(O–Mo–O) for (Mo6O19)2⊂1·(NO3)8 were observed at 956 and 800 cm−1, respectively, which disappeared when forming the new host–guest complex of (SiMo12O40)⊂2·(NO3)8, along with new peaks at 945, 901, 795 cm−1 assignable to SiMo12O404−. As for the conversion from W6O192− to W10O324−, new peaks in the region of 961–434 cm−1 corresponding to W10O324− were observed after heating the sample of the mixture of W6O192− (2 equiv.) and 1·(NO3)12 (Fig. S40†). The peaks at 961, 893 and 804 cm−1 correspond to ν(W–Ot), ν(W–Ob–W), and ν(W–Oc–W) of the [W10O32]4− cluster, respectively.48,66,67
O) and ν(O–Mo–O) for (Mo6O19)2⊂1·(NO3)8 were observed at 956 and 800 cm−1, respectively, which disappeared when forming the new host–guest complex of (SiMo12O40)⊂2·(NO3)8, along with new peaks at 945, 901, 795 cm−1 assignable to SiMo12O404−. As for the conversion from W6O192− to W10O324−, new peaks in the region of 961–434 cm−1 corresponding to W10O324− were observed after heating the sample of the mixture of W6O192− (2 equiv.) and 1·(NO3)12 (Fig. S40†). The peaks at 961, 893 and 804 cm−1 correspond to ν(W–Ot), ν(W–Ob–W), and ν(W–Oc–W) of the [W10O32]4− cluster, respectively.48,66,67
Plausible mechanisms were proposed for the POM condensation/transformation induced cage-to-bowl conversion. In the case of Mo6O192−, two precursors are encapsulated by the cavity of cage 1 and condensation happens after the addition of SiO32−. The resulting SiMo12O404− anion instigates the induced-fit transformation from cage 1 to bowl 2. In fact, when (TBA)2Mo6O19 was treated with Na2SiO3 and heated at 70 °C for 4 days as a control, no SiMo12O404− anion was observed (Fig. S41†), suggesting the vital role of cage 1 in the synthesis of SiMo12O404−. Similarly, no W10O324− or SiW12O404− was observable by heating (TBA)2W6O19 and Na2SiO3 at 70 °C in H2O/CH3CN (v/v, 4/1) even for 4 days (Fig. S42†). Moreover, a remarkable stability of SiMo12O404− hydrolytically protected within the bowl host was confirmed in water, as 1H NMR witnessed negligible change for the (SiMo12O40)⊂2·(NO3)8 complex even after standing at room temperature for one year (Fig. S44 and S45†).
Conclusions
In summary, we have discovered an induced-fit supramolecular transformation driven by in situ POM condensation reactions. Our work represents the first report on inorganic condensation reaction driven supramolecular structure transformation, which may provide new design principles for chemically fueled molecular machines. We also envision that efficient embedding of POMs into adaptive supramolecular hosts could be very promising for the development of functional hybrid materials.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data have been deposited at the CCDC under numbers 2360627, 2380035 and 2380036, and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Author contributions
Q.-F. S. and L.-X. C. conceived and designed this project. L.-X. C. carried out the synthesis, characterization, conducted the experiments and analyzed all the results. Y.-H. H. assisted with the synthesis. L.-P. Z. and Y.-T. C. performed the mass spectroscopy measurement. P.-M. C and X.-Q. G. contributed to the figure production. L.-X. C. and Q.-F. S. wrote the manuscript with input from all the authors.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grants 2022YFA1503300 and 2021YFA1500400), the National Natural Science Foundation of China (Grants 22171262, and 22171264), the National Science and Technology Council (NSTC) of Taiwan (113-2628-M-002-004), the Self-Deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-GH02), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB1170000).Notes and references
- Y. Jin, Q. Zhang, Y. Zhang and C. Duan, Chem. Soc. Rev., 2020, 49, 5561–5600 CAS
.
- D. D. Boehr, R. Nussinov and P. E. Wright, Nat. Chem. Biol., 2009, 5, 789–796 CrossRef CAS PubMed
.
- Z. Dong, Q. Luo and J. Liu, Chem. Soc. Rev., 2012, 41, 7890–7908 CAS
.
- C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012–3035 CAS
.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 Search PubMed
.
- E. G. Percastegui, T. K. Ronson and J. R. Nitschke, Chem. Rev., 2020, 120, 13480–13544 CAS
.
- C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012–3035 CAS
.
- J. Guo, Y.-Z. Fan, Y.-L. Lu, S.-P. Zheng and C.-Y. Su, Angew. Chem., Int. Ed., 2020, 59, 8661–8669 CrossRef CAS
.
- D. Liu, H. Ma, C. Zhu, F. Qiu, W. Yu, L.-L. Ma, X.-W. Wei, Y.-F. Han and G. Yuan, J. Am. Chem. Soc., 2024, 146, 2275–2285 Search PubMed
.
- G. Wu, Y. Chen, S. Fang, L. Tong, L. Shen, C. Ge, Y. Pan, X. Shi and H. Li, Angew. Chem., Int. Ed., 2021, 60, 16594–16599 CrossRef CAS PubMed
.
- R. L. Spicer, H. M. O'Connor, Y. Ben-Tal, H. Zhou, P. J. Boaler, F. C. Milne, E. K. Brechin, G. C. Lloyd-Jones and P. J. Lusby, Chem. Sci., 2023, 14, 14140–14145 Search PubMed
.
- S. Matsuno, M. Yamashina, Y. Sei, M. Akita, A. Kuzume, K. Yamamoto and M. Yoshizawa, Nat. Commun., 2017, 8, 749 Search PubMed
.
- M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251–254 Search PubMed
.
- K. Iizuka, H. Takezawa and M. Fujita, J. Am. Chem. Soc., 2023, 145, 25971–25975 Search PubMed
.
- W. Cullen, M. C. Misuraca, C. A. Hunter, N. H. Williams and M. D. Ward, Nat. Chem., 2016, 8, 231–236 CrossRef CAS PubMed
.
- J. Jiao, Z. Li, Z. Qiao, X. Li, Y. Liu, J. Dong, J. Jiang and Y. Cui, Nat. Commun., 2018, 9, 4423 Search PubMed
.
- K. Wang, J. H. Jordan, X. Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 13712–13721 CAS
.
- Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang and H. C. Zhou, Chem. Soc. Rev., 2019, 48, 4707–4730 CAS
.
- H. Takezawa, K. Shitozawa and M. Fujita, Nat. Chem., 2020, 12, 574–578 Search PubMed
.
- A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CAS
.
- W. Wang, Y.-X. Wang and H.-B. Yang, Chem. Soc. Rev., 2016, 45, 2656–2693 Search PubMed
.
- O. Gidron, M. Jirasek, N. Trapp, M. O. Ebert, X. Zhang and F. Diederich, J. Am. Chem. Soc., 2015, 137, 12502–12505 CAS
.
- R. Zhu, J. Lubben, B. Dittrich and G. H. Clever, Angew. Chem., Int. Ed., 2015, 54, 2796–2800 Search PubMed
.
- S.-P. Zheng, Y.-W. Xu, P.-Y. Su, C.-H. Liu, Y.-H. Huang, Y.-L. Lu, Z.-W. Wei, Z. Jiao, H.-S. Xu and C.-Y. Su, Chin. Chem. Lett., 2024, 35, 108477 Search PubMed
.
- L.-L. Yan, L.-Y. Yao, M. Ng and V. W.-W. Yam, J. Am. Chem. Soc., 2021, 143, 19008–19017 CAS
.
- X.-R. Liu, P.-F. Cui, S.-T. Guo, Y.-J. Lin and G.-X. Jin, J. Am. Chem. Soc., 2023, 145, 8569–8575 CAS
.
- H. J. Yoon, J. Kuwabara, J. H. Kim and C. A. Mirkin, Science, 2010, 330, 66–69 Search PubMed
.
- S. Wang, T. Sawada, K. Ohara, K. Yamaguchi and M. Fujita, Angew. Chem., Int. Ed., 2016, 55, 2063–2066 CAS
.
- D. M. Wood, W. Meng, T. K. Ronson, A. R. Stefankiewicz, J. K. Sanders and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 3988–3992 Search PubMed
.
- A. Walther, I. Regeni, J. J. Holstein and G. H. Clever, J. Am. Chem. Soc., 2023, 145, 25365–25371 Search PubMed
.
- R. Banerjee, S. Bhattacharyya and P. S. Mukherjee, JACS Au, 2023, 3, 1998–2006 CrossRef CAS PubMed
.
- K. Hema, A. B. Grommet, M. J. Bialek, J. Wang, L. Schneider, C. Drechsler, O. Yanshyna, Y. Diskin-Posner, G. H. Clever and R. Klajn, J. Am. Chem. Soc., 2023, 145, 24755–24764 CAS
.
- S. Wang, T. Sawada and M. Fujita, Chem. Commun., 2016, 52, 11653–11656 Search PubMed
.
- P.-M. Cheng, L.-X. Cai, S.-C. Li, S.-J. Hu, D.-N. Yan, L.-P. Zhou and Q.-F. Sun, Angew. Chem., Int. Ed., 2020, 59, 23569–23573 Search PubMed
.
- S. S. Wang and G. Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS
.
- D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 Search PubMed
.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009–6048 Search PubMed
.
- D.-Y. Du, J.-S. Qin, S.-L. Li, Z.-M. Su and Y.-Q. Lan, Chem. Soc. Rev., 2014, 43, 4615–4632 Search PubMed
.
- D. Ravelli, S. Protti and M. Fagnoni, Acc. Chem. Res., 2016, 49, 2232–2242 Search PubMed
.
- B. Kowalewski, J. Poppe, U. Demmer, E. Warkentin, T. Dierks, U. Ermler and K. Schneider, J. Am. Chem. Soc., 2012, 134, 9768–9774 Search PubMed
.
- D. Fenske, M. Gnida, K. Schneider, W. Meyer-Klaucke, J. Schemberg, V. Henschel, A.-K. Meyer, A. Knöchel and A. Müller, ChemBioChem, 2005, 6, 405–413 CAS
.
- S. Brünle, M. L. Eisinger, J. Poppe, D. J. Mills, J. D. Langer, J. Vonck and U. Ermler, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 26497–26504 Search PubMed
.
- C. Molitor, A. Bijelic and A. Rompel, Chem. Commun., 2016, 52, 12286–12289 Search PubMed
.
- W. Xuan, A. J. Surman, Q. Zheng, D. L. Long and L. Cronin, Angew. Chem., Int. Ed., 2016, 55, 12703–12707 Search PubMed
.
- M. Bugnola, R. E. Schreiber, Y. Kaufman, G. Leitus, L. J. W. Shimon and R. Neumann, Eur. J. Inorg. Chem., 2019, 2019, 482–485 CAS
.
- H. Liu, C.-Y. Song, R.-W. Huang, Y. Zhang, H. Xu, M.-J. Li, S.-Q. Zang and G.-G. Gao, Angew. Chem., Int. Ed., 2016, 55, 3699–3703 Search PubMed
.
- Y. Wu, R. Shi, Y. L. Wu, J. M. Holcroft, Z. Liu, M. Frasconi, M. R. Wasielewski, H. Li and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 4111–4118 Search PubMed
.
- K. Uehara, K. Kasai and N. Mizuno, Inorg. Chem., 2007, 46, 2563–2570 Search PubMed
.
- M. Han, J. Hey, W. Kawamura, D. Stalke, M. Shionoya and G. H. Clever, Inorg. Chem., 2012, 51, 9574–9576 CAS
.
- Y. Liu, C. Hu, A. Comotti and M. D. Ward, Science, 2011, 333, 436–440 Search PubMed
.
- D. Prochowicz, A. Kornowicz and J. Lewinski, Chem. Rev., 2017, 117, 13461–13501 CAS
.
- M. Stuckart and K. Y. Monakhov, Chem. Sci., 2019, 10, 4364–4376 Search PubMed
.
- Q. Han, C. He, M. Zhao, B. Qi, J. Niu and C. Duan, J. Am. Chem. Soc., 2013, 135, 10186–10189 CAS
.
- M. A. Moussawi, M. Haouas, S. Floquet, W. E. Shepard, P. A. Abramov, M. N. Sokolov, V. P. Fedin, S. Cordier, A. Ponchel, E. Monflier, J. Marrot and E. Cadot, J. Am. Chem. Soc., 2017, 139, 14376–14379 Search PubMed
.
- M. A. Moussawi, N. Leclerc-Laronze, S. Floquet, P. A. Abramov, M. N. Sokolov, S. Cordier, A. Ponchel, E. Monflier, H. Bricout, D. Landy, M. Haouas, J. Marrot and E. Cadot, J. Am. Chem. Soc., 2017, 139, 12793–12803 CAS
.
- C. Falaise, M. A. Moussawi, S. Floquet, P. A. Abramov, M. N. Sokolov, M. Haouas and E. Cadot, J. Am. Chem. Soc., 2018, 140, 11198–11201 Search PubMed
.
- S.-B. Yu, Q. Qi, B. Yang, H. Wang, D.-W. Zhang, Y. Liu and Z.-T. Li, Small, 2018, 14, 1801037 Search PubMed
.
- X.-F. Li, S.-B. Yu, B. Yang, J. Tian, H. Wang, D.-W. Zhang, Y. Liu and Z.-T. Li, Sci. China:Chem., 2018, 61, 830–835 Search PubMed
.
- M. Yan, X.-B. Liu, Z.-Z. Gao, Y.-P. Wu, J.-L. Hou, H. Wang, D.-W. Zhang, Y. Liu and Z.-T. Li, Org. Chem. Front., 2019, 6, 1698–1704 Search PubMed
.
- L. X. Cai, S. C. Li, D. N. Yan, L. P. Zhou, F. Guo and Q. F. Sun, J. Am. Chem. Soc., 2018, 140, 4869–4876 Search PubMed
.
- S.-C. Li, L.-X. Cai, L.-P. Zhou, F. Guo and Q.-F. Sun, Sci. China:Chem., 2019, 62, 713–718 CrossRef CAS
.
- J. B. Maglic and R. Lavendomme, J. Appl. Crystallogr., 2022, 55, 1033–1044 CrossRef CAS PubMed
.
- S. Mecozzi and J. Rebek Julius, Chem.–Eur. J., 1998, 4, 1016–1022 CrossRef CAS
.
-
M. T. Pope, Heteropoly and isopoly oxometalates, Springer, Berlin, 1983, p. 54 Search PubMed
.
- P.-P. Shen, Y.-N. Chi, Z.-G. Lin and C.-W. Hu, Inorg. Chem. Commun., 2013, 29, 197–200 Search PubMed
.
-
W. G. Klemperer, Tetrabutylammonium Isopolyoxometalates, in Inorganic Syntheses, 1990, pp. 74–85 Search PubMed
.
- H. Xu, Z. Li, B. Liu, G. Xue, H. Hu, F. Fu and J. Wang, Cryst. Growth Des., 2010, 10, 1096–1103 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2360627, 2380035 and 2380036. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08729a |
| This journal is © The Royal Society of Chemistry 2025 |