Open Access Article
Open Access ArticleAlkyl bistriflimidate-mediated electrochemical deaminative functionalization†
Hui
Shu
,
Xiangzhang
Tao
*,
Shengyang
Ni
,
Jiyang
Liu
,
Jia
Xu
,
Yi
Pan
and
Yi
Wang
 *
*
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Collaborative Innovation Center of Advanced Microstructures, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: yiwang@nju.edu.cn; taoxz941006@163.com
First published on 24th December 2024
Abstract
An efficient electrochemical strategy for the deaminative functionalization of alkyl amines has been described. The alkyl bistriflimidates were readily accessed by the treatment of alkyl amines with trifluoromethanesulfonic anhydride and unprecedentedly employed for C–N bond activation. They can be applied to a range of transformations, including borylation, sulfuration, selenation, sulfonation, Additionally, deaminative esterification and amidation can be performed under catalytic base conditions. The protocol features an undivided cell without the use of transition metal- or photo-catalysts and exhibits high conversion and stability in flow reactors.
Introduction
Aliphatic primary amines are prevalent in nature, living organisms, and biologically active molecules.1–7 Serving as fundamental building blocks, they find extensive use in synthesizing complex molecules.8–14 The cleavage of C(sp3)–N bonds to produce alkyl radicals holds great potential in organic synthesis and drug discovery. However, cleaving the C–N bond in a primary amine (C–NH2) poses a significant challenge in organic chemistry. This challenge stems from the poor leaving ability of the NH2 group (C–N BDE = 102 kcal mol−1, pKa = 36) and the free N–H bonds towards oxidation/cross-coupling (N–H BDE = 92 kcal mol−1). In the 1970s, efforts to achieve deamination by sulfonation or pre-functionalization of alkyl amines with 2,4,6-triphenylpyridine salts (Katritzky salts) faced limitations due to strict reaction conditions.15 Recently, Watson and colleagues reported a milestone study using Katritzky salts to access alkyl radicals via a single electron transfer (SET) process.16,17 Subsequent developments of amine-derived Katritzky pyridium salts as carbon radical precursors have emerged.17–35 Meanwhile, isonitriles and alkyl diazo compounds were also used to achieve various deaminative functionalizations (Fig. 1A and B).36,37 In addition, imines could be generated from the corresponding amines with trimethoxybenzaldehyde and applied to a number of photoredox deaminative functionalizations (Fig. 1C).37 Despite these state-of-the-art approaches to achieve deaminative strategies, the pursuit of other readily available surrogates for primary amines remains highly valuable.Electrochemical synthesis has experienced a new trend of research interest in both research and industry owing to its sustainable and practical nature.38–46 In our previous study, Katritzky salts were proven suitable for deaminative functionalization under cell conditions (Fig. 1D).39 However, this strategy could only be applied to secondary alkylamines. Moreover, the expensive pyrylium reagents and the triphenylpyridine by-product restrict the applicability of this method.
To address these issues, we describe a new approach utilizing inexpensive trifluoromethanesulfonic anhydride as an activating reagent. The alkyl bistriflimidates undergo mild electrochemical deaminative functionalization in the absence of stoichiometric reductants or transition metal catalysts. In addition, in the presence of a catalytic amount of base, alkyl bistriflimidates can undergo ionic reactions, such as Ritter-type reactions (Fig. 1E).
Results and discussion
We began our study with electrochemical deaminative borylation using alkyl bistriflimidate (1) and B2cat2 (2) and treated under constant current electrolysis (CCE) (Table 1). After detailed investigations, we found that the desired alkyl boronate 3 could be obtained in 78% yield under an undivided cell set-up with a magnesium anode and nickel foam cathode at a working current of 20 mA (Table 1, entry 1). Other anode materials, such as zinc, iron, and carbon led to a lower yield (entries 2–4). In the case of carbon as the cathode, a lower yield of 36% was observed (entry 5). Notably, the use of nBu4NBF4 instead of TBAI proved to be unfeasible, which indicated that iodide anions were suggested to play an important role in such transformation (entry 6). The presence of air led to a product loss of approximately 15% (entry 7). Control experiments indicate that the electric current is essential (entry 8). Varied current (10 mA and 30 mA) caused decreased yields of the product (entries 9 and 10). The reaction did not occur when B2pin2 was utilized as the boron source (entry 11). Remarkably, activation of the primary alkyl amine with TsCl and preparation as Katritzky salt proved to be impractical under the given reaction conditions, leading to significant challenges in achieving the desired target product (entry 12).| Entry | Modification of standard conditions | Yielda [%] |
|---|---|---|
| a Reaction conditions: 1 (0.3 mmol), 2 (1.2 mmol), TBAI (0.9 mmol), DMA (3.0 mL), Ar, at 25 °C under 20.0 mA constant-current conditions in an undivided cell, 30 min. Crude yields were determined by GC using dodecane as an internal standard. b The isolated yields were determined. | ||
| 1 | None | 88 (78b) |
| 2 | Using Zn(+) instead of Mg(+) | 57 |
| 3 | Using Fe(+) instead of Mg(+) | 69 |
| 4 | Using C(+) instead of Mg(+) | n.d. |
| 5 | Using C(−) instead of Ni(−) | 36 |
| 6 | n Bu4NPF6 instead of nBu4NI | Trace |
| 7 | Under air | 72 |
| 8 | No current | n.d. |
| 9 | 10 mA, 1h | 50 |
| 10 | 30 mA, 20 min | 65 |
| 11 | Using B2pin2 instead of B2Cat2 | n.d. |
| 12 | Using alkyl-NTs2 or alkyl-Katritzky salts instead of 1 | n.d./4 |
With the optimized conditions in hand, the generality of this electrochemical transformation has been evaluated, as shown in Fig. 2. This protocol exhibited good efficiency toward the borylation of alkyl bistriflimidates, showcasing wide functional tolerance and generated borylation products in moderate to high yields. Functional groups, including halides (4-5, 8-9, and 25), trifluoromethyl (6-7), ethers (14, 16), esters (24), aromatic and heterocyclic (11–13, 15, and 17–20), cyanides (31), naphthalene (21), protected alcohols (29), and thioether (32) could be tolerated in the reaction. This underscores the robustness of our electrochemical methodology. Additionally, recognizing the crucial role of polyboronate compounds in organic synthesis and the limitations of direct synthesis methods, this paper outlines the preparation of diverse diboronate compounds through diamines (26 and 30). Secondary alkyl amines were also amenable to this method, as exemplified by 2-amino-4-phenylbutane, which gave product 33 in 45% yield. Cyclic alkylamines, such as cyclohexyl, cyclododecyl and 4-aminotetrahydropyran, also result in moderate yields of the boronated products (34–36).
 | ||
| Fig. 2 Scope of the alkyl bistriflimidates. Conditions: alkyl bistriflimidates (0.5 mmol), 2 (2.0 mmol), TBAI (0.9 mmol), DMA (5.0 mL), under an argon atmosphere, 25 °C, 1 h. a3 h. | ||
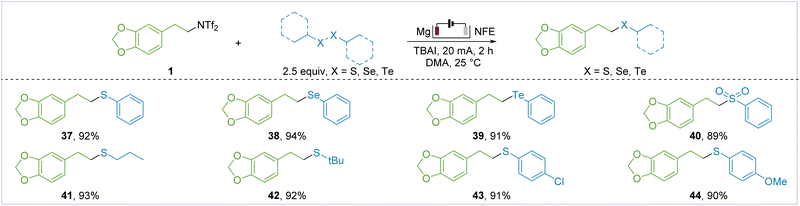
Having successfully established the C–B bond, we explored the possibility of establishing other C–X bonds within this framework. As depicted in Fig. 3, under the standard conditions, we found that both alkyl and aryl sulfides were generated in high yields (37 and 41–44). In addition, we efficiently achieved deaminative selenation (38), telluration (39), and sulfonylation (40), developing a new path for the efficient synthesis of sulfur-containing bioactive molecules.
To further demonstrate the practicality of the deaminative functionalization approach for alkyl bistriflimidates, a number of valuable direct transformations were identified. As illustrated in Fig. 4, the reaction of alkyl bistriflimidates with amides led to the formation of ester compounds (45–48). Notably, alkyl bistriflimidates facilitated deamination Ritter reactions, efficiently producing a diverse array of amide compounds, including alkyl and aryl amides (49–56). Compared to traditional methods, the avoidance of high temperatures and transition-metal catalysts underscores the practical advantages of this approach.47–52 Additionally, to evaluate the compatibility of the deamination process of alkyl bistriflimidates across various methodologies, alternative transformations of alkyl bistriflimidates were explored, as depicted in Fig. 4C. Compound 1 can undergo nucleophilic substitution with aryl primary amine under catalytic amounts of base to afford secondary amine. Alkyl bistriflimidates also participate in reductive cross-coupling reactiona under nickel catalysis, yielding a deaminative allylation product (59). Furthermore, alkyl bistriflimidate 11a is well-suited to photocatalytic conditions, providing 11 with a 66% yield. Using (bpy)Cu(CF3)3 as a trifluoromethylating agent, the deaminative trifluoromethylation product (60) was obtained with moderate yield.
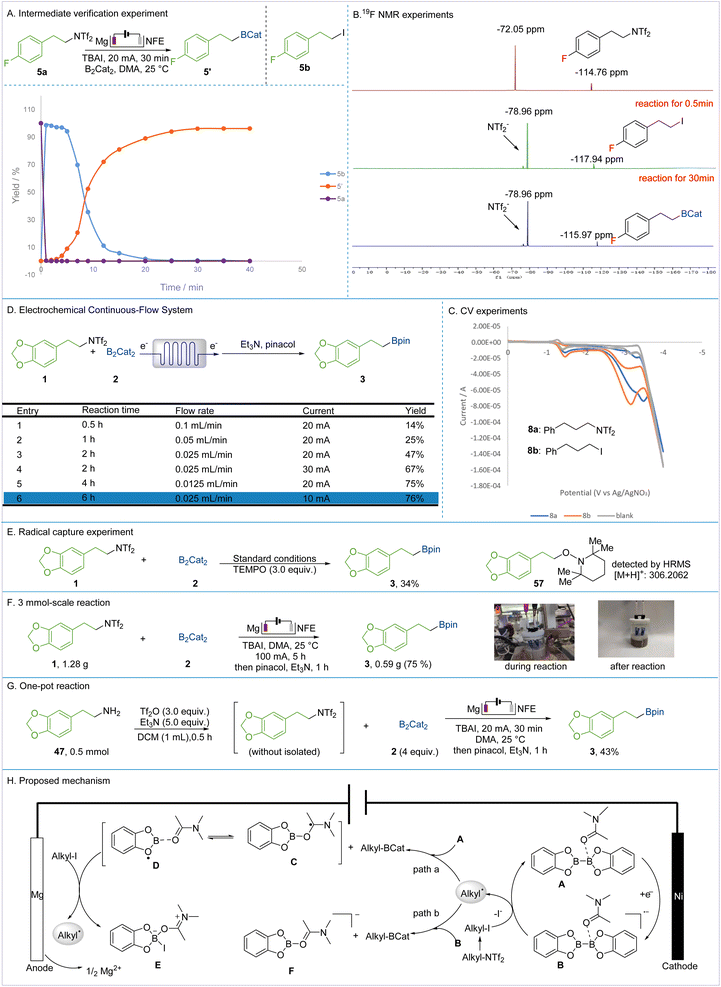
To explore the reaction mechanism, a series of experiments were conducted. The intermediate verification experiment demonstrated that alkyl bistriflimidate (5a) was completely converted to alkyl iodide (5b) within 2 min, followed by the consumption of 5b and its conversion into the corresponding product within 40 min (Fig. 5A). The in situ observation of compound 5bvia19F NMR confirms its role as an intermediate product, which can undergo further transformation to yield the subsequent functional group compound (Fig. 5B).53,54 The radical trapping experiment with TEMPO under standard conditions resulted in the alkyl adduct 57, which was detected by HRMS (Fig. 5E). Additionally, the EPR experiments indicate the formation of radicals (for details, see the ESI†). These experiments indicate that the reaction proceeds via a radical pathway. The CV experiments were performed to verify the species undergoing cathodic reduction. The reduction potential of the alkyl bistriflimidate 8a (−3.6 V) was higher than that of compound 8b (−3.1 V), indicating that the direct reduction of alkyl bistriflimidate to radicals is challenging, whereas 8b is more likely to be reduced to generate alkyl radicals, according to the literature (Fig. 5C).55,56 Next, the reaction was conducted in a flow system, and a microfluidic electrochemistry platform was introduced for scale-up reactions. At a flow rate of 0.025 mL min−1 and an electrolysis current of 10 mA, we obtained a yield of 76% for the product comparable to those obtained in batch (Fig. 5D). To corroborate the applicability of this protocol, a 3 mmol-scale reaction was carried out, yielding the boronated product in 75% within 5 h at 100 mA (Fig. 5F). Furthermore, utilizing a one-pot synthesis approach, the boronated product could be produced in 43% yield, demonstrating the practicality of this method under the given reaction conditions (Fig. 5G).
Based on previous reports and the above experiments, we propose a possible mechanism as shown in Fig. 5H.57–61 The reaction is initiated with rapid iodination of alkyl bistriflimidate to generate alkyl iodide. Meanwhile, DMA with 1 equivalent of B2cat2 forms complex A and undergoes single-electron reduction at the cathode to form radical anion B. Subsequently, this radical may undergo two different paths. In path a, the reaction of the alkyl radical with A gives the alkyl boronate product and C/D, which is eventually oxidized by alkyl iodide to form E and an alkyl radical. In path b, radical–radical cross-coupling of the alkyl radical and B furnishes the alkyl boronate and complex F.
Experimental
General procedure for the synthesis of products 3–32. Condition A
Tetrabutylammonium iodide (TBAI) (332 mg, 0.9 mmol), 2,2′-bis-1,3,2-benzodioxaborole(B2cat2) (477 mg, 2.0 mmol), alkyl bistriflimidates (0.5 mmol) and DMAc (5 mL) were taken in a tube and placed in a glove box. A magnesium plate (52.5 × 8 × 2 mm) was used as the anode, a foamed nickel electrode (52.5 × 8 × 2 mm) was used as the cathode and then the reaction mixture was electrolyzed at a constant current of 20 mA for 30 min. Afterwards, a solution of pinacol (2.0 mmol, 4.0 equiv., 236 mg) in triethylamine (1.0 mL) was added to the electrolyzer cell and the reaction mixture was kept stirring at room temperature for 1 h. The mixture was then quenched with ethyl acetate, dried onto silica gel, and purified by rapid column chromatography.General procedure for the synthesis of products 33–36. Condition B
An oven dried tube flask equipped with a stir bar placed in a glove box was charged with amine (0.5 mmol, 1.0 equiv.), CH2Cl2 (1 mL, 0.5 M) and Et3N (0.28 mL, 1 mmol, 2.0 equiv.). The flask was cooled to −78 °C, and trifluoromethanesulfonic anhydride (0.26 mL, 0.42 g, 1.5 mmol, 3.0 equiv.) was added dropwise. The reaction was stirred vigorously at −78 °C for 0.5 h. Then NaI (135 mg, 0.9 mmol), B2cat2 (477 mg, 2.0 mmol), and DMAc (3 mL) were taken in a tube and placed in a glove box. A magnesium plate (52.5 × 8 × 2 mm) was used as the anode, a foamed nickel electrode (52.5 × 8 × 2 mm) was used as the cathode and then the reaction mixture was electrolyzed at a constant current of 20 mA at 60 °C. Afterwards, a solution of pinacol (2.0 mmol, 4.0 equiv., 236 mg) in triethylamine (1.0 mL) was added to the electrolyzer cell and the reaction mixture was kept stirring at room temperature for 1 h. The mixture was then quenched with ethyl acetate, dried onto silica gel, and purified by rapid column chromatography.Conclusions
In conclusion, we have developed an efficient and operationally simple deaminative protocol for constructing C–X bonds, which complements the diverse functionalization of alkylamines. Under mild electrochemical conditions, deaminative borylation, sulfuration, selenation and sulfonation were shown to be possible applications. Furthermore, scale-up reactions and continuous-flow reactions have demonstrated the applicability of this deaminative borylation strategy. Mechanistic studies indicate that iodide anions play a crucial role in the deaminative process. Additionally, esterification and amidation could be performed under catalytic base conditions.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
H. S., J. L., and J. X. conducted all experiments and characterized the novel compounds. X. T., Y. W., S. N., and Y. P. designed the experiments and wrote the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 22071101, 22271147 and 22471123).Notes and references
- P. RuizCastillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed.
- C. Liu and M. Szostak, Chem.–Eur. J., 2017, 23, 7157–7173 CrossRef CAS PubMed.
- N. A. McGrath, M. Brichacek and J. T. Njardarson, J. Chem. Educ., 2010, 87, 1348–1349 CrossRef CAS.
- F. Mo, G. Dong, Y. Zhang and J. Wang, Org. Biomol. Chem., 2013, 11, 1582–1593 RSC.
- Q. Wang, Y. Su, L. Li and H. Huang, Chem. Soc. Rev., 2016, 45, 1257–1272 RSC.
- S. Plunkett, C. H. Basch, S. O. Santana and M. P. Watson, J. Am. Chem. Soc., 2019, 141, 2257–2262 CrossRef CAS PubMed.
- Y. Liu and H. Ge, Nat. Chem., 2017, 9, 26–32 CrossRef CAS.
- D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Nat. Chem., 2018, 10, 383–394 CrossRef CAS PubMed.
- R. A. Shenvi, D. P. O'Malley and P. S. Baran, Acc. Chem. Res., 2009, 42, 530–541 CrossRef CAS PubMed.
- D. Kong, P. J. Moon and R. J. Lundgren, Nat. Catal., 2019, 2, 473–476 CrossRef CAS.
- Y.-N. Li, F. Xiao, Y. Guo and Y.-F. Zeng, Eur. J. Org Chem., 2021, 2021, 1215–1228 CrossRef CAS.
- C. Laurence, K. A. Brameld, J. Graton, J.-Y. Le Questel and E. Renault, J. Med. Chem., 2009, 52, 4073–4086 CrossRef CAS PubMed.
- Y. Wang, I. Haight, R. Gupta and A. Vasudevan, J. Med. Chem., 2021, 64, 17115–17122 CrossRef CAS PubMed.
- Y. Gao, S. Jiang, N.-D. Mao, H. Xiang, J.-L. Duan, X.-Y. Ye, L.-W. Wang, Y. Ye and T. Xie, Top. Curr. Chem., 2022, 380, 25 CrossRef CAS PubMed.
- P. Müller and M. P. N. Thi, Conversion of Amines into Phenylsulfides and Phenylselenides via Ditosylamides, Helv. Chim. Acta, 1980, 63, 2168–2172 CrossRef.
- C. H. Basch, J. Liao, J. Xu, J. J. Piane and M. P. Watson, J. Am. Chem. Soc., 2017, 139, 5313–5316 CrossRef CAS PubMed.
- F. J. R. Klauck, M. J. James and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 12336–12339 CrossRef CAS PubMed.
- J. Wu, L. He, A. Noble and V. K. Aggarwal, J. Am. Chem. Soc., 2018, 140, 10700–10704 CrossRef CAS PubMed.
- J. Hu, G. Wang, S. Li and Z. Shi, Angew. Chem., Int. Ed., 2018, 57, 15227–15231 CrossRef CAS PubMed.
- X. Jiang, M.-M. Zhang, W. Xiong, L.-Q. Lu and W.-J. Xiao, Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C–N Bond Activation, Angew. Chem., Int. Ed., 2019, 58, 2402–2406 CrossRef CAS PubMed.
- J. Wu, P. S. Grant, X. Li, A. Noble and V. K. Aggarwal, Catalyst-Free Deaminative Functionalizations of Primary Amines by Photoinduced Single-Electron Transfer, Angew. Chem., Int. Ed., 2019, 58, 5697–5701 CrossRef CAS PubMed.
- J. Wang, M. E. Hoerrner, M. P. Watson and D. J. Weix, Nickel-Catalyzed Synthesis of Dialkyl Ketones from the Coupling of N-Alkyl Pyridinium Salts with Activated Carboxylic Acids, Angew. Chem., Int. Ed., 2020, 59, 13484–13489 CrossRef CAS PubMed.
- X. Zeng, W. Yan, S. B. Zacate, A. Cai, Y. Wang, D. Yang, K. Yang and W. Liu, Copper-Catalyzed Deaminative Difluoromethylation, Angew. Chem., Int. Ed., 2020, 59, 16398–16403 CrossRef CAS PubMed.
- C. Wang, R. Qi, H. Xue, Y. Shen, M. Chang, Y. Chen, R. Wang and Z. Xu, Visible-Light-Promoted C(sp3)–H Alkylation by Intermolecular Charge Transfer: Preparation of Unnatural α-Amino Acids and Late-Stage Modification of Peptides, Angew. Chem., Int. Ed., 2020, 59, 7461–7466 CrossRef CAS PubMed.
- Z. Zhang and T. Cernak, The Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp3–sp3 Carbon–Carbon Bonds, Angew. Chem., Int. Ed., 2021, 60, 27293–27298 CrossRef CAS PubMed.
- F. Sandfort, F. Strieth-Kalthoff, F. J. R. Klauck, M. J. James and F. Glorius, Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron Donor–Acceptor Complex, Chem.–Eur. J., 2018, 24, 17210–17214 CrossRef CAS PubMed.
- H. Yue, C. Zhu, L. Shen, Q. Geng, K. J. Hock, T. Yuan, L. Cavallo and M. Rueping, Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling, Chem. Sci., 2019, 10, 4430–4435 RSC.
- C. H. Basch, J. Liao, J. Xu, J. J. Piane and M. P. Watson, Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C–N Bond Activation, J. Am. Chem. Soc., 2017, 139, 5313–5316 CrossRef CAS PubMed.
- S. Plunkett, C. H. Basch, S. O. Santana and M. P. Watson, J. Am. Chem. Soc., 2019, 141, 2257–2262 CrossRef CAS PubMed.
- S.-Z. Sun, C. Romano and R. Martin, Site-Selective Catalytic Deaminative Alkylation of Unactivated Olefins, J. Am. Chem. Soc., 2019, 141, 16197–16201 CrossRef CAS PubMed.
- S.-Z. Sun, Y.-M. Cai, D.-L. Zhang, J.-B. Wang, H.-Q. Yao, X.-Y. Rui, R. Martin and M. Shang, Enantioselective Deaminative Alkylation of Amino Acid Derivatives with Unactivated Olefins, J. Am. Chem. Soc., 2022, 144, 1130–1137 CrossRef CAS PubMed.
- J. Liao, C. H. Basch, M. E. Hoerrner, M. R. Talley, B. P. Boscoe, J. W. Tucker, M. R. Garnsey and M. P. Watson, Deaminative Reductive Cross-Electrophile Couplings of Alkylpyridinium Salts and Aryl Bromides, Org. Lett., 2019, 21, 2941–2946 CrossRef CAS PubMed.
- R. Martin-Montero, V. R. Yatham, H. Yin, J. Davies and R. Martin, Ni-catalyzed Reductive Deaminative Arylation at sp3 Carbon Centers, Org. Lett., 2019, 21, 2947–2951 CrossRef CAS PubMed.
- J. Yi, S. O. Badir, L. M. Kammer, M. Ribagorda and G. A. Molander, Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis, Org. Lett., 2019, 21, 3346–3351 CrossRef CAS PubMed.
- K. M. Baker, D. Lucas Baca, S. Plunkett, M. E. Daneker and M. P. Watson, Engaging Alkenes and Alkynes in Deaminative Alkyl–Alkyl and Alkyl–Vinyl Cross-Couplings of Alkylpyridinium Salts, Org. Lett., 2019, 21, 9738–9741 CrossRef CAS PubMed.
- I. Quirós, M. Martín, M. Gomez-Mendoza, M. J. Cabrera-Afonso, M. Liras, I. Fernández, L. Nóvoa and M. Tortosa, Isonitriles as Alkyl Radical Precursors in Visible Light Mediated Hydro- and Deuterodeamination Reactions, Angew. Chem., Int. Ed., 2024, 63, e202317683 CrossRef PubMed.
- B. D. Dherange, M. Yuan, C. B. Kelly, C. A. Reiher, C. Grosanu, K. J. Berger, O. Gutierrez and M. D. Levin, Direct Deaminative Functionalization, J. Am. Chem. Soc., 2023, 145, 17–24 CrossRef CAS PubMed.
- J. R. Dorsheimer and T. Rovis, Late-Stage Isotopic Exchange of Primary Amines, J. Am. Chem. Soc., 2023, 145, 24367–24374 CrossRef CAS PubMed.
- Y. Liu, X. Tao, Y. Mao, X. Yuan, J. Qiu, L. Kong, S. Ni, K. Guo, Y. Wang and Y. Pan, Electrochemical C–N bond activation for deaminative reductive coupling of Katritzky salts, Nat. Commun., 2021, 12, 6745–6752 CrossRef CAS PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- C. Ma, P. Fang, D. Liu, K.-J. Jiao, P.-S. Gao, H. Qiu and T.-S. Mei, Transition metal-catalyzed organic reactions in undivided electrochemical cells, Chem. Rev., 2021, 12, 12866–12873 CAS.
- K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T.-S. Mei, Site-Selective C–H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis, Acc. Chem. Res., 2020, 53, 300–310 CrossRef CAS PubMed.
- W. Zhang, W. Guan, Y. Wang, S. Lin and K. A. See, Enabling Al sacrificial anodes in tetrahydrofuran electrolytes for reductive electrosynthesis, Chem. Sci., 2023, 14, 13108–13118 RSC.
- C.-Y. Cai, X.-M. Shu and H.-C. Xu, Practical and stereoselective electrocatalytic 1,2-diamination of alkenes, Nat. Commun., 2019, 10, 4953–4960 CrossRef CAS PubMed.
- H. Ding, K. Xu and C.-C. Zeng, Nickel-catalyzed electrochemical Minisci acylation of aromatic N-heterocycles with α-keto acids via ligand-to-metal electron transfer pathway, J. Catal., 2020, 381, 38–43 CrossRef CAS.
- P. Li, Z. Zhu, C. Guo, G. Kou, S. Wang, P. Xie, D. Ma, T. Feng, Y. Wang and Y. Qiu, Nickel-electrocatalysed C(sp3)–C(sp3) cross-coupling of unactivated alkyl halides, Nat. Catal., 2024, 7, 412–421 CrossRef CAS.
- A. Bhattacharya, R. Plata, V. Villarreal, S. Muramulla and J. Wu, An efficient conversion of nitriles to amides: application in the synthesis of N,N-diethyl-m-toluamide (DEET (TM)), Tetrahedron Lett., 2006, 47, 505–506 CrossRef CAS.
- X. Li, Z. Li, H. Deng and X. Zhou, An efficient protocol for the preparation of amides by copper-catalyzed reactions between nitriles and amines in water, Tetrahedron Lett., 2013, 54, 2212–2216 CrossRef CAS.
- S. Davulcu, C. L. Allen, K. Milne and J. M. J. Williams, Catalytic Conversion of Nitriles into Secondary- and Tertiary Amides, ChemCatChem, 2013, 5, 435–438 CrossRef CAS.
- M. P. Doyle, R. J. Bosch and P. G. Seites, Alkyl nitrite-metal halide deamination reactions. 5. In situ generation of nitrosyl halides. Effective product control from nitrosyl chloride diazotization of primary aliphatic amines in N,N-dimethylformamide, J. Org. Chem., 1978, 43, 4120–4125 CrossRef CAS.
- C. J. Cobley, M. van den Heuvel, A. Abbadi and J. G. de Vries, Platinum catalysed hydrolytic amidation of unactivated nitriles, Tetrahedron Lett., 2000, 41, 2467–2470 CrossRef CAS.
- T. Wakabayashi and K. Watanabe, Asymmetric synthesis of 3-substituted dihydroisocarbostyril derivatives, Tetrahedron Lett., 1977, 18, 4595–4598 CrossRef.
- W. Zhang, L. Lu, W. Zhang, Y. Wang, S. D. Ware, J. Mondragon, J. Rein, N. Strotman, D. Lehnherr, K. A. See and S. Lin, Electrochemically driven cross-electrophile coupling of alkyl halides, Nature, 2022, 604, 292–297 CrossRef CAS PubMed.
- T. Fukuyama, M. T. Rahman, H. Mashima, H. Takahashi and I. Ryu, Ionic liquids are not innocent in Pd catalysis. C–H arylation of thiazolium and imidazolium ionic liquids with aryl halides, Org. Chem. Front., 2017, 4, 1863–1866 RSC.
- A. S. Sunagatullina, F. H. Lutter and P. Knochel, Preparation of Primary and Secondary Dialkylmagnesiums by a Radical I/Mg-Exchange Reaction Using sBu2Mg in Toluene, Angew. Chem., Int. Ed., 2022, 61, e202116625 CrossRef CAS PubMed.
- B. Wang, P. Peng, W. Ma, Z. Liu, C. Huang, Y. Cao, P. Hu, X. Qi and Q. Lu, Electrochemical Borylation of Alkyl Halides: Fast, Scalable Access to Alkyl Boronic Esters, J. Am. Chem. Soc., 2021, 143, 12985–12991 CrossRef CAS PubMed.
- L. M. Barton, L. Chen, D. G. Blackmond and P. S. Baran, Electrochemical borylation of carboxylic acids, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2109408118 CrossRef CAS PubMed.
- B. Wang, X. Zhang, Y. Cao, L. Zou, X. Qi and Q. Lu, Angew. Chem., Int. Ed., 2023, 62, e202218179 CrossRef CAS PubMed.
- C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Alkylboronic Esters from Copper-Catalyzed Borylation of Primary and Secondary Alkyl Halides and Pseudohalides, Angew. Chem., Int. Ed., 2012, 51, 528–532 CrossRef CAS PubMed.
- J. Yi, J.-H. Liu, J. Liang, J.-J. Dai, C.-T. Yang, Y. Fu and L. Liu, Alkylboronic Esters from Palladium- and Nickel-Catalyzed Borylation of Primary and Secondary Alkyl Bromides, Adv. Synth. Catal., 2012, 354, 1685–1691 CrossRef CAS.
- A. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers and V. K. Aggarwal, Photoinduced decarboxylative borylation of carboxylic acids, Science, 2017, 357, 283–286 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06773h |
| This journal is © The Royal Society of Chemistry 2025 |