Open Access Article
Open Access ArticleEnhancement of photoinduced reactive oxygen species generation in open-cage fullerenes†
Cristina
Castanyer‡
a,
Çetin
Çelik‡
 b,
Albert
Artigas
b,
Albert
Artigas
 a,
Anna
Roglans
a,
Anna
Roglans
 a,
Anna
Pla-Quintana
a,
Anna
Pla-Quintana
 *a,
Anton J.
Stasyuk
*a,
Anton J.
Stasyuk
 *acd,
Yoko
Yamakoshi
*acd,
Yoko
Yamakoshi
 *b and
Miquel
Solà
*b and
Miquel
Solà
 *a
*a
aInstitut de Quimica Computacional i Catàlisi (IQCC) and Departament de Química, Universitat de Girona, M. Aurèlia Capmany, 69, 17003 Girona, Catalonia, Spain. E-mail: anna.plaq@udg.edu; antony.stasuk@gmail.com; miquel.sola@udg.edu
bDepartment of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 3, CH-8093 Zürich, Switzerland. E-mail: yamakoshi@org.chem.ethz.ch
cFaculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
dDepartament de Farmàcia i Tecnologia Farmacèutica, i Fisicoquímica, Facultat de Farmàcia i Ciències de l’Alimentació & Institut de Química Teòrica i Computacional (IQTCUB), Universitat de Barcelona (UB), Av. Joan XXIII 27-31, Barcelona, Catalonia, Spain
First published on 30th December 2024
Abstract
Photodynamic therapy is an important tool in modern medicine due to its effectiveness, safety, and the ability to provide targeted treatment for a range of diseases. Photodynamic therapy utilizes photosensitizers to generate reactive oxygen species (ROS). Fullerenes can be used as photosensitizers to produce ROS in high quantum yields. Open-cage fullerenes are a subclass of fullerenes characterized by a partially open structure, with one or more openings or apertures. The promising electrochemical properties of open-cage fullerenes motivated us to investigate their use for DNA-cleavage and ROS generation under visible light irradiation through type I electron transfer and type II energy transfer reactions. Our results show that open-cage C60 fullerenes are more efficient for photoinduced cleavage of DNA and ROS generation via both the type I electron transfer and type II energy transfer pathways than pristine C60 or a C60 pyrrolidine derivative without open-cage. The greater efficiency of ROS generation by open-cage C60 fullerene in type I and type II reactions can be attributed to the increased rate of the initial intersystem crossing process, resulting from larger total reorganization energies, as indicated by computationally calculated relative rates using the Marcus equation, and the lower reduction potential of the open-cage derivative 3, as determined by CV, which facilitates a more efficient generation of the corresponding radical anion (C60˙−).
Introduction
Photodynamic therapy (PDT),1–3 discovered over a century ago, has evolved into a well-established treatment for cancer and infections.4–6 PDT involves the use of photosensitizers (PSs),7,8 which are non-toxic dyes activated by light absorption. This activation first forms an excited singlet state of the PS, which then transitions to a long-lived excited triplet state (Scheme 1). In the presence of oxygen, the triplet state undergoes photochemical reactions, producing reactive oxygen species (ROS) that can destroy cancer cells, pathogenic microbes, and unwanted tissue.Fullerene C60 is particularly well-suited PS for PDT due to its ability to generate ROS in high quantum yields.9–11 However, its hydrophobic nature and poor solubility in biological media have hindered the biomedical applications of fullerenes in its pristine form. Therefore, significant efforts have been made over the years to synthesize PSs based on water-soluble fullerene derivatives.12 As one of the highly suited derivatives, polyhydroxy fullerenes (fullerols)13,14 were reported with enhanced aqueous solubility, however, their precise structure often remains unknown. To synthesize water-soluble C60 derivatives with well-defined structures, two synthetic methods were mainly employed; Bingel–Hirsch cycloaddition reaction15,16 and 1,3-dipolar cycloaddition (Prato reaction).17 Using the former reaction, fullerene carboxylic acid derivatives18,19 and the latter, fullerene quaternary ammonium derivatives20,21 were reported as water-soluble derivatives resulting in recent studies with further advanced properties22–27 in combination with biomolecules, including sugars24,25 and peptides,28 polymers,29,30 and nanoparticles.31,32 Despite these intensive reports on C60-based PDT-PS, to date, there have been no studies on open-cage fullerene derivatives reported in this context.
Fullerene-based PSs offer considerable advantages over conventional PSs based on the suitable properties of C60 with (1) high photostability and reduced photobleaching and (2) quantitative singlet oxygen (1O2) generation having a quantum yield of close to 1.9 Notably, C60 follows two photophysical mechanisms for ROS generation – type I electron transfer and type II energy transfer (Scheme 1a), whereas conventional PDT dyes mainly rely on a single mechanism with type II. In type I electron transfer,10,33 the fullerene in its triplet excited state  , which has a relatively high redox potential
, which has a relatively high redox potential  , interacts with an electron donor such as NADH to form the radical anion C60˙−, which further produces O2˙− (Scheme 1b), the ROS which can be subsequently converted to the most toxic ROS, ˙OH, by the Fenton reaction. Alternatively, in the type II energy transfer pathway,
, interacts with an electron donor such as NADH to form the radical anion C60˙−, which further produces O2˙− (Scheme 1b), the ROS which can be subsequently converted to the most toxic ROS, ˙OH, by the Fenton reaction. Alternatively, in the type II energy transfer pathway,  reacts with O2 to generate 1O2, another ROS.9,34 Rates of these two processes can be estimated using the Marcus parabolic model (Scheme 1c).35,36
reacts with O2 to generate 1O2, another ROS.9,34 Rates of these two processes can be estimated using the Marcus parabolic model (Scheme 1c).35,36
Previously, we have observed the generation of O2˙− and ˙OH in aqueous solution of C60 complexed with poly(vinylpyrrolidone) (PVP) by means of electron spin resonance (ESR) spin-trapping methods under visible light irradiation.37,38 More recently, we have reported on the photoinduced oxidative DNA damage and related ROS generations by water-soluble C60-PEG and two isomeric C70-PEG derivatives39 with fulleropyrrolidine skeletons.40 It was found that the photoinduced cleavage of DNA by C60-PEG via type I electron transfer is much higher than that by C70-PEG, whereas the reverse is true for type II energy transfer.30 Computationally estimated relative rates of each step of the type I and type II reactions were able to explain the differences in photoinduced ROS generation of C60-PEG and C70-PEG in water.
Open-cage fullerenes are known as interesting molecules due to their unique shapes and properties. They are obtained by selective cleavage of one or more C–C bonds of the fullerene cage. Once opened, these fullerenes can act as host molecules encapsulating guests within their cavity. In the molecular surgery procedure,41 the fullerene is first opened, then a guest molecule is introduced in the cavity, and finally the structure is closed to generate an endohedral fullerene. Some open-cage derivatives of C60 were found useful as electron accepting materials in organic solar cells.42
In 2018, we prepared bis-fulleroid derivatives by a Rh-catalyzed [2+2+2] cycloaddition of diynes and C60 to yield a cyclohexadiene-fused intermediate, which transformed into a bis-fulleroid through a [4+4]/retro-[2+2+2] rearrangement.43,44 The obtained bis-fulleroid can overcome ring expansion through a photooxygenation process to deliver an open-cage derivative with a 12-membered hole in the C60 cage structure. The same procedure was applied to open an orifice in C70.45 The obtained open-cage C60 derivatives above were incorporated in perovskite solar cells as an electron transport layer (ETL), resulting in solar cells with higher performances than those of previously reported solar cells containing standard PC61BM material as ETL.42 The improved performance of this open cage C60 derivative was attributed, at least in part, to the increased energy levels of the HOMO and LUMO orbitals due to the open cage structure.
The promising electrochemical properties and remarkable stability of these open-cage C60 above prompted us to study these compounds for their photoinduced ROS generation via type I electron transfer and type II energy transfer. To this end, we considered to test on C60 and three C60 derivatives 1–3 shown in Fig. 1. To have more bio-relevant conditions, we prepared water-soluble complexes of C60 and compounds 1–3 with PVP (C60/PVP, P1, P2, and P3) and tested by photoinduced oxidative DNA damages. The generation of responsible ROS (1O2 and O2˙−) by C60/PVP and complexes P1–3 were measured by ESR in the presence of spin-trapping agents. Finally, computational calculations were performed to investigate the relative reaction rate of each step to understand the mechanisms in ROS generation from C60 and 1–3 derivatives.
Results and discussion
Preparation of water-soluble complexes of C60 and C60 derivatives 1–3 with PVP
A fulleropyrrolidine derivative 1 (ref. 46) and two open cage C60 derivatives 2 and 3 (ref. 42 and 44) were synthesized based on the reported procedures. The UV-vis absorption spectra for the three compounds were registered showing the intense absorption in the UV region and lower intensity broad bands in the visible region, with the expected characteristic bands (430 nm for 1; 259 and 325 nm for 2; and 254 and 324 nm for 3, see Fig. S1–S3†). Cyclic voltammetry was also recorded for the three compounds showing first reduction potentials of −1.18 V for 1, −1.26 V for 2 and −1.06 V for 3 (see Fig. S4–S9†). These data altogether also allowed us to experimentally determine the HOMO and LUMO energies for the three compounds (see Table S1†).Since these compounds were not soluble in water, aqueous solutions of C60 and compounds 1–3 were prepared by forming complexes with a neutral polymeric detergent, PVP, as described in the literature.47 Each PVP complex, C60/PVP, P1, P2, or P3, was water-soluble and revealed sufficient absorption intensity in visible wavelength region (Fig. S10†), showing good potentials as PDT-PSs. The characteristic bands of the absorption spectra were not altered by the presence of the PVP especially in the visible region, suggesting that the photophysical processes remain unaffected by the presence of PVP (see Fig. S11–S13†).
Photoinduced ROS (1O2 and O2˙−) generation
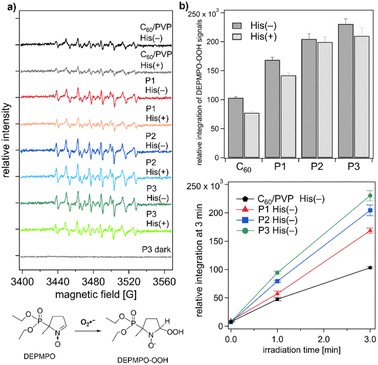
Generation of ROS from the photoexcited C60/PVP and P1–3 complexes were investigated by ESR spin trapping methods. 1O2 generation via type II energy transfer reaction (see Scheme 1) by each compound was measured using a spin-trapping reagent, 4-oxo-TEMP (scheme in Fig. 2a bottom). Peaks corresponding to the 1O2 adduct of 4-oxo-TEMP (4-oxo-TEMPO) were observed in all solutions after irradiation by green LED light (528 nm max) for 10 min (Fig. 2a). Fig. 2b (upper) shows double integration analyses of the ESR signal to estimate the relative amount of 1O2 generation. The results revealed that the generation of 1O2 by bis-fulleroid P2 and open-cage P3 were significantly higher than that of unsubstituted C60, which was in parallel with the absorption intensity at 528 nm varies in the order P1 ≈ P2 > P3 > C60/PVP (Fig. S10†). Despite P1 having relatively higher absorbance at this wavelength, it revealed only slightly higher 1O2 generation than C60. Generation of 1O2 was observed in an irradiation-time-dependent manner in all complexes (Fig. 2b bottom, Fig. S14–S17†), clearly indicating that 1O2 generations were due to the photoexcitation of C60/PVP, P1, P2, and P3. The fact that P2 and P3 show almost identical behaviour can be explained by the facile transformation of 2 into 3 in the presence of light and oxygen.The generation of the other ROS, O2˙−, which can be produced via type I electron transfer pathway (Scheme 1) by photoexcited C60/PVP and complexes P1–3, was investigated in the presence of electron donor NADH using DEPMPO as a spin trapping reagent (scheme in Fig. 3a bottom). Upon irradiation with green LED, all complexes showed O2˙− generation in aqueous media observed as signals of DEPMPO-OOH (Fig. 3a). When the ESR spectra were quantified by double integration, the relative amount of O2˙− produced by each complex was clearly estimated (Fig. 3b). The amount of O2˙− generation was in a similar trend to that of 1O2 generation; P2 and P3 had much higher generation than C60/PVP system and P1 (Fig. 3b). The irradiation-time-dependency was also observed as shown in Fig. 3c and S18–S21.† It is known that O2˙− can be produced not only via the type I pathway but also via1O2, which was once generated through the type II energy transfer pathway, in the presence of electron donors. To detect the O2˙− generated exclusively via the type I electron transfer pathway, ESR experiments in the presence of L-histidine (His), a 1O2 quencher, were performed. As shown in Fig. 3b, the reduced amount of O2˙− generation was observed in all complexes in the presence of His, while P2 showed only a limited amount of decrease (Fig. 3b). This was presumably due to the rapid reaction of P2 with 1O2 to form P3, effectively quenching this species. However, the possibility of a faster rate for the type I pathway reaction relative to the type II pathway cannot be ruled out.
Photoinduced DNA cleavage
In order to evaluate their potential as PDT-PSs, a photo DNA cleavage assay was performed using C60/PVP and P1–3. Supercoiled pBR322 DNA was used as a DNA substrate to observe the DNA cleavage ability of the compounds by quantifying the amount of damaged DNA (form II nicked DNA) in relative to the intact ones (form I). Under visible light irradiation (green LED 527 nm), cleavages of the DNA by all four complexes were observed (Fig. 4 and S23–S26†). P2 and P3 showed stronger DNA cleavage upon photo irradiation than that of C60/PVP and P1. These results were in line with the ROS generation measured by ESR spin trapping methods, where P2 and P3 showed higher generation of both 1O2 and O2˙− than C60/PVP and P1. We also observed that DNA cleavage in absence of NADH was significantly lower in all compounds, suggesting that type I pathway plays a crucial role in photoinduced DNA damage, assuming that 1O2 and O2˙− have an analogous damage power. Noteworthy, generation of form II nicked DNA in the absence of NADH is significantly higher for P3 than the other compounds including C60/PVP, which indicates increased involvement of ROS generation by type II pathway from this complex in relation to others. | ||
| Fig. 4 Photoinduced DNA cleavage by C60/PVP and P1–3 in the absence or presence of NADH as an electron donor. Data is obtained from gels shown in Fig. S11 and S12 in the ESI.† Reaction conditions: pBR322: 25 ng μL−1; C60/PVP or P1–3: 0.125 mM; NADH: 10 mM; in Tris–HCl buffer (pH 8.0, 1 mM EDTA); green LED (527 nm, 45 lm W−1), at room temperature, for 25 min. Electrophoresis: 100 V, 80 min, 1% agarose in 0.5× TBE buffer (pH 8.3). | ||
Quantum chemical modelling
To further investigate the mechanism of ROS generation from considered open cage fullerenes through the type I and type II reactions, computational calculations were performed. Taking into account the moderate size of the studied systems, C60 fullerene as well as derivatives 1–3 were considered without any simplification. The NADH structure was replaced with a model compound represented by one nucleoside containing only nicotinamide as the base (see Fig. S15†). The applicability of such a replacement for the sake of simulation of photoinduced reactive oxygen generation has been previously demonstrated by us.30 The rate of excited state processes, including intersystem crossing (ISC), type II (energy transfer), and type I (electron transfer), both part 1 and part 2 (schemes in Tables 1 and 2), were estimated using the classical Marcus equation:48 | (1) |
 | (2) |
Taking into account that the sizes of 1–3 conjugates are relatively small, the effect of geometrical relaxation in the corresponding excited state can be significant and must be properly accounted for. Indeed, comparison of excited state energies for 1–3 fullerenes in vertical ground state geometries and equilibrium first excited state geometries revealed that the difference was 0.48–0.63 eV and 0.45–0.59 eV for singlet and triplet states, respectively (Table S1, ESI†). Thus, for all studied processes, geometry relaxation in the corresponding excited state was accounted for accordingly.
In accordance with Scheme 1, the photoinduced reactive oxygen species generation is a sequential process consisting of photoexcitation and ISC stages followed by ET or EnT processes. As seen in Fig. S1,† the absorbance of 1–3 derivatives is 1.37 to 1.84 times higher than that of the parental C60 fullerene. Thus, the initial stage of reactive oxygen generation for the systems of interest is expected to be more efficient compared to C60.
Analyses of (ΔG0 + λt) as well as relative rates for ISC and type II energy transfer are summarized in Table 1. The Gibbs energy for triplet-to-singlet oxygen conversion (3O2(3Σg) → 1O2(1Δg)) process was taken from experiment.49
For all studied systems, the ISC reaction occurs in the inverted Marcus region (|ΔG0| > λt). ISC rates for 2 and 3 are predicted to be the fastest among the systems. At the same time, (ΔG + λt) values for C60 and the 1 conjugate are significantly higher, suggesting their slower ISC rates. Table S2 in ESI† contains the individual ΔG0 and λt values for the ISC and type II reaction, for the systems of interest. The type II energy transfer for 1–3 conjugates is characterized by very small (ΔG + λt) values, suggesting a nearly barrierless process with a fast reaction rate. In contrast, the type II reaction in the reference C60 occurs in inverted region and is expected to be slower as compared to 1–3. Taking into account that ISC and 1O2 generation are two linked and consecutive reactions, where the product of the first reaction serves as the substrate for the second one, it can be argued that the rate of the entire process is determined by the rate of the slowest reaction. The experimentally observed more efficient 1O2 generation by P2 and P3 complexes, followed by P1 and C60 (P2 > P3 > P1 > C60/PVP), together with the fact that the absorption intensity at 528 nm varies in the order P1 ≈ P2 > P3 > C60 (and thus does not correctly describe the observed order) allows us to assume that the ISC stage is the rate-determining step. Interestingly, 2 and 3 have relatively large total reorganization energies as compared to 1 and C60 for the ISC step, which translates into relatively low (ΔG0 + λt) values.
The photoinduced ROS generation that proceeds through electron transfer reaction (type I reaction) can be divided into two steps. The first step (part 1) is the reduction of fullerene by the electron transfer from NADH. The second step (part 2) is the reduction of molecular oxygen and generation of the superoxide radical ion O2˙− (see Table 2 footnote). Analyses of (ΔG0 + λt) and relative rates for the initial ISC process and the two subsequent steps of the type I reaction are summarized in Table 2. Individual ΔG0 and λt values for the ISC and both stages of the type I reaction are provided in Table S2, ESI.†
In contrast to the type II reaction, both part 1 and part 2 stages of the type I reaction take place in the normal Marcus region (|ΔG0| < λt). Similar to the generation of 1O2, the type I reaction and the preceding ISC are linked reactions, and the rate-determining stage will be the slowest one. Following the experimental results of O2˙− generation in the presence of the electron donor NADH, the bis-fulleroid P2 and open-cage fullerene P3 demonstrated a superior capability for superoxide generation compared to the closed-cage P1 and the parental C60. Additionally, the P1 conjugate outperformed C60. The observed efficiency of the studied systems towards O2˙− radical anion generation suggested that in the case of the type I reaction, again ISC was the slowest, and thus the rate-determining stage. Experimental support for the relative rates determined for type I part 1 can be found in the determined reduction potentials that vary in the order 3 < 1 < 2.
Conclusions
Four types of water-soluble complexes of C60 and C60 derivatives (1–3) with PVP (C60/PVP and P1–3) were prepared and tested for oxidative damage of DNA and responsible ROS generation under visible light irradiation. While compound 1 is a standard fulleropyrrolidine derivative, compounds 2 and 3 are bis-fulleroid and open-cage fullerene, respectively. Our results show that species 2 and 3 are more efficient than 1 and C60 for both the generation of O2˙−via type I electron transfer pathway and the generation of 1O2 and via type II energy transfer reaction in line with the results of photo DNA cleavage by these compounds. The highly efficient generation of ROS by 2 and 3 in type I and type II reactions was explained by (1) the higher rate for the initial ISC process due to larger total reorganization energies of 2 and 3, as shown by computational results based on Marcus equation, and (2) the reduced reduction potential of open-cage derivative 3, as determined by CV. These altogether allow a more efficient generation of the corresponding anion radical (C60˙−). Since 2 can be easily converted into 3 upon light and oxygen exposure, 3 is the best candidate for PDT identified in this study, highlighting the potential of open-cage derivatives for the development of more efficient photosensitizers in photodynamic therapy.Experimental
General
Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. All 1H and 13C NMR spectra were recorded on a Bruker ASCEND 400 spectrometer equipped with a 5 mm BBFO probe or and Bruker 600 spectrometer equipped with a CryoProbe using CDCl3 as a deuterated solvent. Mass spectrometry analyses were recorded on a Bruker micrOTOF-Q II mass spectrometer (high resolution), equipped with electrospray ion source and a Bruker Daltonics solariX spectrometer.Synthetic methods
For 1O2 measurement, a mixture (50 μL) containing PVP complex of 1–3 (P1–3) or C60 (40 μM) and 4-oxo-TEMP (80 mM) in phosphate buffer (60 mM, pH 7.0) was prepared and O2 was bubbled for 30 s right before placing into the capillary. Each sample solution was then irradiated and the X-band ESR spectrum was recorded immediately.
For O2˙− measurement, a mixture (50 μL) containing PVP complex P1–3 or C60/PVP (40 μM), DETAPAC (1 mM), NADH (10 mM), L-histidine (10 mM), and DEPMPO (113 mM) in phosphate buffer (60 mM, pH 7.0) containing 20% (v/v) DMSO was prepared and O2 was bubbled for 30 s right before placing into the capillary. Each sample solution was then irradiated and the X-band ESR spectrum was recorded immediately.
Computational details
Geometry optimizations was performed employing the range-separated functional from Handy and coworkers' CAM-B3LYP50 and Ahlrichs' Def2-SVP basis set.51,52 The empirical dispersion D3 correction with Becke–Johnson damping was employed.53–55 Vertical excitation energies were calculated with the same functional and basis set using Tamm–Dancoff approximation (TDA) formalism.56 This method was proven to give accurate results for charge transfer process involving fullerenes.57 Solvent effects were estimated using COSMO-like polarizable continuum model58,59 in monopole approximation considering water as solvent. The chosen model demonstrated excellent performance towards the solvation energy prediction for both, neutral and charged fullerene-based systems.60–62 Calculations were performed with the Gaussian 16 (rev. C01)63 and Orca 5.0.3 programs.64 Full computational details including how P1–3 and NADH were modelled can be found in the ESI.†Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Spanish Ministerio de Ciencia, Innovación y Universidades (MCIN/AEI/10.13039/50110001103) (Projects PID2023-147424NB-I00, PID2023-146849NB-I00, RED2022-134939-T, and FPI predoctoral grant to CC) and the Generalitat de Catalunya (Project 2021-SGR-623 and ICREA Academia 2024 prize to MS). AJS is grateful to Poland's high-performance computing infrastructure PLGrid (HPC Centers: ACK Cyfronet AGH) for providing computer facilities and support within computational grant no. PLG/2023/016841. YY is grateful to the Swiss National Science Foundation (projects 173018 and 183660).Notes and references
- M. Overchuk, R. A. Weersink, B. C. Wilson and G. Zheng, Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine, ACS Nano, 2023, 17, 7979–8003 CrossRef CAS PubMed.
- J. Rani and S. Roy, Recent Development of Copper(II) Complexes of Polypyridyl Ligands in Chemotherapy and Photodynamic Therapy, ChemMedChem, 2023, 18, e202200652 CrossRef PubMed.
- M. Piksa, C. Lian, I. C. Samuel, K. J. Pawlik, I. D. W. Samuel and K. Matczyszyn, The role of the light source in antimicrobial photodynamic therapy, Chem. Soc. Rev., 2023, 52, 1697–1722 RSC.
- R. V. Huis In 't Veld, J. Heuts, S. Ma, L. J. Cruz, F. A. Ossendorp and M. J. Jager, Current Challenges and Opportunities of Photodynamic Therapy against Cancer, Pharmaceutics, 2023, 15, 330 CrossRef PubMed.
- M. Kolarikova, B. Hosikova, H. Dilenko, K. Barton-Tomankova, L. Valkova, R. Bajgar, L. Malina and H. Kolarova, Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments, Med. Res. Rev., 2023, 43, 717–774 CrossRef CAS PubMed.
- W. Jiang, M. Liang, Q. Lei, G. Li and S. Wu, The Current Status of Photodynamic Therapy in Cancer Treatment, Cancers, 2023, 15, 585 CrossRef CAS PubMed.
- H. Abrahamse and M. R. Hamblin, New photosensitizers for photodynamic therapy, Biochem. J., 2016, 473, 347–364 CrossRef CAS PubMed.
- R. R. Allison, G. H. Downie, R. Cuenca, X.-H. Hu, C. J. H. Childs and C. H. Sibata, Photosensitizers in clinical PDT, Photodiagn. Photodyn. Ther., 2004, 1, 27–42 CrossRef CAS PubMed.
- J. W. Arbogast, A. P. Darmanyan, C. S. Foote, F. N. Diederich, R. L. Whetten, Y. Rubin, M. M. Alvarez and S. J. Anz, Photophysical properties of sixty atom carbon molecule (C60), J. Phys. Chem., 1991, 95, 11–12 CrossRef CAS.
- J. W. Arbogast, C. S. Foote and M. Kao, Electron transfer to triplet fullerene C60, J. Am. Chem. Soc., 1992, 114, 2277–2279 CrossRef CAS.
- T. Nagano, K. Arakane, A. Ryu, T. Masunaga, K. Shinmoto, S. Mashiko and M. Hirobe, Comparison of Singlet Oxygen Production Efficiency of C60 with Other Photosensitizers, Based on 1268 nm Emission, Chem. Pharm. Bull., 1994, 42, 2291–2294 CrossRef CAS.
- E. Nakamura and H. Isobe, Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience, Acc. Chem. Res., 2003, 36, 807–815 CrossRef CAS PubMed.
- J. Zhu, Z. Ji, J. Wang, R. Sun, X. Zhang, Y. Gao, H. Sun, Y. Liu, Z. Wang, A. Li, J. Ma, T. Wang, G. Jia and Y. Gu, Tumor-Inhibitory Effect and Immunomodulatory Activity of Fullerol C60(OH), Small, 2008, 4, 1168–1175 CrossRef CAS PubMed.
- P. Chaudhuri, A. Paraskar, S. Soni, R. A. Mashelkar and S. Sengupta, Fullerenol–Cytotoxic Conjugates for Cancer Chemotherapy, ACS Nano, 2009, 3, 2505–2514 CrossRef CAS PubMed.
- C. Bingel, Cyclopropanierung von Fullerenen, Chem. Ber., 1993, 126, 1957–1959 CrossRef CAS.
- I. Lamparth and A. Hirsch, Water-soluble malonic acid derivatives of C60 with a defined three-dimensional structure, J. Chem. Soc., Chem. Commun., 1994, 1727–1728 RSC.
- M. Maggini, G. Scorrano and M. Prato, Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines, J. Am. Chem. Soc., 1993, 115, 9798–9799 CrossRef CAS.
- X. Camps and A. Hirsch, Efficient cyclopropanation of C60 starting from malonates, J. Chem. Soc., Perkin Trans. 1, 1997, 1595–1596 RSC.
- M. Brettreich, S. Burghardt, C. Böttcher, T. Bayerl, S. Bayerl and A. Hirsch, Globular Amphiphiles: Membrane-Forming Hexaadducts of C60, Angew. Chem., Int. Ed., 2000, 39, 1845–1848 CrossRef CAS PubMed.
- S. Bosi, T. Da Ros, G. Spalluto, J. Balzarini and M. Prato, Synthesis and Anti-HIV properties of new water-soluble bis-functionalized[60]fullerene derivatives, Bioorg. Med. Chem. Lett., 2003, 13, 4437–4440 CrossRef CAS PubMed.
- A. M. Cassell, C. L. Asplund and J. M. Tour, Self-Assembling Supramolecular Nanostructures from a C60 Derivative: Nanorods and Vesicles, Angew. Chem., Int. Ed., 1999, 38, 2403–2405 CrossRef CAS PubMed.
- T. Komatsu, A. Nakagawa and X. Qu, Structural and Mutagenic Approach to Create Human Serum Albumin-Based Oxygen Carrier and Photosensitizer, Drug Metab. Pharmacokinet., 2009, 24, 287–299 CrossRef CAS PubMed.
- M. Serda, G. Szewczyk, O. Krzysztyńska-Kuleta, J. Korzuch, M. Dulski, R. Musioł and T. Sarna, Developing [60]Fullerene Nanomaterials for Better Photodynamic Treatment of Non-Melanoma Skin Cancers, ACS Biomater. Sci. Eng., 2020, 6, 5930–5940 CrossRef CAS PubMed.
- O. B. Danilov, I. M. Belousova, A. A. Mak, V. P. Belousov, A. S. Grenishin, V. M. Kiselev, A. V. Kris’ko, A. N. Ponomarev and E. N. Sosnov, Generation of singlet oxygen with the use of optically excited fullerenes and fullerene-like nanoparticles, Opt. Spectrosc., 2003, 95, 833–842 CrossRef CAS.
- G. P. Tegos, T. N. Demidova, D. Arcila-Lopez, H. Lee, T. Wharton, H. Gali and M. R. Hamblin, Cationic fullerenes are effective and selective antimicrobial photosensitizers, Chem. Biol., 2005, 12, 1127–1135 CrossRef CAS PubMed.
- L. Huang, M. Terakawa, T. Zhiyentayev, Y.-Y. Huang, Y. Sawayama, A. Jahnke, G. P. Tegos, T. Wharton and M. R. Hamblin, Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials, Nanomed. Nanotechnol. Biol. Med., 2010, 6, 442–452 CrossRef CAS PubMed.
- M. B. Spesia, M. E. Milanesio and E. N. Durantini, Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives, Eur. J. Med. Chem., 2008, 43, 853–861 CrossRef CAS PubMed.
- Y. Ma, L. Persi and Y. Yamakoshi, Synthesis and characterization of water-soluble C60–peptide conjugates, Beilstein J. Org. Chem., 2024, 20, 777–786 CrossRef CAS PubMed.
- S. Aroua, E. G. V. Tiu, M. Ayer, T. Ishikawa and Y. Yamakoshi, RAFT synthesis of poly(vinylpyrrolidone) amine and preparation of a water-soluble C60-PVP conjugate, Polym. Chem., 2015, 6, 2616–2619 RSC.
- K. Liosi, A. J. Stasyuk, F. Masero, A. A. Voityuk, T. Nauser, V. Mougel, M. Solà and Y. Yamakoshi, Unexpected Disparity in Photoinduced Reactions of C60 and C70 in Water with the Generation of O2˙− or 1O2, JACS Au, 2021, 1, 1601–1611 CrossRef CAS PubMed.
- D. Wang, J. Zhao, R. J. Mulder, J. Ratcliffe, C. Wang, B. Wu, J. Wang and X. Hao, Highly aqueously stable C60-polymer nanoparticles with excellent photodynamic property for potential cancer treatment, Smart Med., 2023, 2, e20230033 CrossRef CAS PubMed.
- S.-B. Kim, C.-H. Kim, S.-Y. Lee and S.-J. Park, Carbon materials and their metal composites for biomedical applications: A short review, Nanoscale, 2024, 16, 16313–16328 RSC.
- P. J. Krusic, E. Wasserman, B. A. Parkinson, B. Malone, E. R. Holler Jr, P. N. Keizer, J. R. Morton and K. F. Preston, Electron spin resonance study of the radical reactivity of C60, J. Am. Chem. Soc., 1991, 113, 6274–6275 CrossRef CAS.
- J. W. Arbogast and C. S. Foote, Photophysical properties of C70, J. Am. Chem. Soc., 1991, 113, 8886–8889 CrossRef CAS.
- R. A. Marcus, Electron transfer reactions in chemistry. Theory and experiment, Rev. Mod. Phys., 1993, 65, 599–610 CrossRef CAS.
- R. A. Marcus, Free Energy of Nonequilibrium Polarization Systems. 4. A Formalism Based on the Nonequilibrium Dielectric Displacement, J. Phys. Chem., 1994, 98, 7170–7174 CrossRef CAS.
- Y. Yamakoshi, S. Sueyoshi, K. Fukuhara, N. Miyata, T. Masumizu and M. Kohno, ˙OH and O2˙− Generation in Aqueous C60 and C70 Solutions by Photoirradiation: An EPR Study, J. Am. Chem. Soc., 1998, 120, 12363–12364 CrossRef CAS.
- Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. Nagano, Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2−˙ versus 1O2, J. Am. Chem. Soc., 2003, 125, 12803–12809 CrossRef CAS PubMed.
- K. Liosi, A. Romero-Rivera, O. Semivrazhskaya, C. D. Caniglia, M. Garcia-Borràs, N. Trapp, S. Osuna and Y. Yamakoshi, Site-Selectivity of Prato Additions to C70: Experimental and Theoretical Studies of a New Thermodynamic Product at the dd-[5,6]-Junction, Org. Lett., 2019, 21, 5162–5166 CrossRef CAS PubMed.
- S. Aroua, E. G. V. Tiu, T. Ishikawa and Y. Yamakoshi, Well-Defined Amphiphilic C60-PEG Conjugates: Water-Soluble and Thermoresponsive Materials, Helv. Chim. Acta, 2016, 99, 805–813 CrossRef CAS.
- M. Murata, Y. Murata and K. Komatsu, Surgery of fullerenes, Chem. Commun., 2008, 6083–6094 RSC.
- E. Castro, A. Artigas, A. Pla-Quintana, A. Roglans, F. Liu, F. Perez, A. Lledó, X.-Y. Zhu and L. Echegoyen, Enhanced Open-Circuit Voltage in Perovskite Solar Cells with Open-Cage [60]Fullerene Derivatives as Electron-Transporting Materials, Materials, 2019, 12, 1314 CrossRef CAS PubMed.
- A. Artigas, A. Lledó, A. Pla-Quintana, A. Roglans and M. Solà, A Computational Study of the Intermolecular [2+2+2] Cycloaddition of Acetylene and C60 Catalyzed by Wilkinson's Catalyst, Chem.–Eur. J., 2017, 23, 15067–15072 CrossRef CAS PubMed.
- A. Artigas, A. Pla-Quintana, A. Lledó, A. Roglans and M. Solà, Expeditious Preparation of Open-Cage Fullerenes by Rhodium(I)-Catalyzed [2+2+2] Cycloaddition of Diynes and C60: An Experimental and Theoretical Study, Chem.–Eur. J., 2018, 24, 10653–10661 CrossRef CAS PubMed.
- C. Castanyer, A. Pla-Quintana, A. Roglans, A. Artigas and M. Solà, Unveiling the regioselectivity of rhodium(I)-catalyzed [2+2+2] cycloaddition reactions for open-cage C70 production, Beilstein J. Org. Chem., 2024, 20, 272–279 CrossRef CAS PubMed.
- S. Aroua, W. B. Schweizer and Y. Yamakoshi, C60 Pyrrolidine Bis-carboxylic Acid Derivative as a Versatile Precursor for Biocompatible Fullerenes, Org. Lett., 2014, 16, 1688–1691 CrossRef CAS PubMed.
- Y. N. Yamakoshi, T. Yagami, K. Fukuhara, S. Sueyoshi and N. Miyata, Solubilization of fullerenes into water with polyvinylpyrrolidone applicable to biological tests, J. Chem. Soc., Chem. Commun., 1994, 517–518 RSC.
- R. A. Marcus and N. Sutin, Electron Transfers in Chemistry and Biology, Biochim. Biophys. Acta, 1985, 811, 265–322 CrossRef CAS.
- C. Schweitzer and R. Schmidt, Physical Mechanisms of Generation and Deactivation of Singlet Oxygen, Chem. Rev., 2003, 103, 1685–1758 CrossRef CAS PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- S. Osuna, M. Swart and M. Solà, Dispersion Corrections Essential for the Study of Chemical Reactivity in Fullerenes, J. Phys. Chem. A, 2011, 115, 3491–3496 CrossRef CAS PubMed.
- S. Hirata and M. Head-Gordon, Time-dependent density functional theory within the Tamm–Dancoff approximation, Chem. Phys. Lett., 1999, 314, 291–299 CrossRef CAS.
- P. Besalú-Sala, A. A. Voityuk, J. M. Luis and M. Solà, Evaluation of charge-transfer rates in fullerene-based donor–acceptor dyads with different density functional approximations, Phys. Chem. Chem. Phys., 2021, 23, 5376–5384 RSC.
- A. A. Voityuk and S. F. Vyboishchikov, A simple COSMO-based method for calculation of hydration energies of neutral molecules, Phys. Chem. Chem. Phys., 2019, 21, 18706–18713 RSC.
- A. A. Voityuk and S. F. Vyboishchikov, Fast and accurate calculation of hydration energies of molecules and ions, Phys. Chem. Chem. Phys., 2020, 22, 14591–14598 RSC.
- A. J. Stasyuk, O. A. Stasyuk, M. Solà and A. A. Voityuk, Hypsochromic solvent shift of the charge separation band in ionic donor–acceptor Li+@C60⊂[10]CPP, Chem. Commun., 2019, 55, 11195–11198 RSC.
- A. J. Stasyuk, O. A. Stasyuk, M. Solà and A. A. Voityuk, Electron Transfer in a Li+-Doped Zn-Porphyrin-[10]CPP⊃Fullerene Junction and Charge-Separated Bands with Opposite Response to Polar Environments, J. Phys. Chem. B, 2020, 124, 9095–9102 CrossRef CAS PubMed.
- O. A. Stasyuk, A. J. Stasyuk, M. Solà and A. A. Voityuk, [10]CPP-Based Inclusion Complexes of Charged Fulleropyrrolidines. Effect of the Charge Location on the Photoinduced Electron Transfer, Chem.–Eur. J., 2021, 27, 8737–8744 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. C.01, Wallingford, CT, 2016 Search PubMed.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev. Comput. Mol. Sci., 2022, 12, e1606 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis of 1–3 and preparation of P1–3 complexes with corresponding spectral data, experimental methods for DNA cleavage tests, and ESR spin-trapping methods. Computational details, energies of lowest-lying S1 and T1 excited states estimated in Franck–Condon and relaxed geometries, and Gibbs energy ΔG0, and total reorganization energy λt, for ISC type II, and type I reactions. See DOI: https://doi.org/10.1039/d4sc05428h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |