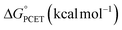Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering mechanisms of proton-coupled electron transfer to a titanium-substituted polyoxovanadate–alkoxide†
Shannon E.
Cooney
a,
S. Genevieve
Duggan
bc,
M. Rebecca A.
Walls
a,
Noah J.
Gibson
d,
James M.
Mayer
 d,
Pere
Miro
d,
Pere
Miro
 *bc and
Ellen M.
Matson
*bc and
Ellen M.
Matson
 *a
*a
aDepartment of Chemistry, University of Rochester, Rochester, NY 14627, USA. E-mail: matson@chem.rochester.edu
bDepartment of Chemistry, University of Iowa, Iowa City, IA 52240, USA
cDepartment of Chemistry, University of South Dakota, Vermillion, SD 57069, USA
dDepartment of Chemistry, Yale University, New Haven, Connecticut 06520, USA
First published on 7th January 2025
Abstract
Metal oxides are promising catalysts for small molecule hydrogen chemistries, mediated by interfacial proton-coupled electron transfer (PCET) processes. Engineering the mechanism of PCET has been shown to control the selectivity of reduced products, providing an additional route for improving reductive catalysis with metal oxides. In this work, we present kinetic resolution of the rate determining proton-transfer step of PCET to a titanium-doped POV, TiV5O6(OCH3)13 with 9,10-dihydrophenazine by monitoring the loss of the cationic radical intermediate using stopped-flow analysis. For this reductant, a 5-fold enhanced rate (kPT = 1.2 × 104 M−1 s−1) is accredited to a halved activation barrier in comparison to the homometallic analogue, [V6O7(OCH3)12]1−. By switching to hydrazobenzene as a reductant, a substrate where the electron transfer component of the PCET is thermodynamically unfavorable (ΔGET = +11 kcal mol−1), the mechanism is found to be altered to a concerted PCET mechanism. Despite the similar mechanisms and driving forces for TiV5O6(OCH3)13 and [V6O7(OCH3)12]1−, the rate of PCET is accellerated by 3-orders of magnitude (kPCET = 0.3 M−1 s−1) by the presence of the Ti(IV) ion. Possible origins of the accelleration are considered, including the possibility of strong electronic coupling interactions between TiV5O6(OCH3)13 with hydrazobenzene. Overall, these results offer insight into the governing factors that control the mechanism of PCET in metal oxide systems.
Introduction
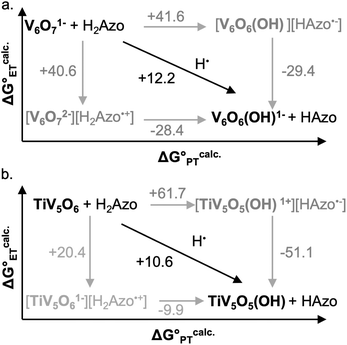
A central reaction invoked in the conversion of small molecules into commodity substrates is the transfer of proton and electron equivalents (i.e. H+/e−, H˙).1 Transfer of proton–electron pairs is described broadly as proton-coupled electron transfer (PCET) which can occur via either stepwise (electron or proton transfer, ET, PT) or concerted mechanisms (concerted proton–electron transfer, CPET; Fig. 1a).2 For small molecule activation, manipulation of the PCET mechanism has been shown to alter product distribution by avoiding unwanted side reactions.3,4 In this capacity, control of the delivery of H-atom equivalents is key for improving product selectivity.5 | ||
| Fig. 1 (a) Square scheme of PCET of a generic metal oxide and (b) cluster complexes studied in this work. | ||
Heterogeneous catalysts, such as transition metal oxides, are promising materials for small molecule activation reactions involving PCET.6,7 The reactivity of metal oxides is dictated by the thermochemistry of the bound H-atom (H˙), presenting as aquo- or hydroxo-ligands, and are quantified by their bond dissociation free energies (BDFEs). In recent years, strides have been made toward understanding the structure–function relationship of the BDFEs, and has resulted in a library of metal oxides capable of (de)hydrogenation of small molecules containing E–H bonds (e.g. E = C, N, O).1,8–10 Knowledge of the thermodynamic properties of these materials allows for the opportunity to tune product selectivity and distribution, though less is known about the pathway of metal oxide mediated PCET.11,12 This is a result of the challenges that arise when investigating the mechanism of PCET on metal oxides due to inefficient methods available to researchers that allow monitoring of potential intermediates, and has resulted in some speculation in the process of the H-atom transfer on extended surfaces.1,8,13
To circumvent the challenges of studying bulk materials, our research team has turned to polyoxovanadate (POV) clusters as model systems for PCET on metal oxide surfaces (Fig. 1b).14 In these multi-metallic assemblies, the arrangement of bridging and terminal oxo sites oxides broadly mimics the surface of metal oxides, rendering them valuable for comparison to colloidal metal oxide nanoparticles.15–17 In addition, these POVs possess Robin and Day Class II delocalized electronic structures, mirroring the electronic structure of nanoscopic and bulk reducible metal oxides with the added benefit of a distinct chemical structure and solubility in organic solvent.18,19 Our group has investigated H-atom uptake for a series of these clusters, with the goal of understanding the structure–function relationship between synthetic modifications of the POV with thermodynamic and kinetic parameters for PCET.20–24 We have shown that terminal vanadyl (VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) sites can accept two H-atom equivalents resulting in a formal oxygen atom (O-atom) vacancy (VIII–OH2), whereas POV containing bridging oxides (2VV–O) prefer to undergo these 2H+/2e− transfers at disparate bridging oxo sites (2VIV–OH).20,21,23 We find that both nucleophilic sites undergo a rate limiting concerted H-atom transfer step, where the thermodynamics of O–H bond formation are dictated by the oxidation state distribution of vanadium ions composing the Lindqvist core.
O) sites can accept two H-atom equivalents resulting in a formal oxygen atom (O-atom) vacancy (VIII–OH2), whereas POV containing bridging oxides (2VV–O) prefer to undergo these 2H+/2e− transfers at disparate bridging oxo sites (2VIV–OH).20,21,23 We find that both nucleophilic sites undergo a rate limiting concerted H-atom transfer step, where the thermodynamics of O–H bond formation are dictated by the oxidation state distribution of vanadium ions composing the Lindqvist core.
Interested in expanding our knowledge of thermodynamic influences by synthetically altering POV clusters, we have recently turned our attention to the study of H-atom uptake in heterometal substituted POV assemblies. Previous work from our laboratory described O-atom defect formation to generate TiV5O5(OH2)(OCH3)13, TiV5O5(OH2), via PCET to a titanium-doped POV cluster, TiV5O6(OCH3)13, TiV5O6, using 9,10-dihydrophenazine (H2Phen) as a source of H-atoms (Scheme 1).25 The incorporation of a TiIV dopant into the Lindqvist assembly results in a change in the mechanism of H-atom uptake at the cluster surface; in the case of the all-vanadium POV cluster V6O7(OCH3)121− (V6O71−), the reaction proceeds via concerted proton–electron transfer (CPET), whereas H-atom uptake at the titanium-doped assembly occurs through an electron transfer–proton transfer (ET–PT) mechanism. This shift in mechanism is attributed to an increase in driving force for ET  from H2Phen upon cation substitution within the Lindqvist core. Indeed, inspection of the cyclic voltammogram (CV) of TiV5O6 in MeCN, shows that the most reducing VV/IV couple (TiV5O6 + e− → TiV5O61−) is anodically shifted in comparison to the relevant VV/IV couple of V6O71− (V6O71− + e− → V6O72−); this change in redox potential renders ET from H2Phen to TiV5O6 thermodynamically accessible
from H2Phen upon cation substitution within the Lindqvist core. Indeed, inspection of the cyclic voltammogram (CV) of TiV5O6 in MeCN, shows that the most reducing VV/IV couple (TiV5O6 + e− → TiV5O61−) is anodically shifted in comparison to the relevant VV/IV couple of V6O71− (V6O71− + e− → V6O72−); this change in redox potential renders ET from H2Phen to TiV5O6 thermodynamically accessible  , whereas ET from H2Phen to V6O71− is endergonic
, whereas ET from H2Phen to V6O71− is endergonic 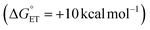 .26
.26
Herein, we report the resolved kinetic data for the rate limiting proton-transfer (PTlimiting), which follows an ET–PTlimiting mechanism from the reaction of TiV5O6 with H2Phen. Acceleration of the PTlimiting, in comparison to rate-determining CPET reactivity for V6O71−, is found to be dictated by lowering the activation barrier such that the stepwise reaction for TiV5O6 is 5-orders of magnitude faster than V6O71−. Additionally, the stepwise reaction can be “turned off”, in favour of a concerted pathway for TiV5O6, by switching to a substrate that is a less potent reductant. Ultimately, this study provides context for changing the mechanism of PCET on metal oxide surfaces through modulation of elementary driving forces  .
.
Results and discussion
Kinetic parameters for rate-limiting proton-transfer to a titanium-doped POV
In our initial report, evidence for the ET–PT mechanism was obtained from low temperature analysis of reaction mixtures by electronic absorption spectroscopy (EAS; Fig. 2). Following addition of substrate, immediate formation of an intermediate is observed, identified as H2Phen˙+ (Fig. 2a).27 Despite attempts to slow the reaction rate (e.g. lowered temperature, change in solvent), we were unable to fully resolve the kinetics of the ET–PT mechanism. Considering this, we turned to stopped flow analysis to experimentally determine the rates of H-atom uptake at TiV5O6.In prior reports describing H-atom uptake at the surface of POV clusters, reaction progress has been monitored by measuring the change in absorption at the VV/IV intervalence charge transfer (IVCT; VIV → VV) band in the near-infrared region of the spectrum (λ = 1000 nm).21,24,28 Following the addition of H2Phen to TiV5O6 (TiIVVVV5IV), the IVCT absorption feature is quenched (<0.6 s). We assign the loss of this feature to the reduction of the neutral TiV5O6 cluster to the mono-anionic assembly, TiV5O61− (TiIVV5IV), which occurs during the initial ET step of H-atom uptake (Fig. 2a). The rapid ET is consistent with fast heterogeneous electron transfer reported for the first VV/IV couple of TiV5O6 (kET = 3.1 × 10−2 cm−1 s−1).26
We shifted our attention to monitoring the kinetics of the rate-limiting PT step by following the loss of the absorption feature corresponding to the intermediate, dihydrophenazinium, H2Phen˙+ (λ = 645 nm; Fig. 2b).27 Experiments reveal that the consumption of H2Phen˙+ over the reaction coordinate has no dependency on the initial concentration of H2Phen (Fig. S1†). This observation can be justified by the anticipated rate expression for the rate-limiting PT step being first order in reduced cluster and first order in H2Phen˙+. The concentration of H2Phen˙+ is determined by the initial ET step and limited under the studied reaction conditions by the concentration of TiV5O6 (see Experimental section for more details). In this experiment, it is likely that quantitative ET occurs between H2Phen and TiV5O6 (Eox1/2 = −0.33 V vs. Fc+/0 and Ered1/2 = −0.29 V vs. Fc+/0, for H2Phen and TiV5O6, respectively;  ), resulting in the formation of equimolar amounts of H2Phen˙+ and TiV5O61−. Second order fitting of the kinetic data can be applied, resulting in a plot of [H2Phen˙+]−1vs. time with a linear relationship to three half-lives (Fig. 2c). The second order rate constant (kPT) can be determined from the slope of the line, kPT = 180 ± 20 M−1 s−1 at 243 K. We note that to resolve the rate of PT, kinetic experiments were conducted at lower temperatures.
), resulting in the formation of equimolar amounts of H2Phen˙+ and TiV5O61−. Second order fitting of the kinetic data can be applied, resulting in a plot of [H2Phen˙+]−1vs. time with a linear relationship to three half-lives (Fig. 2c). The second order rate constant (kPT) can be determined from the slope of the line, kPT = 180 ± 20 M−1 s−1 at 243 K. We note that to resolve the rate of PT, kinetic experiments were conducted at lower temperatures.
The preferred mechanism of PCET is dictated by reaction pathways with the lowest energy barriers. The origin of CPET is due to the preference to couple the ET and PT reactions; in a stepwise mechanism the electron and proton are transferred in two steps, resulting in the formation of charged (i.e. high-energy) intermediates that are often credited with slowing the overall rate of the net H-atom transfer reaction.29–32 However, in the case of TiV5O6, a substantial acceleration of the rate of H-atom uptake is observed in comparison to its homometallic analogue as a consequence of switching to a stepwise mechanism. The experimentally derived second order rate constant (kexp) for H-atom transfer from H2Phen to V6O71− was reported to be 5.1 ± 0.4 M−1 s−1 at 298 K in MeCN.33 Comparison to TiV5O6 reveals that even at reduced temperatures (298 K vs. 243 K), the rate determining step is an order of magnitude faster than V6O71−.
Reaction rates of PCET are proposed to be dependent on a multitude of factors such as driving forces of PT and ET 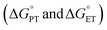 , reorganization energy, as well as tunnelling distances. For example, increasing the driving forces of
, reorganization energy, as well as tunnelling distances. For example, increasing the driving forces of  and
and  may provide a route to achieve enhanced reaction rates.34,35 Considering this, we next examined the driving forces for the rate determining step for both TiV5O6 (PT) and V6O71− (CPET) to assess the origin of the acceleration of reaction rates observed. Using the experimentally derived E1/2 and BDFE(E–H)avg, (E = O, N, respectively) values of TiV5O6 and H2Phen, the Bordwell equation can be used to approximate the pKa (pKapprox.a) of each substrate and obtain
may provide a route to achieve enhanced reaction rates.34,35 Considering this, we next examined the driving forces for the rate determining step for both TiV5O6 (PT) and V6O71− (CPET) to assess the origin of the acceleration of reaction rates observed. Using the experimentally derived E1/2 and BDFE(E–H)avg, (E = O, N, respectively) values of TiV5O6 and H2Phen, the Bordwell equation can be used to approximate the pKa (pKapprox.a) of each substrate and obtain  (see Experimental for more details). For TiV5O6, both
(see Experimental for more details). For TiV5O6, both  and
and  are both small (Table 1 and Fig. 3). This finding is surprising, given that the rate is substantially accelerated for the stepwise pathway. For this reason, activation barriers are valuable when contemplating the origins of the acceleration of PCET.
are both small (Table 1 and Fig. 3). This finding is surprising, given that the rate is substantially accelerated for the stepwise pathway. For this reason, activation barriers are valuable when contemplating the origins of the acceleration of PCET.
| V6O71− | TiV5O6 | |
|---|---|---|
| a Determined in referenced work.23,25 b CPET kinetic parameters. c ET–PTlimiting kinetic parameters. | ||

|
−0.7a | −0.9a |

|
+10a | −0.9a |
|
−2 | −3 × 10−2 |
| k exp @ 298 K (M−1 s−1) | 4.5 × 10−1a | 1.2 × 104 |
| KIE (kH/kD) | 1.7 ± 0.2a | 4.6 ± 0.2 |
| ΔH‡ (kcal mol−1) | 7 ± 1a | 11 ± 2 |
| ΔS‡ (cal mol−1 K−1) | −37 ± 2a | −4 ± 4 |
| ΔG‡ 298 K (kcal mol−1) | 18 ± 1a | 10 ± 2 |
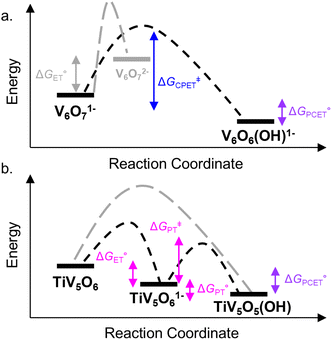 | ||
| Fig. 3 Reaction coordinate diagram for reactivity of V6O71− (top, a) and TiV5O6 (bottom, b) with H2Phen. Activation energy for the CPET process is shown in blue traces. ET–PT free energy changes and PT activation energy are shown in pink traces. Free energy for PCET process is shown in purple. Grey traces show pathways that are not favourable. All values are listed in Table 1. | ||
We next examined the activation parameters of proton uptake via Eyring analysis for TiV5O6 to further evaluate kinetic factors for the disparate mechanisms. The activation parameters of the transition state of the PTlimiting step of H-atom uptake at TiV5O6 were obtained through variable-temperature kinetic analysis. Using experimental conditions described above, kPT values were determined at temperatures ranging from 243 to 283 K (Fig. 2c, 4 and S2†). Enthalpy and entropy of activation (ΔH‡ and ΔS‡) of PT from H2Phen˙+ to TiV5O61− are determined to be 11 ± 2 kcal mol−1 and −4 ± 4 cal mol−1 K−1, respectively. Using the experimentally determined entropy and enthalpy of activation, the free energy of activation (ΔG‡) for proton transfer was determined to be 10 ± 2 kcal mol−1 at 298 K. ΔG‡ for TiV5O6 is ∼8 kcal mol−1 smaller than ΔG‡ for CPET to V6O71−, ΔG‡CPET (Table 1). The reduction in ΔG‡ results in substantial discrepancies in rates between TiV5O6 and the homometallic analogue and can be accredited to the lower intrinsic barriers for the stepwise ET and PT steps of the mechanism. In addition, the energy of the intermediate is expected to scale with the barrier height, and the transition from the charged intermediate to a neutral cluster likely aids in minimizing the activation barrier. For TiV5O6, the overall acceleration of the PCET reactivity is imparted by smaller activation energies (ΔG‡ET and ΔG‡PT) for the stepwise mechanism in comparison to CPET (ΔG‡CPET).36
Thermochemical parameters describing the formation of the transition state of the rate-determining step of a reaction are frequently used to distinguish between mechanisms of PCET to metal oxo complexes.37–41 Generally, a large and negative value for ΔS‡ is indicative of formation of an ordered, hydrogen-bonding donor/acceptor pair in the activated state. This, coupled with a ΔH‡ value that is small and positive has been invoked in CPET mechanisms. Exemplary of this are the activation parameters listed in Table 1 for the reactivity of V6O71− with H2Phen. Considering the established activation parameters for CPET type mechanisms, deviations from these “ideal” values are suggested as a justification when the mechanism is suspected to change. For example, Borovik and coworkers observe a mechanism change from CPET (ΔH‡ = 5 kcal mol−1, ΔS‡ = −49 cal mol−1 K−1, ΔG‡ = 19 kcal mol−1) to PT–ET (ΔH‡ = 14 kcal mol−1, ΔS‡ = −14 cal mol−1 K−1, ΔG‡ = 18 kcal mol−1) upon increasing the basicity of MnIII oxo complex.42 This mechanism assignment is supported by a more positive value of ΔS‡ for the PTlimited process, which the authors attribute to solvation of the ion pair following PT (e.g. Mn complex and anion substrate). In our system, there is a small, negative change in disorder in the generation of the transition state for PT from H2Phen˙+ to TiV5O61−. This ΔS‡ can be rationalized by changes in solvation that are associated with the transition state. In a polar solvent like MeCN, the solvent is expected to arrange around the charged complex, [TiV5O61−][H2Phen˙+]. To achieve the activated complex for proton transfer, changes in charge allocation must occur. The ionic complex in MeCN must rearrange such that there is a new neutral species, which results in reordering of the solvation shell with arranged dipole moments, explaining the small magnitude of ΔS‡.
Kinetic analysis of the addition of deuterated 9,10 dihydrophenazine-d2 (D2Phen) to TiV5O6 reveals a kinetic isotope effect (KIE) for the ET–PTlimiting mechanism. The KIE is obtained by extrapolating the rate at room temperature from activation parameters for PCET to TiV5O6, which are summarized in Table 1. Upon isotope labelling of substrate (98% 2H as determined by 1H NMR spectroscopy), the rate of PT is slowed (kDPT = 2600 M−1 s−1, Fig. S3†), resulting in a KIE of 4.6 ± 0.2. This value supports movement of a proton during the rate determining step of the reaction, in good agreement with the proposed PT-limited mechanism. Notably, the KIE observed in H-atom uptake at the Ti-doped POV cluster is larger than that reported for V6O71− (KIE = 1.7 ± 0.2). While it is possible that this is a result of more extensive tunnelling of the proton in the ET–PT mechanism of the PCET to TiV5O6, we instead propose that the increase in KIE value measured for TiV5O6 is due a small driving force for PT. Thermoneutral proton transfers (ΔG° ≈ 0) have been shown to exhibit larger KIEs.43,44 Indeed, the 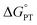 value is found to be small (Table 1).
value is found to be small (Table 1).
Computational insights into free energy changes for PCET to POV with H2Phen
Density functional theory (DFT) calculations were performed on both V6O71− and TiV5O6 to provide insight into consequences of dopant installation on the electronic structure of the assembly. The goal of the calculations is to aid in the elucidation of factors that contribute to the different mechanisms between the two assemblies. For both V6O71− and TiV5O6, spin density maps reveal that the VV center is partially localized, which is in agreement with the assignment of the mixed-valent clusters as Robin and Day Class II systems (Fig. 5).19,45 In the case of V6O71−, the partial localization is induced by the methoxy bridging ligands breaking the ideal Lindqvist Oh core symmetry, whereas the symmetry of TiV5O6 is further broken by the presence of the TiIV ion.Computational support to the assignment of mechanisms of PCET to the homo- and hetero-metallic POV clusters with H2Phen was obtained through DFT calculations. It is important to note that the computational data presented in this work are strictly thermodynamic values and do not consider kinetic values (i.e. activation barriers), vital for explicit mechanistic assignment. Activation barriers associated with ET, PT, and CPET processes are essential in establishing mechanism; however, obtaining these values in open shell multimetallic systems like POVs is nontrivial. Nevertheless, we believe that the thermodynamic data holds merit in conjunction with the experimentally derived kinetic data to propose the most likely pathways of PCET in the presented POVs.
Gibbs free energy differences are determined by optimization of the geometries of the reactants and products of the elementary PCET steps (i.e. PT, ET, CPET) in the gas phase. The values presented in Fig. 6 are representative of the previously proposed rate-determining step, which is suggested to be the formation of the single H-atom transfer product containing V(IV)–OH. This causes the first net H-atom transfer to be slightly uphill, as demonstrated by the positive values reported in Fig. 6. However, the overall reactions are the transfer of two H-atoms (i.e. 2e− + 2H+) and remain downhill. In agreement with the experimentally determined mechanism, calculations suggest that there is thermodynamic preference for CPET to V6O71− (VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → VIV–OH, Fig. 6a). This is because the off diagonal mechanisms (e.g. ΔGcalc.ET = +25.4 kcal mol−1, ΔGcalc.PT = + 38.5 kcal mol−1) are much higher in energy due to the formation of ion paired intermediates.
O → VIV–OH, Fig. 6a). This is because the off diagonal mechanisms (e.g. ΔGcalc.ET = +25.4 kcal mol−1, ΔGcalc.PT = + 38.5 kcal mol−1) are much higher in energy due to the formation of ion paired intermediates.
Considering the thermodynamic landscape provided by the computational analysis in this section, H-atom uptake at TiV5O6 following addition of H2Phen occurs via a sequential ET–PT mechanism (Fig. 6b).46 Thermodynamically, the ET is favored by 2.2 kcal mol−1 over CPET, which is followed by PT to generate the VIV–OH. In the previous section, experimental data suggested that the PT is the rate-determining step, which is in good agreement with the calculated thermodynamic data presented here. We note that the thermodynamic values displayed in Fig. 6 show a greater change in free energy (+25.4 kcal mol−1) than the experimental values listed in Table 1 (+10 kcal mol−1). We attribute this discrepancy in free energy changes due to the complexity of the electronic structures of the POV clusters investigated. Nevertheless, we find the qualitative agreement between the experimental and computational thermodynamic schemes as satisfactory.
The origin of the observed stepwise ET–PT for the TiV5O6 over a CPET mechanism can be explained by differences in the energetics of the frontier molecular orbitals. The LUMO in the titanium doped species is significantly lower in energy thus favoring an initial ET step over CPET (−2.53 eV for V6O71− and −3.47 eV for TiV5O6). As noted by Hammes-Schiffer and co-workers, the use of isodesmic reactions benefit from error cancellation inherent to density functional theory and implicit solvation models.47 In this aspect, we observed a stronger DFT functional dependence in the non-concerted ET–PT over the CPET, likely due the different description of the intermediate VIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O by different exchange–correlation functionals.48 For example, PBE0 functional suggests more favorable thermodynamic values along the ET–PT pathway for TiV5O6, in agreement with the experimental results, while M06 reveals a CPET as the most downhill path (Fig. S4†). The thermodynamic values for PCET to V6O71− are independent on the DFT functional for the VV
O by different exchange–correlation functionals.48 For example, PBE0 functional suggests more favorable thermodynamic values along the ET–PT pathway for TiV5O6, in agreement with the experimental results, while M06 reveals a CPET as the most downhill path (Fig. S4†). The thermodynamic values for PCET to V6O71− are independent on the DFT functional for the VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to VIV–OH transformation, where M06 and PBE0 predict CPET as the energetically favorable route (Fig. S5†). Ultimately, in regimes where the driving forces for stepwise and concerted pathways are comparable, computational analysis must be interpreted cautiously while paying mind to experimental kinetic data, as the DFT for POVs in this work cannot account for activation barriers.
O to VIV–OH transformation, where M06 and PBE0 predict CPET as the energetically favorable route (Fig. S5†). Ultimately, in regimes where the driving forces for stepwise and concerted pathways are comparable, computational analysis must be interpreted cautiously while paying mind to experimental kinetic data, as the DFT for POVs in this work cannot account for activation barriers.
Kinetic investigation with a mild H-atom donor and POV clusters
Experimental and computational evidence discussed in this work suggest that sequential delivery of the proton/electron pair via an ET–PT mechanism to TiV5O6 is a consequence of the exergonic electron transfer step from H2Phen to the cluster. We next became interested in expanding the substrate scope to probe the extent of the influence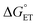 has on ΔG‡, which will alter the mechanism. Accordingly, we aimed to modulate the system to balance
has on ΔG‡, which will alter the mechanism. Accordingly, we aimed to modulate the system to balance 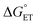 between TiV5O6 and substrate such that PCET can be facilitated via a CPET mechanism. To accomplish this, we investigated PCET from hydrazobenzene (H2Azo) to TiV5O6 (Scheme 2). For H2Azo, the H-atom is similarly sourced from a weak N–H bond (BDFE(N–H)MeCNavg = 60.7 kcal mol−1)2 and the oxidation potential is less accessible to the Ti-doped POV cluster (
between TiV5O6 and substrate such that PCET can be facilitated via a CPET mechanism. To accomplish this, we investigated PCET from hydrazobenzene (H2Azo) to TiV5O6 (Scheme 2). For H2Azo, the H-atom is similarly sourced from a weak N–H bond (BDFE(N–H)MeCNavg = 60.7 kcal mol−1)2 and the oxidation potential is less accessible to the Ti-doped POV cluster (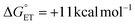 , Fig. S6†). Analysis of the products via1H NMR spectroscopy of the crude reaction mixture of TiV5O6 with H2Azo indicates formation of TiV5O5(OH2) (Fig. S7†), as well as the expected dehydrogenated organic substrate azobenzene (Azo).
, Fig. S6†). Analysis of the products via1H NMR spectroscopy of the crude reaction mixture of TiV5O6 with H2Azo indicates formation of TiV5O5(OH2) (Fig. S7†), as well as the expected dehydrogenated organic substrate azobenzene (Azo).
To probe the mechanism of PCET from H2Azo to TiV5O6, kinetic analyses were performed using EAS (Fig. S8†). Comparison of the averages of the BDFE's of the complexes suggest a  , requiring the addition of excess substrate to drive the reaction to completion. Kinetic analyses of PCET between TiV5O6 with H2Azo was achieved by monitoring reaction progression under pseudo first order reaction conditions at elevated temperature (318 K). Loss of the IVCT bands for the VV/IV transition at 1050 nm allows for facile monitoring of the amount of parent cluster, TiV5O6. Kinetic traces at this wavelength can be fit to an exponential decay function using least squares regression method to obtain the pseudo-first order rate constant, kobs (Fig. S9 and S10†). Varying the concentration of reductant results in a linear relationship with kobs indicating a first-order dependence on [H2Azo] for reactivity with TiV5O6 (Fig. 7). Larger concentrations of reductant were probed for TiV5O6 to rule possible saturation kinetics and showed no levelling effect (Fig. S11†). We attribute the larger error at high concentration to be caused by increased uncertainty due to the faster reaction rates. From the slope of the line, the experimentally derived second order rate constant is obtained, kexp = 0.53 ± 0.05 M−1 s−1 at 318 K. The observed rate of reaction with H2Azo is substantially slower in comparison to its reactivity with H2Phen (kPT = 1.2 × 104 M−1 s−1, 298 K). However, the rate is accelerated in comparison with the homometallic cluster, where H-atom transfer to V6O71− from H2Azo proceeds with kexp = (1.1 ± 0.1) × 10−3 M−1 s−1 at 318 K (Fig. 7 and S12†).
, requiring the addition of excess substrate to drive the reaction to completion. Kinetic analyses of PCET between TiV5O6 with H2Azo was achieved by monitoring reaction progression under pseudo first order reaction conditions at elevated temperature (318 K). Loss of the IVCT bands for the VV/IV transition at 1050 nm allows for facile monitoring of the amount of parent cluster, TiV5O6. Kinetic traces at this wavelength can be fit to an exponential decay function using least squares regression method to obtain the pseudo-first order rate constant, kobs (Fig. S9 and S10†). Varying the concentration of reductant results in a linear relationship with kobs indicating a first-order dependence on [H2Azo] for reactivity with TiV5O6 (Fig. 7). Larger concentrations of reductant were probed for TiV5O6 to rule possible saturation kinetics and showed no levelling effect (Fig. S11†). We attribute the larger error at high concentration to be caused by increased uncertainty due to the faster reaction rates. From the slope of the line, the experimentally derived second order rate constant is obtained, kexp = 0.53 ± 0.05 M−1 s−1 at 318 K. The observed rate of reaction with H2Azo is substantially slower in comparison to its reactivity with H2Phen (kPT = 1.2 × 104 M−1 s−1, 298 K). However, the rate is accelerated in comparison with the homometallic cluster, where H-atom transfer to V6O71− from H2Azo proceeds with kexp = (1.1 ± 0.1) × 10−3 M−1 s−1 at 318 K (Fig. 7 and S12†).
To account for the different number of active sites on the POV clusters, we invoke a statistical factor (n) based on the two H-atoms transferred and number of reactive vanadyls to accurately compare rate constants.33 For V6O71−, all six terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites can accept an H-atom, whereas for TiV5O6, only the four equatorial V
O sites can accept an H-atom, whereas for TiV5O6, only the four equatorial V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are believed to participate in H-atom uptake reactions.23 After accounting for the probability factor associated with the reactive sites on each POV, kPCET values can be determined. The second order rate constant for a rate-limiting H-atom transfer to each POV cluster at 318 K are kPCET = 6.6 × 10−2 M−1 s−1 (TiV5O6) and 9.2 × 10−5 M−1 s−1 (V6O71−). The heterometal-doped POV cluster, TiV5O6, reacts three orders of magnitude faster than the homometallic assembly. This acceleration of the HAT reaction is reminiscent of the previous example employing H2Phen as a reductant, where accelerated rates are a byproduct of the reduced ΔG‡ following an ET–PT mechanism. However, it is important to reiterate that ET from H2Azo to TiV5O6 is endergonic
O bonds are believed to participate in H-atom uptake reactions.23 After accounting for the probability factor associated with the reactive sites on each POV, kPCET values can be determined. The second order rate constant for a rate-limiting H-atom transfer to each POV cluster at 318 K are kPCET = 6.6 × 10−2 M−1 s−1 (TiV5O6) and 9.2 × 10−5 M−1 s−1 (V6O71−). The heterometal-doped POV cluster, TiV5O6, reacts three orders of magnitude faster than the homometallic assembly. This acceleration of the HAT reaction is reminiscent of the previous example employing H2Phen as a reductant, where accelerated rates are a byproduct of the reduced ΔG‡ following an ET–PT mechanism. However, it is important to reiterate that ET from H2Azo to TiV5O6 is endergonic  , thus making a purely ET–PT mechanism unfavourable.
, thus making a purely ET–PT mechanism unfavourable.
We next performed kinetic analyses with deuterium-labelled substrate, D2Azo with the POVs, (99% 2H as determined by 1H NMR spectroscopy). Deuterium labelling of the substrate results in a KIE of 1.7 ± 0.2 for V6O71− (Fig. S13–S16†), whereas no KIE was observed for the titanium-doped cluster (KIE = 1.0 ± 0.2). The lack of a KIE suggests that the rate-limiting step for the reaction between TiV5O6 with H2Azo does not involve a proton. However, in rare examples, KIE values close to origin have been observed for CPET reactions.29,43,49,50 Hammarström and coworkers summarized the effects that could yield a KIE of ∼1 for CPET, which are briefly described as (1) proton transfer potential that are less harmonic than Morse potentials, and (2) proton tunnelling distances that are minimized by thermal distributions.29
Temperature-dependent kinetic analysis was performed on the reactions of H2Azo with TiV5O6 and V6O71− to further probe the mechanism of PCET. As described earlier, from the slope and intercept of the line on the Eyring plot, the activation parameters for PCET to POV clusters can be determined (Fig. 8, S17 and S18,† see Experimental for more details). Interestingly, ΔG‡ for TiV5O6 (ΔG‡ = 18 ± 3 kcal mol−1) and V6O71− (ΔG‡ = 22 ± 3 kcal mol−1) are statistically equivalent (Table 2) at 298 K. Both clusters have activation parameters that are reminiscent to values reported previously for CPET reactions, with large and negative activation entropies and small activation enthalpies.23,38,39,42,51 We attribute the large and negative ΔS‡ to be caused by the need for the cluster and substrate to form an H-bonded pair in the rate-determining CPET. The activation parameters suggest that both POV clusters undergo H-atom uptake via a CPET mechanism with H2Azo.
Using the computational methods described above, we find that the use of H2Azo as a source of H-atom equivalents yields a thermodynamic landscape where the most likely mechanism is CPET for both V6O71− and TiV5O6 (Fig. 9). This is evident upon inspection of the stepwise mechanisms for TiV5O6, where the ET from H2Azo becomes unfavorable (ΔGcalc.ET = +20.4 kcal mol−1). A PT-led mechanism can also be ruled out; the mildly basic cluster is incapable of deprotonating H2Azo, as indicated by the highly endergonic PT determined via computations (ΔGcalc.PT = 61.7 kcal mol−1). We suggest that the thermodynamic theoretical data presented imply that transfer of an H-atom to TiV5O6 from H2Azo occurs by CPET.
Taken together, the data presented in this section suggests that the operative mechanism of H-atom uptake on TiV5O6 from H2Azo is CPET. We find that the mechanism of PCET of the Ti-doped cluster can be predicted and effectively controlled by modulating the free energy barriers for the elementary steps (i.e.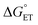 ,
, 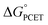 ) of the H-atom transfer reagent. When
) of the H-atom transfer reagent. When 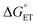 is exergonic, an ET–PT pathway is favored, whereas when
is exergonic, an ET–PT pathway is favored, whereas when 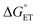 becomes less favorable, a mechanism crossover is encountered to CPET.
becomes less favorable, a mechanism crossover is encountered to CPET.
With experimental support for a CPET mechanism, there remains the subject of the accelerated reaction rate observed for TiV5O6 and H2Azo in comparison to V6O71−. It has been suggested that the rate of CPET may be enhanced by an “imbalanced” or “asynchronous” transition state, which describes the transition state of the H˙ transfer with the H+/e− pair at inequal distances on the reaction coordinate. To measure the extent of the imbalance in the transition state, Srnec and coworkers proposed an asynchronicity factor (η) which is proportional to the free energy changes associated with the proton transfer  and the electron transfer
and the electron transfer  between reduced and oxidized substrates (eqn (1)).52 This value is then approximated by the difference in free energy changes of PT and ET,
between reduced and oxidized substrates (eqn (1)).52 This value is then approximated by the difference in free energy changes of PT and ET, 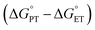 where F is Faraday's constant.53
where F is Faraday's constant.53
 | (1) |
Thus, a larger  would be indicative of more extensive imbalance. For the clusters presented in this work, the titanium-doped POV exhibits more modest changes to the redox potential and pKa contrasted to V6O71− (see Table 1 for values), which results in only minor changes to the
would be indicative of more extensive imbalance. For the clusters presented in this work, the titanium-doped POV exhibits more modest changes to the redox potential and pKa contrasted to V6O71− (see Table 1 for values), which results in only minor changes to the  value. This implies that asynchronicity would not play a substantial role in the acceleration of PCET to TiV5O6.
value. This implies that asynchronicity would not play a substantial role in the acceleration of PCET to TiV5O6.
An alternative explanation for the discrepancy in rates of CPET worth consideration is differences in BDFE's for the first and second H-atom of the aquo-ligand. For TiV5O5(OH2) and the reduced product V6O6(OH2)(OCH3)121−, V6O6(OH2)1−, the reported BDFE values for these reduced clusters are averages, due to the instability of the VIV-OH proposed intermediate. A strong first BDFE is supported by the lack of comproportionation of oxidized and reduced POV in solution.23 If the BDFE of the V–OH is weaker than the BDFE of the V–OH2 for either TiV5O5(OH2) or V6O6(OH2)1−, then this may result in discrepancies in the reaction rate while maintaining a rate-limiting CPET. Since the VIV–OH POV clusters remain elusive, we turn to computational insights into the differences in the first and second O–H bond strengths. The BDFE of the first H-atom (“TiV5O5(OH)”) and the second H-atom (TiV5O5(OH2)) were calculated for both POV clusters of interest (Scheme 3 and Table 3). The calculated values are larger than the experimentally derived values by 5 kcal mol−1. Despite this, there is agreement between the BDFE(O–H)avg values for TiV5O5(OH2) and V6O6(OH2)1− determined experimentally and through computations, and helpful for the observations of the trends for H-atom uptake on the POV.
| BDFE (kcal mol−1) | TiV5O6 | V6O71− |
|---|---|---|
| VIV–OH | 60.2 | 59.1 |
| VIII–OH2 | 71.2 | 70.2 |
| Avg.calc | 65.7 | 64.7 |
| Avg.exp25,33 | 60.1 | 59.9 |
Computations predict that the first BDFE is weak, consistent with a rate-limiting H-atom transfer to form the VIV–OH moiety for both Ti-doped and homometallic clusters. This is consistent with the mechanism proposed based on experimental findings in our 2H+/2e− process to POV clusters.23 The second BDFE is found to be much stronger, by ∼10 kcal mol−1, which would manifest as a fast step in the reaction that cannot be kinetically resolved through experimentation. In addition, identical averages are computed for TiV5O6 and V6O71−. We conclude that while a discrepancy in bond strengths may contribute to the acceleration in reaction rates, our computational model is unable to currently provide appropriate resolution to the BDFE that would support this theory.
Finally, we cannot eliminate the possibility of strong electronic coupling between the H2Azo substrate and TiV5O6. Strong electronic coupling occurs when there is interaction between two molecular orbitals (MOs) resulting in a change of the electron occupancy. Differences in the MOs of TiV5O6vs.V6O71− are highlighted in the computational discussion but can also be visualized in the EAS spectrum of the reduced Ti-POV (Fig. 2). For this cluster, we observe the presence of an IVCT band (V(IV) → Ti(III)). It is possible that the MOs of H2Azo are more strongly coupled to the MOs of TiV5O6 due to the presence of the Ti-ion. The oxidized organic product, Azo, has a weak transition at 440 nm (Fig. S19†) that may couple to TiV5O6. Evidence of this electronic coupling may be observed by analysis of early timepoints for the reaction of the POV with H2Azo via EAS (Fig. S20†). When these two substrates are mixed, there is asymmetry observed in the initial λmax in comparison to identical reaction conditions for V6O71−. We note that the strong coupling effects may provide some perspective to the peculiar KIE value, ∼1. Other groups have shown KIE of ≤1 when strong electronic and vibrational coupling occurs, where stronger coupling effects are more profound in deuterated complexes, making the kD values similar to kH and a KIE of ∼1.54
Conclusions
Understanding the factors that regulate the mechanism of H-atom uptake on metal oxide assemblies afford a unique opportunity to improve the selectivity of products of interfacial reactivity. In this study, we demonstrate the ability to alter the mechanism from stepwise ET–PT to CPET using a TiIV doped POV cluster by modulating the elementary driving forces of the single proton and electron transfer. By “turning on” the stepwise pathway, the reaction rate is accelerated due to lower activation barriers associated with the PTlimiting step. The mechanism of PCET is then changed back to a concerted pathway by switching to a less potent electron reductant. However, despite similar driving forces and activation barriers, an accelerated reaction is observed for the Ti-POV in comparison to the homometallic cluster. The source of the enhanced rates of PCET to TiV5O6 are discussed, including divergences in BDFE(O–H) of the hydroxide clusters as well as potential strong electronic coupling interactions between the titanium-doped POV and substrate. Ongoing efforts from our laboratory seek to investigate the possibility of strong coupling influences on rate as well as explore the effects of other transition metal ions on the mechanism of PCET in doped POV clusters.Experimental
General considerations
All manipulations were carried out in the absence of water and oxygen using standard Schlenk techniques or in a UniLab MBraun inert atmosphere drybox under a dinitrogen atmosphere. All glassware was oven-dried for a minimum of 2 h and cooled in an evacuated antechamber prior to use in the drybox. Solvents were dried and deoxygenated on a glass contour system (Pure Process Technology, LLC) and stored over 3 Å molecular sieves purchased from Fisher Scientific and activated prior to use. Hydrazobenzene was purchased from TCI America and used as received. 2.5 M n-butyllithium in hexanes was purchased from Sigma-Aldrich and used as received. D2O was purchased from Cambridge Isotope Laboratories and used as received. POV clusters V6O71−, TiV5O6 were prepared according to previously reported procedures.19,25,45 9,10-Dihydrophenazine, 9,10-dihydrophenazine-d2, and hydrazobenzene-d2 were generated following literature precedent.23,551H NMR spectra were recorded at 400 MHz or 500 MHz on a Bruker DPX-400 or Bruker DPX-500 spectrometer, respectively, locked on the signal of deuterated solvents. All chemical shifts were reported relative to the peak of the residual H signal in deuterated solvents. CD3CN was purchased from Cambridge Isotope Laboratories, degassed by three freeze–pump–thaw cycles, and stored over fully activated 3 Å molecular sieves. All electrochemistry measurements were performed by using a BioLogic SP-150 Potentiostat and acquired with the EC-Lab software (V11.42). Glassy carbon disc (3 mm, CH Instruments, USA) and a platinum wire was used as working and counter electrode, respectively. A nonaqueous Ag/Ag+ reference electrode with 1 mM AgNO3 and 100 mM [nBu4N][PF6] in acetonitrile (BASi, USA) was used as the reference electrode. All cyclic voltammetry (CV) measurements were carried out at room temperature in a nitrogen-filled glove box and calibrated by Fc0/+ couple at 100 mV s−1. Electronic absorption measurements were recorded at room temperature in anhydrous acetonitrile in a sealed 1 cm quartz cuvette with an Agilent Cary 60 UV-vis spectrophotometer or an Agilent Cary 3500 spectrophotometer. Kinetic experiments were carried out on an Agilient Cary 60 UV-vis spectrophotometer held at desired temperatures using an Unisoku CoolSpek UV cryostat, as well as an Agilent Cary 3500 UV-vis spectrophotometer held at desired temperatures with an integrated Peltier temperature control system.
General procedure for second-order reaction kinetics
In a nitrogen-filled glovebox, stock solutions of TiV5O6 (1.5 mM) and H2Phen (15 mM) in de-gassed, dry acetonitrile were prepared in air-tight syringes and removed. All measurements were performed on a KinetAsyst Double-Mixing Stopped-Flow equipped with a cryostat (TgK Scientific). All measurements were taken in single-mixing mode using a photomultiplier tube. Under active N2 flow, the syringes were attached to the stopped-flow drive syringes after flushing the system with air-free acetonitrile. Electron transfer from H2Phen to TiV5O6 is instantaneous, resulting in equimolar amounts of H2Phen˙+ and TiV5O61− to be formed in solution, allowing for precise second order reactivity to be observed. The reaction coordinate was monitored at 645 nm to observe the loss of H2Phen˙+ over time (λ645nm, ε = 1003 M−1 cm−1, determined in situ). Each trial was repeated in triplicate and an averaged trace was obtained for analysis. The procedure was repeated for each new temperature (243–283 K), and the initial concentration of H2Phen˙+ is in good agreement across the temperature range investigated (Fig. S2†).Concentration of [H2Phen˙+] was calculated from the baseline corrected absorbance (A) by using the molar absorptivity coefficient (ε) at the selected wavelength and the pathlength (b = 1 cm) (eqn (2)).
| [H2Phen˙+] = A × (b × ε)−1 | (2) |
We note that both cluster reactant (TiV5O61−) and product (TiV5O5(OH2) absorb at the selected wavelength, but significantly less than the radical (ε = 80 M−1 cm−1 and 100 M−1 cm−1, respectively). Change in ΔA at 0.75 mM cluster concentration <0.02), therefore the overall change in absorbance from the POV cluster is negligible with respect to the radical species (ΔAcluster ∼ 0) (Fig. 2).
A plot of [H2Phen˙+]−1vs. time reveal a linear relationship for at least 3 half-lives, with excellent fits (R2 ≥ 0.99). The slope of the line is the second order rate constant (kPT) for proton transfer from H2Phen˙+ to TiV5O61− at a given temperature. Uncertainties associated with rates were determined by accounting for 10% of the average value.
The rate expression for HAT to TiV5O6 from H2Phen can be described as (eqn (3)):
| rate = KETkPT[TiV5O61−][H2Phen˙+] | (3) |
To determine the deuterium kinetic isotope effect (KIE), analogous second order reactions were performed under identical conditions, using the deuterium-labelled reductant species 9,10 dideuterophenazine-d2 (D2Phen). The prepared D2Phen used for these reactions was found to be 98% D-labelled by 1H NMR spectroscopy. Uncertainties associated with KIE was determined by accounting for 10% of the average value.
Determination of approximate pKa values from the Bordwell equation
To determine approximate pKa (pKapprox.a) values for TiV5O6 and H2Phen and H2Azo, the Borwell equation is employed (eqn (4)): | (4) |
General procedure for performing pseudo-first-order reaction kinetics
Pseudo-first-order reaction conditions were used to establish the rate expression for the reaction between the POV clusters (TiV5O6 and V6O71−) and the H-atom transfer reagent, H2Azo. Using a UV-vis spectrophotometer with temperature controls set to 318 K, reactions between cluster and excess H2Azo (100–440 eq.) were tracked by monitoring the absorbance at 1050 nm over the reaction coordinate. Final reductant concentrations were varied from 0.02–0.33 M, with a constant concentration of cluster of 0.75 (TiV5O6) or 0.60 mM (V6O71−). Samples of cluster stock solutions in MeCN were loaded in a long-necked quartz cuvette and sealed with a rubber septum and sealed with electrical tape before removing from the glovebox. In a 1 mL syringe (21G needle), a sample of reductant stock solution was measured prior to removal from the glovebox. After equilibrating to 318 K in the spectrophotometer, data acquisition began, and the reductant solution was forcefully injected to ensure efficient sample mixing. Upon the completion of the reaction, the plot of absorbance over time was fit to the following equation by least squares fitting:| At = Ainf + (A0 −Ainf)e−kobst | (5) |
| rate = k[TiV5O6][H2Azo] | (6) |
| rate = k[V6O71−][H2Azo] | (7) |
The slopes of the resultant kobsvs. [H2Azo] plots were normalized for the four (n = 4) possible reactive VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites on the TiV5O6 or for the six (n = 6) possible reactive VV
O sites on the TiV5O6 or for the six (n = 6) possible reactive VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites, as well as the two possible H-atoms which can be transferred from H2Azo, in order to determine the second order rate constant, kPCET (M−1 s −1), such that (eqn (8)):
O sites, as well as the two possible H-atoms which can be transferred from H2Azo, in order to determine the second order rate constant, kPCET (M−1 s −1), such that (eqn (8)):
 | (8) |
With no observed induction period in the pseudo-first order kinetics traces, the y-intercept was held at the origin in all cases. The reported errors are the first significant figure of the difference between the determined slope and the confidence interval maximum. To determine the deuterium kinetic isotope effect (KIE), analogous pseudo-first order reactions were performed under identical conditions, using the deuterium-labelled reductant species Hydrazobenzene-d2 (D2Azo) (Fig. S12–S15†). The prepared D2Azo used for these reactions was found to be 99% D-labelled using 1H NMR spectroscopy. Uncertainties associated with KIE was determined by accounting for 10% of the average value.
General considerations for computationally derived free energies
All density functional theory (DFT) calculations were conducted using the Turbomole 7.3 software package. Geometry optimizations were fully converged using the PBE0 hybrid functional, the resolution of the identity approximation (RI-J), the multipole-accelerated RI-J approximation (MA-RIJ), in conjunction with the m4 grid.56,57 The Aldrich basis set def2-TZVP was used for all atoms.58 Dispersion effects were included using the D3 Grimme correction.59 All calculations were performed in gas phase without any constraints. Harmonic frequency calculations were performed to confirm that the optimized structures were stationary points on the potential energy surface. Partition functions were used in the computation of 298 K thermal contributions to free energy employing the usual ideal-gas, rigid-rotor, harmonic oscillator approximation. All frequencies below 100 cm−1 were replaced to 100 cm−1 when computing Gibbs free energies. Single point calculations including the conductor-like screening model (COSMO) were performed to incorporate solvent effects (tetrahydrofuran, ε = 7.58).60,61 MultiWFN was used to generate the total and partial density of states, spin density, and molecular orbitals.62 Gibbs free reaction energies for elementary PCET steps were calculated according to the following examples (eqn (9)–(11)):| POV + H2Phen → H-POV + HPhen |
| ΔGPCET = GHPOV + GHPhen − GPOV − GH2Phen | (9) |
| POV + H2Phen → POV− + HPhen+ |
| ΔGET = GPOV− + GH2Phen+ − GPOV − GH2Phen | (10) |
| POV + H2Phen → H-POV+ + HPhen− |
| ΔGPT = GHPOV+ + GHPhen− − GPOV − GH2Phen | (11) |
Data availability
All data for this project has been deposited in the main text or ESI.† Additional kinetic traces and computational data can be found in the ESI.†Author contributions
S. E. C. executed experiments involving TiV5O6. S. G. D. performed all computational analysis. M. R. A. W. performed kinetic experiments involving V6O71− and H2Azo. Stopped flow experiments were operated by N. J. G. P. M. and E. M. M. directed the project. Writing and editing of the manuscript was achieved through contribution of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Science Foundation (CHE-2154727). E. M. M. is also a recipient of a Camille Dreyfus Teacher-Scholar Award from the Camille & Henry Dreyfus Foundation, which has provided additional resources to support this work. S. G. D. and P. M. thank the National Science Foundation for their support (NSF CAREER 2145657). All calculations supporting this project were performed on high-performance computing systems at the University of South Dakota funded by NSF MRI award OAC-1626516. Studies at Yale were supported by National Science Foundation award 2403842. N. J. G. acknowledges support through a National Science Foundation Graduate Research Fellowship and a Yale Summer Research Fellowship.References
- J. N. Schrauben, R. Hayoun, C. N. Valdez, M. Braten, L. Fridley and J. M. Mayer, Science, 2012, 336, 1298–1301 CrossRef CAS PubMed.
- R. G. Agarwal, S. C. Coste, B. D. Groff, A. M. Heuer, H. Noh, G. A. Parada, C. F. Wise, E. M. Nichols, J. J. Warren and J. M. Mayer, Chem. Rev., 2022, 122, 1–49 CrossRef CAS PubMed.
- A. Wuttig, M. Yaguchi, K. Motobayashi, M. Osawa and Y. Surendranath, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4585–E4593 CrossRef CAS PubMed.
- A. J. Göttle and M. T. M. Koper, Chem. Sci., 2017, 8, 458–465 RSC.
- X.-H. Yang, A. Cuesta and J. Cheng, Phys. Chem. Chem. Phys., 2021, 23, 22035–22044 RSC.
- E. W. McFarland and H. Metiu, Chem. Rev., 2013, 113, 4391–4427 CrossRef CAS PubMed.
- B. I. Lemon and J. T. Hupp, J. Phys. Chem. B, 1997, 101, 2426–2429 CrossRef CAS.
- J. M. Mayer, J. Am. Chem. Soc., 2023, 145, 7050–7064 CrossRef CAS PubMed.
- C. F. Wise and J. M. Mayer, J. Am. Chem. Soc., 2019, 141, 14971–14975 CrossRef CAS PubMed.
- R. G. Agarwal, H.-J. Kim and J. M. Mayer, J. Am. Chem. Soc., 2021, 143, 2896–2907 CrossRef CAS PubMed.
- M. T. M. Koper, J. Electroanal. Chem., 2011, 660, 254–260 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- M. C. Kessinger, J. Xu, K. Cui, Q. Loague, A. V. Soudackov, S. Hammes-Schiffer and G. J. Meyer, J. Am. Chem. Soc., 2024, 146, 1742–1747 CrossRef CAS PubMed.
- S. Chakraborty, B. E. Petel, E. Schreiber and E. M. Matson, Nanoscale Adv., 2021, 3, 1293–1318 RSC.
- P. Hu, P. Hu, T. D. Vu, M. Li, S. Wang, Y. Ke, X. Zeng, L. Mai and Y. Long, Chem. Rev., 2023, 123, 4353–4415 CrossRef CAS PubMed.
- J. Wang, X. Liu, Z. Du and Y. Xu, Dalton Trans., 2021, 50, 7871–7886 RSC.
- K. Y. Monakhov, W. Bensch and P. Kögerler, Chem. Soc. Rev., 2015, 44, 8443–8483 RSC.
- C. Daniel and H. Hartl, J. Am. Chem. Soc., 2009, 131, 5101–5114 CrossRef CAS PubMed.
- C. Daniel and H. Hartl, J. Am. Chem. Soc., 2005, 127, 13978–13987 CrossRef CAS PubMed.
- A. A. Fertig, W. W. Brennessel, J. R. McKone and E. M. Matson, J. Am. Chem. Soc., 2021, 143, 15756–15768 CrossRef CAS PubMed.
- A. A. Fertig and E. M. Matson, Inorg. Chem., 2023, 62, 1958–1967 CrossRef CAS PubMed.
- S. E. Cooney, A. A. Fertig, M. R. Buisch, W. W. Brennessel and E. M. Matson, Chem. Sci., 2022, 13, 12726–12737 RSC.
- E. Schreiber, A. A. Fertig, W. W. Brennessel and E. M. Matson, J. Am. Chem. Soc., 2022, 144, 5029–5041 CrossRef CAS PubMed.
- E. Schreiber, W. W. Brennessel and E. M. Matson, Chem. Sci., 2023, 14, 1386–1396 RSC.
- S. E. Cooney, M. R. A. Walls, E. Schreiber, W. W. Brennessel and E. M. Matson, J. Am. Chem. Soc., 2024, 146, 2364–2369 CrossRef CAS PubMed.
- L. E. VanGelder and E. M. Matson, J. Mater. Chem. A, 2018, 6, 13874–13882 RSC.
- T. Kwon, J. Y. Koo and H. C. Choi, Cryst. Growth Des., 2019, 19, 551–555 CrossRef CAS.
- S. E. Cooney, E. Schreiber, W. W. Brennessel and E. M. Matson, Inorg. Chem. Front., 2023, 10, 2754–2765 RSC.
- R. Tyburski, T. Liu, S. D. Glover and L. Hammarström, J. Am. Chem. Soc., 2021, 143, 560–576 CrossRef CAS PubMed.
- M. Bourrez, R. Steinmetz, S. Ott, F. Gloaguen and L. Hammarström, Nat. Chem., 2015, 7, 140–145 CrossRef CAS PubMed.
- A. Nilsen-Moe, A. Rosichini, S. D. Glover and L. Hammarström, J. Am. Chem. Soc., 2022, 144, 7308–7319 CrossRef CAS PubMed.
- T. J. Zerk, C. T. Saouma, J. M. Mayer and W. B. Tolman, Inorg. Chem., 2019, 58, 14151–14158 CrossRef CAS PubMed.
- C. Y. M. Peter, E. Schreiber, K. R. Proe and E. M. Matson, Dalton Trans., 2023, 52, 15775–15785 RSC.
- R. Tyburski and L. Hammarström, Chem. Sci., 2022, 13, 290–301 RSC.
- A. Nilsen-Moe, C. R. Reinhardt, P. Huang, H. Agarwala, R. Lopes, M. Lasagna, S. Glover, S. Hammes-Schiffer, C. Tommos and L. Hammarström, Chem. Sci., 2024, 15, 3957–3970 RSC.
- J. M. Mayer and I. J. Rhile, Biochim. Biophys. Acta Bioenerg., 2004, 1655, 51–58 CrossRef CAS PubMed.
- H. Kotani, H. Shimomura, K. Ikeda, T. Ishizuka, Y. Shiota, K. Yoshizawa and T. Kojima, J. Am. Chem. Soc., 2020, 142, 16982–16989 CrossRef CAS PubMed.
- O. Snir, Y. Wang, M. E. Tuckerman, Y. V. Geletii and I. A. Weinstock, J. Am. Chem. Soc., 2010, 132, 11678–11691 CrossRef CAS PubMed.
- J. Amtawong, B. B. Skjelstad, D. Balcells and T. D. Tilley, Inorg. Chem., 2020, 59, 15553–15560 CrossRef CAS PubMed.
- N. Kindermann, C.-J. Günes, S. Dechert and F. Meyer, J. Am. Chem. Soc., 2017, 139, 9831–9834 CrossRef CAS PubMed.
- T. G. Carrell, P. F. Smith, J. Dennes and G. C. Dismukes, Phys. Chem. Chem. Phys., 2014, 16, 11843–11847 RSC.
- T. H. Parsell, M.-Y. Yang and A. S. Borovik, J. Am. Chem. Soc., 2009, 131, 2762–2763 CrossRef CAS PubMed.
- S. J. Edwards, A. V. Soudackov and S. Hammes-Schiffer, J. Phys. Chem. A, 2009, 113, 2117–2126 CrossRef CAS PubMed.
- K.-Y. Wong, J. P. Richard and J. Gao, J. Am. Chem. Soc., 2009, 131, 13963–13971 CrossRef CAS PubMed.
- J. Spandl, C. Daniel, I. Brüdgam and H. Hartl, Angew. Chem., Int. Ed., 2003, 42, 1163–1166 CrossRef CAS PubMed.
- M. Z. Ertem, L. Gagliardi and C. J. Cramer, Chem. Sci., 2012, 3, 1293–1299 RSC.
- R. E. Warburton, A. V. Soudackov and S. Hammes-Schiffer, Chem. Rev., 2022, 122, 10599–10650 CrossRef CAS PubMed.
- G. Rugg, A. Genest and N. Rösch, J. Phys. Chem. A, 2018, 122, 7042–7050 CrossRef CAS PubMed.
- G. A. Parada, Z. K. Goldsmith, S. Kolmar, B. Pettersson Rimgard, B. Q. Mercado, L. Hammarström, S. Hammes-Schiffer and J. M. Mayer, Science, 2019, 364, 471–475 CrossRef CAS PubMed.
- S. D. Glover, G. A. Parada, T. F. Markle, S. Ott and L. Hammarström, J. Am. Chem. Soc., 2017, 139, 2090–2101 CrossRef CAS PubMed.
- P. Mondal, I. Ishigami, E. F. Gérard, C. Lim, S.-R. Yeh, S. P. de Visser and G. B. Wijeratne, Chem. Sci., 2021, 12, 8872–8883 RSC.
- D. Bím, M. Maldonado-Domínguez, L. Rulíšek and M. Srnec, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E10287–E10294 CrossRef PubMed.
- B. D. Groff, B. Koronkiewicz and J. M. Mayer, J. Org. Chem., 2023, 88, 16259–16269 CrossRef CAS PubMed.
- G. F. Manbeck, E. Fujita and J. J. Concepcion, J. Am. Chem. Soc., 2016, 138, 11536–11549 CrossRef CAS PubMed.
- J. Lee, K. Shizu, H. Tanaka, H. Nakanotani, T. Yasuda, H. Kaji and C. Adachi, J. Mater. Chem. C, 2015, 3, 2175–2181 RSC.
- J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 CrossRef CAS.
- O. Treutler and R. Ahlrichs, J. Chem. Phys., 1995, 102, 346–354 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805, 10.1039/P29930000799.
- A. Schäfer, A. Klamt, D. Sattel, J. C. W. Lohrenz and F. Eckert, Phys. Chem. Chem. Phys., 2000, 2, 2187–2193 RSC.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional kinetic traces and computational data. See DOI: https://doi.org/10.1039/d4sc06468b |
| This journal is © The Royal Society of Chemistry 2025 |