Open Access Article
Open Access ArticleSpatially confined transition metals boost high initial coulombic efficiency in alloy anodes†
Haoyu
Fu‡
 a,
Fangchao
Gu‡
a,
Yize
Niu‡
a,
Shuxuan
Liao
a,
Zeyuan
Bu
a,
Haonan
Wang
a,
Dong
Yang
a,
Xiaoshan
Wang
*a and
Qiang
Li
a,
Fangchao
Gu‡
a,
Yize
Niu‡
a,
Shuxuan
Liao
a,
Zeyuan
Bu
a,
Haonan
Wang
a,
Dong
Yang
a,
Xiaoshan
Wang
*a and
Qiang
Li
 *ab
*ab
aCollege of Physics, Weihai Innovation Research Institute, College of Materials Science and Engineering, Qingdao University, Qingdao 266071, China. E-mail: wangxiaoshan1@qdu.edu.cn; liqiang@qdu.edu.cn
bUniversity of Waterloo, Waterloo, Ontario N2L 3G1, Canada
First published on 2nd December 2024
Abstract
Alloy-type materials hold significant promise as high energy density anodes for lithium-ion batteries. However, the initial coulombic efficiency (ICE) is significantly hindered by the poor reversibility of the conversion reaction and volume expansion. Here, the NiO/SnO2 multilayers with a hybrid interface of alloy and transition metal oxides are proposed to generate Ni nanoparticles within confined layers, catalyzing Li2O decomposition and suppressing the coarsening of Sn or Li2O particles. Supported by density functional theory (DFT) calculations and revealed by operando magnetometry, the spatially confined, well maintained Ni active sites lower the energy barrier for Li–O bond rupture and enhance the migration dynamics of Li+. The enhanced reaction kinetics lead to achievement of an impressive ICE of 92.3% and a large capacity of 1247 mA h g−1 with 97% retention after 800 cycles. Furthermore, the NiO/SnO2 anode exhibits excellent electrochemical performances in both Na/K-ion batteries. Notably, when constructed with the same framework, SiO2 also delivers significantly improved lithium storage properties with ultra-high ICEs. This work paves the way for advanced designs of alloy-type anodes that satisfy both ICE and overall electrochemical performance.
Introduction
The rapid growth of new energy vehicles and large-scale energy storage systems has increased the demand for energy storage devices with lithium-ion batteries (LIBs) bearing the majority of this demand.1–3 Among well-studied LIB anode materials, alloy-type materials (e.g., Sn, Si, Ge, etc.) based on alloying/de-alloying reactions have emerged as a promising candidate anode alongside commercial graphite materials due to their high theoretical capacity, low working potential and cost-effectiveness.4–11 These alloy-type anodes typically suffer from drastic volume expansion (>300%), leading to poor electronic/ion conductivity, continuous growth of the solid electrolyte interphase (SEI), and significant performance degradation.12–14 Recently, MOx anodes (M = Sn, Si, etc.) have played a crucial role in advancing energy storage solutions and often exhibit better cycling stability, as the Li2O matrix generated from the conversion reaction (xLi+ + MOx + xe− → M + xLi2O) alleviates expansion stress and reduces volume fluctuations.15–17 However, the poor reversibility of the conversion reaction results in low initial coulombic efficiency (ICE), a critical factor for anode materials as it dictates the ability to mitigate irreversible capacity loss.18 A low ICE reduces energy density of a battery, significantly limiting its practical applicability.19 Therefore, improving the ICE of alloy-type anodes is essential for successful commercial implementation.Two main factors have been identified as the primary determinants of low ICE in alloy-type anodes. First, as discussed above, the irreversible transformation of Li2O results in poor reversibility for the conversion reaction.20,21 Meanwhile, the volume expansion of M (e.g., Sn and Si) during the alloying reaction causes continuous SEI growth, ultimately leading to partial lithium depletion.18 To achieve a high ICE, researchers have attempted to reduce the electrode size and enhance the contact area between M and Li2O.22,23 For example, Park et al. proposed high-entropy silicon anodes by the element screening method, facilitating mass and charge transport and achieving a high ICE of 90.3%.24,25 Li et al. synthesized alloy anodes with a disordered lattice and liquid metal phase to achieve higher metallic conductivity, improving the ICE up to 91%.26 However, the ongoing coarsening of grains, along with the associated volume expansion and electrode fractures, further accelerates the capacity decay. Additionally, transition metals (TMs) could catalyze the decomposition of Li2O and facilitate the phase transition, and are commonly utilized in the construction of alloy-type anodes. However, building alloy-type anodes with small-sized, highly active TMs to achieve an ICE over 90% remains challenging.27–31 Early research demonstrated that small-sized, highly active TMs can be generated in situ from transition metal oxides (TMOs) during the lithiation process.17,32 In this context, hybridizing alloy-type metals with TMOs in a confined space may offer a new approach for achieving high ICE.
Herein, we report magnetron-sputtered NiO/SnO2 multilayers (NSMs) capable of achieving an ultra-high ICE up to 92.3% and a remarkable capacity retention of 97% after 800 cycles. As a self-supporting electrode material, the prepared nanocomposite films have been successfully utilized in quasi-solid state batteries. Operando magnetometry tracks the phase evolution during the charge–discharge process. The tightly wrapped nanolayer structure provides a confined space to stabilize reduced Ni particles, suppressing the coarsening of Sn and Li2O particles. Density functional theory (DFT) calculations revealed that the free energy of Li–O bonds and the migration dynamics of Li+ are significantly influenced by active Ni nanoparticles (NPs), thus facilitating the subsequent delithiation process of Li2O. Furthermore, the NiO/SnO2 anode exhibits decent electrochemical performance in Na/K-ion batteries (SIBs/PIBs), demonstrating great promise as advanced anode materials for next-generation batteries. More interestingly, the superior ICE improvement strategy is universal in NiO/SiO2, CoO/SnO2, and Fe2O3/SnO2 alloy-type anode materials.
Results and discussion
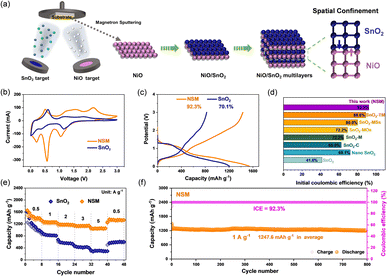
The schematic illustration of the NiO/SnO2 multilayers fabricated by magnetron sputtering is shown in Fig. 1a. Layers of 5 nm NiO and 10 nm SnO2 are alternately sputtered onto the copper electrode to form well-confined NiO/SnO2 interfaces. The synthesized multilayer films are directly used as binder-free anodes for lithium-ion batteries (Fig. 1a). All tested film materials and their preparation conditions are summarized in Table S1.† The X-ray diffraction pattern demonstrates the amorphous nature of the NSM (Fig. S1a†), which is further confirmed by the twisted and disordered lattice pattern of NiO and SnO2 in the corresponding TEM image (Fig. S1b†). The X-ray photoelectron spectroscopy (XPS) survey spectrum of the NSM (Fig. S2a†) confirms the existence of C, O, Sn and Ni. And high-resolution XPS validates the dominant valences states of Ni2+ (Fig. S2b†) and Sn4+ (Fig. S2c†) in the NSM, representing the original high purity film. The cyclic voltammograms (CVs) of the samples versus Li+/Li recorded in the range of 0–3 V at a scan rate of 0.5 mV s−1, are shown in Fig. 1b. In the first negative scan for NSM, SnO2 or NiO combined with Li+ ions shows a reduction peak at around 1.2 V (i.e., SnO2 + 4Li+ + 4e− → Sn + 2Li2O and NiO + 2Li+ + 2e− → Ni + Li2O, respectively).33 The cathodic peaks at around 0.55 V and 0.25 V are attributed to the formation of the solid-electrolyte interface (SEI) layer and the alloying reaction (Sn + xLi+ + 2e− → LixSn).33,34 In the first positive scan, the peaks detected at 0.5 V and 2.2 V are attributed to the dealloying of LixSn alloys and the oxidation of Ni to NiO, respectively.35 Notably, the NSM shows stronger decomposition peaks of Li2O (Sn + 2Li2O → SnO2 + 4Li+ + 4e−) compared to pure SnO2 between 1.0 V and 2.0 V, indicating a significant improvement in the reversibility of the conversion reaction.The electrochemical performance of the NiO/SnO2 multilayer electrode was investigated by using coin-type half cells (LIBs). As shown in Fig. 1c, the specific capacities of the NSM anode for the first charge and discharge are 1543.1 and 1671.9 mA h g−1 respectively, corresponding to a high initial coulombic efficiency (ICE) of 92.3%, as one of the best SnO2-based electrode performances reported in the literature (Fig. 1d). In contrast, the ICEs of pure NiO (Fig. S3†) and SnO2 are only 63.2% and 71.9%, respectively. The rate capability of NSM and SnO2 electrodes at current rates ranging from 0.5 to 5 A g−1 is shown in Fig. 1e. The NSM anode delivers a reversible capacity of 1453.2 mA h g−1 at a current density of 1 A g−1. When the current rate returns to 0.5 A g−1, 91.8% of the original capacity (1333.7 mA h g−1) is retained. In contrast, the SnO2 electrode only exhibits 1038.5 mA h g−1 at 1 A g−1 and retained only 60.6% of its capacity at 0.5 A g−1. Long-term cycling stability was evaluated at a current density of 1 A g−1.Remarkably, the NSM anode exhibits excellent cycling stability (Fig. 1f) with nearly 100% coulombic efficiency and retains 97% of its reversible specific capacity even after 800 deep cycles.
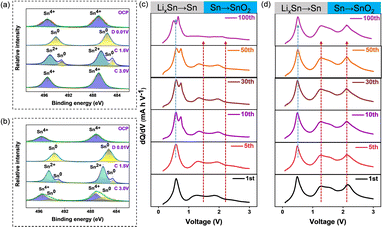
To further understand the remarkable ICE stemming from the high reversibility of the conversion reaction (Li2O + Sn → SnO2 + Li+), ex situ XPS characterization was conducted at different potentials. As shown in Fig. 2a, when gradually charged to 3 V, Sn4+ fully occupies the valence state in the NSM, whereas in bare SnO2 (Fig. 2b), 17% of Sn remains in the Sn0 state along with Sn4+.36 After 50 cycles (Fig. S4†), the Sn0 content increases to 68% in bare SnO2 (Fig. S4a†), while the NSM still shows Sn4+ without any impurities (Fig. S4b†), indicating Sn and LixSn coarsening in bare SnO2 and the high reversibility of the conversion reaction in the NSM electrode. This result is consistent with the differential charge capacity plots (DCPs) observed in the NSM (Fig. 2c). The shift of the dealloying peak (LixSn → Sn) towards higher potential suggests increasing polarization due to the coarsening of Sn and LixSn. Meanwhile, the conversion reaction peaks from 1.0 to 2.0 V disappear after 100 cycles in bare SnO2, indicating the failure of conversion reaction reversibility. Notably, the dealloying peak of the NSM remains stable even after 100 cycles (Fig. 2d), confirming the high reversibility and stability of the conversion/alloying reaction.37,38
To explore the underlying reaction mechanism of the highly reversible and stable conversion reactions in the NSM structure, the phase evolution process during cycling was investigated using operando magnetometry.39–44Fig. 3a and b show CV curves and time-sequenced magnetization of the NSM electrode, along with the potential response during the first three cycles. The periodic magnetization variation reflects the reversible and steady electrochemical processes. The second discharge/charge cycle response is magnified and discussed in detail (Fig. 3c). The peaks and valleys at different voltages in the real-time magnetization variation are labeled as V1 to V9. The lack of magnetization variation indicates that no reaction of NiO occurs from V1 to V2. The pronounced increase in magnetization from V2 to V3 is likely due to the reduction from NiO to Ni. The metallic Ni0 continues to store spin-polarized electrons between V3 and V4, thereby reducing the slope of the magnetization increase.45,46 Followed by the formation and decomposition of the SEI, the magnetization increases (V4 to V5) and then falls (V6 to V7) corresponding to electron transfer.47 As spin-polarized electrons are released from metallic Ni0, a significant increase in magnetization occurs from V7 to V8. The oxidation from Ni to NiO from V8 to V9 induces a rapid decline in magnetization. Thus, the in situ phase evolution of Ni particles in the lithiation process is successfully described by the advanced operando monitoring magnetization, addressing the limitations of the ex situ technique.29,35,48 The size of Ni particles was later quantified using Langevin fitting, as shown in Fig. 3d. The fitted curve matches perfectly with the experimental data, and the calculated diameter of the Ni particle is 1.2 nm. The result proves that the confined multilayers restrict the Ni particle size to the nanoscale, ensuring their uniform dispersion in layers, which provides more electron transport channels. Furthermore, DFT calculations (Fig. 3e) were used to investigate the relationship between Ni NPs and the decomposition of inactive Li2O at a potential of 1.2 V. As shown in Fig. 3e, the Li–O bond breaking process at the Ni/Li2O interface is an exothermic reaction with a total free energy of −2.85 eV, while the Li2O interface exhibits an energy barrier of 1.07 eV. This confirms that the Ni NPs accelerate the delithiation of Li2O, consistent with the highly reversible conversion reactions in the NSM.
The overall morphology and surface microstructure of NSM and SnO2 electrodes were examined after 50 discharge–charge cycles. In the SEM image, the SnO2 electrode exhibits significant volume expansion and a fractured surface (Fig. S5a†). The corresponding staggered lattices observed in STEM (Fig. S6a and b†) are identified as unreacted Sn, SnO2 and Li2O particles. In contrast, the NSM electrode (Fig. S5b†), comprising SnO2 and NiO composites, largely maintains its original structure with minimal changes (Fig. S6d and e†).49 EDX mappings show a uniform distribution of C, O and Sn elements in the NSM electrode after 50 cycles, suggesting no significant atom migration or aggregation during the cycling process. In contrast, the SnO2 electrode shows evident particle aggregation due to the poor distribution of Sn (Fig. S7†).50 Overall, these observations strongly support our experimental results, such as those of operando magnetometry, validating the reversible structural transformation catalysed by Ni NPs in the NSM electrode. Additionally, compared with the thicker SEI film in the SnO2 electrode (Fig. S6c†), the NSM electrode possesses a more uniform SEI film (Fig. S6f†), which reduces the impact of electrolyte corrosion and surface side reactions. Thus, such a synergistic interaction between the internal reversible structure and external SEI protection significantly improves both the cycle stability and rate performance.
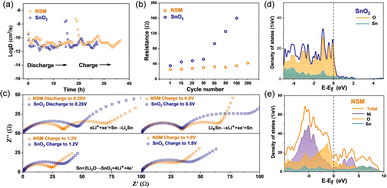
The kinetic behavior of a lithium-ion battery in the whole cycle was investigated using the galvanostatic intermittent titration technique (GITT) method (Fig. 4a).4,51 The diffusion coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) of the NSM is significantly higher than that of SnO2, indicating a faster ion diffusion rate. Electrochemical impedance spectroscopy (EIS) was used to detect changes in internal resistance during the electrochemical process. Fig. 4b illustrates the impedance from the 1st to the 200th cycles. Notably, by the 100th cycle, the impedance of the SnO2 electrode increased fourfold compared to the initial cycle (Table S2†). This drastic rise indicates the electrode's failure to maintain structural integrity due to Li2O deposition and Sn particle agglomeration during the process (Fig. S8†).52 Conversely, the impedance of the NSM shows minimal change even after 200 cycles, implying high structure reversibility during the reaction. For detailed insights, we conducted EIS of both NSM and SnO2 electrodes in the alloying/dealloying voltage range and during the decomposition of Li2O (discharge to 0.25 V and charged to 0.5 V, 1.2 V or 1.8 V, respectively) in a single cycle (Fig. 4c). The equivalent circuit fitting is shown in Fig. S9.† The NSM electrode exhibits lower charge transfer resistance (Rct) in smaller semicircles at high-to-medium frequencies and various potentials compared to bare SnO2, suggesting superior electrical conductivity of the NSM electrode.4 The density of states (DOS) curves further emphasize the conductivity difference between SnO2 and the NSM (Fig. 4d and e). Above the conduction band, the DOS intensity of the NSM is much higher than that of SnO2, indicating a lower energy demand for the electron transition in the NSM, which corresponds to higher conductivity.53 These results demonstrate that the NSM structure significantly enhances Li+ diffusion and charge transfer, thereby markedly improving rate performance.
D) of the NSM is significantly higher than that of SnO2, indicating a faster ion diffusion rate. Electrochemical impedance spectroscopy (EIS) was used to detect changes in internal resistance during the electrochemical process. Fig. 4b illustrates the impedance from the 1st to the 200th cycles. Notably, by the 100th cycle, the impedance of the SnO2 electrode increased fourfold compared to the initial cycle (Table S2†). This drastic rise indicates the electrode's failure to maintain structural integrity due to Li2O deposition and Sn particle agglomeration during the process (Fig. S8†).52 Conversely, the impedance of the NSM shows minimal change even after 200 cycles, implying high structure reversibility during the reaction. For detailed insights, we conducted EIS of both NSM and SnO2 electrodes in the alloying/dealloying voltage range and during the decomposition of Li2O (discharge to 0.25 V and charged to 0.5 V, 1.2 V or 1.8 V, respectively) in a single cycle (Fig. 4c). The equivalent circuit fitting is shown in Fig. S9.† The NSM electrode exhibits lower charge transfer resistance (Rct) in smaller semicircles at high-to-medium frequencies and various potentials compared to bare SnO2, suggesting superior electrical conductivity of the NSM electrode.4 The density of states (DOS) curves further emphasize the conductivity difference between SnO2 and the NSM (Fig. 4d and e). Above the conduction band, the DOS intensity of the NSM is much higher than that of SnO2, indicating a lower energy demand for the electron transition in the NSM, which corresponds to higher conductivity.53 These results demonstrate that the NSM structure significantly enhances Li+ diffusion and charge transfer, thereby markedly improving rate performance.
The schematic diagram of the high electrochemical performance mechanism, particularly in achieving ultra-high ICE for the NSM configuration, is summarized as follows (Fig. 5a): (1) Ni nanoparticles are first in situ generated from NiO and widely distributed in SnO2 multilayers to catalyze the decomposition of Li2O and promote the conversion reaction from Sn/Li2O to SnO2. (2) The multilayer structure effectively stabilizes Ni NP active sites and inhibits the coarsening of Sn and Li2O particles by creating a confined environment. Additionally, it prevents the formation of a thicker and uneven SEI layer, thereby alleviating volume expansion. (3) The nanosized Ni particles derived from the well-designed NSM provide more electron transport channels and facilitate Li+ diffusion and charge transfer, thereby elevating reaction kinetics. The same strategy has been extended to NiO/SiO2, CoO/SnO2 and Fe2O3/SnO2 multilayers to explore their applicability and effectiveness in lithium batteries, as shown in Fig. S10–S14.† It is found that the nanostructure NiO/SiO2 electrode exhibits excellent comprehensive electrochemical performance, achieving an ultrahigh average ICE of 81.2%, significantly surpassing that of pure SiO2. Additionally, the CoO/SnO2 and Fe2O3/SnO2-based electrodes also exhibited remarkable performance, delivering an ultra-high ICE of 91.1% and 90.5% at 500 mA g−1, with high capacities and rate performance, respectively. Moreover, leveraging the structural benefits, the NSM electrode achieves one of the best electrochemical performances reported for sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs), (Fig. S15 to S17; see the ESI† for details). The flexibility and adaptability of this structure make it suitable for various types of batteries, including lithium-ion, sodium-ion, or potassium-ion batteries, enabling outstanding performance across different applications.
To evaluate the practical application prospects of the NSM, quasi-solid-state pouch flexible cells (NSM∣P(VDF-HFP)∣LiCoO2) were assembled as shown in Fig. 5b.54,55 The full battery features an equal size 40 mm cathode and anode. The open circuit voltage of the battery device remains almost unchanged (approximately 3.0 V) at different folding states (Fig. 5c). As another demonstration, a “SnO2” logo comprising 90 LEDs is well illuminated by the flexible battery in both flat and various bending states (Fig. 5d). These electrochemical measurements highlight the key characteristics of flexibility and adaptability, providing a continuous and stable power supply despite changes in its physical shape or bending states. We also assembled coin-type full cells with the NSM and LiNi0.8Co0.1Mn0.1O2 as the anode and cathode separately, demonstrating similarities in the ultra-high capacity and ICE (Fig. S18†). On this basis, the “small and powerful” flexible battery is expected to be a competitive candidate for energy storage and conversion components in next-generation microelectronic devices, as well as in future integrated electronic devices.
Conclusion
In summary, a NiO/SnO2 multilayer anode with confined transition metal oxide (TMO) layers has been developed for Li-ion batteries using an alternating magnetron sputtering method. The designed hierarchical structure of the NSM exhibits an ultrahigh ICE of 92.3% and a large capacity of 1247.6 mA h g−1, retaining ∼97% after 800 cycles. Operando magnetometry directly evidences the evolution of Ni NPs from NiO, and the DFT calculations revealed that the Ni NPs significantly lower the energy barrier for breaking Li–O bonds, thereby facilitating the conversion reaction from Sn/Li2O to SnO2. Additionally, the confined environment in the NSM helps inhibit the coarsening of Sn and Li2O by maintaining the nanoscale of Ni active sites, effectively alleviating volume expansion. Moreover, the well-dispersed Ni NPs profoundly enhance the conductivity and reaction kinetics by providing more electron transport channels and shortening the diffusion distance of Li+ and electrons, respectively. As expected, in a broader range of interfacial systems, we found that NiO/SiO2, CoO/SnO2, and Fe2O3/SnO2 anodes all show significant performance improvements with an ultrahigh ICE of 81.2%, 91.1% and 90.5%, respectively. The multifunctionality and universality of NiO/SnO2 anodes in Na/K-ion batteries and flexible quasi-solid-state full cells highlight their widespread feasibility, offering a promising path towards high-performance energy storage solutions.Data availability
Essential data are provided in the main text and the ESI.† Additional data are available from the authors upon reasonable request.Author contributions
H. Y. Fu: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft. F. C. Gu: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization. Y. Z. Niu, S. X. Liao, Z. Y. Bu, H. N. Wang, and D. Yang: data curation, formal analysis, investigation, visualization. X. S. Wang: funding acquisition, supervision, writing – review & editing. Q. Li: funding acquisition, project administration, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22179066, 92372127 and 22408192), the Natural Science Foundation of Shandong Province (ZR202210060028), the Postdoctoral Fellowship Program of CPSF under Grant GZC20231188, and the Shandong postdoctoral innovation program (SDCX-ZG-202400262).Notes and references
- L. L. Hu, M. H. Jin, Z. Zhang, H. X. Chen, F. B. Ajdari and J. X. Song, Interface-adaptive binder enabled by supramolecular interactions for high-capacity Si/C composite anodes in lithium-ion batteries, Adv. Funct. Mater., 2022, 32, 2111560 CrossRef CAS.
- S. Chae, S. H. Choi, N. Kim, J. Sung and J. Cho, Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries, Angew. Chem., Int. Ed., 2020, 59, 110–135 CrossRef CAS.
- Y. S. Zhu, C. T. Wang, Z. J. Cheng, Q. Yao, J. Su, B. Chen, J. Yang and Y. T. Qian, Bimetallic Bi-Sn microspheres as high initial coulombic efficiency and long lifespan anodes for sodium-ion batteries, Chem. Commun., 2022, 58, 5140–5143 RSC.
- S. Y. Pan, J. W. Han, Y. Q. Wang, Z. S. Li, F. Q. Chen, Y. Guo, Z. S. Han, K. F. Xiao, Z. C. Yu, M. Y. Yu, S. C. Wu, D. W. Wang and Q. H. Yang, Integrating SEI into layered conductive polymer coatings for ultrastable silicon anodes, Adv. Mater., 2022, 34, 2203617 CrossRef CAS.
- M. Q. Peng, K. Shin, L. X. Jiang, Y. Jin, K. Zeng, X. L. Zhou and Y. B. Tang, Alloy-type anodes for high-performance rechargeable batteries, Angew. Chem., Int. Ed., 2022, 61, e202206770 CrossRef CAS PubMed.
- X. W. Zhou, T. Y. Li, Y. Cui, M. L. Meyerson, J. A. Weeks, C. B. Mullins, Y. Jin, Y. Z. Liu and L. K. Zhu, In situ and operando morphology study of germanium-selenium alloy anode for lithium-ion batteries, ACS Appl. Energy Mater., 2020, 3, 6115–6120 CrossRef CAS.
- M. Chen, P. Xiao, K. Yang, B. X. Dong, D. Xu, C. Y. Yan, X. J. Liu, J. T. Zai, C. J. Low and X. F. Qian, Sn anodes protected by intermetallic FeSn2 layers for long-lifespan sodium-ion batteries with high initial coulombic efficiency of 93.8%, Angew. Chem., Int. Ed., 2023, 62, e202219177 CrossRef CAS.
- H. Zhang, K. Tao, X. Zeng, C. Chen, Y. Zhu, T. Han, J. Li and J. Liu, Rationally engineering a binary SnS0.5Se0.5/carbon nest-coated Si nanosphere for a high-performance lithium-ion battery anode, Chem. Sci., 2024, 15, 15769–15775 RSC.
- M. F. Hassan, M. M. Rahman, Z. Guo, Z. Chen and H. Liu, SnO2-NiO-C nanocomposite as a high capacity anode material for lithium-ion batteries, J. Mater. Chem., 2010, 20, 9707–9712 RSC.
- Y. Li, C. Ou, J. Zhu, Z. Liu, J. Yu, W. Li, H. Zhang, Q. Zhang and Z. Guo, Ultrahigh and durable volumetric lithium/sodium storage enabled by a highly dense graphene-encapsulated nitrogen-doped carbon@Sn compact monolith, Nano Lett., 2020, 20, 2034–2046 CrossRef CAS PubMed.
- K. Duan, J. Ning, L. Zhou, S. Wang, Q. Wang, J. Liu and Z. Guo, Synergistic inorganic-organic dual-additive electrolytes enable practical high-voltage lithium-ion batteries, ACS Appl. Mater. Interfaces, 2022, 14, 10447–10456 CrossRef CAS.
- L. Tan, X. X. Lan, J. W. Chen, H. Y. Zhang, R. Z. Hu and M. Zhu, LiF-induced stable solid electrolyte interphase for a wide temperature SnO2-based anode extensible to -50 °C, Adv. Energy Mater., 2021, 11, 2101855 CrossRef CAS.
- Y. Z. Jiang, Y. Li, P. Zhou, Z. Y. Lan, Y. H. Lu, C. Wu and M. Yan, Ultrafast, highly reversible, and cycle-stable lithium storage boosted by pseudocapacitance in Sn-based alloying anodes, Adv. Mater., 2017, 29, 1606499 CrossRef.
- S. Park, S. Kim, J.-A. Lee, M. Ue and N.-S. Choi, Liquid electrolyte chemistries for solid electrolyte interphase construction on silicon and lithium-metal anodes, Chem. Sci., 2023, 14, 9996–10024 RSC.
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Li-ion battery materials: present and future, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- L. Zhang, H. B. Wu and X. W. Lou, Growth of SnO2 nanosheet arrays on various conductive substrates as integrated electrodes for lithium-ion batteries, Mater. Horiz., 2014, 1, 133–138 RSC.
- R. Z. Hu, D. C. Chen, G. Waller, Y. P. Ouyang, Y. Chen, B. T. Zhao, B. Rainwater, C. H. Yang, M. Zhu and M. L. Liu, Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: the effect of nanostructure on high initial reversible capacity, Energy Environ. Sci., 2016, 9, 595–603 RSC.
- L. Sun, Y. X. Liu, J. Wu, R. Shao, R. Y. Jiang, Z. X. Tie and Z. Jin, A review on recent advances for boosting initial coulombic efficiency of silicon anodic lithium ion batteries, Small, 2022, 18, 2102894 CrossRef CAS.
- Y. H. Wan, K. M. Song, W. H. Chen, C. D. Qin, X. X. Zhang, J. Y. Zhang, H. L. Dai, Z. Hu, P. F. Yan, C. T. Liu, S. H. Sun, S. L. Chou and C. Y. Shen, Ultra-high initial coulombic efficiency induced by interface engineering enables rapid, stable sodium storage, Angew. Chem., Int. Ed., 2021, 60, 11481–11486 CrossRef CAS PubMed.
- H. J. Ying, T. T. Yang, P. F. Huang, Z. Zhang, S. L. Zhang, Z. H. Zhang and W. Q. Han, Facile synthesis of hybrid anodes with enhanced lithium-storage performance realized by a “Synergistic Effect”, ACS Appl. Mater. Interfaces, 2022, 14, 35769–35779 CrossRef CAS PubMed.
- J. S. Corsi, S. S. Welborn, E. A. Stach and E. Detsi, Insights into the degradation mechanism of nanoporous alloy-type Li-ion battery anodes, ACS Energy Lett., 2021, 6, 1749–1756 CrossRef CAS.
- L. Zhang, J. Pu, Y. H. Jiang, Z. H. Shen, J. C. Li, J. Y. Liu, H. X. Ma, J. J. Niu and H. G. Zhang, Low interface energies tune the electrochemical reversibility of tin oxide composite nanoframes as lithium-ion battery anodes, ACS Appl. Mater. Interfaces, 2018, 10, 36892–36901 CrossRef CAS.
- J. Y. Wang, L. Liao, H. R. Lee, F. F. Shi, W. Huang, J. Zhao, A. Pei, J. Tang, X. L. Zheng, W. Chen and Y. Cui, Surface-engineered mesoporous silicon microparticles as high-Coulombic-efficiency anodes for lithium-ion batteries, Nano Energy, 2019, 61, 404–410 CrossRef CAS.
- W. Li, Y. Li, J.-H. Wang, S. Huang, A. Chen, L. Yang, J. Chen, L. He, W. K. Pang, L. Thomsen, B. Cowie, P. Xiong, Y. Zhou, G. Jang, D. H. Min, J. S. Byun, L. Xu, J.-Q. Huang, K. C. Roh, S. H. Kang, M. Liu, X. Duan and H. S. Park, Generic synthesis of high-entropy phosphides for fast and stable Li-ion storage, Energy Environ. Sci., 2024, 17, 5387–5398 RSC.
- W. Li, J.-H. Wang, Y. Li, H. Hsueh, X. Liu, Y. Zhao, S. Huang, X. Li, H.-M. Cheng, X. Duan and H. S. Park, Element Screening of High-Entropy Silicon Anodes for Superior Li-Storage Performance of Li-Ion Batteries, J. Am. Chem. Soc., 2024, 146, 21320–21334 CrossRef.
- Y. Li, L. Zhang, H.-Y. Yen, Y. Zhou, G. Jang, S. Yuan, J.-H. Wang, P. Xiong, M. Liu, H. S. Park and W. Li, Single-Phase Ternary Compounds with a Disordered Lattice and Liquid Metal Phase for High-Performance Li-Ion Battery Anodes, Nano-Micro Lett., 2023, 15, 63 CrossRef.
- Y. Li, S. L. Yu, T. Z. Yuan, M. Yan and Y. Z. Jiang, Rational design of metal oxide nanocomposite anodes for advanced lithium ion batteries, J. Power Sources, 2015, 282, 1–8 CrossRef CAS.
- X. H. Wu, W. W. Wu, Y. Zhou, X. S. Huang, W. Chen and Q. Wang, Synthesis and electrochemical performance of SnO2-Fe2O3 composite as an anode material for Na-ion and Li-ion batteries, Powder Technol., 2015, 280, 119–123 CrossRef CAS.
- C. X. Hua, X. P. Fang, Z. X. Wang and L. Q. Chen, Transition-metal-catalyzed oxidation of metallic Sn in NiO/SnO2 nanocomposite, Chem.–Eur. J., 2014, 20, 5487–5491 CrossRef CAS.
- J. Choi, W. S. Kim and S. H. Hong, Highly stable SnO2-Fe2O3-C hollow spheres for reversible lithium storage with extremely long cycle life, Nanoscale, 2018, 10, 4370–4376 RSC.
- R. Z. Hu, Y. P. Ouyang, T. Liang, H. Wang, J. Liu, J. Chen, C. H. Yang, L. C. Yang and M. Zhu, Stabilizing the nanostructure of SnO2 anodes by transition metals: a route to achieve high initial coulombic efficiency and stable capacities for lithium storage, Adv. Mater., 2017, 29, 1605006 CrossRef PubMed.
- W. Wang, J. W. Qin, Z. G. Yin and M. H. Cao, Achieving fully reversible conversion in MoO3 for lithium ion batteries by rational introduction of CoMoO4, ACS Nano, 2016, 10, 10106–10116 CrossRef CAS.
- C. H. Chen, P. J. Perdomo, M. Fernandez, A. Barbeito and C. L. Wang, Porous NiO/graphene composite thin films as high performance anodes for lithium-ion batteries, J. Energy Storage, 2016, 8, 198–204 CrossRef.
- Y. Z. Jiang, Y. Li, P. Zhou, S. L. Yu, W. P. Sun and S. X. Dou, Enhanced reaction kinetics and structure integrity of Ni/SnO2 nanocluster toward high-performance lithium storage, ACS Appl. Mater. Interfaces, 2015, 7, 26367–26373 CrossRef CAS.
- C. Kim, J. W. Jung, K. R. Yoon, D. Y. Youn, S. Park and I. D. Kim, A high-capacity and long-cycle-life lithium ion battery anode architecture: silver nanoparticle-decorated SnO2/NiO nanotubes, ACS Nano, 2016, 10, 11317–11326 CrossRef CAS PubMed.
- X. X. Lan, J. Cui, X. F. Zhang, R. Z. Hu, L. Tan, J. Y. He, H. P. Zhang, X. Y. Xiong, X. F. Yang, S. Q. Wu and M. Zhu, Boosting eeversibility and stability of Li storage in SnO2-Mo multilayers: introduction of interfacial oxygen redistribution, Adv. Mater., 2022, 34, 2106366 CrossRef CAS.
- T. Meng, J. W. Qin, Z. Yang, L. R. Zheng and M. H. Cao, Significantly improved Li-ion diffusion kinetics and reversibility of Li2O in a MoO2 anode: the effects of oxygen vacancy-induced local charge distribution and metal catalysis on lithium storage, J. Mater. Chem. A, 2019, 7, 17570–17580 RSC.
- H. Kim, H. Kim, S. Muhammad, J. H. Um, M. Shah, P. J. Yoo and W. S. Yoon, Catalytic effect of reduced graphene oxide on facilitating reversible conversion reaction in SnO2 for next-generation Li rechargeable batteries, J. Power Sources, 2020, 446, 227321 CrossRef CAS.
- X. Li, L. Zhang, H. Liu, Q. Li and Y. Hou, Magnetic measurements applied to energy storage, Adv. Energy Mater., 2023, 13, 2300927 CrossRef CAS.
- Z. Zhao, W. Ye, F. Zhang, Y. Pan, Z. Zhuo, F. Zou, X. Xu, X. Sang, W. Song, Y. Zhao, H. Li, K. Wang, C. Lin, H. Hu, Q. Li, W. Yang and Q. Li, Revealing the effect of LiOH on forming a SEI using a Co magnetic “probe”, Chem. Sci., 2023, 14, 12219–12230 RSC.
- H. Liu, F. Zou, S. Liao, Y. Pan, Z. Zhao, F. Gu, X. Xu, X. Sang, Y. Han, Z. Bu, L. Qin, Y. Wang, G. Chen, M. Ruan, Q. Li, H. Hu and Q. Li, Reinterpreting the intercalation-conversion mechanism of FeP anodes in lithium/sodium-ion batteries from evolution of the magnetic phase, J. Phys. Chem. Lett., 2024, 15, 4694–4704 CrossRef CAS PubMed.
- F. C. Gu, L. Q. Zhang, Z. H. Li, J. Zhang, Y. Y. Pan, Q. H. Li, H. S. Li, Y. F. Qin and Q. Li, A comparative study of electrochemical and electrostatic doping modulation of magnetism in Fe3O4 via ultracapacitor structure, J. Phys.: Condens. Matter, 2022, 34, 455802 CrossRef CAS.
- Z. H. Li, Y. C. Zhang, X. K. Li, F. C. Gu, L. Q. Zhang, H. J. Liu, Q. T. Xia, Q. H. Li, W. N. Ye, C. Ge, H. S. Li, H. Hu, S. D. Li, Y. Z. Long, S. S. Yan, G. X. Miao and Q. Li, Reacquainting the electrochemical conversion mechanism of FeS2 sodium-ion batteries by operando magnetometry, J. Am. Chem. Soc., 2021, 143, 12800–12808 CrossRef CAS.
- Q. Li, H. S. Li, Q. T. Xia, Z. Q. Hu, Y. Zhu, S. S. Yan, C. Ge, Q. H. Zhang, X. X. Wang, X. T. Shang, S. T. Fan, Y. Z. Long, L. Gu, G. X. Miao, G. H. Yu and J. S. Moodera, Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry, Nat. Mater., 2021, 20, 76–83 CrossRef CAS PubMed.
- F. L. Zhang, Z. H. Li, Q. T. Xia, Q. H. Zhang, C. Ge, Y. X. Chen, X. K. Li, L. Q. Zhang, K. Wang, H. S. Li, L. Gu, S. S. Yan, G. X. Miao and Q. Li, Li-ionic control of magnetism through spin capacitance and conversion, Matter, 2021, 4, 3605–3620 CrossRef CAS.
- X. K. Li, J. Su, Z. H. Li, Z. Q. Zhao, F. L. Zhang, L. Q. Zhang, W. N. Ye, Q. H. Li, K. Wang, X. Wang, H. S. Li, H. Hu, S. S. Yan, G. X. Miao and Q. Li, Revealing interfacial space charge storage of Li+/Na+/K+ by operando magnetometry, Sci. Bull., 2022, 67, 1145–1153 CrossRef CAS.
- H. S. Li, Z. Q. Hu, Q. T. Xia, H. Zhang, Z. H. Li, H. Z. Wang, X. K. Li, F. K. Zuo, F. L. Zhang, X. X. Wang, W. N. Ye, Q. H. Li, Y. Z. Long, Q. Li, S. S. Yan, X. S. Liu, X. G. Zhang, G. H. Yu and G. X. Miao, Operando magnetometry probing the charge storage mechanism of CoO lithium-ion batteries, Adv. Mater., 2021, 33, 2006629 CrossRef CAS PubMed.
- S. Fang, D. Bresser and S. Passerini, Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries, Adv. Energy Mater., 2020, 10, 1902485 CrossRef CAS.
- S. Y. Lee, K. Y. Park, W. S. Kim, S. Yoon, S. H. Hong, K. Kang and M. Kim, Unveiling origin of additional capacity of SnO2 anode in lithium-ion batteries by realistic ex situ TEM analysis, Nano Energy, 2016, 19, 234–245 CrossRef CAS.
- X. S. Zhou, L. J. Wan and Y. G. Guo, Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries, Adv. Mater., 2013, 25, 2152–2157 CrossRef CAS.
- T. Wu, Y. H. Cui, K. Y. Wei, C. Z. Lai, Y. Zhao, S. Ni, Y. J. Chen, X. Gao, Y. X. Cui and C. L. Li, Catalysis of nickel nanodomains on Li-F dissociation for high-capacity fluoride cathodes with prior delithiation ability, Nano Energy, 2022, 103, 107843 CrossRef CAS.
- W. Dong, J. Xu, C. Wang, Y. Lu, X. Liu, X. Wang, X. Yuan, Z. Wang, T. Lin, M. Sui, I. W. Chen and F. Huang, A robust and conductive black tin oxide nanostructure makes efficient lithium-ion batteries possible, Adv. Mater., 2017, 29, 1700136 CrossRef.
- C. Hu, L. Chen, Y. J. Hu, A. P. Chen, L. Chen, H. Jiang and C. Z. Li, Optimizing SnO2-x/Fe2O3 hetero-nanocrystals toward rapid and highly reversible lithium storage, Small, 2021, 17, 2103532 CrossRef CAS.
- Q. Y. Xia, F. Zan, Q. Y. Zhang, W. Liu, Q. C. H. Li, Y. He, J. Y. Hua, J. H. Liu, J. Xu, J. S. Wang, C. Z. Wu and H. Xia, All-solid-state thin film lithium/lithium-ion microbatteries for powering the internet of things, Adv. Mater., 2023, 35, 2200538 CrossRef CAS.
- S. H. Zheng, H. Wang, P. Das, Y. Zhang, Y. X. Cao, J. X. Ma, S. Z. Liu and Z. S. Wu, Multitasking MXene inks enable high-performance printable microelectrochemical energy storage devices for all-flexible self-powered integrated systems, Adv. Mater., 2021, 33, 2005449 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06323f |
| ‡ Co-first-author. |
| This journal is © The Royal Society of Chemistry 2025 |