Open Access Article
Open Access ArticleExploring recent advances and synthesis strategies in conductive polymers and their composites in supercapacitor systems: a comprehensive review
Ahmed Aldulaimia,
Shakir Mahmood Saeedb,
Soumya V. Menon *c,
Ruya Yilmaz Saberd,
Subhashree Raye,
Karthikeyan Jayabalanf,
Aashna Sinhag,
Renu Sharmah,
Waam Mohammed Taher*i and
Mariem Alwanj
*c,
Ruya Yilmaz Saberd,
Subhashree Raye,
Karthikeyan Jayabalanf,
Aashna Sinhag,
Renu Sharmah,
Waam Mohammed Taher*i and
Mariem Alwanj
aDepartment of Pharmacy, Al-Zahrawi University, Karbala, Iraq
bCollege of Pharmacy, Alnoor University, Nineveh, Iraq
cDepartment of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to be University), Bangalore, Karnataka, India. E-mail: v.menon.in@gmail.com; v.soumya@jainuniversity.ac.in
dMedical Device Technology Engineering, Al-Turath University, Al Mansour, Baghdad 10013, Iraq
eDepartment of Biochemistry, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha-751003, India
fDepartment of Chemistry, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India
gSchool of Applied and Life Sciences, Division of Research and Innovation, Uttaranchal University, Dehradun, Uttarakhand, India
hDepartment of Chemistry, University Institute of Sciences, Chandigarh University, Mohali, Punjab, India
iCollege of Nursing, National University of Science and Technology, Dhi Qar, Iraq. E-mail: waam_mohammed@nust.edu.iq
jPharmacy College, Al-Farahidi University, Iraq
First published on 19th November 2025
Abstract
The expansion of industry has led to increased environmental pollution and irreparable damage to the ecosystem. Supercapacitors (hybrid capacitors) have been introduced as renewable energy sources with high power density and energy density. Conducting polymers were introduced as pseudocapacitor electroactive materials. Conducting polymers have advantages, including high stability during alternating charge–discharge cycles, high conductivity, and corrosion resistance. Preparing conductive polymer-based composites with other electroactive materials (MOFs, TMS, C, TMO, and MXene) due to synergistic effects leads to the achievement of high-performance hybrid electrode materials. The electrochemical performance of these composites varied depending on the type of electroactive materials (MOFs, TMS, C, TMO, and MXene), the type of conductive polymer, and the synthesis method. In this study, an attempt was made to provide a basis for researchers to conduct innovative studies by reviewing the synthesis methods, supercapacitor studies conducted on various conductive polymers, and composites based on conductive polymers.
1. Introduction
The gases produced by thermal power plants and fossil fuel combustion have caused irreparable environmental damage.1–3 Therefore, renewable energy sources are essential for the energy supply for industry.3–5 Energy storage devices, including supercapacitors,6 fuel cells,7,8 and various types of lithium batteries9 have been investigated as renewable energy sources over the last decade.10,11 Supercapacitor devices are innovative energy storage systems that operate in a manner intermediate between a battery and a capacitor. In other words, supercapacitors offer the advantages of both a battery and a capacitor simultaneously.12–14 Conventional capacitors have lower energy density than batteries.15–19 Supercapacitors overcome the limitations of other energy storage systems (batteries and capacitors) by combining the characteristics of a battery and a capacitor.20,21 Supercapacitor devices consist of three main components: the current collector,22 electrolyte,23 and electrode material.24 Intelligent selection of each supercapacitor component (current collector, electrolyte, and electrode materials) plays a role in the final behavior of efficient supercapacitor devices.18,25,26 The type of electrode material is influential in determining the type of supercapacitor performance (pseudocapacitor, hybrid capacitors, and EDLC).27–30 Carbon materials, including GO,31 rGO,32 CNT,33 AC,34 and aerogels,35 have EDLC behavior. Metal oxides,36 metal sulfides,37 quantum dots,38 and conducting polymers have pseudocapacitor behavior.39 Pseudocapacitor electrode materials have lower power density than EDLC electrode materials.40,41 To overcome the limitations of pseudocapacitors and EDLCs, the hybrid capacitor device was designed and fabricated, which simultaneously has high energy density and power density.42,43 Conductive polymers, as pseudocapacitors electrode materials, have metal conductive properties and polymer properties.44 Researchers prepared bicomponent or multicomponent composites of CP with other materials (MOFs, TMS, C, and TMO) to make a hybrid supercapacitor device.45–47 Conductive polymers as electroactive materials have limitations, including low stability during the GCD method and volume expansion and contraction, which can be overcome by composites with other electroactive materials, including GO, CNT, etc.48–51 Also, other composites based on conductive polymers with metal oxides or sulfides, MOFs, and quantum dots have been synthesized and reported as two-component or multi-component composites to achieve hybrid electrode materials.52–54 In this study, the performance of conductive polymers and their composites was reviewed as electroactive materials in supercapacitors. In other words, supercapacitor studies conducted on conductive polymer-based composites, such as composites of conductive polymer with carbon materials, MOFs, metal oxides, metal sulfides, and MXene, were reported in this review study. The effect of composite components (composites prepared from conductive polymers with MOF, metal oxides, metal sulfides, carbon materials, and quantum dots) in achieving efficient hybrid electrode materials was discussed and investigated by examining various synthesis methods and other parameters. Therefore, this review provides researchers with a broad perspective for conducting innovative research.2. Energy storage devices
Supercapacitors were referenced by General Electric in 1957, followed by devices patented by SOHIO in 1960–1970. Further developments were reported by NEC, Panasonic (Japan), and other companies in 1996.55–57 Therefore, the supercapacitor has been investigated in many research studies as an efficient and innovative energy storage technology.58 Supercapacitors, as an energy storage system with long lifespan, high power, and excellent performance over a wide temperature range, have many applications in various industries.59 Supercapacitors were used in the electric vehicle, forklift, and crane industries. Also, supercapacitors have been utilized as energy storage sources in various applications, including wind turbines, electronic devices (such as mobile phones and tablets),60 solar energy systems,61 medical devices,62,63 camera flashes,64 and UPS systems.65 Supercapacitor devices possess performance between capacitors and batteries. According to the Ragone diagram, batteries have a higher energy density than capacitors; however, batteries have a lower power density. The performance of batteries in industry is limited due to the low stability of charge and discharge cycles, resource limitations (lithium salt), and expensive storage conditions.66 The classification of supercapacitors into three main groups corresponds to the electrode material.672.1. Electric double layer capacitor (EDLC)
In EDLCs, the specific capacitance corresponds to the storage of electrostatic charge in the interface of the electrode and electrolyte. The performance of EDLCs corresponds to the specific surface area of the electrode materials and has a low energy density.68 EDLCs consist of carbon material types, including activated carbon,69 CNT,70 GO,6 rGO, and MXene.71 The structure of EDLCs was first proposed by Helmholtz as shown in Fig. 1a.72 According to the Helmholtz model, two layers with positive and negative charges were placed between the electrolyte solvent and the electrode, which was similar to a capacitor. The Helmholtz model was then modified by Goey and Chiman in 1910 and 1913, respectively. According to the model proposed by Gouy and Chapman, positive and negative ions were dispersed in the solvent (Fig. 1b). Finally, Stern combined the Gouy and Chapman model with the Helmholtz as shown in Fig. 1c. In Stern's model, ions with opposite charges are located in a region H.732.2. Pseudocapacitors
Pseudocapacitors were introduced to overcome the limitations of EDLC and mass transfer batteries. Pseudocapacitors have higher energy density than EDLCs due to the faradaic reaction.40 Conducting polymers, TMO, TMS, and inorganic quantum dots were introduced as pseudocapacitors. However, pseudocapacitors as electrode material have limitations due to low power density and stability.742.3. Hybrid capacitors
Hybrid devices were developed to achieve an efficient supercapacitor with unique benefits. Hybrid capacitors were a combination of pseudocapacitor materials and EDLC materials, which are designed and manufactured in three main categories: the first category was composites, which include pseudocapacitor/EDLC composites.75,76 The second category consisted of asymmetric devices made from pseudocapacitor materials and EDLC materials.77 The third category is the battery type, which consists of a battery electrode and a supercapacitor electrode.78,793. Conductive polymers
Conducting polymers have the properties of conventional polymers and the conductivity of metals, simultaneously. Conductive polymers have high electrical conductivity due to the π-electron delocalization in conjugated backbones. The type of synthesis method affects the conductivity of these polymers.803.1. Polyacetylene
The first reports on polyacetylene (PA) were published in 1958–1970.81 Polyacetylene (PA) has repeating units (C2H2)n.82 Polyacetylene was introduced as a semiconducting polymer. The conductivity of polyacetylene was investigated by Hideki Shirakawa, Alan MacDiarmid, and Alan Heeger, who received the Nobel Prize in 2000 for achieving these results. Heeger, MacDiarmid, and Shirakawa reported that the conductivity of polyacetylene increases with the addition of an oxidizing agent or a reducing agent.83 The p-doped polymer was synthesized by adding an oxidizing agent (I2), and the n-doped polymer was synthesized by adding a reducing agent (sodium naphthalenide). The conductivity of polyacetylene increased by 11 orders with the addition of an oxidizing agent (I2). However, the stability of polyacetylene decreased with the addition of an oxidizing agent or a reducing agent. Therefore, the preparation of highly conductive and stable polyacetylene has been a challenge for researchers. In recent studies, various derivatives of polyacetylene have been synthesized by different methods.84,853.2. Polythiophene
Polythiophene is a conjugated polymer composed of repeating thiophene units. Polythiophene has unique properties that make it attractive for various applications such as supercapacitors,86 sensors,87,88 non-linear optics,89 photoresists,90 solar cells,91 etc. Polythiophene has high heat resistance, making it suitable for industrial applications. Polythiophene is a soluble polymer with high conductivity; therefore, it has been investigated as an electrode material in many studies. The electrochemical performance of polythiophene can be controlled by doping. Numerous structural derivatives of this polymer have been reported with facile synthesis methods.92 Liu et al. designed and synthesized various morphologies of polythiophene by oxidative polymerization. The morphology of polythiophene was investigated by controlling different synthesis conditions, including reducing agent concentration, catalyst concentration, and oxidizing agent concentration. The results showed that morphologies of spherical, filamentous, and ribbon were obtained by controlling different synthesis conditions.93 Arvas et al. synthesized polythiophene with zigzag morphology using the electropolymerization method. Different dopants were used to modify the morphology of polythiophene, and the use of bromothymol blue dopant resulted in a zigzag morphology. The zigzag morphology increased the specific surface area of polythiophene. Polythiophene and zigzag polythiophene had specific surface areas of 32.629 m2 g−1 and 13.812 m2 g−1, respectively. The zigzag polythiophene electrode material showed a specific capacitance of 443.5 F g−1 at 5 mV s−1. Polythiophenes exist in n-doped and p-doped forms. The specific and conductivity of polythiophene in n-doped form are lower than in p-doped form. Therefore, polythiophenes act as cathodes. N-doped polythiophenes have low stability in the presence of oxygen, so polythiophene derivatives were synthesized to overcome this limitation.94 Undoped polythiophene acts as an insulator or semiconductor. Therefore, doped polythiophene acts as a conductive electrode material. Doped polythiophenes participate in oxidation and reduction reactions. Various dopings are performed on the polythiophene surface, including n-type doping, p-type doping, and polarons/bipolarons. These carriers delocalize the charge on the polythiophene chain, as shown in Fig. 2. In n- and p-type doping, electrons migrate from LUMO and HOMO to the polythiophene skeleton, respectively.95 | ||
| Fig. 2 The electronic band and chemical structures of polythiophene (PT) with (a) p-type doping and (b) n-type doping. | ||
3.3. Polyaniline
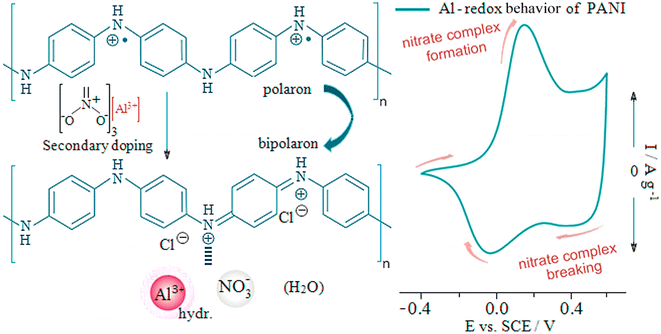
Polyaniline is the perfect electrode material for a supercapacitor, which is synthesized through chemical or electrochemical oxidation of aniline. Electrochemical polymerization works faster than chemical polymerization.96 The electrochemical polymerization synthesis method was oxidant-free and environmentally friendly. Polyaniline exists in three states: reduced (leucoemeraldine), semi-oxidized (emeraldine), and oxidized (pernigraniline). The only electrically conductive form is the protonated form of the base of ameridine, the ameridine salt. The ameridine (E) protonated is produced using the oxidative polymerization of aniline in aqueous acids. Basic sites, including amine and imine in the polymer structure, facilitate protonation of ameridine in acidic solvents. Polyaniline exhibits various physical and chemical properties by applying a potential (−0.2 to +1.0 V). Polyaniline changes from pale yellow leucoemeraldine (L) to the green emeraldine salt/base (E) and finally to the purple pernigraniline form (P). Polyaniline obtained from these three oxidation states performs well in supercapacitor devices. The type of polyaniline synthesis method affects its final morphology. Polyaniline synthesized by electrochemical methods often has a granule-like morphology.97 Polyaniline has shown excellent electrochemical properties, good stability, and ease of synthesis. Therefore, polyaniline has been widely used in the fabrication of supercapacitors. However, polyaniline has limitations such as conductivity variation, processing challenges, mechanical properties, sensitivity to dopants, and environmental contamination. Therefore, the optimal PANI can be determined by selecting the appropriate dopant, the amounts of aniline and oxidant monomer, and other preparation conditions (temperature and time).98 Therefore, polyaniline/other electroactive material composites have been introduced as promising electrode materials. A successful method for improving the performance of polyaniline is to blend it with other polymers that have good mechanical properties. The use of PANI as a conductive filler in other polymers (matrices) has attracted attention due to improved processability and relatively good mechanical properties.99 Polyaniline requires protons for charging and discharging. Therefore, polyaniline has high performance in an acidic electrolyte. The charge storage mechanism of polyaniline in the aqueous aluminum solution is shown in Fig. 3. First, protons from the acidic solution were adsorbed onto nitrogen, forming a positive charge on the polyaniline chain. Then, the anions in the solution were adsorbed onto the polymer to neutralize the positive charge. The diffusion and outflow of ions caused the charge storage in the polyaniline.1003.4. Polypyrrole
Polypyrrole was used as a conductive polymer with environmental stability and high conductivity in energy storage systems and various sensors.101–103 Polypyrrole is a biocompatible heterocyclic polymer.104 Polypyrrole exists as a conductive polymer with a positive charge in its oxidized form. Excessive oxidation of the nitrogen group reduces the polypyrrole's electroactive properties. Polypyrrole electrode materials have electroactive properties only in organic and aqueous solvents. Polypyrrole is synthesized using the polymerization of pyrrole units through various methods. The oldest method of polypyrrole synthesis is the oxidative polymerization of pyrrole in aqueous and organic solvents in the presence of oxidizing agents such as ammonium persulfate.105,106 Polypyrrole synthesis methods include polymerization of photo, vapor phase, ultrasound, and microemulsion. The chemical oxidative method was used in industry despite numerous synthesis methods.107,108 Unlike polythiophene, polypyrrole is not n-doped, so polypyrrole only acts as a cathode. Dense growth of polypyrrole limits access to internal sites of polypyrrole, thus reducing specific capacitance. The charge storage mechanism of polypyrrole occurs in several steps (Fig. 4). Undoped polypyrrole has low conductivity. When polypyrrole is doped with P-type materials, oxidation occurs, which converts the polypyrrole from benzoide to quinoid. Further oxidation accelerates the conversion of the benzoide form to quinoid due to the removal of the p-electron.953.5. Poly(3,4-ethylenedioxythiophene)
Poly(3,4-ethylenedioxythiophene) is known as a bioelectronic conductive polymer.109 There are two different synthesis routes for poly(3,4-ethylenedioxythiophene). The first synthesis route is linear, multi-step, and lengthy, but the second synthesis route involves the addition of a thiophene ring, resulting in a shorter synthesis time; however, it is not economically viable. The advantages of poly(3,4-ethylenedioxythiophene), including optical transparency, biocompatibility, and conductivity, have led to numerous applications in solar cells,110 glass,111 light-emitting diodes,112,113 textile fibers,114 electroluminescence,115 and cathode material in capacitors.116,1173.6. Poly(phenylene vinylene)
Poly(phenylene vinylene) has luminescent properties and is a diamagnetic material with poor conductivity.118,119 The conductivity of this polymer increases by doping with acids, sodium, potassium, and iodine. However, it has poor stability. Poly(phenylene vinylene) has numerous applications, including photovoltaics,120,121 sensors,122 and medical applications.123,1243.7. Polyphenylene and polyparaphenylene
Poly(para-phenylene) is a linear polymer and has poor conductivity. This polymer can be made conductive by doping. Direct and pre-material methods for the synthesis of poly(para-phenylene) were reported.125,126 The synthesis mechanism of the direct method is based on the Scholl method. The direct synthesis method results in powder form oligomers that are not processed. In the precursor method, poly(para-phenylene) derivatives are synthesized from soluble polymers. Microbial oxidative methods or chemical methods were used to synthesize precursors. For example, microbial oxidative benzene was used to prepare precursors for the synthesis of poly(para-phenylene).127 In the chemical method, transition metal catalysts were used to prepare precursors, which had high yields but abundant impurities.128 Chemical synthesis of precursors with cis and trans configurations was used to create poly(para-phenylene) for use in electronic devices.129 The main application of poly(para-phenylene) has been in the aerospace and medical industries.130,1314. The electrochemical energy-storage performance of composites based on CP
Conductive polymers such as PANI,132 PPy,133 and polythiophene derivatives have been recognized as effective high-performance electrode materials.134 However, these electrode materials have limitations, including volume changes during repeated charging/discharging.135 Other electroactive materials, such as various carbon materials (CNT, GO, rGO, CQD, and AC), MOF, and TMO or TMS have been used in supercapacitor systems. Conducting polymers or other electroactive materials alone have a limited role as electrode materials in supercapacitor systems. Therefore, by preparing two-component or multi-component composites of conducting polymers with other electroactive materials, efficient hybrid electrode materials can be achieved.136,1374.1. The electrochemical energy-storage performance of carbon materials/CP composites
Carbon materials (CNT, GO, and AC) are electrode materials with EDLC behavior. Researchers synthesized composites of conductive polymers with other carbon materials to achieve efficient supercapacitor devices, which have high performance as hybrid electrode materials. | ||
| Fig. 5 (a) A general scheme for the preparation of PANI–H2SO4 solution and SWCNTs/PANI composite. (b) The SEM of SWCNTs/PANI and the SWCNTs film. | ||
Polypyrrole demonstrated superior performance compared to polyaniline in composites of CNT and CP as an electrode material. The tubular structure of carbon nanotubes causes them to aggregate and limits their electrochemical performance. By converting MWCNTs into UzMWCNTs, the electrochemical properties are improved. In other words, by converting MWCNTs into UzMWCNTs, it is possible to achieve a higher specific surface area.140,141 Therefore, UzMWCNTs are obtained by transverse and longitudinal modification of multi-walled nanotubes, and can simultaneously exhibit properties of graphene and nanotubes. There are various ways for synthesizing UzMWCNTs, including solid-state reaction, ball milling, electrochemical, etc.142 Theresa et al. used the Hammer method to convert MWCNTs into UzMWCNTs. Then, a 5 wt% solution of UzMWCNTs was prepared and subjected to ultrasonic waves. PPy was mixed with the solution at 0 °C, and the composite of UzMWCNTs and polypyrrole was synthesized after 24 h of stirring. The highly conductive UzMWCNT/PPy hybrid electrode material showed 944 F g−1 at 1 A g−1.143 The morphology of polypyrrole and carbon nanotube-based composites affects their electrochemical behavior. Lin et al. synthesized polypyrrole/carbon nanotubes 3D composites with different morphologies using the electrodeposition method. Different nanocomposites were synthesized by varying the type of polypyrrole deposition method on carbon nanotubes. Polypyrrole with morphologies of nanoparticles, nanowires, and vertical nanowires was deposited on the nanotubes (Fig. 6). Electrochemical analysis was performed for three flexible supercapacitors prepared from these composites. The results confirmed the high conductivity of the flexible supercapacitor prepared from polypyrrole with vertical nanowire arrangement. PPy nanowire/CNTF provided a higher specific surface area than PPy nanoparticles/CNTF due to the wire morphology. The PPy VANA-/CNTF provided higher accessibility of electrolytic ions than the PPy nanowire/CNTF due to porous morphology. Therefore, the conductivity, specific capacitance, and coulombic efficiency of PPy VANA-/CNTF were higher than PPy nanowire/CNTF and PPy nanoparticles/CNTF. The flexible supercapacitor device prepared from the PPy VANA-/CNTF composite recorded the highest specific capacitance (178.14 F g−1 at 0.4 A g−1).144
Mehare et al. synthesized CQD derived from sucrose with different sucrose ratios (10, 15, 25, 30). Then, the composite based on polyaniline and CQD was synthesized through the electrodeposition. According to electrochemical analyses, the composite synthesized from carbon quantum dots with a ratio of 25 has high specific capacitance, high stability, and lower resistance.164 Zhang et al. designed a polyaniline/carbon-based quantum dots/carbon nanofibers electrode using a hierarchical method. The 3D structure of this electrode accelerated the movement of ions. According to the analysis results of electrochemical and chemical, the functional groups of carbon quantum dots increased conductivity by creating multiple active sites for charge transfer.165 Yıldız et al. synthesized the PANI/N-doped CQD composite through in situ polymerization. Investigation of the electrochemical behavior of the composite, PANI, and N-doped CQD confirmed that the electrolyte transfer rate in the composite was improved compared to polyaniline and N-doped CQD. The composite electrode material exhibited 503 F g−1 (5 A g−1) and 91.9% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), respectively.166 Duraisamy et al. used poly(aniline-co-indole) to prepare a composite based on CP and CQD (hybrid electrospray synthesis method). The asymmetric supercapacitor of poly(aniline-co-indole)/CQD and rGO recorded 26.22 W h kg−1. According to the standard Ragone plot of supercapacitors, the standard energy density of supercapacitors is 0.1–100 W h kg−1. The reported energy density of asymmetric devices with CQD-based electrodes is 26.22 W h kg−1, which corresponds to the standard Ragone plot of supercapacitors (Table 1).167
000 cycles), respectively.166 Duraisamy et al. used poly(aniline-co-indole) to prepare a composite based on CP and CQD (hybrid electrospray synthesis method). The asymmetric supercapacitor of poly(aniline-co-indole)/CQD and rGO recorded 26.22 W h kg−1. According to the standard Ragone plot of supercapacitors, the standard energy density of supercapacitors is 0.1–100 W h kg−1. The reported energy density of asymmetric devices with CQD-based electrodes is 26.22 W h kg−1, which corresponds to the standard Ragone plot of supercapacitors (Table 1).167
| Sample | Stability (cycles) | Specific capacitance (or areal specific capacitance) | Current density (or areal current density) | Reference |
|---|---|---|---|---|
| Polyaniline/single-wall carbon nanotube (SWCNTs) | 99% up to 1000 | 463 F g−1 | 10 mA cm−1 | 168 |
| CNT/polyaniline | 91% up to 1000 | 347 F g−1 | 1.7 A g−1 | 169 |
| Ti3C2 MXene/PANI | 90% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
377 F g−1 | 1 A g−1 | 170 |
| Multi-walled carbon nanotubes (MWCNTs)/PANI | — | 248 F g−1 | 0.5 A g−1 | 171 |
| GNPLs/PTh | 84.9% up to 1500 | 673 F g−1 | 0.25 A g−1 | 172 |
| Poly(3-hexylthiophene) (P3HT)/SWCNTs | 80.5% up to 1000 | 245.8 F g−1 | 0.5 A g−1 | 173 |
| Polythiophene (PTP)–CNT | — | 125 F g−1 | 1 A g−1 | 174 |
| Graphene oxide–polythiophene derivative hybrid nanosheet | 91.86% up to 4000 | 296 F g−1 | 0.3 A g−1 | 175 |
| PPy/CNT | 95% up to 5000 | 211 F g−1 | 0.2 A g−1 | 176 |
| Graphene/Polythiophene | 94.5% up to 1500 | 365 F g−1 | 1 A g−1 | 177 |
| GO@PPy | 90% up to 1000 | 1532 mF cm−1 | 0.88 mA cm−1 | 178 |
| GO@PPy | 76% up to 6000 | 0.23 F cm−1 | 1 mV s−1 | 179 |
| N-CQD/PANI | 74.7% up to 2000 | 498 F g−1 | 1 A g−1 | 180 |
| CQDs/PPy | 85.7% up to 2000 | 308 F g−1 | 1 A g−1 | 181 |
| CQDs–PANI | 78.0% up to 1000 | 738.3 F g−1 | 1 A g−1 | 182 |
4.2. The electrochemical energy-storage performance of metal oxide or sulfide/conductive polymers composites
Metal oxides or sulfides have higher energy density than carbon materials.183 TMO or TMS have low conductivity, limited specific surface area, and low stability. Therefore, to achieve efficient electroactive materials, ternary composites of CP, TMO (or TMS), and C have been synthesized. However, bimetallic oxides and sulfides have higher redox potential, higher conductivity, and stability than monometallic oxides and sulfides. Therefore, binary composites of bimetallic oxides or sulfides and conductive polymers perform well as electroactive materials. Preparing binary composites of CP and bimetallic oxides or sulfides as electroactive material is a more economical and industrially feasible method than ternary composites of CP, C, and TMS (or TMO). Shehzad et al. synthesized composites based on conductive polymer (PANI) and NiCo2O4 by hydrothermal method. The synergistic effect of polyaniline and NiCo2O4 resulted in increased conductivity and stability over cycles. The PANI/NiCo2O4 electrode material maintained 100% stability after 5000 cycles.184 Shanmugavalli et al. synthesized NiCo2O4 and NiCo2O4/PANI by solution combustion and physical blending methods, respectively, to achieve a cost-effective synthesis method. According to chemical analyses, the morphology of NiCo2O4/PANI provided special surfaces for electron transport. According to the results of electrochemical analysis of NiCo2O4 and NiCo2O4/PANI, the specific capacitance doubled with the addition of polyaniline (the specific capacitance of NiCo2O4/PANI was twice that of NiCo2O4).185 Xue et al. first synthesized polyaniline using the in situ polymerization method, then designed and synthesized NiCo2O4/polyaniline composite on nickel foam using the hydrothermal method. Electrochemical analysis showed the synergistic effect of polyaniline and NiCo2O4. The composite electrode material based on NiCo2O4 and polyaniline recorded 3108 F g−1 at areal current density 1 mA cm−2.186 Nandi et al. synthesized composites of polythiophene and NiCo2O4 with different ratios (1–1 and 1–2) by the oxidative polymerization method. The results of the chemical analysis showed the porous matrix of polythiophene on NiCo2O4. According to electrochemical analysis, the NiCo2O4/polythiophene composite with a ratio of 1–1 had higher stability than the ratio of 1–2.187 In another study, composites based on polyaniline and NiFe2O4 with different ratios were synthesized through the in situ polymerization. However, by changing the type of metal oxide, the NiFe2O4/polyaniline composite with a ratio of 2–1 had more optimal supercapacitive behavior as an electroactive material.188 The composite of polyaniline and MnCo2O4 synthesized by polymerization showed 185 F g−1.189 Sui et al. synthesized core–shell structures based on polyaniline and MnCo2O4 by two synthesis steps: electrodeposition and hydrothermal. The conductivity resulting from polyaniline and the high specific surface area of MnCo2O4 led to the achievement of electrode materials with a specific capacitance of 1098 F g−1 at 1 A g−1.190 Varma et al. designed and synthesized composites based on MnCO2O4 and polyaniline with different proportions of polyaniline (0–30%) by the co-precipitation. The electrochemical analyses showed the composite with a 20% polyaniline ratio as the optimal ratio (high conductivity and stability). The optimal composite ratio (20%) showed a specific capacitance of 765 F g−1 at a current density of 0.5 A g−1.191 Chen et al. grew a composite based on polyaniline and NiCo2O4 on carbon tissue in three synthesis steps as shown in Fig. 8. The results of electrochemical analyses for the composite and composite components confirmed the synergistic and enhancing effect of polyaniline. The NiCo2O4/PANI electrode material showed a maximum stability of 99.64% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.192
000 cycles.192
Merlin et al. designed and synthesized a nanocomposite of polyaniline and CuCo2O4 as a quasi-capacitor electrode material, which was synthesized through an in situ polymerization method. Electrochemical analyses of the nanocomposite and polyaniline confirmed the increase in specific capacitance and conductivity with the addition of polyaniline. In other words, the active sites for ion transport increased, and the synergistic effect led to the achievement of an efficient electrode material with stability of 98.5% after 5000 cycles.193 The CuCo2O4 electrode materials have low permeability and low stability, which can be improved by compositing with conductive polymers. In recent studies, the preparation of polypyrrole/CuCo2O4 as electroactive materials was reported to achieve efficient electrode materials with high specific capacity and stability. The CuCo2O4/polypyrrole composite electrode showed 912 F g−1 at 2 A g−1.194 Chen et al. synthesized a composite based on polyaniline and ZnCo2O4 using zinc nitrate, cobalt nitrate, and NH4F precursors using two steps (hydrothermal and in situ polymerization). The asymmetric device of polyaniline/ZnCo2O4 composite and AC showed 66.6 W h kg−1.195 Benyoucef et al. synthesized polyaniline/CuO/ZnO/MnO by the co-precipitation method. The high specific surface area of polyaniline/CuO/ZnO/MnO was confirmed by chemical analysis. Therefore, this composite showed the specific capacitance of 451.4 F g−1 at 5.0 mV s−1 in potassium hydroxide electrolyte solution due to high stability and short electron transport path.196 Iqbal et al. also synthesized ternary, quaternary, and monolayer composites of polyaniline, Pr2O, NiO, and Co3O4 through the co-precipitation method. The specific capacitance of the quartet composite (PANI–NiO, PANI–Co3O4) was twice that of the ternary composite (Pr2O3–NiO–Co3O4) and four times that of the binary composites.197 In addition to composites based on bimetallic oxides and conductive polymers, other studies of composite electrode materials based on monometallic oxides and polymers were reported. For example, Shim et al. grew polyaniline/3D CoO on nickel foam by the electrochemical polymerization, which recorded a 2473 F g−1 at 3 A g−1.198 Metal sulfides or bimetallic sulfides are high-performance quasi-capacitor materials that have better electrochemical behavior than TMO. Sulfur is less electronegative than oxygen, so metal sulfides have excellent electrochemical behavior in conductive composites of CP. Li et al. synthesized a composite of PPy (prepared in an ice bath) and nickel–cobalt bimetallic sulfide through in situ polymerization in two steps. According to the results of chemical analyses, the use of the in situ polymerization synthesis method resulted in homogeneous synthesis of the composite, thus increasing the contact between the electrolyte and the composite electrode materials. The asymmetric device of NiCo bi-metal sulfide/polypyrrole composite and AC recorded 44.5 W h kg−1.199 The ammonium ion supercapacitor has high efficiency due to reduced environmental pollution. However, it has limitations that can be overcome by choosing appropriate. The ammonium ion has a low capacitance contribution at the electrode surface compared to common ions such as lithium. A composite based on MoS2 and polyaniline was used to prepare an ammonium ion supercapacitor, which showed 450 F g−1.200 Ulaganathan et al. synthesized electrode materials of MoS2 and polyaniline with different weight ratios of MoS2. Investigation of the electrochemical behavior of the composite in hydrogel electrolyte confirmed the optimal performance with a weight content of MoS2 (5 wt%).201 The musk structure is created by designing and synthesizing composites of TMS, TMO, and CP, which shortens the electron transport path and reduces the contraction and expansion of the polymer chain during charging/discharging. Yang et al. synthesized the MnO2/polyaniline in two steps via oxidative polymerization (Fig. 9). By preparing different ratios of the ternary composite, the ratio PANI (4)/FeS2 (1)/MnO (3) had higher stability.202
Bimetallic sulfides have more favorable electrochemical behavior than monometallic sulfides due to the synergistic effect of two transition metals. Qin et al. synthesized a hybrid based on NiMoS and polyaniline nanotubes by a hydrothermal chemical method, which was grown on polyaniline nanosheets. The increased conductivity of the polyaniline nanotubes and the numerous active sites created by NiMoS led to the achievement of an electroactive layer with 1558 F g−1 at 1 A g−1.203 In another work, electrode materials based on polypyrrole and CuCo2S4 were synthesized on nickel foil. According to chemical and electrochemical analyses, the oxidation states of copper and cobalt increased during charge–discharge, thus recording 1403.21 C g−1 at 1 A g−1 (Table 2).204
| Sample | Stability (cycles) | Specific capacitance (or areal specific capacitance) | Current density (or areal current density) | Reference |
|---|---|---|---|---|
| NiCo2O4/PANI | 86.2% up to 3000 | 561.2 F g−1 | 10 mV s−1 | 205 |
| NiFe2O4/PANI | 93.5% up to 7000 | 334 F g−1 | 1 mA cm−2 | 206 |
| CuMn2O4/PANI | 95% up to 7000 | 1181 F g−1 | 1 A g−1 | 207 |
| Polypyrrole/CuCo2S4 | 90% up to 5000 | 259 F g−1 | 1 A g−1 | 208 |
| ZnBi2O4/PANI | — | 1110.12 F g−1 | 1 A g−1 | 209 |
| CuO/PANI | 75% up to 2000 | 185 F g−1 | 1 A g−1 | 210 |
| PANI–Co3O4 | 84.9% up to 2000 | 1184 F g−1 | 1.25 A g−1 | 211 |
| PANI–Co3O4 | 74.81% up to 3000 | 3105.46 F g−1 | 1 A g−1 | 212 |
| CuCo2O4–PANI | 94%% up to 3000 | 403 C g−1 | 1 A g−1 | 213 |
| Co3O4/PANI | 90%% up to 2000 | 1301 F g−1 | 1 A g−1 | 214 |
| FeCo2O4/PANI | 94.5% up to 5000 | 940 C g−1 | 1 A g−1 | 215 |
| PANI/Fe–Ni codoped Co3O4 | 84%% up to 2000 | 1171 F g−1 | 1 A g−1 | 216 |
| α-MnMoO4/PANI | 84% up to 2000 | 396 F g−1 | 5 mV s−1 | 217 |
| MnO2/PANI | 95% up to 2000 | 687 F g−1 | 5 mV s−1 | 218 |
| α-MnO2/PANI | Excellent cyclic stability | 696.66 F g−1 | 0.5 A g−1 | 219 |
| Cerium oxide/PANI | 90% up to 1000 | 950 mF cm−2 | 10 mA cm−1 | 220 |
| BaNiO2/PANI | 97.9% up to 4000 | 1631 F g−1 | 1 A g−1 | 221 |
| CuCo2S4/PANI | 80.75% up to 3000 | 209 F g−1 | 5 mV s−1 | 222 |
| CuAlO2/PANI | — | 1119.79 F g−1 | 1 A g−1 | 223 |
| SnO2/PANI | 99.71% up to 5000 | 338 F g−1 | 0.1 A g−1 | 224 |
| PANI/CeVO4 | 90% up to 2000 | 1048 F g−1 | 10 mV s−1 | 225 |
| MoS2/PPY | 75.7% up to 3000 | 677 F g−1 | 1 A g−1 | 226 |
| PANI/PbS | 95.5% up to 5000 | 625 F g−1 | 1 A g−1 | 227 |
| MoS2/PANI | 89% up to 2000 | 645 F g−1 | 0.5 A g−1 | 228 |
| polyaniline/MoS2–MnO2 | 94.1% up to 4000 | 479 F g−1 | 5 mV s−1 | 229 |
| NiMnS–PANI | 98% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
976 C g−1 | 7 A g−1 | 230 |
| PPy/PANI/MnO2 | 84% up to 2500 | 136.64 F g−1 | 2 A g−1 | 231 |
4.3. The electrochemical energy-storage performance of metal oxide or sulfide/conductive polymers/carbon materials or MXene composites
Composites of CP and TMO or TMS have low conductivity as quasi-capacitor electrode materials. Therefore, the conductivity is improved by adding carbon materials such as GO, AC, and CQD to composites based on CP and TMO.232 Carbon materials increase the specific surface area and provide active sites for an energy storage system. Innovatively, Jia and colleagues synthesized polyaniline-based carbon through carbonization, then prepared a composite of cobalt oxide/polymer-based carbon. The composite electrode material was self-standing, which enabled its industrial application (no slurry preparation required).233 The addition of graphene oxide to metal sulfide-conducting polymer or metal oxide-conducting polymer composites increases mechanical strength. However, metal oxides or sulfides improve the crystal structure. The composite prepared from polyaniline and GO as electrode material in the study by Rani et al. showed 4800 F g−1 at 1 A g−1.54 Polyaniline caused uniform growth of nickel sulfide (preventing aggregation) in the synthesis of the nickel sulfide/polyaniline/graphene composite. Chang et al. investigated the electrochemical behavior of nickel sulfide/polyaniline/graphene composite. According to analyses of chemical and electrochemical, polyaniline caused uniform growth of nickel sulfide (preventing aggregation) in the synthesis of NiS2/G/PANI composite. Graphene in the NiS2/G/PANI composite structure increased the conductivity.234 Acidic groups of graphene oxide sometimes act as dopants instead of HCL. Thus, preventing corrosion of metal sulfide in the composites based on PANI, TMS, and GO. Batabyal et al. synthesized a composite of MgS, GO and, PANI using HCL as a dopant. The uniform morphology of the MnS/GO/PANI composite improved the electrolyte diffusion rate, and graphene oxide improved the electrical conductivity.235 Vadivel et al. synthesized a composite of FeNiS2, CNT, and polypyrrole in a two-step synthesis process. The CNT in the composite structure acted as a support for polypyrrole, so the PPy/CNT/FeNiS2 composite as a heterogeneous structure showed superior electrochemical performance with a 1541 F g−1 at 2 A g−1.236 Naeem et al. synthesized composites based of NiCoFe2O4, rGO and polypyrrole, using different ratios of nickel and cobalt. First, composites based on NiCoFe2O4 and rGO were synthesized through chemical combustion. Finally, the ternary composite was synthesized by adding polypyrrole through chemical oxidation. The optimal ratio of nickel to cobalt, along with the synergistic effect of the ternary composite, resulted 585 F g−1.237 Kiani et al. designed and synthesized an asymmetric device made of positive and negative electrodes. The negative electrode was a composite based on polypyrrole and titanium dioxide on carbon fabric by electrochemical deposition. Then it was plunged into the MXene solution in several steps. To prepare the positive electrode, the carbon fabric was immersed in sulfuric acid, nitric acid, and KMnO4 solution. The electrochemical behavior of the negative electrode was investigated by several steps of immersion in MXene solution (Fig. 10). The asymmetric device showed 87% stability after 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.238
000 cycles.238
Jiang et al. prepared a core–shell structure based on polypyrrole, MnO2, Fe3O4 in layered structures of graphene oxide using an industrial and cost-effective method. The four-component composite with a three-dimensional structure provided a high specific surface area for electron transport. The combination of two different metal oxides produced a high faradaic property. The synergistic effect of metal oxides with graphene oxide resulted in high-performance electrode materials.239 Shaheen et al. synthesized AgNiO/rGO/PANI composite using a simple hydrothermal method. The synergistic effect and high specific surface area led to 1375.55 F g−1 at 0.5 A g−1.240 Composites of carbon materials, conductive polymer, and TMO with different percentages of conductive polymer were synthesized and investigated to achieve efficient electrode materials. Subbramaniyan et al. synthesized a quaternary hybrid of GO, MnO2, MoO3, and different ratios of polyaniline. The composite with the optimal ratio of polyaniline showed 596 F g−1 at 1 A g−1.241 Atram et al. synthesized the carbon nanofibers/NiFe2S4/polyaniline composite in two steps. First, a hybrid based on carbon nanofibers and NiFe2S4 was synthesized using electrospinning, and then polyaniline was added by in situ polymerization. The synergistic effect of the pseudocapacitor materials (NiFe2S4/PANI) and the EDLC materials (CNF) improved the supercapacitor performance, and the electrode materials showed 645 F g−1 at 1 A g−1.242 Umair et al. synthesized an asymmetric supercapacitor device from PANI@CoNbS composite and PANI@AC composite. The quasi-capacitive performance of the PANI@CoNbS electrode, along with the high specific surface area of the PANI@AC electrode, resulted in an efficient asymmetric device (35 W h kg−1) (Table 3).243
| Sample | Stability (cycles) | Specific capacitance (or areal specific capacitance) | Current density (or areal current density) | Reference |
|---|---|---|---|---|
| Co3O4/polyaniline/graphene | 94% up to 3000 | 476 F g−1 | 2 A g−1 | 244 |
| MnS/PANI/CNT | 85% up to 1000 | 325 F g−1 | 1 A g−1 | 245 |
| ZnCoOx/C–PANI | 80% up to 5000 | 1055 F g−1 | 1 A g−1 | 246 |
| PANI–rGO–CoS | 90% up to 1000 | 431 F g−1 | 0.5 A g−1 | 247 |
| PANI–rGO–Co3S4 | 81.7% up to 5000 | 767 F g−1 | 1 A g−1 | 248 |
| GO/PANI/CuCo2O4 | 84.25% up to 5000 | 312.72 F g−1 | 1 A g−1 | 249 |
| PANI/SnS2@CNTs | 83.6% up to 6000 | 891 F g−1 | 20 mV s−1 | 250 |
| PANI/CNT/e-MoS2 | 80% up to 4000 | 532 F g−1 | 1 A g−1 | 251 |
| NiCo2S4/PANI/CNT | 80.13% up to 5000 | 1290 mF cm−1 | 2 mA cm−1 | 252 |
| Cs/CNTs/PANI | 87.5% up to 5000 | 767 F g−1 | 1 A g−1 | 253 |
| CS/GM/Fe3O4/PANI | 99.8% up to 5000 | 1513.4 F g−1 | 4 A g−1 | 254 |
| La2S3/PANI/N–rGO | 94.87% up to 5000 | 2311.2 F g−1 | 5 A g−1 | 255 |
| As3Mo8V4/PANI/rGO | 85.7% up to 5000 | 1295 F g−1 | 1 A g−1 | 256 |
| VO2/CNT@PANI | 88.2% up to 5000 | 354.2 F g−1 | 0.5 A g−1 | 257 |
| CuS/C@PANI | 89.86% up to 3000 | 425.53 F g−1 | 1 A g−1 | 258 |
| PANI/GO/CuFe2O4 | 88% up to 3500 | 614.76 F g−1 | 1 A g−1 | 259 |
| Mn3O4/PANI/G | 97% up to 3000 | 1240 F g−1 | 2 A g−1 | 260 |
| PANI–GO–Mn3O4 | 89% up to 4000 | 460 F g−1 | 1 A g−1 | 261 |
| MoSx–PANI@RGO | 88% up to 5000 | 1365 F g−1 | 1 A g−1 | 262 |
| CF/Ni3S2@PANI | 93.4% up to 2500 | 318 F g−1 | 1 A g−1 | 263 |
| NiSe2/rGO/PANI | 100% up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
657.36 C g−1 | 1 A g−1 | 264 |
| PANI/nTiO2/AC | 72% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
827 F g−1 | 10 mV s−1 | 265 |
| NiO/g-C3N4/CNTs/TiO2 | — | 362.12 F g−1 | 1 A g−1 | 266 |
| rGO@Fe2O3/CuO/PANI | 96% up to 1000 | 1210 F g−1 | 1 A g−1 | 267 |
| α-Fe2O3/SnO2/rGO | 98.7% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
821 F g−1 | 1 A g−1 | 268 |
| CNTs-PANI/CoNi(PO4)2 | 100% up to 5000 | 2136 F g−1 | 1.5 A g−1 | 269 |
| PPy/MnO2/CC | 91% up to 5000 | 324.5 mF cm−2 | 2.5 mA cm−2 | 270 |
| CC/MnO2/PPy | 96.46% up to 8000 | 123.96 F g−1 | 10 mV s−1 | 271 |
| g-C3N4/V2O5/PANI | 78% up to 2000 | 880 F g−1 | 1 A g−1 | 272 |
| rGO/PANI/ZnO | 97% up to 3000 | 1546 F g−1 | 2 mV s−1 | 273 |
| MoO3/PPy/rGO | 85% up to 6000 | 412.3 F g−1 | 0.5 A g−1 | 274 |
| V2O5/PPy/GO | 83% up to 3000 | 750 F g−1 | 5 A g−1 | 275 |
| V2O5/f-CNT/PPy | 83% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1266 mF cm−2 | 1 mA cm−1 | 276 |
| MnO2/PANI–GCN | 82% up to 1000 | 318 F g−1 | 1 A g−1 | 277 |
4.4. The electrochemical energy-storage performance of MOF/conductive polymers composites and MOF/conductive polymers/TMO or TMS composites
Metal–organic frameworks were prepared from metal ions linked by organic ligands. The connection between metal ions and organic ligands was established through coordination bonds. MOF are porous structures with high specific surface areas, thus providing active sites for electron transport.278 The redox properties of metals combined with organic ligands offer high potential for energy storage. Two-dimensional metal–organic frameworks provide a shorter path for electron transfer and electrochemical activity. Metal–organic frameworks made of cobalt, zinc, zirconium, and nickel have been reported in supercapacitor studies. Johnson et al. synthesized Co-MOF/polyaniline composite by in situ oxidative polymerization. The Co-MOF/PANI composite electrode material with a high specific surface area and conductivity recorded 504 F g−1 at 1 A g−1.279 Ebenezer et al. investigated the behavior Ni-MOF/polyaniline composite in detail. The electrochemical analyses showed that the specific capacitance of the PANI/Ni-MOF was twice that of polyaniline and Ni-MOF. Despite the faradaic behavior of Ni-MOF and polyaniline, the composite electrode materials had high performance as electroactive materials and retained 99% specific capacitance up to 5000 cycles.280 Maheshwari et al. synthesized copper MOF derived from plastic waste residues. Despite the high specific surface area of the copper metal framework, its conductivity was low. Composites based on conductive polymers (polyaniline and polypyrrole) and copper metal–organic framework were designed and synthesized to improve conductivity and increase the electron transfer rate. According to the electrochemical performance results of composites based on conductive polymers and copper metal–organic framework, additional channels for ion transport were created by the addition of conductive polymer.281 Hybrids of MOF and CP have been synthesized to prevent polymer aggregation and achieve high conductivity and specific surface area. However, the addition of metal oxide increases stability and mechanical strength. Yang et al. synthesized a three-component of polyaniline/zinc oxide/cobalt metal organic framework in two steps, as shown in Fig. 11. The high conductivity of conductive polymers, the high specific surface area of the MOF, and the mechanical strength of zinc oxide resulted in efficient hybrid electrode materials with 458.9 F g−1 at 1 A g−1.282Boopathiraja et al. synthesized a composite of polypyrrole, MOF, and zinc oxide by the hydrothermal method. The supercapacitive behavior was improved by changing the type of conductive polymer (polypyrrole instead of polyaniline), and 1181 F g−1 at 1 A g−1 was recorded.283 The g-C3N4 is a carbon-based structure where the electron-donating properties of nitrogen accelerate electron transfer. Composites of g-C3N4 and CP have been synthesized to prevent the aggregation of two-dimensional g-C3N4 structures.284,285 Fu et al. synthesized a quaternary composite of PANI/g-C3N4/Ni-MOF/nickel oxide in three steps. First, a composite of PANI and g-C3N4 was synthesized through chemical oxidation. Second, it was attached to a nickel MOF, and finally, nickel oxide was injected. The polyaniline in the composite structure prevented the g-C3N4 sheets from overlapping, and the metal oxide increased the stability. Therefore, the quaternary composite-based electrode material with high specific surface area, high stability, and multiple active sites showed 2420 F g−1 at 5 A g−1.286 Other carbon materials, including GO, G, and AC, were incorporated as electroactive components in composites of CP, MOF, and CNT. Rani et al. synthesized composites based on polyaniline, metal–organic framework, and graphene oxide by the hydrothermal method. The synergistic effect of highly conductive carbon materials, cobalt MOF with multiple active sites, and polyaniline as a plate separator resulted 290 F g−1 at 1 A g−1.287 Some et al. first synthesized GO by the Hummers' method to manufacture a metal–organic framework of zinc/rGO, and polypyrrole composite. Then, combined polypyrrole and graphene oxide by a chemical method. Finally, a ternary composite was synthesized by adding imidazole and zinc nitrate to the solution through a hydrothermal method. The ternary composite with multiple sites and high surface area for electron transfer recorded 175 F g−1 at 1 A g−1.288
4.5. The MXene/CP composites for supercapacitors
MXenes are two-dimensional materials with high surface area. MXenes were synthesized from transition metals, carbides, and nitrides.289 These materials have advantages, including thermal stability and high mechanical strength, which outperform other 2D materials (graphene). Therefore, MXenes have good performance in sensors, batteries, supercapacitors, and water purification. MXenes have metallic conductivity and hydrophilic properties, which make their performance unique. In other words, MXenes have the properties of being stable like ceramics and conducting like metals. However, MXene sheets tend to aggregate during long cycles. Therefore, MXene-based composites were designed and synthesized. The conductive polymer is placed between the MXene plates in MXene/CP composites to prevent the plates from agglomerating. Jin et al. synthesized the MXene/polyaniline composite by a hydrothermal method. The BET analyses showed that the specific surface area increased with the addition of MXene. Therefore, the ion diffusion was facilitated. Polyaniline acted as a coupling agent between the MXene layers in the composite, thus facilitating charge transfer between the layers. The MXene/PANI showed 563 F g−1 at 0.5 A g−1.290 Singh et al. first prepared polyaniline through in situ polymerization and Ti3C2Tx by the mild-etching method. Then, the composite based on Ti3C2Tx and polyaniline was synthesized through the polymerization (Fig. 12). The graphite current collector was used to investigate the electrochemical performance of Ti3C2Tx/PANI. This study was compared with similar studies conducted with other current collectors (Ni and C cloth). The use of a graphite current collector for Ti3C2Tx/polyaniline composite resulted in reduced resistance and increased stability. The Ti3C2Tx/polyaniline composite showed 854 F g−1 at 1 A g−1.291Zhang et al. synthesized (PANI)/MXene composites by the in situ polymerization. This synthesis method, the high surface area of MXene increases the electronegativity, which promotes the polymerization of aniline on the MXene surface. The high surface area of MXene improved the electrochemical activity of the PANI/MXene composite. The asymmetric device of (PANI)/MXene recorded 11.25 W h kg−1.292 Wang et al. synthesized multilayer MXene. Functional of MXene provided conditions for the synthesis of composites with different ratios of MXene and polyaniline. The functional groups of MXene provided active nucleation sites for the growth of polyaniline on MXene. The MXene/PANI electrode material with the optimal ratio showed 222 F g−1.293 The MXene/polyaniline composite can be grown on carbon fabric, thereby preventing the accumulation of MXene. Yang and co-workers grew MXene and polyaniline-based composites on carbon cloth. The synthesis and design of a 2D/0D/1D structure improved the electron transfer rate and composite homogeneity. Therefore, the composite grown on carbon cloth showed areal specific capacitance 1347 mF cm−2.294 Bae et al. synthesized (Ti3C2Tx)/polyaniline composites by Ti3AlC2 etching followed via polymerization. The electrochemical analysis of the (Ti3C2Tx)/polyaniline composite showed 458.3 F g−1 at 5 mV s−1.295 Wang et al. sulfonated polyaniline to prepare a 3D composite structure based on PANI and MXene. Sulfonation of polyaniline accelerated the redox reaction and also caused the disordered growth of polyaniline on MXene. The irregular growth of polyaniline between the MXene sheets resulted in a three-dimensional structure that facilitates the ions movement. The composite based on MXene and PANI showed 512.45 F g−1.296 Hou et al. synthesized Ti3C2Tx/polyaniline film by the suction filtration method. The polyaniline increased the spacing of Ti3C2Tx sheets as indicated by the chemical analysis. Therefore, the rate of ion transport increased, and the Ti3C2Tx/polyaniline film as an efficient electroactive material showed 272.5 F g−1 at 1 A g−1 (Table 4).297
| Sample | Stability (cycles) | Specific capacitance (or areal specific capacitance) | Current density (or areal current density) | Reference |
|---|---|---|---|---|
| Ti2CTx@polyaniline | 97.54% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
635 F g−1 | 1 A g−1 | 298 |
| MXene–CNT/PANI | 93% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
429.4 F g−1 | 1 A g−1 | 299 |
| MXene/PANI | 98% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
336 F g−1 | 1 A g−1 | 300 |
| MXene/polyaniline | 84.6% up to 5000 | 645.7 F g−1 | 1 A g−1 | 301 |
| MXene/PANI | 86.5% up to 5000 | 523.8 F g−1 | 1 A g−1 | 302 |
| MXene/PANI | 86% up to 5000 | 190.8 F g−1 | 1 A g−1 | 303 |
| Ti3C2/PANI-NTs-1 | 86% up to 4000 | 596.6 F g−1 | 1 A g−1 | 304 |
| MXene/PANI | 81.6% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
361.9 mF cm−3 | 6 mA cm−3 | 305 |
| Ti3C2Tx/PANI | 96.4% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
385 F g−1 | 10 V s−1 | 306 |
| MXene/PANI | 89.4% up to 9000 | 483.1 F g−1 | 1 A g−1 | 307 |
| PANI@MXene–CNTs | 92% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
463 F g−1 | 5 mV s−1 | 308 |
| MXene/PANI | 71.6% up to 3000 | 327 F g−1 | 1 A g−1 | 309 |
| Ti3C2Tx MXene/polypyrrole | 86.8% up to 6000 | 563.8 F g−1 | 0.5 A g−1 | 310 |
| Ti3C2–MXene/polypyrrole | 73.68% up to 4000 | 458 F g−1 | 2 mV s−1 | 311 |
| PPy@MXHCNF | 92.8% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
567.5 F g−1 | 1 A g−1 | 312 |
| Ti3C2 MXenes/polypyrrole | 83.33% up to 4000 | 184.36 F g−1 | 2 mV s−1 | 313 |
| PANI/MXene | 86% up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
322 F g−1 | 0.5 A g−1 | 314 |
5. Conclusion and future prospects
The development of industry and the depletion of fuel resources have been discussed as a global challenge. The supercapacitor has been introduced as a suitable technology to replace fossil fuels. Conductive polymers as electrode materials have metallic conductivity and polymer properties simultaneously. The instability of conductive polymers during long cycles is due to the volume change of polymer chains, thus limiting the performance of CP in supercapacitor systems. High-stability hybrid compounds were achieved by preparing composites based on conductive polymers and other electroactive materials. Composites prepared from conductive polymers with C, TMO, MOF, and TMS were reported as a high-performance hybrid compound in recent studies. Despite the optimal performance of composites based on conductive polymers, conductive polymers have low processability and cannot be used in large quantities in wide areas. Therefore, some important challenges related to conductive polymer-based composites include industrial scalability and standardization of mass production, and the toxic effects of conductive polymers on the environment, which have prevented the widespread adoption of polymer-based composites as electrode materials. In addition, high production cost, material incompatibility, sensitivity to high temperatures, low solubility in solvents, and the inability to process this material directly in the melt state are considered challenges for conductive polymers. Industrial production of conductive polymer-based composites on a scale beyond laboratory environments may result in changes to their electrochemical properties. Therefore, this requires large investments and technological development that may not be economically justified. High production costs may prevent widespread adoption of conductive polymer-based composites in the global market. Researchers' awareness of the advantages and disadvantages of conductive polymer-based composite electrode materials will be effective in the intelligent development of this technology. Much effort and research must be made to achieve high-performance electrode materials of conductive polymer-based composites. Researchers can overcome energy supply constraints in industry by designing and synthesizing conductive polymer-based composites with greater solubility, lower toxicity, and cost-effectiveness.Conflicts of interest
The authors have no conflict of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors gratefully acknowledge the University of JAIN (Deemed to be University) and University of Nursing for the financial support for this research.References
- P. Es' haghi, H. Seddighi, K. Shayesteh and N. Omrani, Chem. Rev. Lett., 2024, 7, 1042–1052 Search PubMed.
- S. Gautam, S. Rialach, S. Paul and N. Goyal, RSC Adv., 2024, 14, 14311–14339 RSC.
- O. A. Al-Basri, A. A. Mohammed, A. A. Salman, M. A. Kadhom and E. A. Yousif, Chem. Res. Technol., 2025, 2, 90–98 Search PubMed.
- N. Norouzi and S. Talebi, Chem. Rev. Lett., 2020, 3, 38–52 CAS.
- N. Kumar, R. Aepuru, S.-Y. Lee and S.-J. Park, Mater. Sci. Eng., R, 2025, 163, 100932 CrossRef.
- E. Vessally, R. M. Rzayev, A. A. Niyazova, T. Aggarwal and K. E. Rahimova, RSC Adv., 2024, 14, 40141–40159 RSC.
- T. Abedin, J. Pasupuleti, J. K. S. Paw, Y. C. Tak, M. Mahmud, M. P. Abdullah and M. Nur-E-Alam, J. Power Sources, 2025, 640, 236769 CrossRef CAS.
- L. Wenkai, X. Zhiyong and Z. Haodong, RSC Adv., 2024, 14, 7172–7194 RSC.
- J. Wu, J. Li and X. Yao, Adv. Funct. Mater., 2025, 35, 2416671 CrossRef CAS.
- T. H. Karakoc, C. O. Colpan, S. Ekici and O. Yetik, Journal of Green Energy, 2025, 22, 1402 Search PubMed.
- P. Kumar, K. Kumar, N. Adhikary and E. L. Tesfaye, Sci. Rep., 2025, 15, 13953 CrossRef CAS PubMed.
- M. A. Dar, S. Majid, M. Satgunam, C. Siva, S. Ansari, P. Arularasan and S. R. Ahamed, Int. J. Hydrogen Energy, 2024, 70, 10–28 CrossRef CAS.
- Y. Wang, T. Xu, K. Liu, M. Zhang, X. M. Cai and C. Si, Aggregate, 2024, 5, e428 CrossRef CAS.
- P. Gaikwad, N. Tiwari, R. Kamat, S. M. Mane and S. B. Kulkarni, Mater. Sci. Eng., B, 2024, 307, 117544 CrossRef CAS.
- J. Li, C. Liu, R. Momen, J. Cai, X. Hu, F. Zhu, H. Liu, L. Xu, W. Deng and H. Hou, Coord. Chem. Rev., 2024, 517, 216018 CrossRef CAS.
- V. Surendran and V. Thangadurai, ACS Appl. Energy Mater., 2024, 7, 1873–1881 CrossRef CAS.
- X. Xu, X. Han, L. Lu, F. Wang, M. Yang, X. Liu, Y. Wu, S. Tang, Y. Hou and J. Hou, J. Power Sources, 2024, 603, 234445 CrossRef CAS.
- J. Zia and M. Tejaswini, RSC Adv., 2025, 15, 9055–9080 RSC.
- A. I. Saber, H. K. Dabis, N. M. A. Alsultany, H. M. H. Abdulwahab, A. S. Mansoor, N. S. Abd and F. Alimola, Chem. Rev. Lett., 2025, 8, 639–658 CAS.
- F. Alimola and N. Arsalani, J. Alloys Compd., 2025, 181190 Search PubMed.
- F. Alimola, N. Arsalani and I. Ahadzadeh, Mater. Chem. Phys., 2024, 319, 129293 CrossRef CAS.
- P. Molaiyan, M. Abdollahifar, B. Boz, A. Beutl, M. Krammer, N. Zhang, A. Tron, M. Romio, M. Ricci and R. Adelung, Adv. Funct. Mater., 2024, 34, 2311301 CrossRef CAS.
- S. Lan, C. Yu, J. Yu, X. Zhang, Y. Liu, Y. Xie, J. Wang and J. Qiu, Small, 2024, 2309286 Search PubMed.
- K. Dissanayake and D. Kularatna-Abeywardana, J. Energy Storage, 2024, 96, 112563 CrossRef.
- X. Liu, N. Ostrovsky-Snider, M. Lo Presti, T. Kim, G. Guidetti and F. G. Omenetto, ACS Biomater. Sci. Eng., 2024, 10, 5390–5398 CrossRef CAS PubMed.
- Y. Weng, N. Tan, Z. Cao, B. Huang, B. Lu, H. Liu, X. You, J. Lv, Y. Guo and L. Tang, J. Energy Storage, 2025, 118, 116259 CrossRef.
- S. Khan, S. Chand and C. Chakraborty, Chem. Eng. J., 2025, 164232 CrossRef CAS.
- Q. Liu, T. Wang, D. Jia, P. Ren and D. Wu, Adv. Funct. Mater., 2025, 2500016 CrossRef CAS.
- S. Jha, Y. Qin, Y. Chen, Z. Song, L. Miao, Y. Lv, L. Gan and M. Liu, J. Mater. Chem. A, 2025, 13, 15101–15110 RSC.
- S. B. Aziz, P. O. Hama, D. M. Aziz, N. M. Sadiq, H. J. Woo, M. F. Kadir, R. T. Abdulwahid, B. A. Al-Asbahi, A. A. Ahmed and J. Hassan, J. Energy Storage, 2025, 114, 115841 CrossRef.
- M. A. Hossain, K. Sheikh, M. S. Islam Sagar, K. R. Hossain, X. Yao, C. Bai and X. Wang, Chem. Rev. Lett., 2023, 6, 461–478 CAS.
- Z. H. Hussein, F. F Karam and N. Rahi Mashkur, Chem. Rev. Lett., 2025, 8, 128–136 Search PubMed.
- F. Mashkoor, M. Shoeb, S. Zhu, J. Ahmed, S. M. Noh and C. Jeong, Surf. Interfaces, 2025, 62, 106198 CrossRef CAS.
- S. N. Ndung'u, T. Nyahanga, E. Kinuthia, A. Ndiritu, S. Kirkok and J. Kirimi, J. Chem. Technol., 2025, e229562 Search PubMed.
- G. A. Tafete, N. G. Habtu, M. K. Abera, T. A. Yemata, A. K. Shibeshi and N. W. Kebede, Sustainable Development Research in Materials and Renewable Energy Engineering: Advancements of Science and Technology, 2025, pp. 127–157 Search PubMed.
- P. V. Patale, S. R. Mathapati and J. L. Somawanshi, J. Chem. Lett., 2024, 5, 206–220 Search PubMed.
- J. Khan, A. Ahmed and A. A. Al-Kahtani, Mater. Adv., 2025, 6, 3344–3354 RSC.
- G. B. Pour and L. F. Aval, Electrochem. Commun., 2025, 107874 CrossRef.
- M. F. Jimoh, G. S. Carson, M. B. Anderson, M. F. El-Kady and R. B. Kaner, Adv. Funct. Mater., 2025, 35, 2405569 CrossRef CAS.
- Z. Zhu, Y. Bu, C. Gu and X. Wang, J. Eur. Ceram. Soc., 2025, 117423 CrossRef CAS.
- D. Dake, N. Raskar, V. Mane, R. Sonpir, K. Gattu and B. Dole, Material Science for Future Applications: Emerging Development and Future Perspectives, 2025 Search PubMed.
- Z. Hou, L. Chang, W. Yang, R. Yang, A. Wei, K. Cai and S. Luo, J. Energy Storage, 2024, 100, 113550 CrossRef.
- A. Samage, M. Halakarni, H. Yoon and N. S. Kotrappanavar, Carbon, 2024, 219, 118774 CrossRef CAS.
- M. Goyal, K. Singh and N. Bhatnagar, Prog. Org. Coat., 2024, 187, 108083 CrossRef CAS.
- V. Golovakhin, V. I. Litvinova, A. Manakhov, A. R. Latypova, O. N. Novgorodtseva, A. V. Ukhina, A. V. Ishchenko, A. S. Al-Qasim, E. A. Maksimovskiy and A. G. Bannov, Mater. Today Commun., 2024, 39, 109163 CrossRef CAS.
- J. E. Ogbu and C. I. Idumah, Polym.-Plast. Technol. Mater., 2024, 63, 939–974 CAS.
- K. Chattopadhyay, A. Basak, G.-B. Lee, M. Mandal, C. Nah and D. K. Maiti, ACS Appl. Energy Mater., 2024, 7, 8683–8693 CrossRef CAS.
- Z. Çıplak, J. Electron. Mater., 2022, 51, 1077–1088 CrossRef.
- D. Gui, C. Liu, F. Chen and J. Liu, Appl. Surf. Sci., 2014, 307, 172–177 CrossRef CAS.
- A. K. Sharma, Y. Sharma, R. Malhotra and J. Sharma, Adv. Mater. Lett., 2012, 3, 82–86 CrossRef CAS.
- N. M. Yousif and M. R. Balboul, Russ. J. Electrochem., 2024, 60, 1133–1152 CrossRef.
- D. Geetha, ECS J. Solid State Sci. Technol., 2025, 14(7), 076002 CrossRef.
- A. Soleimani, H. G. Taleghani and M. S. Lashkenari, Ternary RGO/PANI/UCNT nanohybrid for high performance electrochemical supercapacitors, Research Square, 2024, DOI:10.21203/rs.3.rs-4589783/v1.
- K. Batool, M. Rani, S. M. Osman, M. Sillanpää, R. Shafique, S. Khan and M. Akram, Diamond Relat. Mater., 2024, 143, 110904 CrossRef CAS.
- B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Springer Science & Business Media, 2013 Search PubMed.
- Z. S. Iro, C. Subramani and S. Dash, Int. J. Electrochem. Sci., 2016, 11, 10628–10643 CrossRef CAS.
- P. Pattathil, N. Sivakumar and T. S. Sonia, Capacitor to Supercapacitor: An Introduction, in Nanostructured Ceramic Oxides for Supercapacitor Applications, ed. A. Balakrishnan and K. R. V. Subramanian, CRC Press, Boca Raton, 2014, ch. 1, pp. 1–10 Search PubMed.
- A. González-Banciella, D. Martinez-Diaz, J. Artigas-Arnaudas, M. V. Vázquez, M. Sánchez and A. Ureña, J. Alloys Compd., 2025, 181139 CrossRef.
- F. Alimola, N. Arsalani and I. Ahadzadeh, Electrochim. Acta, 2022, 417, 140283 CrossRef CAS.
- S. Sahoo, S. Ratha, C. S. Rout and S. K. Nayak, J. Mater. Sci., 2022, 57, 4399–4440 CrossRef CAS.
- V. D. Nithya, J. Electron. Mater., 2025, 1–20 Search PubMed.
- R. Aslani and H. Namazi, J. Ind. Eng. Chem., 2022, 112, 335–347 CrossRef CAS.
- R. Aslani and H. Namazi, React. Funct. Polym., 2022, 170, 105101 CrossRef CAS.
- U. Okoroanyanwu, A. Bhardwaj and J. J. Watkins, ACS Appl. Mater. Interfaces, 2023, 15, 13495–13507 CrossRef CAS PubMed.
- K. O. Oyedotun and B. B. Mamba, Inorg. Chem. Commun., 2024, 113154 CrossRef CAS.
- B. Hashemzadeh, L. Edjlali, P. Delir Kheirollahi Nezhad and E. Vessally, Chem. Rev. Lett., 2021, 4, 232–238 Search PubMed.
- F. Alimola, N. Arsalani and R. Aslani, J. Organomet. Chem., 2025, 123893 CrossRef CAS.
- R. A. Khellouf, V. Cyriac, C. Bubulinca and V. Sedlarik, Energy Environ. Mater., 2025, e70023 CrossRef CAS.
- Y. Aishan, Q. Chen, T. Saito and A. Muto, Mater. Res. Express, 2025, 12, 045601 CrossRef CAS.
- V. Aswathi and P. Sreeja, J. Colloid Interface Sci., 2025, 137637 CrossRef CAS PubMed.
- A. S. Etman, J. Halim and J. Rosen, J. Energy Storage, 2022, 52, 104823 CrossRef.
- R. Burt, G. Birkett and X. Zhao, Phys. Chem. Chem. Phys., 2014, 16, 6519–6538 RSC.
- S. B. Aziz, P. O. Hama, R. T. Abdulwahid, D. M. Aziz, P. A. Mohammed, M. H. Hamsan, R. M. Abdullah and M. F. Kadir, Emergent Mater., 2025, 1–26 Search PubMed.
- F. Ran, M. Hu, S. Deng, K. Wang, W. Sun, H. Peng and J. Liu, RSC Adv., 2024, 14, 11482–11512 RSC.
- A. R. Ferdous, S. S. Shah, A. Hussain, A. A. Mirghni, Y. P. Hardianto and M. A. Aziz, Sustainable Mater. Technol., 2025, e01296 CrossRef CAS.
- R. Aslani, F. Alimola and N. Arsalani, J. Alloys Compd., 2025, 1033, 181190 CrossRef CAS.
- V. Thirumal, B. Babu, J. Kim, K. Yoo and S. H. Lee, J. Alloys Compd., 2025, 1022, 179956 CrossRef CAS.
- K. Chinnaiah, K. Kannan, Y.-S. Chen and K. Gurushankar, J. Phys. Chem. Solids, 2025, 196, 112310 CrossRef CAS.
- F. Ahmad, M. A. Khan, U. Waqas, S. M. Ramay and S. Atiq, RSC Adv., 2023, 13, 25316–25326 RSC.
- C. I. Awuzie, Mater. Today: Proc., 2017, 4, 5721–5726 Search PubMed.
- C. Chiang, M. Druy, S. Gau, A. Heeger, E. Louis, A. G. MacDiarmid, Y. Park and H. Shirakawa, J. Am. Chem. Soc., 1978, 100, 1013–1015 CrossRef CAS.
- S. C. Rasmussen, ChemPlusChem, 2020, 85, 1412–1429 CrossRef CAS PubMed.
- L. D. Foyle, G. E. Hicks, A. A. Pollit and D. S. Seferos, J. Phys. Chem. Lett., 2021, 12, 7745–7751 CrossRef CAS PubMed.
- X. Bai, H.-S. Lee, J.-E. Han, H. N. Murthy and S.-Y. Park, Horticulturae, 2025, 11, 612 CrossRef.
- H. M. Bergman and T. M. Swager, J. Am. Chem. Soc., 2025, 147, 12392–12396 CrossRef CAS PubMed.
- D. Xiao, B. Sun, Y. Liu, E. Jiao, X. Wang, X. Chen, X. Cheng, K. Guo, K. Yuan and H. Zhang, Polym. Polym. Compos., 2025, 33, 09673911241304848 Search PubMed.
- M. Hong, P. U. Do, C. H. Lee and Y. D. Park, Appl. Surf. Sci., 2025, 692, 162679 CrossRef CAS.
- M. R. Jalali Sarvestani, Med. Med. Chem., 2024, 1, 84–90 Search PubMed.
- A. M. Soliman, M. Abd El Aleem Ali Ali El-Remaily, M. S. Kamel, A. El-Araby and E. K. Shokr, Sci. Rep., 2025, 15, 1611 CrossRef CAS PubMed.
- X. Hu, J. A. Lawrence III, J. Mullahoo, Z. C. Smith, D. J. Wilson, C. R. Mace and S. W. Thomas III, Macromolecules, 2017, 50, 7258–7267 CrossRef CAS.
- L. Ye, H. Ke and Y. Liu, Trends Chem., 2021, 3, 1074–1087 CrossRef CAS.
- A. Kausar, Conducting Polymer Based Nanocomposites, Elsevier, Cambridge, MA, USA, 2021, pp. 129–156 Search PubMed.
- R. Liu and Z. Liu, Chin. Sci. Bull., 2009, 54, 2028–2032 CrossRef CAS.
- S. Yazar, M. B. Arvas and K. Gürkan, J. Mater. Sci., 2024, 59, 10936–10952 CrossRef CAS.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 RSC.
- Z. Li and L. Gong, Materials, 2020, 13, 548 CrossRef CAS PubMed.
- K. Ajeel and Q. Kareem, Journal of Basrah Researches (Sciences), 2019, 45, 2 Search PubMed.
- Z. Jabarzadeh, M. R. Jalali Sarvestani, S. Arabi, M. Mahboubi-Rabbani and S. Ahmadi, Chem. Rev. Lett., 2025, 8, 576–588 CAS.
- S. Khammarnia, J. Saffari and M.-S. Ekrami-Kakhki, Chem. Rev. Lett., 2025, 8, 741–750 Search PubMed.
- M. J. Vujković, M. Etinski, B. Vasić, B. Kuzmanović, D. Bajuk-Bogdanović, R. Dominko and S. Mentus, J. Power Sources, 2021, 482, 228937 CrossRef.
- D. Balan, B. Singh, A. Sheokand and D. Mohan, J. Mater. Sci.: Mater. Electron., 2025, 36, 1–14 CrossRef.
- A. Hefnawy, J. El Nady, A. Hassan, F. Mahgoub, S. Ebrahim, R. A. Emanfaloty and A. Elshaer, J. Alloys Compd., 2025, 1019, 179240 CrossRef CAS.
- E. Avcu Altıparmak, S. Yazar and T. Bal-Demirci, Small Methods, 2025, 9, 2401140 CrossRef PubMed.
- Y. Liu, K. Luo, W. Xing, W. Yin, J. Feng, S. Pi, Z. Kang, J. Liang, L. Tang and W. Tang, Angew. Chem., Int. Ed., 2025, 64, e202501797 CrossRef CAS PubMed.
- J. Vícha, L. s. Münster, F. Latečka, M. Martínková, Z. Víchová, O. e. Vašíček and P. Humpolíček, ACS Sustainable Chem. Eng., 2025, 13(22), 8435–8446 CrossRef.
- K. A. Milakin, O. Taboubi, M. Lhotka, J. Hromádková, J. Hodan, O. Pop-Georgievski and P. Bober, Emergent Mater., 2025, 1–13 Search PubMed.
- A. L. Pang, A. Arsad and M. Ahmadipour, Polym. Adv. Technol., 2021, 32, 1428–1454 CrossRef CAS.
- A. Saidfar, M. Alizadeh and S. Pirsa, J. Chem. Lett., 2020, 1, 39–46 Search PubMed.
- D. Mantione, I. Del Agua, A. Sanchez-Sanchez and D. Mecerreyes, Polymers, 2017, 9, 354 CrossRef PubMed.
- Q. Zeng, X. Liu, L. Wang, S. Li, X. Xie, G. Liu and Z. Liu, Chem. Phys., 2025, 588, 112488 CrossRef CAS.
- S. Sindhu, K. N. Rao, S. Ahuja, A. Kumar and E. Gopal, Mater. Sci. Eng., B, 2006, 132, 39–42 CrossRef CAS.
- L. Zhou, M. Yu, X. Chen, S. Nie, W. Y. Lai, W. Su, Z. Cui and W. Huang, Adv. Funct. Mater., 2018, 28, 1705955 CrossRef.
- H. Nam, H. Cho, H. Lee, H. J. Son and C. Yun, J. Ind. Eng. Chem., 2025, 152, 256–265 CrossRef CAS.
- A. S. Ghouri, R. Aslam, S. Siddiqui and S. K. Sami, J. Coat. Technol. Res., 2025, 1–19 Search PubMed.
- Y.-H. Kim, S.-H. Lee, J. Noh and S.-H. Han, Thin Solid Films, 2006, 510, 305–310 CrossRef CAS.
- Y. Jing and H. Okuzaki, ACS Appl. Polym. Mater., 2025, 7, 4955–4962 CrossRef CAS.
- J. Carlberg and O. Inganäs, J. Electrochem. Soc., 1997, 144, L61 CrossRef CAS.
- J. Chorbacher, J. Klopf, A. Friedrich, M. Fest, J. S. Schneider, B. Engels and H. Helten, Angew. Chem., Int. Ed., 2025, 64, e202416088 CrossRef CAS PubMed.
- J. Banerjee and K. Dutta, Chem. Pap., 2021, 1–13 CAS.
- C. Li, M. Liu, N. G. Pschirer, M. Baumgarten and K. Mullen, Chem. Rev., 2010, 110, 6817–6855 CrossRef CAS PubMed.
- Z. a. Tan, R. Tang, E. Zhou, Y. He, C. Yang, F. Xi and Y. Li, J. Appl. Polym. Sci., 2008, 107, 514–521 CrossRef CAS.
- S. Perumal, R. Atchudan, K. Krukiewicz, A. Banaś, D. R. Kumar, H. Lee and W. Lee, J. Taiwan Inst. Chem. Eng., 2025, 172, 106103 CrossRef CAS.
- A. M. Díez-Pascual and A. L. Díez-Vicente, ACS Appl. Mater. Interfaces, 2014, 6, 10132–10145 CrossRef PubMed.
- Y. Guo, R. Liu, L. Zhou, H. Zhao, F. Lv, L. Liu, Y. Huang, H.-W. Zhang, C. Yu and S. Wang, Nano Today, 2020, 35, 100969 CrossRef CAS.
- L. Wan, H. Zhou, H. Zhou, J. Gu, C. Wang, Q. Liao, H. Gao, J. Wu and X. Huo, Polymers, 2025, 17, 1237 CrossRef CAS PubMed.
- S.-K. Qian, Y.-M. Shen, J.-Z. Shen and G.-P. Cao, Mater. Today Commun., 2025, 112475 CrossRef CAS.
- D. G. Ballard, A. Courtis, I. M. Shirley and S. C. Taylor, J. Chem. Soc. Chem. Commun., 1983, 954–955 RSC.
- A. Abdulkarim, K.-P. Strunk, R. Bäuerle, S. Beck, H. Makowska, T. Marszalek, A. Pucci, C. Melzer, D. Jänsch and J. Freudenberg, Macromolecules, 2019, 52, 4458–4463 CrossRef CAS.
- K.-P. Strunk, A. Abdulkarim, S. Beck, T. Marszalek, J. Bernhardt, S. Koser, W. Pisula, D. Jänsch, J. Freudenberg and A. Pucci, ACS Appl. Mater. Interfaces, 2019, 11, 19481–19488 CrossRef CAS PubMed.
- B. Ikizer, C. W. Lawton and N. Orbey, Polymer, 2021, 228, 123945 CrossRef CAS.
- S. Cai, D. Yan, X. Chen, H. Yang, C. Chen, X. Li, Y. Yan and H. Ren, Polym. Compos., 2024, 45, 1391–1404 CrossRef CAS.
- J. Banerjee, K. Dutta, M. A. Kader and S. K. Nayak, Polym. Adv. Technol., 2019, 30, 1902–1921 CrossRef CAS.
- Y. Huang, H. Li, Z. Wang, M. Zhu, Z. Pei, Q. Xue, Y. Huang and C. Zhi, Nano Energy, 2016, 22, 422–438 CrossRef CAS.
- A. R. Peringath, M. A. Bayan, M. Beg, A. Jain, F. Pierini, N. Gadegaard, R. Hogg and L. Manjakkal, J. Energy Storage, 2023, 73, 108811 CrossRef.
- W. Luo, Y. Ma, T. Li, H. K. Thabet, C. Hou, M. M. Ibrahim, S. M. El-Bahy, B. B. Xu and Z. Guo, J. Energy Storage, 2022, 52, 105008 CrossRef.
- C. Zhao, X. Jia, K. Shu, C. Yu, G. G. Wallace and C. Wang, J. Mater. Chem. A, 2020, 8, 4677–4699 RSC.
- M. Vinayagam, R. S. Babu, A. Sivasamy and A. de Barros, Diamond Relat. Mater., 2025, 154, 112165 CrossRef CAS.
- F. Li, J. Shi and X. Qin, Chin. Sci. Bull., 2010, 55, 1100–1106 CrossRef CAS.
- F. Liu, H. Ge, F. Gao, J. Li, M. Li, Y. Liu, J. Zhang, M. Li, Y. Wang and M. Zhu, Batteries Supercaps, 2025, 2500063 Search PubMed.
- T. M. Alharbi, J. Taibah Univ. Sci., 2024, 18, 2310885 CrossRef.
- D. K. Patel, S.-Y. Won, T. V. Patil, S. D. Dutta, K.-T. Lim and S. S. Han, Int. J. Biol. Macromol., 2024, 265, 131025 CrossRef CAS PubMed.
- A. Bozeya, Y. F. Makableh, L. A. Al-Mezead and R. Abu-Zurayk, Polym. Bull., 2024, 81, 1707–1727 CrossRef CAS.
- S. Simon and L. V. Theresa, Mater. Adv., 2025, 6, 2002–2015 RSC.
- S. Huang, D. Bi, Y. Xia and H. Lin, ACS Appl. Energy Mater., 2023, 6, 856–864 CrossRef CAS.
- A. K. Tawade, S. N. Tayade, D. P. Dubal, S. S. Mali, C. K. Hong and K. K. K. Sharma, Chem. Eng. J., 2024, 492, 151843 CrossRef CAS.
- M. Ates and C. Alperen, Iran. Polym. J., 2023, 32, 1241–1255 CrossRef CAS.
- H. Khanari, M. S. Lashkenari and H. Esfandian, Int. J. Hydrogen Energy, 2024, 68, 27–34 CrossRef CAS.
- A. Umar, F. Ahmed, N. Ullah, S. A. Ansari, S. Hussain, A. A. Ibrahim, H. Qasem, S. A. Kumar, M. A. Alhamami and N. Almehbad, Electrochim. Acta, 2024, 479, 143743 CrossRef CAS.
- C. Arumugam, S. K. Kandasamy, K. Gunasekaran, K. Somasundaram and K. P. Eswaramoorthi, AIP Conf. Proc., 2021, 2387, 090003 CrossRef CAS.
- S. S. Suranshe, A. Patil, T. Deshmukh and J. Chavhan, Electrochim. Acta, 2023, 450, 142277 CrossRef CAS.
- S. Porgar, Chem. Res. Technol., 2025, 2, 170–181 Search PubMed.
- R. Aslani and H. Namazi, Int. J. Pharm., 2023, 636, 122804 CrossRef CAS PubMed.
- X. Liang, L. Zhao, Q. Wang, Y. Ma and D. Zhang, Nanoscale, 2018, 10, 22329–22334 RSC.
- M. Al-Badri and M. Albdiry, J. Mater. Sci.: Mater. Electron., 2022, 33, 675–682 CrossRef CAS.
- A. Aphale, K. Maisuria, M. K. Mahapatra, A. Santiago, P. Singh and P. Patra, Sci. Rep., 2015, 5, 14445 CrossRef CAS PubMed.
- L. Xu, M. Jia, Y. Li, S. Zhang and X. Jin, RSC Adv., 2017, 7, 31342–31351 RSC.
- M. R. Jalali Sarvestani and P. Gholami Dastnaei, Med. Med. Chem., 2024, 1, 31–35 Search PubMed.
- A. B. Adam, M. Y. Abubakar and D. Abubakar, Med. Med. Chem., 2024, 1, 115–128 Search PubMed.
- S. Abrahi Vahed, Med. Med. Chem., 2024, 1, 14–19 Search PubMed.
- R. A. Omer, A. Sdiq, A. F. Qader, M. Salih, E. Abdulkareem, H. Ismail, R. Rashid and U. Raheja, Chem. Rev. Lett., 2025, 8, 612–627 Search PubMed.
- S. Sharanappa, S. Vijaykumar, D. Suresh, A. B. Shbil, H. Ganesha, S. Veeresh, Y. Nagaraju and H. Devendrappa, J. Energy Storage, 2023, 74, 109371 CrossRef.
- C. Zhou, G. Liu, F. Wang, H. Liu, J. Nai, J. Hao, Z. Sui, Z. Yang and W. Xu, J. Alloys Compd., 2024, 992, 174618 CrossRef CAS.
- B. Chen, Q. Yang, Y. Yang, J. Chen, B. Yan, Y. Gu, R. Fu and S. Chen, J. Power Sources, 2025, 633, 236407 CrossRef CAS.
- M. D. Mehare, A. D. Deshmukh and S. Dhoble, J. Nanosci. Nanotechnol., 2020, 20, 3785–3794 CrossRef CAS PubMed.
- Y. Zou, Y. Bu, X. Zhou, M. Hu and M. Zhang, Dalton Trans., 2025, 54, 3722–3732 RSC.
- B. Getiren, H. Altınışık, Z. Çıplak, F. Soysal and N. Yıldız, Synth. Met., 2023, 298, 117451 CrossRef CAS.
- E. Dhandapani, N. Duraisamy and R. Rajedran, ACS Appl. Polym. Mater., 2023, 5, 7420–7432 CrossRef CAS.
- V. Gupta and N. Miura, J. Power Sources, 2006, 157, 616–620 CrossRef CAS.
- C. Meng, C. Liu and S. Fan, Electrochem. Commun., 2009, 11, 186–189 CrossRef CAS.
- X. Wang, Y. Wang, D. Liu, X. Li, H. Xiao, Y. Ma, M. Xu, G. Yuan and G. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 30633–30642 CrossRef CAS PubMed.
- S.-Y. Lee, J.-I. Kim and S.-J. Park, Energy, 2014, 78, 298–303 CrossRef CAS.
- A. ur Rahman, H. Noreen, Z. Nawaz, J. Iqbal, G. Rahman and M. Yaseen, New J. Chem., 2021, 45, 16187–16195 RSC.
- A. Shokry, M. Karim, M. Khalil, S. Ebrahim and J. El Nady, Sci. Rep., 2022, 12, 11278 CrossRef CAS PubMed.
- A. K. Thakur, M. Majumder, R. B. Choudhary and S. N. Pimpalkar, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 149, 012166 Search PubMed.
- A. Alabadi, S. Razzaque, Z. Dong, W. Wang and B. Tan, J. Power Sources, 2016, 306, 241–247 CrossRef CAS.
- X. Lu, H. Dou, C. Yuan, S. Yang, L. Hao, F. Zhang, L. Shen, L. Zhang and X. Zhang, J. Power Sources, 2012, 197, 319–324 CrossRef CAS.
- J. Melo, E. N. Schulz, C. Morales-Verdejo, S. Horswell and M. Camarada, Int. J. Electrochem. Sci., 2017, 12, 2933–2948 CrossRef CAS PubMed.
- Y. He, X. Ning and L. Wan, Polym. Bull., 2022, 79, 9075–9091 CrossRef CAS.
- M. Barakzehi, M. Montazer, F. Sharif, T. Norby and A. Chatzitakis, Electrochim. Acta, 2019, 305, 187–196 CrossRef CAS.
- Q. Wang, H. Wang, D. Liu, P. Du and P. Liu, Synth. Met., 2017, 231, 120–126 CrossRef CAS.
- X. Jian, H.-m. Yang, J.-g. Li, E.-h. Zhang, L.-l. Cao and Z.-h. Liang, Electrochim. Acta, 2017, 228, 483–493 CrossRef CAS.
- Z. Zhao and Y. Xie, J. Power Sources, 2017, 337, 54–64 CrossRef CAS.
- F. M. Omotola, O. O. Olutayo and E. A. Stella, Chem. Res. Technol., 2025, 2, 99–107 Search PubMed.
- M. F. Shehzad, H. M. Abo-Dief, H. A. Elzilal, T. R. Aldhafeeri, S. K. Ali and M. Faizan, J. Indian Chem. Soc., 2025, 101847 CrossRef CAS.
- V. Shanmugavalli and K. Vishista, Mater. Res. Express, 2019, 6, 045021 CrossRef.
- Y. Li, Z. Zhang, Y. Chen, H. Chen, Y. Fan, Y. Li, D. Cui and C. Xue, Appl. Surf. Sci., 2020, 506, 144646 CrossRef CAS.
- U. Basak, P. Ghosh, D. P. Chatterjee, G. Mahapatra, A. Banerjee and A. K. Nandi, J. Mater. Chem. A, 2025, 13, 7813–7833 RSC.
- B. Senthilkumar, K. V. Sankar, C. Sanjeeviraja and R. K. Selvan, J. Alloys Compd., 2013, 553, 350–357 CrossRef CAS.
- S. Panahi and M. Es' haghi, Can. J. Chem., 2018, 96, 477–483 CrossRef CAS.
- Z. Li, Y. Sui, J. Qi, F. Wei, Y. He, Q. Meng, Y. Ren, X. Zhang, Z. Zhan and Z. Sun, Compos. Interfaces, 2020, 27, 631–644 CrossRef CAS.
- P. S. Shukla, A. Agrawal, A. Kumar, A. Gaur and G. D. Varma, Journal of Energy Storage, 2025, 105, 114782 CrossRef.
- C. Pan, Z. Liu, W. Li, Y. Zhuang, Q. Wang and S. Chen, J. Phys. Chem. C, 2019, 123, 25549–25558 CrossRef CAS.
- S. Rajkumar, E. Elanthamilan, J. P. Merlin, I. J. D. Priscillal and I. S. Lydia, Sustainable Energy Fuels, 2020, 4, 5313–5326 RSC.
- Z. Zhao, L. Zheng, H. Li, Z. He, D. Han, J. Shi, B. Xu and H. Wang, Nanotechnology, 2022, 33, 155606 CrossRef CAS PubMed.
- X. Chen and J. Cai, Dalton Trans., 2022, 51, 16587–16595 RSC.
- N. Boutaleb, G. M. Al-Senani, S. D. Al-Qahtani, A. Benyoucef and B. D. Alkoudsi, Colloids Surf., A, 2025, 718, 136867 CrossRef CAS.
- M. N. ur Rehman, T. Munawar, M. S. Nadeem, F. Mukhtar, A. Maqbool, M. Riaz, S. Manzoor, M. N. Ashiq and F. Iqbal, Ceram. Int., 2021, 47, 18497–18509 CrossRef.
- S. Sahoo, G. Dhakal, W. K. Kim, Y. R. Lee and J.-J. Shim, J. Energy Storage, 2023, 73, 109061 CrossRef.
- T. Yu, S. Li, L. Zhang, F. Li, H. Pan and D. Zhang, J. Energy Storage, 2024, 87, 111427 CrossRef.
- J. Dai, C. Yang, Y. Xu, X. Wang, S. Yang, D. Li, L. Luo, L. Xia, J. Li and X. Qi, Adv. Mater., 2023, 35, 2303732 CrossRef CAS PubMed.
- M. Madeshwaran, K. Rajni and M. Ulaganathan, Mater. Today Chem., 2024, 42, 102390 CrossRef CAS.
- K. Li, Z. Li, J. Cui, B. Zhou, W. Dong, B. Zhang and C. Yang, ChemistrySelect, 2024, 9, e202304564 CrossRef CAS.
- Y. Ye, X. Guo, Y. Ma, Q. Zhao, Y. Sui, J. Song, W. Ma, P. Zhang and C. Qin, J. Electroanal. Chem., 2021, 897, 115588 CrossRef CAS.
- Q. Wu, Y. Zhang, Y. Lin, W. Wei, G. Liu, X. Cui, M. Su, H. Jiang, T. Wu and X. Li, ACS Appl. Mater. Interfaces, 2023, 15, 46971–46981 CrossRef CAS PubMed.
- X. Li, H. Xie, Y. Feng, Y. Qu, L. Zhai, H. Sun, X. Liu and C. Hou, J. Appl. Polym. Sci., 2023, 140, e54580 CrossRef CAS.
- R. Nagaraj, K. Aruchamy, D. Mondal, S. K. Nataraj and D. Ghosh, J. Electroanal. Chem., 2019, 851, 113482 CrossRef CAS.
- A. S. Almalki, J. Mater. Sci.: Mater. Electron., 2024, 35, 581 CrossRef CAS.
- S. Ramesh, K. Karuppasamy, H. Yadav, Y.-J. Lee, A. Sivasamy, A. Kathalingam, H.-S. Kim, J.-H. Kim and H. S. Kim, J. Energy Storage, 2023, 67, 107518 CrossRef.
- T. Mehmood, A. B. Ali, A. Kumar, S. Gouadria, J. Makasana, S. Ballal, K. Chennakesavulu, J. Nanda, R. Chaudhary and A. D. Oza, J. Alloys Compd., 2025, 180887 CrossRef CAS.
- M. B. Gholivand, H. Heydari, A. Abdolmaleki and H. Hosseini, Mater. Sci. Semicond. Process., 2015, 30, 157–161 CrossRef CAS.
- Z. Hai, L. Gao, Q. Zhang, H. Xu, D. Cui, Z. Zhang, D. Tsoukalas, J. Tang, S. Yan and C. Xue, Appl. Surf. Sci., 2016, 361, 57–62 CrossRef CAS.
- Y. Fan, H. Chen, Y. Li, D. Cui, Z. Fan and C. Xue, Ceram. Int., 2021, 47, 8433–8440 CrossRef CAS.
- F. S. Omar, A. Numan, N. Duraisamy, M. M. Ramly, K. Ramesh and S. Ramesh, Electrochim. Acta, 2017, 227, 41–48 CrossRef CAS.
- X. Ren, H. Fan, J. Ma, C. Wang, M. Zhang and N. Zhao, Appl. Surf. Sci., 2018, 441, 194–203 CrossRef CAS.
- S. Rajkumar, E. Elanthamilan, J. P. Merlin and A. Sathiyan, J. Alloys Compd., 2021, 874, 159876 CrossRef CAS.
- M. Usman, M. Adnan, M. T. Ahsan, S. Javed, M. S. Butt and M. A. Akram, ACS Omega, 2021, 6, 1190–1196 CrossRef CAS PubMed.
- J. Yesuraj, V. Elumalai, M. Bhagavathiachari, A. S. Samuel, E. Elaiyappillai and P. M. Johnson, J. Electroanal. Chem., 2017, 797, 78–88 CrossRef.
- A. N. Naveen and S. Selladurai, Mater. Sci. Semicond. Process., 2015, 40, 468–478 CrossRef.
- Y. V. Naik, R. Naik, H. Nagaswarupa, J. H. Kim, J.-H. Jung, N. T. N. Truong and G. Koyyada, Inorg. Chem. Commun., 2025, 114808 CrossRef CAS.
- H. Y. Kalyon, Y. F. Karasan and M. Gencten, Nanotechnology, 2025, 36, 215402 CrossRef CAS PubMed.
- A. Qamar, A. Kumar, F. Alharbi, J. Makasana, M. Rekha, G. S. Kumar, M. A. Al-Anber, S. N. Das, R. R. Chaudhary and A. D. Oza, J. Indian Chem. Soc., 2025, 101771 CrossRef CAS.
- K. Kavya, K. Kalawat, P. Kour, S. Kour and A. Sharma, Mater. Res. Bull., 2025, 184, 113270 CrossRef.
- E. Bukhsh, A. Kumar, A. Yadav, A. S. Alqarni, R. Sharma, G. C. Sharma, V. K. Pandey, S.-C. Kim and V. Mishra, J. Alloys Compd., 2025, 1022, 179441 CrossRef CAS.
- N. Awoke, G. Beyene, F. Tolassa, M. Asfaw, P. M. Ejikeme, A. C. Nwanya and F. I. Ezema, ChemistrySelect, 2025, 10, e01289 CrossRef CAS.
- S. Abirami, E. Kumar, B. Vigneshwaran and P. Vijayalakshmi, Electrochim. Acta, 2025, 146512 Search PubMed.
- L. Li, Z. Wei, J. Liang, J. Ma and S. Huang, Results Chem., 2021, 3, 100205 CrossRef CAS.
- A. Gamal, M. Shaban, M. BinSabt, M. Moussa, A. M. Ahmed, M. Rabia and H. Hamdy, Nanomaterials, 2022, 12, 817 CrossRef CAS PubMed.
- Q. Chen, F. Xie, G. Wang, K. Ge, H. Ren, M. Yan, Q. Wang and H. Bi, Ionics, 2021, 27, 4083–4096 CrossRef CAS.
- H. Heydari, M. Abdouss, S. Mazinani, A. M. Bazargan and F. Fatemi, J. Energy Storage, 2021, 40, 102738 CrossRef.
- A. Mindil, H. Hassan, M. W. Iqbal, A. M. Afzal, N. Amri and N. Hadia, Mater. Chem. Phys., 2023, 306, 128077 CrossRef CAS.
- P. Elumalai, J. Charles and L. J. Kennedy, Ionics, 2024, 30, 7397–7420 CrossRef CAS.
- A. B. Adam, K. M. Mahmood, M. Y. Abubakar and F. S. Umar, Chem. Res. Technol., 2025, 2, 27–37 Search PubMed.
- J. Wang, G. Xiao, T. Zhang, S. Hao, Z. Jia and Y. Li, J. Alloys Compd., 2021, 863, 158071 CrossRef CAS.
- Y. Guo, J. Chang, L. Hu, Y. Lu, S. Yao, X. Su, X. Zhang, H. Zhang and J. Feng, ChemSusChem, 2024, 17, e202301148 CrossRef CAS PubMed.
- K. Yasoda, S. Kumar, M. Kumar, K. Ghosh and S. Batabyal, Mater. Today Chem., 2021, 19, 100394 CrossRef CAS.
- M. Premkumar and S. Vadivel, J. Energy Storage, 2023, 69, 107948 CrossRef.
- N. Nabeel, A. Jain, K. C. Juglan and S. Naeem, Trans. Electr. Electron. Mater., 2025, 1–16 Search PubMed.
- E. Azizi, J. Arjomandi, H. Shi and M. A. Kiani, J. Energy Storage, 2024, 75, 109665 CrossRef.
- Y. Fu, Y. Dong, X. Zhang, H. Niu, C. Qin and X. Jiang, J. Mater. Sci., 2025, 1–18 Search PubMed.
- S. Aslam, F. Shaheen, R. Ahmad, S. M. Ali and Q. Huang, J. Energy Storage, 2024, 85, 111065 CrossRef.
- R. Kalpana and P. Subbramaniyan, Int. Res. J. Multidiscip. Technovation, 2024, 6, 40–50 Search PubMed.
- R. R. Atram, V. M. Bhuse, R. G. Atram, C.-M. Wu, P. Koinkar and S. B. Kondawar, Mater. Chem. Phys., 2021, 262, 124253 CrossRef CAS.
- A. M. Afzal, M. W. Iqbal, M. Imran, H. Umair, S. M. Wabaidur, E. A. Al-Ammar, S. Mumtaz and E. H. Choi, ECS J. Solid State Sci. Technol., 2023, 12, 051003 CrossRef CAS.
- P. Haldar, J. Mater. Sci.: Mater. Electron., 2020, 31, 7905–7915 CrossRef CAS.
- S. Hema and D. Geetha, ECS J. Solid State Sci. Technol., 2025, 14, 051006 CrossRef.
- B. Zhou, Z. Li, D. Qin, Q. Zhang, M. Yu and C. Yang, J. Alloys Compd., 2023, 956, 170327 CrossRef CAS.
- H. Heydari and M. B. Gholivand, J. Mater. Sci.: Mater. Electron., 2017, 28, 3607–3615 CrossRef CAS.
- A. G. Tabrizi, N. Arsalani, Z. Naghshbandi, L. S. Ghadimi and A. Mohammadi, Int. J. Hydrogen Energy, 2018, 43, 12200–12210 CrossRef CAS.
- S. Verma, V. K. Pandey and B. Verma, Synth. Met., 2022, 286, 117036 CrossRef CAS.
- Z. Zhang, L. Feng, P. Jing, X. Hou, G. Suo, X. Ye, L. Zhang, Y. Yang and C. Zhai, J. Colloid Interface Sci., 2021, 588, 84–93 CrossRef CAS PubMed.
- M. Jasna, M. M. Pillai, A. Abhilash, P. Midhun, S. Jayalekshmi and M. Jayaraj, Carbon Trends, 2022, 7, 100154 CrossRef CAS.
- X. Cheng, D. Wang, H. Ke, Y. Li, Y. Cai and Q. Wei, Compos. Commun., 2022, 30, 101073 CrossRef.
- X. Hong, X. Wang, Y. Li, C. Deng and B. Liang, Electrochim. Acta, 2022, 403, 139571 CrossRef CAS.
- M. G. Hosseini, E. Shahryari and P. Yardani Sefidi, J. Appl. Polym. Sci., 2021, 138, 50976 CrossRef CAS.
- M. Sadiq, M. Islam, M. Moharam, E. A. M. Saleh and S. U. Asif, J. Mater. Sci.: Mater. Electron., 2024, 35, 1011 CrossRef CAS.
- Y. Song, Z. Su, Z. Zhao, S. Lin and D. Wang, Ceram. Int., 2021, 47, 21367–21372 CrossRef CAS.
- C. Chen, S. Wei, Q. Zhang, H. Yang, J. Xu, L. Chen and X. Liu, J. Colloid Interface Sci., 2024, 664, 53–62 CrossRef CAS PubMed.
- Q. Liu, S. Zhang and Y. Xu, Nanomaterials, 2020, 10, 1034 CrossRef CAS PubMed.
- A. K. Ghasemi, M. Ghorbani, M. S. Lashkenari and N. Nasiri, Electrochim. Acta, 2023, 439, 141685 CrossRef CAS.
- P. Haldar, S. Biswas, V. Sharma, A. Chowdhury and A. Chandra, Appl. Surf. Sci., 2019, 491, 171–179 CrossRef CAS.
- R. Boddula, R. Bolagam and P. Srinivasan, Ionics, 2018, 24, 1467–1474 CrossRef CAS.
- S. P. Lonkar, V. Gupta, S. M. Alhassan and A. Schiffer, Energy Storage, 2023, 5, e416 CrossRef CAS.
- L. Wang, M. Bo, Z. Guo, H. Li, Z. Huang, H. Che, Z. Feng, Y. Wang and J. Mu, J. Colloid Interface Sci., 2020, 577, 29–37 CrossRef CAS PubMed.
- E. Harini, D. Rani, M. Afshan, M. Pahuja, N. Chaudhary, S. Rani, S. A. Siddiqui, S. Das, S. Sharangi and R. Ghosh, Chem. Eng. J., 2024, 498, 155112 CrossRef.
- G. Singh, Y. Kumar and S. Husain, Energy Technol., 2023, 11, 2200931 CrossRef CAS.
- N. Farooq, P. Kallem, M. I. Khan, A. M. Qureshi, A. Shanableh and A. U. Rehman, J. Mater. Res. Technol., 2023, 26, 7127–7136 CrossRef CAS.
- T. Abdullah, S. I. Shamsah, I. A. Shaaban, M. Akhtar and S. Yousaf, Synth. Met., 2023, 299, 117472 CrossRef CAS.
- M. Geerthana, S. Prabhu, S. Harish, M. Navaneethan, R. Ramesh and M. Selvaraj, J. Mater. Sci.: Mater. Electron., 2022, 33, 8327–8343 CrossRef CAS.
- M. R. Abdul Karim, W. Shehzad, M. Atif, E. u. Haq and Z. Abbas, Energy Environ., 2024, 0958305X231221260 Search PubMed.
- Y. Chen, H. He, M. Liu, H. Xu, H. Zhang, X. Zhu and D. Yang, Nanomaterials, 2025, 15, 641 CrossRef CAS PubMed.
- S. Ma, W. Wang, R. Huang, J. Hou, X. Wang, X. Che, Q. Ren, Y. Li and C. Hou, Appl. Organomet. Chem., 2024, 38, e7497 CrossRef CAS.
- V. Aswathi and P. Sreeja, J. Energy Storage, 2025, 107, 114993 CrossRef.
- S. Abbas, Z. M. Elqahtani, G. Yasmeen, S. Manzoor, S. Manzoor, M. Al-Buriahi, Z. Alrowaili and M. N. Ashiq, J. Korean Ceram. Soc., 2023, 60, 127–140 CrossRef CAS.
- H. Deng, J. Huang, Z. Hu, X. Chen, D. Huang and T. Jin, ACS Omega, 2021, 6, 9426–9432 CrossRef CAS PubMed.
- P. Asen and S. Shahrokhian, Int. J. Hydrogen Energy, 2017, 42, 21073–21085 CrossRef CAS.
- J. P. Jyothibasu, M.-Z. Chen, Y.-C. Tien, C.-C. Kuo, E.-C. Chen, Y.-C. Lin, T.-C. Chiang and R.-H. Lee, Catalysts, 2021, 11, 980 CrossRef CAS.
- P. Chahal, S. L. Madaswamy, S. C. Lee, S. M. Wabaidur, V. Dhayalan, V. K. Ponnusamy and R. Dhanusuraman, Fuel, 2022, 330, 125531 CrossRef CAS.
- Z. Qin, Y. Xu, L. Liu, M. Liu, H. Zhou, L. Xiao, Y. Cao and C. Chen, RSC Adv., 2022, 12, 29177–29186 RSC.
- R. Srinivasan, E. Elaiyappillai, E. J. Nixon, I. S. Lydia and P. M. Johnson, Inorg. Chim. Acta, 2020, 502, 119393 CrossRef CAS.
- T. Ebenezer, I. Johnson, W. Galeb and J. S. K. Arockiasamy, Electrochim. Acta, 2024, 507, 145130 CrossRef.
- P. Dubey, V. Shrivastav, S. Sundriyal and P. H. Maheshwari, ACS Appl. Nano Mater., 2024, 7, 18554–18565 CrossRef CAS.
- D. Qin, B. Zhou, Z. Li and C. Yang, J. Mol. Struct., 2024, 1309, 138140 CrossRef CAS.
- A. Revathi, D. J. Williams, D. Sudha and R. Boopathiraja, J. Mater. Sci.: Mater. Electron., 2023, 34, 1175 CrossRef CAS.
- S.-X. Zhou, X.-Y. Tao, J. Ma, L.-T. Guo, Y.-B. Zhu, H.-L. Fan, Z.-S. Liu and X.-Y. Wei, Vacuum, 2018, 149, 175–179 CrossRef CAS.
- A. Atta, R. Altuijri, N. Al-Harbi and M. Abdelhamied, ECS J. Solid State Sci. Technol., 2025, 14, 043015 CrossRef CAS.
- S. Kumar, P.-H. Weng and Y.-P. Fu, Mater. Today Chem., 2023, 28, 101385 CrossRef CAS.
- M. Rani, B. Zaheer, F. Sajid, A. Ibrahim, A. A. Shah and A. D. Chandio, J. Inorg. Organomet. Polym. Mater., 2025, 1–15 Search PubMed.
- N. Saxena, M. P. Bondarde, K. D. Lokhande, M. A. Bhakare, P. S. Dhumal and S. Some, Chem. Phys. Lett., 2024, 856, 141605 CrossRef CAS.
- O. Salim, K. Mahmoud, K. Pant and R. Joshi, Mater. Today Chem., 2019, 14, 100191 CrossRef CAS.
- Y. Li, P. Kamdem and X.-J. Jin, J. Alloys Compd., 2021, 850, 156608 CrossRef CAS.
- N. Tyagi, G. Sharma and M. K. Singh, Polymer, 2025, 326, 128328 CrossRef.
- X. Wang, D. Zhang, H. Zhang, L. Gong, Y. Yang, W. Zhao, S. Yu, Y. Yin and D. Sun, Nano Energy, 2021, 88, 106242 CrossRef CAS.
- W. Bai, Z. Yong, S. Wang, X. Wang, C. Li, F. Pan, D. Liang, Y. Cui and Z. Wang, J. Energy Storage, 2023, 71, 108053 CrossRef.
- W. L. Liu, Y. Q. Guo, T. Lin, H. C. Peng, Y. P. Yu, F. Yang and S. Chen, J. Alloys Compd., 2022, 926, 166855 CrossRef CAS.
- T. Chen, M. Li, Y. Li, S. Song, J. Kim and J. Bae, Mater. Sci. Eng., B, 2023, 290, 116354 CrossRef CAS.
- G. Ma, W. Bai, X. Zhou, X. Guan, S. Zhang, W. Wu, C. Li and S. Wang, Chem. Eng. J., 2024, 496, 153730 CrossRef CAS.
- W. Luo, Y. Wei, Z. Zhuang, Z. Lin, X. Li, C. Hou, T. Li and Y. Ma, Electrochim. Acta, 2022, 406, 139871 CrossRef CAS.
- J. Fu, J. Yun, S. Wu, L. Li, L. Yu and K. H. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 34212–34221 CrossRef CAS PubMed.
- Y.-Z. Cai, Y.-S. Fang, W.-Q. Cao, P. He and M.-S. Cao, J. Alloys Compd., 2021, 868, 159159 CrossRef CAS.
- A. VahidMohammadi, J. Moncada, H. Chen, E. Kayali, J. Orangi, C. A. Carrero and M. Beidaghi, J. Mater. Chem. A, 2018, 6, 22123–22133 RSC.
- B. Chen, Q. Song, Z. Zhou and C. Lu, Adv. Mater. Interfaces, 2021, 8, 2002168 CrossRef CAS.
- T. He, X. Li, B. Sun, L. Lin, F. Guo, G. Diao, Y. Piao and W. Zhang, RSC Adv., 2024, 14, 13685–13693 RSC.
- Z. Li, J. Li, B. Wu, H. Wei, H. Guo, Z. M. El-Bahy, B. Liu, M. He, S. Melhi and X. Shi, J. Mater. Sci. Technol., 2024, 203, 201–210 CrossRef CAS.
- W. Wu, C. Wang, C. Zhao, D. Wei, J. Zhu and Y. Xu, J. Colloid Interface Sci., 2020, 580, 601–613 CrossRef CAS PubMed.
- A. Zhang, Y. Wang, H. Yu and Y. Zhang, Materials, 2025, 18, 2277 CrossRef CAS PubMed.
- J. Wang, D. Jiang, M. Zhang, Y. Sun, M. Jiang, Y. Du and J. Liu, J. Mater. Chem. A, 2023, 11, 1419–1429 RSC.
- Y. Zou, H. Liu, G. Liu, B. Yang, J. Li, S. Wang, K. Xie, C. Wang and S. Iqbal, J. Energy Storage, 2025, 120, 116373 CrossRef.
- X. Wu, W. Hu, J. Qiu, B. Geng, M. Du and Q. Zheng, J. Alloys Compd., 2022, 921, 166062 CrossRef CAS.
- L. Xu, W. Wang, Y. Liu and D. Liang, Gels, 2022, 8, 798 CrossRef CAS PubMed.
- W. Luo, Y. Sun, Y. Han, J. Ding, T. Li, C. Hou and Y. Ma, Electrochim. Acta, 2023, 441, 141818 CrossRef CAS.
- D. Wei, W. Wu, J. Zhu, C. Wang, C. Zhao and L. Wang, J. Electroanal. Chem., 2020, 877, 114538 CrossRef CAS.
- I. Pathak, D. Acharya, K. Chhetri, P. C. Lohani, T. H. Ko, A. Muthurasu, S. Subedi, T. Kim, S. Saidin and B. Dahal, Chem. Eng. J., 2023, 469, 143388 CrossRef CAS.
- W. Wu, D. Wei, J. Zhu, D. Niu, F. Wang, L. Wang, L. Yang, P. Yang and C. Wang, Ceram. Int., 2019, 45, 7328–7337 CrossRef CAS.
- S. Maity, S. Bera, A. Kapuria, A. Debnath, S. Das and S. K. Saha, Mater. Today Chem., 2025, 45, 102690 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |