Open Access Article
Open Access ArticleNovel bakuchiol derivatives inhibit the migration and invasion of non-small cell lung cancer by suppressing epithelial-to-mesenchymal transition
Meng-Fan Xua,
Ke Zhonga,
Jing Zhua,
Jie Chena,
Fang Liuab,
Feng Dingab,
Cheng-Zhu Wu*ab and
Long Zhao *ab
*ab
aSchool of Pharmacy, Bengbu Medical University, Bengbu, Anhui, China. E-mail: wuchengzhu0611@bbmu.edu.cn; zhaolong@bbmu.edu.cn
bAnhui Province Biochemical Pharmaceutical Engineering Technology Research Center, Bengbu, Anhui, China
First published on 19th November 2025
Abstract
Recently, bakuchiol and its derivatives have been widely investigated owing to their anticancer activity. However, there are few studies on the inhibition of epithelial–mesenchymal transition (EMT) by bakuchiol and its derivatives. Besides, thiosemicarbazones (TSCs) have also been demonstrated to exhibit potential EMT-inhibitory effects. Hence, the cytotoxic activity of novel bakuchiol derivatives containing thiosemicarbazone moieties was evaluated against two non-small lung cancer cell lines. Among them, compound 2f demonstrated the highest activity, with IC50 values of 2.23 and 5.55 µM against PC9 and H1975 cells, respectively. Compound 2f effectively suppresses tumor cell proliferation by inducing cell cycle arrest and promoting apoptosis. Additionally, various evidence after treatment with compound 2f in PC9 cells, including the inhibition of migration and invasion, the enhancement of E-cadherin expression and reduction of vimentin levels, indicated the role of bakuchiol derivatives in inhibiting EMT. These findings suggest that bakuchiol derivatives could be positioned as potential candidates for cancer treatment.
Introduction
EMT is a complex biological process that is crucial in various physiological and pathological contexts, such as embryonic development, wound healing, fibrosis, and cancer metastasis.1 During EMT, epithelial cells undergo programmed changes, which enable them to lose their characteristic cell–cell adhesion and polarity and acquire mesenchymal-like properties, enhancing their ability to invade surrounding tissues and migrate to new locations.2–4 In addition, these are accompanied by changes in epithelial cell markers, such as a decrease in cytokeratins and E-cadherin and increase in N-cadherin, vimentin and snail.5,6 This transition allows epithelial cells to become more motile, invasive, and resistant to apoptosis, characteristics that are crucial for cancer cells to metastasize and spread to distant organs.7 EMT is regulated by a complex network of signaling pathways and transcription factors, and its dysregulation has been implicated in the progression of various cancers.8 Therefore, developing new compounds that can be used to suppress EMT is essential for developing targeted therapies to inhibit cancer metastasis and improve patient outcomes.Non-small cell lung cancer (NSCLC) accounts for the majority of all lung cancers, which encompasses various subtypes, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma.9,10 NSCLC typically originates from the epithelial cells lining the airways and is frequently linked to a history of smoking or exposure to carcinogens.11,12 In the context of NSCLC, EMT has been implicated in promoting the invasiveness and metastatic potential of cancer cells.13,14
Bakuchiol, a natural compound found in the seeds of the Psoralea corylifolia plant, has been demonstrated to play an important role in preventing the development of lung cancer cells.15–18 Studies have shown that by inducing apoptosis and cell cycle arrest, bakuchiol can effectively restrict the growth and spread of lung cancer cells.15 Moreover, bakuchiol has shown antioxidant and anti-inflammatory properties, which could help in decreasing inflammation and oxidative stress linked to the development of cancer.19–22 Additionally, bakuchiol has been found to inhibit the process of EMT in lung cancer cells.16
TSCs are a class of organic compounds that have gained attention for their potential application in anti-tumor.23–27 TSCs have shown synergistic effects when combined with conventional chemotherapy drugs, enhancing their efficacy and reducing drug resistance.28–32 Alternatively, TSCs function as iron chelators, exerting anti-tumour and anti-metastatic effects by upregulating Ndrg-1. Ndrg-1 suppresses TGF-β-induced EMT in tumour cells by stabilizing E-cadherin/β-catenin adhesion complexes and enhancing intercellular adhesion.33 In 2020, Liu et al. reported the synthesis of a series of TSC-based lead compounds, which exhibited excellent antiproliferative activity against MGC803 cells and inhibited EMT.34 Consequently, by employing the molecular hybridization strategy, we hope to link TSCs with bakuchiol via a condensation reaction and further explore their application in the treatment of NSCLC (Fig. 1). In this work, a series of novel bakuchiol derivatives were designed and synthesized to enhance the anti-cancer activity of bakuchiol. Their cytotoxicity was evaluated against two non-small cell lung cancer cell lines. Furthermore, we found that the bakuchiol derivatives exhibited good inhibition of migration and invasion, which was manifested by inhibiting EMT.
 | ||
| Fig. 1 Molecular hybridization strategy for the design of bakuchiol derivatives.16,32,34 | ||
Results and discussion
Chemistry
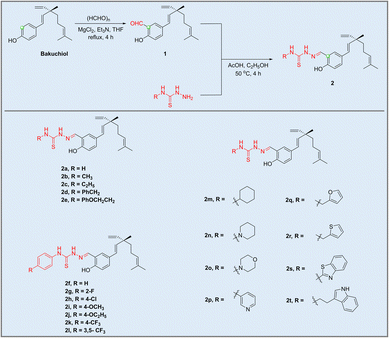
As depicted in Scheme 1, bakuchiol derivatives (2a–2t) were designed and synthesized via the condensation reaction between 2-formyl-bakuchiol 1 and different commercially available substituted thiosemicarbazides. Notably, a series of alkyl, substituted benzene and heterocyclic rings were considered in this study, showcasing the versatility and potential of these derivatives in further applications. The chemical structures of the bakuchiol derivatives were characterized using 1H-NMR, 13C-NMR and HR-ESI-MS (see SI).Biological evaluation
| Compound | PC9 | H1975 |
|---|---|---|
| a Positive control. | ||
| 1 | 22.42 | 13.77 |
| 2a | 18.56 | 15.75 |
| 2b | 38.12 | >100 |
| 2c | 14.84 | 15.84 |
| 2d | 4.94 | 13.08 |
| 2e | 14.16 | 11.55 |
| 2f | 2.23 | 5.55 |
| 2g | 8.57 | 34.44 |
| 2h | 8.75 | 12.16 |
| 2i | 7.46 | 18.15 |
| 2j | 10.12 | 20.43 |
| 2k | 6.88 | 37.80 |
| 2l | 16.39 | 18.28 |
| 2m | 28.02 | 25.96 |
| 2n | 30.21 | 67.41 |
| 2o | 20.48 | 34.67 |
| 2p | 10.80 | 12.56 |
| 2q | 11.14 | 14.02 |
| 2r | 17.49 | 15.41 |
| 2s | 78.88 | >100 |
| 2t | 11.35 | 16.75 |
| Bakuchiol | 4.73 | 11.82 |
| Alkannina | 5.62 | 5.27 |
| Compound | AmI-12 |
|---|---|
| 2f | 19.00 ± 1.46 |
| Alkannin | 14.50 ± 2.08 |
Anti-proliferative activity of compound 2f
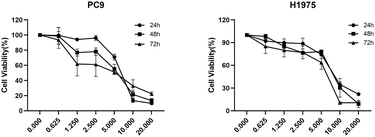
The remarkable findings presented in Fig. 2 unveil the compelling concentration- and time-dependent anti-proliferative potential of compound 2f against the PC9 and H1975 human lung cancer cell lines. Notably, this study also revealed the varying sensitivity of these two cell lines to the growth-inhibiting effects of compound 2f, with PC9 cells emerging as particularly susceptible to its remarkable action.Effects of compound 2f on cell cycle phase arrest
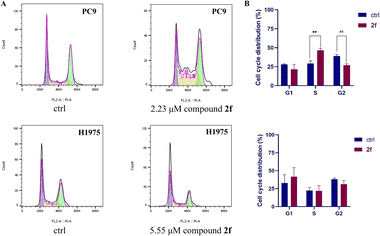
The effects of compound 2f on cell cycle phase arrest in PC9 and H1975 cells were evaluated at its IC50 by flow cytometry. After treatment for 24 h with compound 2f, the cells were harvested and analyzed by flow cytometry. The results indicated that compound 2f can prolong the S phase and shorten the G1 and G2 phases in PC9 cells compared with the control group. The cell cycle phase arrest of compound 2f in H1975 cells is different from that in PC9 cells, where compound 2f prolongs the G1 phase and shortens the S and G2 phases. The above-mentioned results suggested that compound 2f induces cell cycle phase arrest in PC9 and H1975 cells (Fig. 3).Compound 2f-induced apoptosis in PC9 cells
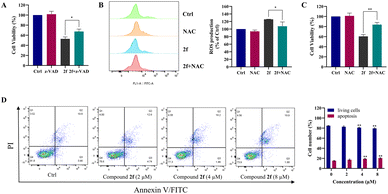
To functionally confirm the engagement of apoptotic pathways, we utilized a chemical inhibition strategy with z-VAD-FMK. The cell viability of PC9 cells was rescued after co-treatment with compound 2f and z-VAD-FMK, which demonstrates that the suppression of proliferation is contingent on the induction of apoptosis (Fig. 4A). To further elucidate the role of reactive oxygen species (ROS) in cell death, N-acetylcysteine (NAC), a known ROS scavenger, was employed to assess the intracellular ROS levels. As depicted in Fig. 4B, treatment with compound 2f (4 µM) alone resulted in a 126% increase in intracellular ROS. However, this elevation was significantly reduced to 107% upon co-treatment with NAC (p < 0.05). Furthermore, NAC exhibited a protective effect against compound 2f-induced cell death in PC9 cells (Fig. 4C, p < 0.01), supporting the involvement of ROS in the cytotoxic mechanism. Apoptosis was quantified via annexin V/PI dual staining, followed by flow cytometric analysis. Treatment of PC9 cells with compound 2f for 24 h resulted in a dose-dependent increase in apoptotic cell death. As illustrated in Fig. 4D, the percentage of apoptotic cells reached 17.2%, 19.0%, and 20.6% at concentrations of 2, 4, and 8 µM, respectively. These results indicated that compound 2f induced apoptosis in the PC9 cells.Compound 2f inhibits the migration and invasion of PC9 cells
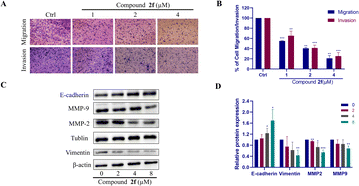
Given that most cases of lung cancer are detected only in the final stages of the disease, high mortality is strongly associated with metastasis.37 Migration and invasion and are critical processes involved in tumour metastasis, with cancer cells breaking away from the primary tumour site and spreading to distant organs. Understanding how compounds can affect these processes can help in developing targeted therapies to inhibit metastasis and improve patient outcomes.38 Hence, we examined the impact of compound 2f on the capabilities of migration and invasion in PC9 cells. The migration and invasion ability of the PC9 cells was reduced as the concentration of compound 2f increased. In conclusion, compound 2f has concentration-dependent ability to dramatically suppress PC9 cell migration and invasion (Fig. 5A, B, 63S and 64S). Moreover, higher levels of MMP2 and MMP9 have been linked to greater cell invasion and migratory capacities.39 The results of western blotting demonstrated the down-regulation of MMP2 and MMP9 expression, supporting the proposal that compound 2f might prevent PC9 cells from migrating and invading. Furthermore, to assess the potential impact of compound 2f on EMT, western blotting also was performed to analyse the expression of mesenchymal markers (vimentin) and epithelial markers (E-cadherin). We found that the expression of E-cadherin was upregulated and the expression of vimentin was downregulated after treatment with compound 2f in PC9 cells. The findings demonstrated that compound 2f successfully suppressed the EMT process of PC9 cells (Fig. 5C and D).Compound 2f inhibits the migration and invasion of PC9 cells induced by TGF-β
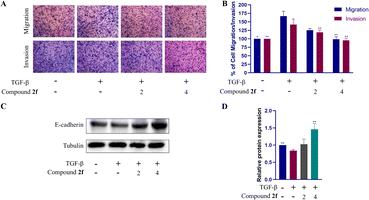
Migration and invasion are closely linked to EMT because EMT is a process that enables epithelial cells to acquire migratory and invasive properties, allowing them to metastasize to distant sites in the body.3,40,41 Besides, TGF-β, which is a key regulator of EMT, can induce EMT, promoting the migration and invasion of cancer cells in the stage of tumour progression. Therefore, following TGF-β induction, the inhibitory effects of bakuchiol derivatives on their migration and invasion were investigated, which are closely related with the effects of bakuchiol derivatives on EMT.42,43We examined the effects of compound 2f on the migration and invasion of PC9 cells induced by TGF-β. The migration and invasion ability of PC9 cells was significantly enhanced after induction with 30 ng per mL TGF-β. Compared with the control group, the enhancement amplitude of migration and invasion was 1.7-fold and 1.4-fold, respectively. The results indicated that TGF-β successfully induced the migration and invasion of PC9 cells. After treatment with compound 2f, the migration and invasion ability of the PC9 cells was dramatically inhibited in a dose-dependent manner (Fig. 6A, B, S65 and S66). The above-mentioned data suggested that compound 2f inhibited the migration and invasion of PC9 cells induced by TGF-β associated with EMT. To examine the impact of compound 2f on EMT caused by TGF-β, we used western blotting to assess the E-cadherin expression levels. The findings demonstrated that following TGF-β stimulation, E-cadherin expression declined and compound 2f restored the expression of E-cadherin. Finally, compound 2f can reverse the TGF-β-induced EMT process (Fig. 6C and D).
In vivo antitumor efficacy and biosafety
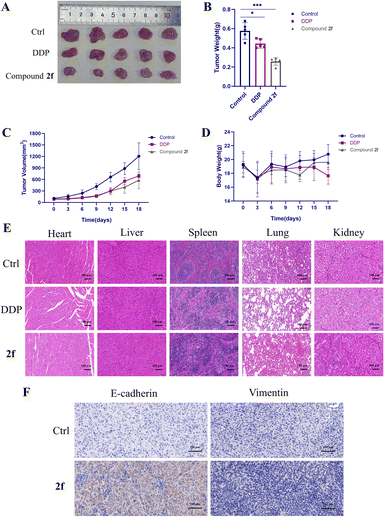
To further assess the in vivo antitumor efficacy of compound 2f, we established a Balb/c nude mouse model with subcutaneous PC9 tumours. When the size of the tumour reached 100 mm3, the nude mice were randomly assigned to three groups, as follows: saline, DDP, and compound 2f. Compared with the control group, compound 2f showed a more obvious tumour inhibition effect and weaker weight change in the nude mice after three times intraperitoneal administration (Fig. 7C). After 18 days of treatment, all the nude mice were killed by neck removal to remove their tumours and organs. Compared with the control group, compound 2f can effectively inhibit tumour growth and has less effect on the body weight of the nude mice. The DDP group also showed similar tumour suppression but had a higher impact on the body weight of the nude mice (Fig. 7A–D). The results of the histopathological examination showed that compound 2f had no obvious damage to the organs of the nude mice (Fig. 7E). Then, we examined the expression of E-cadherin and vimentin in the xenografts by IHC. Similar to the results in the in vitro study, IHC analysis revealed that compound 2f can effectively inhibit the progression of EMT, where the expression of E-cadherin significantly increased, while the expression of vimentin diminished (Fig. 7F). The above-mentioned result indicated that bakuchiol derivatives might reverse TGF-β-induced EMT by modulating the E-cadherin and vimentin levels. In conclusion, these findings revealed that compound 2f has excellent anti-tumour activity in vivo.Conclusions
In this study, we aimed to enhance the activity of bakuchiol derivatives through molecular hybridization. We successfully introduced TSCs with different substituents at the α-position of the hydroxyl group through Friedel–Crafts acylation and condensation reactions. A series of bakuchiol derivatives (2a–2t) containing thiosemicarbazone moieties were designed, synthesized and evaluated for their anti-cancer activities in vitro against two human lung cancer cell lines (PC9 and H1975 cells). The bakuchiol derivatives with benzene rings in TSCs exhibited better cytotoxicity compared with alkyl- and heterocyclic-substituted TSC bakuchiol derivatives. Especially, compound 2f demonstrated significant inhibition of PC9 and H1975 cell proliferation in vitro, along with highly effective anti-tumour effects in vivo. Besides, histopathological assessment of major organs revealed no apparent treatment-related lesions following the administration of compound 2f. Coupled with the absence of significant body weight loss, these findings suggest a favorable preliminary safety profile for compound 2f at the tested doses. The cell cycle results also indicated that compound 2f can cause cell cycle phase arrest. Moreover, in conjunction with its pro-apoptotic effects, these results suggest that compound 2f exerts its antiproliferative activity through both cell cycle disruption and apoptosis induction. Compound 2f significantly inhibited the invasion and migration of PC9 cells induced by TGF-β. Additionally, it was observed that compound 2f suppressed EMT by regulating the expression of E-cadherin and vimentin. These findings suggest that bakuchiol derivatives have potential as anti-tumour agents in the discovery and development of anti-NSCLC drugs.Experimental
Materials and characterization
All commercially available compounds were used without further purification. TLC plates and column chromatography silica gel (300–400 mesh) were purchased from Qingdao Haiyang Chemical Co. NMR spectra were recorded on a Bruker Avance II (300 MHz or 400 MHz) instrument. HRMS spectra were obtained using a Thermo Scientific LTQ Orbitrap XL mass spectrometer (Bruker, Bremerhaven, Germany).General procedure for the synthesis of compound 1
To a solution of bakuchiol (3 g, 11.7 mmol) in anhydrous THF (18 mL), MgCl2 (2.2 g, 23.4 mmol), (HCHO)n (1.1 g, 35.1 mmol) and Et3N (3.2 mL, 23.4 mmol) were added, and the reaction was kept at 70 °C for 4 h. After the reaction was finished, the reaction was quenched with 10% hydrochloric acid solution. The product was extracted three times with ethyl acetate (30 mL). The organic layer was combined and dried over anhydrous Na2SO4. Then, the solvent was removed under reduced pressure. The residue was purified by flash chromatography on silica gel to afford pure compound 1 using petroleum/ethyl acetate (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
General procedure for the synthesis of compounds 2a–2t
To a solution of compound 1 (1.0 equiv.) and different substituted thiosemicarbazides (1.2 equiv.) in EtOH, acetic acid (0.01 equiv.) was added. The reaction was kept at room temperature for 4 h. When compound 1 was completely consumed, the reaction was quenched with aq NaHCO3. The product was extracted three times with ethyl acetate (30 mL). The combined organic layer was dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by flash chromatography on silica gel to afford pure compound 2 using petroleum/ethyl acetate.The characterization 1H NMR, 13C NMR, and HR-ESI-MS data of the target compounds are shown in the SI.
Biological evaluation
The H1795 and PC9 cell lines were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Gibco RPMI-1640 was purchased from Thermo Fisher Scientific (Shanghai, China). Fetal bovine serum (FBS) was purchased from Yikesai Biotechnology (Taicang) Co., Ltd. RIPA lysis buffer and BCA protein assay kit were purchased from Beyotime (Hangzhou, China). TGF-β1 was purchased from Sino Biological (Beijing, China). Anti-E-cadherin, anti-vimentin, and β-actin antibodies were purchased from Proteintech (Wuhan, China). Anti-MMP9 antibody was purchased from Abcam (Cambridge, MA, UK). Anti-MMP2 and tubulin antibodies were purchased from ABclonal (Wuhan, China).
MTT assay
In the MTT assay, all cells were seeded in 96 well plates and 5 × 103 cells per well. After culturing overnight, various concentrations (0, 6.25, 12.5, 25, and 50 µM) of the derivatives were added to the cells and cultured for 48 h. Then, MTT solution (5 mg mL−1) was added to each well and incubated at 37 °C for 4 h. The medium was removed and DMSO was added to dissolve formazan. The absorbance was measured at 490 nm using a microplate spectrophotometer (Synergy HT, BioTek, Vermont, USA).Cell cycle analysis
Cells were seeded in 6 well plates at a concentration of 3 × 105 cells per well and incubated overnight. Following this, the cells were treated with compound 2f and incubated for 24 h. Subsequently, the cells were digested and washed 1–2 times with pre-cooled phosphate-buffered saline (PBS). To fix the cells, 1 mL of 70% ethanol solution was added, and the samples were incubated at 4 °C overnight. After fixation, the cells were centrifuged and washed again with PBS. Propidium iodide (PI) was then added for staining, and the samples were incubated at 37 °C for 30 min. The cell cycle distribution was subsequently analyzed using flow cytometry.Transwell migration and invasion assay
The migration and invasion experiment of tumor cells was performed using Transwell chambers (24 wells, 8 µm pore size, and 6.5 mm diameter polycarbonate membranes that were coated with 1 mg per mL fibronectin). The cells were seeded onto each upper well at a concentration of 2 × 105 PC9 cells and different concentrations of compound 2f (0, 1, 2 and 4 µM) were added. The bottom chambers of the Transwell contained 600 µL of 10% FBS medium. Then, the cells were cultured for 24 h, fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet solution for 20 min. Finally, the migrating and invasive cells were recorded under a light microscope at 10× magnification.Transwell migration and invasion with TGF-β-induced assay
The migration and invasion experiment of tumor cells was performed using Transwell chambers (24 well, 8 µm pore size, 6.5 mm diameter polycarbonate membranes that were coated with 1 mg per mL fibronectin). The cells were seeded onto each upper well at a concentration of 2 × 105 PC9 cells, treated with TGF-β (30 ng mL−1), and different concentrations of compound 2f were added. The bottom chambers of the Transwell contained 600 µL of 10% FBS medium. Then, the cells were cultured for 24 h, fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet solution for 20 min. Finally, the migrating and invasive cells were recorded under a light microscope at 10× magnification.Western blotting
Western blotting analysis was carried out as previously described.44 PC9 cells were cultured in 6 well plates at a concentration of 3 × 106 cells. The cells were treated with TGF-β (30 ng mL−1) and different concentrations of compound 2f (0, 1, 2, and 4 µM) and incubated for 24 h. After that, the cells were washed and lysed. Proteins were extracted and quantified. Proteins were separated by SDS-PAGE and transferred to PVDF membrane. The PVDF membrane was blocked with 5% non-fat milk and incubated with primary antibodies (E-cadherin, vimentin, MMP-2 and MMP-9) at 4 °C overnight. After washing with Tris-buffered saline/Tween 20 buffer, the PVDF membranes were incubated with the secondary antibodies for 2 h at room temperature. Finally, protein bands were recorded using a chemiluminescent gel imaging system (Bio-Rad, Hercules, CA, USA). Anti-tubulin and anti-β-actin were used as internal controls.In vivo antitumor efficacy
Female Balb/c nude mouse (16–20 g) around four weeks old were purchased from Qinglongshan Biotechnology (Jiangsu) Co., Ltd. All mouse experiments were approved by the Ethical Committee of Bengbu Medical University (IACUC BBMU 2023-166). Estrogen has been demonstrated to play a promotive role in the tumorigenesis and progression of NSCLC. Given that PC9 cells exhibit significantly higher tumour formation rates in female nude mice compared to males, we employed female Balb/c nude mice for the evaluation of in vivo antitumor efficacy in this study.45 We evaluated the in vivo anticancer efficacy of compound 2f using a non-small cell lung cancer xenograft model. Tumour-bearing mice were randomly divided into three groups (n = 5/group) when the size of the tumour reached about 100 mm3. The mice in different groups were intraperitoneally injected with saline, DDP (2 mg kg−1), and compound 2f (20 mg kg−1) every 3 days. At approximately day 18, the average tumour volume reached 1000 mm3 and the experiment was terminated. The body weight and tumour volume were monitored every three days. The size of the tumour was calculated using the following formula: volume of tumour = 1/2 × width2 × length. All mice were anaesthetised with isoflurane and sacrificed on the final day of therapy through cervical dislocation. The tumours in different groups were harvested for measuring their size. Additionally, the organs (heart, liver, spleen, lung, and kidney) were harvested for the evaluation of organ injury in nude mice by staining with H&E and imaged.Immunohistochemistry assay
The expression of E-cadherin and vimentin in the mouse tumour tissues was evaluated by immunohistochemistry (IHC). The tumour tissues in each group were isolated and fixed with 4% paraformaldehyde.Statistical analysis
All data were analyzed using GraphPad Prism software version 8.0 and expressed as the mean ± SD from three independent experiments. The Student's t-test was used for statistical comparison between two groups. *p < 0.05, **p < 0.01, ##p < 0.01 and ***p < 0.001 were considered statistically significant.Author contributions
Meng-Fan Xu contributed to the synthesis of compounds, in vitro experiments, western blot and data curation. Ke Zhong and Jing Zhu contributed to the in vivo experiments and western blotting. Jie Chen contributed to the experimental methodology, synthesis of compounds and data analysis. Fang Liu contributed to the funding acquisition, resources, guidance and supervision of the in vivo experiments. Feng Ding contributed to the experimental design and supervision of the in vitro experiment. Cheng-Zhu Wu and Long Zhao contributed to the conceptualization, supervision, funding acquisition and writing. All authors approved the final version of the manuscript and are responsible for all aspects of the work.Conflicts of interest
The authors report no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ra04625d.Acknowledgements
This work was financially supported by the Foundation of Bengbu Medical College (2022byzd013), the 512 Talent Cultivation Plan of Bengbu Medical College (By51202202); and the Natural Science Research Project of the Education Department of Anhui, China (KJ2021A0788).Notes and references
- J. Yang, P. Antin, G. Berx, C. Blanpain, T. Brabletz, M. Bronner, K. Campbell, A. Cano, J. Casanova, G. Christofori, S. Dedhar, R. Derynck, H. L. Ford, J. Fuxe, A. G. Herreros, G. J. Goodall, A. Hadjantonakis, R. J. Y. Huang, C. Kalcheim, R. Kalluri, Y. B. Kang, Y. Khew-Goodall, H. Levine, J. S. Liu, G. D. Longmore, S. A. Mani, J. Massagué, R. Mayor, D. McClay, K. E. Mostov, D. F. Newgreen, M. A. Nieto, A. Puisieux, R. Runyan, P. Savagner, B. Stanger, M. P. Stemmler, Y. Takahashi, M. Takeichi, E. Theveneau, J. P. Thiery, E. W. Thompson, R. A. Weinberg, E. D. Williams, J. H. Xing, B. H. P. Zhou, G. J. Sheng and EMT International Association (TEMTIA), Nat. Rev. Mol. Cell Biol., 2020, 21, 341 CrossRef CAS PubMed.
- R. Fontana, A. Mestre-Farrera and J. Yang, Annu. Rev. Pathol., 2024, 19, 133 CrossRef CAS PubMed.
- Y. H. Huang, W. Q. Hong and X. W. Wei, J. Hematol. Oncol., 2022, 15, 129 CrossRef PubMed.
- H. L. Ang, C. D. Mohan, M. K Shanmugam, H. C. Leong, P. Makvandi, K. S. Rangappa, A. Bishayee, A. P. Kumar and G. Sethi, Med. Res. Rev., 2023, 43, 1141 CrossRef CAS PubMed.
- S. Brabletz, H. Schuhwerk, T. Brabletz and M. P. Stemmler, EMBO J., 2021, 40, e108647 CrossRef CAS PubMed.
- P. Debnath, R. S. Huirem, P. Dutta and S. Palchaudhuri, Biosci. Rep., 2022, 42, BSR20211754 CrossRef CAS.
- D. D. Li, L. Y. Xia, P. Huang, Z. D. Wang, Q. W. Guo, C. C. Huang, W. D. Leng and S. S. Qin, Cell Prolif., 2023, 56, e13423 CrossRef PubMed.
- N. Ebrahimi, M. S. Manavi, F. Faghihkhorasani, S. S. Fakhr, F. J. Baei, F. F. Khorasani, M. M. Zare, N. P. Far, F. Rezaei-Tazangi, J. Ren, R. J. Reiter, N. Nabavi, A. R. Aref, C. Chen, Y. N. Ertas and Q. Lu, Cancer Metastasis Rev., 2024, 43, 457 CrossRef CAS PubMed.
- S. K. Jha, G. D. Rubis, S. R. Devkota, Y. L. Zhang, R. Adhikari, L. A. Jha, K. Bhattacharya, S. Mehndiratta, G. Gupta, S. K. Singh, N. Panth, K. Dua, P. M. Hansbro and K. R. Paudel, Ageing Res. Rev., 2024, 97, 102315 CrossRef CAS PubMed.
- P. X. Chen, Y. H. Liu, Y. K. Wen and C. C. Zhou, Cancer Commun., 2022, 42, 937 CrossRef PubMed.
- R. L. Siegel, K. D. Miller, H. E. Fuchs and A. Jemal, Ca-Cancer J. Clin., 2022, 72, 7 CrossRef PubMed.
- S. D. Deas, N. Huprikar and A. Skabelund, Curr. Opin. Pulm. Med., 2017, 23, 167 CrossRef PubMed.
- R. Manshouri, E. Coyaud, S. T. Kundu, D. H. Peng, S. A. Stratton, K. Alton, R. Bajaj, J. J. Fradette, R. Minelli, M. D. Peoples, A. Carugo, F. J. Chen, C. Bristow, J. J. Kovacs, M. C. Barton, T. Heffernan, C. J. Creighton, B. Raught and D. L. Gibbons, Nat. Commun., 2019, 10, 5125 CrossRef PubMed.
- Y. X. Li, C. Sun, Y. G. Tan, H. Y. Zhang, Y. C. Li and H. W. Zou, Int. J. Biol. Sci., 2021, 17, 635 CrossRef CAS PubMed.
- Z. Chen, K. Jin, L. Y. Gao, G. D. Lou, Y. Jin, Y. P. Yu and Y. J. Lou, Eur. J. Pharmacol., 2010, 643, 170 CrossRef CAS PubMed.
- D. E. Lee, E. H. Jang, C. Bang, G. L. Kim, S. Y. Yoon, D. H. Lee, J. Koo, J. H. Na, S. Lee and J. H. Kim, Arch. Biochem. Biophys., 2021, 709, 108969 CrossRef CAS.
- M. X. Lin, Q. Xu, Y. Luo, G. H. Liu and P. F. Hou, J. Biochem. Mol. Toxicol., 2023, 37, e23401 CrossRef CAS PubMed.
- R. Majeed, M. V. Reddy, P. K. Chinthakindi, P. L. Sangwan, A. Hamid, G. Chashoo, A. K. Saxena and S. Koul, Eur. J. Med. Chem., 2012, 49, 55 CrossRef CAS PubMed.
- A. Kumar, G. Sawhney, R. K. Nagar, N. Chauhan, N. Gupta, A. Kaul, Z. Ahmed, P. Sangwan, P. S. Kumar and G. Yadav, Int. Immunopharmacol., 2021, 91, 107264 CrossRef CAS.
- H. B. Yao, H. S. Almoallim, S. A. Alharbi and H. Feng, Appl. Biochem. Biotechnol., 2024, 196, 3456 CrossRef CAS PubMed.
- H. X. Liu, W. Guo, H. Guo, L. Zhao, L. Yue, X. Li, D. Y. Feng, J. N. Luo, X. Wu, W. X. Cui and Y. Qu, Front. Pharmacol, 2020, 11, 712 CrossRef CAS PubMed.
- A. Cariola, M. E. Chami, J. Granatieri and L. Valgimigli, Food Chem., 2023, 405, 134953 CrossRef CAS PubMed.
- H. Mehmood, M. Musa, S. Woodward, M. S. Hossan, T. D. Bradshaw, M. Haroon, A. Nortcliffe and T. Akhtar, RSC Adv., 2022, 12, 34126 RSC.
- X. Zhao and D. R. Richardson, Biochim. Biophys. Acta, Rev. Cancer, 2023, 1878, 188871 CrossRef CAS PubMed.
- X. H. Jiang, L. A. Fielding, H. Davis, W. Carroll, E. C. Lisic and J. E. Deweese, Int. J. Mol. Sci., 2023, 24, 12010 CrossRef CAS PubMed.
- J. Kopecka, A. Barbanente, D. Vitone, F. Arnesano, N. Margiotta, P. Berchialla, M. Niso, C. Riganti and C. Abate, Pharmacol. Rep., 2023, 75, 1588 CrossRef CAS.
- P. Heffeter, V. F. S. Pape, É. A. Enyedy, B. K. Keppler, G. Szakacs and C. R. Kowol, Antioxid. Redox Signaling, 2019, 30, 1062 CrossRef CAS PubMed.
- O. Farsa, V. Ballayová, R. Žáčková, P. Kollar, T. Kauerová and P. Zubáč, Int. J. Mol. Sci., 2022, 23, 9813 CrossRef CAS PubMed.
- X. G. Bai, Y. Y. Zheng and J. X. Qi, Front. Pharmacol, 2022, 13, 1018951 CrossRef CAS PubMed.
- F. Miglioli, M. D. Franco, J. Bartoli, M. Scaccaglia, G. Pelosi, C. Marzano, D. Rogolino, V. Gandin and M. Carcelli, Eur. J. Med. Chem., 2024, 276, 116697 CrossRef CAS PubMed.
- V. Pape, S. Tóth, A. Füredi, K. Szebényi, A. Lovrics, P. Szabó, M. Wiese and G. Szakács, Eur. J. Med. Chem., 2016, 117, 335 CrossRef CAS PubMed.
- B. Hu, B. Wang, B. Zhao, Q. Guo, Z. H. Li, X. H. Zhang, G. Y. Liu, Y. Liu, Y. Tang, F. Luo, Y. Du, Y. X. Chen, L. Y. Ma and H. M. Liu, MedChemComm, 2017, 8, 2173–2180 RSC.
- D. Lane, T. Mills, N. Shafie, A. Merlot, R. S. Moussa, D. Kalinowski, Z. Kovacevic and D. Richardson, Biochim. Biophys. Acta, 2014, 1845, 166–181 CAS.
- X. H. Zhang, B. Wang, Y. Y. Tao, Q. Ma, H. J. Wang, Z. X. He, H. P. Wu, Y. H. Li, B. Zhao, L. Y. Ma and H. M. Liu, Eur. J. Med. Chem., 2020, 199, 112349 CrossRef CAS.
- J. Yang, L. J. Wang, J. J. Liu, L. Zhong, R. L. Zheng, Y. Xu, P. Ji, C. H. Zhang, W. J. Wang, X. D. Ling, L. L. Li, Y. Q. Wen and S. Y. Yang, J. Med. Chem., 2012, 55, 10685–10699 CrossRef CAS PubMed.
- C. J. Xu, Y. F. Han, S. C. Xu, R. X. Wang, M. Yue, Y. Tian, X. F. Li, Y. F. Zhao and P. Gong, Eur. J. Med. Chem., 2020, 186, 111867 CrossRef CAS PubMed.
- B. C. Bade and C. S. D. Cruz, Clin. Chest Med., 2020, 41, 1 CrossRef.
- S. Krieg, S. I. Fernandes, C. Kolliopoulos, M. Liu and S. M. Fendt, Cancer Discov., 2024, 14, 934 CrossRef CAS PubMed.
- N. Johansson, M. Ahonen and V. M. Kähäri, Cell. Mol. Life Sci., 2000, 57, 5 CrossRef CAS PubMed.
- T. Nagai, T. Ishikawa, Y. Minami and M. Nishita, J. Biochem., 2020, 167, 347 CrossRef CAS PubMed.
- V. Aggarwal, C. A. Montoya, V. S. Donnenberg and S. Sant, iScience, 2021, 24, 102113 CrossRef CAS PubMed.
- H. Schuhwerk and T. Brabletz, Semin. Cancer Biol., 2023, 97, 86 CrossRef CAS PubMed.
- J. H. Lee and J. Massagué, Semin. Cancer Biol., 2022, 86, 136 CrossRef CAS.
- J. Chen, L. Zhao, M. F. Xu, D. Huang, X. L. Sun, Y. X. Zhang, H. M. Li and C. Z. Wu, J. Enzym. Inhib., 2024, 39, 2292006 CrossRef PubMed.
- N. Svoronos, A. Perales-Puchalt, M. J. Allegrezza, M. R. Rutkowski, K. K. Payne, A. J. Tesone, J. M. Nguyen, T. J. Curiel, M. G. Cadungog, S. Singhal, E. B. Eruslanov, P. Zhang, J. Tchou, R. Zhang and J. R. Conejo-Garcia, Cancer Discov., 2017, 7, 72–85 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |