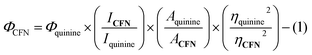Open Access Article
Open Access ArticleDevelopment of a chromene-based fluorescent and colorimetric sensor for the sensitive detection of calcium ions in a complex medium: a detailed DFT and experimental approach
Kainat Khurshid,
Kainat Gul,
Hafiz Muhammad Junaid,
Alam Shabbir,
Umar Farooq* and
Sohail Anjum Shahzad *
*
Department of Chemistry, COMSATS University Islamabad, Abbottabad Campus, University Road, Abbottabad 22060, Pakistan. E-mail: umarf@cuiatd.edu.pk; sashahzad@cuiatd.edu.pk
First published on 19th November 2025
Abstract
Calcium plays a key role in various biochemical processes and cellular functions in the human body; its imbalance can cause severe hyper- and hypo-calcemic medical conditions. In this research work, we developed a novel AIE-active chromene-based fluorescent sensor, CFN (DMF, λexc = 330 nm, λem = 476 nm), through an easy Knoevenagel condensation reaction with solvatochromic characteristics. The synthesized CFN sensor exhibited excellent fluorescence enhancement with a blue shift in 90% aqueous media (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, H2O/DMF, λexc = 330 nm, λem = 410 nm). Blue shift in the fluorescence spectrum indicates the formation of H-aggregates, which is further supported by dynamic light scattering (DLS) results. The CFN sensor shows a large Stokes shift of 120 nm in pure DMF and 54 nm in water/DMF. The CFN sensor was employed for extremely selective and sensitive colorimetric detection of Ca2+ ions via chelation-enhanced fluorescence quenching (CHEQ) mechanism. The calculated detection and quantification limits were 10.5 nM and 35 nM, respectively. Furthermore, UV-Vis spectroscopy, fluorescence spectroscopy, and 1H NMR titration experiments were carried out to validate the interaction mechanism between CFN and Ca2+ ions. The application of CFN for the detection of Ca2+ ions in real samples were accessed through real-time fluorescence titration analyses in human plasma, commercial milk, and different water samples. The portable CFN sensor-coated strips were developed for on-site sensing of Ca2+ ions, and a logic gate was designed to mimic it as a point-of-need calcium ion sensor.
1, H2O/DMF, λexc = 330 nm, λem = 410 nm). Blue shift in the fluorescence spectrum indicates the formation of H-aggregates, which is further supported by dynamic light scattering (DLS) results. The CFN sensor shows a large Stokes shift of 120 nm in pure DMF and 54 nm in water/DMF. The CFN sensor was employed for extremely selective and sensitive colorimetric detection of Ca2+ ions via chelation-enhanced fluorescence quenching (CHEQ) mechanism. The calculated detection and quantification limits were 10.5 nM and 35 nM, respectively. Furthermore, UV-Vis spectroscopy, fluorescence spectroscopy, and 1H NMR titration experiments were carried out to validate the interaction mechanism between CFN and Ca2+ ions. The application of CFN for the detection of Ca2+ ions in real samples were accessed through real-time fluorescence titration analyses in human plasma, commercial milk, and different water samples. The portable CFN sensor-coated strips were developed for on-site sensing of Ca2+ ions, and a logic gate was designed to mimic it as a point-of-need calcium ion sensor.
1. Introduction
Calcium is one of the most abundant minerals in the human body, which is crucial for biological activities and human life.1,2 It plays significant roles in body functioning, such as blood coagulation, muscle movement, metabolism, hormone secretion, fertilization, enzyme functions, gene transcription, apoptosis, and neurotransmission, which can be mainly controlled through sequential and three-dimensional variations in Ca2+ ions.3 The safe calcium concentration level in blood serum is 2.12 to 2.55 mM, with 1.16 to 1.32 mM in active form, inside the human body for optimum biological functions. The concentration of calcium above this optimum level causes hypercalcemia, and below this level, it causes hypocalcemia.4 A reduction in the albumin level in blood serum also reduces the level of Ca2+ ions in biological systems. Significantly low concentrations of Ca2+ ions are also observed in pseudohypoparathyroidism, malnutrition, hypoparathyroidism, excretory system disorders, and vitamin D deficiency. Major causes of hypercalcemia include renal disorders, hyperparathyroidism, thyrotoxicosis, cancer, osteoporosis, high vitamin D intake, and sarcoidosis. The dysregulation of Ca2+ ion concentration in the body nerve cells is also closely related to a few neurodegenerative disorders,5 such as Parkinson's disease (PD), Alzheimer's disease (AD), and Huntington's disease (HD).6,7 To avoid these health risks for human beings, calcium levels in aqueous samples must be effectively monitored. Hence, procedures for the qualitative and quantitative analysis of Ca2+ ions in naturally and biologically occurring bodies should be accurate, less time-consuming, and economical. In this regard, innovative and high-performance sensing techniques that would allow the determination of its minimal concentration in real samples, such as food quality assessment, drinking water, human plasma analysis, medical diagnosis, and biological fluid analysis are required.A range of techniques has been employed to determine calcium in various samples, which include titration, complexation-based spectroscopy (CBS), atomic absorption spectrometry (AAS), inductively coupled plasma mass spectrometry (ICPMS), ion selective electrode-based electrochemical methods, and high-performance liquid chromatography (HPLC).8–12 Though these approaches are quite sensitive and accurate, they require special personnel, costly instruments, and tedious sample treatment, and they suffer from the inability to perform on-spot analysis, urging the scientists to develop other methods. Thus, cost-effective, simple, sensitive techniques that can timely identify calcium on site are necessary.13,14
Fluorescence-based sensing techniques, being the least invasive, have shown promising results for their accuracy and applicability.15 Calcium has been detected using many fluorescent sensors, which include calcium detection via mechanisms such as resonance electron transfer, chelate fluorescence, and aggregation-induced emission (AIE).16 Some notable examples of calcium sensors are Fura-2 and Fluo-4, which have been utilized for quick and sensitive calcium detection. Certain typical organic materials show a significant aggregation-caused quenching (ACQ) phenomenon.17 Exciplex or excimer induced by noncovalent strong interactions, such as dipole–dipole interaction and π–π stacking, results in the ACQ phenomenon. ACQ limits the practical uses of fluorophores commonly employed as solid films in real-sample analysis.18
Tang et al. (2001) discovered a phenomenon known as aggregation-induced emission (AIE), which is the opposite of ACQ.19 AIE-active organic compounds are not capable of any emission in an organic solvent, while in a poor solvent such as water, they show enhanced emission due to aggregation. However, aggregation-induced emission (AIE) is a characteristic phenomenon observed in organic compounds in which weakly emissive fluorophores exhibit exceptional emission upon aggregation.20,21 To induce these features, rotatable moieties are incorporated in the structure of such fluorophores via C–C, C–N, and N–N type single bonds.22,23
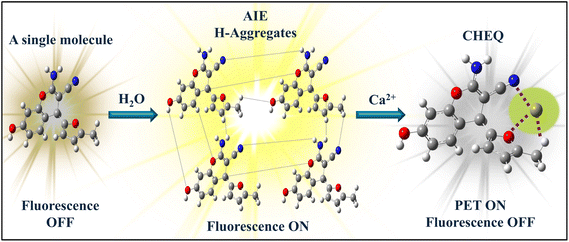
AIE-based fluorescence sensors enable the concurrent and localized study of calcium ions in living cells, tackling previous challenges and expanding practical applications, particularly in a complex medium. Furthermore, H aggregates typically lead to fluorescence quenching because of their high aggregation levels, which can reduce the effectiveness of fluorescence-based sensing techniques.24 However, highly emissive H-aggregates are also reported25,26 among which some are known to be aligned in a herringbone fashion.27 Our designed sensor also exhibits a non-conventional photophysical behavior in its aggregated state. In this sensor, the restricted intramolecular motion and restricted intramolecular rotations contribute towards high quantum yields by enhancing radiative decay pathways despite the blue-shift caused by H-aggregates owing to the reorganization of the excited energy level to increase the energy gap. In fact, AIE originates as a result of rigidity and restriction of intramolecular motions (RIM) upon the formation of aggregates. The restriction of intramolecular motions (RIM) prevents non-radiative decay pathways and enhances luminescence. Major types of aggregates formed through self-assembly of fluorescent dyes are known as J- and H-type aggregates. These aggregates are formed based on specific types of molecular arrangements that offer distinct spectral shifts in their absorption and emission spectra compared to their monomeric forms of fluorophores. Head-to-tail fashion arrangement of fluorophore molecules forms J-aggregates that shift the absorption and emission spectrum towards longer wavelengths compared to the monomer molecules. Sharp absorption and narrow fluorescence bands with strong intensity are characteristic features of J-aggregates. However, head-to-head (plane-to-plane or face-to-face stacking) arrangement of fluorophore molecules leads to the creation of H-aggregates. Interestingly, absorption and emission spectra of H-aggregates of fluorophores are shifted towards shorter wavelengths (blue-shift) with suppressed or no fluorescence emission properties. In H-aggregates, a shift towards a shorter wavelength in the absorption and emission spectra of the molecules is observed due to the perpendicular orientation of transition dipoles of the fluorophores.
A well-documented theory suggested the splitting of an excited electronic state into two exciton states, lower and higher energy exciton states.28 These low- and high-energy exciton states are attributed to antiparallel and parallel dipoles, respectively. Usually, H-aggregates are characterized by observing a hypsochromic or blue shift in the absorption band owing to allowed transitions to the higher energy exciton state. A shift towards a shorter wavelength in the absorption band occurs due to forbidden transition to the lower energy exciton state with reduced transition probability. This reduced transition probability to the lower energy exciton state is responsible for the quenching of fluorescence emission in H-aggregates. However, deformation in transition dipoles allows the transitions to a lower energy exciton state, and consequently, fluorescence emission originates from the lower exciton state (Fig. 1). Interestingly, a computational study on special circumstances of H-aggregates reveals that unusual fluorescence emission occurs directly from the second exited state (S2) to the ground energy state (S0), not from the first excited singlet state (S1), as S1 is considered a dark state.29 This kind of unusual fluorescence emission from H-aggregates is linked to an exceptional violation of Kasha's rule.
 | ||
| Fig. 1 Splitting of an excited electronic state into lower and higher exciton states in H-aggregates. | ||
In J-aggregates, a red-shifted spectrum is obtained as a result of the allowed electronic transition from the lower-energy exciton state to the ground state. In H-aggregates, the higher-energy exciton state becomes permissible upon a co-facial/parallel arrangement of transition dipole moments of fluorophores to each other, resulting in a blue-shifted spectrum.30 Kasha's exciton model also predicts a shift towards a shorter wavelength in the absorption spectrum of the H-aggregates as a result of forbidden transition to the lower-energy exciton states.30,31 Generally, broad-band spectral appearance of H-aggregates is characterized with no fluorescence emission and low extinction coefficient. However, some recent studies reveal the creation of fluorescent H-aggregates and challenge the conventional understanding of H-aggregates as the only non-emissive species. For example, Modi et al. reported that H-aggregates of fluorinated pyridyl-azo dye exhibited a sharp absorption band with a higher extinction coefficient and demonstrated rare blue-shifted fluorescence emission in a range of organic solvents with large Stokes shift.32 Fluorophore-like Rhodamine B is known to form highly fluorescent H-aggregates in polar solvents.33 Some other organic fluorophores such as Rhodamine 6G,34 fluorescein, eosin Y,35 cyanine derivatives,30 thioflavin-T (ref. 36) and coumarin dyes37 exhibited fluorescent H-aggregates. It is well known that H-aggregates are formed by plane-to-plane stacking of the molecules; however, H-aggregates of imperfectly stacked molecules offer unusual fluorescence emissions.37–42
In our case, with the increase in the contents of the aqueous medium, the plane of the chromene-based sensor molecules starts stacking in a twisted manner. Transition dipoles of the molecules are twisted through imperfect parallel stacking of molecules. As a result, unusual fluorescence emission occurs directly from the second exited state (S2) to the ground energy state (S0), not from the first excited singlet state (S1), as S1 is considered a dark state. As an extension of our research efforts, we aimed at developing novel fluorescent sensors.43–46 We present the design and synthesis of a novel furan and nitrile-substituted chromene-based fluorescence sensor, CFN, which demonstrates aggregation-induced emission with a large blue-shift. The blue-shift in emission illustrates the formation of H-aggregates. This sensor exhibits favorable traits such as a straightforward synthesis procedure, high synthetic yield, interesting photophysical properties, and a unique molecular structure for selective sensing of calcium ions. The structural feature of the CFN sensor induces solvatochromic and aggregation properties. The aggregation-induced emission (AIE) character of the CFN sensor makes it a suitable candidate for the fabrication of solid strips, which is used for the on-site sensing of biologically important minerals. Hence, the CFN sensor is utilized for the selective sensing of calcium through a chelation-induced fluorescence enhancement (CHEF) mechanism. A combination of spectroscopic techniques such as fluorescence, UV-visible, DLS, and NMR titration experiments was employed to elucidate the sensing mechanism, which is responsible for selective sensing of calcium. Furthermore, all these experimental findings are supported through DFT calculations. Real-time applications of the CFN sensor were confirmed by experiments on real human plasma, commercial milk, and different water samples. The portable CFN sensor-coated strips were developed, and the logic gate was also designed to mimic it as a worth-applying tool for the point-of-need calcium ion sensor.
2. Experimental work
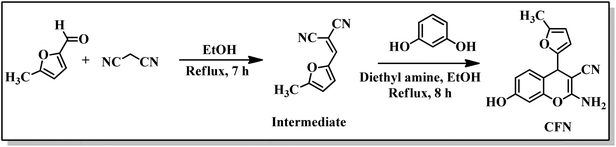
2.1. Synthesis of the CFN sensor
In a well-dried and nitrogen-flushed round-bottom flask, 4.0 mmol malononitrile (0.27 g) was taken, and 5-methyl-2-furaldehyde (0.33 g, 0.3 mL), dissolved in 5 mL ethanol, was poured into it. TLC was used to monitor the course of the reaction over a seven-hour duration. Both reactants were consumed, and the intermediate was formed by the Knoevenagel condensation. With diethyl amine serving as the base and ethanol as a solvent, resorcinol (4.0 mmol, 0.45 g) was added to the intermediate. The progress of reaction was observed using TLC and refluxed for an additional 8 h. Subsequently, the crude mixture was thoroughly washed with 20 mL of distilled water and filtered. A brown residue was obtained on filter paper, which was washed again with water and dried at room temperature. Recrystallization from ethanol afforded the fluorophore CFN. The product was further purified by a filtration column using ethyl acetate and n-hexane as eluent. The desired compound CFN was obtained in 90% yield. 1H NMR (400 MHz, DMSO-d6): δ 9.71 (s, 1H, OH), 6.93 (d, 1H, J = 8.4 Hz), 6.89 (s, 2H, NH2), 6.53 (dd, 2H, J = 8.3, 2.2 Hz), 6.38 (d, 1H, J = 2.1 Hz), 5.98 (d, 2H, J = 2.9 Hz), 4.66 (s, 1H), 2.15 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 161.3 (C), 157.8 (C), 155.6 (C), 151.3 (C), 149.5 (C), 130.0 (1C), 120.9 (C), 112.7 (C), 111.6 (C), 106.7 (2 × C), 102.7 (C), 53.8 (C), 32.2 (C), 13.7 (C); DEPT (100 MHz, DMSO-d6): δ 130.0 (C), 112.7 (C), 106.7 (2 × C), 102.7 (C), 34.2 (C), 13.7 (C).2.2. Theoretical studies
| Eint = Ec − (Es + Ea) | (1) |
The interaction energy is represented by Eint, the complex energy by Ec, the sensor energy by Es, and the analyte energy by Ea. A higher Eint value at a point indicates a thermodynamically stable sensor–analyte interaction. The Eint value, 305.04 kcal mol−1, for CFN and Ca2+ present at ‘site I’ is most favorable for complexation.
2.3. Quantum yield calculation
The quantum yield of CFN and CFN@Ca2+ complex with respect to quinine sulfate (Φ = 0.54, 0.1 M H2SO4) as a standard has been quantified using eqn (1):ΦCFN = quantum yield of CFN. Φquinine = quantum yield of standard quinine sulfate. ACFN = absorbance of CFN. Aquinine = absorbance of standard quinine sulfate. ηCFN = refractive index of the sample's solvent. ηquinine = refractive index of the standard's solvent (0.1 M H2SO4).
3. Results and discussion
3.1. Chemistry of CFN
The CFN sensor was synthesized via Knoevenagel condensation using commercially available 5-methyl-2-furaldehyde, diethyl amine, malononitrile, and resorcinol (Scheme 1). Initially, methyl-2-furaldehyde was treated with malononitrile to access the intermediate using Knoevenagel condensation reaction conditions. Treatment of the resulting intermediate with resorcinol using diethyl amine as a base in the presence of ethanol under reflux conditions afforded the desired fluorophore CFN in good yields. The progress of reaction was monitored using TLC. Recrystallization from ethanol afforded the fluorophore CFN in 90% yield.NMR spectroscopy was used to characterize the structure of the synthesized product CFN. The 1H NMR spectrum of the CFN sensor shows a characteristic signal at δ 9.71, corresponding to the hydroxyl (OH) proton, the multiplet in the range of 5.99 to 5.93 ppm and the singlet at 2.16 ppm are assigned to the furan ring protons and methyl group, respectively, confirming the incorporation of the 5-methylfuran-2-yl moiety into the chromene framework. Additionally, the 13C NMR spectrum exhibited fifteen peaks that are attributed to fifteen unique carbon atoms of the synthesized compound. Seven peaks that appeared in the DEPT135 NMR spectrum demonstrate the presence of hydrogen-attached carbon atoms that confirmed the formation of desired compound CFN. The details about the procedure, instruments, titration experiments, and reagents are provided in the SI (SI-1). The SI also contains the characterization data (S25–S42).
3.2. Comparison with previous sensors
Calcium ion sensing has been a topic of research for many years, which leads to a great collection of scientific results in this domain. Despite all the great contributions of previous research, there is always room for improvement, and great work done previously acts as a steppingstone for those improvements. CFN is a new effort for improvement in the field of fluorescent sensors. Calcium ion sensors developed earlier are mentioned in Table S1, which depict the type of studies performed and parameters mentioned. Many of those sensors have a better LOD than CFN; however, some lack ease of synthesis to access fluorophore, pH studies, real sample tests, binding efficiency record and underlying mechanism discussion. Among all the sensors mentioned, Ankireddy et al. developed a most efficient Ca2+ sensor with all its excellent properties; however, it is a quenching-based sensor leaving the room for noise or false positivity.54 Some sensors were organelle specific and were not tested in water and milk samples such as that developed by Hong et al., Shen et al., Zhang et al., Cao et al., and Gomes et al.55–59 Some other sensors either have a specific pH range for calcium ion detection needs and an extra step of buffer addition such as in the case of Hong et al. and Kacmaz et al.55,60 Some of the mentioned sensors are not fully selective such as that by Gomes et al. and Cao et al.58,59 In comparison to all these CFN sensor is an easy to synthesize, highly selective, super sensitive sensor applicable in a wide range of pH, temperature and type of samples.3.3. Photophysical properties based on structure–property relationships
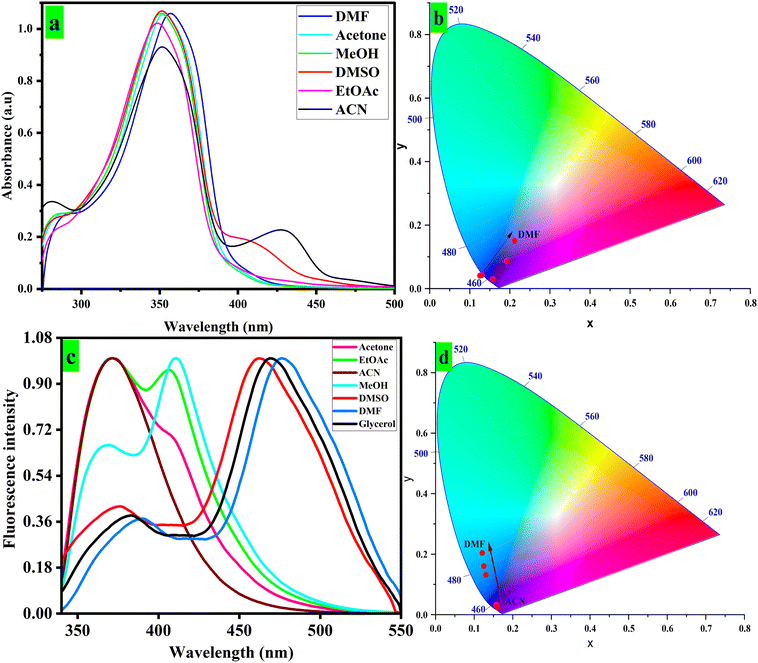 | ||
| Fig. 2 Absorption spectra of fluorophore CFN in different solvents (a), CIE diagram for absorption spectra (b), emission spectra of fluorophore CFN (c), and CIE diagram for emission spectra (d). | ||
Similarly, the CFN exhibits maximum emission at different wavelengths in different polarity solvents such as ACN (370 nm), acetone (373 nm), EtOAc (409 nm), MeOH (410 nm), DMSO (462 nm), glycerol (469 nm) and DMF (476 nm) depending on solvent viscosity, their interactions with the CFN fluorophore and their respective dielectric constants (Fig. 2c). Moreover, the CIE diagram illustrates the changes in emission wavelengths (Fig. 2d). The ground- and excited-state emissions of fluorophores are impacted by solvent polarity, resulting in a change in absorption and emission properties.62 Additionally, the free-rotating phenyl rings attached to the developed sensors impart various optical properties in response to changes in the solvent polarity. The study of Stokes shift, dipole moments and charge translations such as twisted intramolecular charge transfer (TICT) and intermolecular charge transfer (ICT) in sensor molecules is made possible by solvatochromism.63 As shown in Fig. 2, CFN displayed a red-shift in absorption bands within the range of 348 nm to 356 nm with the increase in solvent polarity. The π–π* transition attributed to the bathochromic shift of 8 nm for CFN was observed in absorption spectra when moving from low- to high-polarity solvents. As evident from the emission spectrum, the more proclaimed positive solvatochromic behavior is probably due to extended π conjugation in the molecular structure. The CFN sensor displayed intense emission bands at 476 nm in DMF. Furthermore, a 106 nm bathochromic shift was observed when moving from low- to high-polarity solvents. For example, emission of CFN is observed at 370 nm in acetone compared to 476 nm emission maxima in DMF.
Generally, under high polar conditions, fluorophores encounter solvent relaxation, resulting in longer wavelength emissions due to vibrational energy at the excited state being transferred to surrounding molecules.62 The notable changes in fluorescence intensity and wavelength (λmax) are also observed due to the impact of polar solvents on dipole moments of excited fluorophores.64 The photophysical properties of the sensors that have been developed are summarized in Table S2. The quantification of emission changes for the sensor due to solvent polarity was further done using the Lippert–Mataga plot, which displayed linear relationships (R2 = 0.795) between Stokes shifts and polarizability (Δf) (Fig. S3a).65 These plots were further considered to investigate solvatochromism via the change in dipole moments (Δµ) among the excited and ground states, which gave 9.7 D for CFN. The dipole moment (Δµ) values infer that the developed sensor can discriminate between solvents of varying polarity. The detailed structure–property relationship studies prompted the application of the developed material for biological and environmentally crucial metal ions. Further optical studies were carried out in DMF due to the substantial quantum yield and Stokes shift in fluorescence emission observed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture was chosen for aggregation studies. However, with a further change in water contents (20–80%), a significant increase in absorption was observed for CFN with a steep increase in absorbance from 80% to 90% water fraction (Fig. 3a). Further addition of water did not change the absorption spectra, and the Mie scattering effect characterized by a level-off tail was noted (Fig. 3a). The absorption bands are changed slightly with water fractions (0–90%) in DMF due to the solvent polarity effect, and the overall linear response of the CFN sensor was observed with the increase in water fraction (Fig. 3b).67
H2O mixture was chosen for aggregation studies. However, with a further change in water contents (20–80%), a significant increase in absorption was observed for CFN with a steep increase in absorbance from 80% to 90% water fraction (Fig. 3a). Further addition of water did not change the absorption spectra, and the Mie scattering effect characterized by a level-off tail was noted (Fig. 3a). The absorption bands are changed slightly with water fractions (0–90%) in DMF due to the solvent polarity effect, and the overall linear response of the CFN sensor was observed with the increase in water fraction (Fig. 3b).67
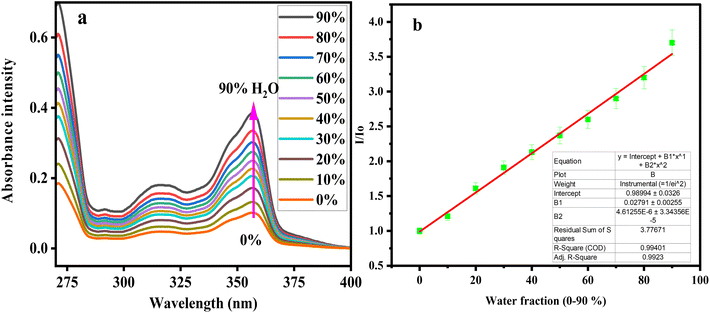 | ||
| Fig. 3 UV-Vis absorption spectra (a) and linear-fitting regression line (b) calculated after gradual mixing of water fractions (0–90%) in a 30 µM DMF solution of the CFN sensor. | ||
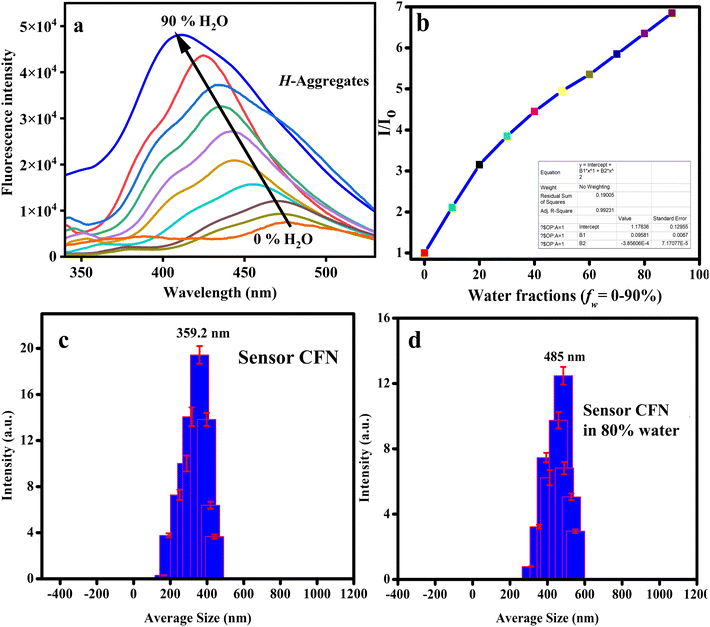
Various amounts of water (fw = 0–90%) were added to the DMF solution of the sensor to conduct thorough emission experiments. Despite extended π-conjugation, CFN showed comparatively weak emissions in DMF caused by non-radiative decay routes of free rotation of phenyl rings. However, with the addition of water, the emission intensity of CFN (30 µM) significantly increased by shifting from 476 nm to 410 nm (Fig. 4a). These absorption changes with increased water proportions suggest the H-aggregate formation.68 Moreover, with the increase in water fractions (0–90%) the emission enhancement of about 5 times for CFN was seen with a blue-shift of 66 nm, further indicating the formation of H-aggregates.
The quantum yield of CFN also increased from Φ = 0.175 to Φ = 0.420. The CFN shows a Stokes shift of 54 nm at 90% water fraction (Fig. S2b). The higher water content results in the formation of well-organized aggregates. The aggregate formation was characterized through the monitoring of particle size via dynamic light scattering (DLS) analysis (Fig. S3b). The particle size kept increasing from 359 nm to 485 nm upon increasing the water content from 0% to 80% (Fig. 4c and d). The free rotations of substituted phenyl rings (RIR) in the molecules are also suppressed, resulting in non-radiative decay pathways and enhanced fluorescence.69
Using a polynomial fit plot, the determination of the correlation between emission intensity and the addition of water fraction in DMF solvent was made possible (Fig. 4b). The materials showing enhanced fluorescence through the AIE mechanism ought to exhibit consistent behavior in a viscous environment.70
3.4. Photosensitive detection of biologically important nutrient calcium
The CFN sensor (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF, 9
DMF, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
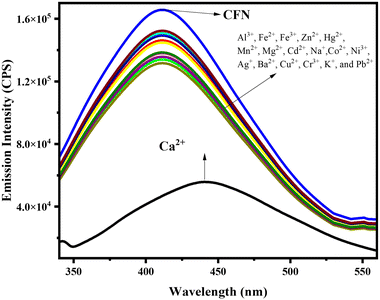
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 30 µM), with λexc = 330 nm, Sw = 3/3, and λemission = 410 nm, possesses unique structural features with nitrogen heteroatoms, which uniquely show complexation with metal ions. This characteristic prompted us to evaluate the detection capabilities of the sensor against various metal ions in various media, including food, water, and industrial effluents. We tested the specific concentrations (1000 nM) of a range of metal cations with competing anions including Cu2+, Cr3+, Fe3+/2+, Mn2+, Mg2+, Al2+, Zn2+, Ni3+, Ca2+, Co2+, Ag+, Na+, Pb2+, K+, Hg2+, Ba2+, SCN−, I−, F−, Cl−, Br−, SO42−, NO2−, CN−, HSO3−, OAc−, OH−, NO32−, HS−, and BrO− with an AIE-active CFN sensor. The emission spectra were obtained in H2O
1, v/v, 30 µM), with λexc = 330 nm, Sw = 3/3, and λemission = 410 nm, possesses unique structural features with nitrogen heteroatoms, which uniquely show complexation with metal ions. This characteristic prompted us to evaluate the detection capabilities of the sensor against various metal ions in various media, including food, water, and industrial effluents. We tested the specific concentrations (1000 nM) of a range of metal cations with competing anions including Cu2+, Cr3+, Fe3+/2+, Mn2+, Mg2+, Al2+, Zn2+, Ni3+, Ca2+, Co2+, Ag+, Na+, Pb2+, K+, Hg2+, Ba2+, SCN−, I−, F−, Cl−, Br−, SO42−, NO2−, CN−, HSO3−, OAc−, OH−, NO32−, HS−, and BrO− with an AIE-active CFN sensor. The emission spectra were obtained in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (9
DMF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 30 µM, λexcitation = 330 nm, Sw = 3/3), as shown in Fig. 5.
1, v/v, 30 µM, λexcitation = 330 nm, Sw = 3/3), as shown in Fig. 5.
The CFN sensor displayed an intense peak at 410 nm upon the addition of a wide range of metal ions. However, a significant quenching of fluorescence emission was observed at 444 nm for Ca2+ (500 nM). This indicates distinct and preferential complexation of the sensors with calcium ions. Additionally, a noticeable change in color from pale yellow to dark yellow with the addition of 1 µM calcium further indicated the utility of the developed sensor for naked-eye detection of the analyte in daylight (Fig. S4).
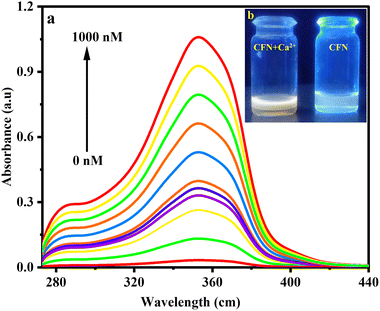
Given the identical absorption and color changes of the AIE-based CFN sensor for the oxidation state (Ca2+). Ca2+-focused UV-visible absorption experiments were conducted by analyzing the change in the absorption spectrum of CFN by gradually increasing the amount of Ca2+ (0–1000 nM) (Fig. 6). As shown in Fig. 6, the intensity of the absorption peaks of CFN at 356 nm was progressively increased. A substantial change in the color of CFN was observed after the addition of Ca2+ ions under a UV 365 nm lamp (inset Fig. 6b). The absorption intensity reached its maximum concentration (1000 nM) in a solution containing H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (9
DMF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), indicating stronger analyte-sensor complexation. Further fluorescence emission experiments were conducted to investigate the sensitivity of the AIE-based CFN sensor to calcium after the absorption studies. The emission spectra of the sensor were taken before and after complexation with the analyte (Fig. 7). With the increase in Ca2+ concentration, the fluorescence emission of CFN steadily decreased starting from 410 nm emission maxima and achieving the highest quenching at 1000 nM at 444 nm emission maxima, leading to a bathochromic shift of 34 nm (Fig. 7). The quantum yield of CFN decreased from Φ = 0.420 to Φ = 0.035.
1 v/v), indicating stronger analyte-sensor complexation. Further fluorescence emission experiments were conducted to investigate the sensitivity of the AIE-based CFN sensor to calcium after the absorption studies. The emission spectra of the sensor were taken before and after complexation with the analyte (Fig. 7). With the increase in Ca2+ concentration, the fluorescence emission of CFN steadily decreased starting from 410 nm emission maxima and achieving the highest quenching at 1000 nM at 444 nm emission maxima, leading to a bathochromic shift of 34 nm (Fig. 7). The quantum yield of CFN decreased from Φ = 0.420 to Φ = 0.035.
 | ||
Fig. 7 Fluorescence emission spectra (a) and the corresponding 3D Stern–Volmer plot (b) of the CFN sensor upon the consistent addition of Ca2+ (0–1000 nM) in aqueous solutions (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 9 DMF = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
The sensitivity of the AIE fluorescence-based CFN sensor was confirmed by calculating the limit of detection (LOD) using the established IUPAC procedure.
The LOD of 10.5 nM with CFN was found against Ca2+, which is significantly lower than the permissible calcium level of 2.12–2.62 mmol L−1 by WHO recommendations in dietary supplements and previously reported literature (Table S2).71
The results align with the absorption experiments, supporting the CFN–Ca2+ complex, which is supported by atomic radius, charges, and binding energy of calcium metal ion. In Fig. 7b, 3D SV plot demonstrates a selective quenching against Ca2+ ion. A linear relationship between the fluorescence intensity and the analyte concentration was found by calculation using the Stern–Volmer plot. With a significant correlation coefficient value of 0.999, it suggests a single chelation-enhanced quenching mechanism for CFN (Fig. S5). The SV binding constants (Ksv), value for Ca2+ were determined to be 1.76 × 10−7 M−1 with CFN (Fig. S5). Additionally, CFN exhibited stable emission upon exposure to 100 equivalents of interfering species, thus showing good selectivity towards the Ca2+ analyte (Fig. S6). The sensitivity of the developed sensor was further implied by the continuous variation method, and the output was plotted to elucidate its binding stoichiometry. The solvent system H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (9
DMF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with a fixed concentration of CFN was utilized to analyze various mole fractions (0–1.0) of the analyte. The results suggested a 1
1, v/v) with a fixed concentration of CFN was utilized to analyze various mole fractions (0–1.0) of the analyte. The results suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation stoichiometry between metal ions and the developed highly selective AIE sensor (Fig. S7). The fluorescence results were corroborated by density functional theory (DFT) studies (vide infra). Hence, after thorough UV-visible absorption and fluorescence emission experiments, the CFN sensor was found to be a suitable choice for sensitive and selective monitoring of Ca2+ in aqueous media (H2O
1 complexation stoichiometry between metal ions and the developed highly selective AIE sensor (Fig. S7). The fluorescence results were corroborated by density functional theory (DFT) studies (vide infra). Hence, after thorough UV-visible absorption and fluorescence emission experiments, the CFN sensor was found to be a suitable choice for sensitive and selective monitoring of Ca2+ in aqueous media (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF).
DMF).
3.5. Analysis of counter ions and reversibility
The specific chelation of Ca2+ with CFN was further examined through counter-experiments. Aqueous solutions of various calcium salts, including CaSO4, CaCl2, CaF2, and CaI2 were added to the sensor solution, followed by emission analysis (Fig. S8). The results established the selectivity of CFN even in the presence of counter ions. In addition, reversibility is an important parameter of a sensor for the development of real-sample analysis. The reversible detection of calcium was achieved by the addition of ethylene diamine tetraacetic acid (EDTA, 0–3 equivalents) to a metal-sensor complex (1 µM Ca2+) in an optimized solvent medium (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, H2O
1, H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF) (Fig. S9). The original fluorescence intensity was restored outstandingly, thus successful release of calcium from the complex was confirmed.
DMF) (Fig. S9). The original fluorescence intensity was restored outstandingly, thus successful release of calcium from the complex was confirmed.
3.6. Evaluation of pH, photostability and response time
For ensured real-time application of CFN, its behavior under various pH conditions and photostability was assessed. The emission response of CFN (10 µM) was taken across a pH range of 1–14 (Fig. S10). Significant quenching in response to the presence of the analyte was observed over the pH range (5.0–11.0), indicating the suitability of the observed pH range for Ca2+ sensing (Fig. S11). Sensor response time was evaluated for real-time applicability of the sensor. The sensor was mixed with a 500 nM Ca2+ solution, and a fluorescence change was monitored over 0–60 seconds time periods. Significant quenching after 10 seconds was observed for CFN up to 60 seconds (Fig. S12a). Similarly, the quenching response of CFN was tested under different temperatures from 28 °C to 38 °C, and the results remained promising as evident from Fig. S12b. These findings demonstrated the potential of the sensor as a reliable tool for the sensitive, selective, and rapid on-site detection of Ca2+ in aqueous media.Another vital aspect of practical applications of fluorescence-based sensors is its photostability. Following exposure to high energy excitation radiation, the emission of the sensor was observed, in both the presence and the absence of analytes in a selected H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF solvent combination (Fig. S13). The promising stability of the developed sensor was confirmed under the tentative conditions by no disruption in emission and sensing response.
DMF solvent combination (Fig. S13). The promising stability of the developed sensor was confirmed under the tentative conditions by no disruption in emission and sensing response.
3.7. Mechanism of the CFN sensor for Ca2+ detection
Job's plot was drawn based on fluorescence titration experiments at different mole fractions, which showcased the binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pointing towards the nature of chelates formed (Fig. S7). In the case of CFN, calcium ions showed best interaction with the nitrogen of the –CN group, oxygen of furan, and hydrogen of methyl group. The interaction is followed by fluorescence quenching due to the chelation-enhanced quenching (CHEQ) mechanism.21
1, pointing towards the nature of chelates formed (Fig. S7). In the case of CFN, calcium ions showed best interaction with the nitrogen of the –CN group, oxygen of furan, and hydrogen of methyl group. The interaction is followed by fluorescence quenching due to the chelation-enhanced quenching (CHEQ) mechanism.21
1H NMR titration was carried out to further validate the sensitive detection of Ca2+ via fluorescence quenching (S44–S46). The 1H NMR assay was conducted before and after the titration with 0.5 and 1.0 equivalent of Ca2+ from CaCl2 in DMSO-d6. The NH2 proton signals of CFN exhibited a significant downfield shift (Fig. 8), which suggests efficient complexation. The signal broadening is likely due to the paramagnetic nature of Ca2+. The complexation of CFN@Ca2+ changes the overall electronic environment over the structure of CFN, which alters the proton NMR signals. Moreover, a significant change in the chemical shift of the –OH group in the structure of CFN was observed. This demonstrates that the complexation of CFN@Ca2+ induced ICT that reduces the charge density over the oxygen atom of the –OH group, which increases the acidity of this group. Due to this, the OH group of CFN easily deprotonates or starts to exchange its proton with the solvent, which is not only responsible for a downfield chemical shift, but also quenches the intensity of the signal. The proposed metal-sensor complexation mechanism is depicted in Fig. 9.
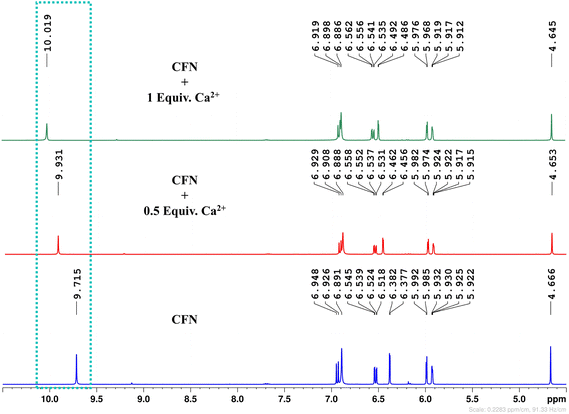 | ||
| Fig. 8 1H NMR titration spectra of CFN upon the addition of 0.5 and 1.0 equivalents of calcium ions in DMSO-d6. | ||
The reduction in the H–L gape of the CFN sensor was observed due to the overlapping of molecular orbitals of CFN with the atomic orbital of Ca2+. Moreover, the charge density distribution in the CFN sensor and its complex with Ca2+ was evaluated to identify the variation in their HOMO–LUMO energies. The H–L charge density was distributed over the sensor molecule before its interaction with the analyte. Nonetheless, the HOMO–LUMO charge density of the CFN sensor was slightly over Ca2+ ions after its interaction with the CFN sensor. Moreover, the entire characterization of the sensitivity/selectivity of CFN extensively depends on the DOS (density of state) analysis. DOS analysis was performed to evaluate the formation of new energy levels. The DOS results confirmed the formation of new energy levels (virtual energy states) and a sufficient reduction in the H–L gap in the CFN sensor–Ca2+ from 4.95 eV to 4.06 eV, which showed the significant sensitivity of CFN for Ca2+ ions, the eventual detection goal (Fig. S17b & c). DOS is one of the supreme DFT analyses to examine the sensitivity of the sensor.
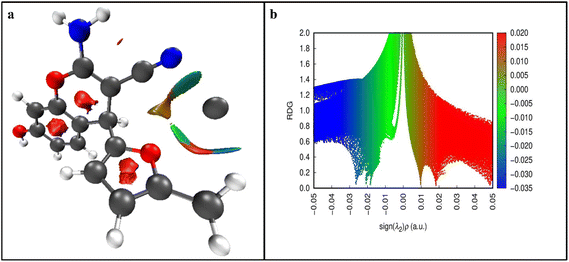 | ||
| Fig. 10 (a) 3D NCI isosurface plot of the CFN–Ca2+complex. (b) 2D RDG-NCI graph of the CFN–Ca2+ complex. | ||
To further understand the weak interactions and to evaluate the BCPs (bond critical points) of bonds between CFN and Ca2+ ions, QTAIM studies were enacted using the Multiwfn program. Three bond critical points were obtained using QTAIM theoretical calculations (Fig. 11).
The presence of weak interactions (NCIs) between CFN and Ca2+ is represented through resulting parameter values such as ∇2 ρ(r) and ρ(r) in the range of 0.0149 to 0.0171 a. u. and 0.081 to 0.091 a. u. Correspondingly, and the other BCP values represent the parameters of NCIs given in Table S3.
4. Analytical applications
4.1. Determination of Ca2+ in human plasma
Accurate detection of Ca2+ levels in real samples is vital because of the crucial role of Ca2+ in various biological processes, highlighting the suggested sensor's effectiveness. Its cytotoxicity was evaluated to ensure that the CFN sensor is suitable for biological applications (Fig. S19). Human plasma (Ceruloplasmin, Human Plasma) samples were purchased from Sigma-Aldrich (Product No. 239799, Description: Ceruloplasmin, Human Plasma, CAS 9031-37-2, from human plasma, is involved in serum copper transport and plays a protective role against oxygen radicals generated by iron and ascorbate), and 30 µM of CFN was added to different amounts of human plasma in double-distilled water (0–50 µL mL−1). The solution (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 1
H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) was used to record the fluorescence spectra, as shown in Fig. S20a.
9 v/v) was used to record the fluorescence spectra, as shown in Fig. S20a.
The results indicated a gradual decrease in fluorescence emission as a proportion of human plasma increased. Subsequently, different amounts of calcium were added to human plasma, and the resulting emission spectra were analyzed. The fluorescence emission spectra of CFN show a continuous decrease with the increase in Ca2+ concentration from 0 to 500 nM, as shown in Fig. S20b. Then 50 µL of human plasma was found to contain 300 nM of Ca2+, calculated by comparing the change in emission spectra and plotting a standard linear graph before and after spiking with Ca2+. The findings demonstrate the suitability of the CFN sensor for biological environments in quantifying Ca2+.
4.2. Recovery experiments of water and milk samples
The real-time applicability of CFN for monitoring the calcium ion concentration in river water and tap water samples was investigated. Water samples from Kunhar River, Garhi Habibullah, normal tap water from COMSATS University Islamabad, Abbottabad Campus, and commercial tetra pack milk of Olpers Pakistan from Shaheen Chemists Abbottabad were collected in glass vials and were diluted to suitable concentrations in double-distilled water for further analysis, performed without any delay. After removing impurities with a 0.2 µm syringe filter, the samples were spiked with different amounts of Ca2+. The fluorescence experiments were performed in triplicate with low to high spiking concentrations of 350 nM to 1000 nM. The graphs were plotted for all three types of samples (Fig. S21). Spike and recovery measurements were performed from the calibration curve of analyte concentration versus fluorescence results. The recovery percentages range from 102% to 109.8% with its relative standard deviation up to 2.37, showcasing the strong interaction of the sensor and the analyte (Tables S4–S6).4.3. Portable handheld fluorescent films
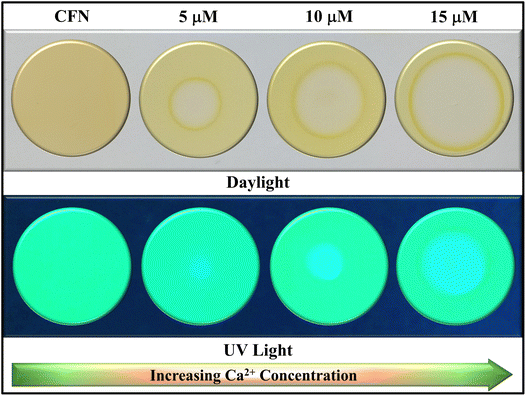
Modernization with uncontrolled industrialization and mounting environmental pollution have created an urgent need for a straightforward, onsite monitoring mechanism for Ca2+ monitoring. Building on the promising sensing capabilities of CFN, we developed portable, reliable, and cost-effective sensor-loaded fluorescent films. A 30 µM solution in THF was prepared, and thin-layer chromatography plates were dipped into the solution to fabricate these sensing films, which were dried at 35 °C for 20 minutes in a drying oven. The films appeared brownish in daylight while exhibiting a green color under 254 nm UV light (Fig. 12).When 10 µM Ca2+ was applied to these active fluorescent films, changes in color and absorption were observed as brown turns to greyish shade under daylight and light green turning to light blue under UV, respectively (Fig. 12). The results confirmed the effectiveness of the sensor-coated TLC films against the Ca2+ analyte as a convenient, efficient, potable and straightforward solution for onsite monitoring.
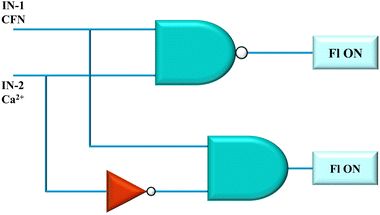
4.4. Fabrication of logic devices
Digital computing has modernized and simplified fluorescence sensing by converting fluorescence emission signals into chemically encoded information through the use of logic gates.73 Combining innovation and encouraging CFN sensor spectroscopic results, we developed a logic gate framework. A logic circuit consisting of NAND, AND, and NOT gates is used in the suggested configuration. Input-1 (IN-1) and input-2 (IN-2) are the corresponding input signals from CFN and Ca2+. Fig. 13 shows an employed setup consisting of an output signal (OUT) at an emission spectrum of 444 nm.The threshold for the detection of Ca2+ was set by the emission peak at 420 nm. The signals at 420 nm wavelength or higher were categorized as “ON” or “1”, while those below this were marked as “OFF” or “0”. Therefore, the logic gate demonstrated “OFF” when the complex CFN–Ca2+ was supplied as input and reverted to “ON” when only CFN was the input because of the use of a NAND gate. A truth table based on the logic circuit results that corresponded to fluorescent emission was drawn (Table S7). In conclusion, our research indicates that these sensors could be effectively integrated into contemporary electronic devices, facilitating easier and more efficient analytical procedures.
5. Conclusion
In conclusion, a novel chromene-based AIE-active and solvatochromic CFN sensor was synthesized and characterized by NMR spectroscopy. The CFN sensor with novel structural features and structure–property relationship demonstrated solvatochromism, large Stokes shift (120 nm), and aggregation-induced emission enhancement (AIE). The CFN sensor was employed for highly specific and sensitive colorimetric sensing of Ca2+ in an aqueous solution (λem = 444 nm, DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 1
H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) in the presence of potential interferents. Chelation-enhanced fluorescence quenching (CHEQ) is involved as a primary sensing mechanism in the sensing of Ca2+ ions. The CFN@Ca2+ complexation was confirmed through titration NMR analysis, dynamic light scattering (DLS) analysis, and DFT studies. A continuous variation method confirmed 1
9 v/v) in the presence of potential interferents. Chelation-enhanced fluorescence quenching (CHEQ) is involved as a primary sensing mechanism in the sensing of Ca2+ ions. The CFN@Ca2+ complexation was confirmed through titration NMR analysis, dynamic light scattering (DLS) analysis, and DFT studies. A continuous variation method confirmed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between the CFN and Ca2+ ion. Moreover, the detection and quantification limit were found to be 10.5 nM and 35 nM, respectively. Furthermore, the CFN sensor was employed for the quantification of Ca2+ ions in real samples, including human plasma, river water, tap water and commercial milk using spike recovery measurements. Additionally, a portable, cost-effective fluorescent TLC strip was fabricated for effective on-site monitoring of Ca2+ ions. Finally, a logic gate was constructed for the electronic monitoring of Ca2+ ions.
1 binding stoichiometry between the CFN and Ca2+ ion. Moreover, the detection and quantification limit were found to be 10.5 nM and 35 nM, respectively. Furthermore, the CFN sensor was employed for the quantification of Ca2+ ions in real samples, including human plasma, river water, tap water and commercial milk using spike recovery measurements. Additionally, a portable, cost-effective fluorescent TLC strip was fabricated for effective on-site monitoring of Ca2+ ions. Finally, a logic gate was constructed for the electronic monitoring of Ca2+ ions.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The scientific data supporting this article have been included as part of the supplementary information (SI). Supplementary information: absorption and emission spectra, DLS analysis, theoretical studies and NMR spectra. See DOI: https://doi.org/10.1039/d5ra06005b.Acknowledgements
The author (S. A. Shahzad) is very grateful to Pakistan Science Foundation for providing financial assistance via Grant No. PSF/NSFC-II/Eng/KP-COMSATS-Abt (12).References
- L. Li, X. Ma, W. Dong, P. Miao and Y. Tang, Bioconjugate Chem., 2018, 29, 1021–1024 CrossRef CAS PubMed.
- M. W. Berchtold, H. Brinkmeier and M. Muntener, Physiol. Rev., 2000, 80, 1215–1265 CrossRef CAS PubMed.
- J. Zheng, X. Zeng and S. Wang, Sci. China Life Sci., 2015, 58, 1–5 Search PubMed.
- J. Pepe, L. Colangelo, F. Biamonte, C. Sonato, V. C. Danese, V. Cecchetti, M. Occhiuto, V. Piazzolla, V. De Martino and F. Ferrone, Endocrine, 2020, 69, 485–495 CrossRef CAS PubMed.
- G. Zündorf and G. Reiser, Antioxid. Redox Signaling, 2011, 14, 1275–1288 CrossRef PubMed.
- M. Salek-Maghsoodi, Z. Golsanamlu, S. Sadeghi-Mohammadi, M. Gazizadeh, J. Soleymani and R. Safaralizadeh, RSC Adv., 2022, 12, 31535–31545 RSC.
- M. P. Mattson, Aging Cell, 2007, 6, 337–350 CrossRef CAS PubMed.
- S. Takano, H. Kaji, F. Hayashi, K. Higashiguchi, S. Joukei, Y. Kido, J. Takahashi and K. Osawa, Anal. Chem. Insights, 2012, 7, ACI.S9681 CrossRef PubMed.
- A. B. Borle and F. N. Briggs, Anal. Chem., 1968, 40, 339–344 CrossRef CAS PubMed.
- H. Vaessen and C. G. van de Kamp, Z. Lebensm. Unters. Forsch., 1990, 190, 199–204 CrossRef CAS PubMed.
- K. P. Jochum, D. Scholz, B. Stoll, U. Weis, S. A. Wilson, Q. Yang, A. Schwalb, N. Börner, D. E. Jacob and M. O. Andreae, Chem. Geol., 2012, 318, 31–44 CrossRef.
- H. Kaila, M. Ambasana, R. Thakkar, H. Saravaia and A. Shah, Indian J. Pharmaceut. Sci., 2010, 72, 592 CrossRef CAS PubMed.
- W. Zhang, H. Zhang and A. Zhou, Microchem. J., 2019, 147, 215–223 CrossRef CAS.
- S. Erdemir, O. Kocyigit and S. Malkondu, J. Photochem. Photobiol., A, 2022, 425, 113713 CrossRef CAS.
- Y. Wu, Y. Zhang, Z. Xu, X. Guo, W. Yang, X. Zhang, Y. Liao, M. Fan and D. Zhang, Biosensors, 2022, 12, 917 CrossRef CAS PubMed.
- X. Li, C. Pan, J. Cao, Z. Liu, Z. Zhu, C. Yan, W. Zhao, W.-H. Zhu and Q. Wang, Biomaterials, 2022, 289, 121778 CrossRef CAS PubMed.
- K. Khurshid, S. Saeed, M. A. Assiri, A. Shabbir and S. A. Shahzad, Spectrochim. Acta, Part A, 2025, 126421 CrossRef CAS PubMed.
- J. Yang, M. Fang and Z. Li, Aggregate, 2020, 1, 6–18 CrossRef.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741, 10.1039/B105159H.
- Muqadas, A. Shabbir, M. A. Assiri, M. Khan and S. A. Shahzad, J. Mol. Liq., 2025, 427, 127399 CrossRef CAS.
- Muqadas, A. Shabbir, M. Khan, M. A. Assiri, A. M. Khan and S. A. Shahzad, Microchem. J., 2025, 214, 114129 CrossRef CAS.
- Y. Yang, D. Deng, X. Deng, Z. Chen and S. Pu, Molecules, 2022, 27, 4740 CrossRef CAS PubMed.
- C. Zhu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, ACS Appl. Bio Mater., 2018, 1, 1768–1786 CrossRef CAS PubMed.
- J. Heo, D. P. Murale, H. Y. Yoon, V. Arun, S. Choi, E. Kim, J. S. Lee and S. Kim, Aggregate, 2022, 3, e159 CrossRef CAS.
- C. Wu, M. DeLong, Z. Vardeny and J. Ferraris, Synth. Met., 2003, 137, 939–941 CrossRef CAS.
- R. Kabe, H. Nakanotani, T. Sakanoue, M. Yahiro and C. Adachi, Adv. Mater., 2009, 21, 4034–4038 CrossRef CAS.
- J. Gierschner, L. Lüer, B. Milián-Medina, D. Oelkrug and H.-J. Egelhaaf, J. Phys. Chem. Lett., 2013, 4, 2686–2697 CrossRef CAS.
- F. C. Spano, Accounts Chem. Res., 2010, 43, 429–439 CrossRef CAS PubMed.
- S. Basak, N. Nandi, K. Bhattacharyya, A. Datta and A. Banerjee, Phys. Chem. Chem. Phys., 2015, 17, 30398–30403 RSC.
- N. Ryu, Y. Okazaki, E. Pouget, M. Takafuji, S. Nagaoka, H. Ihara and R. Oda, Chem. Commun., 2017, 53, 8870–8873 RSC.
- M. Kasha, Radiat. Res., 2012, 178, AV27–AV34 CrossRef CAS PubMed.
- J. Modi, S. Patel, G. K. Mahato, B. Mahto and G. Chandra, ChemPhotoChem, 2024, 8, e202300284 CrossRef CAS.
- S. M. Radiul, J. Chowdhury and S. Hazarika, J. Mol. Struct., 2023, 1275, 134606 CrossRef CAS.
- V. Martínez Martínez, F. López Arbeloa, J. Bañuelos Prieto and I. López Arbeloa, J. Phys. Chem. B, 2005, 109, 7443–7450 CrossRef PubMed.
- S. De, S. Das and A. Girigoswami, Spectrochim. Acta, Part A, 2005, 61, 1821–1833 CrossRef PubMed.
- N. H. Mudliar and P. K. Singh, Chem.–Eur. J., 2016, 22, 7394–7398 CrossRef CAS PubMed.
- M. Cigan, J. Donovalová, V. Szöcs, J. Gaspar, K. Jakusová and A. Gaplovsky, J. Phys. Chem., 2013, 117, 4870–4883 CrossRef CAS PubMed.
- S. Chakraborty, P. Debnath, D. Dey, D. Bhattacharjee and S. A. Hussain, J. Photochem. Photobiol., A, 2014, 293, 57–64 CrossRef CAS.
- A. K. Chibisov, G. V. Zakharova and H. Görner, Phys. Chem. Chem. Phys., 1999, 1, 1455–1460 RSC.
- A. K. Chibisov, G. V. Zakharova and H. Görner, Phys. Chem. Chem. Phys., 2001, 3, 44–49 RSC.
- C. Peyratout and L. Daehne, Phys. Chem. Chem. Phys., 2002, 4, 3032–3039 RSC.
- U. Rösch, S. Yao, R. Wortmann and F. Würthner, Angew. Chem., 2006, 118, 7184–7188 CrossRef.
- K. Khurshid, S. A. Shahzad, M. A. Assiri, A. Shabbir, T. Javid and H. Irshad, RSC Adv., 2024, 14, 21682–21691 RSC.
- A. Shabbir, S. A. Shahzad, A. Y. A. Alzahrani, Z. A. Khan, M. Yar and W. Rauf, Spectrochim. Acta, Part A, 2025, 327, 125414 CrossRef CAS PubMed.
- A. Shabbir, M. M. Iqbal, M. A. Assiri, T. Javid, A. Pervaiz, A. H. Khan, A. Hassan and S. A. Shahzad, Spectrochim. Acta, Part A, 2025, 333, 125895 CrossRef CAS PubMed.
- A. Majeed, S. A. Shahzad, M. A. Assiri, K. O. Khan, H. Rabale and A. Shabbir, Spectrochim. Acta, Part A, 2025, 125802 CrossRef CAS PubMed.
- M. J. Frisch, et al., Gaussian 09; Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
- Muqadas, A. Shabbir, M. A. Assiri, M. Khan and S. A. Shahzad, J. Mol. Liq., 2025, 427, 127399 CrossRef CAS.
- M. Khalid, A. Ali, S. Asim, M. N. Tahir, M. U. Khan, L. C. C. Vieira, A. F. de la Torre and M. Usman, J. Phys. Chem. Solids, 2021, 148, 109679 CrossRef CAS.
- R. Dennington, T. Keith and J. Millam, KS: Dennington, Semichem Inc., Shawnee Mission, 2009 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graph., 1996, 14, 33–38 CrossRef CAS PubMed.
- B. Mennucci, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 386–404 CAS.
- C. F. Matta and R. J. Boyd, An Introduction to the Quantum Theory of Atoms in Molecules, 2007, 1, 1–34 Search PubMed.
- S. R. Ankireddy and J. Kim, Sens. Actuators, B, 2018, 255, 3425–3433 CrossRef CAS.
- S. T. Hong, M. S. Kim, B. R. Kim, E. J. Lee, Y. U. Yoon, K. C. Paik, M. S. Han, E. S. Kim and B. R. Cho, Talanta, 2022, 244, 123408 CrossRef CAS PubMed.
- Y. Shen, S. Zhou, W. He, J. Gao, Y. Yang, K. Xiao and Z. Yao, J. Photochem. Photobiol., A, 2023, 442, 114771 CrossRef CAS.
- H. Zhang, C. Yin, T. Liu, Y. Zhang and F. Huo, Spectrochim. Acta, Part A, 2017, 180, 211–216 CrossRef CAS PubMed.
- L. J. Gomes, M. Outis, C. S. Gomes, A. C. Tomé and A. J. Moro, Molecules, 2024, 29, 527 CrossRef CAS PubMed.
- L. Cao, C. Jia, Y. Huang, Q. Zhang, N. Wang, Y. Xue and D. Du, Tetrahedron Lett., 2014, 55, 4062–4066 CrossRef CAS.
- S. Kacmaz, K. Ertekin, O. Oter, D. Mercan, E. Cetinkaya and E. Celik, J. Lumin., 2014, 147, 265–272 CrossRef CAS.
- A. S. Tanwar, S. Hussain, A. H. Malik, M. A. Afroz and P. K. Iyer, ACS Sens., 2016, 1, 1070–1077 CrossRef CAS.
- M. Haidekker, T. Brady, D. Lichlyter and E. Theodorakis, Bioorg. Chem., 2005, 33, 415–425 CrossRef CAS PubMed.
- J. Palion-Gazda, B. Machura, T. Klemens, A. Szlapa-Kula, S. Krompiec, M. Siwy, H. Janeczek, E. Schab-Balcerzak, J. Grzelak and S. Maćkowski, Dyes Pigm., 2019, 166, 283–300 CrossRef CAS.
- W. Liu, Y. Wang, J. Yang, X. Li, X. Wang and L. Ma, Dyes Pigm., 2020, 175, 108149 CrossRef CAS.
- F. Han, L. Chi, W. Wu, X. Liang, M. Fu and J. Zhao, J. Photochem. Photobiol., A, 2008, 196, 10–23 CrossRef CAS.
- S. Saeed, K. Khurshid, M. A. Assiri, A. Shabbir and S. A. Shahzad, Dyes Pigm., 2025, 239, 112813 CrossRef CAS.
- H. Auweter, H. Haberkorn, W. Heckmann, D. Horn, E. Lüddecke, J. Rieger and H. Weiss, Angew. Chem., Int. Ed., 1999, 38, 2188–2191 CrossRef CAS PubMed.
- H. Wu, L. Xue, Y. Shi, Y. Chen and X. Li, Langmuir, 2011, 27, 3074–3082 CrossRef CAS PubMed.
- M. Yang, D. Xu, W. Xi, L. Wang, J. Zheng, J. Huang, J. Zhang, H. Zhou, J. Wu and Y. Tian, J. Org. Chem., 2013, 78, 10344–10359 CrossRef CAS PubMed.
- M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC.
- P. E. Johnson, H. C. Lukaski and T. D. Bowman, J. Nutr., 1987, 117, 501–507 CrossRef CAS PubMed.
- L. Konečný, Z. Lomozová, G. Karabanovich, J. Roh, K. Vávrová and P. Mladěnka, J. Biol. Inorg. Chem., 2024, 1–16 Search PubMed.
- A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574–586 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |