Open Access Article
Open Access ArticleCorrosion mechanism of Cr-rich nickel-based alloys in a ternary molten salt: morphology analysis and first-principles study of Cl adsorption
Wenhao Zhaoa,
Dongkai Nib,
Wenjie Qia,
Jing Dingb,
Jianfeng Lub,
Weilong Wangb,
Xiaolan Wei*a and
Shule Liu *b
*b
aSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, PR China. E-mail: xlwei@scut.edu.cn
bSchool of Materials Science and Engineering, Sun Yat-sen University, Guangzhou High Education Mega Center, Guangzhou 510006, PR China. E-mail: liushle@mail.sysu.edu.cn
First published on 19th November 2025
Abstract
The corrosion of metal materials by chloride molten salts has limited their high-temperature application as heat transfer/storage media, while the composition of alloys strongly affects the corrosiveness of molten salts. This work combines experiments and first-principles calculations to study the long-term corrosion mechanism at the interface between nickel-based alloys and NaCl–CaCl2–MgCl2 chloride molten salts. The findings indicate the considerable depletion of chromium within the metallic stratum situated beneath the corrosion oxide layer of chromium-enriched alloys. The corrosion morphology shows that the Cr-rich alloys Hastelloy C-276 and Hastelloy X produce many holes in their cross-section, with MgCr2O4 formed on their surface, while Hastelloy B-2 has fewer stable oxidation products on its surface and no MgCr2O4 is generated. First-principles calculations focusing on Cl atom adsorption suggest that the reaction process of Cl atoms on the surface of doped Cr (Fe or Mo)–Ni (111) crystals is the key to Cr loss, and the results show that the doped Cr–Ni (111) crystal surface exhibited a larger adsorption energy for Cl, higher charge transfer amount, and lower desorption energy for the corrosion product CrCl4. The segregation energy difference of transferring a doped Cr atom from the second layer to the top surface layer is only 0.04 eV, while the adsorbed Cl is located around Cr on the Cr–Ni (111) crystal surface, indicating continuous Cr loss during the corrosion process. Therefore, if Cl atoms in molten salts are prevented from forming an adsorption layer on the alloy surface, the corrosion of Cr-rich nickel alloys in chloride molten salts can be reduced.
1. Introduction
Concentrating solar power (CSP) plants can provide low-cost power and are potential power plants for sustainable development in the future. The optimal operational temperature for the forthcoming generation of concentrating solar power (CSP) technology is in excess of 700 °C. Molten salts are frequently utilized as heat transfer fluid in CSP systems. In comparison to commercial nitrates, chloride molten salts exhibit analogous thermophysical properties, superior operating temperature, enhanced thermal stability, and reduced cost, thereby making them a prospective medium for heat storage and promising fluid for the next generation of concentrated solar power (CSP) systems.1 However, the strong corrosion of metals by chloride molten salts at high temperatures limits their application.Metal structural materials such as nickel- and iron-based alloys contain other metallic elements (Mo, Cr, W, etc.) to enhance their corrosion resistance, strength and other properties,2 which characterize the structural stability of these materials under corrosion. However, controversy remains regarding the impact of these elements on the corrosion of alloys in high temperature molten salts, particularly in regard to the role of chromium. In a study conducted by Wang et al.,3 the corrosion of pure Fe and pure Cr in ZnCl2 molten salt was examined. The findings indicated that metal Cr exhibited a comparatively reduced corrosion degree in comparison to Fe. Vignarooban et al.4 investigated the corrosion behavior of three nickel-based alloys, Hastelloy N, C276 and C22, in NaCl–KCl–ZnCl2 ternary chloride molten salt. The findings indicated that the Cr element could serve as a protective measure for the alloy. However, Hastelloy N, which exhibited the lowest Cr content, demonstrated a significant degree of corrosion. Thus, it can be surmised that the Cr element in the alloy serves to provide protection in the NaCl–KCl–ZnCl2 molten salt.
Liu et al.5 conducted a comparative analysis of the corrosion behavior of an Ni–Mo–Cr alloy exposed to two distinct environments, liquid salt and vaporized chloride molten salt. Their findings revealed that the alloy exhibited significant Cr depletion on the corrosion surface within the molten salt, with the depth of Cr depletion in molten salt steam being nearly twice that observed in liquid salt. In the study of the corrosion behavior of ferritic-martensitic P91 and stainless steel SS304 in NaCl–KCl–MgCl2 molten salt, Gong et al.6 observed the emergence of numerous missing cracks in the cross-sectional analysis of SS304 specimens that had been subjected to corrosion in molten salt for 1400 h. However, this phenomenon was not observed in the case of P91. Liu et al.7 found that the corrosion layer of Cr-rich alloy exhibits obvious Cr deficiency phenomenon, while the alloy with less Cr does not, and it is speculated that more Cr in alloys has an adverse effect on the loss of chromium during corrosion. Although the above-mentioned studies and others have found that some Cr-containing metals exhibit Cr loss in different chloride molten salts, there are still insufficiencies in the research on the loss of alloy elements of candidate structural materials in chloride molten salts, and the results of various metal elements seem to be contradictory.8,9
Existing studies have focused on corrosion experiments of alloys in molten salts, while the drivers of elemental loss and atomic-scale mechanisms are still poorly understood. First-principles calculation based on quantum mechanics is a powerful tool for studying microscopic corrosion mechanisms and has been applied in recent years in the study of corrosion mechanisms in fluoride molten salts. Yu et al.10 investigated the segregation behavior of some transition metals on the surface of Ni (111) and concluded that the elemental segregation behavior is closely related to the surface energy difference and elastic potential energy between the elemental alloy bodies. Yin et al.11 simulated the effect of F atom adsorption on the segregation of Cr on the surface of nickel in a fluoride salt environment by first principle calculations, and found that the adsorption of F atoms would significantly lead to the segregation of Cr on its surface. Nam et al. used the first-principles molecular dynamics (FPMD) method to calculate the diffusion rate and local structure of Cr0, Cr2+ and Cr3+ in LiF–BeF2 and LiF–NaF–KF molten salts, and found that with an increase in the valence state charge, the coordination number of Cr in the molten salts increased and the diffusion rate slowed down.12 Austin et al.13 studied the 0–6 valence coordination structure of Mo in LiF–NaF–KF molten salt. The higher valence Mo will form dimers or trimers in the molten salt, and the 4 valence Mo is the highest valence state that can exist stably in the molten salt. The higher valence Mo is easy to overflow the system in a gaseous state. Chahal used FPMD to study the effect of ZrF4 mole fraction and temperature on the coordination structure of the LiF–NaF–ZrF4 system.14 It was found that the average coordination number decreased with an increase in composition and temperature, which was consistent with the results of Raman spectroscopy. In addition, many researchers have determined the thermophysical properties and microstructure of F–LiNaK molten salt by calculation.15–19 Startt et al.20 simulated and calculated the effect of possible anion–cation interactions in chloride and fluoride salts on Cr segregation on the Ni (100) surface.
In general, the existing research lacks the interpretation of the nature of Cr loss from the perspective of adsorption and reaction and the difference in the segregation of elements in the alloy in molten salt corrosion. The influence of trace elements in alloys on the corrosion mechanisms in molten salts is still poorly understood, especially with regard to the effect of chloride salts.21 The impact of chloride adsorption on the segregation and dissolution of Cr, Fe, and Mo on the alloy surface is still not fully elucidated.
Herein, to gain better insight into the corrosion of Cr-rich nickel-based alloys in chloride-ion molten salts, the long-term corrosion behavior of two Cr-rich alloys and one Cr-poor nickel-based alloy in NaCl–MgCl2–CaCl2 salts is investigated. The reaction process of Cl atoms adsorbed on the surface of doped Cr (Fe or Mo)–Ni (111) crystals was also systematically determined using first-principles calculations. The microscopic mechanism of the loss is further revealed. This work provides insight into the development of methods to inhibit the corrosion of nickel-based alloys in chloride molten salts.
2. Experimental
2.1 Materials
The ternary NaCl–MgCl2–CaCl2 eutectic molten salt (39.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39.1
39.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.4 wt%) was prepared by using commercially available NaCl (purity ≥99.5 wt%), MgCl2 (purity ≥99 wt%) and CaCl2 (purity ≥96 wt%).7 The three salts were initially subjected to drying at 120 °C, after which they were combined in an alumina crucible in accordance with the prescribed ratio. The crucible was positioned within a muffle furnace, maintained at a temperature of 250 °C, and subsequently subjected to a 3 h drying procedure. Afterwards, drying was continued at 600 °C for 2 h. Once the mixture cooled to room temperature, the desired NaCl–MgCl2–CaCl2 ternary chloride salt (TCS) was obtained by crushing the material. The main thermophysical properties of TCS are listed in Table 1.22
21.4 wt%) was prepared by using commercially available NaCl (purity ≥99.5 wt%), MgCl2 (purity ≥99 wt%) and CaCl2 (purity ≥96 wt%).7 The three salts were initially subjected to drying at 120 °C, after which they were combined in an alumina crucible in accordance with the prescribed ratio. The crucible was positioned within a muffle furnace, maintained at a temperature of 250 °C, and subsequently subjected to a 3 h drying procedure. Afterwards, drying was continued at 600 °C for 2 h. Once the mixture cooled to room temperature, the desired NaCl–MgCl2–CaCl2 ternary chloride salt (TCS) was obtained by crushing the material. The main thermophysical properties of TCS are listed in Table 1.22
| Sample | Melting point/°C | Fusion enthalpy/J g−1 | Specific heat capacity (cp)/J g−1 K−1 | Density (ρ)/g cm−3 | Thermal conductivity (λ)/W m−1 K−1 |
|---|---|---|---|---|---|
| TCS | 427.5 | 190.1 | 1.112 (600 °C) | 2.349 (550 °C) | 0.404 (480 °C) |
Three types of alloys, Hastelloy X (HX), Hastelloy C-276 (HC-276) and Hastelloy B-2 (HB-2), were used for the corrosion experiments in this study. The main compositions of these alloys are shown in Table 2. Each alloy sample was mechanically divided into a 40 mm × 15 mm × 1 mm piece and the sample surface was polished with 10 µm SiC sandpaper before the experiment. Subsequently, the surface of each sample was polished to a mirror finish using a 2.5 µm diamond-based polish. Following a washing process involving deionized water and anhydrous ethanol, the samples were dried in an oven for future use.
| Alloy | Ni | Cr | Fe | Mo | Other |
|---|---|---|---|---|---|
| Hastelloy X | 42–52 | 20.5–23.0 | 17.0–20.0 | 8.0–10.0 | Co ≤ 1.0 |
| Hastelloy C-276 (HC-276) | 51–59 | 14.5–16.5 | 4.0–7.0 | 15.0–17.0 | W 3.0–4.5 Co ≤ 2.5, Mn ≤ 1.0, V 0 ≤ 0.35 |
| Hastelloy B-2 | 65–70 | 0.4–0.7 | 1.6–2.0 | 26.0–30.0 | Co ≤ 3.0 |
2.2 Corrosion and analysis method
The treated alloy was placed in a crucible, with 300 g of TCS added to completely submerge the alloy, and then the crucible was covered with a lid. The crucible was placed in a muffle furnace, ramping the temperature to 600 °C (temperature above the TCS eutectic at which the salt remains stable) at 5 °C min−1, and held for 35 days. Following the completion of the aforementioned corrosion experiments, the alloy samples were treated in accordance with the methodology outlined in the ASTM G1-03 standards.23Physical analyses of the specimens before and after corrosion were carried out using a multi-position auto-sampling X-ray diffractometer (XRD; X'pert Powder, Panacor, The Netherlands). The morphology of the specimen cross-section was analyzed by scanning electron microscopy, prior to and following the corrosion process (SEM; Hitachi Model 3700N, Japan), and the cross-section morphology was analyzed by using polymer embedding, where the sample to be characterized was encased in resin and polished to expose its cross-section. Concurrently, an energy dispersive spectrometer (EDS) was employed to ascertain the distribution of elements within the specimen cross-section, thereby discerning the diminution of alloying elements within the specimen cross-section.
2.3 Computational method and details
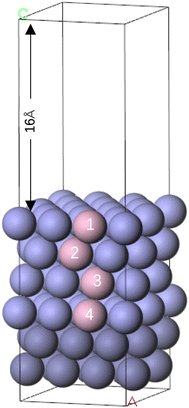
To explore the microscopic mechanism of the loss of alloying elements during the corrosion process of Cr-rich nickel-based alloy materials in NaCl–MgCl2–CaCl2 salt, the first-principles method based on density functional theory (DFT) was used to systematically calculate the reaction process of Cl atoms adsorbed on the surface of doped Cr (Fe or Mo)–Ni (111) crystals. The Cl atoms are in the form of Cl− ions, which are formed through the process of oxidation by dissolved O2 in the molten salt.The bulk structure of Ni crystals was first constructed to carve out the Ni surface model. Based on the face-centered cubic (fcc) single cell of Ni crystal, the k-points are set to 25 × 25 × 25. The calculated lattice constant of the Ni crystal is 3.518 Å. The surface energy and surface work function of three low-index crystal planes in the Ni cell were calculated, and the low-index crystal plane with a stable crystal was selected as the research target. Then, an Ni-based alloy crystal surface containing Cr, Fe or Mo was created by replacing a single Ni atom with Cr, Fe or Mo, respectively. Among them, the Ni atoms in the bottom two layers of the model are fixed, and in the four layers from the surface to the inside, one Ni atom in each layer is replaced and numbered by Cr, Fe or Mo atoms, respectively (see Fig. 1).
All first-principles calculations were performed using density functional theory (DFT) and Born–Oppenheimer approximation, which are implemented in the Vienna Ab Initio Simulation Package (VASP).24–26 The exchange–correlation functional was treated with the generalized gradient approximation (GGA) and the Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional,27 and the electron–ion interactions were approximated by pseudopotentials developed using the projector augmented wave (PAW) method.28,29 The valence electrons considered in the calculation are as follows: Cl (3s23p5), Ni (3d84s2), Fe (3d64s2), Cr (3d54s1), and Mo_sv (4p64d55s1). The energy cutoff value for the plane wave was set to 450 eV, and the energy convergence threshold of the self-consistent iteration is 10−6 eV. The convergence criterion for geometric optimization is that the force acting on each atom is reduced to below 0.01 eV Å−1, which is the limit of the optimization. In all structural relaxation calculations, the first-order Methfessel–Paxton smear method was used with a smear width of 0.2 eV. The gamma method was used to construct the inverted space K points, and a 5 × 5 × 1 grid was used to divide the Brillouin zone. Furthermore, all calculations were conducted using spin-polarized density functional theory to accurately depict the behavior of unpaired electrons in the valence shell.
3. Results and discussion
3.1 Morphology of the cross-section and element distribution
Fig. 2 illustrates the morphology of the cross-section and element distribution of the HX, HC-276 and HB-2 alloy samples corroded in the TCS at 600 °C for 35 days.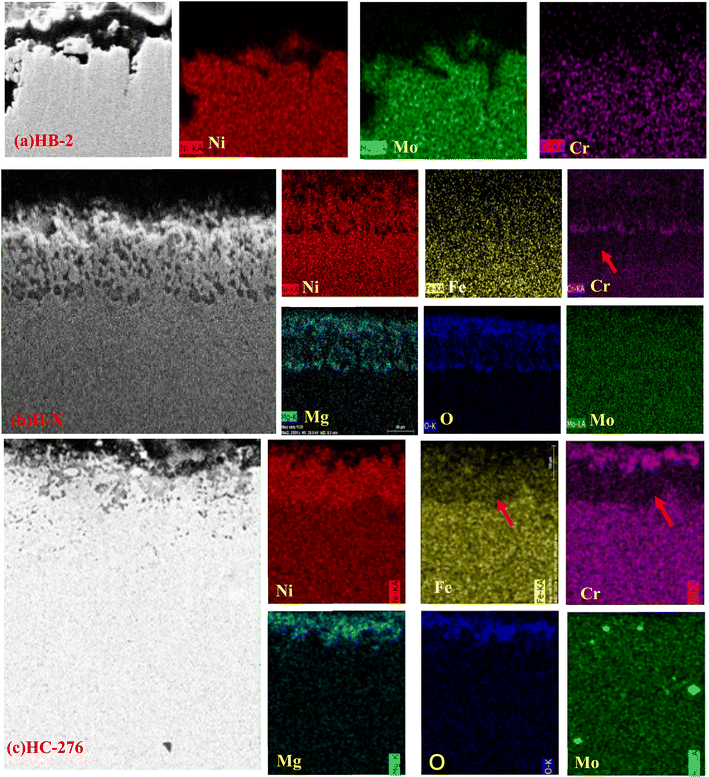 | ||
| Fig. 2 SEM images of cross-section and the elemental distributions of (a) HB-2, (b) H-X and (c) HC-276. | ||
Fig. 2 shows that the region of O distribution on the alloys surface is relatively thin, especially on HC276 and HB-2. This might be due to the short corrosion time or the insufficiency supply of oxygen, which may come from the water vapor or the air7 during the corrosion process. The Mo distributions in the corrosion layer and metal matrix are not much different from each other. The Mg element is distributed in the same area with O and Cr in the alloys, which suggests that some compounds are formed with them.
The elemental distributions shown in Fig. 2 indicate that there are Cr and Fe loss zones between the oxide layer and the metal matrix of the Cr-rich alloys HX and HC-276. In the Cr loss area of HX and HC-276, the content of Ni is more than that of the metal matrix, which may be due to the increase in Ni relative content in this area resulting from the loss of Cr and Fe. This phenomenon of Cr loss is consistent with the observations in other Cr-rich nickel-based alloys.5–7
3.2 Phase structure analysis of corrosion products
The X-ray diffraction patterns of the corrosion products covered on the surfaces of the HC-276, HB-2 and HX alloy samples after the corrosion experiment are presented in Fig. 3(a)–(c), respectively. | ||
| Fig. 3 XRD patterns of (a) HB-2, (b) HC-276 and (c) HX pre-corrosion and post-treatment after corrosion for 35 days in NaCl–MgCl2–CaCl2 molten salt at 600 °C. | ||
Fig. 3 shows that the three alloys retain their phase structure to a large extent after 35 days of corrosion. There is no obvious diffraction peak of oxide product in HB-2. The diffraction peaks of the corrosion product from HC-276 are relatively small. The peaks of corrosion product in HX are mainly attributed to MgCr2O4, combining the cross-sectional line scan and element distribution map images of EDS (Fig. S1 of the SI). The corrosion product MgCr2O4 was also observed on the surface of Inconel625 alloy corroded in TCS.7
Based on the EDS and XRD analysis, it is speculated that the reactions related to Cr loss occur during the corrosion process of Cr-rich alloys in TCS, as shown in Fig. 4.
The Gibbs free energy changes of the reactions in Fig. 4 are all negative,7 indicating that the chlorides can be oxidized by the O2 molecules dissolved in the molten salt, forming Cl2. The formed Cl2 may diffuse into the interior of the metal along the corrosion crack, causing the continuous loss of Cr during corrosion.
Cl atoms may also be generated and adsorbed onto the metal surface at the microscopic level, promoting the subsequent chlorination corrosion reaction. To further explore the role of Cl atoms in corrosion reactions, we established an M-doped nickel crystal structure, where M standards for the doped metal elements. After that, the first-principles structural optimization method was used to calculate the chlorination reaction process and its energy changes and reveal the mechanism of Cr loss in Cr-rich alloys during the corrosion process.
3.3 Microanalysis of corrosion mechanism
As listed in Table 3, the results calculated in this work are close to the literature values. The Ni (111) crystal plane has the highest work function and the lowest surface energy, and is the most stable low-index crystal plane, suggesting that the Ni (111) plane has the highest exposure probability and preferential corrosion in molten salt. Therefore, the Ni (111) surface is identified as the primary surface for molten salt ion adsorption in this study.
The adsorption energy of one Cl atom at various sites on the Ni (111) surface and the M–Ni (111) surface was calculated as follows:
| Eadsorption = Eslab-adsorbate − (Eslab + Eadsorbate) | (1) |
The adsorption energy difference of one Cl atom at various sites on the Ni (111) surface and the M–Ni (111) surface was calculated to ascertain the most stable adsorption site for the Cl atom on the crystal plane. This was then used to determine the initial site of corrosion. Fig. 5 illustrates the adsorption sites of the Ni (111) surface and the M–Ni (111) surface. The adsorption energies of Cl atoms at different sites on Ni (111) or M–Ni (111) surfaces were calculated and presented in Table 5.
Before calculating the adsorption energy, we verified the stability of the M–Ni system by calculating the formation energy, as follows:
| Eform = EM–Ni − ENi − µCr + µNi | (2) |
| Cr–Ni | Fe–Ni | Mo–Ni | |
|---|---|---|---|
| Eform (eV) | −0.09 | −0.31 | −0.10 |
| Adsorption site | TOP | HCP | FCC | TOP1 | HCP1 | HCP2 | FCC1 | FCC2 |
|---|---|---|---|---|---|---|---|---|
| Ni (111) | −1.517 | −2.013 | −2.064 | |||||
| Cr–Ni (111) | −1.964 | −2.238 | −2.000 | −2.328 | −2.033 | |||
| Fe–Ni (111) | −1.675 | −2.063 | −2.017 | −2.121 | −2.059 | |||
| Mo–Ni (111) | −2.273 | −2.250 | −1.957 | −2.338 | −1.968 |
The formation energies of all the doped systems are negative, indicating that the doping of Fe, Cr, and Mo in the Ni matrix is thermodynamically feasible.
During the structure optimization calculation, it was found that when Cl is adsorbed on the bridge site of the Ni (111) surface, it is easy to slip to the FCC site; when Cl is adsorbed on the TOP2 and bridge sites of the M–Ni (111) surface, it is also easy to slip to the FCC1 site, indicating that the TOP2 and bridge sites cannot adsorb Cl.
According to the values of the adsorption energy shown in Table 5, one Cl atom is preferentially adsorbed at the FCC1 site closest to Mo, Cr or Fe in M–Ni (111), and the FCC site in Ni (111). The adsorption energy of Cl at the FCC1 site of the M–Ni (111) surface is −2.328 eV (Cr), −2.121 eV (Fe) and −2.338 eV (Mo), respectively, which is larger than that of −2.064 eV at the FCC site on the Ni (111) surface. This means that doping Mo, Cr or Fe in the Ni (111) plane may promote Cl adsorption and probability of the chlorination reaction.
According to the adsorption energy of Cl, Mo has the strongest adsorption capacity for one Cl. However, experiments show that Cr is most likely to be lost after corrosion. Considering that the adsorption of Cl is the early stage of corrosion, the adsorption of multiple Cl, the transfer of charge after Cl adsorption, the desorption of products, and the migration of doped atoms in Ni crystal should also be considered.
| Ni (111) | Cr–Ni (111) | Fe–Ni (111) | Mo–Ni (111) | |
|---|---|---|---|---|
| a In table, M refers to the doped atoms Cr, Fe, Mo; the positive value means charge loss, and the negative means charge gain; ‘tot’ is the algebraic sum of the charge transfer amounts of the three Ni atoms adjacent to the adsorbed Cl at FCC, or that of two Ni atoms and a Cr (Fe or Mo) atom adjacent to the adsorbed Cl at FCC1 site. | ||||
| Ni | +0.126 | +0.065 | +0.086 | +0.067 |
| M | — | +0.743 | +0.488 | +0.757 |
| Cl | −0.457 | −0.491 | −0.486 | −0.474 |
| tot | −0.079 | +0.382 | +0.174 | +0.417 |
As shown in Table 6, doping the Cr (Fe or Mo) atom promotes the charge transfer from the metal atoms to Cl because the charge gain of Cl in Cr (Fe or Mo)–Ni (111) is larger than that in Ni (111). On the Cr–Ni (111) surface, the Ni adjacent to the adsorbed Cl at the FCC1 site loss +0.065e0 charge, which is much less than that of +0.126e0 charge. This means that when the Cl is adsorbed at the site of FCC1, the doped Cr will play a protective role for Ni, and the same role for Fe or Mo.
Table 6 shows the MCl-type adsorption and that its preferential adsorption site of Cl on the M–Ni (111) surface is FCC1 site. In the case of MCl2-type adsorption, further structural optimization found that if one Cl is placed at the FCC1 position, the other Cl will be located at the HCP1 position opposite to the FCC1 position because the energy of this type of adsorption is the lowest. The MCl3-type adsorption is the case that three Cl atoms are dispersed at three FCC1 sites and maintain symmetry, where the Cl atoms will slightly deviate from the FCC1 site, which is caused by the repulsive coulombic force and the van der Waals forces between Cl atoms. In the case of MCl4-type adsorption, three Cl atoms are placed at the FCC1 sites, and the fourth Cl atom is placed at TOP site of the M atom. After structural optimization, the Cl atoms will move slightly from the original adsorption site because of atom repulsion. The optimized configurations of MCl2, MCl3 and MCl4 on the M–Ni (111) surface are shown in Fig. S2 of the SI and the corresponding charge transfer amounts obtained are listed in Table 7, where the positive or negative values mean charge loss or gain, respectively.
| Doped atom | MCl2 | MCl3 | MCl4 | |||||
|---|---|---|---|---|---|---|---|---|
| M | Cl(HCCP) | Cl(FCC) | M | Cl(FC)C | M | Cl(FCC) | Cl (TOP) | |
| Cr | +0.831 | −0.483 | −0.481 | +0.916 | −0.481 | +0.984 | −0.481 | −0.484 |
| Fe | +0.550 | −0.473 | −0.474 | +0.637 | −0.466 | — | — | — |
| Mo | +0.976 | −0.470 | −0.464 | +0.953 | −0.462 | +0.946 | −0.460 | −0.465 |
Table 7 shows that Cr is easier to transfer charge to Cl, regardless of MCl2-type, MCl3-type or MCl4-type adsorption. In CrCl4-type adsorption, Cr lost the most charge of +0.984e0 and four Cl obtained the most total charge of −1.927e0 (−0.481 × 3 + 0.484) compared to the other types of adsorption. The difference in Cr loss charge and total gain charge of four Cl is −0.943e0 (+0.984–1.927), indicating that four Cl atoms around Cr need to obtain more charge from the surrounding Ni atoms, and then promote corrosion.
In the case of MCl3-type adsorption, the magnitude of the three types of Cl total charge gain is −1.443e0 (Cr) > −1.398e0 (Fe) > −1.386e0 (Mo), which is greater than the charge loss of Cr (+0.916e0), Fe (+0.637e0) and Mo (+0.953e0), respectively. In the case of MCl2-type adsorption, the total charge gain of two types of Cl from doped atoms is −0.964e0 (Cr) > −0.947e0 (Fe) > −0.934e0 (Mo), which is also greater than the charge loss of Cr (+0.831e0) and Fe (+0.550e0). In MoCl2-type adsorption, the total charge gain of two Cl is −0.934e0, which is smaller than the charge loss of Mo (+0.976e0), indicating that that the excess charge lost by Mo has been transferred to the surrounding Ni to protect Ni.
By evaluating the differential charge density, the charge transfer near the doped atom can be visually displayed while a Cl atom is adsorbed. The calculation of the differential charge density is based on the following equation:
| Δρ = ρtot − ρslab − ρCl | (3) |
Fig. 6(a)–(c) show that three Cl atoms are chemically adsorbed on the M–Ni (111) surfaces around the doped M atom. The electron cloud around Cl around Cr (Fe or Mo) accumulates significantly, while the electron cloud around of Cr (Fe or Mo) dissipates, and the electron cloud aggregation area is pointing to the doped Cr (Fe or Mo) atom, rather than Ni atoms, indicating that Cr (Fe or Mo) is bonded with Cl atoms, respectively and form MCl3 corrosion products. The interaction between Ni and Cl around the doped atoms is weak, and no chloride formation of Ni is around the doped atoms.
Fig. 6(d)–(f) show the horizontal cross section at the Cl atom. The darker the red color, the greater the charge density. It can be seen that the red color of Cl near the doped Cr (Fe or Mo) is darker, indicating that the charge density between the Cl and doped Cr (Fe or Mo) is higher, and chemical bonds are formed between Cl and Cr (Fe or Mo).
Structural optimization calculations of other types of adsorption such as MCl2-type and MCl4-type were also carried out. The calculated h and the desorption energy of MCl2 and MCl4 are also listed in Table 8. The formula for the calculation of the desorption energy is as follows:
| Edesorption = Eout − Eslab + adsorbate | (4) |
| Products | CrCl2 | CrCl3 | CrCl4 | FeCl2 | FeCl3 | MoCl2 | MoCl3 | MoCl4 |
|---|---|---|---|---|---|---|---|---|
| h/Å | 0.317 | 0.479 | 0.602 | 0.162 | 0.242 | 0.164 | 0.289 | 0.401 |
| Edesorption/eV | 4.023 | 3.883 | 3.342 | 4.455 | 4.685 | 6.418 | 5.147 | 4.243 |
Table 8 shows that the h of the Cr atom in CrCl4 is 0.602 Å, which is the highest in the compounds of MCl2, MCl3 or MCl4, indicating that CrCl4 is most likely to leave the Ni (111) crystal plane. The minimum desorption energy of 3.342 eV of CrCl4 among the compounds also supports the above-mentioned inference. The boiling point of CrCl4 is 600 °C,22 and thus when the Cr-rich alloys were corroded at 600 °C, the CrCl4 formed may leave the metal surface in the form of gas.
The above-mentioned studies show that the doped Cr–Ni (111) crystal surface exhibited a larger adsorption energy for Cl, higher charge transfer amount of absorbed Cl from Cr, and lower desorption energy of corrosion product CrCl4. These results well explain the preferred reaction of Cr and Cl atoms when the M–Ni (111) crystal plane adsorbs Cl atoms. However, it is also necessary to understand the migration ability of doped Cr, Fe or Mo atoms from the inside of the Ni crystal to the surface layer, which is related to the continuous loss of Cr during the corrosion process of the Cr-rich Ni-based alloys.
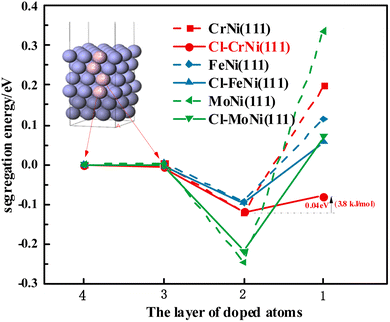 | ||
| Fig. 8 Segregation energy of different Ni atom stacking layers with Cr, Fe and Mo on Ni (111) surface. | ||
Fig. 8 shows that in the doped Cr–Ni (111) crystal, compared to unadsorbed Cl, the segregation energy of Cr in the first layer decreases from 0.199 eV to −0.080 eV when Cl is adsorbed on the surface. In crystals doped with Fe–Ni (111) and Mo–Ni (111), the segregation energies of Fe and Mo in the first layer decrease after the surface adsorption of Cl, but their values are all greater than 0.0 eV.
The difference between the segregation energy of the 1st and the 2nd layer doped Cr (Fe or Mo) atom is shown in Table 9.
| Surface | Cr–Ni (111) | Fe–Ni (111) | Mo–Ni (111) | Cl–Cr–Ni (111) | Cl–Fe–Ni (111) | Cl–Mo–Ni (111) |
|---|---|---|---|---|---|---|
| Segregation/eV | 0.319 | 0.208 | 0.582 | 0.040 | 0.152 | 0.291 |
According to Table 9 and Fig. 8, the results show that the segregation energy difference of Cr from the sub-outer layer to the surface layer is only 0.040 eV after the adsorption of Cl compared to 0.319 eV before Cl adsorption, which is 0.040 eV. The Cr atom can overcome this energy difference easily and move to the first layer. This suggests that the Cr atom tends to remain in the bulk phase of Ni when the Cr–Ni (111) surface does not adsorb Cl; after the adsorption of Cl, Cr tends to move to the surface to approach Cl atom, such that the subsequent reaction continues. To some extent, this explains the reason for the preferential loss of Cr when the Cr-rich nickel-based alloy is corroded in the chloride molten salt.
4. Conclusion
In this work, the corrosion behavior of the Cr-rich alloys Hastelloy C-276 and Hastelloy X and Cr-poor alloy Hastelloy B-2 in ternary NaCl–MgCl2–CaCl2 molten salts was studied via both experiments and first-principles calculations to systematically illustrate the reaction process of Cl atoms adsorbed on the surface of doped Cr (Fe or Mo)–Ni (111) crystals to explore the mechanism of corrosion. Cr loss was observed in the cross-section of Hastelloy C-276 and Hastelloy X after long-term corrosion in the molten salt, while this phenomenon was not observed in Hastelloy B-2. The MgCr2O4 protective layer produced by the preferential loss of chromium is the reason why the alloy is more resistant to corrosion.The first-principles calculations on Cl adsorption show that the Cr–Ni (111) surface exhibits stronger activity than the Fe or Mo doping surface in terms of Cl adsorption, charge transfer, corrosion product desorption, and reduced segregation energy Cl migration from the inner layer of the crystal to the surface layer. These findings indicate that Cl adsorption on the Cr–Ni (111) surface is a key driving force of Cr loss in Cr-rich alloys. This suggests that the corrosion of Cr-rich nickel-based alloys in chloride molten salts can be inhibited if the contact between the chlorine atoms in the molten salts and the metal surface can be reduced. For example, a possible corrosion prevention strategy for alloys in molten salts is via the pre-generation of an MgCr2O4 protective layer. The proposed mechanism of corrosion of Cr-rich nickel alloys in chloride molten salts from several perspectives in this work would provide insights for mitigating the corrosion problem in chloride molten salt applications.
Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available upon request.Supplementary information: the element distribution map images of EDS and adsorption configurations. See DOI: https://doi.org/10.1039/d5ra04910e.
Acknowledgements
The authors would like to acknowledge the financial support provided by the National Natural Science Foundation of China (No. 52036011 and No. U22A20213).References
- W. Ding, F. Yang, A. Bonk and T. Bauer, Sol. Energy Mater. Sol. Cells, 2021, 223, 110979–110988 CrossRef CAS.
- S. S. Raiman and S. Lee, J. Nucl. Mater., 2018, 511, 523–535 CrossRef CAS.
- X. Wang, H. Yin, W. Liu, G. Yu, J. He, Z. Tang and L. Yan, Mater. Corros., 2020, 71, 931–937 CrossRef CAS.
- K. Vignarooban, P. Pugazhendhi, C. Tucker, D. Gervasio and A. M. Kannan, Sol. Energy, 2014, 103, 62–69 CrossRef CAS.
- Q. Liu, Z. Wang, W. Liu, H. Yin, Z. Tang and Y. Qian, Corros. Sci., 2021, 180, 109183–109188 CrossRef CAS.
- Q. Gong, A. Hanke, F. Kessel, A. Bonk, T. Bauer and W. Ding, Sol. Energy Mater. Sol. Cells, 2023, 253, 112233 CrossRef CAS.
- B. Liu, X. Wei, W. Wang, J. Lu and J. Ding, Sol. Energy Mater. Sol. Cells, 2017, 170, 77–86 CrossRef CAS.
- M. Alkhamis, Master's Degree Dissertation, University of Arizona, 2016.
- J. E. Indacochea, J. L. Smith, K. R. Litko, E. J. Karell and A. G. Raraz, Oxid. Met., 2001, 55, 1–16 CrossRef CAS.
- Y. Yu, W. Xiao, J. Wang and L. Wang, Phys. Chem. Chem. Phys., 2016, 18, 26616–26622 RSC.
- Y.-R. Yin, C.-L. Ren, H. Han, J.-X. Dai, H. Wang, P. Huai and Z.-Y. Zhu, Phys. Chem. Chem. Phys., 2018, 20, 28832–28839 RSC.
- H. O. Nam, A. Bengtson, K. Vortler, S. Saha, R. Sakidja and D. Morgan, J. Nucl. Mater., 2014, 449, 148–157 CrossRef CAS.
- A. D. Clark, W. L. Lee, A. R. Solano, T. B. Williams, G. S. Meyer, G. J. Tait, B. C. Battraw and S. D. Nickerson, J. Phys. Chem. B, 2021, 125, 211–218 CrossRef CAS PubMed.
- R. Chahal, S. Roy, M. Brehm, S. Banerjee, V. Bryantsev and S. T. Lam, JACS Au, 2022, 2, 2693–2702 CrossRef CAS PubMed.
- B. A. Frandsen, S. D. Nickerson, A. D. Clark, A. Solano, R. Baral, J. Williams, J. Neuefeind and M. Memmott, J. Nucl. Mater., 2020, 537, 152219–152233 CrossRef CAS.
- D. Sprouster, G. Zheng, S.-C. Lee, D. Olds, C. Agca, J. McFarlane and B. Khaykovich, ACS Appl. Energy Mater., 2022, 5, 8067–8074 CrossRef CAS.
- M. Salanne, C. Simon, P. Turq and P. A. Madden, J. Fluorine Chem., 2009, 130, 38–44 CrossRef CAS.
- S.-C. Lee, Y. Zhai, Z. Li, N. P. Walter, M. Rose, B. J. Heuser and Y. Zhang, J. Phys. Chem. B, 2021, 125, 10562–10570 CrossRef CAS.
- C. S. Sona, M. A. Khanwale, C. S. Mathpati, A. Borgohain and N. K. Maheshwari, Appl. Therm. Eng., 2014, 70, 451–461 CrossRef CAS.
- J. Startt, R. Dingreville, S. S. Raiman and C. Deo, Appl. Surf. Sci., 2021, 563, 149836–149847 CrossRef CAS.
- S. Guo, J. Zhang, W. Wu and W. Zhou, Prog. Mater. Sci., 2018, 97, 448–487 CrossRef CAS.
- X. Wei, X. Zhang, J. Ding, W. Wang and J. Lu, Energy Procedia, 2019, 158, 773–778 CrossRef CAS.
- ASTM G1-03. Standard practice for preparing, cleaning, and evaluating corrosion test specimens, 2011 corrosion test specimens, the ASTM website, https://www.astm.org, 2011.
- G. Kresse and J. Hafner, Phys. Rev. B:Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B:Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- P. E. Blochl, Phys. Rev. B:Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B:Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- R. Tran, Z. Xu, B. Radhakrishnan, D. Winston, W. Sun, K. A. Persson and S. P. Ong, Sci. Data, 2016, 3, 160080 CrossRef CAS PubMed.
- H. B. Michaelson, J. Appl. Phys., 1977, 48, 4729–4733 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |