Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Analysis of the surface structure and electrochemical properties of functionalized carbon nanodots as environmentally friendly corrosion inhibitors for maraging steel in hydrochloric acid
Jyothi M. Alphonsoabc,
Ronald Aquin Nazareth*bc,
Geetha M. Pinto *b and
Sudhakar Y. N.
*b and
Sudhakar Y. N. *d
*d
aDepartment of Chemistry, Mahatma Gandhi Memorial College, Udupi-574102, Karnataka, India. E-mail: jyothialphonso305@gmail.com
bDepartment of Chemistry, St. Aloysius (Deemed to be University), Mangaluru-575003, Karnataka, India. E-mail: ronald.nazareth@staloysius.edu.in; geetha_pinto@staloysius.edu.in
cDepartment of Chemistry, Mangalore University, Mangalagangotri, Karnataka, India
dDepartment of Chemistry, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, 576104, India. E-mail: sudhakar.yn@manipal.edu
First published on 18th November 2025
Abstract
Maraging steel, although valued for its strength and durability, is highly prone to corrosion under acidic conditions. This work introduces functionalized carbon nanodots (FCNDs), synthesized from phenylalanine and citric acid, as novel, eco-friendly corrosion inhibitors for maraging steel in 1.0 M hydrochloric acid. Electrochemical techniques, including Tafel polarization and EIS, were used to evaluate the inhibition performance at various FCND concentrations and temperatures. The results clearly show an increase in the inhibition efficiency with increasing FCND dosage, although elevated temperatures and acid strengths reduce the effectiveness. Adsorption studies confirmed a Langmuir-type behavior governed mainly by physisorption. This study not only introduces carbon nanodots as a promising class of green corrosion inhibitors but also establishes their potential applicability in protecting high-strength maraging steel components exposed to acidic industrial environments.
1. Introduction
Metals and their alloys serve as indispensable components in modern engineering because of their robust mechanical characteristics, ease of fabrication, and broad commercial availability. These materials find application in sectors such as civil infrastructure, automotive production, industrial equipment, and transportation systems. However, in hostile environments, metals are susceptible to degradation through corrosion—a natural but destructive phenomenon that gradually diminishes their structural integrity.1The widespread occurrence of metal corrosion has significant implications, not only in terms of economic loss but also concerning health and safety within the industrial and public sectors. Although this process cannot be completely avoided, its progression can be effectively managed through suitable mitigation strategies.2 Corrosion typically results from electrochemical reactions between a metallic surface and its surrounding medium. For example, the corrosion of iron leads to the formation of reddish-brown hydrated ferric oxide, a product of electron and mass transfer at the metal–electrolyte interface.3 There are multiple techniques to avoid corrosion, such as cathodic and anodic protection, alloying, surface coating, and the use of chemical inhibitors. Among these methods, the use of corrosion inhibitors is widely adopted because of their cost-effectiveness and relative ease of application.4,5 In recent years, graphene oxide-based materials have garnered significant attention as potential corrosion-resistant coatings because of their large surface area, thermal stability, stable mechanical properties, and impermeability to water, oxygen molecules, and ions.6 Many inhibitors work by adhering to the metal surface, forming a barrier that limits direct interaction with corrosive agents. Organic inhibitors containing elements such as nitrogen, sulfur, or oxygen are particularly efficient owing to their ability to coordinate with metal ions and form stable protective films.7,8
Corrosion can occur in various forms, depending on the environmental conditions and the nature of the exposed material. Typical classifications include erosion–corrosion, selective leaching, stress corrosion cracking, uniform deterioration, localized pitting, grain boundary corrosion, corrosion in crevices, and galvanic effects.9–11 One effective approach to reduce corrosion is the use of metallic alloys. These materials often experience selective surface enrichment of more noble elements, which enhances their corrosion resistance. A particularly notable alloy in this context is maraging steel, which is characterized by excellent strength, toughness, and dimensional stability due to its unique microstructure.12 Maraging steels are distinct from conventional steels in that their hardness does not originate from the carbon content but rather from the precipitation of intermetallic phases during the thermal aging process. This aging, which occurs within a martensitic matrix, significantly enhances the mechanical properties while maintaining good formability and weldability.13,14
In recent years, growing environmental concerns and regulatory restrictions on toxic organic inhibitors have led to increased interest in environmentally benign alternatives. Among these materials, carbon dots (CDs)—a category of zero-dimensional nanostructures—have garnered attention for their promising characteristics. With average sizes below 20 nm (although sometimes reaching up to 60 nm), CDs offer features such as strong photoluminescence, high water solubility, a large surface area, tunable functional groups, and excellent biocompatibility.15–17 These carbon-based nanomaterials were first discovered during the purification of carbon nanotubes by Xu and colleagues,18 and since then, their potential applications have expanded into fields such as biomedicine, sensing, and electrochemistry. The corrosion inhibition properties of CDs have recently been explored, with encouraging results. For example, Shuyun Cao et al. demonstrated that nitrogen-doped CDs (N-CDs) provided 97.8% inhibition efficiency for carbon steel in sulfuric acid at 298 K when only 30 mg L−1 of the inhibitor was used.19 Similarly, Mingjun Cui and coworkers investigated the performance of CDs on Q235 steel in hydrochloric acid and reported a significant reduction in corrosion rates.20 In another study, Dongping Yang et al. synthesized CDs using imidazole ionic liquids and citric acid and reported that the inhibitors were more effective in 1 M HCl than in 3.5 wt% NaCl solutions, reflecting their performance across various corrosive environments.21 Further studies revealed the role of heteroatom doping in enhancing inhibition. Vandana Saraswat et al. reported an inhibition efficiency of 98.64% on mild steel using nitrogen and sulphur codoped CDs at 200 mg L−1 and 303 K.22 Hongyu Cen and team examined the protection of aluminum alloys in hydrochloric acid and achieved 85.9% efficiency with just 5 mg L−1 N−1, S-CD.23 Additionally, Subash Padhan et al. introduced copper into nitrogen-doped CDs and demonstrated high inhibition even at low concentrations, emphasizing the beneficial effects of metal ion doping.24 Despite these promising advances, research involving CDs as corrosion inhibitors for maraging steel remains scarce. Given the strategic importance of maraging steels in aerospace and high-performance structural applications, there is a compelling need to explore sustainable inhibition strategies tailored for such materials. The present study addresses this gap by investigating the use of functionalized carbon nanodots as corrosion inhibitors for maraging steel, with a focus on their adsorption behavior and protective performance in corrosive environments.
2. Experimental
2.1 Materials and chemicals
M250-grade maraging steel was obtained from a local shop, and phenylalanine and citric acid were procured from Merck.2.2 Method
The study utilized an aged M250-grade maraging steel sample shaped into a cylindrical rod. The elemental composition (wt%) of the 18Ni 250-grade maraging steel is shown in Table 1.| Element | % composition |
|---|---|
| Ni | 18.19 |
| Co | 7.84 |
| Mo | 4.82 |
| Ti | 0.52 |
| C | 0.015 |
| Fe | Balance |
The functionalized carbon nanodot (FCND) inhibitor was synthesized on the basis of previously reported procedures.23 Fifteen milliliters of water was used to dissolve phenylalanine and citric acid, which were then hydrothermally processed for eight hours at 200 °C in an autoclave lined with Teflon. After the suspension naturally cooled to room temperature, it was filtered. The carbon nanodots were designated according to the mole fraction of phenylalanine used in the mixture. For this work, P75, containing 75 mol% phenylalanine and 25 mol% citric acid, was selected (Scheme 1).
 | ||
| Scheme 1 Illustration of the initial reaction steps during the synthesis of carbon nanodots from citric acid and phenylalanine.25 | ||
2.3 Electrochemical study
The corrosive medium was prepared by dissolving hydrochloric acid to obtain a 1.0 M concentration, which was used to prepare inhibitor (FCND) solutions of varying concentrations. Electrochemical experiments were carried out with a saturated calomel electrode (SCE) as the reference, a platinum electrode as the counter, and a maraging steel sample as the working electrode assembled in a Pyrex glass cell. The electrochemical measurements were conducted using an ACM Instrument electrochemical workstation (Gill AC, Serial No. 1726, UK).The maraging steel sample was sectioned from a larger plate and mounted in epoxy resin, leaving a defined area exposed for electrochemical testing. The exposed surface was initially abraded with Emery papers ranging from 150 to 2000 grit and then subjected to fine polishing on a polishing wheel using an alumina slurry to achieve a mirror-like finish. The polished sample was subsequently cleaned with acetone, rinsed thoroughly with double-distilled water, and finally conditioned by washing with the test electrolyte solution. The effective surface area of the maraging steel electrode exposed during the EIS and PDP measurements was precisely maintained at 0.92 cm2. Measurements were taken by applying a sinusoidal voltage of 10 mV at the system's open circuit potential (OCP), covering a frequency range from 100 kHz to 0.01 Hz. The resulting impedance profiles were examined through Nyquist plots, and the charge transfer resistance (Rct) was determined from the size of the notable semicircular section of the plots. To ensure the reliability of the data, each experiment was repeated at least three times, with the average values of the key parameters being used for interpretation.
2.4 Characterization techniques
Using scanning electron microscopy in conjunction with energy-dispersive X-ray spectroscopy (SEM-EDS), the surface morphology of the maraging steel samples was investigated both before and after they were exposed to an acidic environment, both with and without the inhibitor. Instruments from 7610FPLUS, Jeol, Japan, and GEMINI 300, Carl Zeiss, Germany, were used for the analyses. UV-visible absorbance readings were recorded via a SYSTRONICS PC-based double beam Spectrophotometer 2202, with Serial No. 445. Infrared spectroscopy measurements via Fourier transform were conducted with a PerkinElmer Spectrum IR 10.7.2. The fluorescence properties were evaluated via two instruments: a Horiba spectrofluorometer (Model: FLUOROMAX_PLUS_C_0844G-3324-FMPLUS R928P) and an FLS1000 system from Edinburgh Instruments, UK. Topographical imaging through atomic force microscopy (AFM) was carried out in tapping mode using the Flex-Axiom system from NanoSurf, Switzerland.3. Results and discussion
3.1 Characterization
The UV-visible absorption profile of the prepared FCNDs, measured between 200 and 500 nm, revealed a strong absorption band at 210 nm and a secondary feature at approximately 250 nm. A gradual absorption extension into the visible range was also noted, likely arising from π–π* electronic transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C moieties and n–π transitions linked to C
C moieties and n–π transitions linked to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities,26 as depicted in Fig. 1.
O functionalities,26 as depicted in Fig. 1.
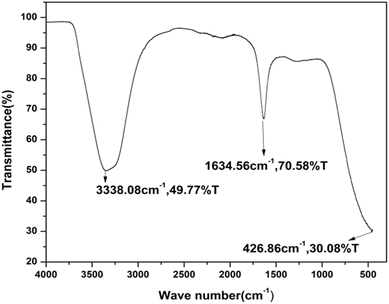
The FTIR spectra provide strong evidence for the attachment of amino acid-derived functional groups to the surface of the carbon dots. The distinct and intense absorption band observed within the range of 1600–1800 cm−1 is attributed to the vibrational stretching modes of carbonyl-related groups, including carboxylate, carbonyl, and amide functionalities. Furthermore, a broad absorption feature appearing between 3200 and 3500 cm−1 indicates the presence of hydroxyl (–OH) and amine (–NH2) groups, confirming the surface functionalization of the synthesized C-dots, as presented in Fig. 2.27
A systematic photoluminescence (PL) study was performed to better understand the optical behavior of the synthesized FCNDs using a range of excitation wavelengths (Fig. 3). As the excitation wavelength decreased from 384 nm to 280 nm, the PL intensity decreased significantly; however, the emission wavelength remained constant. This wavelength-independent PL response is in stark contrast to many carbon-based nanomaterials reported earlier,28–30 which typically show a redshift in emission with increasing excitation wavelength. The consistent emission feature observed here is likely due to the highly uniform size distribution and minimal surface defects present in the FCNDs.31
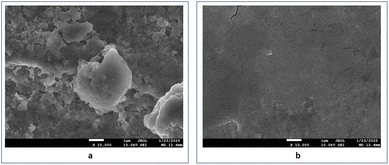 | ||
| Fig. 5 SEM images of aged maraging steel welds after being immersed in 1.0 M hydrochloric acid: (a) without the presence of FCND and (b) with the presence of FCND. | ||
In Fig. 6a, the carbon signal is minimal (1.65 wt%), which aligns with the trace amount of carbon naturally present in maraging steel. No nitrogen signal was detected, whereas oxygen dominated the spectrum (98.35 wt%), implying substantial oxidation and corrosion on the metal surface due to the aggressive acidic environment. In contrast, Fig. 6b shows a notable increase in the carbon content (6.84 wt%) and a detectable amount of nitrogen (2.46 wt%), with oxygen reduced slightly to 90.70 wt%. The higher carbon concentration confirmed that the phenylalanine-derived carbon dots were successfully deposited onto the steel surface. The appearance of nitrogen in this set suggests that nitrogen-containing functional groups from the amino acid precursor remained attached to the surface, likely contributing to the formation of a protective barrier. These elemental differences highlight the role of carbon dots in mitigating acid-induced corrosion by establishing a chemically interactive coating on the steel substrate.
3.2 Corrosion inhibition study
Electrochemical investigations, potentiodynamic polarization (PDP) and EIS analyses, were carried out at various temperatures: 303 K, 308 K, 313 K and 318 K.3.3 DC electrochemical techniques – potentiodynamic polarization (PDP) studies using Tafel extrapolation
Polished alloy samples were immersed in corrosive media with varying concentrations and subjected to different temperature conditions ranging from 303 K to 318 K. After achieving a stable open circuit potential (OCP), the samples underwent polarization by adjusting the potential from −250 mV to +250 mV relative to the OCP at a scan rate of 1 mV s−1. The resulting current–potential curves were examined via the Tafel extrapolation technique to obtain important polarization parameters. Potentiodynamic polarization curves for the corrosion of maraging steel in 1.0 M HCl media with various concentrations of the FCND inhibitor at 303 K, 308 K, 313 K and 318 K are shown in Fig. 7.A slight variation in the corrosion potential (Ecorr) suggests that the functionalized carbon nanodots (FCNDs) exhibit the characteristics of a mixed-type corrosion inhibitor. This implies that FCNDs influence both anodic metal dissolution and cathodic hydrogen evolution processes during corrosion. With increasing concentrations of FCNDs, a notable decline in corrosion current density (icorr) is observed, indicating enhanced corrosion resistance. The electrochemical parameters, including icorr, Ecorr, and Tafel slopes (βa and βc), are summarized in Table 2.
| Temperature (K) | Inhibitor concentration (ppm) | Ecorr (mV/SCE) | βa (mV) | −βc (mV) | Icorr (mA cm−2) ± SD | γcorr (mm per year) | η (%) ± SD |
|---|---|---|---|---|---|---|---|
| 303 | Blank | −316.3 | 113.0 | 164.9 | 6.8147 ± 0.0006 | 92.071 | |
| 100 | −313.4 | 97.4 | 160.4 | 3.8056 ± 0.0051 | 51.432 | 44.12 ± 0.02 | |
| 200 | −305.32 | 94.4 | 162.3 | 3.6909 ± 0.0009 | 49.875 | 45.84 ± 0.04 | |
| 300 | −290.38 | 87.6 | 145.3 | 3.2688 ± 0.0020 | 43.211 | 52.04 ± 0.05 | |
| 400 | −284.44 | 88.9 | 141.8 | 1.8413 ± 0.0107 | 24.236 | 73.07 ± 0.11 | |
| 308 | Blank | −313.76 | 126.9 | 171.9 | 5.5086 ± 0.0072 | 74.411 | |
| 100 | −303.41 | 130.7 | 175.7 | 4.8580 ± 0.0044 | 65.604 | 11.82 ± 0.02 | |
| 200 | −289.51 | 120.0 | 154.5 | 4.5368 ± 0.0021 | 64.237 | 17.57 ± 0.06 | |
| 300 | −283.71 | 97.6 | 158.7 | 3.0387 ± 0.0263 | 41.314 | 44.76 ± 0.05 | |
| 400 | −279.99 | 98.4 | 179.7 | 1.5523 ± 0.0060 | 21.227 | 71.67 ± 0.06 | |
| 313 | Blank | −289.11 | 157.1 | 193.4 | 5.9348 ± 0.0053 | 80.146 | |
| 100 | −288.85 | 141.7 | 192.3 | 5.3796 ± 0.0133 | 72.789 | 9.16 ± 0.05 | |
| 200 | −285.54 | 141.9 | 171.5 | 4.7804 ± 0.0134 | 64.721 | 19.25 ± 0.05 | |
| 300 | −284.64 | 106.1 | 166.4 | 4.4148 ± 0.0089 | 59.542 | 25.67 ± 0.06 | |
| 400 | −282.38 | 130.2 | 209.2 | 2.3610 ± 0.0221 | 31.989 | 60.44 ± 0.04 | |
| 318 | Blank | −290.25 | 175.8 | 205.4 | 6.6067 ± 0.0064 | 89.217 | |
| 100 | −286.45 | 152.3 | 160.2 | 5.8225 ± 0.0123 | 78.023 | 12.07 ± 0.10 | |
| 200 | −282.96 | 164.6 | 207.5 | 5.4950 ± 0.0079 | 74.298 | 16.73 ± 0.06 | |
| 300 | −281.4 | 140.5 | 187.4 | 3.7153 ± 0.0140 | 50.045 | 43.86 ± 0.14 | |
| 400 | −279.1 | 138.2 | 201.9 | 3.0226 ± 0.0035 | 40.246 | 54.16 ± 0.05 |
The variability in βc values is minimal. This suggests that the inhibitor does not affect the hydrogen evolution pathway. Inhibition is achieved solely through adsorption, which blocks the surface.35 Changes in the inhibitor concentration have little effect on the βa and βc Tafel slopes, which remain largely unchanged, indicating that the fundamental anodic and cathodic reaction pathways are not significantly altered by the presence of FCNDs.36,37 Instead, the observed inhibition is attributed to the surface coverage or barrier effect offered by the adsorbed FCNDs. This protective layer limits the access of corrosive species to the metal surface, thereby suppressing both anodic and cathodic reactions. On the basis of these observations, FCNDs can be classified as mixed-type inhibitors,38 functioning primarily through adsorption and the formation of a passive protective film on the alloy surface.
Adding error bars to the primary graph increased the certainty of the data trend. By displaying error bars for each data point, the plot provides visual evidence of how consistent the experimental measurements are. The very small spread of the bars shows that the variation in the data is minimal, indicating that the trend observed between the inhibitor concentration and inhibition efficiency is dependable and not due to random measurement fluctuations (Fig. 8).
 | ||
| Fig. 8 Error bars of the inhibition efficiency versus the inhibitor concentration, indicating minimal experimental error. | ||
The slopes of the anodic and cathodic branches of the Tafel curves change as the concentration of FCNDs increases. These findings suggest that the FCNDs affect both anodic and cathodic processes, reducing the degree of oxidation of the metal as well as the degree of hydrogen evolution on the steel surface.
The corrosion rate (mm per year), CR, was calculated via eqn (1):
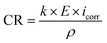 | (1) |
The constant k is 3.270 × 10−3 mm g (mA cm year)−1, the alloy's equivalent weight is denoted by E, its corrosion current density is quantified in units of mA cm−2 and symbolized by icorr, and its density is expressed in units of kg m−3 marked by ρ. To determine the corrosion inhibition efficiency η (%), eqn (2) was used.
 | (2) |
3.4 AC electrochemical techniques–electrochemical impedance spectroscopy (EIS) analysis
The Nyquist plots highlighted the effects of varying doses of FCNDs synthesized from citric acid and phenylalanine on the corrosion behavior of maraging steel in 1.0 M HCl at 303 K. The values of the impedance obtained with and without the inhibitor are denoted as Rctinh and Rctuninh, respectively. An increase in Rct under inhibited conditions suggests a significant retardation of the charge transfer mechanism at the metal–solution interface, which is indicative of protective film formation by the FCNDs. This increase is typically linked to a greater interfacial barrier, possibly due to a denser inhibitor layer and/or a decrease in the local dielectric constant, both of which result in a reduced double-layer capacitance (Cdl).39The effectiveness of inhibition (η%) was determined via the relationship outlined below:
 | (3) |
The electrical double layer capacitance, Cdl, at the interface was determined via the following equation:
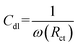 | (4) |
3.5 Interpretation of the Nyquist plot
Electrochemical impedance spectroscopy (EIS) was used to assess the corrosion characteristics of maraging steel in a hydrochloric acid environment, both without and with functionalized carbon nanodots (FCNDs), at four distinct temperatures: 303 K, 308 K, 313 K, and 318 K. The related Nyquist plots are shown in Fig. 9. In the impedance diagram, the X-direction corresponds to Z′, the real impedance, whereas the Y-direction reflects Z″, the imaginary counterpart. The impedance spectra display semicircular arcs, which are indicative of charge transfer processes at the metal–electrolyte interface. This semicircular nature is commonly associated with electrochemical systems undergoing corrosion. The appearance of capacitive loops at high frequencies and inductive loops at lower frequencies is typical for these systems, whether inhibited or uninhibited. A clear trend emerges from the plots: as the FCND concentration increases, the diameter of the semicircle becomes larger. This expansion reflects a higher charge transfer resistance, implying improved resistance to corrosion. The protective benefit is linked to the adsorption of FCNDs onto the surface of the steel, creating an insulating layer that restricts the interaction between the metal and the corrosive environment.The blank sample (represented by the black curve), which contains no inhibitor, has the smallest semicircle and the lowest impedance values, indicating rapid corrosion. As the FCND concentration increases from 100 ppm (red curve) to 400 ppm (green curve), the size of the semicircles significantly increases. This observation suggests that higher concentrations of FCNDs enhance the inhibition efficiency by strengthening the surface barrier. Furthermore, the slightly flattened shape of the semicircles suggests surface irregularities and nonuniform adsorption, leading to frequency dispersion—an effect commonly observed on solid metal surfaces with heterogeneous morphology.41 The elevated Rct values in the presence of FCNDs support the conclusion that these nanodots act as effective corrosion inhibitors by reducing the rate of electrochemical reactions on the steel surface.42–44
Table 3 clearly shows that as the inhibitor concentration increases, the CPE values tend to decrease, whereas the Rct values increase. The decrease in the CPE can be linked to an increase in the electrical double layer thickness or a change in the local dielectric constant, indicating that the FCND operates through adsorption at the metal/solution interface.45 As the inhibitor concentration increased, more FCND particles adsorbed on the surface, resulting in lower CPE values and a thicker film.
| Temperature (K) | Inhibitor concentration (ppm) | Rct (Ω cm2) ±SD | Cdl (mF cm−2) ± SD | Rsol (Ω cm2) | CPE, Ω−1 cm−2 | η (%) |
|---|---|---|---|---|---|---|
| 303 | Blank | 15.28 ± 0.03 | 8.94 ± 0.006 | 1.207 | 0.99 | |
| 100 | 27.11 ± 0.02 | 4.975 ± 0.005 | 1.64 | 0.93 | 43.65 | |
| 200 | 28.42 ± 0.03 | 3.037 ± 0.006 | 1.169 | 0.91 | 46.24 | |
| 300 | 32.13 ± 0.05 | 2.925 ± 0.005 | 1.349 | 0.83 | 52.45 | |
| 400 | 56.70 ± 0.10 | 1.387 ± 0.006 | 1.2 | 0.76 | 73.06 | |
| 308 | Blank | 7.235 ± 0.003 | 16.847 ± 0.040 | 1.649 | 1.13 | |
| 100 | 8.178 ± 0.007 | 6.526 ± 0.005 | 1.238 | 1,12 | 11.56 | |
| 200 | 8.800 ± 0.020 | 5.719 ± 0.026 | 1.563 | 1.11 | 17.93 | |
| 300 | 13.090 ± 0.017 | 5.638 ± 0.005 | 1.357 | 0.85 | 44.62 | |
| 400 | 25.200 ± 0.100 | 2.177 ± 0.006 | 1.269 | 0.84 | 71.27 | |
| 313 | Blank | 5.027 ± 0.022 | 4.944 ± 0.006 | 1.249 | 1.14 | |
| 100 | 5.576 ± 0.004 | 4.929 ± 0.010 | 1.676 | 1.13 | 9.61 | |
| 200 | 6.257 ± 0.006 | 5.846 ± 0.005 | 1.646 | 1.11 | 19.48 | |
| 300 | 6.745 ± 0.005 | 3.155 ± 0.005 | 1.935 | 1.05 | 25.24 | |
| 400 | 12.700 ± 0.100 | 3.238 ± 0.027 | 1.294 | 1.08 | 60.61 | |
| 318 | Blank | 3.787 ± 0.006 | 11.687 ± 0.104 | 1.473 | 1.20 | |
| 100 | 4.337 ± 0.006 | 8.942 ± 0.034 | 1.732 | 1.15 | 12.67 | |
| 200 | 4.538 ± 0.052 | 8.044 ± 0.036 | 1.588 | 1.12 | 16.04 | |
| 300 | 6.724 ± 0.005 | 6.945 ± 0.045 | 2.117 | 1.08 | 43.61 | |
| 400 | 8.395 ± 0.005 | 5.831 ± 0.052 | 1.447 | 1.00 | 54.84 |
Fig. 10 shows the five-element circuit used for impedance simulation. The element Rs represents the bulk resistance of the medium. The resistance linked to charge transfer is denoted by Rct. The inductance is modeled via the pair RL and L. A constant phase element, Q, is connected across the Rt and RL branches to account for nonideal capacitive behavior. The polarization resistance Rp can be calculated from eqn (5):
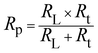 | (5) |
In the equivalent circuit, no ideal capacitor is included. Instead, a constant phase element (CPE) is used to represent the nonideal capacitive behavior of the electrode interface. The CPE accounts for effects such as surface roughness, heterogeneity, and nonuniform current distribution, which prevent the system from behaving like an ideal capacitor.44,46 The impedance of the CPE varies with frequency and is expressed as (eqn (6)):
 | (6) |
3.6 Interpretation of the Bode plot
Electrochemical impedance measurements were utilized to obtain Bode plots—both magnitude and phase angle—to evaluate the protective behavior of functionalized carbon nanodots (FCNDs) on maraging steel under acidic conditions. These tests were conducted in a corrosive medium using an open circuit potential (OCP) containing 1 M hydrochloric acid at 30 °C. The results are illustrated in Fig. 11. The phase angle response shows a noticeable upward trend with increasing FCND concentration, reaching a maximum at the most effective inhibitor dosage. This increase in the phase angle suggests improved surface coverage and more capacitive behavior, indicative of the formation of a stable and uniform inhibitor film at the metal–electrolyte interface. In the Bode magnitude plot (Fig. 10b), a single linear region is evident across the frequency range for both the uninhibited and inhibited systems. This behavior is characteristic of systems dominated by one primary electrochemical process. The polarization resistance (Rp), which reflects the resistance to charge movement and is related to the integrity of the inhibitor layer, is inferred from the difference in impedance between the higher and lower ends of the frequency spectrum. A greater separation implies greater resistance to corrosion, as it accounts for the combined effects of surface passivation, metal dissolution, and the barrier characteristics of the adsorbed FCND layer.47 The observed increase in the phase angle and Rp values with increasing FCND loading highlights the material's capacity to hinder electrochemical activity and reinforces its potential as an effective corrosion inhibitor. | ||
| Fig. 11 (a) Bode phase and (b) magnitude plots for the alloy in 1.0 M HCl at 30 °C with varying FCND concentrations. | ||
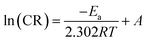 | (7) |
The term Ea indicates the corrosion activation energy, R is the gas constant, and A is the preexponential term in the Arrhenius equation. The straight-line trend observed in the Arrhenius plots confirms that the corrosion rate decreases with increasing temperature, which is consistent with thermally activated processes. The calculated slopes of these plots were used to estimate the Ea values, which are detailed in Table 4. A comparison between the blank and inhibited systems revealed that the activation energy was greater when the inhibitor (FCND) was present. This suggests that the FCND molecules adsorb onto the steel surface, forming a protective barrier that increases the energy requirement for corrosion to occur.48 The elevated Ea values indicate a shift toward reduced metal dissolution in the presence of the inhibitor. Additionally, activation energy values exceeding 20 kJ mol−1 typically point toward a physisorption mechanism, where the interaction between the inhibitor molecules and the metal surface is primarily through weak, noncovalent forces. This is consistent with the behavior observed here. The data also indicate that as the concentration of FCND increases, the corresponding activation energy increases, which implies enhanced surface coverage and increased inhibition efficiency. However, at higher temperatures, partial desorption of the physically adsorbed inhibitor may occur, slightly reducing the protection and leading to variations in the Ea values.49
| Inhibitor concentration (ppm) | Ea (kJ mol−1) | ΔHa(kJ mol−1) | ΔSa(kJ mol−1) | Ea − ΔHa (kJ mol−1) |
|---|---|---|---|---|
| Blank | 14.48 | 11.98 | −168.19 | 2.5 |
| 100 | 21.78 | 19.21 | −148.48 | 2.57 |
| 200 | 19.34 | 17.03 | −155.88 | 2.31 |
| 300 | 20.86 | 18.31 | −153.80 | 2.55 |
| 400 | 35.15 | 33.12 | −110.65 | 2.03 |
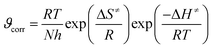 | (8) |
| Ea − ΔHa = RT | (9) |
This small difference confirms the validity of transition state theory in this system and suggests a unimolecular reaction pathway for the dissolution process.50 This mechanism implies that the reaction proceeds via a single activated complex without the need for simultaneous collision between multiple species.
The notably low values of activation entropy (ΔS≠), observed both in the absence and presence of the inhibitor, suggest that the creation of the activated complex in the rate-limiting step is characterized by an associative mechanism rather than a dissociative mechanism. This results in a decrease in disorder as the system moves from the reactants to the activated complex51.
Langmuir adsorption isotherm, linear graph of  against C
against C
 | (10) |
Temkin adsorption isotherm equation, linear graph of θ vs. log C
 | (11) |
Frumkin adsorption isotherm, linear graph of  vs. log C
vs. log C
 | (12) |
Freundlich isotherm, linear graph of log θ vs. log C
 | (13) |
Fig. 13 shows the linear fits corresponding to the adsorption isotherms described by eqn (9)–(12). The coefficient of determination (R2) values for each isotherm are summarized in Table 5. Among these isotherms, the Langmuir isotherm has the highest R2 value, indicating that it best represents the adsorption behavior of the system. However, the linear plots do not pass through the origin and have an average correlation coefficient of 0.9235, suggesting a slight deviation from the ideal Langmuir adsorption for the FCND molecules. The positive enthalpy of adsorption suggests that the process of adsorption is endothermic.50 On the basis of the Langmuir model, the adsorption sites on the metal surface have consistent energy levels, enabling the creation of a monolayer of inhibitor molecules while disregarding any interactions among the adsorbed species.53 The adsorption equilibrium constant (Kads) was calculated via the Langmuir isotherm. The relationship between Kads and the standard free energy of adsorption  is represented by eqn (14).
is represented by eqn (14).
ΔG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = −2.303RT log(55.5Kads) = −2.303RT log(55.5Kads)
| (14) |
 | ||
| Fig. 13 Adsorption isotherms for FCND at various temperatures in 1 M HCl: (a) Langmuir, (b) Temkin, (c) Freundlich, and (d) Frumkin models. | ||
| Temp. (K) | Langmuir Cinh vs. Cinh/θ | Frumkin, θ/1 − θ vs. log C | Temkin, log C vs. θ | Freundlich, log C vs. log θ | |||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | Kads (mol L−1) | R2 | ΔG0ads (kJ mol−1) | ΔH0ads (kJ mol−1) | ΔS0ads (J K−1 mol−1) | R2 | R2 | R2 | |
| 303 | 1.103 | 5.865 × 103 | 0.7065 | −32.34 | −9.17 | −197.52 | 0.8739 | 0.9234 | 0.9313 |
| 308 | 1.311 | 2.358 × 103 | 0.9805 | −32.71 | −10.89 | −197.52 | 0.9222 | 0.9404 | 0.9251 |
| 313 | 1.594 | 2.227 × 103 | 0.8138 | −31.92 | −13.25 | −197.52 | 0.8483 | 0.9110 | 0.7989 |
| 318 | 1.574 | 1.661 × 103 | 0.7864 | −31.75 | −13.08 | −197.52 | 0.5918 | 0.6099 | 0.6337 |
In this study, the measured enthalpy of adsorption  was found to be less than 20 kJ mol−1, indicating that the adsorption of the inhibitor on weld-aged maraging steel occurs primarily through physical adsorption (physisorption) and is an exothermic process. The negative value of the Gibbs free energy of adsorption
was found to be less than 20 kJ mol−1, indicating that the adsorption of the inhibitor on weld-aged maraging steel occurs primarily through physical adsorption (physisorption) and is an exothermic process. The negative value of the Gibbs free energy of adsorption  suggests that the adsorption process proceeds spontaneously with the coating of the inhibitor on the alloy surface, which is found to be inert. Additionally, the notably large negative entropy change
suggests that the adsorption process proceeds spontaneously with the coating of the inhibitor on the alloy surface, which is found to be inert. Additionally, the notably large negative entropy change  implies that a reduction in randomness occurs during the shift from reactants to the adsorbed complex on the surface of the alloy, which is consistent with the typical entropy reduction observed in adsorption phenomena.7
implies that a reduction in randomness occurs during the shift from reactants to the adsorbed complex on the surface of the alloy, which is consistent with the typical entropy reduction observed in adsorption phenomena.7
In acidic media, positively charged FCNDs also experience electrostatic attraction with negatively charged metal surfaces, which supports physisorption. At elevated temperatures, the adsorption shifts to be dominated by physisorption, as evidenced by the observed adsorption enthalpy  values below 20 kJ mol−1. This synergistic effect suppresses both anodic and cathodic processes, effectively slowing corrosion and extending the service life of steel.
values below 20 kJ mol−1. This synergistic effect suppresses both anodic and cathodic processes, effectively slowing corrosion and extending the service life of steel.
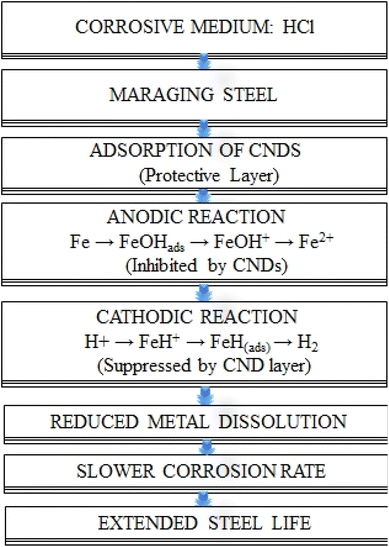
The corrosion process of maraging steel in an acidic environment includes both anodic and cathodic reactions. The schematic representation of the inhibition mechanism is as follows:
At the anode, iron interacts with water to produce an adsorbed iron hydroxide compound while releasing protons and electrons:
| (i) Fe + H2O ⇌ FeOHads + H+ + e− | (15) |
The adsorbed iron hydroxide then undergoes a rate-determining step, leading to the formation of FeOH+ ions:
| (ii) FeOHads → FeOH+ + e− | (16) |
These ions further react with protons to produce ferrous ions Fe2+ and water:
| (iii) FeOH+ + H+ → Fe2+ + H2O | (17) |
In the reduction process on the cathodic side, hydrogen ions adsorb onto the metal surface, leading to the formation of FeH+ species:
| (iv) Fe + H+ → FeH+ (ads) | (18) |
Following this, electron transfer reduces FeH+ to produce adsorbed atomic hydrogen:
| (v) FeH+ + e− → FeH (ads) | (19) |
The adsorbed hydrogen then combines with additional protons and electrons to form molecular hydrogen gas, which is released from the surface:
| (vi) FeH (ads) + H+ +e− → Fe + H2 | (20) |
This series of reactions leads to the dissolution of metal at the anode and the release of hydrogen gas at the cathode, which defines the corrosion process in an acidic setting.
4. Conclusion
This study verified that carbon nanodots derived from phenylalanine and citric acid serve as effective corrosion inhibitors for maraging steel in a 1.0 M hydrochloric acid environment. Electrochemical impedance and polarization analyses indicate that these nanodots create an adsorbed protective layer on the metal surface, significantly decreasing its interaction with the acidic medium. The inhibition efficiency increased to 73% and improved further with increasing concentrations of the nanodots, demonstrating a clear dependence of corrosion protection on the inhibitor dosage.Conflicts of interest
There is no conflict to declare.Data availability
Data will be shared upon request to the authors.Acknowledgements
The authors sincerely acknowledge Dr P. A. Suchethan, Associate Professor, Department of Chemistry, Tumkur University, India for his valuable guidance, insightful ideas, and helpful suggestions that greatly contributed to this work.References
- J. K. Odusote, et al., Inhibition efficiency of gold nanoparticles on corrosion of mild steel, stainless steel and aluminum in 1 M HCl solution, Mater. Today: Proc., 2021, 38, 578–583 CAS.
- M. G. Fontana and N. D. Greene, Corrosion Engineering, McGraw-Hill, New York, 1978 Search PubMed.
- E. McCafferty, Introduction to Corrosion Science, Springer New York, New York, NY, 2010, DOI:10.1007/978-1-4419-0455-3.
- B. E. A. Rani and B. B. J. Basu, Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview, Int. J. Corros., 2012, 1–15 CrossRef.
- V. S. Sastri, Green Corrosion Inhibitors-Theory and Practice, Wiley, 2012 Search PubMed.
- M. S. Darris, et al., Evaluation and correlation of self-healing functionalized silane-based hybrid nanocomposite materials as corrosion inhibitor, J. Mol. Liq., 2023, 391, 122770 CrossRef CAS.
- B. S. Sanatkumar, J. Nayak and A. Nityananda Shetty, Influence of 2-(4-chlorophenyl)-2-oxoethyl benzoate on the hydrogen evolution and corrosion inhibition of 18 Ni 250 grade weld aged maraging steel in 1.0 M sulfuric acid medium, Int. J. Hydrogen Energy, 2012, 37, 9431–9442 CrossRef CAS.
- M. S. Darris, Electrochemical, kinetical and computational insights of inhibitor encapsulated self-healing nanocontainer for triple layered protection of steel from corrosion, J. Mol. Liq., 2025, 437, 128325 CrossRef CAS.
- N. E. Berry, “Thermogalvanic” Corrosion, vol. 2, 1946 Search PubMed.
- R. W. Revie and H. H. Uhlig, Corrosion and Corrosion Control, John Wiley & Sons, New Jersey, 2025 Search PubMed.
- S. V. Yadla, V. Sridevi, M. V. V. C. Lakshmi and S. P. Kiran Kumari, A Review On Corrosion Of Metals And Protection, Int. J. Eng. Sci. Adv. Technol., 2012, 2(3), 637–644 Search PubMed.
- R. C. Newman and K. Sieradzki, Metallic Corrosion, Science, 1994, 263, 1708–1709 CrossRef CAS PubMed.
- C. Menapace, I. Lonardelli and A. Molinari, Phase transformation in a nanostructured M300 maraging steel obtained by SPS of mechanically alloyed powders, J. Therm. Anal. Calorim., 2010, 101, 815–821 CrossRef CAS.
- W. Sha and Z. Guo, Introduction to maraging steels, in Maraging Steels, Elsevier, 2009, pp. 1–16, DOI:10.1533/9781845696931.1.
- S. Sagbas and N. Sahiner, Carbon dots: preparation, properties, and application, in Nanocarbon and its Composites, Elsevier, 2019, pp. 651–676, DOI:10.1016/B978-0-08-102509-3.00022-5.
- R. Liu, D. Wu, X. Feng and K. Müllen, Bottom-Up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology, J. Am. Chem. Soc., 2011, 133, 15221–15223 CrossRef CAS PubMed.
- F. Yuan, et al., Shining carbon dots: Synthesis and biomedical and optoelectronic applications, Nano Today, 2016, 11, 565–586 CrossRef CAS.
- X. Xu, et al., Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- S. Cao, et al., Nitrogen-doped carbon dots as high-effective inhibitors for carbon steel in acidic medium, Colloids Surf., A, 2021, 616, 126280 CrossRef CAS.
- M. Cui, S. Ren, Q. Xue, H. Zhao and L. Wang, Carbon dots as new eco-friendly and effective corrosion inhibitor, J. Alloys Compd., 2017, 726, 680–692 CrossRef CAS.
- D. Yang, et al., Functionalization of citric acid-based carbon dots by imidazole toward novel green corrosion inhibitor for carbon steel, J. Cleaner Prod., 2019, 229, 180–192 CrossRef CAS.
- V. Saraswat and M. Yadav, Improved corrosion resistant performance of mild steel under acid environment by novel carbon dots as green corrosion inhibitor, Colloids Surf., A, 2021, 627, 127172 CrossRef CAS.
- H. Cen, X. Zhang, L. Zhao, Z. Chen and X. Guo, Carbon dots as effective corrosion inhibitor for 5052 aluminum alloy in 0.1 M HCl solution, Corros. Sci., 2019, 161, 108197 CrossRef CAS.
- S. Padhan, T. K. Rout and U. G. Nair, N-doped and Cu, N-doped carbon dots as corrosion inhibitor for mild steel corrosion in acid medium, Colloids Surf., A, 2022, 653, 129905 CrossRef CAS.
- S. Chahal, N. Yousefi and N. Tufenkji, Green Synthesis of High Quantum Yield Carbon Dots from Phenylalanine and Citric Acid: Role of Stoichiometry and Nitrogen Doping, ACS Sustain. Chem. Eng., 2020, 8, 5566–5575 CrossRef CAS.
- J. Tian, R. Liu, Z. Liu, C. Yu and M. Liu, Boosting the photocatalytic performance of Ag2CO3 crystals in phenol degradation via coupling with trace N-CQDs, Chin. J. Catal., 2017, 38, 1999–2008 CrossRef CAS.
- S. Pandit, P. Behera, J. Sahoo and M. De, In Situ Synthesis of Amino Acid Functionalized Carbon Dots with Tunable Properties and Their Biological Applications, ACS Appl. Bio Mater., 2019, 2, 3393–3403 CrossRef CAS PubMed.
- A. B. Bourlinos, et al., Surface Functionalized Carbogenic Quantum Dots, Small, 2008, 4, 455–458 CrossRef CAS PubMed.
- S. Zhuo, M. Shao and S.-T. Lee, Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- J. Zhou, et al., An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs), J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed.
- S. Ghosh, et al., Photoluminescence of Carbon Nanodots: Dipole Emission Centers and Electron–Phonon Coupling, Nano Lett., 2014, 14, 5656–5661 CrossRef CAS PubMed.
- S. Chahal, N. Yousefi and N. Tufenkji, Green Synthesis of High Quantum Yield Carbon Dots from Phenylalanine and Citric Acid: Role of Stoichiometry and Nitrogen Doping, ACS Sustain. Chem. Eng., 2020, 8, 5566–5575 CrossRef CAS.
- N. Alinejadian, F. Nasirpouri, J. Yus and B. Ferrari, Reduction-based engineering of three-dimensional morphology of Ni-rGO nanocomposite, J. Mater. Sci. Eng. B, 2021, 271, 115259 CrossRef CAS.
- N. Alinejadian, S. H. Kazemi, F. Nasirpouri and I. Odnevall, Electrodeposited nano-Ni/reduced graphene oxide composite film of corrugated surface for high voltammetric sensitivity, Mater. Chem. Phys., 2023, 297, 127288 CrossRef CAS.
- N. Shet, R. Nazareth and P. A. Suchetan, Corrosion inhibition of 316 stainless steel in 2 M HCl by 4-{[4-(dimethylamino)benzylidene]amino}-5-methyl-4H-1,2,4-triazole-3-thiol, Chem. Data Collect., 2019, 20, 100209 CrossRef CAS.
- B. El Mehdi, B. Mernari, M. Traisnel, F. Bentiss and M. Lagrenée, Synthesis and comparative study of the inhibitive effect of some new triazole derivatives towards corrosion of mild steel in hydrochloric acid solution, Mater. Chem. Phys., 2003, 77, 489–496 CrossRef CAS.
- S. Kertit and B. Hammouti, Corrosion inhibition of iron in 1 M HCl by 1-phenyl-5-mercapto-1,2,3,4-tetrazole, Appl. Surf. Sci., 1996, 93, 59–66 CrossRef CAS.
- D. Jayaperumal, Effects of alcohol-based inhibitors on corrosion of mild steel in hydrochloric acid, Mater. Chem. Phys., 2010, 119, 478–484 CrossRef CAS.
- M. Lebrini, et al., Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: Electrochemical, X-ray photoelectron spectroscopy and theoretical studies, Appl. Surf. Sci., 2007, 253, 9267–9276 CrossRef CAS.
- K. F. Khaled, The inhibition of benzimidazole derivatives on corrosion of iron in 1 M HCl solutions, Electrochim. Acta, 2003, 48, 2493–2503 CrossRef CAS.
- T. Jeena, M. P. Geetha, P. A. Suchetan, N. Ronald and K. Amrutha, Influence of cobalt doping on chemical and green synthesized magnesium oxide nanoparticles for enhanced photocatalytic evaluation, adsorption studies, antimicrobial analysis and corrosion inhibition study, Inorg. Chem. Commun., 2023, 157, 111232 CrossRef CAS.
- S. T. Arab, Inhibition action of thiosemicabazone and some of it is ρ-substituted compounds on the corrosion of iron-base metallic glass alloy in 0.5 M H2SO4 at 30 °C, Mater. Res. Bull., 2008, 43, 510–521 CrossRef CAS.
- C. Verma, M. A. Quraishi and A. Singh, 2-Amino-5-nitro-4,6-diarylcyclohex-1-ene-1,3,3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1 M HCl: Experimental and theoretical studies, J. Mol. Liq., 2015, 212, 804–812 CrossRef CAS.
- N. Alinejadian, S. H. Kazemi and I. Odnevall, SLM-processed MoS2/Mo2S3 nanocomposite for energy conversion/storage applications, Sci. Rep., 2022, 12, 5030 CrossRef CAS PubMed.
- M. A. Quraishi and J. Rawat, Influence of iodide ions on inhibitive performance of tetraphenyl-dithia-octaaza-cyclotetradeca-hexaene (PTAT) during pickling of mild steel in hot sulfuric acid, Mater. Chem. Phys., 2001, 70, 95–99 CrossRef CAS.
- N. Alinejadian, et al., Importance of the micro-lattice structure of selective laser melting processed Mo/Mo(x)S(x + 1) composite: Corrosion studies on the electrochemical performance in aqueous solutions, Mater. Today Chem., 2022, 26, 101219 CrossRef CAS.
- G. M. Pinto, J. Nayak and A. N. Shetty, Corrosion inhibition of 6061 Al–15vol. pct. SiC(p) composite and its base alloy in a mixture of sulphuric acid and hydrochloric acid by 4-(N,N-dimethyl amino) benzaldehyde thiosemicarbazone, Mater. Chem. Phys., 2011, 125, 628–640 CrossRef CAS.
- I. A. Alkadir Aziz, et al., Insights into Corrosion Inhibition Behavior of a 5-Mercapto-1, 2,4-triazole Derivative for Mild Steel in Hydrochloric Acid Solution: Experimental and DFT Studies, Lubricants, 2021, 9, 122 CrossRef CAS.
- F. Bouhlal, et al., Electrochemical and Thermodynamic Investigation on Corrosion Inhibition of C38 Steel in 1 M Hydrochloric Acid Using the Hydro-Alcoholic Extract of Used Coffee Grounds, Int. J. Corros., 2020, 1–14 Search PubMed.
- A. Hamdy and N. S. El-Gendy, Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium, Egypt. J. Pet., 2013, 22, 17–25 CrossRef.
- A. S. Fouda, F. E. Heakal and M. S. Radwan, Role of some thiadiazole derivatives as inhibitors for the corrosion of C-steel in 1 M H2SO4, J. Appl. Electrochem., 2009, 39, 391–402 CrossRef CAS.
- V. Pinto, G. M. Pinto, L. D. Kateel and A. Thomas, Green approach to corrosion inhibition of mild steel in hydrochloric acid using extract from the Pericarp of the fruit Tamarindus indica, Biointerface Res. Appl. Chem., 2023, 13, 1–21 Search PubMed.
- N. A. Negm, F. M. Ghuiba and S. M. Tawfik, Novel isoxazolium cationic Schiff base compounds as corrosion inhibitors for carbon steel in hydrochloric acid, Corros. Sci., 2011, 53, 3566–3575 CrossRef CAS.
- S. John and A. Joseph, Electro analytical, surface morphological and theoretical studies on the corrosion inhibition behavior of different 1,2,4-triazole precursors on mild steel in 1 M hydrochloric acid, Mater. Chem. Phys., 2012, 133, 1083–1091 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |