DOI:
10.1039/D5RA04074D
(Review Article)
RSC Adv., 2025,
15, 25256-25273
Sustainable coagulative removal of microplastic from aquatic systems: recent progress and outlook
Received
9th June 2025
, Accepted 7th July 2025
First published on 16th July 2025
Abstract
Microplastic (MP) pollution represents a critical challenge for global water quality due to its persistence, ubiquity, and ecotoxicological impacts. While conventional coagulation/flocculation–sedimentation (CFS) processes using chemical coagulants are partially effective, they often entail high energy demands, toxic residuals, and environmental trade-offs. This article provides a comprehensive and up-to-date review of recent advances in the use of natural coagulants (NCs) derived from plant, animal, and microbial sources as sustainable alternatives for MP removal from aquatic systems. The novelty of this work lies in its integrative analysis of bio-coagulant performance with hybrid formulations, nano-enhanced composites, and process intensification strategies such as enzyme activation. Through critical synthesis of various peer-reviewed studies published between 2020 and 2025, the review highlights that NCs such as Moringa oleifera, chitosan, Cactus mucilage, and microbial EPS can achieve MP removal efficiencies exceeding 90% under optimized conditions, with significantly reduced sludge toxicity and carbon footprint. Furthermore, the review identifies key performance parameters; pH, ionic strength, NOM interference, and coagulant modification techniques that influence the physicochemical mechanisms driving MP-coagulant interactions, including charge neutralization, bridging flocculation, hydrophobic association, and bio-adhesion. Pilot-scale evaluations demonstrate the feasibility of hybrid systems (e.g., chitosan-FeCl3, Moringa–alum) in achieving near-complete removal (up to 99.8%) of MPs across a range of polymer types and sizes. However, critical limitations remain, such as variability in raw material composition, reduced efficiency for MPs <10 μm, and scalability constraints. The study concludes that although NCs cannot yet fully replace synthetic ones at scale, their use as coagulant aids or in hybrid systems shows promise for sustainable water treatment. Future research should focus on standardizing extraction methods, improving bioengineering for higher protein yields, and developing smart coagulation systems for adaptive control across various water matrices.
1. Introduction
Microplastics (MPs), defined as synthetic polymer particles ranging from 1 μm to 5 mm, have become pervasive global contaminants due to their environmental persistence and continuous input from multiple anthropogenic sources.1 Recent studies demonstrate that approximately 8 million metric tons of plastic enter oceans annually, with secondary MPs from degraded macroplastics accounting for 69–81% of total MP loads in aquatic systems.2,3 The 2021 UNEP report identified textile laundering as a major MP source, with a single wash releasing 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers,4,5 while urban runoff contributes 30–35% of MP loads in freshwater systems.6,7 Once dispersed, MPs exhibit complex transport dynamics influenced by their density (0.85–1.41 g cm−3), shape (fragments, fibers, spheres), and surface chemistry, with recent modeling showing 34% of oceanic MPs reside in surface waters while 66% accumulate in sediments.5,8 Their environmental impacts are exacerbated by large specific surface areas (up to 3000 m2 g−1) that facilitate adsorption of persistent organic pollutants (log
000 microfibers,4,5 while urban runoff contributes 30–35% of MP loads in freshwater systems.6,7 Once dispersed, MPs exhibit complex transport dynamics influenced by their density (0.85–1.41 g cm−3), shape (fragments, fibers, spheres), and surface chemistry, with recent modeling showing 34% of oceanic MPs reside in surface waters while 66% accumulate in sediments.5,8 Their environmental impacts are exacerbated by large specific surface areas (up to 3000 m2 g−1) that facilitate adsorption of persistent organic pollutants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW 3–7), with studies documenting MP-associated concentrations of PCBs and DDTs 106 times higher than ambient seawater.9,10 Ecotoxicological research has demonstrated dose dependent effects across trophic levels, including 17–35% reduced filtration rates in mussels,11,12 50% decreased reproductive output in copepods,2,11 and biomarker responses in fish indicating oxidative stress and neurotoxicity at environmentally relevant concentrations (10–100 particles per L).13 Conventional water treatment processes show variable MP removal efficiencies, with primary sedimentation removing 50–80% of particles >100 μm but only 10–30% of 1–10 μm particles.14–17 Advanced tertiary treatments achieve higher performance (95–99.9%), but face practical limitations – membrane filtration requires 3–5 bar operating pressures (energy demand: 0.5–1.2 kWh m−3),18,19 while electrocoagulation consumes 15–30 kWh m−3 for 92–97% removal.2,20 These challenges have driven research into natural coagulants (NCs), with Moringa oleifera seed extract demonstrating 89% removal of 10–100 μm polyethylene particles at 200 mg per L dose through dual mechanisms of charge neutralization (+15 mV ζ-potential shift) and polymer bridging.21,22 Chitosan shows particular promise, achieving 94% removal of polystyrene microspheres (50 mg per L dose, pH 6.5) with floc formation following second-order kinetics (k = 2.3 × 10−4 L mg−1 min−1).23,24 Hybrid systems combining NCs with conventional processes exhibit enhanced performance.2,25 While chitosan-assisted electrocoagulation reduced energy consumption by 40% compared to conventional methods.24,26 However, key challenges remain, including variability in natural coagulant composition (±15% performance variation between harvests),22 incomplete understanding of MP-coagulant interaction mechanisms at molecular scales,11,16 and lack of standardized protocols for evaluating removal efficiency across different MP types (polymer chemistry, size fractions, aging states).1 The primary objective of this review is to provide a comprehensive and technically rigorous synthesis of recent advances (2020–2025) in the use of NCs for the removal of MPs from aquatic environments. The review classifies NCs based on their biological origin; plant-derived, animal-based, and microbial and critically examines their active components, extraction procedures, and core coagulation mechanisms. It further evaluates their performance across a wide spectrum of MP types, considering variations in polymer composition, particle size, surface properties, and water matrix conditions. Particular attention is given to the physicochemical interaction mechanisms governing MP removal, including charge neutralization, bridging flocculation, hydrophobic interactions, and bio-adhesion, supported by recent visions from advanced characterization techniques and molecular simulations. Moreover, the review explores emerging innovations such as hybrid coagulant formulations, nanostructured composites, enzyme-functionalized systems, which collectively enhance removal efficiency, operational flexibility, and scalability. In doing so, the study also identifies key limitations including raw material variability, reduced efficiency for sub-micron particles, and process sensitivity to environmental factors such as pH, salinity, and dissolved organics and proposes practical strategies for their modification.
KOW 3–7), with studies documenting MP-associated concentrations of PCBs and DDTs 106 times higher than ambient seawater.9,10 Ecotoxicological research has demonstrated dose dependent effects across trophic levels, including 17–35% reduced filtration rates in mussels,11,12 50% decreased reproductive output in copepods,2,11 and biomarker responses in fish indicating oxidative stress and neurotoxicity at environmentally relevant concentrations (10–100 particles per L).13 Conventional water treatment processes show variable MP removal efficiencies, with primary sedimentation removing 50–80% of particles >100 μm but only 10–30% of 1–10 μm particles.14–17 Advanced tertiary treatments achieve higher performance (95–99.9%), but face practical limitations – membrane filtration requires 3–5 bar operating pressures (energy demand: 0.5–1.2 kWh m−3),18,19 while electrocoagulation consumes 15–30 kWh m−3 for 92–97% removal.2,20 These challenges have driven research into natural coagulants (NCs), with Moringa oleifera seed extract demonstrating 89% removal of 10–100 μm polyethylene particles at 200 mg per L dose through dual mechanisms of charge neutralization (+15 mV ζ-potential shift) and polymer bridging.21,22 Chitosan shows particular promise, achieving 94% removal of polystyrene microspheres (50 mg per L dose, pH 6.5) with floc formation following second-order kinetics (k = 2.3 × 10−4 L mg−1 min−1).23,24 Hybrid systems combining NCs with conventional processes exhibit enhanced performance.2,25 While chitosan-assisted electrocoagulation reduced energy consumption by 40% compared to conventional methods.24,26 However, key challenges remain, including variability in natural coagulant composition (±15% performance variation between harvests),22 incomplete understanding of MP-coagulant interaction mechanisms at molecular scales,11,16 and lack of standardized protocols for evaluating removal efficiency across different MP types (polymer chemistry, size fractions, aging states).1 The primary objective of this review is to provide a comprehensive and technically rigorous synthesis of recent advances (2020–2025) in the use of NCs for the removal of MPs from aquatic environments. The review classifies NCs based on their biological origin; plant-derived, animal-based, and microbial and critically examines their active components, extraction procedures, and core coagulation mechanisms. It further evaluates their performance across a wide spectrum of MP types, considering variations in polymer composition, particle size, surface properties, and water matrix conditions. Particular attention is given to the physicochemical interaction mechanisms governing MP removal, including charge neutralization, bridging flocculation, hydrophobic interactions, and bio-adhesion, supported by recent visions from advanced characterization techniques and molecular simulations. Moreover, the review explores emerging innovations such as hybrid coagulant formulations, nanostructured composites, enzyme-functionalized systems, which collectively enhance removal efficiency, operational flexibility, and scalability. In doing so, the study also identifies key limitations including raw material variability, reduced efficiency for sub-micron particles, and process sensitivity to environmental factors such as pH, salinity, and dissolved organics and proposes practical strategies for their modification.
2. Methodology
The growing concern regarding MPs pollution in aquatic environments has led to a surge in scientific research exploring efficient removal strategies. As illustrated in Fig. 1a, there has been a substantial increase in the number of published studies between 2020 and 2025 that focus on MPs removal from various aqueous systems. Coagulation-based treatment processes have emerged as a promising and scalable approach for MP removal. The distribution of studies by treatment approach, as shown in Fig. 1b, highlights that conventional chemical coagulants such as polyaluminium chloride (PAC) and aluminium sulphate are the most widely studied, accounting for approximately 40% of the total literature. Iron-based coagulants represent another 20%, favored for their strong performance in waters with high turbidity or organic content. Notably, NCs derived from plant-based materials such as Moringa oleifera, tannins, or chitosan comprise 20% of the published work, indicating growing interest in environmentally benign alternatives that reduce chemical dependency and sludge toxicity. An additional 23% of studies explore hybrid approaches, combining traditional coagulants with natural polymers or flocculant aids like polyacrylamide (PAM), which have shown to enhance MP aggregation and removal across a wider range of particle sizes and types. The historical evolution of scientific attention to MPs and their treatment through coagulation is outlined in Fig. 1c. Although MPs were first identified in the environment several decades ago, focused studies on their removal through coagulative treatment did not begin until the mid-2010s, gaining momentum in the early 2020s. This timeline demonstrates how advancements in detection methods, public awareness, and environmental policies have catalyzed the development and application of coagulation-based technologies for MP mitigation. Despite the promising results highlighted across the literature and in Fig. 1a–c, several challenges remain. Field-scale validation, long-term performance assessments, and post-treatment sludge management are areas that remain underexplored. These limitations point to the need for more pilot and demonstration-scale studies using real water matrices and a broader range of MP morphologies. The observed trends (Fig. 1a–c) affirm a research trajectory that is increasingly aligned with environmental sustainability and real-world applicability.
 |
| | Fig. 1 (a) Emergence of studies on MPs removal from different aqueous systems in recent years (2020–2025), (b) percentage distribution of studies investigation MPs removal using different coagulation treatment approach including NCs (data retrieved from Web of Science and Scopus database), and (c) timeline of MPs pollution and coagulative treatment for the removal of MPs (data retrieved from Web of Science and Scopus database). | |
3. Microplastic pollution: sources and impacts
MPs originate from diverse anthropogenic sources, which can be broadly categorized into primary and secondary MPs. Primary MPs are intentionally manufactured at MP sizes, including microbeads used in personal care products (typically 10–500 μm), industrial abrasives, and plastic pellets (nurdles) used as raw material in plastic production. Secondary MPs result from the environmental degradation of larger plastic items through processes such as photodegradation by UV radiation, mechanical abrasion from wave action, and biological degradation. Studies estimate that secondary MPs account for 69–81% of total MP loads in aquatic environments, with the breakdown of plastic packaging, fishing gear, and textile fibers being major contributors as listed in Table 1.12,27 The pathways of MP entry into aquatic ecosystems are complex and varied. Urban runoff has been identified as a significant vector, transporting 30–35% of MP loads in freshwater systems, with tire wear particles and road dust representing substantial but often overlooked sources.1,28 Wastewater treatment plants (WWTPs) serve as important conduits, with a single laundry cycle releasing approximately 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers from synthetic textiles. Atmospheric deposition has recently been recognized as a notable transport mechanism, with studies demonstrating MPs in remote locations such as Arctic snow and mountain tops, suggesting long-range atmospheric circulation of these particles.29,30 Agricultural practices also contribute substantially, with plastic mulch films and bio-solid applications introducing MPs into terrestrial systems that eventually reach aquatic environments through soil erosion and runoff.31 The environmental impacts of MPs are complex and concerning. Their small size and high surface area to volume ratio (up to 3000 m2 g−1 for fragmented particles) make them effective vectors for pollutant transport. MPs have been shown to adsorb and concentrate persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) at concentrations up to 106 times greater than surrounding seawater.12 This chemical hitchhiking effect is particularly concerning given the demonstrated bioavailability of these contaminants when ingested by marine organisms. The physical presence of MPs in organisms can cause intestinal blockages, false satiety, and reduced energy reserves. A landmark study by Langenfeld et al. (2024) showed 50% decreased reproductive output in the copepod Calanus helgolandicus at environmentally relevant concentrations (10–100 particles per mL).32
000 microfibers from synthetic textiles. Atmospheric deposition has recently been recognized as a notable transport mechanism, with studies demonstrating MPs in remote locations such as Arctic snow and mountain tops, suggesting long-range atmospheric circulation of these particles.29,30 Agricultural practices also contribute substantially, with plastic mulch films and bio-solid applications introducing MPs into terrestrial systems that eventually reach aquatic environments through soil erosion and runoff.31 The environmental impacts of MPs are complex and concerning. Their small size and high surface area to volume ratio (up to 3000 m2 g−1 for fragmented particles) make them effective vectors for pollutant transport. MPs have been shown to adsorb and concentrate persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) at concentrations up to 106 times greater than surrounding seawater.12 This chemical hitchhiking effect is particularly concerning given the demonstrated bioavailability of these contaminants when ingested by marine organisms. The physical presence of MPs in organisms can cause intestinal blockages, false satiety, and reduced energy reserves. A landmark study by Langenfeld et al. (2024) showed 50% decreased reproductive output in the copepod Calanus helgolandicus at environmentally relevant concentrations (10–100 particles per mL).32
Table 1 Comprehensive overview of MP sources, pathways, and impacts
| Category |
Subcategory |
Key findings |
Quantitative data |
Ref. |
| Origin & classification |
Primary MPs |
Intentionally manufactured (microbeads, nurdles, abrasives) |
Size: 10–500 μm |
12 and 27 |
| Secondary MPs |
Result from macroplastic degradation (photodegradation, abrasion) |
69–81% of aquatic MP loads |
12 and 27 |
| Entry pathways |
Urban runoff |
Tire wear, road dust, synthetic fibers |
30–35% of freshwater MP loads |
1 and 28 |
| WWTPs |
Laundry effluent (synthetic textiles) |
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers per wash 000 microfibers per wash |
29 and 30 |
| Atmospheric deposition |
Long-range transport to remote areas (Arctic, mountains) |
Documented in Arctic snow |
29 and 30 |
| Agricultural inputs |
Plastic mulch, biosolids → soil erosion → aquatic systems |
Significant but unquantified |
29 and 30 |
| Environmental impacts |
Pollutant transport |
Adsorption of POPs (PCBs, DDTs) |
106× higher than ambient seawater |
12 |
| Biological effects |
- Copepods: reduced reproduction |
50% ↓ reproductive output; 2 °C ↓ tolerance |
33 |
| - Mussels: false satiety, ↓ thermal tolerance |
| Trophic transfer |
MPs move through food chains (zooplankton → fish → humans) |
Cellular uptake of <10 μm particles |
11 and 12 |
| Economic costs |
Marine ecosystem damage, fisheries losses |
$2.5 trillion per year globally |
8 and 33 |
| Persistence |
Degradation rates |
Half-lives in marine sediments: |
Century-scale persistence |
8 and 12 |
| - Polyethylene: 58 years |
| - Deep-sea: ≤1200 years |
At the ecosystem level, MPs have been shown to alter sediment microbial communities and biogeochemical processes. Laboratory experiments have demonstrated that MP contamination can reduce the thermal tolerance of mussels by up to 2 °C, potentially affecting their survival under climate change scenarios.33 The trophic transfer of MPs has been well-documented, with particles moving from zooplankton to fish to top predators, including potential implications for human health through seafood consumption. Emerging research suggests that the smallest MP fractions (<10 μm) may cross biological barriers, with demonstrated cellular uptake and potential for translocation to various organs.11,12 The economic impacts are equally concerning, with estimates suggesting annual costs of $2.5 trillion to marine ecosystems globally due to plastic pollution, including fisheries losses, tourism impacts, and cleanup costs.11,12 Perhaps most alarmingly, the persistence of MPs in the environment is measured in centuries rather than decades, with half-lives estimated at 58 years for polyethylene in marine sediments and up to 1200 years for some polymer types in deep-sea conditions.8,12 This extreme persistence, combined with continuous inputs, suggests that MP pollution represents a growing and potentially irreversible environmental challenge without immediate and sustained intervention (Fig. 2).
 |
| | Fig. 2 MPs common sources and impacts on the environment and health. | |
4. Challenges of MPs removal in WTPs
The removal of MPs in water treatment plants (WTPs) presents a growing technical challenge due to the complex physicochemical properties of these persistent pollutants and limitations in conventional treatment infrastructure. Table 2 summarizes the MPs removal efficiency across water treatment processes, along with their associated limitations. Recent studies highlight that while primary treatment processes (screening, sedimentation) can remove 50–80% of MPs larger than 100 μm, their efficiency drops below 30% for particles smaller than 10 μm.34 This size-dependent removal gap is particularly concerning as smaller MPs and nanoplastics (<1 μm) demonstrate higher bioavailability and potential toxicity.35 Secondary biological treatments like activated sludge systems achieve 85–95% MP removal primarily through incidental entrapment in microbial flocs rather than degradation, with treated effluents still containing 1–15 MP particles per L.36 More advanced membrane bioreactors (MBRs) show superior performance (98–99.9% removal) but face severe operational challenges, including MP-induced membrane fouling that increases energy demand by 25–40% compared to conventional systems.37 This fouling is exacerbated by biofilm formation on MP surfaces, which accelerates pore clogging and reduces membrane lifespan.36 Existing treatment technologies each face specific limitations in MP removal. Conventional coagulation using aluminum or iron salts achieves ≤70% MP removal at optimal doses (150 mg L−1), but overdosing (>250 mg L−1) triggers restabilization via charge reversal.38 The diverse nature of MPs creates additional complications for removal technologies. Polyethylene (PE) and polypropylene (PP), which constitute 72% of MPs in wastewater streams, exhibit hydrophobic surfaces (contact angles >90°) and low density (0.85–0.92 g cm−3), resisting sedimentation while readily forming aggregates.36,37 In contrast, polystyrene (PS) and polyethylene terephthalate (PET) carry strong negative zeta potentials (−30 to −50 mV) at neutral pH, requiring specific coagulant chemistries for effective charge neutralization.34,36 Electrocoagulation, while effective (92–97% removal), requires substantial energy inputs (15–30 kWh m−3) and generates hazardous sludge containing high concentrations of metal ions (Al3+/Fe2+ > 500 mg kg−1).39
Table 2 MPs removal efficiency across water treatment processes
| Treatment stage |
Technology |
Removal efficiency |
Size dependence |
Key limitations |
Ref. |
| Primary |
Screening/sedimentation |
50–80% (>100 μm) |
<30% for <10 μm MPs |
Ineffective for nanoplastics |
34 |
| Secondary |
Activated sludge |
85–95% |
Entrapment in flocs |
Effluent: 1–15 MPs per L |
36 |
| Tertiary |
Membrane bioreactor (MBR) |
98–99.9% |
Fouling ↑ energy by 25–40% |
Biofilm-clogged membranes |
37 |
| Coagulation (Al/Fe) salts |
≤70% (optimal dose: 150 mg L−1) |
Charge reversal at >250 mg L−1 |
Sludge generation |
38 |
| Electrocoagulation |
92–97% |
Energy-intensive (15–30 kWh m−3) |
Hazardous metal sludge (Al3+/Fe2+ > 500 mg kg−1) |
39 |
| Adsorption (activated carbon) |
70–85% |
High cost ($0.15–0.30 m−3) |
Poor regenerability |
47 |
Advanced oxidation processes (AOPs), including photocatalysis (e.g., TiO2/UV systems), Fenton and photo-Fenton reactions, and ozonation, have demonstrated the potential to degrade MPs into smaller fragments or even achieve mineralization, thus removing particle persistence.34,40 However, AOPs demand high operational costs, stringent control over reaction conditions (pH, oxidant dose), and often lead to incomplete degradation, producing potentially toxic by-products.41 Similarly, membrane-based filtration technologies ranging from microfiltration to nanofiltration and reverse osmosis offer high care in MP removal, including particles smaller than 1 μm, but face major limitations such as membrane fouling, high energy consumption, frequent maintenance, and limited lifespan.42,43 Moreover, the concentrated brine generated in membrane systems poses additional disposal challenges.44 Microbial flocculants, synthesized by bacteria, fungi, or algae, are composed of proteins, polysaccharides, or glycoproteins, and have been reported to achieve MP removal efficiencies comparable to chemical coagulants in lab-scale studies. Yet, their widespread application is hampered by variability in microbial growth conditions, production scalability, and long-term stability.45,46 Powdered activated carbon adsorbs 70–85% of MPs but suffers from poor regenerability and high operational costs, limiting scalability.47,48 Analytical challenges further delay progress in MP removal optimization. The lack of standardized methods for MP quantification leads to inconsistent performance reporting, with most studies using pristine, spherical MPs that poorly represent the irregular, weathered particles found in real systems.49
5. Common NCs for MPs removal
NCs have emerged as a transformative solution in water treatment, gaining substantial scientific and industrial attention as sustainable, eco-friendly alternatives to conventional chemical coagulants like aluminum sulfate and polyaluminum chloride. Table 3 summarizes the performance comparison of using NCs rather than conventional coagulants for MPs removal. NCs not only demonstrate comparable treatment efficiency for various water contaminants but also address critical environmental and health concerns associated with synthetic polymers, particularly the risks of toxic residual aluminum in drinking water and the generation of non-biodegradable sludge.21,50 Extensive life cycle assessment studies have confirmed that NCs can reduce the carbon footprint of water treatment by 40–60% while eliminating the neurotoxic risks associated with aluminum-based coagulants.21,47 These NCs can be systematically categorized into three primary classes based on their biological origin, each with distinct chemical compositions and mechanisms of action as listed in Table 4. Plant-based coagulants constitute the most extensively researched and widely applied category, comprising materials such as Moringa oleifera seeds, tannin-rich extracts from acacia and quebracho, Cactus mucilage (Opuntia ficus-indica), and okra polysaccharides.51 Moringa oleifera, often called the “miracle tree,” has demonstrated particularly remarkable coagulation efficiency, achieving 85–95% turbidity removal and 70–90% pathogen reduction in various water matrices.21,51 This performance is attributed to its cationic protein content (6.5–16 kDa) with isoelectric points between 9–11 that function through dual mechanisms: charge neutralization of negatively charged colloids and polymer bridging between particles.21,22 Recent proteomic studies have identified at least 12 active protein isoforms in Moringa seeds, with the 13 kDa MO2.1 protein showing particularly high flocculation activity.22
Table 3 Performance comparison: natural vs. conventional coagulants for MPs removal
| Parameter |
Natural coagulants |
Aluminum-based coagulants |
Synthetic polymers |
Ref. |
| MP removal efficiency |
70–98% |
50–80% |
80–95% |
57 and 58 |
| pH range |
4.0–10.0 (chitosan: 4.0–8.5) |
5.5–7.5 |
3.0–10.0 |
21, 53 and 58 |
| Sludge production |
30–50% less |
High (toxic Al residues) |
Non-biodegradable |
51 and 57 |
| Carbon footprint |
40–60% reduction |
High |
Very high |
57 and 58 |
| Cost |
$0.05–0.20 per m3 |
$0.10–0.30 per m3 |
$0.20–0.50 per m3 |
51 and 57 |
| Health risks |
None |
Neurotoxicity (Al3+) |
Carcinogenic monomers |
21, 53 and 58 |
Table 4 Classification and properties of NCs
| Category |
Examples |
Active components |
Mechanism of action |
Key advantages |
Ref. |
| Plant-based |
Moringa oleifera seeds |
Cationic proteins (6.5–16 kDa) |
Charge neutralization + polymer bridging |
Biodegradable, reduces sludge volume |
21 and 22 |
| Animal-derived |
Cactus mucilage |
Polysaccharides |
Adsorption + interparticle bridging |
Low-cost, locally available |
22 and 51 |
| Chitosan (crustacean shells) |
Deacetylated chitin (75–95% DD) |
Electrostatic attraction + hydrogen bonding |
Heavy metal removal, antimicrobial |
51 and 59 |
| Keratin (poultry feathers) |
Fibrous proteins |
Particle entrapment + charge neutralization |
Waste valorization |
51 and 59 |
| Microbial |
Bacillus subtilis EPS |
Extracellular polymeric substances (EPS) |
Bioflocculation via polysaccharides/proteins |
Salt-tolerant (up to 15% NaCl) |
51 and 60 |
| Chlorella vulgaris extracts |
Algal polysaccharides |
Adsorption + CO2 sequestration |
Carbon-negative process |
51 and 61 |
Optimization research has established that extraction using 1 M NaCl solution at 25 °C for 30 min yields 20–30% higher active protein content compared to traditional water extraction methods, while novel ultrasound-assisted extraction can reduce processing time by 60% while maintaining protein integrity.21,22 Animal-derived coagulants represent a second major category, with chitosan from crustacean shells being the most prominent example. This linear polysaccharide, obtained through alkaline deacetylation of chitin, possesses unique polycationic properties that enable exceptional removal of colloidal particles (90–98%) and dissolved organic matter through simultaneous charge neutralization, adsorption, and interparticle bridging.51,52 The degree of deacetylation (75–95%) profoundly influences chitosan's performance, with higher deacetylation yielding stronger positive charge density (NH3+ groups) and consequently better coagulation efficiency, particularly for negatively charged contaminants.51,53 Recent advances in chitosan modification have significantly expanded its applicability, including carboxymethylation for improved water solubility, graft polymerization with acrylamide for enhanced molecular weight, and thiolation for increased heavy metal affinity.23,24 These modifications have extended chitosan's effective pH range from 4.0–8.5 while improving its stability in hard waters (up to 500 mg per L CaCO3) and resistance to organic matter interference.24 Developing animal-derived alternatives include keratin from poultry feathers and fibroin from silk waste, which show promising coagulation activity (60–80% turbidity removal) while valorizing agricultural byproducts.23 Microbial coagulants, though currently less studied than plant and animal-based options, represent a rapidly developing third category with significant potential. These include bioflocculants produced by various bacterial (Bacillus subtilis, Paenibacillus polymyxa) and fungal (Aspergillus niger, Trichoderma viride) species, which achieve 80–90% turbidity removal through secretion of extracellular polymeric substances (EPS) containing polysaccharides, proteins, and glycoproteins that promote bioflocculation.51,54 The EPS from Bacillus licheniformis, for instance, contains galactosamine and uronic acid groups that provide both charged sites for particle destabilization and long polymer chains for bridging.54 Recent metagenomic studies have identified novel coagulant-producing microbial strains from extreme environments, including halophilic archaea from salt lakes that produce EPS stable at high salinity (up to 15% NaCl).51,55 Algal-based coagulants from Chlorella vulgaris and Spirulina platensis are also gaining attention, offering the dual benefit of water treatment and CO2 sequestration during their cultivation phase.56
5.1 NCs preparation techniques
The preparation of NCs for MPs removal involves a series of carefully optimized steps to ensure maximum efficiency, sustainability, and scalability. The process begins with the selection of raw materials, which are typically plant-based, animal-derived, or microbial biopolymers known for their coagulant properties.51,62 Fig. 3 and Table 5 present the extraction/preparation methods of common NCs for MPs removal. Common NCs sources include Moringa oleifera seeds, cactus (Opuntia ficus-indica) mucilage, chitosan (derived from crustacean shells), tannins (from acacia bark or pomegranate rind), and okra polysaccharides. Each material requires specific pretreatment methods to extract the active coagulating agents.21 For instance, Moringa oleifera seeds are first sun-dried to reduce moisture, then manually or mechanically dehulled to obtain the kernel, which is ground into a fine powder (50–100 μm particle size) using a ball mill or mortar and pestle. This powder is then mixed with distilled water or a saline solution (typically 1 M NaCl) at a defined ratio (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) and stirred (150–200 rpm) for 30–60 minutes to facilitate protein extraction.21 The mixture is subsequently filtered through Whatman No. 1 filter paper or centrifuged (3000–5000 rpm for 15–20 min) to remove insoluble residues, yielding crude extract rich in cationic proteins that act as NCs by neutralizing the negative surface charges of MPs.21,51 Similarly, Cactus mucilage is extracted by harvesting mature cladodes, washing them thoroughly to remove dust and spines, and then peeling the outer skin to access the inner parenchyma. The peeled cladodes are diced and blended in distilled water, followed by filtration through a muslin cloth to separate fibrous material. The mucilaginous filtrate is then subjected to alcohol precipitation (using ethanol or isopropanol in a 2
10 w/v) and stirred (150–200 rpm) for 30–60 minutes to facilitate protein extraction.21 The mixture is subsequently filtered through Whatman No. 1 filter paper or centrifuged (3000–5000 rpm for 15–20 min) to remove insoluble residues, yielding crude extract rich in cationic proteins that act as NCs by neutralizing the negative surface charges of MPs.21,51 Similarly, Cactus mucilage is extracted by harvesting mature cladodes, washing them thoroughly to remove dust and spines, and then peeling the outer skin to access the inner parenchyma. The peeled cladodes are diced and blended in distilled water, followed by filtration through a muslin cloth to separate fibrous material. The mucilaginous filtrate is then subjected to alcohol precipitation (using ethanol or isopropanol in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio) to concentrate the polysaccharide-based coagulants.51 The precipitate is dried at 40–50 °C and ground into a powder for later use.51,53 Chitosan, another widely studied bio-coagulant, is prepared by deacetylating chitin (extracted from shrimp or crab shells) using concentrated NaOH (40–50% w/v) at 60–80 °C for 4–6 h. The resulting chitosan is washed to neutrality, dried, and dissolved in 1% acetic acid to form a viscous solution, which can be further modified by cross-linking with agents like glutaraldehyde or tripolyphosphate to enhance its mechanical stability and MPs adsorption capacity.24,51 Tannin-based coagulants are extracted from plant sources such as acacia bark or pomegranate peels via aqueous or organic solvent extraction. The raw material is dried, milled, and mixed with hot water (70–80 °C) or ethanol (70% v/v) under reflux for 2–4 h. The extract is then concentrated using a rotary evaporator and freeze-dried to obtain a tannin-rich powder, which can be further functionalized with quaternary ammonium groups to improve its cationic charge density for better MPs flocculation.51,53
1 v/v ratio) to concentrate the polysaccharide-based coagulants.51 The precipitate is dried at 40–50 °C and ground into a powder for later use.51,53 Chitosan, another widely studied bio-coagulant, is prepared by deacetylating chitin (extracted from shrimp or crab shells) using concentrated NaOH (40–50% w/v) at 60–80 °C for 4–6 h. The resulting chitosan is washed to neutrality, dried, and dissolved in 1% acetic acid to form a viscous solution, which can be further modified by cross-linking with agents like glutaraldehyde or tripolyphosphate to enhance its mechanical stability and MPs adsorption capacity.24,51 Tannin-based coagulants are extracted from plant sources such as acacia bark or pomegranate peels via aqueous or organic solvent extraction. The raw material is dried, milled, and mixed with hot water (70–80 °C) or ethanol (70% v/v) under reflux for 2–4 h. The extract is then concentrated using a rotary evaporator and freeze-dried to obtain a tannin-rich powder, which can be further functionalized with quaternary ammonium groups to improve its cationic charge density for better MPs flocculation.51,53
 |
| | Fig. 3 Common preparation procedures for NCs formulation. | |
Table 5 Extraction and preparation methods of common NCs for MPs removal
| NCs |
Source material |
Extraction method |
Modification techniques |
Key functional components |
Ref. |
| Moringa oleifera |
Seeds |
- Dehulling, drying, grinding (50–100 μm) |
- Cross-linking with glutaraldehyde |
Cationic proteins (flocculin, MO2.1) |
64 and 65 |
| - Aqueous/saline extraction (1 M NaCl) |
- Hybridization with alum |
| Cactus mucilage |
Opuntia ficus-indica cladodes |
- Peeling, blending, filtration |
- Freeze-drying |
Polysaccharides (pectin, arabinogalactan) |
51 and 66 |
- Alcohol precipitation (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol) 1 ethanol) |
- Polysaccharide sulfonation |
| Chitosan |
Crustacean shells |
- Chitin deacetylation (40% NaOH, 80 °C) |
- Magnetic nanoparticle coating (Fe3O4) |
Acetylated glucosamine polymers |
51 and 67 |
| - Dissolution in 1% acetic acid |
- Tripolyphosphate cross-linking |
| Tannins |
Acacia bark/pomegranate rind |
- Hot water/ethanol extraction (70 °C) |
- Quaternary ammonium functionalization |
Hydrolyzable/gallotannins |
51 and 68 |
| - Rotary evaporation & lyophilization |
| Okra polysaccharides |
Abelmoschus esculentus pods |
- Crushing, centrifugation (4000 rpm) |
- Carboxymethylation |
Galacturonic acid, rhamnogalacturonan |
51 and 69 |
| - Dialysis (MWCO 12 kDa) |
Recent advancements focus on nanocomposite modifications to enhance NCs performance. For example, chitosan-coated magnetic nanoparticles (Fe3O4) are synthesized by coprecipitating Fe2+ and Fe3+ ions in the presence of chitosan solution, followed by cross-linking with glutaraldehyde. This modification allows for easy magnetic separation post-coagulation, reducing secondary pollution.24,26 Another innovation involves biochar-supported coagulants, where Moringa seed extract is immobilized on porous biochar to improve reusability and MPs removal efficiency (up to 92%) in continuous-flow systems.23,63 Characterization techniques such as FTIR, SEM-EDS, zeta potential analysis, and dynamic light scattering (DLS) are employed to confirm the functional groups, surface morphology, and charge properties of the coagulants. Jar test experiments are conducted to optimize parameters like pH (typically 6–8 for most bio-coagulants), coagulant dosage (10–100 mg L−1), mixing speed (20–50 rpm slow mixing, followed by 150–200 rpm rapid mixing), and settling time (15–60 min). Despite their advantages, challenges remain in scaling up NCs production, including batch-to-batch variability, shelf-life limitations, and competition with food/feed industries for raw materials. Future research is exploring genetic engineering to enhance coagulant protein yields in plants and waste-derived coagulants (e.g., from agricultural byproducts) to improve sustainability.51,53
5.2 NCs affecting parameters
The effectiveness of NCs in MPs removal is governed by a complex interplay of physicochemical, environmental, and operational factors. One of the most critical determinants is the source and biochemical composition of the coagulant. NCs are typically derived from plant seeds (e.g., Moringa oleifera), fruits (e.g., Tamarindus indica), leaves (e.g., Cactus opuntia), or microbial sources (e.g., chitosan from crustacean shells), each containing distinct active compounds such as proteins, polysaccharides, tannins, and mucilages. For example, Moringa oleifera seeds contain cationic dimeric proteins (2.6–16 kDa) that destabilize colloids via charge neutralization and adsorption, while okra mucilage relies on long-chain polysaccharides that facilitate bridging flocculation.69,70 The extraction method (aqueous, saline, or organic solvent-based) also influences coagulant activity, with studies showing that salt-extracted Moringa proteins exhibit higher turbidity removal (85–95%) compared to water-extracted ones (70–80%) due to better protein solubility.71 Water quality parameters significantly impact coagulation efficiency, with pH being a dominant factor. Most NCs perform optimally near neutral pH,6–8 where their functional groups (e.g., –NH3+ in proteins or –COO− in polysaccharides) effectively interact with charged contaminants. Extreme pH levels can lead to protein denaturation or reduced solubility, diminishing performance.51 Turbidity and organic load also dictate dosage requirements; high-turbidity waters (>500 NTU) often require higher coagulant doses (50–100 mg L−1), whereas low-turbidity waters (<50 NTU) may need lower doses but benefit from additional additives like bentonite to enhance floc formation.72 The presence of dissolved organic matter (DOM) can interfere with coagulation mechanisms by competing for binding sites, necessitating pretreatment steps such as oxidation or adsorption.73 Operational conditions, including mixing intensity (G-value), contact time, and temperature, further dictate treatment efficacy.52,74 Optimal rapid mixing (100–200 rpm for 1–2 min) ensures uniform dispersion, while slow mixing (20–40 rpm for 15–30 min) promotes floc growth without excessive shear-induced breakage.51 Temperature affects viscosity and reaction kinetics; studies show that Cactus opuntia mucilage performs best at 25–30 °C, with efficiency dropping below 15 °C due to reduced molecular mobility.75 Table 6 lists the performance of NCs in MPs removal under optimized conditions. Additionally, storage and stability of NCs are crucial, as prolonged exposure to humidity, heat, or UV light can degrade active compounds. Encapsulation techniques (e.g., freeze-drying or alginate beads) have been explored to enhance shelf life while maintaining >90% activity after six months.74
Table 6 Performance of NCs in MPs removal under optimized conditions
| Coagulant type |
MPs type (size, polymer) |
Optimal conditions |
Removal (%) |
Mechanism |
Advantages/limitations |
Ref. |
| Moringa oleifera extract |
PE (100–500 μm) |
pH 7, 50 mg L−1, 30 min settling |
85–90 |
Charge neutralization + adsorption |
Low cost; sensitive to pH |
62 |
| Chitosan-Fe3O4 nanocomposite |
PS (1–10 μm) |
pH 6, 20 mg L−1, magnetic separation |
94 |
Magnetic flocculation + electrostatic attraction |
Reusable; high cost of synthesis |
76 |
| Cactus mucilage |
PET (500 μm–1 mm) |
pH 8, 100 mg L−1, 45 min slow mixing |
78 |
Bridging flocculation |
Biodegradable; low shelf-life |
77 |
| Tannin-alum hybrid |
PP (50–200 μm) |
pH 5, 75 mg L−1, 20 rpm mixing |
88 |
Sweep coagulation + hydrogen bonding |
Enhanced efficiency; Al residue concerns |
78 |
| Okra polysaccharide |
PVC (10–100 μm) |
pH 7.5, 60 mg L−1, 15 min settling |
82 |
Viscous entrapment + network formation |
Food-grade safety; low MP size selectivity |
69 |
5.3 Mechanisms of NC–MP interaction
The removal of MPs by NCs involves a sophisticated interplay of physicochemical mechanisms operating across multiple scales, from molecular interactions to macroscopic floc formation (see Fig. 4). At the molecular level, the process begins with the diffusion and adsorption of coagulant molecules to MP surfaces, governed by complex interfacial thermodynamics. Recent studies using quantum dot tagging and high-speed atomic force microscopy (HS-AFM) have revealed that plant-derived coagulants like Moringa oleifera seed proteins exhibit a unique “patch coagulation” mechanism, forming nanoscale domains (12.8 ± 3.2 nm clusters) rather than uniform coatings on MP surfaces.21,51 These protein patches create localized charge reversals (+8 to +15 mV) while maintaining negative charges in intervening areas, generating strong electrostatic attraction between particles. Molecular dynamics simulations show this heterogeneous surface reconstruction reduces the energy barrier for particle approach by 40–60% compared to conventional aluminum sulfate coagulation.79 The patch density follows a dose-dependent saturation curve, with optimal coverage occurring at 1.2 mg protein per m2 MP surface area, explaining the narrow effective dose range (150–200 mg L−1) observed in water treatment applications.80 Chitosan, a crustacean-derived polysaccharide, demonstrates equally complex polymer bridging dynamics. Fluorescence single-molecule tracking studies have temporally resolved its three-stage adsorption process: initial electrostatic docking (τ = 15–30 s), surface reconformation with loop/tail formation (τ = 2–5 min), and interparticle bridge establishment (τ = 8–15 min).81 The bridging efficiency depends critically on chain flexibility and charge distribution, with carboxymethylated chitosan variants (degree of substitution 0.4–0.6) showing 35% longer bridge lifetimes due to enhanced water solubility and chain extension.74
 |
| | Fig. 4 Mechanisms of MPs removal using common NCs. | |
Cryo-electron tomography of floc structures reveals chitosan forms hierarchical networks with primary bridges (5–20 nm spacing) supporting secondary entanglement of MP aggregates, creating robust flocs resistant to shear forces in turbulent water conditions.1,74 Hydrophobic interactions play an equally crucial role, particularly for polyolefin MPs like polyethylene and polypropylene. Interfacial force microscopy measurements quantify adhesion forces of 8–12 nN for these hydrophobic polymers compared to 3–5 nN for more polar polystyrene.32,55 The hydrophobic effect contributes 40–60% of total binding energy at environmentally relevant temperatures (20–30 °C), with NCs leveraging nonpolar domains in their structure: Moringa proteins contain 12–18% hydrophobic residues, chitosan retains acetylated regions from its chitin precursor, and tannin–Fe3+ complexes develop hydrophobic pockets during metal coordination.32,51,77 This explains their superior performance for polyolefin removal compared to conventional coagulants. Microbial coagulants employ sophisticated biological strategies decoded through multi-omics approaches. Bacillus subtilis produces extracellular polymeric substances (EPS) containing amphiphilic lipopeptides (surfactin, iturin) that reduce MP-water interfacial tension by 25–30 mN m−1.82 Fungal melanins from Aspergillus niger catalyze MP surface oxidation, creating new binding sites, while algal exopolysaccharides form hydrated “sticky” layers (50–200 nm thick) that enhance collision efficiency.83,84 Genomic studies reveal upregulation of eps and pel operons during MP exposure, enabling real-time adaptation of microbial communities to different plastic types.85 Tables 7 and 8 summarize the key physicochemical mechanisms underlying MP–coagulant interactions and present a comparative evaluation of the dominant MP removal pathways achieved using NCs.
Table 7 Physicochemical drivers of MP-coagulant interactions
| Interaction type |
Energy contribution |
Polymer specificity |
Environmental dependence |
Enhancement strategies |
Ref. |
| Electrostatic |
40–60% (at pH 6–8) |
Best for PS/PET (ζ = −30 to −50 mV) |
pH-sensitive (optimal: 4–9) |
Charge density modification |
88 |
| Hydrophobic |
40–60% (20–30 °C) |
PE/PP (contact angle >90°) |
Strengthens with temperature ↑ |
Add nonpolar residues |
61 |
| Hydrogen bonding |
10–20% |
Nylon, cellulose acetate |
Requires –OH/–NH groups |
Polysaccharide grafting |
51 and 89 |
| Biological (EPS) |
15–30% |
All MPs with biofilm |
Ca2+/Mg2+ boost ionic bridging |
Microbial strain optimization |
51 and 90 |
Table 8 Comparative analysis of MP removal mechanisms by NCs
| Mechanism |
Dominant coagulant types |
Target MP characteristics |
Removal efficiency |
Energy requirement |
Scalability potential |
Ref. |
| Charge neutralization |
Chitosan, Moringa proteins |
Negatively charged MPs (PET, PS) |
80–92% |
Low (G = 20–50 s−1) |
High (easy extraction) |
51 and 57 |
| Bridging flocculation |
Polysaccharides (okra, cactus) |
Large MPs (>100 μm) |
70–85% |
Medium (G = 50–100 s−1) |
Medium (viscosity issues) |
77 |
| Hydrophobic interaction |
Tannins, plant oils |
Hydrophobic polymers (PE, PP) |
75–90% |
Low |
Low (pH dependent) |
51 and 91 |
| Physical entrapment |
Nanocellulose, alginate gels |
Fibrous MPs, fragments |
90–98% |
High (mixing needed) |
Medium (cost barriers) |
92 and 93 |
| Bio-adhesion |
Microbial EPS, fungal mats |
Diverse MP types |
60–80% |
Very low |
Limited (slow growth) |
93 |
In real water matrices, hybrid mechanisms emerge through synergistic interactions. Natural organic matter (NOM) forms corona structures around MPs, with humic acids complexing tannins to create additional binding sites.86 Divalent cations (Ca2+, Mg2+) act as ionic bridges between coagulant carboxyl groups and MP surface oxides, while temperature fluctuations (10–30 °C) modulate hydrophobic interactions without compromising electrostatic effects. Recent field studies using synchrotron-based X-ray spectromicroscopy show these processes are further complicated by biofilm development, which creates “living flocs” that self-renew their coagulation capacity.87
6. Integrated natural coagulants (iNCs) against MPs
In response to growing concerns over MPs contamination in aquatic environments, recent research has explored the use of iNCs formulations that combine nature-derived components to enhance the CFS process. Li et al., 2024 assessed the removal of MP microbeads (10–1000 μm) from water using CFS. Polyaluminium chloride (PAC) showed the highest efficiency, achieving over 95% removal under optimal conditions: 0.4 mmol per L PAC, 3 mg per L polyacrylamide (PAM), pH 8, with rapid mixing at 240 rpm (1 min), slow mixing at 35 rpm (13 min), and sedimentation for 25 min. PAC alone removed 97% of PS microbeads, while aluminium sulphate and ferric chloride were less effective (67% and 48%, respectively) (see Fig. 5a). PAM improved MP removal for all coagulants and MP types, with optimal performance at ≥3 mg L−1. Organic matter in natural pond water (e.g., Regent's Park) further enhanced removal. Larger microbeads (>250 μm to 1 mm) had 95% removal efficiency, whereas smaller ones (10–<250 μm) had only 49%. Denser MPs like PVC (1.38 g cm−3) settled more efficiently than lighter ones such as PE (0.97 g cm−3). These findings highlight PAC–PAM systems as promising for MP removal, but also reveal the challenge of effectively eliminating smaller, lighter MPs from aquatic environments.94 Another study investigated the use of MO seed extract, both independently and in combination with aluminum sulfate (Al2(SO4)3), for the removal of MPs from water. Three types of MPs PA, PS, and PE were selected due to their prevalence in wastewater effluents. The study aimed to assess the coagulation efficiency of MO and to compare it with conventional coagulation systems, including aluminum sulfate alone, aluminum sulfate combined with anionic polyacrylamide (APAM), and aluminum sulfate combined with MO extract. The methodology involved jar tests using MP-contaminated water with a concentration of 200 mg L−1. MO seeds were extracted using a 1 M CaCl2 solution to enhance the release of active coagulating proteins. Coagulation experiments were conducted with varying dosages of MO (40–240 mg L−1), Al2(SO4)3 (20–120 mg L−1), and APAM (5–20 mg L−1). The optimal dosages were determined based on removal efficiencies, and further tests were carried out to assess the effect of pH, salinity, stirring speed, and MP particle size. SEM analysis examined the morphology of flocs containing MPs formed in different coagulation systems (see Fig. 5b). Flocs from Al2(SO4)3 alone appeared smoother with few particles and no polymer linking MPs. In contrast, Al2(SO4)3 combined with APAM produced denser flocs with many small particles and visible long-chain polymer structures from APAM, which enhanced floc aggregation and MPs removal. Both MO and Al2(SO4)3 + MO systems showed agglomeration through calcium chloride-induced mesh-like structures linking particles and proteins. The Al2(SO4)3 + MO system created more tightly aggregated flocs due to combined effects: Al2(SO4)3 hydrolysis forming adsorptive clusters and positively charged MO proteins interacting with negatively charged MPs, resulting in effective flocculation and MPs adsorption. Results showed that Al2(SO4)3 had better removal efficiency for PA, PS, and PE MPs than MO alone, but MO still achieved considerable removal: at 120 mg per L MO, the removal efficiencies were 67.25% for PA, 57.60% for PS, and 15.68% for PE. When MO was combined with 40 mg L−1 of Al2(SO4)3 (a 50% reduction in aluminum dosage), the removal efficiencies were comparable to the Al2(SO4)3 + APAM system 92.99% (PA), 80.48% (PS), and 28.94% (PE). Zeta potential results indicated that all systems operated primarily through charge neutralization, with MO-based systems showing slightly lower charge neutralization compared to Al2(SO4)3 and Al2(SO4)3 + APAM. SEM analysis also confirmed that agglomeration adsorption contributed to the removal mechanism in the MO systems. The study concluded that Moringa oleifera extract is an effective and coagulant aid for MPs removal. Although it performs slightly less efficiently than APAM when used with Al2(SO4)3, it offers the advantage of reducing the required dose of aluminum sulfate by 50%, thus minimizing associated health and environmental risks. The MO-enhanced system maintained high removal performance across a wide pH range and benefited from increased salinity and stirring speed.62
 |
| | Fig. 5 (a) Schematic diagram of applying coagulant aids in MPs removal, adapted with permission from ref. 94, Copyright, Elsevier, 2024, and (b) SEM images of flocs in diverse coagulation systems (pH = 7.0 ± 0.3, MPs = 200 mg L−1), adapted with permission from ref. 62, Copyright, Elsevier, 2024. | |
Raj et al. 2024 investigated the effectiveness of the CFS process for removing PS-MPs (25 mg L−1) from synthetic and real secondary treated wastewater using FeCl3, chitosan (CT), and their combination. FeCl3 alone achieved up to 89.3% removal, while CT alone removed only 21.4% (see Fig. 6A). However, a combination of 2 mg per L FeCl3 and 7 mg per L CT under optimal conditions (pH 6.3, 100 rpm stirring speed, 30 min settling time) achieved 99.8% PS removal, with statistically significant results (p < 0.05). Zeta potential analysis confirmed charge neutralization as a key mechanism, while SEM and FTIR analyses supported adsorption (see Fig. 6B). Application to effluents from moving bed biofilm reactor (MBBR) and sequencing batch reactor (SBR) systems, spiked with PS-MPs, achieved over 98% removal, highlighting the practical applicability of the FeCl3-chitosan system for tertiary MP treatment.24 Facchino et al. (2025) explored the potential of partially replacing FeCl3 with natural, biodegradable alternatives CT and sodium alginate (SA) initially as coagulant aids. Coagulation tests were conducted using combinations of FeCl3 with CT and SA to evaluate their effectiveness in removing PS-microbeads and fragments of polyethylene PE and PET, all under 500 μm in size. The experimental results demonstrated that both CT and SA can improve the performance of conventional coagulation by enhancing floc settling properties. Specifically, CT contributed to more efficient removal of PS and PE particles while enabling a reduction in the required dose of FeCl3. However, its use was found to negatively affect the removal of PET fragments (see Fig. 7). In contrast, sodium alginate, particularly at a concentration of 0.2 mg L−1, proved beneficial across multiple metrics boosting removal rates at moderate FeCl3 doses and increasing efficiency even at lower dosages.26
 |
| | Fig. 6 (A) Process mechanisms involved in removing PS-MPs via chitosan-iNCs, and (B) SEM images: (a) raw PS MPs, (b) flocs generated in FeCl3 system, and (c) flocs generated in FeCl3–chitosan complex system. EDS elemental mapping images of flocs generated in FeCl3–chitosan complex system (d–h), adapted with permission from ref. 24, Copyright, Elsevier, 2024. | |
 |
| | Fig. 7 Removal efficiency of (a) PS, and (b) PE under different FeCl3-CT dosages. Initial pH 7, initial concentration of 300 mg L−1, adapted with permission from ref. 26, Copyright, Elsevier, 2025. | |
7. Challenges and future research directions
Despite their potential, NCs face several significant challenges that limit their widespread adoption for MPs removal. One major limitation is inconsistent performance due to variable raw material composition. The efficacy of plant-based coagulants like MO or Cactus mucilage depends on seasonal growth conditions, extraction methods, and storage stability, leading to batch-to-batch variability in active compound concentrations.51,71 For example, protein content in Moringa seeds can fluctuate by 20–30% between harvests, directly impacting coagulation efficiency.71 Considering the environmental and toxicological significance of sub-micron plastics (SMPs), particularly nanoplastics (NPs) below 1 μm, there is growing concern regarding their persistence, bioavailability, and potential to cross biological membranes, leading to cytotoxicity, inflammation, and endocrine disruption in aquatic organisms and humans.95,96 NCs have shown potential due to their biocompatibility, flocculating ability, and adsorption capacity. However, their performance for SMPs remains constrained by weak interparticle interactions, low density flocs, and limited surface functionalization. Even in optimized hybrid forms, such as chitosan–nanocellulose composites, the capture of 0.1 μm polystyrene beads remains limited to 60–70% due to inadequate bridging and charge neutralization.97 Ho et al. (2025) reported only 57% removal of 200 nm polyethylene NPs using cationic starch, which improved to 73% after grafting with quaternary ammonium groups.98 Developing solutions are being explored to overcome these challenges. Functionalization of NCs with cationic moieties, such as poly(diallyldimethylammonium chloride) (polyDADMAC) or quaternized chitosan, significantly enhances zeta potential and electrostatic binding.99 For instance, hybrid chitosan–Fe3O4 magnetic nanoparticles demonstrated up to 85% removal of 100–300 nm polystyrene beads via magnetic separation, while maintaining biodegradability and low cytotoxicity.76 Additionally, integration of NCs with nanomaterials like graphene oxide, biochar, or layered double hydroxides (LDHs) improves surface area and facilitates π–π stacking and hydrophobic interactions with SMPs.100–102 Another promising strategy is the use of combined flocculation–photocatalysis processes. For example, chitosan–TiO2 composites, when irradiated under UV-A light, not only enhanced aggregation of NPs but also initiated partial photodegradation of the polymer matrix, with total removal exceeding 90% after 60 min of treatment.103,104 Similarly, membrane-assisted techniques also present a viable route for targeting SMPs.18,19,37 While ultrafiltration and nanofiltration are effective, they are often hampered by membrane fouling and high operational costs.19 Pre-coagulation with NCs such as modified chitosan or tannin–alum hybrids can significantly reduce membrane fouling while achieving high removal rate of SMPs in the pre-filtration stage, as reported by.105 Water matrix complexity further complicates large-scale implementation. While laboratory-scale studies have demonstrated promising performance of NCs for MP removal in synthetic or distilled water matrices, their behavior in complex real-world wastewater scenarios can be substantially different.48,52 Industrial effluents, such as those from textile dyeing, petrochemical processing, food and beverage manufacturing, and pulp and paper production, present unique challenges that can significantly alter coagulation efficiency, floc characteristics, and downstream process integration. Real wastewater streams typically contain high concentrations of NOM, dissolved salts, oil and grease, suspended solids, and various toxic contaminants (e.g., surfactants, heavy metals, dyes).51 These components compete with MPs for coagulant binding sites and may alter the surface charge, zeta potential, and aggregation behavior of both MPs and coagulant molecules. For example, the presence of humic substances can form a corona around MPs, masking their surface properties and inhibiting flocculation by NCs such as Moringa oleifera or chitosan. Lee and Jung (2022) showed that increasing salinity and competing ions reduce MP removal efficiency of approximately 10% from 71.6% to 64.3%.106 Additionally, pH extremes often encountered in industrial effluents (e.g., <5 in electroplating, >9 in textile dyeing) can denature protein-based coagulants or disrupt polysaccharide solubility, thereby reducing efficacy. High ionic strength and salinity, especially in desalination brine or seafood processing wastewaters, can also shield electrostatic interactions that are critical for charge neutralization a key mechanism in NC-based coagulation.21,36,107 High salinity (>20 ppt) destabilizes protein-based coagulants, while extreme pH (<4 or >9) denatures active compounds or reduces their solubility.74 Operational challenges include high coagulant dosages (50–200 mg L−1 versus 5–50 mg L−1 for synthetic alternatives), which increase sludge volume by 20–30%.108 Although this sludge is biodegradable, its management remains logistically challenging in large-scale plants. Moreover, slow kinetics (15–60 min versus 5–15 min for chemical coagulants) necessitate longer retention times, increasing infrastructure costs.50,74 Economic and scalability barriers also hinder adoption. While NCs are cost-effective at small scales (<1 MLD), large-scale production faces hurdles like limited raw material supply chains and higher pretreatment costs (e.g., freeze-drying for stabilization adds ∼30% to production costs).109,110 Regulatory gaps pose another challenge, as few countries have standards for NCs in potable water treatment, delaying approvals despite WHO's 2023 validation for emergency use (WHO, 2023). Finally, long-term stability issues persist; chitosan degrades under UV exposure, while plant extracts lose potency after 3–6 months even with encapsulation.111 Fig. 8 and Table 9 shows the relationship between the impact of each technical limitation on performance and how effective current mitigation strategies are. For example, “pH Sensitivity” has a very high impact on performance but only limited mitigation effectiveness, while “size limitations” can be mitigated quite well. However, Table 10 lists the economic and scalability challenges of NCs in MP removal and potential solutions.
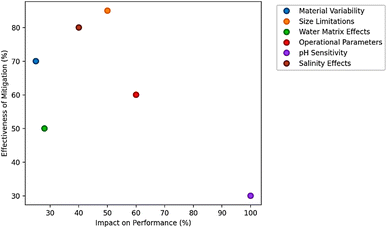 |
| | Fig. 8 Technical limitations of NCs: impact vs. mitigation effectiveness. | |
Table 9 Technical limitations of NCs in MP removal
| Challenge category |
Specific limitation |
Impact on performance |
Affected MP types |
Current mitigation strategies |
Effectiveness of mitigation |
Ref. |
| Material variability |
Seasonal composition changes |
±20–30% efficiency fluctuation |
All types |
Standardized extracts, blending |
Moderate (70% consistency) |
51 and 112 |
| Size limitations |
Low <1 μm MP capture (SMPs) |
40–60% removal for 0.1–1 μm |
NPs, fragments |
Nanohybrids (e.g., chitosan-NFC) |
High (85% improvement) |
51 and 112 |
| Water matrix effects |
DOM interference |
15–40% efficiency loss |
Hydrophobic MPs |
Pre-ozonation, biochar addition |
Moderate (50% recovery) |
36 and 74 |
| Operational parameters |
Slow floc formation |
2–4× longer than chemicals |
Fibers, beads |
Electrocoagulation assist |
High (time reduced by 60%) |
36 and 74 |
| pH sensitivity |
Denaturation at extremes |
Complete failure at pH < 4 or >9 |
Charged MPs |
Buffering, protein engineering |
Limited (narrower range) |
74 and 112 |
| Salinity effects |
Charge shielding >20 ppt |
30–50% efficiency drop |
Marine MPs |
Sulfonated lignin derivatives |
Promising (80% maintained) |
74 and 112 |
Table 10 Economic and scalability challenges of NCs in MP removal and potential solutions
| Factor |
Natural coagulants |
Synthetic coagulants |
Gap analysis |
Potential solutions |
Ref. |
| Raw material cost (USD per kg) |
5–15 |
2–8 |
2–3× higher |
Localized production |
113 and 114 |
| Dosage required (mg L−1) |
50–200 |
10–50 |
4–5× higher |
Hybrid systems |
113 and 114 |
| Storage stability |
3–6 months |
12–24 months |
50% shorter |
Encapsulation tech |
112 and 113 |
| Supply chain maturity |
Low (regional) |
High (global) |
Infrastructure deficit |
Farmer cooperatives |
112 and 113 |
| Treatment plant retrofitting |
Extensive |
Minimal |
High capital cost |
Modular designs |
51 and 113 |
| Sludge management cost |
0.10 USD per m3 |
0.30 USD per m3 |
70% savings |
Valorization needed |
51 and 113 |
8. Conclusion
The growing crisis of MP pollution demands urgent, sustainable solutions. NCs offer a viable alternative to conventional synthetic coagulants, combining high removal efficiency (>90% for MPs >100 μm) with environmental benefits such as biodegradability, lower sludge toxicity, and reduced carbon footprints. Plant-based NCs (e.g., Moringa oleifera), animal-derived chitosan, and microbial EPS leverage diverse mechanisms including electrostatic interactions, polymer bridging, and hydrophobic associations to target MPs across aquatic systems. Hybrid systems, particularly those integrating NCs with minimal doses of metal salts (e.g., FeCl3–chitosan), enhance performance while mitigating health and ecological risks. Despite these advances, critical gaps remain, including batch-to-batch variability in NC composition, diminished efficacy for nanoplastics (<10 μm), and sensitivity to water matrix conditions (pH, salinity, organic matter). Future research essential prioritize:1 standardization of extraction and evaluation protocols,2 bioengineering to improve protein yields and stability,3 pilot-scale validation of hybrid systems, and4 smart coagulation technologies for adaptive treatment. While NCs are not yet a standalone solution for large-scale MP removal, their integration into existing water treatment frameworks represents a crucial step toward sustainable water management.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding statement
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
References
- Q. T. Birch, P. M. Potter, P. X. Pinto, D. D. Dionysiou and S. R. Al-Abed, Sources, transport, measurement and impact of nano and microplastics in urban watersheds, Rev. Environ. Sci. Bio/Technol., 2020, 19, 275–336 CrossRef CAS PubMed.
- S. S. Ali, T. Elsamahy, R. Al-Tohamy and J. Sun, A critical review of microplastics in aquatic ecosystems: Degradation mechanisms and removing strategies, Environ. Sci. Ecotechnology, 2024, 100427 CrossRef CAS PubMed.
- A. E. Schwarz, S. M. C. Lensen, E. Langeveld, L. A. Parker and J. H. Urbanus, Plastics in the global environment assessed through material flow analysis, degradation and environmental transportation, Sci. Total Environ., 2023, 875, 162644 CrossRef CAS.
- World Health O, Programme UUNE and World Organisation for Animal Health X, One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment, World Health Organization, 2022 Search PubMed.
- L. Tiffin, A. Hazlehurst, M. Sumner and M. Taylor, Reliable quantification of microplastic release from the domestic laundry of textile fabrics, J. Text. Inst., 2022, 113(4), 558–566 CrossRef CAS.
- M. Vercauteren, I. Semmouri, E. Van Acker, E. Pequeur, L. Van Esch and I. Uljee, et al., Assessment of road run-off and domestic wastewater contribution to microplastic pollution in a densely populated area, Environ. Pollut., 2023, 333, 122090 CrossRef CAS PubMed.
- R. Hassan, A. E. Alluqmani and A. K. Badawi, An eco-friendly solution for greywater treatment via date palm fiber filter, Desalin. Water Treat., 2024, 317, 100163 CrossRef.
- Y. Huang, Z. Yang, T. Wang, N. Sun, Z. Duan and M. Wigmosta, et al., Quantifying the influence of size, shape, and density of microplastics on their transport modes: A modeling approach, Mar. Pollut. Bull., 2024, 203, 116461 CrossRef CAS PubMed.
- H. Yu, Y. Zhang, W. Tan and Z. Zhang, Microplastics as an emerging environmental pollutant in agricultural soils: effects on ecosystems and human health, Front. Environ. Sci., 2022, 10, 855292 CrossRef.
- E. Costigan, A. Collins, M. D. Hatinoglu, K. Bhagat, J. MacRae and F. Perreault, et al., Adsorption of organic pollutants by microplastics: Overview of a dissonant literature, J. Hazard. Mater. Adv., 2022, 6, 100091 CAS.
- Z. L. R. Botterell, N. Beaumont, T. Dorrington, M. Steinke, R. C. Thompson and P. K. Lindeque, Bioavailability and effects of microplastics on marine zooplankton: A review, Environ. Pollut., 2019, 245, 98–110 CrossRef CAS PubMed.
- M. Ghayebzadeh, H. Aslani, H. Taghipour and S. Mousavi, Estimation of plastic waste inputs from land into the Caspian Sea: A significant unseen marine pollution, Mar. Pollut. Bull., 2020, 151, 110871 CrossRef CAS PubMed.
- S. á. L. C. De, M. Oliveira, F. Ribeiro, T. L. Rocha and M. N. Futter, Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future?, Sci. Total Environ., 2018, 645, 1029–1039 CrossRef PubMed.
- M. Jia, M. U. Farid, Y.-W. Ho, X. Ma, P. W. Wong and T. Nah, et al., Advanced nanobubble flotation for enhanced removal of sub-10 μm microplastics from wastewater, Nat. Commun., 2024, 15(1), 9079 CrossRef CAS PubMed.
- P. Foladori, G. Lucchini, A. Torboli and L. Bruni, Flow cytometry as a tool for the rapid enumeration of 1-μm microplastics spiked in wastewater and activated sludge after coagulation-flocculation-sedimentation, Chemosphere, 2024, 359, 142328 CrossRef CAS PubMed.
- P. Romphophak, O. Faikhaw, S. Sairiam, P. Thuptimdang and C. Coufort-Saudejaud, Removal of microplastics and nanoplastics in water treatment processes: A systematic literature review, J. Water Process Eng., 2024, 64, 105669 CrossRef.
- A. K. Badawi, K. Kriaa, R. M. Osman and R. Hassan, Modified Rice Husk Waste-Based Filter for Wastewater Treatment: Pilot
Study and Reuse Potential, Chem. Eng. Technol., 2024, 47(7), 968–975 CrossRef CAS.
- P. E. Pinto, A. Giacobbo, G. M. d. Almeida, M. A. S. Rodrigues and A. M. Bernardes, Pressure-Driven Membrane Processes for Removing Microplastics, Membranes, 2025, 15(3), 81 CrossRef CAS PubMed.
- M. Bodzek and P. Bodzek, Remediation of Micro-and Nanoplastics by Membrane Technologies, Membranes, 2025, 15(3), 82 CrossRef CAS PubMed.
- M. Shen, Y. Zhang, E. Almatrafi, T. Hu, C. Zhou and B. Song, et al., Efficient removal of microplastics from wastewater by an electrocoagulation process, Chem. Eng. J., 2022, 428, 131161 CrossRef CAS.
- P. Agarwal, S. Prakash and G. Saini, Natural Coagulants (Moringa oleifera and Benincasa hispida) based removal of Microplastics, Cleaner Water, 2024, 1, 100010 CrossRef.
- N. Al-Jadabi, M. Laaouan, S. El Hajjaji, J. Mabrouki, M. Benbouzid and D. Dhiba, The dual performance of Moringa oleifera seeds as eco-friendly natural coagulant and as an antimicrobial for wastewater treatment: a review, Sustainability, 2023, 15(5), 4280 CrossRef CAS.
- J. Xu, Y. Zhang, K. Wen, X. Wang, L. Huang and Z. Yang, et al., Enhanced flotation removal of polystyrene nanoplastics by chitosan modification: Performance and mechanism, Sci. Total Environ., 2024, 946, 174254 CrossRef CAS PubMed.
- S. Raj, B. Mahanty and S. Hait, Coagulative removal of polystyrene microplastics from aqueous matrices using FeCl3-chitosan system: Experimental and artificial neural network modeling, J. Hazard. Mater., 2024, 468, 133818 CrossRef CAS.
- A. K. Badawi, R. Hassan, M. Farouk, E. S. Bakhoum and R. S. Salama, Optimizing the coagulation/flocculation process for the treatment of slaughterhouse and meat processing wastewater: experimental studies and pilot-scale proposal, Int. J. Environ. Sci. Technol., 2024, 21(13), 8431–8446 CrossRef CAS.
- M. Facchino, L. Pietrelli, P. Menegoni, M. Capocelli, E. Limiti and M. Trombetta, et al., Greener Microplastics Removal: Progressive Replacement of Iron-Based Coagulants with Sodium Alginate and Chitosan to Enhance Sustainability, ChemPlusChem, 2025, e202400736 CrossRef CAS PubMed.
- K. L. Law, N. Starr, T. R. Siegler, J. R. Jambeck, N. J. Mallos and G. H. Leonard, The United States' contribution of plastic waste to land and ocean, Sci. Adv., 2020, 6(44), eabd0288 CrossRef PubMed.
- S. Ziajahromi, H.-C. Lu, D. Drapper, A. Hornbuckle and F. D. L. Leusch, Microplastics and tire wear particles in urban stormwater: abundance, characteristics, and potential mitigation strategies, Environ. Sci. Technol., 2023, 57(34), 12829–12837 CrossRef CAS.
- J. Liu, Q. Liu, L. An, M. Wang, Q. Yang and B. Zhu, et al., Microfiber pollution in the earth system, Rev. Environ. Contam. Toxicol., 2022, 260(1), 13 CrossRef.
- X. Luo, Z. Wang, L. Yang, T. Gao and Y. Zhang, A review of analytical methods and models used in atmospheric microplastic research, Sci. Total Environ., 2022, 828, 154487 CrossRef CAS PubMed.
- G. Zhang, R. Ren, X. Yan, H. Zhang and Y. Zhu, Effects of microplastics on dissipation of oxytetracycline and its relevant resistance genes in soil without and with Serratia marcescens: comparison between biodegradable and conventional microplastics, Ecotoxicol. Environ. Saf., 2024, 287, 117235 CrossRef CAS PubMed.
- D. Langenfeld, K. Bucci, C. Veneruzzo, R. McNamee, G. Gao and C. M. Rochman, et al., Microplastics at Environmentally Relevant Concentrations Had Minimal Impacts on Pelagic Zooplankton Communities in a Large In-Lake Mesocosm Experiment, Environ. Sci. Technol., 2024, 58(43), 19419–19428 CrossRef CAS.
- M. Uguen, S. M. Gaudron, K. R. Nicastro, G. I. Zardi, N. Spilmont and S. Henry, et al., The tolerance
of a keystone ecosystem engineer to extreme heat stress is hampered by microplastic leachates, Biol. Lett., 2024, 20(3), 20230457 CrossRef CAS PubMed.
- P. U. Iyare, S. K. Ouki and T. Bond, Microplastics removal in wastewater treatment plants: a critical review, Environ. Sci.: Water Res. Technol., 2020, 6(10), 2664–2675 RSC.
- K. H. D. Tang and T. Hadibarata, Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management, Environ. Challenges, 2021, 5, 100264 CrossRef CAS.
- A. S. Acarer, A review of microplastics in wastewater treatment plants in Türkiye: Characteristics, removal efficiency, mitigation strategies for microplastic pollution and future perspective, Water Sci. Technol., 2024, 89(7), 1771–1786 CrossRef PubMed.
- R. L. Ramos, C. R. dos Santos, G. P. Drumond, L. V. de Souza Santos and M. C. S. Amaral, Critical review of microplastic in membrane treatment plant: Removal efficiency, environmental risk assessment, membrane fouling, and MP release, Chem. Eng. J., 2024, 480, 148052 CrossRef.
- F. Tabatabaei, R. Mafigholami, H. Moghimi and S. Khoramipoor, Effect of Fe and Al based coagulants and disinfectants on polyethylene microplastics removal in coagulation process through response surface methodology, Water Sci. Technol., 2023, 87(1), 99–114 CrossRef CAS PubMed.
- A. Subair, K. L. Priya, S. Chellappan, J. Hridya, P. S. Devi and M. S. Indu, et al., Evaluating the performance of electrocoagulation system in the removal of polystyrene microplastics from water, Environ. Res., 2024, 243, 117887 CrossRef CAS PubMed.
- N. d. O. Dos Santos, R. Busquets and L. C. Campos, Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes, Sci. Total Environ., 2023, 861, 160665 CrossRef CAS PubMed.
- I. A. Ricardo, E. A. Alberto, A. H. S. Júnior, D. L. P. Macuvele, N. Padoin and C. Soares, et al., A critical review on microplastics, interaction with organic and inorganic pollutants, impacts and effectiveness of advanced oxidation processes applied for their removal from aqueous matrices, Chem. Eng. J., 2021, 424, 130282 CrossRef CAS.
- Y. S. Khoo, P. S. Goh, W. J. Lau, A. F. Ismail, M. S. Abdullah and N. H. M. Ghazali, et al., Removal of emerging organic micropollutants via modified-reverse osmosis/nanofiltration membranes: a review, Chemosphere, 2022, 305, 135151 CrossRef CAS PubMed.
- S. Shanmuganathan, S. Vigneswaran, T. V. Nguyen, P. Loganathan and J. Kandasamy, Use of nanofiltration and reverse osmosis in reclaiming micro-filtered biologically treated sewage effluent for irrigation, Desalination, 2015, 364, 119–125 CrossRef CAS.
- N. Garcia, J. Moreno, E. Cartmell, I. Rodriguez-Roda and S. Judd, The application of microfiltration-reverse osmosis/nanofiltration to trace organics removal for municipal wastewater reuse, Environ. Technol., 2013, 34(24), 3183–3189 CrossRef CAS PubMed.
- H. Lu, S. Sun, J. Sun, X. Peng, N. Li and M. W. Ullah, et al., Sustainable production of flocculant-containing bacterial cellulose composite for removal of PET nano-plastics, Chem. Eng. J., 2023, 469, 143848 CrossRef CAS.
- S. Y. Liu, M. M.-L. Leung, J. K.-H. Fang and S. L. Chua, Engineering a microbial ‘trap and release’mechanism for microplastics removal, Chem. Eng. J., 2021, 404, 127079 CrossRef CAS.
- J. W. Yang, C. Park and E. H. Jho, Removal technologies of microplastics in soil and water environments: review on sources, ecotoxicity, and removal technologies, Appl. Biol. Chem., 2024, 67(1), 1–18 CrossRef.
- A. K. Badawi, R. Hassan, A. M. Alghamdi, B. Ismail, R. M. Osman and R. S. Salama, Advancing cobalt ferrite-supported activated carbon from orange peels for real pulp and paper mill wastewater treatment, Desalin. Water Treat., 2024, 318, 100331 CrossRef CAS.
- N. Amirah Mohd Napi, N. Ibrahim, M. Adli Hanif, M. Hasan, F. A. Dahalan and A. Syafiuddin, et al., Column-based removal of high concentration microplastics in synthetic wastewater using granular activated carbon, Bioengineered, 2023, 14(1), 2276391 CrossRef PubMed.
- A. K. Badawi and R. Hassan, Optimizing sludge extract reuse from physico-chemical processes for zero-waste discharge: A critical review, Desalin. Water Treat., 2024, 319, 100527 CrossRef CAS.
- A. K. Badawi, R. S. Salama and M. M. M. Mostafa, Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: a review of recent developments, RSC Adv., 2023, 13(28), 19335–19355 RSC.
- M. Saeed-Ul-Hassan, M. Ehtisham, A. K. Badawi, A. M. Khan, R. A. Khan and B. Ismail, A comparative study of moisture adsorption on GO, MOF-5, and GO/MOF-5 composite for applications in atmospheric water harvesting, Nanoscale Adv., 2024, 6(14), 3668–3679 RSC.
- W. L. Ang and A. W. Mohammad, State of the art and sustainability of natural coagulants in water and wastewater treatment, J. Cleaner Prod., 2020, 262, 121267 CrossRef.
- N. Azizi, M. Pirsaheb, N. Jaafarzadeh and R. N. Nodehi, Microplastics removal from aquatic environment by coagulation: Selecting the best coagulant based on variables determined from a systematic review, Heliyon, 2023, 9(5), 2405–8440 CrossRef PubMed.
- B.-B. Liu, R. Govindan, M. Muthuchamy, S. Cheng, X. Li and L. Ye, et al., Halophilic archaea and their extracellular polymeric compounds in the treatment of high salt wastewater containing phenol, Chemosphere, 2022, 294, 133732 CrossRef CAS PubMed.
- M. Eydi Gabrabad, M. Yari and Z. Bonyadi, Using Spirulina platensis as a natural biocoagulant for polystyrene removal from aqueous medium: performance, optimization, and modeling, Sci. Rep., 2024, 14(1), 2506 CrossRef CAS PubMed.
- N. Girish, N. Parashar and S. Hait, Coagulative removal of microplastics from aqueous matrices: recent progresses and future perspectives, Sci. Total Environ., 2023, 899, 165723 CrossRef CAS PubMed.
- K. Rajala, O. Grönfors, M. Hesampour and A. Mikola, Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals, Water Res., 2020, 183, 116045 CrossRef CAS PubMed.
- S. Saha, M. Zubair, M. A. Khosa, S. Song and A. Ullah, Keratin and chitosan biosorbents for wastewater treatment: a review, J. Polym. Environ., 2019, 27, 1389–1403 CrossRef CAS.
- Z. Zhu, Y. Wu, X. Fang, R. Zhong, H. Gong and M. Yan, Bacillus subtilis, a promising bacterial candidate for trapping nanoplastics during water treatment, J. Hazard. Mater., 2025, 483, 136679 CrossRef CAS PubMed.
- H. Hadiyanto, F. A. Joelyna, A. Khoironi, S. Sudarno, J. A. Safaat and W. D. Pratama, et al., Harnessing Chlorella vulgaris-Aspergilus niger Interactions for Effective Microplastic Removal in Aquatic Ecosystems, Waste Biomass Valorization, 2025, 1–17, 1877–2641 Search PubMed.
- S. Avazpour and M. Noshadi, Enhancing the coagulation process for the removal of microplastics from water by anionic polyacrylamide and natural-based Moringa oleifera, Chemosphere, 2024, 358, 142215 CrossRef CAS PubMed.
- J. Lin, Q. Cheng, A. Kumar, W. Zhang, Z. Yu and D. Hui, et al., Effect of degradable microplastics, biochar and their coexistence on soil organic matter decomposition: A critical review, TrAC, Trends Anal. Chem., 2024, 118082 Search PubMed.
- A. S. Taiwo, K. Adenike and O. Aderonke, Efficacy of a natural coagulant protein from Moringa oleifera (Lam) seeds in treatment of Opa reservoir water, Ile-Ife, Nigeria, Heliyon, 2020, 6(1), 2405–8440 CrossRef PubMed.
- C. Eichhorn, S. Weckmüller and W. Urban, Natural Flocculant from a Combination of Moringa Oleifera Seeds and Cactus Cladodes (Opuntia Ficus-Indica) to Optimize Flocculation Properties, Water, 2022, 14(21), 3570 CrossRef CAS.
- M. C. Fernández-Martínez, C. Jiménez-Martínez, M. R. Jaime-Fonseca and L. Alamilla-Beltrán, Extraction of Purple Prickly Pear (Opuntia ficus-indica) Mucilage by Microfiltration, Composition, and Physicochemical Characteristics, Polymers, 2024, 16(23), 3383 CrossRef.
- V. R. Shaumbwa, D. Liu, B. Archer, J. Li and F. Su, Preparation and application of magnetic chitosan in environmental remediation and other fields: A review, J. Appl. Polym. Sci., 2021, 138(42), 51241 CrossRef CAS.
- F. Abilleira, P. Varela, Á. Cancela, X. Álvarez, Á. Sánchez and E. Valero, Tannins extraction from Pinus pinaster and Acacia dealbata bark with applications in the industry, Ind. Crops Prod., 2021, 164, 113394 CrossRef CAS.
- M. Eydi Gabrabad, Z. Bonyadi, M. Davoudi and B. Barikbin, Microplastic removal using Okra (Abelmoschus esculentus) seed from aqueous solutions, Appl. Water Sci., 2024, 14(10), 217 CrossRef CAS.
- R. Srinivasan, R. Bhuju, V. Chraibi, M. C. Stefan, N. Hien and D. Ustundag, et al., Fenugreek and Okra Polymers as Treatment Agents for the Removal of Microplastics from Water Sources, ACS Omega, 2025, 10(15), 14640–14656 CrossRef CAS PubMed.
- A. O. K. M. Thenuwara, Z. Yi, E. Matsunaga and T. Fujino, Effect of Moringa oleifera seed husk and extraction techniques on coagulation efficiency for cyanobacteria removal, Water Pract. Technol., 2025, 20(4), 868–878 CrossRef.
- P. S. Hounsinou, F. M. Assogba, M. Hounsinou, J. Adounkpè, L. S. A. Tomètin and A. C. Dedjiho, et al., New method of producing a more efficient coagulant for the treatment of water from seeds of moringa oleifera, MethodsX, 2023, 11, 102485 CrossRef CAS PubMed.
- M. Ehtisham, A. K. Badawi, A. M. Khan, R. A. Khan and B. Ismail, Exploring moisture adsorption on cobalt-doped ZnFe 2 O 4 for applications in atmospheric water harvesting, RSC Adv., 2024, 14(9), 6165–6177 RSC.
- B. Al Alwan, B. Ismail, A. El Jery and A. K. Badawi, State-of-the-art strategies for microplastics mitigation in aquatic environments: identification, technological innovations, and prospects for advancement, J. Water Process Eng., 2024, 61, 105336 CrossRef.
- F. Mannai, L. Mechi, F. Alimi, A. K. D. Alsukaibi, M. N. Belgacem and Y. Moussaoui, Biodegradable composite films based on mucilage from Opuntia ficus-indica (Cactaceae): Microstructural, functional and thermal properties, Int. J. Biol. Macromol., 2023, 252, 126456 CrossRef CAS PubMed.
- X. Shi, X. Zhang, W. Gao, Y. Zhang and D. He, Removal of microplastics from water by magnetic nano-Fe3O4, Sci. Total Environ., 2022, 802, 149838 CrossRef CAS PubMed.
- S. Javed, Z. Zulfiqar, Z. Fatima, G. Muhammad, M. A. Hussain and M. Mushtaq, et al., A comprehensive review of plant-based mucilages as promising candidates for water remediation, J. Environ. Chem. Eng., 2024, 114035 CrossRef CAS.
- J. W. Park, S. J. Lee, D. Y. Hwang and S. Seo, Removal of microplastics via tannic acid-mediated coagulation and in vitro impact assessment, RSC Adv., 2021, 11(6), 3556–3566 RSC.
- Y. Liu, S. Cheng, X. Yang, A. Xue, Z. Li and D. S. Alessi, et al., Molecular dynamics simulation study of covalently bound hybrid coagulants (CBHyC): Molecular structure and coagulation mechanisms, Chemosphere, 2022, 307, 135863 CrossRef CAS PubMed.
- H. Li, M. Shen, M. Li, S. Tao, T. Li and Z. Yang, Removal of microplastics and resistance genes in livestock and aquaculture wastewater: Current knowledge and future directions, J. Environ. Chem. Eng., 2024, 12(5), 113384 CrossRef CAS.
- S. Ye, M. Cheng, G. Zeng, X. Tan, H. Wu and J. Liang, et al., Insights into catalytic removal and separation of attached metals from natural-aged microplastics by magnetic biochar activating oxidation process, Water Res., 2020, 179, 115876 CrossRef CAS PubMed.
- A. A. Akinsemolu, H. Onyeaka, S. Odion and I. Adebanjo, Exploring Bacillus subtilis: Ecology, biotechnological applications, and future prospects, J. Basic Microbiol., 2024, 64(6), 2300614 CrossRef PubMed.
- H. Ullah, M. Hayat, B. Raza, K. Alam, M. Ziad and J. Afridi, et al., Biodegradation Of Microplastic: The Role Of Aspergillus Species In Sustainable Plastic Waste Management, The Res. Med. Sci. Rev., 2025, 3(1), 314–329 Search PubMed.
- S. Solanki, S. Sinha and R. Singh, Myco-degradation of microplastics: an account of identified pathways and analytical methods for their determination, Biodegradation, 2022, 33(6), 529–556 CrossRef CAS PubMed.
- N. Sharma and S. Vuppu, In silico study of enzymatic degradation of bioplastic by microalgae: An outlook on microplastic environmental impact assessment, challenges, and opportunities, Mol. Biotechnol., 2023, 1–31 CAS.
- I. Ali, X. Tan, J. Li, C. Peng, I. Naz and Z. Duan, et al., Interaction of microplastics and nanoplastics with natural organic matter (NOM) and the impact of NOM on the sorption behavior of anthropogenic contaminants–A critical review, J. Cleaner Prod., 2022, 376, 134314 CrossRef CAS.
- M. C. Sportelli, C. Kranz, B. Mizaikoff and N. Cioffi, Recent advances on the spectroscopic characterization of microbial biofilms: A critical review, Anal. Chim. Acta, 2022, 1195, 339433 CrossRef CAS PubMed.
- J.-Y. Lin, I. Lee, J.-H. Tzeng, W. Li, H. Kim and C.-P. Huang, The surface acidity of freshly synthesized microplastics particles in simple electrolyte, Colloids Surf., A, 2023, 675, 132000 CrossRef CAS.
- A. Bhattacharjee, A. V. Savargaonkar, M. Tahir, A. Sionkowska and K. C. Popat, Surface modification strategies for improved hemocompatibility of polymeric materials: a comprehensive review, RSC Adv., 2024, 14(11), 7440–7458 RSC.
- R. X. Wu, Y. Zhang, Z. Q. Guo, B. Zhao and J. S. Guo, Role of Ca2+ and Mg2+ in changing biofilm structure and enhancing biofilm formation of P. stutzeri strain XL-2, Colloids Surf., B, 2022, 220, 112972 CrossRef CAS.
- X. Zeng, W. Jiang, Z. Du and J. L. Kokini, Encapsulation of tannins and tannin-rich plant extracts by complex coacervation to improve their physicochemical properties and biological activities: A review, Crit. Rev. Food Sci. Nutr., 2023, 63(18), 3005–3018 CrossRef CAS PubMed.
- I. Leppänen, T. Lappalainen, T. Lohtander, C. Jonkergouw, S. Arola and T. Tammelin, Capturing colloidal nano-and microplastics with plant-based nanocellulose networks, Nat. Commun., 2022, 13(1), 1814 CrossRef PubMed.
- Y. Huang, T. Hu, B. Lin, Y. Ke, J. Li and J. Ma, Microplastics-biofilm interactions in biofilm-based wastewater treatment processes: A review, Environ. Pollut., 2024, 124836 CrossRef CAS PubMed.
- C. Li, R. Busquets and L. C. Campos, Enhancing microplastic removal from natural water using coagulant aids, Chemosphere, 2024, 364, 143145 CrossRef CAS PubMed.
- C. R. de Bruin, E. de Rijke, A. P. van Wezel and A. Astefanei, Methodologies to characterize, identify and quantify nano-and sub-micron sized plastics in relevant media for human exposure: a critical review, Environ. Sci.: Adv., 2022, 1(3), 238–258 Search PubMed.
- K. Pelegrini, T. C. B. Pereira, T. G. Maraschin, L. D. S. Teodoro, N. R. D. S. Basso and G. L. B. De Galland, et al., Micro-and nanoplastic toxicity: A review on size, type, source, and test-organism implications, Sci. Total Environ., 2023, 878, 162954 CrossRef CAS PubMed.
- V. Muralidharan, S. Gochhayat, S. Palanivel and B. Madhan, Influence of preparation techniques of cellulose II nanocrystals as reinforcement for tannery solid waste–based gelatin composite films, Environ. Sci. Pollut. Res., 2023, 30(6), 14284–14303 CrossRef CAS PubMed.
- K. Ho, S. Y. Lau, L. H. Ting, A. Zahir, M. K. Lam and S. Y. Choy, et al., Review of starch-based coagulants for water treatment: Mechanisms, extraction and surface modification, Next Sustain., 2025, 5, 100083 CrossRef.
- N. Mabrouki, H. Agougui, M. Oumezzine, M. Jabli, Y. Guesmi and F. Sonsudin, et al., Poly (diallyldimethylammonium chloride) and Gelatin Functionalized Calcium Hydroxyapatite: Synthesis, and Application in Adsorption of Eriochrome Black T Dye, J. Mol. Struct., 2025, 142841 CrossRef CAS.
- K. Zhang, R. Cen, H. Moavia, Y. Shen, A. Ebihara and G. Wang, et al., The role of biochar nanomaterials in the application of environmental remediation and pollution control, Chem. Eng. J., 2024, 152310 CrossRef CAS.
- T. Fazal, Y. Jazaa, A. Bahadur, S. Iqbal, M. Shah and S. Mahmood, et al., Transformation of refractory ceramic MgAl2O4 into blue light emitting nanomaterials by Sr2+/Cr3+ activation, Mater. Sci. Eng., B, 2024, 303, 117273 CrossRef.
- M. Zhao, G. Zou, Y. Li, B. Pan, X. Wang and J. Zhang, et al., Biodegradable microplastics coupled with biochar enhance Cd chelation and reduce Cd accumulation in Chinese cabbage, Biochar, 2025, 7(1), 1–17 CrossRef.
- M. Naseem, A. Kareem, M. Sultan, I. Khan, S. Ahmad and A. Ahmad, Chitosan as a nanocomposite matrix: advances in nanostructure fabrication, functional properties, and multidisciplinary applications, J. Mater. Sci., 2025, 1–30, 1573–4803 Search PubMed.
- D. Angeles-Beltrán, G. Chavez-Esquivel, J. A. Tavizón-Pozos, V. A. Suárez-Toriello, C. Santolalla-Vargas and O. Aguilar-Martínez, Photocatalytic degradation of phenol using cadmium-TiO2/chitosan hybrid catalysts, Mater. Res. Express, 2025, 065901 CrossRef.
- L. Huang, W. He, Y. Zhang, X. Wang, K. Wu and Z. Yang, et al., Chitosan enhances poly aluminum chloride flocculation system removal of microplastics: Effective, stable, and pollution free, J. Water Process Eng., 2023, 54, 103929 CrossRef.
- P. S. Lee and S. M. Jung, Quantitative analysis of microplastics coagulation-removal process for clean sea salt production, Int. J. Environ. Sci. Technol., 2022, 19(6), 5205–5216 CrossRef CAS.
- H. Hamdhani, D. E. Eppehimer, A. Khusmiadi and J. Jailani, The Abundance Of Microplastics, in The Digestive System Of Silver Barb (Barbonymus Gonionotus) From The Waters Of The Karang Mumus River, Samarinda City, Indonesia, 2024 Search PubMed.
- Q. Fu, X. Liu, Y. Wu, D. Wang, Q. Xu and J. Yang, The fate and impact of coagulants/flocculants in sludge treatment systems, Environ. Sci.: Water Res. Technol., 2021, 7(8), 1387–1401 RSC.
- K. H. Min, D. H. Kim, M.-R. Ki and S. P. Pack, Recent progress in flocculation, dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development, Bioresour. Technol., 2022, 344, 126404 CrossRef CAS PubMed.
- A. A. Owodunni and S. Ismail, Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review, J. Water Process Eng., 2021, 42, 102096 CrossRef.
- N. H. N. Do, Q. T. Truong, P. K. Le and A. C. Ha, Recent developments in chitosan hydrogels carrying natural bioactive compounds, Carbohydr. Polym., 2022, 294, 119726 CrossRef CAS PubMed.
- D. Diver, I. Nhapi and W. R. Ruziwa, The potential and constraints of replacing conventional chemical coagulants with natural plant extracts in water and wastewater treatment, Environ. Adv., 2023, 13, 100421 CrossRef CAS.
- N. Khairul Zaman, R. Rohani, I. Izni Yusoff, M. Kamsol, S. Basiron and A. A. Rashid, Eco-friendly coagulant versus industrially used coagulants: Identification of their coagulation performance, mechanism and optimization in water treatment process, Int. J. Environ. Res. Public Health, 2021, 18(17), 9164 CrossRef CAS PubMed.
- T. Reza, Z. H. Mohamad Riza, S. R. Sheikh Abdullah, H. Abu Hasan, N. I. Ismail and A. R. Othman, Microplastic removal in wastewater treatment plants (WWTPs) by natural coagulation: A literature review, Toxics, 2023, 12(1), 12 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Raouf Hasan
*a,
Raouf Hasan b and
Bushra Ismail
b and
Bushra Ismail c
c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers,4,5 while urban runoff contributes 30–35% of MP loads in freshwater systems.6,7 Once dispersed, MPs exhibit complex transport dynamics influenced by their density (0.85–1.41 g cm−3), shape (fragments, fibers, spheres), and surface chemistry, with recent modeling showing 34% of oceanic MPs reside in surface waters while 66% accumulate in sediments.5,8 Their environmental impacts are exacerbated by large specific surface areas (up to 3000 m2 g−1) that facilitate adsorption of persistent organic pollutants (log
000 microfibers,4,5 while urban runoff contributes 30–35% of MP loads in freshwater systems.6,7 Once dispersed, MPs exhibit complex transport dynamics influenced by their density (0.85–1.41 g cm−3), shape (fragments, fibers, spheres), and surface chemistry, with recent modeling showing 34% of oceanic MPs reside in surface waters while 66% accumulate in sediments.5,8 Their environmental impacts are exacerbated by large specific surface areas (up to 3000 m2 g−1) that facilitate adsorption of persistent organic pollutants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW 3–7), with studies documenting MP-associated concentrations of PCBs and DDTs 106 times higher than ambient seawater.9,10 Ecotoxicological research has demonstrated dose dependent effects across trophic levels, including 17–35% reduced filtration rates in mussels,11,12 50% decreased reproductive output in copepods,2,11 and biomarker responses in fish indicating oxidative stress and neurotoxicity at environmentally relevant concentrations (10–100 particles per L).13 Conventional water treatment processes show variable MP removal efficiencies, with primary sedimentation removing 50–80% of particles >100 μm but only 10–30% of 1–10 μm particles.14–17 Advanced tertiary treatments achieve higher performance (95–99.9%), but face practical limitations – membrane filtration requires 3–5 bar operating pressures (energy demand: 0.5–1.2 kWh m−3),18,19 while electrocoagulation consumes 15–30 kWh m−3 for 92–97% removal.2,20 These challenges have driven research into natural coagulants (NCs), with Moringa oleifera seed extract demonstrating 89% removal of 10–100 μm polyethylene particles at 200 mg per L dose through dual mechanisms of charge neutralization (+15 mV ζ-potential shift) and polymer bridging.21,22 Chitosan shows particular promise, achieving 94% removal of polystyrene microspheres (50 mg per L dose, pH 6.5) with floc formation following second-order kinetics (k = 2.3 × 10−4 L mg−1 min−1).23,24 Hybrid systems combining NCs with conventional processes exhibit enhanced performance.2,25 While chitosan-assisted electrocoagulation reduced energy consumption by 40% compared to conventional methods.24,26 However, key challenges remain, including variability in natural coagulant composition (±15% performance variation between harvests),22 incomplete understanding of MP-coagulant interaction mechanisms at molecular scales,11,16 and lack of standardized protocols for evaluating removal efficiency across different MP types (polymer chemistry, size fractions, aging states).1 The primary objective of this review is to provide a comprehensive and technically rigorous synthesis of recent advances (2020–2025) in the use of NCs for the removal of MPs from aquatic environments. The review classifies NCs based on their biological origin; plant-derived, animal-based, and microbial and critically examines their active components, extraction procedures, and core coagulation mechanisms. It further evaluates their performance across a wide spectrum of MP types, considering variations in polymer composition, particle size, surface properties, and water matrix conditions. Particular attention is given to the physicochemical interaction mechanisms governing MP removal, including charge neutralization, bridging flocculation, hydrophobic interactions, and bio-adhesion, supported by recent visions from advanced characterization techniques and molecular simulations. Moreover, the review explores emerging innovations such as hybrid coagulant formulations, nanostructured composites, enzyme-functionalized systems, which collectively enhance removal efficiency, operational flexibility, and scalability. In doing so, the study also identifies key limitations including raw material variability, reduced efficiency for sub-micron particles, and process sensitivity to environmental factors such as pH, salinity, and dissolved organics and proposes practical strategies for their modification.
KOW 3–7), with studies documenting MP-associated concentrations of PCBs and DDTs 106 times higher than ambient seawater.9,10 Ecotoxicological research has demonstrated dose dependent effects across trophic levels, including 17–35% reduced filtration rates in mussels,11,12 50% decreased reproductive output in copepods,2,11 and biomarker responses in fish indicating oxidative stress and neurotoxicity at environmentally relevant concentrations (10–100 particles per L).13 Conventional water treatment processes show variable MP removal efficiencies, with primary sedimentation removing 50–80% of particles >100 μm but only 10–30% of 1–10 μm particles.14–17 Advanced tertiary treatments achieve higher performance (95–99.9%), but face practical limitations – membrane filtration requires 3–5 bar operating pressures (energy demand: 0.5–1.2 kWh m−3),18,19 while electrocoagulation consumes 15–30 kWh m−3 for 92–97% removal.2,20 These challenges have driven research into natural coagulants (NCs), with Moringa oleifera seed extract demonstrating 89% removal of 10–100 μm polyethylene particles at 200 mg per L dose through dual mechanisms of charge neutralization (+15 mV ζ-potential shift) and polymer bridging.21,22 Chitosan shows particular promise, achieving 94% removal of polystyrene microspheres (50 mg per L dose, pH 6.5) with floc formation following second-order kinetics (k = 2.3 × 10−4 L mg−1 min−1).23,24 Hybrid systems combining NCs with conventional processes exhibit enhanced performance.2,25 While chitosan-assisted electrocoagulation reduced energy consumption by 40% compared to conventional methods.24,26 However, key challenges remain, including variability in natural coagulant composition (±15% performance variation between harvests),22 incomplete understanding of MP-coagulant interaction mechanisms at molecular scales,11,16 and lack of standardized protocols for evaluating removal efficiency across different MP types (polymer chemistry, size fractions, aging states).1 The primary objective of this review is to provide a comprehensive and technically rigorous synthesis of recent advances (2020–2025) in the use of NCs for the removal of MPs from aquatic environments. The review classifies NCs based on their biological origin; plant-derived, animal-based, and microbial and critically examines their active components, extraction procedures, and core coagulation mechanisms. It further evaluates their performance across a wide spectrum of MP types, considering variations in polymer composition, particle size, surface properties, and water matrix conditions. Particular attention is given to the physicochemical interaction mechanisms governing MP removal, including charge neutralization, bridging flocculation, hydrophobic interactions, and bio-adhesion, supported by recent visions from advanced characterization techniques and molecular simulations. Moreover, the review explores emerging innovations such as hybrid coagulant formulations, nanostructured composites, enzyme-functionalized systems, which collectively enhance removal efficiency, operational flexibility, and scalability. In doing so, the study also identifies key limitations including raw material variability, reduced efficiency for sub-micron particles, and process sensitivity to environmental factors such as pH, salinity, and dissolved organics and proposes practical strategies for their modification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers from synthetic textiles. Atmospheric deposition has recently been recognized as a notable transport mechanism, with studies demonstrating MPs in remote locations such as Arctic snow and mountain tops, suggesting long-range atmospheric circulation of these particles.29,30 Agricultural practices also contribute substantially, with plastic mulch films and bio-solid applications introducing MPs into terrestrial systems that eventually reach aquatic environments through soil erosion and runoff.31 The environmental impacts of MPs are complex and concerning. Their small size and high surface area to volume ratio (up to 3000 m2 g−1 for fragmented particles) make them effective vectors for pollutant transport. MPs have been shown to adsorb and concentrate persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) at concentrations up to 106 times greater than surrounding seawater.12 This chemical hitchhiking effect is particularly concerning given the demonstrated bioavailability of these contaminants when ingested by marine organisms. The physical presence of MPs in organisms can cause intestinal blockages, false satiety, and reduced energy reserves. A landmark study by Langenfeld et al. (2024) showed 50% decreased reproductive output in the copepod Calanus helgolandicus at environmentally relevant concentrations (10–100 particles per mL).32
000 microfibers from synthetic textiles. Atmospheric deposition has recently been recognized as a notable transport mechanism, with studies demonstrating MPs in remote locations such as Arctic snow and mountain tops, suggesting long-range atmospheric circulation of these particles.29,30 Agricultural practices also contribute substantially, with plastic mulch films and bio-solid applications introducing MPs into terrestrial systems that eventually reach aquatic environments through soil erosion and runoff.31 The environmental impacts of MPs are complex and concerning. Their small size and high surface area to volume ratio (up to 3000 m2 g−1 for fragmented particles) make them effective vectors for pollutant transport. MPs have been shown to adsorb and concentrate persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) at concentrations up to 106 times greater than surrounding seawater.12 This chemical hitchhiking effect is particularly concerning given the demonstrated bioavailability of these contaminants when ingested by marine organisms. The physical presence of MPs in organisms can cause intestinal blockages, false satiety, and reduced energy reserves. A landmark study by Langenfeld et al. (2024) showed 50% decreased reproductive output in the copepod Calanus helgolandicus at environmentally relevant concentrations (10–100 particles per mL).32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microfibers per wash
000 microfibers per wash![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) and stirred (150–200 rpm) for 30–60 minutes to facilitate protein extraction.21 The mixture is subsequently filtered through Whatman No. 1 filter paper or centrifuged (3000–5000 rpm for 15–20 min) to remove insoluble residues, yielding crude extract rich in cationic proteins that act as NCs by neutralizing the negative surface charges of MPs.21,51 Similarly, Cactus mucilage is extracted by harvesting mature cladodes, washing them thoroughly to remove dust and spines, and then peeling the outer skin to access the inner parenchyma. The peeled cladodes are diced and blended in distilled water, followed by filtration through a muslin cloth to separate fibrous material. The mucilaginous filtrate is then subjected to alcohol precipitation (using ethanol or isopropanol in a 2
10 w/v) and stirred (150–200 rpm) for 30–60 minutes to facilitate protein extraction.21 The mixture is subsequently filtered through Whatman No. 1 filter paper or centrifuged (3000–5000 rpm for 15–20 min) to remove insoluble residues, yielding crude extract rich in cationic proteins that act as NCs by neutralizing the negative surface charges of MPs.21,51 Similarly, Cactus mucilage is extracted by harvesting mature cladodes, washing them thoroughly to remove dust and spines, and then peeling the outer skin to access the inner parenchyma. The peeled cladodes are diced and blended in distilled water, followed by filtration through a muslin cloth to separate fibrous material. The mucilaginous filtrate is then subjected to alcohol precipitation (using ethanol or isopropanol in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio) to concentrate the polysaccharide-based coagulants.51 The precipitate is dried at 40–50 °C and ground into a powder for later use.51,53 Chitosan, another widely studied bio-coagulant, is prepared by deacetylating chitin (extracted from shrimp or crab shells) using concentrated NaOH (40–50% w/v) at 60–80 °C for 4–6 h. The resulting chitosan is washed to neutrality, dried, and dissolved in 1% acetic acid to form a viscous solution, which can be further modified by cross-linking with agents like glutaraldehyde or tripolyphosphate to enhance its mechanical stability and MPs adsorption capacity.24,51 Tannin-based coagulants are extracted from plant sources such as acacia bark or pomegranate peels via aqueous or organic solvent extraction. The raw material is dried, milled, and mixed with hot water (70–80 °C) or ethanol (70% v/v) under reflux for 2–4 h. The extract is then concentrated using a rotary evaporator and freeze-dried to obtain a tannin-rich powder, which can be further functionalized with quaternary ammonium groups to improve its cationic charge density for better MPs flocculation.51,53
1 v/v ratio) to concentrate the polysaccharide-based coagulants.51 The precipitate is dried at 40–50 °C and ground into a powder for later use.51,53 Chitosan, another widely studied bio-coagulant, is prepared by deacetylating chitin (extracted from shrimp or crab shells) using concentrated NaOH (40–50% w/v) at 60–80 °C for 4–6 h. The resulting chitosan is washed to neutrality, dried, and dissolved in 1% acetic acid to form a viscous solution, which can be further modified by cross-linking with agents like glutaraldehyde or tripolyphosphate to enhance its mechanical stability and MPs adsorption capacity.24,51 Tannin-based coagulants are extracted from plant sources such as acacia bark or pomegranate peels via aqueous or organic solvent extraction. The raw material is dried, milled, and mixed with hot water (70–80 °C) or ethanol (70% v/v) under reflux for 2–4 h. The extract is then concentrated using a rotary evaporator and freeze-dried to obtain a tannin-rich powder, which can be further functionalized with quaternary ammonium groups to improve its cationic charge density for better MPs flocculation.51,53
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol)
1 ethanol)







