Open Access Article
Open Access ArticleRecent advances in the synthesis and versatile applications of transition metal complexes featuring Schiff base ligands
Radhika Malav
and
Sriparna Ray
 *
*
Catalytic Applications Laboratory, Department of Chemistry, School of Physical and Biological Sciences, Faculty of Science, Technology and Architecture, Manipal University Jaipur, Dehmi Kalan, Jaipur 303007, Rajasthan, India. E-mail: sriparna.ray@gmail.com; sriparna.ray@jaipur.manipal.edu
First published on 4th July 2025
Abstract
Among the different ancillary ligands, Schiff base (SB) ligands are considered ubiquitous owing to their ease of synthesis and varied applications. When these ligands are utilized to form transition metal complexes, they can modulate the steric and electronic environment of metal ions. These transition metal complexes exhibit specific bioactivity and catalytic activity. Some bioactive SB complexes exhibit excellent anticancer activity against various cell lines, anthelminthic activity, anti-inflammatory activity and anti-oxidant activity. Antimicrobial activity against common Gram-negative and Gram-positive bacteria was observed for several transition metal complexes, which can thus be utilized in the pharmaceutical sector. In addition to their diverse bioactivities, transition metal complexes have various catalytic applications. Specifically, different carbon–carbon cross-coupling reactions and the oxidation of different organic functional groups have been successfully catalyzed by different SB-transition metal complexes. Herein, this review provides a comprehensive account of the versatile applications of different SB-transition metal complexes, including those based on earth-abundant metals.
1 Introduction
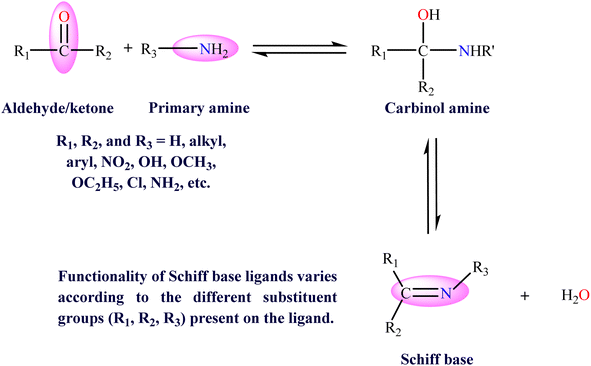
The discovery and application of new molecules with diverse functions have been a continuing quest in modern-day chemistry. Among these compounds, Schiff bases (SBs), along with their transition metal complexes, have attracted considerable attention owing to their significant versatility and wide variety of applications.1 Schiff base ligands, resulting from the condensation reaction of carbonyl moieties and primary amines,2 own a characteristic imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) bond (Fig. 1) that imparts distinctive properties, such as electronic and steric effects, to the resulting complexes.3 In addition, their structural flexibility has enabled a wide range of applications. These SB ligands, when coordinated with metal ions, form metal complexes, which demonstrate enhanced characteristics, including improved reactivity, stability, and specific spectroscopic properties. These integral characteristics have led to an array of applications spanning multiple disciplines, ranging from catalysis to pharmacological applications.4 Over the last couple of years, numerous reports have highlighted their applications in biology in terms of their anticancer,5 antioxidant,6 antibacterial, antifungal,7 anthelminthic,8 antitubercular,9 anti-inflammatory,10 antiviral, and antimalarial activities. Moreover, these complexes successfully functioned as catalysts in several reactions, such as cross-coupling reactions,11 reduction reaction of ketones, oxidation of organic compounds,12 aldol reaction, and hydrosilation.13 Furthermore, SB complexes have been utilized as corrosion inhibitors,14 in dye degradation,15,16 in pigment synthesis, and for other purposes. In addition, various SB metal complexes have been utilized as a methodological platform for stereoselective electrochemical functionalization of amino acids and in metal-catalyzed asymmetric reactions.17,18 The ability to modify Schiff base ligands allows for the design of catalysts with specific characteristics suited to different substrates and reaction conditions, making them versatile tools in many organic syntheses.19,20
N–) bond (Fig. 1) that imparts distinctive properties, such as electronic and steric effects, to the resulting complexes.3 In addition, their structural flexibility has enabled a wide range of applications. These SB ligands, when coordinated with metal ions, form metal complexes, which demonstrate enhanced characteristics, including improved reactivity, stability, and specific spectroscopic properties. These integral characteristics have led to an array of applications spanning multiple disciplines, ranging from catalysis to pharmacological applications.4 Over the last couple of years, numerous reports have highlighted their applications in biology in terms of their anticancer,5 antioxidant,6 antibacterial, antifungal,7 anthelminthic,8 antitubercular,9 anti-inflammatory,10 antiviral, and antimalarial activities. Moreover, these complexes successfully functioned as catalysts in several reactions, such as cross-coupling reactions,11 reduction reaction of ketones, oxidation of organic compounds,12 aldol reaction, and hydrosilation.13 Furthermore, SB complexes have been utilized as corrosion inhibitors,14 in dye degradation,15,16 in pigment synthesis, and for other purposes. In addition, various SB metal complexes have been utilized as a methodological platform for stereoselective electrochemical functionalization of amino acids and in metal-catalyzed asymmetric reactions.17,18 The ability to modify Schiff base ligands allows for the design of catalysts with specific characteristics suited to different substrates and reaction conditions, making them versatile tools in many organic syntheses.19,20
The aim of this review is to provide an extensive overview of the versatile applications of SB ligands, along with their transition metal complexes, demonstrating their fundamental roles in addressing challenges and advancing various fields of study. The present work will provide a comprehensive analysis of the synthetic strategies employed for the formation of Schiff bases and metal complexes. This review will not only serve as a compilation of current advancements, but it will also act as a source of inspiration for researchers aiming to capitalise on the fundamental adaptability of metal–SB complexes. Thus, this study aims to gain a deeper understanding of how these substances can be used to address challenging obstacles and drive scientific progress by revealing the underlying principles and mechanisms that govern their behaviour.
2 Diverse applications of Schiff base metal complexes
2.1 In anticancer studies
Cancer is a complex group of diseases characterized by the uncontrolled growth and division of cells. It can occur in nearly any organ or tissue in the body and spread to other parts of the body through the process known as metastasis. Cancer is initiated by genetic mutations that accumulate over time, leading to disruptions in the normal regulation of cell growth, division, and death. This disease is still a major public health issue around the world and is one of the prime causes of mortality in both developed and developing nations.21 Presently, the medication for cancer mainly comprises chemotherapy and surgery. Furthermore, the medicinal effects of the existing chemotherapeutic pills are inadequate and have several adverse effects.22–24 The discovery of more effective cancer therapy drugs has been a key focus for many research groups over the past few decades. In this regard, several SB complexes have been designed and investigated for anticancer properties in recent years.25The cytotoxic activity of SB and metal complexes was researched by A. Cyril et al. in 2022.26 An equimolar amount of phenylamine and 3-ethoxy-2-hydroxy benzaldehyde in ethanol was mixed together at an ambient temperature for 2 h. This resulted in the formation of a precipitate of ligand (L1) with 76% yield.27,28 Co, Cu, and Zn-complexes were prepared by the addition of ethanolic solutions of the respective metal chlorides to a Schiff base solution in ethanol. After 5 h of refluxing, varied colored precipitates of metal complexes were obtained on cooling (Fig. 2).29 The obtained compounds were characterized using various techniques, including, UV-Vis, FTIR, 1H-NMR, 13C-NMR, and TG analysis. The spectral data clearly indicated that all the metal chelates exhibited distorted octahedral geometry, with the exception of the Zn(II) complex, which exists in a square planar configuration.
The SB ligand and complexes were tested for cytotoxicity analysis, using the MTT assay method in human cervical cancer cells (HeLa Cells).30,31 All the samples were dissolved in DMSO, and cisplatin was used as the standard. Although the ligand was significantly less active, the complexes exhibited anticancer activity at a concentration of 1 μg ml−1 or higher. It was observed that as the concentration of the samples increased from 1 to 500 μg ml−1, the cell inhibition percentage also increased. The results were examined using cell inhibition, stated as IC50 values, and are reported in Table 1. The obtained IC50 estimates for the complexes were notably greater than those of the standard drug. Notably, the Co complex exhibited better cytotoxicity than the Zn or Cu complexes.
| Compounds | IC50 (g ml−1) |
|---|---|
| L1 | 188.3 |
| [CoCl2·L1·2H2O] | 25.51 |
| [CuCl2·L1·2H2O] | 53.35 |
| [ZnL1(H2O)2] | 55.99 |
| Cisplatin | 13.00 |
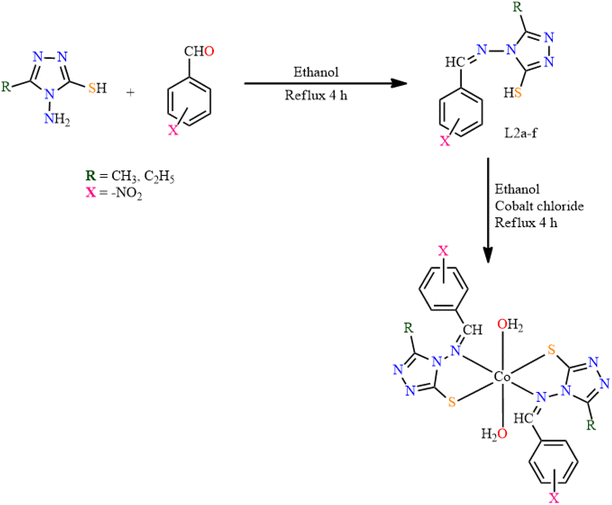
In 2021, triazole-based SB cobalt complexes were synthesized by Deodware et al. and their anticancer activity was evaluated.32 2/3/4-Nitrobenzaldehyde was reacted with substituted mercaptotriazoles in ethanol to form the Schiff base ligands, L2(a–f). Six distinctive cobalt complexes were obtained by reacting the ligands with cobalt chloride in ethanol. The mixtures were refluxed for 4 h, which produced colored solids of cobalt complexes (Fig. 3). In the FTIR spectra of ligands, the most significant peak appeared at 1580–1602 cm−1, which resembles the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group. On complexation, this peak appeared at a lower frequency of 1525–1593 cm−1, which proved the coordination of azomethine nitrogen to the metal ion in all complexes. The 1HNMR spectra of the ligands showed a signal at δ 10.62–11.07 ppm due to the azomethine proton. The thermal decomposition of [Co(L2a–f)2]2H2O was studied, which exhibited four steps of decomposition.
N group. On complexation, this peak appeared at a lower frequency of 1525–1593 cm−1, which proved the coordination of azomethine nitrogen to the metal ion in all complexes. The 1HNMR spectra of the ligands showed a signal at δ 10.62–11.07 ppm due to the azomethine proton. The thermal decomposition of [Co(L2a–f)2]2H2O was studied, which exhibited four steps of decomposition.
All compounds were evaluated for in vitro anticancer activity using the Sulforhodamine B stain (SRB) assay against various cancer cell lines, including lung (NCI–H226), breast (MCF-7), ovary (OVCAR-3) and prostate (PC-3).33,34 Adriamycin was employed as a positive control for these investigations. It was perceived that all the synthesized compounds are exceedingly active against the breast cancer cell line (MCF-7). The overall study data are presented in Table 2.
| Compounds | Sample conc. (mole) | Average values for percentage growth | |||
|---|---|---|---|---|---|
| MCF-7 | NCI-H226 | PC-3 | OVCAR-3 | ||
| L2a | 10−4 | 25.7 | 67.6 | 61.3 | 73.4 |
| [Co(L2a)2]2H2O | 10−4 | −16.3 | 46.1 | 59.4 | 33.8 |
| L2b | 10−4 | 17 | 73.5 | 72.5 | 90.9 |
| [Co(L2b)2]2H2O | 10−4 | 13.9 | 49.6 | 23.3 | 40.4 |
| L2c | 10−4 | 15.3 | 77.2 | 85.4 | 85.4 |
| [Co(L2c)2]2H2O | 10−4 | 32.8 | 64.7 | 41.2 | 41.2 |
| L2d | 10−4 | 31.7 | 81.9 | 83.5 | 113.4 |
| [Co(L2d)2]2H2O | 10−5 | 21.9 | 19.6 | 21.4 | 17.7 |
| 10−4 | −7.9 | — | — | — | |
| L2e | 10−4 | 14.4 | 81.0 | 84.9 | 98.4 |
| [Co(L2e)2]2H2O | 10−5 | 22.9 | 47.2 | 42.2 | 6.5 |
| 10−4 | −4.6 | — | — | — | |
| L2f | 10−5 | 29.4 | 87.9 | 76.8 | 82.1 |
| 10−4 | 19.7 | — | — | — | |
| [Co(L2f)2]2H2O | 10−5 | 17.7 | 22.3 | 49.3 | 3.7 |
| 10−4 | −7.0 | — | — | — | |
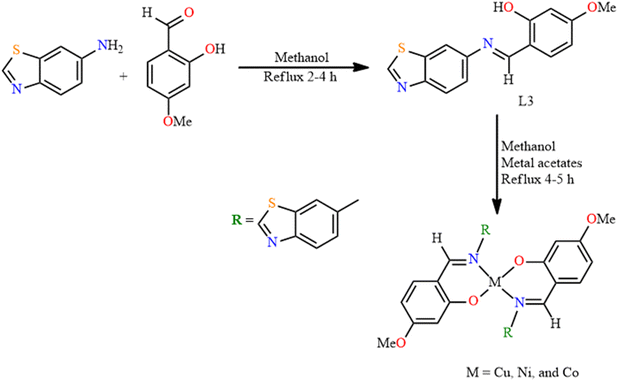
Shivaraj and co-workers in 2020 designed the synthesis of benzothiazole-based SB transition metal complexes and consequently explored the biological action of the synthesized compounds.35 Ligand, L3 was produced by adding a hot solution of 4-methoxy salicylaldehyde in methanol to a solution of benzothiazole-6-amine. After appropriate work-up, metal complexes of Co, Ni, and Cu were prepared by adding metal acetates to a methanolic solution of Schiff base L3 (Fig. 4). The infrared spectra of L3 indicated strong vibrations at 1525 and 1622 cm−1, corresponding to the benzothiazole ring and ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, respectively. This ν
NH, respectively. This ν![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHstret band was shifted within the range of 1603–1610 cm−1 for the Co, Ni, and Cu complexes, owing to the coordination of the SB-nitrogen to the metal ion. In contrast, there is no shifting in frequency of the benzothiazole ring, which highlights the absence of interactions between the
NHstret band was shifted within the range of 1603–1610 cm−1 for the Co, Ni, and Cu complexes, owing to the coordination of the SB-nitrogen to the metal ion. In contrast, there is no shifting in frequency of the benzothiazole ring, which highlights the absence of interactions between the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH group of the benzothiazole ring and the metal ions. The UV-Vis spectra of the aforementioned compounds were verified in L3 by the presence of the n–π* transition of
NH group of the benzothiazole ring and the metal ions. The UV-Vis spectra of the aforementioned compounds were verified in L3 by the presence of the n–π* transition of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH groups. In addition, two bands at 349 and 257 nm appeared due to the π–π* transition of the aromatic moiety, which were slightly shifted on complexation.
NH groups. In addition, two bands at 349 and 257 nm appeared due to the π–π* transition of the aromatic moiety, which were slightly shifted on complexation.
The cytotoxicity of L3 and its complexes was examined using different cancer cell lines through an MTT assay: A549 lung cancer cell (adenocarcinomic human alveolar basal epithelial cells), HeLa (cervical cancer cell), and MCF-7 (breast cancer cells). Table 3 illustrates the IC50 results of compounds in comparison to the standard drug cisplatin. The IC50 value of the copper complex revealed increased activity compared to other metal complexes and ligands. Additionally, these compounds demonstrated greater efficacy against HeLa cells relative to A-549 and MCF-7 cell lines.
| Compounds | IC50 (g ml−1) | ||
|---|---|---|---|
| HeLa | A549 | MCF-7 | |
| L3 | 61 ± 0.17 | 62 ± 0.13 | 67 ± 0.15 |
| Cu(L3)2 | 32 ± 0.42 | 41 ± 0.16 | 44 ± 0.17 |
| Ni(L3)2 | 35 ± 0.23 | 44 ± 0.12 | 48 ± 1.34 |
| Co(L3)2 | 37 ± 1.34 | 45 ± 0.34 | 49 ± 0.64 |
| Cisplatin | 29 ± 0.54 | 30 ± 0.42 | 28 ± 0.36 |
According to the reported data, it can be concluded that the anticancer activity of different complexes varied depending on the substituents. From the published reports, it was found that complexes having a benzothiazole system35 exhibit increased activity for copper complexes. Contrarily, cobalt complexes [CoCl2 L1 2H2O] with bulky substituents, such as the ethoxy group, show better activity towards HeLa cells.26
2.2 In anthelminthic studies
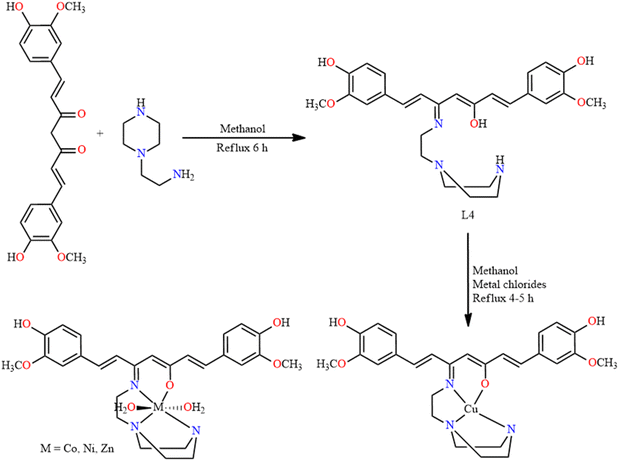
Helminths are parasitic worms, which include roundworms, flukes, tapeworms, etc., that infect humans and animals, causing a variety of diseases. Developing effective treatments for helminth infections is important for public health, especially in regions where these infections are endemic. Certain SB metal complexes have been linked to antihelminth properties in recent years.36 It is notable that research in this field is ongoing, and the effectiveness of SB metal complexes as anthelminthic agents depends on the specific complex, the type of helminth and the host organism.N. Nishat and co-workers in 2018 designed the synthesis of curcumin-based SB and its transition metal complexes, followed by an investigation of their biological potential in anthelminthic activity.37 Initially, a hot methanolic solution of curcumin was stirred for an hour. To this solution, amino ethyl piperazine was added. The SB ligand (L4) was obtained as a brownish powder with 74% yield. Methanolic solutions of metal chlorides (Co, Cu, Ni and Zn) were added to the L4 solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) to form the corresponding SB–metal complexes. Consequently, the metal complexes were obtained in different yields and colours (Fig. 5). The compounds were extensively analyzed using different techniques.
1 molar ratio) to form the corresponding SB–metal complexes. Consequently, the metal complexes were obtained in different yields and colours (Fig. 5). The compounds were extensively analyzed using different techniques.
The anthelminthic activity of the new ligand (L4) and its metallic complexes was examined against the Indian earthworm (Pheretima posthuma) at two different concentrations (0.20 and 0.50% w/v).38 The standard drug Albendazole was used for comparison. Among all synthesized metal complexes, Cu complex showed enhanced anthelminthic potential,39 while Ni showed the least anthelminthic activity. Also, upon complexation, the anthelminthic activity increased compared to L4, which includes an azomethine moiety.40 Furthermore, the lipophilic character of metal ions promotes their passage across the lipid layer of the cell membrane, improving their activity. Hence, the time of paralysis or death of the worm was noted and illustrated in Table 4.
| Time taken for paralysis and death of worms | |||
|---|---|---|---|
| Compounds | Concentration (% w/v) | Paralysis (min) | Death (min) |
| L4 | 0.20 | 6.6 | 8.3 |
| 0.50 | 5.8 | 7.4 | |
| [Co(H2O)2L4] | 0.20 | 4.2 | 6.3 |
| 0.50 | 3.9 | 5.6 | |
| [Ni(H2O)2L4] | 0.20 | 5.3 | 6.5 |
| 0.50 | 4.2 | 5.9 | |
| [CuL4] | 0.20 | 2.9 | 5.1 |
| 0.50 | 2.4 | 3.9 | |
| [Zn(H2O)2L4] | 0.20 | 3.2 | 6.2 |
| 0.50 | 2.9 | 4.6 | |
Manjunath et al. (2017) synthesised and characterised antipyrine-based SB transition metal complexes41 and investigated their anthelminthic activity. An ethanolic mixture of two substituted coumarins was added to a stirred solution of 4-aminoantipyrine to form the SB ligands, L5a (74%) and L5b (72%), respectively. Here, 2–3 drops of HCl acted as a catalyst (Fig. 6). From the prepared SB ligands, metal complexes of Co, Ni, and Cu were synthesized. An EtOH solution of L5(a–b) was added to an aqueous ethanolic solution of hydrated metal chlorides [M = Cu, Co, and Ni] and heated for 2 h. Consequently, CH3COONa was added to the above mixture and refluxed for 3 h, which precipitated the corresponding metal complexes from the solution (Fig. 7). The elemental studies revealed that metal complexes have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal
2 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand stoichiometry. In the 1HNMR spectra of L5a, a signal at 11.7 ppm was attributed to the phenolic –OH. In addition, proton signals at 9.44, 2.4, and around 1.6 to 1.8 ppm correspond to the –HC
ligand stoichiometry. In the 1HNMR spectra of L5a, a signal at 11.7 ppm was attributed to the phenolic –OH. In addition, proton signals at 9.44, 2.4, and around 1.6 to 1.8 ppm correspond to the –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, N–CH3 and C–CH3 groups of coumarin and pyrazoline moieties, respectively. Similar shifts were also observed in the case of L5b.
N, N–CH3 and C–CH3 groups of coumarin and pyrazoline moieties, respectively. Similar shifts were also observed in the case of L5b.
An anthelminthic study was performed utilizing the synthesized compounds against Pheretima posthuma. This Indian earthworm was selected for study owing to its functional and structural resemblance to the abdominal worms of humans.42 The Co, Ni and Cu complexes demonstrated promising anthelminthic activity with respect to both the ligands and the standard drug Albendazole. During the study, the period required for worm paralysis and death was estimated, which is presented in Table 5. Copper complexes took the least time to paralyze and disinfect worms.
| Compounds | Time taken for paralysis and death of worms | |
|---|---|---|
| Paralysis (min) | Death (min) | |
| L5a | 10 | 161 |
| L5b | 8 | 16 |
| Co(L5a)2 | 7 | 13 |
| Co(L5b)2 | 9 | 12 |
| Ni(L5a)2 | 10 | 15 |
| Ni(L5b)2 | 3 | 16 |
| Cu(L5a)2 | 7 | 10 |
| Cu(L5b)2 | 8 | 10 |
| Blank | No effect for 10 h | — |
| Albendazole | 10 | 17 |
Metal complexes of a ligand system based on chromene were synthesized and anthelminthic properties were assessed by Prabhakar and co-workers in 2015.43 Firstly, 4-methyl-7-hydroxycoumarin was produced from a previously reported procedure by Ahluwalia. Utilizing this synthesized compound, 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde was formed by a procedure reported by Kulkarni et al. in 2009.44 The Schiff base ligand, L6, was synthesized by adding an ethanolic solution of 8-formyl-7-hydroxy-4-methylcoumarin and phenylmethanamine under acid-catalyzed conditions. On refluxing the mixture for 5–6 h, a light orange solid of L6 precipitated with 88% yield. Co, Ni, and Cu complexes were obtained by adding an ethanolic solution of MCl2·xH2O (M = Co, Ni, Cu) to the L6 solution, followed by heating for 1 h. In order to obtain the final product, the mixture was refluxed for 3 h after the addition of CH3COONa. The metal complexes were obtained in 61, 66, and 62% yields for the Co, Ni, and Cu complexes, respectively (Fig. 8). In the IR spectra of L6, a characteristic band at 1622 cm−1 was ascribed to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), which was shifted to a lower frequency (1605–1601 cm−1) in the transition metal complexes, indicating the chelation via nitrogen of the azomethine group. Interestingly, the mass spectra of the Co, Ni, and Cu complexes exhibited M + 1 peaks at m/z values of 681, 680, and 685, respectively, corresponding to their molecular weight.
N), which was shifted to a lower frequency (1605–1601 cm−1) in the transition metal complexes, indicating the chelation via nitrogen of the azomethine group. Interestingly, the mass spectra of the Co, Ni, and Cu complexes exhibited M + 1 peaks at m/z values of 681, 680, and 685, respectively, corresponding to their molecular weight.
All synthesized compounds were investigated against Pheretima posthuma for their anthelminthic activity at two different concentrations, 2 and 10 μg ml−1. Albendazole was used as a positive control and the time of death and paralysis of the earthworm was recorded, as shown in Table 6. Interestingly, Cu(L6)2·2H2O was found to be a more potent anthelminthic agent, while the nickel complex was found to be the least potent.
| Compounds | Time taken for paralysis and death of worms | ||
|---|---|---|---|
| Concentration (% μg ml−1) | Paralysis (min) | Death (min) | |
| L6 | 2 | 20.10 ± 0.02 | 24.30 ± 0.12 |
| 10 | 9.21 ± 0.21 | 14.29 ± 0.11 | |
| Co(L6)2·2H2O | 2 | 12.14 ± 0.14 | 19.50 ± 0.02 |
| 10 | 7.40 ± 0.04 | 10.12 ± 0.03 | |
| Ni(L6)2·2H2O | 2 | 16.10 ± 0.25 | 21.20 ± 0.09 |
| 10 | 9.13 ± 0.01 | 15.51 ± 30.00 | |
| Cu(L6)2·2H2O | 2 | 10.27 ± 0.03 | 16.41 ± 0.06 |
| 10 | 5.39 ± 0.22 | 9.31 ± 0.01 | |
| Albendazole | 10 | 3.48 ± 0.06 | 7.25 ± 0.14 |
2.3 In anti-inflammatory studies
Inflammation is a vital part of the body's defence mechanism. However, when it becomes chronic or excessive, it can contribute to the development of various diseases. It is regarded as a key physiological defence mechanism that assists the body in protecting itself from infections, burns, allergies, toxic chemicals and other harmful stimuli. Currently used anti-inflammatory medications have serious negative effects. Consequently, the development of effective anti-inflammatory drugs with fewer side effects is highly required. In recent years, many SBs and their transition metal complexes have displayed anti-inflammatory properties in various in vivo and in vitro studies. Also, different metals can exhibit variable levels of effectiveness and mechanisms of action.45 Further research in this field is ongoing to understand the specific anti-inflammatory mechanism of these metal complexes and to identify the most effective complexes for clinical use.In 2021, an anti-inflammation study was carried out by Saritha et al. by utilizing transition metal complexes, including a Schiff base ligand.46 A methanolic solution of a substituted ketone was mixed with a warm aqueous solution of semicarbazide, followed by the addition of sodium acetate. After appropriate work-up, an orange-red solid of the ligand (L7) was obtained with a 78% yield.47 Mn, Co, Ni, Cu, and Zn-complexes were prepared by adding the SB ligand to a MeOH solution of metal chlorides. Ammonia solution was added to adjust the pH (Fig. 9). Varied colored metal complexes were obtained with a 65–78% yield after refluxing. The electronic data of L7 exhibited two distinctive bands at 423 and 288 nm, which correspond to the n–π* transition of azomethine and π–π* transition of the benzene ring, respectively. In the spectra of metal complexes, the representative bands shifted to higher energy, which indicated the involvement of SB in coordination.
An in vitro anti-inflammatory experiment was performed using the synthesized ligand and complexes, employing the inhibition of albumin denaturation method at varying concentrations.48 The inhibitory action demonstrated that protein denaturation is a concentration-dependent activity. Thus, as the concentration rises, the degree of inhibition also improves. Among the different tested samples, Zn complexes showed the highest percentage of inhibition when compared to other metals and also compared to the ligand, as shown in Table 7.49 Additionally, because of the imine moiety and phenolic group, transition metal complexes of the L7 ligand displayed high anti-inflammatory activity in contrast to the standard drug. The aforementioned complexes were also tested for their antibacterial and antifungal efficacy, out of which the Zn complex showed exceedingly prominent activity in both cases.
| Protein denaturation (% of inhibition) | ||||
|---|---|---|---|---|
| Compounds | Different concentrations (μg ml−1) | |||
| 25 μg ml−1 | 50 μg ml−1 | 75 μg ml−1 | 100 μg ml−1 | |
| L7 | 8.22 | 19.47 | 21.67 | 23.1 |
| [Mn(L7)2]H2O | 22.38 | 48.0 | 53.7 | 58.48 |
| [Co(L7)2]H2O | 24.16 | 24.8 | 42.4 | 43.5 |
| [Ni(L7)2]H2O | 13.17 | 16.6 | 26.25 | 30.96 |
| [Cu(L7)2]H2O | 10.8 | 44.4 | 54.15 | 62.6 |
| [Zn(L7)2]H2O | 44.6 | 57.5 | 80.5 | 92.2 |
| Diclofenac sodium | 75.5 | 100.0 | 100.0 | 100.0 |
The anti-inflammatory or antiphlogistic activity of azomethines and the derived transition metal complexes was examined by Ramadhan and co-workers in 2016.50 Two discrete SB ligands were synthesized by adding 4-chloro-2-toluidine and 3-chloro-2-toluidine to an ethanolic solution of salicylaldehyde under acidic conditions. After completion of the reaction, followed by the required work-up, orange and pale-yellow crystals of L8a (77%) and L8b (77%) were obtained. Metal–SB complexes of iron, cobalt and nickel have been synthesized by mixing an equimolar ratio of L8a and L8b with the respective dehydrated metal acetates in ethanol. The metal complexes were isolated in yields ranging from 60 to 85% (Fig. 10). The IR spectrum showed a broad band in compounds L8a and L8b at around 1620 cm−1 and 1618 cm−1, respectively, which can be attributed to the azomethine group. In the UV-visible spectra of complexes, new bands were formed and an associated shifting of ligand bands was observed. These data indicated the formation of complexes through the coordination of SBs to the metal ions.
The synthesized complexes were evaluated for their activity against the stimulation of inflammation by sub-planter insertion of fresh hen egg albumin in mice paws. 1 h before the induced inflammation, a single oral dose of the compound (80 mg kg−1) was administered to the mice. For comparison, aspirin was utilised as a positive control and distilled water served as the negative control. On completion of the experiment, the paw size of the mice was measured at various time intervals of 120, 200, and 260 min as well as at zero time. According to the study, it was observed that Fe complexes showed better potential against inflammation as compared to Co and Ni complexes. This enhanced activity of Fe may be owing to the fact that Fe is more extensively dispersed in the living system than Co and Ni. Also, Fe has better redox properties than the other metals, allowing it to interact with the free radicals and H2O2 produced during the inflammation process. Though the Co complexes exhibited necessary activity against inflammation, the mice died 24 h later. Hence, Co complexes are believed to have lethal consequences in addition to their anti-inflammatory effects. The overall study outcome is demonstrated in Table 8.
| Compounds | Changes in the paw size in cm × 10−1 at different time periods | |||
|---|---|---|---|---|
| 0 min | 120 min | 200 min | 260 min | |
| Control | 0 ± 2.19 | 14.2 ± 1.44 | 12.0 ± 1.82 | 11.3 ± 1.31 |
| Aspirin | 0 ± 1.35 | 10.9 ± 1.17 | 7.71 ± 0.97 | 6.72 ± 0.73 |
| Fe(L8a)2 | 0 ± 1.75 | 7.03 ± 0.15 | 4.03 ± 1.09 | 4.32 ± 0.17 |
| Fe(L8b)2 | 0 ± 0.96 | 3.76 ± 2.41 | 1.83 ± 1.34 | 0.41 ± 2.41 |
| Ni(L8a)2 | 0 ± 2.81 | 7.53 ± 0.36 | 4.89 ± 0.61 | 3.97 ± 0.26 |
| Ni(L8b)2 | 0 ± 1.36 | 5.87 ± 3.81 | 8.79 ± 2.30 | 4.59 ± 0.19 |
| Co(L8a)2 | 0 ± 2.81 | 5.72 ± 1.12 | 3.60 ± 0.74 | 2.53 ± 1.68 |
| Co(L8b)2 | 0 ± 2.52 | 2.74 ± 0.94 | 3.30 ± 2.08 | 0.03 ± 0.11 |
2.4 In antioxidant studies
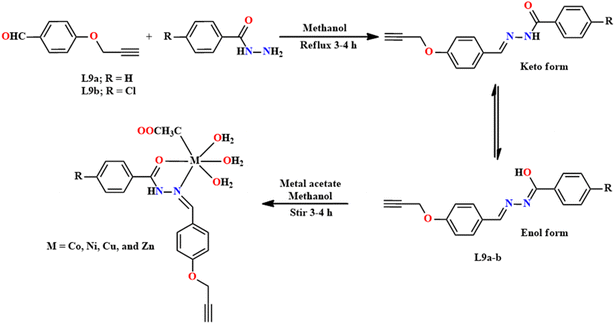
Antioxidants are substances that inhibit the process of unwanted biological oxidation (a chemical reaction that produces free radicals). Currently, the field of free radical chemistry has generated considerable interest.51 Free radical-based ROS (Reactive Oxygen Species) are created by our body via several endogenous mechanisms and exposure to various physicochemical conditions. To maintain a proper physiological role, a balance between antioxidants and free radicals is mandatory. Free radicals significantly damage proteins, DNA, and lipids and cause a wide array of diseases in humans. Synthetic antioxidants, including butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), have recently been shown to be hazardous to human health. Consequently, the quest for non-toxic and effective compounds with antioxidant activities has intensified in recent years. SB metal complexes have shown promising results in scavenging ROS and defending cells from oxidative stress. The metal centres present in this set of complexes, such as Mn, Fe, Cu, etc., undergo redox reactions, thus effectively neutralizing the effect of ROS.52The antioxidant activity of mononuclear complexes, including Co, Ni, Cu, and Zn with hydrazone-based ligands, was reported by Yadav et al. in 2021.53 4-Prop-2-ynyloxy-benzaldehyde was reacted with benzoic acid hydrazide and 4-chlorobenzoic acid hydrazide to synthesize L9(a–b) ligands, respectively (Fig. 11). Similarly, 2-benzyloxy naphthalene-1-carbaldehyde was reacted with benzoic acid hydrazide and 4-chlorobenzoic acid hydrazide to synthesize ligand L9(c–d) (Fig. 12), respectively. The resultant mixtures were refluxed for 3–4 h, where CH3COOH acted as a catalyst. After an appropriate work-up, white solids of ligands were obtained with a 82–86% yield. Metallic complexes comprising Co, Ni, Cu, and Zn were obtained by combining an equimolar amount of metal acetates with SB ligands in methanol. In the FTIR spectrum, strong peaks were observed at 1585–1575 cm−1 (ν![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHstret) in L9a–d, which shifted to 1562–1550 cm−1 on complexation, indicating the coordination of nitrogen to the concerned metal ions. Following a similar pattern, in the NMR spectra of the complexes, the signals due to carbonyl groups and azomethine groups shifted downfield. The mass, NMR, FT-IR spectra and elemental analysis confirmed that ML9a–d(CH3COO)·3H2O complexes were formed.
NHstret) in L9a–d, which shifted to 1562–1550 cm−1 on complexation, indicating the coordination of nitrogen to the concerned metal ions. Following a similar pattern, in the NMR spectra of the complexes, the signals due to carbonyl groups and azomethine groups shifted downfield. The mass, NMR, FT-IR spectra and elemental analysis confirmed that ML9a–d(CH3COO)·3H2O complexes were formed.
The free radical scavenging antioxidant effect of the above-mentioned compounds was evaluated for their antioxidant reactivity with the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical at various dilutions using ascorbic acid as a standard.54 It was discovered that as the concentration increases, the percentage of scavenging activity also increases. In a reported work, the antioxidant activity of L9(a–d) was found to be in the order of L9b > L9d > L9a > L9c. This can be explained by the enhancement of interaction through the electron-withdrawing groups. Thus, these ligands can act as electron donors for DPPH radicals or as hydrogen radical abstractors. Among the different synthesized metal complexes, Cu complexes exhibit excellent scavenging activity with the lowest IC50 values, while the Zn complexes show the least activity. Thus, based on the results of antioxidant studies, these complexes offer a promising treatment for the cure of pathological disorders caused by oxidative stress.
The Fe, Ni, and Pd complexes of pyridine-based SB ligands were prepared and investigated by Bursal and co-workers in 2021, and the antioxidative mechanism of all the compounds formed was investigated.55 L10 was obtained through a condensation reaction between o-vanillin and substituted amine under refluxing conditions.56 From the synthesized ligand, metallic complexes of Fe, Ni, and Pd were developed by reacting the metal chlorides with the corresponding ligand. On an appropriate workup, black to brown-colored metal complexes were formed in 78–86% yield (Fig. 13). The 1H-NMR of L10 showed the presence of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N proton at δ 8.51 ppm, which shifted to δ 8.47 ppm in the Pd complex because of the coordination of azomethine nitrogen with the Pd ion.
N proton at δ 8.51 ppm, which shifted to δ 8.47 ppm in the Pd complex because of the coordination of azomethine nitrogen with the Pd ion.
The above-mentioned compounds were explored for their antioxidant reactivity, which was evaluated in vitro using DPPH and FRAP (Reducing Potential Method).57 BHA, ascorbic acid (vitamin C), BHT, and α-tocopherol (vitamin E) were used as standards. The entire study concluded that the DPPH free radical scavenging degrees of SB, Fe, and Pd complexes were comparable to the standards. However, the scavenging degree of the nickel complex was estimated to be less than that of the standards. Samples with more efficient antioxidant ability have a lower IC50 value. IC50 values were in the following order: BHT > BHA > Pd complex > vitamin E > SB > Fe complex > vitamin C > Ni complex. Overall, the L10 and its Ni complex exhibited superior antioxidant and reducing potential compared to different samples and standards (vitamin C and E). Notably, the scavenging degree of iron and nickel complexes was lower compared to that of Pd, L10 and the standards.
2.5 In antibacterial and antifungal studies
Metal complexes of SB have garnered significant interest in the field of medicinal chemistry owing to their potential antimicrobial activities. The SB ligands and complexes have many biological applications in addition to their catalytic properties and industrial applications in organic synthesis.58 When these SB coordinate with metals (like Co, Ni, Cu, Zn, etc.), they form SB metal complexes. The high propensity of Schiff bases for chelation with transition metals is used to prepare their complexes. Metal ions can considerably modify the electronic properties of the Schiff base, leading to improved biological activities.59 Their ability to disrupt microbial cells through various mechanisms, coupled with the versatility of these ligands, makes them attractive candidates for further development in medicinal chemistry.60 Ongoing research is crucial to fully exploit their potential and translate these findings into successful clinical applications.The antimicrobial activity of quinoline-centred SB and derived metal complexes was assessed by Mamatha Rani and Kavitha in 2024.61 Here, 2-chloro-3-formylquinoline-5-carbonitrile was formed by a previously reported procedure involving Vilsmeier–Haack reaction. To the solution of the aforementioned quinoline, acetic acid and water were added. Afterwards, the resulting mixture was decanted into cold water and after appropriate work-up, 3-formyl-2-hydroxyquinoline-5-carbonitrile was obtained. From the above-prepared substrate, L11 was obtained by reacting it with tosylhydrazine in ethanol along with a few drops of acetic acid. Moreover, Cu, Ni, and Co complexes were prepared through the reaction of SB with the corresponding metal chloride in ethanol in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively. This solution was allowed to stir for an interval of 30 min. Consequently, to maintain the alkaline conditions, 5% NaOH was added to this mixture until it attained a pH of 8 (Fig. 14). The X-ray diffraction studies confirmed the polycrystalline configuration of L11 and complexes. The FTIR spectra of L11 showed the C
1 ratio, respectively. This solution was allowed to stir for an interval of 30 min. Consequently, to maintain the alkaline conditions, 5% NaOH was added to this mixture until it attained a pH of 8 (Fig. 14). The X-ray diffraction studies confirmed the polycrystalline configuration of L11 and complexes. The FTIR spectra of L11 showed the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –OH stretching frequency at 1662 and 3168 cm−1, respectively. The electronic spectra of SB displayed transition bands at 376 and 317 nm, corresponding to n → π* and π → π* transitions.
N and –OH stretching frequency at 1662 and 3168 cm−1, respectively. The electronic spectra of SB displayed transition bands at 376 and 317 nm, corresponding to n → π* and π → π* transitions.
The above-mentioned compounds were evaluated for their efficacy as antimicrobials against various bacterial (Bacillus subtilis and Escherichia coli) and fungal species (Trichoderma reesei and Candida albicans) employing the agar well diffusion technique. Ciprofloxacin and ketoconazole were used as controls for this experiment. Interestingly, the SB ligand showed better antimicrobial potential against B. subtilis and T. reesei. The overall data from the study are depicted in Table 9.
| Compounds | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| B. subtilis | E. coli | T. reesei | C. albicans | |
| L11 | 36 | 34 | 32 | 34 |
| CuL112 | 29 | 33 | 28 | 29 |
| NiL112 | 25 | 26 | 25 | 24 |
| CoL112 | 28 | 37 | 26 | |
| Ciprofloxacin | 22 | 25 | — | — |
| Ketoconazole | — | — | 23 | 22 |
Al-Qadsy and co-workers in 2023 synthesized and characterized the thiazole-based ligand along with its nickel and zinc complexes.62 For applications, the synthesized compounds were investigated for their antimicrobial efficiency. In this study, the ligand (L12) was synthesized using two different methods, including a conventional method63 and a green microwave-assisted method.64 Using the traditional pathway, a condensation reaction occurs between o-hydroxybenzaldehyde and 5-(4-bromophenyl)thiazol-2-amine. An appropriate work-up yielded 89% of the greenish-yellow ligand. Following a green route, the above-mentioned reactants were dissolved in an equivalent amount of water and PEG and heated in a microwave for 3 min. After work-up, a 90% yield of a similar colored ligand was obtained. Furthermore, Ni and Zn complexes of L12 were formed by adding the aqueous solution of metal salts [Ni(NO3)2 and ZnCl2] to a DMF solution of the ligand. As a result, light brown powder of Ni complex (55%) and dark yellow powder of Zn complex (70%) were obtained (Fig. 15). The electronic spectra of L12 comprise peaks within a range of 267–286 nm, attributed to the π–π* transition of phenyl and thiazole rings. In the 1HNMR spectra of L12, a singlet at δ 9.70 ppm was observed, consistent with the hydrogen of the imine group of the ligand. A singlet of hydrogen of the thiazole ring appears at δ 7.22 ppm.
All aforementioned compounds were analyzed with respect to six strains of microorganisms, namely S. aureus, E. faecalis, methicillin-resistant S. aureus (MRSA) (Gram-positive) and E. coli, P. aeruginosa, K. pneumoniae (Gram-negative). After investigation, the MIC of all the compounds were recorded and are presented in Table 10. Based on the results, it can be concluded that all Ni complexes displayed the highest antibacterial potential against all bacterial strains compared to L12, Zn-complex and the reference (streptomycin). The difference in bioactivity between L12 and its Ni and Zn-complexes is the coordination of the metal ion to L12.
| Compound | S. aureus | MRSA | E. faecalis | P. aeruginosa | E. coli | K. pneumoniae |
|---|---|---|---|---|---|---|
| L12 | 7.81 | 1.95 | 1.95 | 3.91 | 1.95 | 7.81 |
| [Ni(L12)2(NO3)2H2O] | 7.81 | 1.95 | 1.95 | 3.91 | 1.95 | 7.81 |
| [Zn(L12)2(Cl2)2H2O] | 125 | 31.25 | 125 | 62.5 | 62.5 | 62.5 |
| Streptomycin | 31.25 | 1.25 | 15.63 | 31.25 | 31.25 | 15.63 |
Synthesis and antibacterial assessment of surfactant-based SB transition metal complexes were investigated by Adhikari et al. in 2022.65 To perform the ligand synthesis, an ethanolic solution of dodecylamine was mixed with a solution of pyrrole-2-carboxaldehyde. Subsequently, the temperature was lowered via a slow diffusion process.66 A reddish-brown solid (L13) was gained (75% yield). Later, to a stirred solution of L13, an ethanolic solution of metal chloride (NiCl2·6H2O and ZnCl2) was added dropwise. Upon constant stirring, Ni and Zn complexes were obtained with 68 and 70% yields, respectively (Fig. 16). The 1H NMR spectra of L13 displayed a peak at δ = 8.041 ppm, which was assigned to the azomethine proton, confirming the synthesis of SB. In the Zn complex, this signal was shifted downfield (δ = 8.486 ppm), signifying the involvement of the azomethine group in the complex formation. Compounds were also analyzed using NMR, UV, FT-IR, and mass spectra.
The assessment of antibacterial activity for the prepared compounds was carried out against five infective pathogens, including E. coli, K. pneumoniae, S. aureus, P. aeruginosa, Enterococci. From the results, it can be concluded that all compounds hold good antibacterial strength. Enhanced MIC was noted for the Ni-complex against all microorganisms. The Ni-complex illustrated good antibacterial potency against S. aureus. The investigation also unveiled that Zn-complex was more effective against P. aeruginosa, (MIC 0.0122 μg μL−1). The experimental results of the tested compounds are shown in Table 11.
| Compounds | MIC (μg ml−1) | ||||
|---|---|---|---|---|---|
| E. coli | K. pneumoniae | P. aeruginosa | Enterococci | S. aureus | |
| Dodecylamine | 0.7812 | 0.0488 | 0.0976 | 0.3906 | 0.1953 |
| L13 | 0.0976 | 0.1953 | 0.1953 | 0.3906 | 0.0976 |
| [Ni(L13)Cl·3H2O] | 0.0122 | 0.0122 | 0.0488 | 0.0122 | 0.0488 |
| [Zn(L13)Cl·3H2O] | 0.0122 | 0.0122 | 0.0122 | 0.0976 | 0.0976 |
2.6 In catalysing carbon–carbon cross-coupling reactions
Since the inception in the 19th century, the formation of carbon–carbon bonds has been a significant technique in the repertoire of synthetic organic chemists. In the arena of C–C couplings, numerous different ideas are currently in development with the objective of achieving simple and feasible methodologies for forming carbon–carbon bonds under balanced conditions with excellent yields. These include the quest for novel catalytic procedures, usage of alternative metal catalysts, and utility of new substrates, among others. The metal complexes of SB have been explored as catalysts in various C–C bond-forming reactions (Fig. 17). The unique steric and electronic environment present around the metal centre significantly influences catalytic activity. Among the notable chemical conversions catalyzed by distinct metal complexes of SB are numerous forms of C–C coupling mechanisms, such as Mizoroki–Heck reaction,67 Hiyama,68,69 Suzuki–Miyaura reaction,70,71 Negishi,72,73 Sonogashira,74,75 Kumada-Corriu,76 Stille reaction,77,78 and more. The number of medications that are obtained through the use of palladium-catalysed reactions at some point in their manufacturing has continuously increased over the last few decades, with the primary three reaction types as Suzuki, Sonogashira, and Heck.79Kargar and colleagues in 2022 designed a tetradentate SB ligand and its palladium complex (Pd) and explored the synthesized compounds in a coupling reaction, namely Suzuki coupling.80 L14 was obtained as a result of a condensation reaction involving 3-ethoxy-2-hydroxybenzaldehyde and 4-methyl-o-phenyldiamine (89% yield). The formed ligand was utilized in the synthesis of a Pd(II) complex by adding L14 to a palladium acetate solution in methanol. After 4–5 days, dark red crystals of Pd complex were obtained (61% yield) (Fig. 18). In FTIR spectra, a peak at 1616 cm−1 was detected for the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety in L14, which was altered to a lower wavenumber (1589 cm−1) in PdL14. In the 1H NMR spectrum of L14, the phenolic protons were assigned at δ = 12.95 and 13.02 ppm.
N moiety in L14, which was altered to a lower wavenumber (1589 cm−1) in PdL14. In the 1H NMR spectrum of L14, the phenolic protons were assigned at δ = 12.95 and 13.02 ppm.
The synthesized L14 and PdL14 were tested for catalytic efficacy in Suzuki coupling, which was achieved using aryl halides and phenylboronic acid at 70 °C. 0.01 mmol of the catalyst was used with KOH (base) and ethanol (solvent). After an appropriate interval of time, a varied number of cross-coupled products for different substituted aryl halides were produced in 47–99% yields. The aryl halides used for the experiment include aromatic chlorides, iodides, and bromides with several substituents (R ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, –OCH3, –CH3, –CN, COCH3, and NO2) as functional groups.
H, –OCH3, –CH3, –CN, COCH3, and NO2) as functional groups.
Synthesis of the ligand and its dihydrazone-based di–copper complex was carried out by Adam in 2019 and the synthesized metal complex was efficiently used in a catalytic reaction (Sonogashira).81 L15 was synthesized by mixing an aqueous solution of sodium 3-formyl-4-hydroxybenzenesulfonate and succinyldihydrazide. L15 was obtained as a pale yellow solid in 89% yield. An aqueous solution of Cu(OAc)2·H2O was added to L15, heated for 3 h at 80 °C, resulting in the formation of a green solution. After a suitable work-up, a 69% yield of the required product was obtained (Fig. 19).
The designed metal complex was assessed for its utility in a palladium-free Sonogashira reaction under a nitrogen atmosphere. The coupling reaction was performed between halobenzene [X = Br and I] and phenylacetylene. Various conditions were optimized for carrying out the reaction, including the base, solvent, temperature and time. After a detailed investigation, it was concluded that K2CO3 base and EtOH solvent were suitable for the coupling reaction at 80 °C for 20 h with a 0.02 mmol catalyst. In the absence of the catalyst, no sufficient yield of the product was obtained. An increased yield of the catalytic product was obtained with an increasing catalyst amount [0.01, 0.02, 0.05]; however, when 0.10 mmol of the catalyst was loaded, the catalytic yield was reduced.
Analysis of the synthesized nickel Schiff base complex in Kumada–Corriu cross-coupling reaction was performed by Kuchtanin and the group in 2016.82 Formation of the ligand was previously reported by Tamizh and the group by reacting salicylaldehyde and 2-aminophenol in acetonitrile.83 The Ni complex was synthesized by a reaction between L16 and Ni(OAc)2·4H2O. Red to red-brown powder or crystals of products were obtained in 70–80% yield (Fig. 20). In the UV-Vis spectra of Ni(II) complexes, a single band at 2200 cm−1 and a second, more intense band near 2300 cm−1 were observed. The ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) band in the FTIR spectra of L16 was observed at 1625 cm−1, which shifts to a lower frequency on complexation.
N) band in the FTIR spectra of L16 was observed at 1625 cm−1, which shifts to a lower frequency on complexation.
Some of the synthesized complexes were used to evaluate their catalytic efficiency in the Kumada–Corriu reaction, which is nevertheless one of the highly desirable methods for making diphenyl.84,85 4-Bromoanisole and PhMgBr were chosen as model substrates, and the reaction was carried out for 24 h in THF with 2 mol% the catalyst. All the complexes provided good yields of catalytic products. Nonetheless, complexes containing the imidazole moiety exhibited lower catalytic activity. This may be owing to the fact that the hydrogen atom bonded to nitrogen might be deactivated by PhMgBr, causing structural modification. Overall, the catalytic products were obtained in a 42–81% yield with different substituted aryl bromides. Additionally, the catalytic effectiveness of commercially available Ni(dppp)Cl2 was also tested in this coupling, which gave a moderate yield of the product (57%).
2.7 In catalytic oxidation of organic compounds
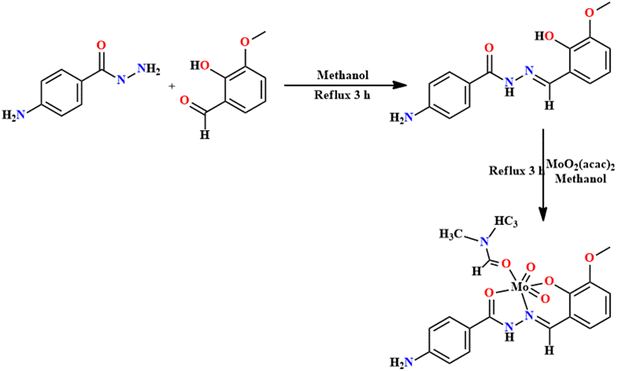
SB-transition metal complexes have demonstrated a variety of uses in the oxidation and reduction of organic compounds,86 as efficient catalysts, thereby producing useful compounds or degrading harmful ones.87 They often exhibit good catalytic utility in a variety of reactions, for instance, oxidation of alcohols to aldehydes or ketones,88 oxidation of alkenes to form epoxides, oxidation of alkanes,89 and oxidation of sulfides to sulfones or sulfoxides,90 etc. The development of SB transition metal complexes for oxidation reactions is an active area of research, and researchers continue to explore new ligands and metal complexes to enhance catalytic activity, selectivity, and efficiency in various organic transformations.In 2021, Kargar et al. designed a dioxomolybdenum complex of a Schiff base and carried out the selective oxidation of benzyl alcohols using the synthesized complex.91 Methanolic solutions of 3-methoxysalicylaldehyde and 4-aminobenzaldehyde were mixed, followed by 3 h of refluxing. After the complete workup, 81% yield of L17 was obtained. The Mo-complex was prepared by reacting a methanolic mixture of MoO2(acac)2 and L17 in an equimolar ratio. Finally, an orange-colored metal complex (73% yield) was obtained after cooling (Fig. 21). In the 1HNMR spectra of the ligand, NH appeared at δ = 11.33 ppm and phenolic–OH at 11.73 ppm, which are absent in the Mo salt. Correspondingly, the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH that appeared at δ = 8.56 ppm in L17 was shifted downfield at δ = 8.76 ppm, indicating the reduction of electronic density following the coordination of imine nitrogen with the metal atom. In IR, the ν
NH that appeared at δ = 8.56 ppm in L17 was shifted downfield at δ = 8.76 ppm, indicating the reduction of electronic density following the coordination of imine nitrogen with the metal atom. In IR, the ν![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH peaks of L17 and the molybdenum complex were observed at 1602 and 1604 cm−1, respectively.
NH peaks of L17 and the molybdenum complex were observed at 1602 and 1604 cm−1, respectively.
The catalytic activity of the synthesized Mo complex was explored in the selective oxidation of simple organic compounds.92 The 0.0006 mmol catalyst was utilized in the reaction between benzylic alcohol and urea hydrogen peroxide (UDP) in acetonitrile to perform the oxidation reaction. The catalyst, MoO2(L17)·DMF is inactive with no support of the oxidant. Reactions of a varied number of substituted benzylic alcohols were performed under the aforementioned conditions, which gave the respective benzaldehyde products in 88–92% yield.
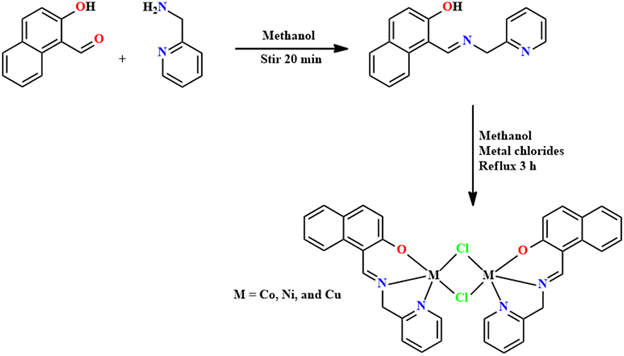
The synthesis of SB complexes of Co, Ni, and Cu was reported by Sengottuvelan and co-workers in 2020.93 The prepared complexes were assessed for catalytic efficiency in the aerobic oxidation of alcohols. The SB ligand (L18) was synthesized by adding 2-picolylamine to 1-formyl-2-naphthol. This synthesized L18 was used to form the complexes, mainly of Co, Ni, and Cu. Respective metal chlorides, dissolved in methanol, were added to the formed solution of L18. Brown-green precipitates of metal complexes were obtained in 50, 56, and 53% yields for Co, Ni, and Cu complexes, respectively (Fig. 22). The UV-Vis spectra of complexes show absorption bands in the range of 265–286 nm, which might be assigned to the π–π* transition. A band was observed owing to ligand-to-metal charge transfer at 316–376 nm. The absorption frequencies at 1640, 1587 and 1638 cm−1 in Co, Ni and Cu complexes, respectively, signify the presence of an azomethine group.94
The catalytic oxidation of n-octanol and benzyl alcohol was carried out with 10 mol% of catalyst [(ML18)2(Cl)2], K2CO3, 10 mol% NMI and 5 mol% TEMPO in CH3CN at room temperature. The initial testing revealed that the presence of TEMPO (cocatalyst) is essential for this oxidation. Interestingly, the transformation of benzyl alcohol was just 16–31% in 24 h with NMI and complexes. It was noted that when 5 mol% TEMPO and K2CO3 (6 mmol) were introduced into the reaction, the yield slightly improved to 22–41%. Furthermore, a catalytic system of complexes, TEMPO, and NMI was evaluated for the oxidation of n-octanol under optimized conditions, and the conversion was only 18–34%.
Tahir and research group (2017) performed the catalytic oxidation of cyclohexane using the dithiocarbazate-based SB metal complexes.95 To an ethanolic solution of S-methyldithiocarbazate, diacetyl pyridine was introduced. The formed mixture was kept at room temperature for 12 h after 1 h of heating. A yellow solid product of L19 was separated with a 60% yield. To prepare the desired metal complexes, the SB ligand was dissolved in an equimolar amount of DCM and EtOH and the resultant solution was poured into a solution of specific metal chlorides (such as Co, Cu, Ni, Fe, Mn, and Zn) to obtain the corresponding metal complexes (Fig. 23). In the 1H NMR of the SB ligand, the signal at 12.70 ppm was observed because of the N–H proton. Interestingly, no peak was observed near 4.00 ppm due to the formation of thione, revealing the absence of the S–H proton. Interestingly, the IR spectra revealed bands near 240 and 370 cm−1, which could be attributed to the stretching frequencies of metal-N and metal-S bonding.
The innovative catalysts have been evaluated for the oxidation of cyclohexane. A solution of cyclohexane and 30% H2O2 in acetonitrile was mixed, and 0.09 mmol of catalyst was added to the mixture at 70 °C. It was observed that the Cu and Fe complexes are highly functioning catalysts for cyclohexane oxidation (30% transformation). These catalysts exhibit high selectivity for cyclohexanone and cyclohexanol (98%), which was enhanced with time and after 3 h of the reaction, a steady state was achieved.
2.8 In catalytic transfer hydrogenation reactions of ketones
Many organic transformations involve transfer hydrogenation reactions under catalytic conditions. This process involves the transfer of hydrogen from a donor molecule (usually isopropanol or formic acid) to a substrate (e.g., ketone), rather than using molecular hydrogen (H2). Schiff base transition metal complexes are used to facilitate the transfer hydrogenation reactions of ketones.96 There exists a vast potential for the usage of hydrogenated products in various aspects. Transfer hydrogenation (TH) processes, catalyzed by transition metal complexes, are one of the ecologically benign practices to substitute conventional hydrogenation reactions. Transition metal complexes, predominantly of noble metals, have been investigated.97–99Buldurun and Özdemir in 2020 derived Ru(II) complexes including pyridine-based Schiff bases.100 Additionally, the catalytic utility of synthesized complexes was inspected in the transfer hydrogenation of ketones. In this investigation, SB ligands, L20(a–d), were prepared through the condensation of 6-t-butyl-3-ethyl-2-amino-4,5-dihydrothieno[2,3-c]pyridine-3,6(7H)-dicarboxylate with different substituted benzaldehydes. Furthermore, the Schiff bases reacted with [RuCl2(p-cymene)]2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively, after which an orange precipitate of metal complexes was formed in 72–78% yield (Fig. 24). The electronic spectra of Ru(L20a–d) illustrated signals at 220–287 and 304–398 nm, which were attributed to n → π* and π → π* transitions, respectively. Interestingly, in the FTIR spectra of Ru(L20a–d), an extra band at 465–461 cm−1 was attributed to ν(Ru–Cl).
1 ratio, respectively, after which an orange precipitate of metal complexes was formed in 72–78% yield (Fig. 24). The electronic spectra of Ru(L20a–d) illustrated signals at 220–287 and 304–398 nm, which were attributed to n → π* and π → π* transitions, respectively. Interestingly, in the FTIR spectra of Ru(L20a–d), an extra band at 465–461 cm−1 was attributed to ν(Ru–Cl).
The catalytic TH reaction of acetophenone derivatives was studied using the synthesized Ru(L20a–d) (0.001 mmol) to obtain the corresponding secondary alcohols. Benzophenone was used as an ideal substrate to evaluate the functioning of the catalyst using i-PrOH as the solvent. No reaction was observed in the absence of the base. The addition of sodium or potassium hydroxide resulted in a better conversion to the respective alcoholic products, with a reaction time of 8 h. However, weak bases, such as Cs2CO3, Na2CO3, KOBut and K2CO3, were found to be less efficient. Among the strong bases, potassium hydroxide was found to be superior. The complexes, Ru(L20a–d), facilitated a catalytic conversion in the range 70–100%, which is listed in Table 12.
| Substrate | Base | Catalyst | Yield |
|---|---|---|---|
| Acetophenone | KOH | L20a–d | 81–95% |
| m-Methoxyacetophenone | KOH | L20a–d | 79–90% |
| p-Bromoacetophenone | KOH | L20a–d | 70–87% |
| p-Methoxyacetophenone | KOH | L20a–d | 71–88% |
| Benzophenone | KOH | L20a–d | 80–100% |
Satheesh et al., in 2019, synthesized half-sandwich complexes of ruthenium(II) bearing multidentate SB ligands and utilized these compounds in catalytic transfer hydrogenation of ketones.101 Initially, 2-(3,4-dimethoxyphenyl) ethanamine was stirred at room temperature for half an hour. To the former solution, o-formyl phenol or 2-hydroxy acetyl benzene was added dropwise to the obtained SB ligands (L21a–b). After completion, a yellow precipitate of L21(a–b) was obtained.102 Ru(II) complexes were synthesized by reacting L21(a–b) and [Ru(p-cymene)Cl2]2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, respectively. Finally, orange-red solids of the desired compounds were obtained in 80–85% yield (Fig. 25). Single-crystal X-ray diffraction confirmed the molecular structures of the complexes.
1 molar ratio, respectively. Finally, orange-red solids of the desired compounds were obtained in 80–85% yield (Fig. 25). Single-crystal X-ray diffraction confirmed the molecular structures of the complexes.
The synthesized complexes were used to evaluate their catalytic activity in the TH reaction of ketones using isopropyl alcohol as a hydrogen source. 1-Phenylethanone was used as a substrate for the optimization studies. The reaction was facilitated using 0.1–0.5 mol% of the catalyst at 85 °C. The time taken for the completion of the reaction varied according to the substrate used. Remarkably, the TH reaction was efficacious even in the absence of a base using this catalytic system, which was an exceptional scenario in TH catalysis. Different secondary alcohols were obtained using different substituted ketones in 77–99% yield.
3 Summary and future scope
Herein, a brief overview of the synthesis of various Schiff base ligands from a variety of aldehydes and amines is presented. Additionally, the preparation of their transition metal complexes, along with their different activities and thereby versatile applications, is elaborated. SB transition metal complexes have demonstrated remarkable versatility by facilitating antimicrobial, anti-inflammatory, anticancer, anthelminthic activities, as well as catalytic activity in C–C cross-coupling reactions. The unique structural properties of Schiff bases, coupled with the diverse coordination chemistry with transition metals, provide a robust platform for the development of novel therapeutic and catalytic agents. These complexes have shown significant promise in preclinical studies, offering candidates for different disease-associated drugs. The antimicrobial activity of Schiff base complexes is particularly notable, with several studies reporting strong efficacy against a broad spectrum of bacterial and fungal pathogens. Similarly, the anticancer potential of these complexes has been underscored by their ability to trigger apoptosis and constrain cell proliferation in various cancer cell lines. Also, these compounds exhibit effective catalytic activity in C–C cross-coupling reactions, transfer hydrogenation reactions, and in the oxidation of different functional groups in organic compounds. In the realm of organic synthesis, SB metal complexes have proven to be efficient and versatile catalysts in C–C cross-coupling reactions, a cornerstone process in the synthesis of complex organic molecules.Both biological efficacy and catalytic performance are crucially directed by the interaction of donor atoms, conjugation, steric environment, and substituents present on the Schiff base ligands. These flexible ligands, when combined with metal ion moieties, produce multifunctional complexes with strong, specific functionalities. This emphasises how essential and logical SAR-based design is for applications involving therapeutic purposes and green catalysis. Further elucidation of the mechanisms underlying the biological activities of Schiff base complexes will be crucial. Continued structural optimization through rational design and high quantity screening could enhance the efficacy and selectivity of these complexes. This approach may involve modifying SB ligands and exploring a wider range of transition metals. Advanced spectroscopic and computational techniques can be employed to gain deeper insights into their modes of action at the molecular level. In the context of catalysis, there is a growing interest in developing sustainable and environmentally friendly catalytic processes. Schiff base complexes could be designed to facilitate green chemistry principles, such as reducing the use of hazardous solvents and improving catalyst recyclability. In conclusion, Schiff base transition metal complexes represent a potentially versatile group of compounds, with a variety of applications, ranging from medicine to catalysis. Continued research and development in this field hold great potential for advancing science and technology, ultimately contributing to improved healthcare and sustainable industrial processes, which in turn result in beneficial economic and environmental impacts. Hence, a detailed discussion of the existing transition metal complexes of Schiff base ligands is extremely useful for all researchers to carry forward new investigative studies involving green reaction conditions. Volatile organic solvents are frequently used in traditional syntheses. Although greener alternatives, such as solvent-free conditions, supercritical CO2, ionic liquids, and water, are becoming more popular, scaling these techniques for industrial catalysis still presents significant challenges. While underutilised energy-efficient methods, such as microwave synthesis, have the potential to lower energy consumption and boost reaction efficiency, they are not standardised for the preparation of metal complexes. There is limited understanding about the direct impact of ligand modifications on sustainability, recyclability, and catalytic performance. For biological applications, structural optimisation of SB-transition metal complexes is essential for lowering toxicity towards normal cells. These ligands frequently form hydrophobic complexes, which reduces their solubility in tissues and blood. Their toxicity may increase because of the build-up in non-target organs and decreased bioavailability caused by this poor solubility. Therefore, to achieve the proper lipophilic–hydrophilic balance for safe and efficient circulation, logical design, such as the introduction of a hydrophilic group, is crucial. In summary, careful ligand design advances metal complexes towards clinical viability by improving efficacy and safety, thereby unlocking their medicinal potential. By addressing these gaps, Schiff base metal complexes will transition from expensive laboratory experiments to reliable, eco-friendly platforms with practical applications in both biomedical and catalytic utility.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Author contributions
SR: conceptualization, supervision and project administration, and writing – review and editing. RM: writing (including all figures) – original draft, review and editing.Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.Acknowledgements
SR is grateful for the financial support of the Manipal University Jaipur Endowment Grant no. EF/2017-18/QE04-15 and MRB Grant no. DOR/MRB/2023/SG-07.References
- A. M. Abu-Dief and I. M. A. Mohamed, A review on versatile applications of transition metal complexes incorporating Schiff bases, Beni-Suef Univ. J. Basic Appl. Sci., 2015, 4(2), 119–133, DOI:10.1016/j.bjbas.2015.05.004.
- L. P. Nitha, R. Aswathy, N. E. Mathews, B. S. Kumari and K. Mohanan, Synthesis, spectroscopic characterisation, DNA cleavage, superoxidase dismutase activity and antibacterial properties of some transition metal complexes of a novel bidentate Schiff base derived from isatin and 2-aminopyrimidine, Spectrochim. Acta, Part A, 2014, 118, 154–161, DOI:10.1016/j.saa.2013.08.075.
- R. Malav and S. Ray, Carbon-carbon cross coupling reactions assisted by Schiff base complexes of Palladium, cobalt and copper: A brief overview, Inorg. Chim. Acta, 2023, 551, 121478, DOI:10.1016/j.ica.2023.121478.
- D. Y. Ma, L. X. Zhang, X. Y. Rao, T. L. Wu, D. H. Li. Li and X. Q. Xie, Synthesis, characterization, luminescence, antibacterial, and catalytic activities of a palladium(II) complex involving a Schiff base, J. Coord. Chem., 2013, 66(9), 1486–1496, DOI:10.1080/00958972.2013.783699.
- S. Ramakrishnan, D. Shakthipriya, E. Suresh, V. S. Periasamy, M. A. Akbarsha and M. Palaniandavar, Ternary Dinuclear Copper(II) Complexes of a Hydroxybenzamide Ligand with Diimine Coligands: the 5,6-dmp Ligand Enhances DNA Binding and Cleavage and Induces Apoptosis, Inorg. Chem., 2011, 50, 6458–6471, DOI:10.1021/ic1024185.
- M. H. Khalil and F. O. Abdullah, Synthesis, Characterization, and Anticancer and Antioxidant Activities of Novel Complexes of Palladium and Organic Schiff-Base Ligand, Bull. Chem. Soc. Ethiop., 2024, 38(3), 605–613, DOI:10.4314/bcse.v38i3.5.
- R. Malav and S. Ray, Biological Utility of Palladium, Cobalt and Copper Schiff Base Complexes: New Approach towards Nanomedicines, NanoWorld J., 2022, 8(S1), S89–S96, DOI:10.17756/nwj.2022-s1-017.
- M. Manjunath, V. H. Naik, A. K. D. Kulkarni and S. A. Patil, DNA cleavage, antimicrobial, anti-inflammatory anthelmintic activities, and spectroscopic studies of Co(II), Ni(II), and Cu(II) complexes of biologically potential coumarin Schiff bases, J. Coord. Chem., 2011, 64(24), 4264–4275, DOI:10.1080/00958972.2011.621082.
- R. Malav and S. Ray, Recent developments on the synthesis of copper and cobalt-Schiff base complexes and their assessment as anti-tuberculosis drugs, Chem. Pap., 2024, 78, 4623–4646, DOI:10.1007/s11696-024-03425-2.
- B. Sandhya, D. Giles, V. Mathew, G. Basavarajaswamy and R. Abraham, Synthesis, pharmacological evaluation and docking studies of coumarin derivatives, Eur. J. Med. Chem., 2011, 46, 4696–4701, DOI:10.1016/j.ejmech.2011.07.013.
- R. Malav, S. Yadav and S. Ray, Catalytic efficacy of Schiff base-palladium complexes in important C–C cross coupling reactions: a brief overview, Proc. Indian Natl. Sci. Acad., 2024, 90, 563–575, DOI:10.1007/s43538-024-00241-w.
- T. Mallat and A. Baiker, Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts, Chem. Rev., 2004, 104, 3037–3058, DOI:10.1021/cr0200116.
- M. B. Lachachi, T. Benabdallah, P. M. Aguiar, M. H. Youcef, A. C. Whitwooda and J. M. Lynam, Synthesis of a series of new platinum organometallic complexes derived from bidentate Schiff base ligands and their catalytic activity in the hydrogenative and dehydrogenative silylation of styrene, Dalton Trans., 2015, 44(26), 11919–11928, 10.1039/C5DT01407G.
- C. Verma and M. A. Quraishi, Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications, Coord. Chem. Rev., 2021, 446, 214105, DOI:10.1016/j.ccr.2021.214105.
- A. A. A. Awad, O. A. M. Ali and D. A. F. Nassar, Degradation of dye wastewater by using cobalt, copper and nickel Schiff base complexes as catalysts: spectral, molecular modelling, catalytic activity and metal removal from aqueous solution, Int. J. Environ. Anal. Chem., 2023, 103, 9562–9581, DOI:10.1080/03067319.2021.2014466.
- M. K. Ghosh, S. Pathak and T. K. Ghorai, Synthesis of Two Mononuclear Schiff Base Metal (M = Fe, Cu) Complexes: MOF Structure, Dye Degradation, H2O2 Sensing, and DNA Binding Property, ACS Omega, 2019, 4, 16068–16079, DOI:10.1021/acsomega.9b02268.
- T. V. Magdesieva, Ni(II)Schiff-base Complexes as Chiral Electro auxiliaries and Methodological Platform for Stereoselective ElectrochemicalFunctionalizationof Amino Acids, Chem. Rec., 2021, 21(9), 2178–2192, DOI:10.1002/tcr.202100019.
- M. Hayashi, Progress of Chiral Schiff Bases with C1 Symmetry in Metal-Catalyzed Asymmetric Reactions, Chem. Rec., 2016, 16(6), 2712–2739, DOI:10.1002/tcr.201600091.
- V. K. Juyal, A. Pathak, M. Panwar, S. C. Thakuri, O. Prakash, A. Agrwal and V. Nand, Schiff base metal complexes as a versatile catalyst: A review, J. Organomet. Chem., 2023, 999, 122825, DOI:10.1016/j.jorganchem.2023.122825.
- P. Ghanghas, A. Choudhary, D. Kumar and K. Poonia, Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications, Inorg. Chem. Commun., 2021, 130, 108710, DOI:10.1016/j.inoche.2021.108710.
- B. P. Bandgar, S. S. Gawande, R. G. Bodade, J. V. Totre and C. N. Khobragade, Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents, Bioorg. Med. Chem., 2010, 18, 1364–1370, DOI:10.1016/j.bmc.2009.11.066.
- M. J. Clarke, F. Zhu and D. R. Frasca, Non-Platinum Chemotherapeutic Metallopharmaceuticals, Chem. Rev., 1999, 99(9), 2511–2533, DOI:10.1021/cr9804238.
- P. J. Dyson and G. Sava, Metal-based antitumour drugs in the post genomic era, Dalton Trans., 2006, 1929–1933, 10.1039/B601840H.
- T. Thirunavukkarasu, H. A. Sparkes, K. Natarajan and V. G. Gnanasoundari, Synthesis, characterization and biological studies of a novel Cu(II) Schiff base complex, Inorg. Chim. Acta, 2018, 473, 255–262, DOI:10.1016/j.ica.2018.01.006.
- Z. C. Liu, B. D. Wang, B. Li, Q. Wang, Z. Y. Yang, T. R. Li and Y. Li, Crystal structures, DNA-binding and cytotoxic activities studies of Cu(II) complexes with 2-oxo-quinoline-3-carbaldehyde Schiff-bases, Eur. J. Med. Chem., 2010, 45, 5353–5361, DOI:10.1016/j.ejmech.2010.08.060.
- G. Senthamilselvan, B. S. Asma, A. Dhanalakshmi and A. Cyril, Potentially Active Metal of Cobalt, Copper and Zinc Complexes Derived from Schiff Base Ligand of 3-ethoxy-2-hydroxy-benzaldehyde and Aniline for their Anticancer Activity, J. Adv. Sci. Res., 2022, 13(2), 76–84, DOI:10.55218/JASR.202200000.
- S. Suwanboon, P. Amornpitoksuk, A. Haidoux and J. C. Tedena, Structural and optical properties of undoped and aluminium doped zinc oxide nanoparticles via precipitation method at low temperature, J. Alloys Compd., 2008, 462, 335–339, DOI:10.1016/j.jallcom.2007.08.048.
- R. K. Das, N. Gogoi and U. Bora, Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract, Bioprocess Biosyst. Eng., 2011, 34, 615–619, DOI:10.1007/s00449-010-0510-y.
- K. Vidhya, M. Saravanan, G. Bhoopathi, V. P. Devarajan and S. Subanya, Structural and optical characterization of pure and starch-capped ZnO quantum dots and their photocatalytic activity, Appl. Nanosci., 2015, 5, 235–243, DOI:10.1007/s13204-014-0312-7.
- A. Golcu, M. Tumer, H. Demirelli and R. A. Wheatley, Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity, Inorg. Chim. Acta, 2005, 358, 1785–1797, DOI:10.1016/j.ica.2004.11.026.
- A. A. A. Aziz, A. N. M. Salem, M. A. Sayed and M. M. Aboaly, J. Mol. Struct., 2012, 1010, 130–138, DOI:10.1016/j.molstruc.2011.11.043.
- S. A. Deodware, U. B. Barache, U. B. Chanshetti, D. J. Sathe, U. P. Ashok, S. H. Gaikwad and S. P. Kollur, Newly synthesized triazole-based Schiff base ligands and their Co(II) complexes as antimicrobial and anticancer agents: Chemical synthesis, structure and biological investigations, Results Chem., 2021, 3, 100162, DOI:10.1016/j.rechem.2021.100162.
- V. Vichai and K. Kirtikara, Sulforhodamine B colorimetric assay for cytotoxicity screening, Nat. Protoc., 2006, 1(3), 1112–1116, DOI:10.1038/nprot.2006.179.
- M. R. Grever, S. A. Schepartz and B. A. Chabner, The National Cancer Institute: cancer drug discovery and development program, Semin. Oncol., 1992, 19(6), 622–638 CAS.
- N. Vamsikrishna, S. Daravath, N. Ganji, N. Pasha and Shivaraj, Synthesis, structural characterization, DNA interaction, antibacterial and cytotoxicity studies of bivalent transition metal complexes of 6 aminobenzothiazole Schiff base, Inorg. Chem. Commun., 2020, 113, 107767, DOI:10.1016/j.inoche.2020.107767.
- R. Rao, K. R. Reddy and K. N. Mahendra, Synthesis, characterization, antibacterial, antifungal and anthelmintic activities of a new 5-nitroisatin Schiff base and its metal complexes, Bulg. Chem. Commun., 2014, 46(1), 11–17 CAS.
- A. Kareem, M. S. Khan, S. A. A. Nami, S. A. Bhat, A. U. Mirza and N. Nishat, Curcumin derived Schiff base ligand and their transition metal complexes: Synthesis, spectral characterization, catalytic potential and biological activity, J. Mol. Struct., 2018, 1167, 261–273, DOI:10.1016/j.molstruc.2018.05.001.
- E. O. Ajaiyeob, P. A. Onocha and O. T. Olarenwaju, In vitro Anthelmintic Properties of Buchholzia coriaceae and Gynandropsis gynandra Extracts, Pharm. Biol., 2001, 39(3), 217–220, DOI:10.1076/phbi.39.3.217.5936.
- H. Zafar, A. Kareem, A. Sherwani, O. Mohammad, M. A. Ansari, H. M. Khan and T. A. Khan, Synthesis and characterization of Schiff base octaazamacrocyclic complexes and their biological studies, J. Photochem. Photobiol., B, 2015, 142, 8–19, DOI:10.1016/j.jphotobiol.2014.10.004.
- C. T. Prabhakara, S. A. Patil, A. K. D. Kulkarni, V. H. Naik, M. Manjunatha, S. M. Kinnal and P. S. Badami, Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2 one Schiff base, J. Photochem. Photobiol., B, 2015, 148, 322–332, DOI:10.1016/j.jphotobiol.2015.03.033.
- M. Manjunath, A. K. D. Kulkarni, G. B. Bagihalli, S. Malladi and S. A. Patil, Bio-important antipyrine derived Schiff bases and their transition metal complexes: Synthesis, spectroscopic characterization, antimicrobial, anthelmintic and DNA cleavage investigation, J. Mol. Struct., 2017, 1127, 314–321, DOI:10.1016/j.molstruc.2016.07.123.
- L. C. Garg and C. K. Atal, Indian J. Pharm. Sci., 1963, 59, 240–245 Search PubMed.
- C. T. Prabhakara, S. A. Patil, A. D. Kulkarni, V. H. Naik, M. Manjunatha, S. M. Kinnal and P. S. Badami, Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2 one Schiff base, J. Photochem. Photobiol., B, 2015, 148, 322–332, DOI:10.1016/j.jphotobiol.2015.03.033.
- A. K. Kulkarni, S. A. Patil and P. S. Badami, Synthesis, characterization, DNA cleavage and in vitro antimicrobial studies of La(III), Th(IV) and VO(IV) complexes with Schiff bases of coumarin derivatives, Eur. J. Med. Chem., 2009, 44, 2904–2912, DOI:10.1016/j.ejmech.2008.12.012.
- Q. U. A. Sandhu, M. Pervaiz, A. Majid, U. Younas, Z. Saeed, A. Ashraf, R. R. M. Kham, S. Ullah, F. Ali and S. Jelani, Review: Schiff base metal complexes as anti-inflammatory agents, J. Coord. Chem., 2023, 76, 1094–1118, DOI:10.1080/00958972.2023.2226794.
- T. J. Saritha and P. Metilda, Synthesis, spectroscopic characterization and biological applications of some novel Schiff base transition metal (II) complexes derived from curcumin moiety, J. Saudi Chem. Soc., 2021, 25(6), 101245, DOI:10.1016/j.jscs.2021.101245.
- M. B. Ferrari, S. Capacchi, F. Bisceglie, G. Pelosi and P. Tarasconi, Synthesis and characterization of square planar nickel(II) complexes with p-fluorobenzaldehyde thiosemicarbazone derivatives, Inorg. Chim. Acta, 2001, 312, 81–87, DOI:10.1016/S0020-1693(00)00339-X.
- Y. Mizushima and M. Kobayashi, Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins, J. Pharm. Pharmacol., 1968, 20, 169–173, DOI:10.1111/j.2042-7158.1968.tb09718.x.
- S. S. Sakat, A. R. Juvekar and M. N. Gambhire, Invitro Anti-inflammatory Activities of Methanol Extract of Oxalis Corniculata Linn, Int. J. Pharm. Pharm. Sci., 2010, 2(1), 146–155 Search PubMed.
- U. H. Ramadhan, H. M. Haddad and Z. G. Ezaria, Synthesis of Schiff Bases Complexes as Anti-inflammatory Agents, World J. Pharm. Pharm. Sci., 2016, 5(10), 98–108 CAS.
- R. O. Awolope, I. P. Ejidike and H. S. Clayton, Schiff base metal complexes as a dual antioxidant and antimicrobial agents, J. Appl. Pharm. Sci., 2023, 13(03), 132–140, DOI:10.7324/JAPS.2023.91056.
- W. A. Zoubi, A. A. S. Al-Hamdani and M. Kaseem, Synthesis and antioxidant activities of Schiff bases and their complexes: a review, Appl. Organomet. Chem., 2016, 30(10), 810–817, DOI:10.1002/aoc.3506.
- M. Yadav, S. Sharma and J. Devi, Designing, spectroscopic characterization, biological screening and antioxidant activity of mononuclear transition metal complexes of bidentate Schiff base hydrazones, J. Chem. Sci., 2021, 133, 21, DOI:10.1007/s12039-020-01854-6.
- Y. Chen, M. Wang, R. T. Rosen and C. T. Ho, 2,2-Diphenyl-1-picrylhydrazyl Radical-Scavenging Active Components from Polygonum multiflorum Thunb, J. Agric. Food Chem., 1999, 47, 2226–2228, DOI:10.1021/jf990092f.
- E. Bursal, F. Turkan, K. Buldurun, N. Turan, A. Aras, N. Colak, M. Murahari and M. C. Yergeri, Transition metal complexes of a multidentate Schiff base ligand containing pyridine: synthesis, characterization, enzyme inhibitions, antioxidant properties, and molecular docking studies, BioMetals, 2021, 34, 393–406, DOI:10.1007/s10534-021-00287-z.
- B. Geeta, K. Shravankumar, P. M. Reddy, E. Ravikrishna, M. Sarangapani, K. K. Reddy and V. Ravinder, Binuclear cobalt(II), nickel(II), copper(II) and palladium(II) complexes of a new Schiff-base as ligand: Synthesis, structural characterization, and antibacterial activity, Spectrochim. Acta, Part A, 2010, 77, 911–915, DOI:10.1016/j.saa.2010.08.004.
- A. Abdulmelik, B. Ercan, A. Yusuf, F. Turkan, A. Huseyin and K. Omer, Polyphenolic Content, Antioxidant Potential and Antimicrobial Activity of Satureja boissieri, Iran. J. Chem. Chem. Eng., 2018, 37, 209–219, DOI:10.30492/ijcce.2018.32113.
- S. Chandra and L. K. Gupta, Spectroscopic and biological studies on newly synthesized nickel(II) complexes of semicarbazones and thiosemicarbazones, Spectrochim. Acta, Part A, 2005, 62, 1089–1094, DOI:10.1016/j.saa.2005.04.005.
- N. Raman, J. D. Raja and A. Sakthivel, Synthesis, spectral characterization of Schiff base transition metal complexes: DNA cleavage and antimicrobial activity studies, J. Chem. Sci., 2007, 119(4), 303–310, DOI:10.1007/s12039-007-0041-5.
- T. Y. Fonkui, M. I. Ikhile, D. T. Ndinteh and P. B. Njobeh, Microbial activity of some heterocyclic Schiff bases and metal complexes: A review, Trop. J. Pharm. Res., 2018, 17(12), 2507–2518, DOI:10.4314/tjpr.v17i12.29.
- R. M. Rani and P. Kavitha, Synthesis, characterization and antimicrobial activity of Schiff base ligand and metal complexes, Indian J. Chem., 2024, 63(3), 281–285, DOI:10.56042/ijc.v63i3.6539.
- I. Al-Qadsy, W. S. Saeed, A. A. Al-Owais, A. Semlali, A. Alrabie, L. A. S. Al-Faqeeh, M. ALSaeedy, A. Al-Adhreai, A.-B. Al-Odayni and M. Farooqui, Antimicrobial Activity of Novel Ni(II) and Zn(II) Complexes with (E)-2-(((5-Bromothiazol-2-yl)imino)methyl)phenol Ligand: Synthesis, Characterization and Molecular Docking Studies, Antibiotics, 2023, 12, 1634, DOI:10.3390/antibiotics12111634.
- Mahmood-ul-Hassan, Z. H. Chohan and C. T. Supuran, Antibacterial Zn(II) Compounds of Schiff Bases Derived From Some Benzothiazoles, Main Group Met. Chem., 2002, 25(5), 291–296, DOI:10.1515/MGMC.2002.25.5.291.
- B. A. Al-Hiyari, A. K. Shakya, R. R. Naik and S. Bardaweel, Microwave-Assisted Synthesis of Schiff Bases of Isoniazid and Evaluation of Their Anti-Proliferative and Antibacterial Activities, Molbank, 2021, 2021, M1189, DOI:10.3390/M1189.
- J. Adhikari, A. Bhattarai and N. K. Chaudhary, Synthesis, characterization, physicochemical studies, and antibacterial evaluation of surfactant-based Schiff base transition metal complexes, Chem. Pap., 2022, 76, 2549–2566, DOI:10.1007/s11696-022-02062-x.
- R. C. Maurya, P. Patel and S. Rajput, Synthesis and characterization of Mixed-Ligand Complexes of Cu(II), Ni(II), Co(II), Zn(II), Sm(III), and U(VI)O2, with a Schiff Base Derived from the Sulfa Drug Sulfamerazine and 2,2′-Bipyridine, Synth. React. Inorg. Met.-Org. Chem., 2003, 33, 801–816, DOI:10.1081/SIM-120021647.
- A. P. S. Andrade, L. M. Arantes, J. Y. Kadooca, R. L. Carvalho, A. de. Fatima and A. A. Sabino, Palladium Complexes with Tetradentate Schiff Bases or their Corresponding Amines: Synthesis and Application in Heck Reactions, ChemistrySelect, 2016, 5, 886–890, DOI:10.1002/slct.201600244.
- Y. Hatanaka and T. Hiyama, Cross-Coupling of Organosilanes with Organic Halides Mediated by Palladium Catalyst and Tris(diethy1amino)sulfonium Difluorotrimethylsilicate, J. Org. Chem., 1998, 53, 918–920, DOI:10.1021/jo00239a056.
- S. E. Denmark and C. S. Regens, Palladium-Catalyzed Cross-Coupling Reactions of Organosilanols and Their Salts: Practical Alternatives to Boron- and Tin-Based Methods, Acc. Chem. Res., 2008, 41(11), 1486–1499, DOI:10.1021/ar800037p.
- M. M. Tamizh and R. Karvembu, Synthesis of triethylphosphite complexes of nickel(II) and palladium(II) with tridentate Schiff base ligand for catalytic application in carbon–carbon coupling reactions, Inorg. Chem. Commun., 2012, 25, 30–34, DOI:10.1016/j.inoche.2012.08.016.
- A. Ratnam, M. Bala, R. Kumar, U. P. Singh and K. Ghosh, Design and syntheses of a new family of palladium complexes derived from tridentate ligands and their application as catalysts for Suzuki-Miyaura cross-coupling reactions, J. Organomet. Chem., 2018, 856, 41–49, DOI:10.1016/j.jorganchem.2017.12.017.
- A. Gavryushin, C. Kofink, G. Manolikakes and P. Knochel, Efficient Cross-Coupling of Functionalized Arylzinc Halides Catalyzed by a Nickel Chloride−Diethyl Phosphite System, Org. Lett., 2005, 7(22), 4871–4874, DOI:10.1021/ol051615+.
- A. King, N. Okukado and E. I. Negish, Highly General Stereo-, Regio-, and Chemo-selective Synthesis of Terminal and Internal Conjugated Enynes by the Pd -catalysed Reaction of Alkynylzinc Reagents with Alkenyl Halides, J. Chem. Soc. Chem. Commun., 1977, 683–684, 10.1039/C39770000683.
- S. M. Islam, S. Mondal, A. S. Roy, P. Mondal, M. Mobarak, D. Hossain and P. Pandit, Synthesis and characterization of a polymer-anchored palladium(II) Schiff base complex and its catalytic efficiency in phosphine-free Sonogashira coupling reactions, Transition Met. Chem., 2010, 35, 305–313, DOI:10.1007/s11243-010-9328-3.
- F. Mohajer, M. M. Heravi, V. Zadsirjan and N. Poormohammad, Copper-free Sonogashira cross-coupling reactions: an overview, RSC Adv., 2021, 11, 6885–6925, 10.1039/D0RA10575A.
- R. J. P. Corriu and J. P. Masse, Activation of Grignard Reagents by Transition-metal Complexes. A New and Simple Synthesis of trans-Stilbenes and Polyphenyls, J. Chem. Soc. Chem. Commun., 1972,(3), 144, 10.1039/C3972000144A.
- J. K. Stille, The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524, DOI:10.1002/anie.198605081.
- B. Xu and B. A. Arndtsen, Palladium-Catalyzed Stille-Type Coupling of N-Acyl Iminium Ions with Distannanes: A Multicomponent Synthesis of α-Amidostannanes, ACS Catal., 2014, 4, 843–846, DOI:10.1021/cs401164z.
- K. Hong, M. Sajjadi, J. M. Suh, K. Zhang, M. Nasrollahzadeh, H. W. Jang, R. S. Varma and M. Shokouhimehr, Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions, ACS Appl. Nano Mater., 2020, 3, 2070–2103, DOI:10.1021/acsanm.9b02017?ref=pdf.
- H. Kargar, M. F. Mehrjardi, R. B. Ardakani, K. S. Munawar, M. Bahadori and M. Moghadam, Synthesis, Spectral Characterization, and Theoretical Investigation of Pd(II) Complex Incorporating Unsymmetrical Tetradentate Schiff Base Ligand and its Application in Suzuki Miyaura Cross-Coupling Reaction, Inorg. Chem. Res., 2022, 6, 76–83, DOI:10.22036/icr.2022.337714.1128.
- M. S. S. Adam, Sustainable dipolar homo-dicopper (II) dihydrazone complex as a catalyst for Sonogashira cross couplings, J. Organomet. Chem., 2019, 903, 120985, DOI:10.1016/j.jorganchem.2019.120985.
- V. Kuchtanin, L. Klešcíková, M. Šoral, R. Fischer, Z. Ruzicková, E. Rakovsky, J. Moncol and P. Segla, Nickel(II) Schiff base complexes: Synthesis, characterization and catalytic activity in Kumada–Corriu cross-coupling reactions, Polyhedron, 2016, 117, 90–96, DOI:10.1016/j.poly.2016.05.037.
- M. M. Tamizh, K. Mereiter, K. Kirchner, B. R. Bhat and R. Karvembu, Synthesis, crystal structures and spectral studies of square planar nickel(II) complexes containing an ONS donor Schiff base and triphenylphosphine, Polyhedron, 2009, 28, 2157–2164, DOI:10.1016/j.poly.2009.04.021.
- Y. Kiso, K. Yamamoto, K. Tamao and M. Kumada, Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes, J. Am. Chem. Soc., 1972, 94(12), 4374–4376, DOI:10.1021/ja00767a075.
- G. T. Venkanna, S. Tammineni, H. D. Arman and Z. J. Tonzetich, Synthesis, Characterization, and Catalytic Activity of Nickel(II) Alkyl Complexes Supported by Pyrrole−Diphosphine Ligands, J. Organomet. Chem, 2013, 32, 4656–4663, DOI:10.1021/om400630q.
- M. Lashanizadegan, H. A. Ashari, M. Sarkheil, M. Anafcheh and S. Jahangiry, New Cu(II), Co(II) and Ni(II) azo-Schiff base complexes: Synthesis, characterization, catalytic oxidation of alkenes and DFT study, Polyhedron, 2021, 200, 115148, DOI:10.1016/j.poly.2021.115148.
- R. Malav, R. K. Sharma and S. Ray, Versatile applications of cobalt and copper complexes of biopolymeric Schiff base ligands derived from chitosan, Int. J. Biol. Macromol., 2025, 301, 140338, DOI:10.1016/j.ijbiomac.2025.140338.
- T. Mallat and A. Baiker, Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts, Chem. Rev., 2004, 104, 3037–3058, DOI:10.1021/cr0200116.
- M. H. Ardakania and A. Naeimi, Efficient and Selective Oxidation of Hydrocarbons with tert Butyl Hydroperoxide Catalyzed by Copper(II) Unsymmetrical Schiff Base Complex Immobilized into Mesoporous MCM-41, Inorg. Chem. Res., 2022, 6, 176–181, DOI:10.22036/j10.22036.2023.408510.1148.
- E. Kadwa, H. B. Friedrich and M. D. Bala, Base metal Schiff base complexes applied as catalysts for the oxidation of n-octane, Inorg. Chim. Acta, 2017, 463, 112–117, DOI:10.1016/j.ica.2017.04.032.
- H. Kargar, M. F. Mehrjardi, R. B. Ardakani, K. S. Munawar, M. Ashfaq and M. N. Tahir, Selective oxidation of benzyl alcohols to benzaldehydes catalyzed by dioxomolybdenum Schiff base complex: synthesis, spectral characterization, crystal structure, theoretical and computational studies, Transition Met. Chem., 2021, 46, 437–455, DOI:10.1007/s11243-021-00460-w.
- M. Karman, M. Wera and G. Romanowski, Chiral cis-dioxidomolybdenum(VI) complexes with Schiff bases possessing two alkoxide groups: Synthesis, structure, spectroscopic studies and their catalytic activity in sulfoxidation and epoxidation, Polyhedron, 2020, 187, 114653, DOI:10.1016/j.poly.2020.114653.
- V. Thamilarasana, P. Revathi, A. Praveena, J. Kim, V. Chandramohan and N. Sengottuvelan, Synthesis and characterization of dimeric Schiff base CoII,NiII,CuII complexes for their catalytic application of aerobic oxidation of alcohol and interaction with biomolecules, Inorg. Chim. Acta, 2020, 508, 119626, DOI:10.1016/j.ica.2020.119626.
- W. E. Marsh, K. C. Patel, W. E. Hatfield and D. Hodgson, Magnetic Interactions in Chloro-Bridged Copper(II) Dimers. Structural and Magnetic Characterization of Bis(μ-chloro)bis[chloro(N,N,N’-triethylethylenediamine)copper(II)], [Cu(Et3en)C1]2, Inorg. Chem., 1983, 22, 511–515, DOI:10.1021/IC00145A028.
- A. A. Alshaheri, M. I. M. Tahir, M. B. A. Rahman, T. Begum and T. A. Saleh, Catalytic Oxidation of Cyclohexane Using Transition Metal Complexes of Di thiocarbazate Schiff Base, Chem. Eng. J., 2017, 327, 423–430, DOI:10.1016/j.cej.2017.06.116.
- P. Muniyappan, V. Paranthaman and V. Galmari, Ru(II) complexes containing NOO donors of tridentate Schiff base ligands: Synthesis, characterization, crystal structure and catalytic activity in transfer hydrogenation of ketones, Chem. Inorg. Mat., 2023, 1, 100003, DOI:10.1016/j.cinorg.2023.100003.
- G. D. Yadav and R. K. Mewada, Selective hydrogenation of acetophenone to 1-phenyl ethanol over nanofibrous Ag-OMS-2 catalysts, Catal. Today, 2012, 198, 330–337, DOI:10.1016/j.cattod.2012.06.028.
- N. Turan and K. Buldurun, Synthesis, characterization and antioxidant activity of Schiff base and its metal complexes with Fe(II), Mn(II), Zn(II), and Ru(II) ions: Catalytic activity of ruthenium(II) complex, Eur. J. Chem., 2018, 9(1), 22–29, DOI:10.5155/eurjchem.9.1.22-29.1671.
- T. Bereta, W. Tylus and A. M. Trzeciak, New palladium(II) complexes with ferrocenyl Schiff bases in the hydrogenation of aromatic ketones, Polyhedron, 2022, 225, 116075, DOI:10.1016/j.poly.2022.116075.
- K. Buldurun and M. Özdemir, Ruthenium(II) complexes with pyridine-based Schiff base ligands: Synthesis, structural characterization and catalytic hydrogenation of ketones, J. Mol. Struct., 2020, 127266, DOI:10.1016/j.molstruc.2019.127266.
- C. E. Satheesh, P. N. S. Kumar, P. Raghavendra, R. Karvembu, A. Hosamani and M. Nethaji, Half-sandwich Ru (II) complexes containing (N, O) Schiff base ligands: Catalysts for base-free transfer hydrogenation of ketones, Appl. Organomet. Chem., 2019, 33(10), e5111, DOI:10.1002/aoc.5111.
- C. E. Satheesh, P. R. Kumar, P. Sharma, K. Lingaraju, B. S. Palakshamurthy and H. Rajanaikan, Synthesis, characterization and antimicrobial activity of new paladium and nickel complexes containing Schiff bases, Inorg. Chim. Acta, 2016, 442, 1–9, DOI:10.1016/j.ica.2015.11.017.
| This journal is © The Royal Society of Chemistry 2025 |