Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CO2 responsive materials in oilfield engineering: synthesis, mechanisms, and applications
Qiang Li†
,
Xuanze Zhu†
,
Jiuyi Chen
and
Xionghu Zhao
 *
*
Petroleum College, China University of Petroleum-Beijing at Karamay, Karamay 834000, China
First published on 30th June 2025
Abstract
The increasing demand for oil and gas resources, coupled with growing concerns over the environmental impact of conventional chemical agents, has heightened the need for sustainable alternatives. CO2 responsive materials, which utilize CO2 as an environmentally friendly stimulus, have emerged as promising solutions for improving chemical performance while minimizing environmental impact in petroleum engineering. This review systematically examines the functional groups, response mechanisms, and synthesis strategies of CO2 responsive polymers in oil and gas operations, with particular emphasis on their applications in drilling and reservoir engineering. The review explores the relationship between the reversibility of CO2 responsive materials and their environmental adaptability, focusing on applications in cementing, oil–water separation, gas channeling plugging, viscosity modification, and enhanced oil recovery. By evaluating response mechanisms and environmental adaptability, this work offers valuable insights into the optimization of CO2 responsive materials for practical use in petroleum operations. Additionally, challenges such as response sensitivity and long-term stability are critically explored, and potential solutions and strategies are proposed. The findings aim to support the low-carbon transformation of the oil industry and promote the adoption of sustainable practices in hydrocarbon extraction.
1 Introduction
Energy is the lifeblood of modern society, supporting both global economic growth and social progress.1 Global energy consumption has surged from less than 4 billion tons of oil equivalent in 1965 to nearly 14 billion tons in 2018.2 Fossil energy has consistently dominated the global energy mix, accounting for over 83% of total energy consumption, with oil and gas resources making up more than 50% of this share.3 Studies have shown that human dependence on fossil energy sources will continue to increase over the next 20 years, with demand for oil and natural gas expected to grow at an average annual rate of 0.7% and 1.2%, respectively.4,5 The growth in oil and gas consumption is significantly positively correlated with crude oil extraction, however, the extraction process itself contributes 15–40% of global greenhouse gas emissions, primarily in the form of CO2.6 With the continuous accumulation of greenhouse gases, a series of major problems have arisen, including sea level rising, ocean acidification, and global warming. Climate and environmental issues are related to the common destiny of mankind.7–9 In response to these challenges, CO2 responsive materials have emerged, driven by efforts to improve energy efficiency and promote renewable energy development. These materials offer the potential to transform CO2 from an environmental burden into an engineering advantage and to facilitate the transition to smarter and more sustainable oil and gas development technologies.10,11CO2 responsive materials are a class of smart materials that produce reversible responses to changes in CO2 concentration. These materials achieve this functionality by incorporating CO2 sensitive groups into polymer chains.12–14 CO2 responsive materials utilize CO2 as a stimulating factor, eliminating the need for exogenous chemical additives. They primarily combine multiple materials through protonation/deprotonation mechanisms to achieve variable performance regulation.15,16 In the 2010s, CO2 responsive materials began to be applied to wellbore sealing and fluid viscosity enhancement on a small scale.17,18 As global energy demand rises and conventional shallow fossil resources are gradually depleted, oil exploration is expanding into complex reservoirs, such as low-permeability and ultra-deep formations. Consequently, the application scope of CO2 responsive materials is also broadening.19 The latest applications of CO2 responsive materials in oil and gas fields are mainly concentrated in drilling, cementing, oil production, enhanced oil recovery and oil–water separation. In the cementing stage, CO2 responsive materials can be triggered to repair cement microcracks through a self-repairing response. These materials are also applied in Carbon Capture, Utilization, and Storage (CCUS) technology to seal leaking layer;20–22 during the fracturing stage, the viscosity of the fracturing fluid in the formation can be enhanced by combining CO2 responsive materials with surfactants and foams. Once the proppant migrates to the designated location, the fluid viscosity can be rapidly reduced by injecting N2, thus minimizing reservoir damage.23,24 For emulsions produced during oil and gas extraction, CO2 responsive materials can trigger the hydrophilic/hydrophobic dynamic switching of the emulsions to disrupt the emulsification interface and promote oil droplets aggregation, thus enhancing the efficiency of the oil–water separation.25
Recent research on the application of CO2 responsive materials in petroleum industry has shown significant growth, particularly in drilling and reservoir engineering. Publications from the Google Scholar database are analyzed to assess the current research status of CO2 responsive materials, with the statistical period spanning from 2021 to 2025. A customized query was employed to search for relevant articles, incorporating keywords such as CO2 responsive materials, drilling fluids, fracturing fluids, oil–water separation, plugging profile control, enhanced oil recovery, and CCUS. A total of 65 valid documents were retrieved (Fig. 1). The findings reveal that the primary application areas of CO2 responsive materials in oilfield are enhanced oil recovery, fracturing, and sealing, with increasing research focus on CCUS and oil–water separation in recent years. The main types of CO2 responsive materials that have been utilized include surfactants, gels, foams, and nanoparticles.
The gradual application of environmentally responsive smart materials in the oil field provides new ideas for solving complex formation challenges. In recent years, CO2 responsive materials have become a research hotspot in the field of oilfield smart materials due to their environmental friendliness and controllability. H. Liu et al.13 systematically sorted out the chemical properties of CO2 responsive polymers, conducted in-depth analysis from the response mechanism at the molecular level to the synthesis path, and explored the potential application direction of CO2 responsive gels based on their self-assembly characteristics. Yang et al.26 focused on the application progress of CO2 responsive materials in the separation field, and innovatively proposed the feasibility of their synergistic effect with surfactants in oil–water separation and unconventional oil and gas development, providing an important reference for functional design. Jansen-van Vuuren et al.27 comprehensively summarizes the preparation, properties and applications of CO2 responsive gels. However, existing reviews have yet to fully address the emerging applications of CO2 responsive materials in oil and gas extraction, while their engineering adaptability under complex reservoir conditions remains to be systematically integrated.28 The value and novelty of this review lies in its key differences from existing reviews on this topic: (1) a brief description of the existing CO2 responsive polymer action mechanisms and synthesis methods that are widely used in petroleum engineering; (2) the progress in the application of gels, surfactants, membranes, and nanoparticles related to CO2 response in drilling engineering, reservoir engineering, and produced fluid treatment; (3) a comprehensive categorization and outlook for the last five years of the specific applications of CO2 responsive materials in the field of oil and gas, which provides support for the green transformation of the oil and gas industry.
The purpose of this review is to systematically sort out the development of CO2 responsive materials in oil and gas engineering over the past five years, focusing on the suitability of their response mechanisms for the oilfield environment, and their engineering contributions to extreme reservoirs and low-carbon targets. This paper emphasizes the material systems such as gels, foams, and membranes prepared based on CO2 responsive functional groups, provides an overview of the current status and challenges of their field applications in oilfields. In this work, the first part after the introduction summarizes in detail the common CO2 sensitive groups and synthesis methods for CO2 responsive polymers. The second section reviews the current status of CO2 responsive materials in drilling and reservoir engineering. Finally, the prospects and challenges of CO2 responsive materials in the oil and gas field are discussed. Fig. 2 illustrates the structure of this review.
2 CO2 responsive polymer synthesis
2.1 CO2 responsive groups
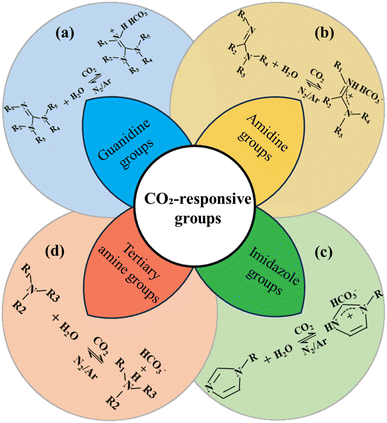
CO2 responsive functional groups play a crucial role in the design of smart polymeric materials, as they facilitate dynamic and reversible interactions with carbon dioxide. These interactions enable precise control over material properties in response to various environmental stimuli, thereby enhancing CO2 sensitivity and intelligent functionality. Traditionally, the specific response mechanism can be described as Brønsted acid-base theory.29 Based on the nature of their CO2 responsive functional groups, CO2 responsive polymers can be classified into four types: guanidine group, amidine group, imidazole group and tertiary amine group (Fig. 3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)–NH2) in the presence of CO2
NH)–NH2) in the presence of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) when CO2 dissolves in water, CO2 hydrates to form H+/HCO3− (CO2 + H2O ⇌ H+ + HCO3−), the guanidinium group protonates to form R–NH–C(
when CO2 dissolves in water, CO2 hydrates to form H+/HCO3− (CO2 + H2O ⇌ H+ + HCO3−), the guanidinium group protonates to form R–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)–NH3+, which drives hydrophilic swelling of the polymer. Conversely, when subjected to N2 purging and heating, the guanidinium group deprotonates to restore hydrophobic contraction (Fig. 3a).30 This mechanism can be applied in petroleum engineering to realize intelligent control of fluid flow.31,32 CO2 injection induces protonation-driven viscosity surge or gelation to block high-permeability channels, redirecting fluids to low-permeability zones; CO2 withdrawal restores low-viscosity states, minimizing reservoir damage.33,34 Reacting cyanamide (NH2CN) with amine-functionalized supports (e.g., aminated silica/carbon) in toluene/xylene at 110–130 °C for 6–12h under N2 is a recommended industrial synthesis method. Catalysts like AlCl3 accelerate imine intermediate formation, yielding surface-grafted guanidine. These materials exhibit superior performance in high- temperature and high salinity reservoirs, however, their pH sensitivity and synthetic complexity require further optimization to broaden their applications.
NH)–NH3+, which drives hydrophilic swelling of the polymer. Conversely, when subjected to N2 purging and heating, the guanidinium group deprotonates to restore hydrophobic contraction (Fig. 3a).30 This mechanism can be applied in petroleum engineering to realize intelligent control of fluid flow.31,32 CO2 injection induces protonation-driven viscosity surge or gelation to block high-permeability channels, redirecting fluids to low-permeability zones; CO2 withdrawal restores low-viscosity states, minimizing reservoir damage.33,34 Reacting cyanamide (NH2CN) with amine-functionalized supports (e.g., aminated silica/carbon) in toluene/xylene at 110–130 °C for 6–12h under N2 is a recommended industrial synthesis method. Catalysts like AlCl3 accelerate imine intermediate formation, yielding surface-grafted guanidine. These materials exhibit superior performance in high- temperature and high salinity reservoirs, however, their pH sensitivity and synthetic complexity require further optimization to broaden their applications.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)–NH2, Fig. 3b), a strongly basic nitrogen-rich functional unit, exhibits highly efficient CO2 responsive behavior due to its planar conjugated structure and reversible protonation. In aqueous media, CO2 hydrates to form H+ and HCO3−. The amidine group forms a protonated cation (–NH–C(
NH)–NH2, Fig. 3b), a strongly basic nitrogen-rich functional unit, exhibits highly efficient CO2 responsive behavior due to its planar conjugated structure and reversible protonation. In aqueous media, CO2 hydrates to form H+ and HCO3−. The amidine group forms a protonated cation (–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)–NH3+) by trapping the H+, which significantly enhances the hydrophilicity and induces the polymer to solubilize. After removal of the CO2 (e.g., via N2 blowing or heating), the deprotonation restores the hydrophobic state, driving material shrinkage or precipitation. The response is efficient and reversible under mild conditions (pH = 7–9, room temperature), with fast kinetics and broad pH adaptability, making the amidine-based polymers ideal for smart materials applications. The CO2 responsive behavior of amidine-based polymers was first reported by Y. Liu et al.35 Subsequent studies have refined their responsive design through atom transfer radical polymerization (ATRP) of functional monomers, such as N-amidinododecylacrylamide.36 However, the synthetic complexity and hydrolysis sensitivity of amidine-based polymers limit their scale-up applications--nitrile substrates (e.g., acrylonitrile copolymers) react with anhydrous HCl in ethanol at 0–5 °C, followed by amine addition at 25 °C for 4 h, where yields amidinium salts hydrolyzed to free amidine using NaOH. In order to improve the stability of amidine polymers, it is necessary to optimize their chemical structure through molecular design, such as incorporating rigid backbones or protective groups, to expand their potential in engineering applications, including CO2 flooding and oil–water separation.37,38
NH)–NH3+) by trapping the H+, which significantly enhances the hydrophilicity and induces the polymer to solubilize. After removal of the CO2 (e.g., via N2 blowing or heating), the deprotonation restores the hydrophobic state, driving material shrinkage or precipitation. The response is efficient and reversible under mild conditions (pH = 7–9, room temperature), with fast kinetics and broad pH adaptability, making the amidine-based polymers ideal for smart materials applications. The CO2 responsive behavior of amidine-based polymers was first reported by Y. Liu et al.35 Subsequent studies have refined their responsive design through atom transfer radical polymerization (ATRP) of functional monomers, such as N-amidinododecylacrylamide.36 However, the synthetic complexity and hydrolysis sensitivity of amidine-based polymers limit their scale-up applications--nitrile substrates (e.g., acrylonitrile copolymers) react with anhydrous HCl in ethanol at 0–5 °C, followed by amine addition at 25 °C for 4 h, where yields amidinium salts hydrolyzed to free amidine using NaOH. In order to improve the stability of amidine polymers, it is necessary to optimize their chemical structure through molecular design, such as incorporating rigid backbones or protective groups, to expand their potential in engineering applications, including CO2 flooding and oil–water separation.37,38In conclusion, tertiary amine group is predominant in the field of intelligent drive modulation, particularly for CO2-triggered viscosity enhancement, gelation plugging and plugging profile control due to its low cost, ease of synthesis and reversible response at room temperature. The guanidine and amidine groups offer distinct advantages in high-salt reservoir plugging and high-temperature micellar viscosification respectively, but are limited by energy consumption and stability. The imidazole group has been expanded for applications in high-temperature CO2 capture and environmental protection treatment through ionic liquid design. Based on the characteristics of these response groups, a variety of highly efficient, environmentally adaptable, and multifunctional CO2 responsive technologies have been developed and applied in engineering. Table 1 presents a comparison of the advantages and limitations of four CO2 responsive groups.
| Group type | Response mechanism | Advantages | Limitations | Primary applications | Ref. |
|---|---|---|---|---|---|
| Guanidine | CO2-triggered protonation enhances hydrophilicity; reversible hydrophobic recovery under high temperature | High thermal and salt tolerance; rapid response | Requires heating for reversal; complex synthesis; hydrolysis risk | High-temperature gas channeling plugging; CO2 selective membranes | 30 and 45 |
| Amidine | Reversible protonation/deprotonation at ambient conditions | Fast kinetics; broad pH compatibility; synergized effects with surfactants | Limited adaptability in alkaline environments; long-term stability | High-temperature micellar viscosity enhancement; smart foam flooding | 35 and 46 |
| Imidazole | Protonation via nitrogen lone pairs enables hydrophilic/hydrophobic switching | Biocompatibility; multifunctional design potential; high-temperature resistance | pH sensitivity; complex synthesis; high cost | High-temperature CO2 capture; ionic liquid-based oil displacement | 39, 40 and 47 |
| Tertiary amine | CO2-induced quaternary ammonium salt formation and dissociation | Low-cost synthesis; ambient reversibility; environmental adaptability | High concentration; performance degradation in complex reservoirs | Plugging profile control; viscoelastic micelle systems; mobility control | 42 and 48 |
2.2 Synthesis method of CO2 responsive polymer
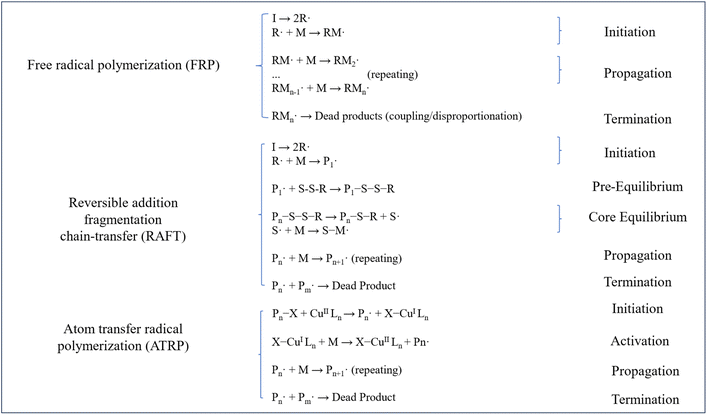
The synthesis of CO2 responsive polymers focuses on incorporating functional groups into polymer structure to achieve reversible interactions with CO2. Several researchers have classified synthesis approaches into two categories: pre-modification and post-modification methods.49,50 Pre-modification methods consist of various polymerization techniques, including free radical polymerization (FRP), reversible addition–fragmentation chain transfer (RAFT), atom transfer radical polymerization (ATRP), and nitroxide-mediated radical polymerization (NMP).13,51 In contrast, post-modification methods primarily rely on post polymerization modification techniques, such as click chemistry and other related reactions to introduce CO2 responsive groups.52 Fig. 4 demonstrates the possible reaction chains in terms of main synthesis methods, illustrating the basic industrially practical synthesis chains and fundamental parts of responsive groups including FRP, RAFT, and ATRP.Fowler et al.54 reported the preparation of CO2 switchable polystyrene and poly methyl methacrylate (PMMA) latexes by FRP using the cationic switchable surfactants, showing the fact that the aggregation behavior of those latexes largely depended on the amount of initiator, surfactant and polymer concentration in the system. Mihara and coworkers reported the example of redispersible CO2 switchable latexes by indicating that addition and removal of CO2 led to redispersion and coagulation of the latexes.55 The imidazole-functionalized initiator on each polymer chain underwent CO2-mediated protonation, thereby enhancing colloidal stabilization of the latex particles. This behavior underscores the potential of CO2 responsive moieties in designing switchable surfactants for emulsion-based applications.12 The CO2 switchable amidine-functionalized latex developed by Zhu et al. demonstrated a sustainable pathway for emulsion recycling in coating industries, eliminating the need for traditional chemical stabilizers.56 They then designed a reactive surfactant that can dynamically control latex morphology, in line with the principles of circular economy and further advancing green polymerization technology. Currently, the primary challenges associated with FRP include the complexity of the process due to the stringent deoxygenation requirements during synthesis and the broad molecular weight distribution of the resulting products.57,58 Nevertheless, owing to its cost-effectiveness, FRP remains an indispensable method for industrial-scale polymer synthesis. Emerging trends emphasize the synergistic integration of FRP with other polymerization techniques to develop more efficient stimulus-responsive synthesis methods.59
RAFT polymerization has been effectively utilized to synthesize CO2 switchable polymers, exemplified by the preparation of dual CO2-and temperature-responsive block copolymers such as poly (diethylaminoethyl methacrylate)-block-poly (N-isopropylacrylamide) (PDEAEMA-b-PNIPAM).61 RAFT polymerization technology is currently advancing toward the development of multi-responsive systems (e.g., dual pH/CO2-responsive polymers) and sustainable practices (e.g., green solvents, enzyme-mediated RAFT)62 to mitigate the instability and potential toxicity associated with the process.63,64 Simultaneously, its integration with machine learning for predicting polymerization kinetics is expected to further enhance the performance of responsive materials.65,66
Huo et al.70 demonstrated a CO2 regulated self-assembly behavior of an amphiphilic terpolymer. The CO2 responsive polymer was integrated with other polymer assemblies through ATRP technology to achieve precise control over the assembly structure.12 Simultaneously, the CO2 responsiveness of the assembly was significantly enhanced. The versatility of CO2 responsive polymers, particularly block copolymers synthesized via ATRP, stems from three inherent advantages of the ATRP methodology (1) its capacity to design complex morphologies (e.g., micelles, vesicles): with precise stimuli-responsiveness;60 (2) compatibility with surface-initiated polymerizations (SI-ATRP) for functional coatings; (3) broad monomer applicability, ranging from hydrophobic styrene to hydrophilic aminoethyl methacrylates.66,71 ATRP is favored for its superior scalability and sustainability, however, its industrial application has lagged due to limitations such as high catalyst cost and slow reaction kinetics.
The synthesis of CO2 responsive polymers is primarily achieved through FRP,RAFT and ATRP. FRP demonstrates industrial viability due to its cost-effectiveness, though it exhibits broad molecular weight distributions. RAFT enables precise structure control for complex functionalities through tailored block sequences, while ATRP excels in constructing sophisticated topological structures despite requiring metal catalysts. Table 2 systematically compares these methods in terms of molecular weight regulation, monomer compatibility, and petroleum engineering applications, providing critical guidance for selecting appropriate technologies across different operational scenarios.
| Content | FRP | Synthetic methods | ATRP |
|---|---|---|---|
| RAFT | |||
| Reaction mechanism | Radical chain reaction; initiation, propagation, and termination via free radicals | Chain transfer to RAFT agent controls polymer growth; reversible transfer between active and dormant chains | Reversible redox equilibrium between active radical and dormant species; transition metal catalyst mediates activation/deactivation |
| Molecular weight control | Poor, broad | Excellent, narrow | Excellent, narrow |
| Reaction conditions | Mild conditions (ambient to 120 °C); requires minimal oxygen exclusion | Similar to FRP but oxygen exclusion is necessary; moderate temperature (60–90 °C) | Metal catalysts; oxygen-sensitive (degassing required); moderate temperature (60–100 °C) |
| Monomer compatibility | Wide range of monomers | Compatible with diverse monomers | Works well with vinyl monomers |
| Cost | Low | Moderate | High, due to expensive catalysts and ligands |
| Reaction time | Fast | Moderate | Moderate to slow |
| Advantages | Simple and cost-effective; suitable for large-scale production | Excellent molecular weight control; ability to synthesize complex structure | Precise control over polymer structure; versatile for various polymer structure |
| Limitation | Poor molecular weight control; limited end-group functionality | RAFT agent residues may affect final properties | High sensitivity to oxygen and requires rigorous deoxygenation |
| Oilfield applications | Viscosity enhancers, high-temp and salt resistance additives | High-temp fracturing fluids, fluid loss control | Smart gels, Nanocarriers |
| Ref. | 72 and 73 | 74, 75 and 76 | 77 and 78 |
3 Application of CO2 responsive materials in drilling engineering
Drilling engineering, serving as a critical link across the entire lifecycle of oil and gas resource development, faces numerous challenges that threaten operational efficiency and reservoir integrity. These challenges include formation fluid erosion, wellbore instability, fracturing fluids performance failure and complex oil–water mixture treatment. CO2 responsive materials, characterized by their environmentally triggered dynamic response mechanisms, provide innovative solutions across multiple fronts. These materials can significantly enhance cementing strength by forming adaptive, self-healing barriers that respond to CO2 exposure, thereby improving long-term wellbore stability. Additionally, they enable intelligent regulation of fracturing fluids by modulating viscosity and fluid behavior in real time under varying CO2 concentrations, optimizing fracture propagation and proppant placement. Furthermore, CO2 responsive materials facilitate efficient oil–water separation by altering interfacial properties, allowing for rapid phase demulsification and improved treatment of produced fluids. These advancements not only address critical challenges in drilling operations but also contribute to sustainable and cost-effective oilfield development.3.1 Cementing
Cementing refers to the process of injecting cement slurry into the wellbore and casing annulus to form a sealing layer, thereby supporting the wellbore structure to prevent collapse and protecting the casing from corrosion. It is a key technology to ensure drilling safety, optimize production capacity, and maintain long-term stable oil and gas production. In cementing operation, CO2 responsive materials facilitate rapid densification and performance optimization of the cement matrix through controlled carbonation reactions. Simultaneously, their responsive properties enable the self-repair of microcracks, thereby enhancing the acid corrosion resistance of conventional cements and extending the wellbore service life (Fig. 5).In an earlier study, cement crack samples were exposed to CO2 saturated water, revealing that CO2 reacted with Ca2+ in the cement matrix to form calcium carbonate, which filled the cracks and formed a dense layer, resulting in the recovery of peak strength compared to untreated samples.79 Subsequently, a cement system resistant to CO2/H2S corrosion was developed by compounding mineral binders with ordinary portland cement.80 CO2 responsive materials have also been applied to wellbore surface coatings, providing adaptive corrosion protection in curing environments and enhancing wellbore corrosion resistance.81 J. Zhang et al.82 significantly improved the corrosion resistance of cement by introducing CO2 responsive microspheres, which undergo molecular chain crosslinking and membrane reconfiguration in the acidic environment of CO2 to form a dense barrier. This barrier effectively blocks the penetration of corrosive media and inhibits the acid-base reactions of cement hydration products, reducing corrosion rates by 70% compared to conventional cement materials. Microcracks often form in cement during the cementing process and are difficult to repair. To address this, Xie et al.73 developed a CO2 responsive hydrogel by FRP with acrylic acid and diethylaminoethyl methacrylate as monomers. This hydrogel can trigger the antipolyelectrolyte effect upon exposure to CO2, enabling it to swell and fill microcracks. Experimental results demonstrated that cement containing 0.3% hydrogel exhibited a 1361% strength growth rate after 56 days of repair. Gong et al.83 added graphene oxide (GO) to cement slurry, triggered the “carbon dot effect” in supercritical CO2 (ScCO2) environment, induced the hydration product Ca(OH)2 to rapidly carbonize into CaCO3, and formed a high-polymerization C–S–H gel. Ultimately, the porosity of cement was reduced by 43%, the compressive strength growth rate increased by 14%, and the microstructure of cement was significantly optimized, improving its impermeability and durability in cementing operations.
3.2 Fracture
Fracturing fluid is the key medium for fracture and sand-carrying in hydraulic fracturing. Its core function is to form a diversion fracture network and support reservoir transformation through high-pressure injection. The current main fracturing fluids for low-permeability reservoirs include natural guar or slick water with low concentrations of polyacrylamide.84 However, conventional fracturing fluids in low permeability reservoirs encounter several challenges, including polymer residue impairing flow conductivity, environmental risks of chemical breakers and instability at high temperature. CO2 responsive viscoelastic fracturing fluids overcome these limitations through CO2-triggered self-assembly of surfactants, forming worm-like micelles and enabling intelligent switching, thereby avoiding the irreversible degradation associated with conventional fluids.23CO2 responsive fracturing technology continues to enhance the system performance through the integration of molecular structure design and environmental triggering mechanisms. Early studies focused on the anionic surfactant system, which constructs a dynamic biomimetic baryonic structure via CO2 protonation, forming a worm-like micellar network, that achieves a viscosity of 25 mPas and a gel-breakage fluid viscosity of 3.2 mPas at 70 °C. However, this system exhibited limited adaptability to high temperatures.85 Sun et al.86 improved the temperature resistance of CO2 responsive fracturing fluid to 120 °C (26.2 mPas) by incorporating amphoteric betaine with amine-based surfactants. Moreover, M. W. Gao et al.87 developed an innovative composite system introducing a temperature-pressure-CO2 triple response mechanism. The self-assembled structure changes dynamically with the environment. It is an elastic gel at room temperature and pressure (Fig. 6a). After the injection of CO2, the protonation is enhanced to form longer and harder worm-like micelles (Fig. 6b). As the temperature rises, the degree of protonation decreases and the micelles shorten (Fig. 6c). The increase in CO2 partial pressure causes CO2 to continue to dissolve and the viscosity to further increase (Fig. 6d). Experiments have shown that the system still maintains an effective viscosity of 30 mPas at 140 °C (Fig. 6e). Moreover, the gel-breaking fluid achieves a remarkable oil displacement efficiency of approximately 40% through spontaneous imbibition mechanisms (Fig. 6f), thus advancing development of synergistic fracturing-oil repulsion technology.
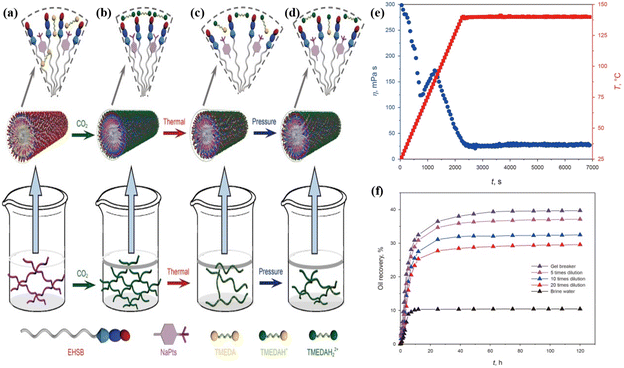 | ||
| Fig. 6 Schematic illustration of self-assembly mechanism of the smart fluid induced by CO2, thermal and pressure. (a) Elastic gel, initial state without CO2 at 25 °C and atmosphere; (b) stronger elastic gel, after CO2-response at 25 °C and atmosphere; (c) worm-like micelle, after CO2-response at 80 °C and atmosphere; (d) stronger worm-like micelle, after CO2-response at 80 °C and 3.0 MPa; (e) apparent viscosity (170 s−1) and temperature as a function of time for the smart fluids with CO2 at 3.5 MPa; (f) oil recovery of spontaneous imbibition experiments for gel breaking fluids and brine water at 80 °C. This figure has been reproduced from ref. 87 permission from Elsevier, copyright (2023). | ||
Current research focuses on achieving precise control of fracturing fluid viscosity through CO2 response mechanisms; however, its sand-carrying capacity under extreme conditions requires further optimization. Surfactant molecules can be induced to self-assemble into worm-like micelles under CO2 stimulation, where the entanglement of micelles forms a transient three-dimensional network that significantly enhances viscosity. Simultaneously, the incorporation of polymers further improves fluid stability and optimizes sand-carrying capacity. Samuel et al.88 developed the S-Gel 38 system, which can maintain a viscosity of more than 100 cP and achieve a proppant suspension time of more than 1 hour at a high temperature of 135 °C by introducing S-Gel 38 polymer. Its 15 gpt system can maintain a viscosity of 70 cP within two hour. The system significantly improves the permeability recovery effect through a controllable gel-breaking mechanism. Field applications have shown that this technology has successfully reduced the amount of acid fracturing fluid used by 50%, while the surface tension of the gel-breaking fluid is stably controlled at 28 mN m−1, showing excellent engineering applicability. Traditional foam fracturing fluids generally have problems such as weak suspended sand-carrying capacity, short foam half-life, and residual gel damage to the reservoir. To address the above problems, Zheng et al.89 developed a recyclable CO2 responsive VES-CO2 foam fracturing fluid system in a supercritical CO2 environment based on CO2 responsive surfactants oleamido propyl dimethylamine (DOAPA) and sodium benzenesulfonate (NaSDS). The study showed that under CO2 stimulation, DOAPA and NaSDS synergistically formed a worm-like micelle network (Fig. 7a), which increased the zero shear viscosity of the foaming fluid from 12 mPas to 2869.69 mPas (Fig. 7b). It also extends the foam drainage half-life to 3720 s, which was significantly better than the traditional system (Fig. 7c). After N2 is injected, it can quickly replace CO2 to achieve rapid gel breaking. The system viscosity can be reversibly switched between 2869 mPas (CO2 stimulation) and 2.2 mPas (gel breaking). After 4 cycles, the performance remained stable, and the core damage rate was only 8.08% (Fig. 7d–f). However, the foam stability of this system in high-temperature reservoirs still faces challenges, and the subsequent focus needs to be on optimizing its temperature adaptability to expand its engineering adaptability under extreme conditions.
 | ||
| Fig. 7 (a) Self-assembled structures of surfactant aggregates in CO2 responsive DOAPA-NaSDS systems; (b) shear-dependent viscosity characteristics of foam formulations under varying flow conditions; (c) salt-induced modifications in foam drainage kinetics; (d) pressure-responsive proppant transport performance of CO2-activated viscoelastic foams; (e) thermal and pressure stability evaluation of nitrogen-containing foams under reservoir conditions (60 °C, 8 MPa); (f) reversible viscosity modulation through gas switching (CO2/N2) in smart surfactant systems. This figure has been reproduced from ref. 89 permission from Elsevier, copyright (2025). | ||
3.3 Oil–water separation
A large amount of oil–water mixtures produced by oil development and production contain oils, heavy metals and toxic organic matter, with complex components and difficult to handle.90 Although traditional physical and biological combined processes can be handled in stages, they still face problems such as the stability of the emulsion system and the residue of chemical demulsifiers. Traditional oil–water separation materials rely on static wettability changes and cannot effectively handle complex emulsion systems. In contrast, CO2 is easy to remove as a gas and has no residue, which meets the needs of green separation, and it can be reversibly acid-base responsive when dissolved in water.91 CO2 responsive materials can undergo reversible changes in wettability or charge state upon exposure to CO2, enabling intelligent control of separation behavior.CO2 responsive membrane materials achieve self-cleaning and oil phase desorption by CO2-triggered dynamic reversal of surface wettability. When CO2 is injected, a chemical reaction occurs on the membrane surface, altering its wettability from lipophilic to hydrophilic and enabling efficient selective passage of the aqueous phase. Upon switching to N2 injection, CO2 is physically expelled, causing the membrane surface to revert to a hydrophobic state and preferentially permeate the oil phase (Fig. 8). Qi et al.92 developed a CO2 responsive nanofiber membrane polyacrylonitrile-co-poly(diethylaminoethyl methacrylate (PAN-co-PDEAEMA)) based on electrostatic spinning technology. Under normal conditions, the tertiary amine groups in the DEAEMA chain segments on the membrane surface were not protonated and showed hydrophobicity. However, upon prolonged exposure to CO2, the tertiary amine groups on the membrane undergo protonation, transforming the membrane into a superhydrophilic state. This switchability of the nanofibrous membrane stems from the interaction of CO2-induced protonation and hierarchical nanostructures: the PDEAEMA chains extend and increase the surface roughness Ra from 2.99 to 6.48 nm, enabling water permeation while retaining oil due to the hydrophilic/oleophobic properties. Crucially, deprotonation restored the original hydrophobic state of the membrane after 30 min of N2 treatment, completely reversing the separation process and confirming the membrane's reversible O/W switching ability. Inspired by the capillary force in nature, Y. Wang et al.93 fabricated CO2-responsive membranes by the capillary force self-assembly (CFCS) method, which utilizes CO2 to trigger the hydrophilic–hydrophobic switching property of polymers. The scalable preparation mechanism relies on manipulating capillary force to drive the homogeneous adhesion of poly(diethylaminoethyl methacrylate-co-methyl methacrylate (PMMA-co-PDEAEMA) copolymers onto polyester fabric within a 150 μm gap, enabling large-area membrane production up to 3600 cm2. This process ensures uniform distribution of CO2 responsive tertiary amine groups, as validated by SEM showing consistent surface roughness and EDX mapping confirming homogeneous N element distribution. The membranes achieved more than 99.3% separation of simulated multiphase emulsions such as n-butane, silicone oil, and toluene. Moreover, they demonstrated excellent self-cleaning efficiency of up to 99.5% for all emulsion systems through CO2/N2 switching and maintained stable performance after 20 reuse cycles, offering a novel approach for the large-scale production of stimuli-responsive membranes.
Conventional CO2 responsive membrane materials often face the challenges such as slow deprotonation and high energy consumption when processing complex double emulsions (O/W/O or W/O/W), making it difficult to achieve efficient separation. To address these limitations, H. Liu et al.94 used a two-step coating method to prepare fibers with a primary photothermal responsive coating and a secondary CO2 responsive coating, and converted the fibers into dual CO2/photothermal responsive films by industrial means. CO2 stimulation protonates polymethyl methacrylate (PMMA)-co-poly (2-(diethylamino)) ethyl methacrylate (PDEAEMA), rendering the membrane surface superhydrophilic and allowing the permeation of the aqueous phase. Moreover, near-infrared (NIR) light was able to trigger the photothermal effect of graphene oxide (GO), locally heating the membrane to 140 °C and inducing rapid deprotonation of PDEAEMA to restore lipophilicity within 1 min, allowing the oil phase to pass through efficiently. Compared to traditional CO2 membranes relying on nitrogen purge or overall heating, this material reduces the deprotonation time by 95% and achieves precise separation of double emulsions with an efficiency exceeding 99.6%. Similarly, D. Yan et al.95 achieved continuous separation of complex ternary mixtures of heavy oil, water, and light oil by leveraging the protonation–deprotonation transition of PDEAEMA, providing valuable insights for the development of novel membranes with switchable wettability. Despite these advancements, the continuous separation of multicomponent mixtures remains a significant challenge, necessitating breakthroughs in the integration of multiple response mechanisms.
In summary, CO2 responsive materials achieve environmentally adaptive functionality through precise molecular design, enabling intelligent responsiveness to CO2 stimuli. These materials have been widely applied in various aspects of oil and gas operations, including cementing, fracturing, and oil–water separation. In cementing, they enhance wellbore integrity by forming self-healing barriers in response to CO2 exposure. In fracturing, they enable real-time viscosity regulation, improving fluid efficiency and fracture control. Additionally, in oil–water separation, they facilitate efficient phase demulsification, optimizing produced fluid treatment. The main chemical compositions of these materials, along with their key application are detailed in Table 3.
| Application | Key chemicals | Temperature tolerance | Beneficial effects | Ref. |
|---|---|---|---|---|
| Cement slurry | Self-synthesized new materials environment responsive microsphere (ERPM) | — | Corrosion depth reduced by 70% and compressive strength reduction by <12% | 82 |
| Polypropylene calcium salt-dimethylaminoethyl methacrylate hydrogel (Ca-PAD) | — | The self-repair strength reached 1361% in 56 days, and the volume repair rate increased to 61.7% in 14 days | 73 | |
| Graphene oxide (GO) | — | The compressive strength growth rate reaches 2.9 Mpa per day, the porosity decreases by 43% | 83 | |
| Fracturing fluid | Sodium dodecyl sulfate (SDS), 2,6,10-trimethyl-2,6,10-triazaundecane (TMTAD) | 70 °C | The viscosity at 70 °C is 25 mPas, the viscosity after breaking is 3.2 mPas, and the clay anti-swelling rate is 91.3% | 85 |
| Erucic acid amide hydroxypropyl sulfobetaine (EAHSB), erucic acid amide propyl dimethylamine (EKO) | 120 °C | The viscosity is 26.2 mPas at 120 °C, and the core damage rate is only 7.48% | 86 | |
| N-Erucylamidopropyl-N,N-dimethyl-3-ammonio-2-hydroxy-1-propane-sulfonate (EHSB), N,N,N′,N′-tetramethyl-1,3-propanediamine (TMEDA) | 140 °C | The viscosity at 140 °C is 30 mPas, the permeability damage rate is 3.33%, and the oil displacement efficiency of the gel breaking fluid is 40% | 87 | |
| A cationic micropolymer S-gel 38 | 135 °C | Maintains viscosity above 100 mPas at 135 °C, proppant suspension time over 1 hour | 88 | |
| Oleylamide propyl dimethylamine (DOAPA), sodium benzenesulfonate (NaSbS) | 60 °C | The proppant settling velocities in CO2 were 2.34 cm min−1, the fracturing fluid performance did not change after 4 cycles of CO2/N2 | 89 | |
| Oil–water separation membrane | Poly(diethylaminoethyl methacrylate) (PDEAEMA) | — | Separation efficiency > 99%, performance is stable in pH = 2–12, and salt resistance reaches 10% | 92 |
| Poly(diethylaminoethyl methacrylate-co-methyl methacrylate) (PMMA-co-PDEAEMA) | — | The CFCS method is used for synthesis, which is conducive to large-scale production, and the self-cleaning rate >99.5% | 93 | |
| Graphene oxide (GO), poly(diethylaminoethyl methacrylate) (PDEAEMA) | — | Effectively separate double emulsions, separation efficiency > 99.6% | 94 | |
| Poly(vinyltrimethoxysilane)-co-poly(N,N-dimethylaminoethyl methacrylate) (PVTMS-co-PDMAEMA) | — | The continuous separation efficiency of heavy oil–water-light oil mixture reached 99.9%, exhibits stable performance after repeated use | 95 |
4 Application of CO2 responsive materials in reservoir engineering
The primary challenge in reservoir engineering is to precisely regulate the complex subsurface seepage flow to achieve efficient and sustainable resource exploitation. However, the inherent heterogeneity of reservoirs, the difficulty in controlling gas flow, and the persistent risk of sealing leakage have long constrained the effectiveness and applicability of conventional materials. These limitations not only reduce recovery efficiency but also increase operational risks and costs. In this context, CO2 responsive materials, with their environmentally triggered adaptive properties, offer a transformative solution. By dynamically responding to changes in reservoir conditions, these materials can effectively seal gas channeling, minimize leakage risks, and enhance oil recovery. Moreover, they play a pivotal role in advancing CCUS technologies, which have gained increasing prominence in recent years as a critical strategy for mitigating carbon emissions. Through their intelligent adaptability and multifunctional capabilities, CO2 responsive materials can help increase oil and gas production while reducing the negative environmental impacts of hydrocarbon extraction.4.1 Gas channeling plugging
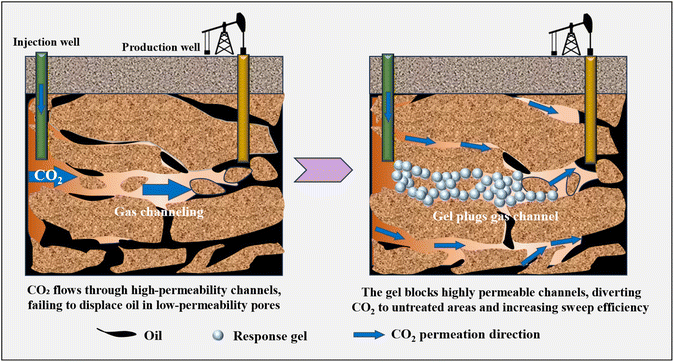
As global demand for crude oil continues to increase, the exploitation of low- permeability hydrocarbon resources has become increasingly crucial.96 Although CO2 flooding technology can significantly enhance oil recovery by reducing viscosity and increasingly solubility, it tends to preferentially flow through high-permeability channels in heterogeneous reservoirs, leading to instability and reduced efficiency at the displacement front.97 Technologies such as water-alternating-gas (WAG) injection and foam/gel sealing have been employed to regulate CO2 flow. However, traditional gel materials often exhibit poor injectability and insufficient long-term blocking performance under extreme conditions. In recent years, intelligent responsive materials, such as CO2 responsive gels and foams, have been continuously optimized to achieve dynamic and adaptive gas plugging by sensing the reservoir's CO2 environment, thereby triggering phase transitions and enhancing performance.98–101 Fig. 9 compares the performance of conventional gels and CO2 responsive gels in plugging gas channeling, focusing on plugging capacity, injectability and cost-effectiveness. Particle gel has good injectability due to its small particle size and good fluidity, but its plugging ability is weak. Foam gel has less damage to the reservoir, but it also has the problem of insufficient plugging ability. CO2 responsive gel has strong plugging ability and low damage to the reservoir due to its intelligent response characteristics. However, its production process still needs to be further optimized to reduce the cost.To address the gas channeling problem caused by fractures during CO2 flooding in ultra-low-permeability reservoirs, Du et al.102 reported a coupled system of CO2 responsive gel particles (CRPGP) and worm-like micelles (CTWM). After exposure to CO2, CTWM transformed from a spherical to a worm-like structure and formed a dense network with CRPGP through hydrophobic interactions, which increased the viscosity by 225 times and the plugging efficiency by 99.2%. In order to solve the challenge of poor injectability during the gel plugging process, Gu et al.103 synthesized a CO2 responsive microgel based on chitosan. After exposure to CO2, the gel particle size of this material can shrink rapidly, significantly improving the injection performance, and its flow properties can be adjusted by injecting N2. Based on the traditional gel swelling-bridging plugging mechanism, M. L. Shao& Liu104 developed a core–shell structured CO2 responsive nanoparticle blocking agent, whose particle size can expand from 96 nm to 221 nm, effectively plugging high permeability channels. The rigid styrene component in the plugging agent limits its excessive expansion, thereby preventing plugging failure caused by shear.
Traditional foams have also been reported to be used to plug gas channeling due to their good injectability, but there are challenges such as poor foam stability and low plugging strength. Q. Gao et al.105 synthesized a CO2 responsive foam (CRF) using sodium lauryl ether sulfate (LES) and diethylenetriamine (DETA), which modulates the solution viscosity through CO2/N2 stimulation, achieving a balance between low injection pressure and high plugging performance. Experimental results demonstrated that the half-life of CRF was 13 times longer than that of conventional CO2 foam and exhibited a stronger resistance factor in high-permeability cores. X. Huang et al.106 designed a CO2 responsive polymer PAD-H by introducing a hydrophobic structure containing polyether chains. The tertiary amine groups in PAD-H are protonated in CO2, generating electrostatic repulsion and forming a three-dimensional network structure through hydrophobic association. This network structure significantly enhances the strength of the gel, increasing the plugging success rate to over 95% (Fig. 10). Although foam gels and polymer gels exhibit certain advantages, their effectiveness still requires validation through large-scale field applications.107
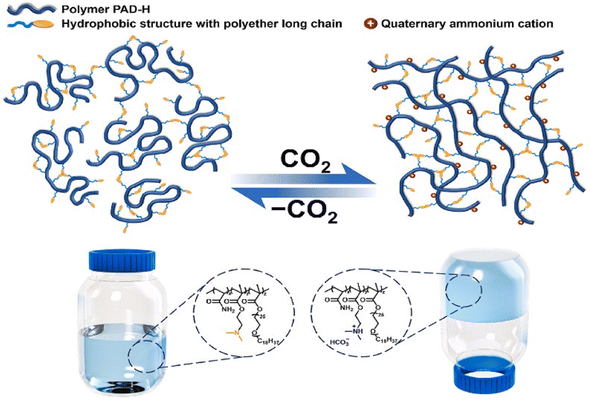 | ||
| Fig. 10 Schematic diagram of CO2 responsive viscosity-increasing mechanism of PAD-H. This figure has been reproduced from ref. 106 permission from Elsevier, copyright (2024). | ||
4.2 Enhanced oil recovery
CO2 responsive materials can dynamically adjust fluid viscosity based on CO2 concentration, inhibiting the flow of plugging gels in high-permeability formations and thereby enhancing the sweep efficiency of displacement media.34,108 Additionally, CO2 responsive materials can also act as surfactants to continuously reduce oil–water interfacial tension, and promote the stripping of residual oil from the formation109,110 Fractured reservoirs generally have the problem of low CO2 displacement efficiency. CO2 responsive gel can achieve the synergistic effect of “plugging high permeability layers and displacing low permeability layers”. During the injection state, low-viscosity fluids enter high-permeability fractured formation, where the smart materials in the fluid respond upon contact with CO2, increasing viscosity and gradually exhibiting viscoelastic gel properties. This transformation effectively blocks CO2 gas channels in fractures and pore channels. Since the gas channel is sealed, CO2 can easily penetrate and diffuse in the low-permeability reservoir, improving the sweep efficiency of CO2 and ultimately enhancing the oil and gas recovery (Fig. 11).Recent studies have shown that surfactant-based CO2 responsive gel systems have shown significant potential in improving oil recovery. A reversible system was constructed based on the long-chain tertiary amine surfactant N,N-dimethyl erucamide tertiary-amine(DMETA), and the viscosity was reversibly switched through the self-assembly of worm-like micelles (WLMs) triggered by CO2, ultimately enhancing the oil recovery rate by 21.7%.33 Xin et al.111 developed a CO2 responsive gel system using long-chain alkylamidopropyl dimethyl tertiary amine, which expands the sweep efficiency by uniformly displacing the front edge and reduced the viscosity of crude oil, increasing the oil recovery by 23.92%. Similarly, Fang et al.112 further designed an irreversible hydrogel based on a long-chain tertiary amine surfactant (HXB-2), which formed a three-dimensional worm-like cross-linked network (Fig. 12a and b) through carboxyl protonation and electrostatic adsorption of bicarbonate. The viscosity of the 0.5wt% solution after CO2 triggering reached 1117 mPas (Fig. 12c), and showed elastic response (Fig. 12d). The viscosity could still remained 4 times the initial value at high temperature (Fig. 12e), and the viscosity was only partially restored after N2 treatment (Fig. 12f), proving its irreversibility. Core experiments show that during alternating water and gas injection, the displacement pressure increased from 0.416 MPa to 2.423 MPa, the maximum seepage resistance reached 29.45 MPa min cm−3, and enhance oil recovery by 24.6% (Fig. 12g and h). After secondary CO2 flooding, the oil recovery increased to 89.23%, and the plugging rate reached 94.1%. Surfactant-based CO2 responsive materials have the advantages of being mild, safe and economical. In the future, their large-scale production and on-site application can be promoted by further optimizing the synthesis process.
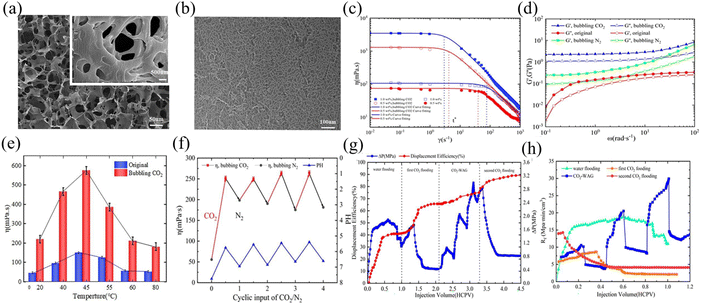 | ||
| Fig. 12 (a) SEM image of hydrogel following CO2 induction; (b) TEM image of hydrogel following CO2 induction; (c) the viscosity-shear rate relationship of samples at 25 °C with CO2 bubbling or non-bubbling. The dotted line represents the position of the critical shear rate; (d) the dynamic moduli of 0.5 wt% samples before and after CO2 uptake on the angular frequency at 25 °C; (e) CO2 switchable thickening performance during the CO2/N2 bubbling cycle at 25 °C (concentration: 0.5 wt%); (f) the apparent viscosity of 0.5 wt% sample with CO2 and without CO2 at different temperatures (g) relationship between oil recovery and ΔP with HCPV; (h) relationship between seepage resistance and HCPV under different injection methods. This figure has been reproduced from ref. 112 permission from Elsevier, copyright (2025). | ||
The previous paragraph has introduced the application of CO2 responsive materials in chemical flooding such as surfactant flooding and polymer flooding to enhance oil recovery (EOR). In fact, common EOR methods also include thermal flooding, other gas flooding and so on. In recent years, these technologies have shown remarkable results in improving oil recovery through innovative combination with CO2 responsive materials.113 Tian et al.114 used acrylamide (AAm), N,N-dimethylaminoethyl acrylamide (DMAEMA) and [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (SBMA) as raw materials, thermal and CO2 dual-responsive smart polymer microgels (SPMs) were synthesized by solution copolymerization and cross-linking technology. When the temperature is higher than 65 °C, the SBMA unit swells due to the thermal induction of the SBMA unit to destroy the intramolecular electrostatic effect; when encountering CO2, the tertiary amine group of the DMAEMA unit is protonated to produce electrostatic repulsion, causing the microgel to swell secondary. This technology has firstly promoted the application of CO2 responsive materials in thermal flooding. In the future, in scenarios such as steam flooding, the synergistic effect of temperature sensitivity and CO2 response can be further used to complete the plugging of high-permeability channels and dynamic profile adjustment, thereby improving the recovery rate.115 Additionally, gases like N2 and CH4 can potentially combine with CO2 responsive materials to enhance oil recovery. For instance, N2 can be integrated with CO2 responsive foams to regulate foam stability via gas switching in water-alternating-gas injection, enabling plugging of high-permeability channels. In CH4 miscible flooding, CO2 responsive materials can adjust their swelling degree according to gas composition changes, optimizing fluid mobility control.116,117 Overall, CO2 responsive materials show enormous potential in EOR. Future research should focus on integrating multiple EOR methods.
4.3 CO2 geological storage
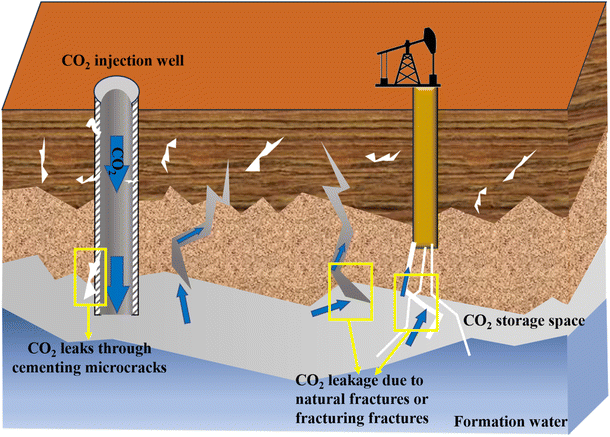
Greenhouse gas emissions from industrial activities, including oil and gas exploration and development, have intensified global warming and triggered ecological disasters such as glacier melting and sea level rise, prompting countries to accelerate their pursuit of carbon neutrality. As a key technology to achieve this goal, carbon sequestration can achieve long-term storage by injecting CO2 into deep underground rock formations, including abandoned oil and gas reservoirs, saline layers, and coal seam.10,118 However, natural cracks in the formation or defects in the wellbore may cause CO2 leakage, posing environmental and safety risks (Fig. 13). Although traditional leak prevention methods such as cementing or artificial barriers are widely used, they face problems which involve material corrosion and delayed repair.31,119 CO2 responsive materials are born out of demand. When CO2 leakage alters local pH or ion concentrations, CO2 responsive materials can rapidly trigger swelling, solidification or mineralization reactions, autonomously forming a dense barrier within the leakage pathway. Additionally, it can synergistically grow with the surrounding rock formations, providing a smarter and more reliable technical means for carbon sequestration.120Currently, the CO2 responsive materials sealing mechanism for porous or fractured media primarily involves two processes: (1) the gelation reaction, which forms a physical barrier to block fluid flow; (2) the introduction of a CO2 sensitive particle suspension system that undergoes a phase transition upon CO2 exposure, gradually forming a dense stacking structure that seals the pore network.121 H. Wu et al.122 achieved dynamic sealing by combining acrylamide (AM) and N-(3-(dimethylamino)propyl) methylacrylamide (DMAPMA) as monomers and polyethylene amide (PEI) as a cross-linking agent to form a three-dimensional porous network. CO2 triggers the protonation of tertiary amines, inducing a sol–gel phase transition, which increases the subsequent water injection plugging efficiency to 96.2%. Methyl methacrylate-based gel is another typical CO2 responsive plugging material. It can expand 20 times in volume at low pH values and maintain structural stability, especially at high temperature and high pressure.123 In addition, the CO2 responsive polymer microspheres can dissolve or cross-link when exposed to CO2, enhancing the plugging ability through a dual mechanism of physical plugging and chemical bonding.124 Y. Zhao. et al.110 used a two-step method to synthesize a CO2 responsive dual-network gel system, in which 2-acrylamido-2-methylpropane sulfonic acid (AMPS) formed the first layer of rigid inner network, and acrylamide (AM) and polyethyleneimine (PEI) synthesized the second layer of flexible outer network. This dual-network structure significantly improved the mechanical ability and resistance to CO2 flushing of the gel, and improved the problems of poor injectability and plugging performance of traditional gels, thereby improving the success rate of carbon sequestration. S. Chen et al.125 experimentally compared the sealing performance of three individual systems (polymer gel, CO2 responsive foam, and CO2 responsive thickened polymer), as well as their combinations. It was found that the polymer gel demonstrated the highest plugging efficiency with 86.13%, but its injection performance was relatively poor. In contrast, the CO2 responsive thickened polymer exhibited the lowest plugging performance at 23.7%, but when combined with the foam, this hybrid system could plug CO2 gas channeling with an efficiency of 95%. Currently, CO2 responsive sealing materials require further improvements in acid resistance, long-term stability and environmental friendliness.126 Future research should focus on the development of smart gels and the investigation of synergistic effects of relevant additives on gel performance and plugging efficiency, with the goal of enhancing the mechanical strength and corrosion resistance of the gel, thereby broadening its range of applications.
In summary, CO2 responsive materials can intelligently adjust fluid viscosity, wettability, and plugging strength based on CO2 concentration in the reservoir. These materials have been widely applied in gas channeling plugging, enhance oil recovery, and CCUS, providing precise, long-lasting, and stable solutions for the development of complex oil and gas reservoirs and carbon sequestration. The specific response mechanisms and main results of CO2 responsive materials in reservoir engineering applications are summarized in Table 4.
| Application | Key chemicals | Mechanism | Beneficial effects | Ref. |
|---|---|---|---|---|
| Plug gas channeling | CO2 responsive gel particles (CRPGP),CO2 responsive wormlike micelles (CTWM) | CO2 triggers the hydrophobicity of the micellar gel system | The viscosity of the micelle system increased by 225 times, and the plugging efficiency reached 99.2% | 102 |
| Chitosan (CS), N-(3-(dimethylamino)propyl) methylacrylamide (DMAPMA) | CO2-induced protonation of chitosan amino groups and reconstruction of the hydrophobic network | The sol/gel state of the system can be reversibly switched by injecting N2/CO2 | 103 | |
| Styrene (st), dimethylaminoethyl methacrylate (DMAEMA), acrylamide (AM) | Nanoparticles enhance plugging strength and stability | The system performance can remain stable under high temperature and high salt conditions | 104 | |
| Lauryl ether sulfate sodium (LES), diethylenetriamine (DETA) | CO2 triggers the formation of worm-like micelles and dynamically regulates viscosity to block gas channeling | CO2 responsive foam exhibits a half-life 13 times longer than that of conventional foams | 105 | |
| N-(2-(methylpropenoxy) ethyl)-N,N-dimethyloctadecane ammonium bromide(Hb) | CO2-triggered protonation of tertiary amines synergizes viscosity enhancement | The system viscosity is increased by 360 times, and the plugging efficiency is >95% | 106 | |
| Enhanced oil recovery | N,N-dimethyl octylamide-propyl tertiary amine (DOAPA),sodium p-toluene sulfonate (SPTS) | CO2-triggered protonation reaction caused the spherical micelles to transform into worm-like micelles to form highly viscoelastic gels | Mixing 4.4 wt% DOAPA and 2.0 wt% SPTS can enhance oil recovery by 20% | 34 |
| N,N-dimethyl erucamide tertiary-amine (DMETA) | CO2 induces protonation of the solution to form worm-like micelles and reduce crude oil viscosity and interfacial tension | Viscosity can be switched reversibly, and the oil recovery is enhanced by 21.7% | 33 | |
| Long-chain alkyl acid amidopropyl dimethyl tertiary amine | CO2 triggers worm micelle network to plug fractures | The oil recovery of low permeability core enhanced from 39.78% to 63.7% | 111 | |
| Z-2-(3-(docos-13-amido)propyl) dimethylammonium) propanoate (HXB-2) | CO2 induces surfactant protonation to form micelles to improve sweep efficiency | The viscosity increased by 4.53 times after CO2 bubbling, and the oil recovery enhanced by 23.53% | 112 | |
| Carbon sequestration | Acrylamide (AM) and N-[3-(dimethylamino) propyl] methacrylamide (DMAPMA) | Protonation for sol–gel phase transition | The gel tensile strength reaches 0.65 N and the bonding force reaches 4264 Pa | 122 |
| Methyl methacrylate gels | Protonation and solvation reactions of gel molecular chains | Stable structure under high temperature and pressure | 123 | |
| Crylamido-2-methylpropane sulfonic acid (AMPS), acrylamide (AM),polyethyleneimine (PEI) | CO2 responsive expansion to seal cracks | The double network structure improves the gel's resistance to CO2 flushing and mechanical strength | 110 | |
| Chromium stabilizer, sulfate foaming agent, foam stabilizer | Synergistic blocking of CO2 responsive foam, gel and polymer | The CO2 responsive thickening polymer and foam synergistic plugging system has the best CO2 storage effect | 125 |
5 Prospects and challenges
CO2 responsive materials have demonstrated significant potential in hydrocarbon extraction due to their environmentally friendly nature and reversible regulation capabilities. Unlike traditional materials that rely on chemical additives, CO2 serves as a natural triggering medium, activated by in situ concentration or external gas injection, effectively avoiding chemical residue pollution and reducing operational costs. Current advancements have highlighted their application across multiple scenarios in the field of petroleum engineering. For cementing engineering, these materials enhance corrosion resistance and prolong wellbore integrity through dynamic carbonation control of cement matrices. In reservoir stimulation, they optimize proppant transport and placement by intelligently modulating the viscosity of fracturing fluids in response to CO2 exposure. Additionally, in produced fluid treatment, they facilitate efficient oil–water separation by altering interfacial wettability, enhancing phase demulsification and improving treatment efficiency.A particularly promising application in the use of CO2 responsive gels, which can precisely plug fractures and pores due to their good injectability and plugging strength. CO2 response gels can improve the sweep efficiency of CO2 flooding in low-permeability reservoirs by plugging gas channels in high-permeability formations, ultimately achieving the goal of enhancing oil recovery. However, their full potential remains underexplored, particularly in addressing complex reservoir heterogeneities. Moreover, current research predominantly focuses on plugging and displacement functions, with limited exploration of their broader role in carbon capture and utilization. As the global energy industry accelerates its transition under the “Dual Carbon” strategy, the development of next-generation intelligent material systems that seamlessly integrate reservoir adaptability with carbon sequestration capabilities will be essential for achieving both enhanced recovery and sustainable resource management.
Despite their promising prospects, the practical application of CO2 responsive materials remains to face multiple challenges across three dimensions in oil and gas operations (Fig. 14). Technically, their responsive behavior is constrained by narrow environmental pH ranges, leading to performance degradation in acidic gas reservoirs or alkaline waterflooding zones. Most practiced systems require high CO2 concentrations for activation, limiting sensitivity in low-concentration environments like depleted reservoirs. The complex synthesis processes and poor high-salinity tolerance further restrict field applicability. Economically, industrial-scale production incurs high costs (e.g., specialized monomers for guanidine groups), while balancing CO2 diffusion efficiency with long-term material stability elevates operational maintenance pressures. Environmentally, potential CO2 leakage from inadequate plugging and limited adaptability to high-salinity formations pose ecological challenges.
 | ||
| Fig. 14 Challenges and opportunities of CO2 responsive materials in oilfield extraction: a tripartite analysis of technical, economic, and environmental dimensions. | ||
To address these challenges, future research should focus on three complementary dimensions. In the technical dimension, multi-stimulus systems that combine CO2 responsiveness with light, magnetic fields, or temperature to help materials adapt to different reservoir conditions are to be developed. Simultaneously, innovating microgel structures with precise pore and surface designs to improve CO2 diffusion and material stability is pressing. In the environmental dimension, using eco-friendly designs to reduce ecological impact and combining carbon sequestration with leakage prevention through CO2 mineralization to boost carbon fixation efficiency are necessary. In the economic dimension, generating income from carbon trading via CCUS-certified applications is of great importance, thus lowering costs with scalable polymerization techniques to build a sustainable model is also required at the same time. By aligning material innovation with engineering practices, CO2 responsive materials can provide sustainable solutions for the green development of complex hydrocarbon reservoirs. These advancements will enable the petroleum industry to dynamically balance the dual objectives of enhancing oil recovery and achieving carbon neutrality, paving the way for a more sustainable and efficient future in hydrocarbon resource utilization.
6 Conclusions
This review underscores the promising potential of CO2 responsive materials in oil and gas engineering, highlighting key advancements in the past five years. It examines the compatibility of material response mechanisms with reservoir environments, and emphasizing their engineering value under extreme conditions and low-carbon objectives.(1) CO2 responsive materials utilize functional groups such as guanidine, amidine, imidazole, and tertiary amine to undergo reversible protonation reactions with CO2, inducing molecular conformational changes that modulate physicochemical properties like viscosity and wettability. Additionally, FRP, featured as low cost, compatibility with large-scale processes but uncontrolled architecture, batch-to-batch variability, and thermal instability; RAFT, featured as its tolerance to functional groups/protic media, ideal for stimuli-responsive polymers but purification challenges due to sulfur residues; ATRP, featured as high-fidelity surface grafting but metal contamination, oxygen sensitivity, and costly catalyst removal enable the responsiveness of polymers precisely tailored, granting these materials environmentally adaptive properties.
(2) CO2 responsive materials enable intelligent control of key processes in oil and gas production by triggering phase transitions and structural reorganization upon CO2 exposure. They are widely applied in drilling and reservoir engineering, significantly enhancing wellbore stability during drilling operations and improving oil recovery. Adaptive plugging systems increase sweep efficiency and mitigate gas channeling, while dynamic wettability inversion effectively addresses complex emulsification challenges in oil–water separation.
(3) Despite their revolutionary potential, challenges remain in the practical deployment of CO2 responsive materials, including stability under extreme conditions, limited response ranges, and issues with scalability and process compatibility. Future research should focus on optimizing synthesis methods for high-temperature, high-pressure-resistant polymers to facilitate the large-scale adoption of CO2 responsive materials in intelligent drilling and CCUS.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Qiang Li: writing (original draft), investigation, visualization; Xuanze Zhu: writing (original draft), investigation, visualization; Jiuyi Chen: writing (original draft); Xionghu Zhao: writing (review& editing), project administration, supervision, validation.Conflicts of interest
There is no conflict to declare.Acknowledgements
This work was supported by the Research Foundation of China University of Petroleum-Beijing at Karamay (No. XQZX20250032).References
- Q. Li, D.-Y. Zhu, G.-Z. Zhuang and X.-L. Li, Pet. Sci., 2025, 22(5), 1977–1996 CrossRef CAS
.
- J.-P. Rodrigue, The Geography of Transport Systems, Routledge, 2020 Search PubMed
.
- S. A. Alnuaim, Energy Transition or Energy Advancement, SPE Annual Technical Conference and Exhibition, 2023 Search PubMed
.
- Q. Li, Doctoral Thesis, Sorbonne Université, 2023 Search PubMed
.
- Y. L. Zhukovskiy, D. E. Batueva, A. D. Buldysko, B. Gil and V. V. Starshaia, Energies, 2021, 14(17), 5268 CrossRef CAS
.
- L. Malka, F. Bidaj, A. Kuriqi, A. Jaku, R. Roçi and A. Gebremedhin, Energy, 2023, 272, 127107 CrossRef CAS
.
- X. Ye, Z. Yu, T. Xu, Y. Zhang and L. Guo, Energy, 2024, 310, 133306 CrossRef CAS
.
- S. Zendehboudi, M. Seyyedattar, N. M. Cata Saady, O. Mohammadzadeh, A. Mamudu and D. Heagle, Energy Fuels, 2025, 39, 5987–6025 CrossRef CAS
.
- M. S. Masnadi, H. M. El-Houjeiri, D. Schunack, Y. Li, J. G. Englander, A. Badahdah, J.-C. Monfort, J. E. Anderson, T. J. Wallington, J. A. Bergerson, D. Gordon, J. Koomey, S. Przesmitzki, I. L. Azevedo, X. T. Bi, J. E. Duffy, G. A. Heath, G. A. Keoleian, C. McGlade, D. N. Meehan, S. Yeh, F. You, M. Wang and A. R. Brandt, Science, 2018, 361, 851–853 CrossRef CAS PubMed
.
- A. Bashir, M. Ali, S. Patil, M. S. Aljawad, M. Mahmoud, D. Al-Shehri, H. Hoteit and M. S. Kamal, Earth Sci. Rev., 2024, 249, 104672 CrossRef CAS
.
- R. Matsumoto and T. Tabata, Appl. Sci., 2022, 12, 3738 CrossRef CAS
.
- A. Darabi, P. G. Jessop and M. F. Cunningham, Chem. Soc. Rev., 2016, 45, 4391–4436 RSC
.
- H. Liu, S. Lin, Y. Feng and P. Theato, Polym. Chem., 2017, 8, 12–23 RSC
.
- Z. Zhu, Y. Song, Q. Gao and C. Wang, Front. Earth Sci., 2023, 10–2022, 1053307 CrossRef
.
- L. Fu, M. Wei, K. Liao, M. Qianli, M. Shao, F. Gu, Y. Fan, L. Longjie and H. Yanfeng, J. Pet. Sci. Eng., 2022, 219, 111088 CrossRef CAS
.
- G. C. Maitland, Curr. Opin. Colloid Interface Sci., 2000, 5, 301–311 CrossRef CAS
.
- Y. Feng, Z. Chu, C. A. Dreiss, Y. Feng, Z. Chu and C. A. Dreiss, Smart Wormlike Micelles: Des. Charact, Appl., 2015, pp. 79–91 Search PubMed
.
- J. D. Mangadlao, P. Cao and R. C. Advincula, J. Pet. Sci. Eng., 2015, 129, 63–76 CrossRef CAS
.
- H. Wang, H. Huang, W. Bi, G. Ji, B. Zhou and L. Zhuo, Nat. Gas Ind. B, 2022, 9, 141–157 CrossRef
.
- S. H. Hajiabadi, M. Khalifeh, R. Van Noort and P. H. Silva Santos Moreira, ACS Omega, 2023, 8, 23320–23345 CrossRef CAS PubMed
.
- Y. Yong, S. Zhang, C. Xiaopeng, L. Qi, L. Guangzhong, C. Zhang, L. Zongyang, D. Zhang and W. Zheng, Adv. Pet. Explor. Dev., 2024, 51, 1247–1260 CrossRef
.
- L. Cai, J. Wu, M. Zhang, K. Wang and Z. Yang, ACS Omega, 2024, 9, 44293–44303 CrossRef CAS PubMed
.
- A. Bakhsh, L. Zhang, H. Wei, A. Shaikh, N. Khan, Z. Khan and R. Shaoran, Processes, 2022, 10, 885 CrossRef CAS
.
- X. Huang, M. Zhang and X. Su, J. Mol. Liq., 2023, 389, 122842 CrossRef CAS
.
- X. Li, X. Liu, J. Qi, Y. Yang, J. Wang, S. Dai and H. Lu, Chemphysmater, 2023, 2, 217–224 CrossRef
.
- Z. Yang, C. He, H. Sui, L. He and X. Li, J. CO2 Util., 2019, 30, 79–99 CrossRef CAS
.
- R. D. Jansen-van Vuuren, S. Naficy, M. Ramezani, M. Cunningham and P. Jessop, Chem. Soc. Rev., 2023, 52, 3470–3542 RSC
.
- D. Rahmatabadi, I. Ghasemi, M. Baniassadi, K. Abrinia and M. Baghani, J. Mater. Res. Technol., 2022, 21, 3970–3981 CrossRef CAS
.
- S. Lin, J. Shang, X. Zhang and P. Theato, Macromol. Rapid Commun., 2018, 39, 1700313 CrossRef PubMed
.
- L. Phan, D. Chiu, D. J. Heldebrant, H. Huttenhower, E. John, X. Li, P. Pollet, R. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539–545 CrossRef CAS
.
- Y. Ding, Y. Zhao, X. Wen, Y. Liu, M. Feng and Z. Rui, Gels, 2023, 9, 936 CrossRef CAS PubMed
.
- Y. Xiong, J. Liu, X. Yi, B. Xiao, D. Wu, B. Wu and C. Gao, J. Energy Eng., 2024, 121(7), 1759–1780 CrossRef
.
- H. Shen, Z. Yang, X. Li, Y. Peng, M. Lin, J. Zhang and Z. Dong, Fuel, 2021, 292, 120306 CrossRef CAS
.
- Y. Zhang, M. Gao, Q. You, H. Fan, W. Li, Y. Liu, J. Fang, G. Zhao, Z. Jin and C. Dai, Fuel, 2019, 241, 442–450 CrossRef CAS
.
- Y. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958–960 CrossRef CAS PubMed
.
- Q. Yan, R. Zhou, C. Fu, H. Zhang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923–4927 CrossRef CAS PubMed
.
- B. Jiang, Y. Zhang, X. Huang, T. Kang, S. J. Severtson, W.-J. Wang and P. Liu, Ind. Eng. Chem. Res., 2019, 58, 15088–15108 CrossRef CAS
.
- Q. Wu and Y. Xu, J. Pet. Sci. Eng., 2021, 206, 108985 CrossRef CAS
.
- Z. Zheng, C. Liu and W. Qiao, Eur. J. Lipid Sci. Technol., 2015, 117, 1673–1678 CrossRef CAS
.
- J. Y. Quek, P. J. Roth, R. A. Evans, T. P. Davis and A. B. Lowe, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 394–404 CrossRef CAS
.
- F. Barzagli, F. Mani and M. Peruzzini, Energy Environ. Sci., 2009, 2, 322–330 RSC
.
- M. Zeng, M. Huo, Y. Feng and J. Yuan, Macromol. Rapid Commun., 2018, 39, 1800291 CrossRef PubMed
.
- Z. Wang, B. Bai, Y. Long and L. Wang, SPE J., 2019, 24, 2398–2408 CrossRef CAS
.
- D. Rahmatabadi, M. A. Yousefi, S. Shamsolhodaei, M. Baniassadi, K. Abrinia, M. Bodaghi and M. Baghani, Adv. Intell. Syst. Comput., 2500113 Search PubMed
.
- A. Roy, H. E. Holmes, L. S. Baugh, D. C. Calabro, J. Leisen, S. Seth, Y. Ren, S. C. Weston, R. P. Lively and M. Finn, Chem. Mater., 2024, 36, 4393–4402 CrossRef CAS
.
- D. J. Chun, A. Mccord, N. Mokhtari-Nori, S. Dai, J. Z. Larese, L. L. Daemen, B. S. Lokitz and S. M. Kilbey, Macromolecules, 2024, 57, 11177–11189 CrossRef CAS
.
- J. Sun, Z. Xiu, L. Li, K. Lv, X. Zhang, Z. Wang, Z. Dai, Z. Xu, N. Huang and J. Liu, Petroleum, 2024, 10, 11–18 CrossRef
.
- M. A. Marzabad, S. Chattopadhyay, S. Hietala, N. Nonappa, R. Marek and O. Jurček, Chem.–Eur. J., 2025, 31, e202500748 CrossRef CAS PubMed
.
- R. Zhang, H. Lu, S. Ji, X. Lin, Z. Yang and Z. Zhang, Colloids Surf., A, 2025, 713, 136538 CrossRef CAS
.
- A. Galib, M. M. Islam, M. A. Mahamud, M. A. Rahman, M. S. Hossan, H. Ahmad, M. A. J. Miah, M. M. Rahman and M. A. Alam, Polym. Bull., 2025, 82(8), 1–24 CrossRef
.
- P. Schattling, I. Pollmann and P. Theato, React. Funct. Polym., 2014, 75, 16–21 CrossRef CAS
.
- S. Lin and P. Theato, Macromol. Rapid Commun., 2013, 34, 1118–1133 CrossRef CAS PubMed
.
- K. Matyjaszewski, T. P. Davis, et al., Handbook of Radical Polymerization, Wiley Online Library, 2002, vol. 922 Search PubMed
.
- C. I. Fowler, C. M. Muchemu, R. E. Miller, L. Phan, C. O'Neill, P. G. Jessop and M. F. Cunningham, Macromolecules, 2011, 44, 2501–2509 CrossRef CAS
.
- M. Mihara, P. Jessop and M. Cunningham, Macromolecules, 2011, 44, 3688–3693 CrossRef CAS
.
- Q. Zhang, W.-J. Wang, Y. Lu, B.-G. Li and S. Zhu, Macromolecules, 2011, 44, 6539–6545 CrossRef CAS
.
- A. R. Shirin-Abadi, A. Darabi, P. G. Jessop and M. F. Cunningham, Polymer, 2016, 106, 303–312 CrossRef
.
- A. Darabi, A. R. Shirin-Abadi, P. G. Jessop and M. F. Cunningham, Macromolecules, 2015, 48, 72–80 CrossRef CAS
.
- N. Che, S. Yang, H. Kang, R. Liu, Z. Li, Z. Liu, P. Li, X. Qu and Y. Huang, Polym. Chem., 2014, 5, 7109–7120 RSC
.
- C. Barner-Kowollik, Handbook of RAFT Polymerization, John Wiley & Sons, 2008 Search PubMed
.
- A. Feng, C. Zhan, Q. Yan, B. Liu and J. Yuan, Chem. Commun., 2014, 50, 8958–8961 RSC
.
- B. A. Abel, M. B. Sims and C. L. McCormick, Macromolecules, 2015, 48, 5487–5495 CrossRef CAS
.
- X. Guo, T. Zhang, Y. Wu, W. Shi, B. Choi, A. Feng and S. H. Thang, Polym. Chem., 2020, 11, 6794–6802 RSC
.
- G. Moad, Polym. Chem., 2017, 8, 177–219 RSC
.
- X. Jiang, C. Feng, G. Lu and X. Huang, Polymer, 2015, 64, 268–276 CrossRef CAS
.
- W. Yuan, J. Shen and H. Zou, RSC Adv., 2015, 5, 13145–13152 RSC
.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed
.
- Q. Yan, J. Wang, Y. Yin and J. Yuan, Angew. Chem., 2013, 125(19), 5070–5073 CrossRef PubMed
.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS
.
- M. Huo, H. Du, M. Zeng, L. Pan, T. Fang, X. Xie, Y. Wei and J. Yuan, Polym. Chem., 2017, 8, 2833–2840 RSC
.
- Q. Zhang and S. Zhu, ACS Macro Lett., 2014, 3, 743–746 CrossRef CAS PubMed
.
- M. A. Haruna, E. Nourafkan, Z. Hu and D. Wen, Energy Fuels, 2019, 33, 1637–1648 CrossRef CAS
.
- T. Xie, H. Zhang, Y. Bai, K. Mei, Y. Zheng, C. Zhang and X. Cheng, Constr. Build. Mater., 2024, 435, 136651 CrossRef CAS
.
- J. Huang, J. Xu, K. Chen, T. Wang, C. Cui, X. Wei, R. Zhang, L. Li and X. Guo, Ind. Eng. Chem. Res., 2015, 54, 1564–1575 CrossRef CAS
.
- Y. Lili, W. Aijia, J. Guancheng, A. Tian and Z. Zhengguo, Drill. Fluid Complet. Fluid, 2022, 39, 23–28 Search PubMed
.
- J. Zhao, B. Yang, J. Mao, Y. Zhang, X. Yang, Z. Zhang and Y. Shao, Energy Fuels, 2018, 32, 3039–3051 CrossRef CAS
.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS
.
- M. Shao, X. Yue, T. Yue and J. He, J. Polym. Sci., 2020, 58, 519–527 CrossRef CAS
.
- O. Contreras, M. Alsaba, G. Hareland, M. Husein and R. Nygaard, J. Energy Resour. Technol., 2016, 138, 032906 CrossRef
.
- G. Lende, J. A. Clausen and A. J. Kvassnes, Evaluation of new innovative cement blend for enhanced CO2 and H2S resistance, SPE/IADC International Drilling Conference and Exhibition, 2021 Search PubMed
.
- J. Wang, J. Tang, H. Zhang, Y. Wang, H. Wang, B. Lin, J. Hou and H. Zhang, Corros. Sci., 2021, 180, 109194 CrossRef CAS
.
- J. Zhang, C. Wang and Z. Peng, Cem. Concr. Res., 2021, 143, 106397 CrossRef CAS
.
- P. Gong, K. Huang, Y. Huang, Y. Wu, K. Mei, T. Wei, C. Zhang and X. Cheng, Constr. Build. Mater., 2024, 449, 138268 CrossRef CAS
.
- C. P. Zhang, S. Liu, Z. Y. Ma and P. G. Ranjith, Fuel, 2021, 305, 121431 CrossRef CAS
.
- A. Shaikh, C. Dai, Y. Sun, Q. You, A. S. Qureshi, G. Zhao, V. Foutou, A. Bakhsh, N. Khan, Z. Abro and M. Zhao, J. Pet. Sci. Eng., 2022, 208, 109674 CrossRef CAS
.
- N. Sun, M. Gao, J. Liu, G. Zhao, F. Ding, Q. You and C. Dai, Colloids Surf., A, 2023, 665, 131247 CrossRef CAS
.
- M.-W. Gao, M.-S. Zhang, H.-Y. Du, M.-W. Zhao, C.-L. Dai, Q. You, S. Liu and Z.-H. Jin, Pet. Sci., 2023, 20, 982–992 CrossRef CAS
.
- M. M. Samuel, Z. Al-Jalal, N. Nurlybayev, M. Farouk, Z. Xiao, C. Kang Kang and L. Huanming, Novel carbon dioxide (CO2) foamed fracturing fluid, an innovative technology to minimize carbon footprint, Middle East Oil, Gas and Geosciences Show, 2023 Search PubMed
.
- N. Zheng, J. Zhu, Z. Yang, C. Song, X. Li, Y. Long, L. Yi, J. Lai and J. Ge, Fuel, 2025, 385, 134190 CrossRef CAS
.
- L. A. Mokif, H. K. Jasim and N. A. Abdulhusain, Mater. Today Proc., 2022, 49, 2671–2674 CrossRef CAS
.
- X. Li, L. Wang, H. Lu and B. Wang, Sep. Purif. Technol., 2021, 254, 117566 CrossRef CAS
.
- Z. Qi, Y. Liu, Q. Gao, D. Tao, Y. Wang, J. Guo and Y. Yu, React. Funct. Polym., 2023, 182, 105481 CrossRef CAS
.
- Y. Wang, S. Yang, J. Zhang, Z. Chen, B. Zhu, J. Li, S. Liang, Y. Bai, J. Xu, D. Rao, L. Dong, C. Zhang and X. Yang, Nat. Commun., 2023, 14, 1108 CrossRef CAS PubMed
.
- H. Liu, Y. Wang, B. Zhu, H. Li, L. Liang, J. Li, D. Rao, Q. Yan, Y. Bai, C. Zhang, L. Dong, H. Meng and Y. Zhao, Adv. Mater., 2024, 36, 2311013 CrossRef CAS PubMed
.
- D. Yan, Y. Zhao, S. Zhang, X. Wang and X. Ning, Chem. Eng. J., 2024, 493, 152679 CrossRef CAS
.
- W.-L. Kang, B.-B. Zhou, M. Issakhov and M. Gabdullin, Pet. Sci., 2022, 19, 1622–1640 CrossRef CAS
.
- Y.-Q. Zhang, S.-L. Yang, L.-F. Bi, X.-Y. Gao, B. Shen, J.-T. Hu, Y. Luo, Y. Zhao, H. Chen and J. Li, Pet. Sci., 2025, 22, 255–276 CrossRef CAS
.
- F. Li, Y. Luo, X. Luo, L. Wang and R. D. Nagre, J. Pet. Sci. Eng., 2016, 146, 103–110 CrossRef CAS
.
- F. Zhao, P. Wang, S. Huang, H. Hao, M. Zhang and G. Lu, J. Pet. Sci. Eng., 2020, 192, 107336 CrossRef CAS
.
- S. Li, Z. Wang, S. Li and D. Liu, Energy Fuels, 2024, 38, 20384–20396 CrossRef CAS
.
- Y. Chen, X. Lin, R. Zhang, Z. Yang, N. Wang, L. Wang, H. Lu and Z. Huang, Energy Fuels, 2024, 38, 22365–22375 CrossRef CAS
.
- D. Du, B. Chen, W. Pu, X. Zhou, R. Liu and F. Jin, Colloids Surf., A, 2022, 650, 129546 CrossRef CAS
.
- Y. Gu, H. Tu and M. Zhou, Energy Fuels, 2024, 38, 6182–6194 CrossRef CAS
.
- J. G. M. L. Shao, M. J. Cheng and X. Q. Liu, J. Dispersion Sci. Technol., 2025, 1–12 CAS
.
- Q. Gao, X. Xu, S. Liu, A. O. Mmbuji and Y. Wu, Energy Fuels, 2024, 38, 3755–3768 CrossRef CAS
.
- X. Huang, M. Zhang, X. Su and Y. Feng, Polymer, 2024, 306, 127227 CrossRef CAS
.
- B. Ding, A. Kantzas and A. Firoozabadi, Water Resour. Res., 2024, 60(12), 1–16 CrossRef
.
- S. Zhao, L. Liu, Z. Li, X. Fan, Z. Wang, M. Xing and F. Li, ACS Omega, 2025, 10(14), 14362–14372 CrossRef CAS PubMed
.
- O. Massarweh and A. S. Abushaikha, Petroleum, 2022, 8, 291–317 CrossRef
.
- Y. Zhao, X. Wen, Z. Rui and Y. Ding, Chem. Eng. J., 2024, 498, 155187 CrossRef CAS
.
- Y. Xin, B. Li, Z. Li, Z. Li, B. Wang, X. Wang, M. Zhang and W. Li, Sep. Purif. Technol., 2025, 353, 128475 CrossRef CAS
.
- P. Fang, Q. Zhang, M. Wu, C. Zhou, Z. Yang, H. Yu, Z. Ji, L. Yi, W. Jiang, X. Chen, Y. Gao, M. Zhou and M. Cao, Sep. Purif. Technol., 2025, 359, 130526 CrossRef CAS
.
- B. Hascakir, J. Pet. Sci. Eng., 2017, 154, 438–441 CrossRef CAS
.
- Q. Tian, P. Han, B. Li and Y. Feng, J. Appl. Polym. Sci., 2020, 137, 48305 CrossRef CAS
.
- R. Nguele, B. A. Omondi, S. Yamasaki, S. Mandai, Y. Sugai and K. Sasaki, Colloids Surf., A, 2021, 622, 126688 CrossRef CAS
.
- C. Sambo, N. Liu, R. Shaibu, A. A. Ahmed and R. G. Hashish, Geoenergy Sci. Eng., 2023, 221, 111185 CrossRef
.
- Y. Wang, X. Han, J. Li, R. Liu, Q. Wang, C. Huang, X. Wang, L. Zhang and R. Lin, Energy Fuels, 2023, 37, 2539–2568 CrossRef CAS
.
- G. Zhang, P. G. Ranjith, Z. Li, M. Gao and Z. Ma, J. Pet. Sci.
Eng., 2021, 207, 109093 CrossRef CAS
.
- R. Gholami, A. Raza and S. Iglauer, Earth Sci. Rev., 2021, 223, 103849 CrossRef CAS
.
- W. Hu, Z. Lun, H. Wang, C. Zhao, X. Zhou, Z. Meng, P. Zhu, D. Zhang and J. Zou, Energy Fuels, 2024, 38, 17441–17457 CrossRef CAS
.
- L. Quan and M. Mirabolghasemi, Exploration of CO2-sensitive chemicals as potential sealing agents for subsurface CO2 storage, SPE Western Regional Meeting, 2024 Search PubMed
.
- H. Wu, Y. Lou, X. Zhai, Z. Li and B. Liu, ACS Omega, 2023, 8, 35066–35076 CrossRef CAS PubMed
.
- I. Anwar, M. Meng, J. W. Carey, R. Gilbertson, A. Zandanel, N. Himmelberg, W. Zhang, E. Tao, C. Neil and R. Nair, Experimental Investigation on CO2-sensitive Gel-type Sealant for Sealing Leaky Carbon Sequestration Wellbores, SPE Annual Technical Conference and Exhibition, 2024 Search PubMed
.
- X. Zheng, Z. Mei, X. Zhou, S. Chen and N. Lai, Arabian J. Sci. Eng., 2023, 48, 16783–16790 CrossRef CAS
.
- S. Chen, J. Li, S. Chen, J. Qin, G. Wang, Y. Yi, L. Zhang, R. Wang and D. Zhu, Energy Fuels, 2023, 37, 4401–4412 CrossRef CAS
.
- D. Zhu, S. Peng, S. Zhao, M. Wei and B. Bai, Energy Fuels, 2021, 35, 4711–4742 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |