Open Access Article
Open Access ArticleRemoval of tetracycline antibiotic activity in water by stable cubic phase barium stannate-perovskite nanoparticles under energy-efficient blue light LED irradiation†
Nehal Ashok Waghchoure ,
Atul Patel
,
Atul Patel ,
Laxman G. Raikar
,
Laxman G. Raikar ,
Jemi Gandhi,
Kampurath Poduvattil Jayadevan
,
Jemi Gandhi,
Kampurath Poduvattil Jayadevan * and
Halan Prakash
* and
Halan Prakash *
*
Energy and Environmental Chemistry Laboratory, Department of Chemistry, Birla Institute of Technology and Science, Pilani, K K Birla Goa Campus, Zuarinagar, Goa 403726, India. E-mail: halanprakash@goa.bits-pilani.ac.in; jayadev@goa.bits-pilani.ac.in
First published on 1st July 2025
Abstract
Photocatalysis is promising for the degradation of antibiotic pollutants in water, which are known to cause the emergence of harmful antibiotic-resistant bacteria (ARB). Importantly, photocatalytic degradation of antibiotic pollutants in water with loss of antibacterial activity in an energy-efficient manner needs to be explored. Here, for the first time, we report degradation of tetracycline (TC), a known antibiotic pollutant in water, with removal of its antibiotic activity by cubic-barium stannate (BaSnO3, BSO) perovskite photocatalytic nanoparticles under a blue LED light (367 nm) irradiation, (BSO/LED) system. XRD results revealed a single cubic phase of BSO with a crystallite size of 35 nm. FESEM and TEM images showed that BSO had cubic block-like morphology. BET analysis revealed the mesopores nature of BSO with a surface area of 1.1 m2 g−1. XPS confirmed the existence of Ba+2 and Sn+4 ions. The bandgap energy of BSO was determined to be ∼3.2 eV. Importantly, photolysis of BSO under blue LED light caused complete degradation of TC in water, with a pseudo-first-order rate constant (kobs) value determined as 0.0824 min−1. Radical scavenging and ESR results revealed O2˙− and holes were profoundly generated in the BSO/LED system, causing degradation of TC along with antibiotic activity removal. Electrical energy per order (EEO) was determined as 13.63 kWh per m3 per order, revealing that the BSO/LED system is energy-efficient. Further, BSO was reused four times, highlighting its stability. Degradation byproducts were predicted to be non-toxic by ecological structure activity relationship analysis. Thus, the study discloses an effective photocatalytic degradation of TC along with removal of its antibacterial activity that is needed to prevent hazardous ARB, by a stable non-lead-perovskite nano-photocatalyst under an energy-efficient LED source.
1. Introduction
Antibiotics are used to treat infectious diseases in humans and animals.1–4 However, antibiotic waste leads to the contamination of water bodies.3 Water contaminated with antibiotics has been reported to cause the emergence of antibiotic-resistant bacteria (ARBs), which can cause severe disease.5–8 Antibiotics such as tetracycline (TC) are highly consumed.9,10 Importantly, TC is not completely metabolised by animals and humans.11,12 Thus, TCs are excreted, and their presence in sewage wastewater has been reported, which is a serious problem for the environment and the health of humans and animals.13–15 Notably, the presence of TC, even in drinking water sources, has been reported.16 In the aquatic environment, TC has a long half-life (34–329 h),17,18 which could lead to accumulation and cause the spreading of ARBs into the environment.19 Hence, effective degradation of TC in water is urgently needed.The degradation of antibiotics in water has been reported by various methods such as ozonation,20 UV photolysis,21 Fenton/photo-Fenton,22 and electrochemical process.23 Photocatalysis is a promising method for the effective degradation of organic micropollutants.24–26 Photoexcitation of photocatalysts produces excited electron and hole pairs, which are known to generate robust redox reactive species that induce degradation of organic pollutants in water.27–30 Nanocrystalline perovskites are a class of promising photocatalytic materials.24,31,32 Earlier, mainly lead-based perovskites, having a formula MPbO3, where M = alkaline earth metals, were reported to show excellent photocatalysis.33 However, lead-based perovskites have a limitation due to the toxicity of the lead.34 Hence, the development of non-lead (Pb) based perovskite photocatalysts has gained significant attention. Barium stannate, BaSnO3 (BSO), is emerging as a non-lead (Pb) based perovskite photocatalyst.35–37 Earlier, the synthesis of BSO photocatalyst has been reported by different methods like combustion technique,38 chemical coprecipitation,39 wet chemicals,40 sol–gel,41,42 and hydrothermal.43 The chemical co-precipitation method has advantages such as low cost, repeatability, high yield, high product purity, and no need of organic solvents.44 Earlier, photocatalytic degradation of antibiotics in water by modified BaSnO3-based photocatalysts, such as tungsten-modified BaSnO3, heterojunction between BaSnO3 with photocatalysts like TiO2, copper ferrites and others were reported.,45–47 Tables S4 and S5.† Importantly, the ability of BaSnO3 nanoparticles to degrade an antibiotic pollutant with the removal of its antibacterial activity after photocatalytic treatment needs to be explored. Moreover, earlier BaSnO3-based photocatalysis were investigated using a conventional xenon lamp.36 Importantly, a xenon lamp emits wide wavelengths of light in the UV and visible ranges, and appropriate filters are needed for photocatalysis.48 Thus, a large amount of light is wasted, and xenon lamp sources consume very high power. Notably, bare BaSnO3 has poor light absorption above 400 nm.49,50 Hence, BaSnO3 photocatalytic activity with light delivered from energy-efficient light sources in the UVA region needs to be explored.51–53
It is important to note that light-emitting diodes (LEDs) with UVA light are energy efficient and are emerging as an efficient light source.54,55 Currently, UVA-LED (367 nm emission maxima), with a wall plug efficiency of 0.046, is commercially available in the market. The wall-plug efficiency of the LED increases as the UVA radiation maximum increases.56–58 Importantly, the photo excitation of bare BaSnO3 (BSO) photocatalysts under blue light LED (UVA) irradiation for the photocatalytic degradation of antibiotics micropollutants in water, with the elimination of antibiotic activity and reuse of BSO photocatalyst, needs to be evaluated.
Therefore, for the first time, we aimed to study the photocatalytic degradation of antibiotic pollutant TC by BSO under blue light LED irradiation (BSO/LED system). A blue LED (367 nm), which closely corresponds to the absorption band of BSO (3.3 eV) was used for irradiation. The BSO obtained by co-precipitation method and characterized by different techniques such as X-ray diffraction spectroscopy (XRD), Field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), higher resolution transmission electron microscopy (HR-TEM), Brunauer–Emmett–Teller (BET), selected area electron diffraction (SAED), UV visible spectroscopy, Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The effect of the initial concentration of TC and BSO photocatalyst, the effect of pH, electron spin resonance (ESR), electrochemical analysis, photocatalytic degradation mechanism, degradation byproducts and ecological structure activity relationships (ECOSAR) analysis and antibacterial activity before and after BSO/LED system treatment was presented. The reusability of the BSO photocatalyst was performed and ICP-OES analysed the stability of the BSO photocatalyst. The EEO value for the degradation of TC by the BSO/LED system was determined.
2. Experiments
2.1 Materials
The precursor, namely, barium nitrate [Ba(NO3)2] (98.5% pure, Sigma-Aldrich), stannic chloride [SnCl4] (98% pure, Sigma-Aldrich), sodium hydroxide (NaOH) (Merck), and tetracycline (TCI make) were used in the experiments. The chemicals like methanol, formic acid, and acetonitrile required for HPLC analysis were purchased from Finar Chemicals, and were of HPLC grade. Sodium chloride, sodium bicarbonate, sodium hydrogen phosphate, sulphuric acid, sodium hydroxide, iron(III) nitrate nonahydrate, and humic acid were supplied by Thermo Scientific. The above precursors were directly used without any further purification.2.2 Synthesis of BSO
The aqueous solution of Ba(NO3)2 was prepared by dissolving Ba(NO3)2 in 25 ml distilled water. The solution of SnCl4 was prepared by dissolving SnCl4 in 10 ml of distilled water. The aqueous solution of SnCl4 was added dropwise in the above Ba(NO3)2 solution, stirring for 1 h. After the formation of the homogenous solution, the aqueous solution of NaOH was added to it and stirred for 30 min. This solution was heated at 70 °C for 3 h with constant stirring until the precipitate was formed. The obtained precipitate was transferred to a Petri dish and dried at 80 °C for another 3 h. This dry residue was crushed using a pestle-mortar, transferred in an alumina crucible, heated to 200 °C in a muffle furnace for 2 h, followed by washing with 15 ml of distilled water, dried for 30 minutes at 80 °C, and then heated for two hours at 200 °C. The purpose of the washing stage was to remove the contaminants such as carbonates, nitrates, salt, and chlorine ions. The above process was repeated three times, and powder was calcined and annealed at 500 °C and 800 °C each for 2 h, respectively and allowed to cool at ambient temperature. The powder thus obtained was further used for the analysis.2.3 Characterization
XRD measurements (Bruker D8) with Cu Kα radiation (λ = 1.542 Å) from 20° to 80° with a step size of 0.02 and a step time of 0.6 s. The crystallite size of BSO was calculated to be 35 nm from the Scherrer eqn (1).
 | (1) |
A spectrophotometer (Shimadzu UV-2450) was used to analyse the UV-visible diffuse reflectance spectra (UV-DRS), with BaSO4 as a reference. The measurement range for the spectra was 200–800 nm. The bandgap energy of BSO was calculated from Tauc's eqn (2) and was determined to be 3.2 eV.
| αhν = A(hν − Eg)n. | (2) |
A 532 nm Nd-YAG laser 3.2 mW was used as the laser source for recording Raman spectra (LAB RAM HR Horiba France). The Quanta FEG 250 was used to do FESEM analysis and EDS readings. The voltage for the SEM and EDS experiments was 20 kV. LEICA EM ACE 200 was used for gold sputtering. Transmission Electron Microscopy (TEM) (Fig. 1e) was carried out using FEI Tecnai G2 S-Twin, 200 kV. Thermo Fisher Scientific (UK) K-Alpha X-ray Photoelectron Spectrometer was used to conduct X-ray photoelectron spectroscopy (XPS).
Electron Spin Resonance (ESR) was recorded using a JEOL ESR Spectrometer. Spin-trapping agents, including DMPO, TEMP, and TEMPO, were used for the analysis. The conditions employed were magnetic centre field = 336 mT, microwave power = 0.995 mW, and microwave frequency of 9.440 GHz. Samples were taken out and examined at the proper intervals.
A BioLogic SP-150e electrochemical workstation was used to carry out the electrochemical analysis. The Mott–Schottky was plotted using electrochemical impedance spectroscopy (EIS) and chronoamperometric measurements, which were performed using a three-electrode electrochemical cell. The Mott–Schottky plots were plotted using EIS measurements, with a reference electrode (Ag/AgCl) having a potential between −2.0 V and −0.4 V with a frequency range of 100 kHz to 10 Hz. The BSO was prepared in ethanol as the working electrode with Nafion acting as a binder. After that, it was applied to a 1 cm2 region on fluorine-doped tin oxide (FTO) glass slides. The reference electrode was Ag/AgCl (saturated KCl), while the counter electrode was graphite. For each photoelectrochemical experiment, 15 ml of 0.1 M Na2SO4 electrolyte solution, pH = 5.8 and an LED light source (367 nm) were used.
2.4 Photocatalytic degradation experiments
Deionised water was used in the overall photocatalytic experiments. A photocatalytic degradation experiment was conducted in an 80 ml quartz glass reactor at room temperature (25 °C). A desirable amount of TC and BSO were mixed, and the solution was stirred for 30 minutes in the dark. After reaching adsorption equilibrium, 500 μl of the solution was taken in a micro-centrifuge tube, which was captioned as 0 min reading and was later centrifuged, and supernatant was used for the analysis. The LED light was switched on and the samples were collected at a specific time interval. An ultraviolet-A light-emitting diode (UVA LED) with a maximum wavelength (λmax) of 367 nm was used for the degradation experiment. The irradiance spectra of 367 nm LED light is shown in Fig. S5.† The distance between the LED light and the quartz was 0.5 cm, and an external fan was used to keep the LEDs cool. The solution was stirred continuously throughout the experiment, and 0.5 ml solution was withdrawn at a 3 min time interval in a 1 ml micro-centrifuge tube containing 10 μl of methanol as a quencher. This solution was centrifuged at 5000 rpm for 10 min, and the supernatant solution was analysed using high-performance liquid chromatography (HPLC Shimadzu technologies). The Phenomenex Kinetex EVO C18 2.6 μm, 4.6 × 100 mm column was used for HPLC. An isocratic technique was used with a mobile phase of acetonitrile and formic acid (0.1%) in water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) at a flow rate of 0.8 ml min−1. The limit of detection was found to be 0.0944 μg L−1 for TC with an R-square value of 0.999 (Fig. S1†).
90) at a flow rate of 0.8 ml min−1. The limit of detection was found to be 0.0944 μg L−1 for TC with an R-square value of 0.999 (Fig. S1†).
The following eqn (4) and (5) were used to compute the photocatalytic degradation efficiency (φ).
 | (3) |
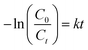 | (4) |
To analyse the dynamics data, the first-order model was used:
Where φ is the degradation efficiency, C0 (mg L−1) and Ct (mg L−1) are the concentrations at the initial and final (after t min), and k is the first order rate constant (min−1). The chemical oxygen demand (COD) was performed to analyse the organic content after the degradation of TC. Initially, the COD before degradation was 9 mg L−1, which was reduced to 4 mg L−1 with an equilibrium time of 45 min for 80 ml TC solution with 0.4 g L−1 of BSO catalyst. The reusability of the BSO catalyst was performed by centrifugation and reuse.
A scavenging experiment was performed using isopropyl alcohol (IPA) as a HO˙ radical scavenger, sodium azide (NaN3) as a 1O2 scavenger, ethylenediamine tetra acetic acid (EDTA) as a hole (h+) scavenger, and 1,4-benzoquinone (BQ) as a superoxide radical anion (O2˙−) scavengers. Spin-trapping agents such as TEMP in water and DMPO in water were employed for the analysis of singlet oxygen, and HO˙, respectively. BSO and DMPO in methanol was used to identify O2˙− radical.59 The aqueous BSO and TEMPO were used to detect holes (h+).
2.5 Toxicity assessment
2.6 Electrical energy determination
The electrical energy order (EEO) was calculated to find the electrical energy required to degrade the contaminant in a water unit. It was calculated as discussed below
 | (5) |
 | (6) |
From eqn (5) and (6) EEO can be modified into
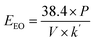 | (7) |
Using the above formula, the EEO of the LEDs was calculated as 13.63 kWh per m3 per order.
3. Results and discussion
3.1 Characterization of BSO
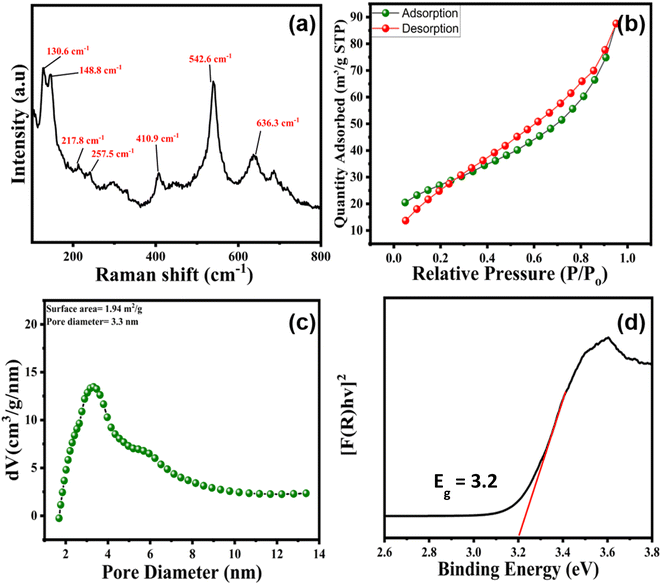 | ||
| Fig. 3 (a) Raman spectra of pure BSO, (b) nitrogen adsorption–desorption isotherms, (c) BJH pore-size distributions of BSO, and (d) Tauc plot to determine the bandgap of BSO. Minor tick in Fig. 3d. | ||
The nitrogen adsorption–desorption isotherm of BSO corresponds to a type IV isotherm with an H4 type of hysteresis loop, revealing mesoporous material with narrow-slit pores, as per the IUPAC categorisation of hysteresis loops.72 The surface area, total pore volume, and pore diameter (BJH) were calculated to be 1.10 m2 g−1, 0.0031 cm3 g−1, and 3.16 nm, respectively, (Fig. 3b and c).73,74 The surface area obtained in the present work was slightly lower compared to the earlier suggested report, where BaSnO3 was obtained via microwave-assisted hydrothermal treatment.75 Importantly, the Tauc plot reveals that BSO has an absorption band edge/tail around 390 nm, and the bandgap energy (Eg) of BSO was calculated to be around 3.2 eV (Fig. 3d).76
 | ||
| Fig. 4 XPS of bare BSO, (a) XPS survey of BSO, (b) the core-level splitting of peaks of Ba, (c) the core-level splitting of peaks of Sn, and (d) the core-level splitting of peaks of O. | ||
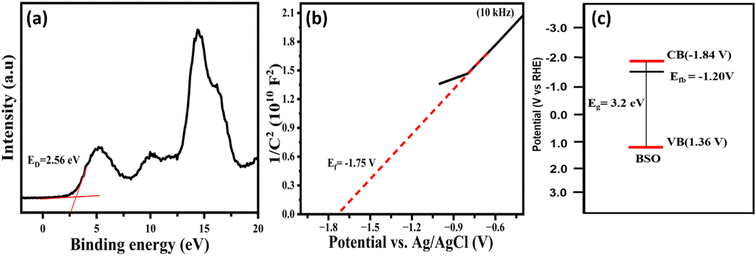
Further, the energy difference between the Fermi level and the valence band (Ed) was determined to be about 2.56 V from the XPS valence band spectrum of BSO, Fig. 5a. The Fermi level (flat band potential Ef) was determined to be −1.75 V (vs. Ag/AgCl) from the Mott–Schottky plot (Fig. 5b), and corresponding Ef value was – 1.20 V (vs. NHE). Thus, the position of the valence band was determined to be 1.36 V (vs. NHE), based on the eqn (Vb = Ed + Ef).82 Further, the position of the conduction band was determined to be −1.84 V (vs. NHE) based on the equation ECB = EVB − Eg, where Eg is the band gap energy. The band structure of BSO highlighting the position of VB, CB, and Ef is presented in Fig. 5c. A light-emitting diode (LED) that has an emission profile (Fig. S3,† λmax = 367 nm, 3.3 eV), and strongly overlaps with the absorption (band gap energy of 3.2 eV) of the BSO, was used to evaluate the ability of the BSO photocatalyst (BSO/LED) system to degrade organic pollutant, as discussed below.
3.2 Photocatalysis
 | ||
| Fig. 9 (a) Effect of initial pH on tetracycline degradation, and (b) kobs vs. pH; [TC] = 10 mg L−1, [BSO] = 0.4 g L−1. | ||
Interestingly, at highly alkaline pH, the degradation was not significantly reduced compared to acidic conditions at pH 3. This result indicates that the positively charged holes may significantly attract the negatively charged TC molecule under alkaline conditions and cause appreciable degradation, Fig. 9b. Overall, these results reveal that the BSO/LED system has good degradation ability over a wide pH range.89
Further, the effect of common inorganic anions in water, viz., Cl−, NO3−, HCO3−, SO42−, and PO42− and the effect of organic matter (humic acid, HA) on the degradation of TC by the BSO/LED system was explored. Fig. 10b shows the impact of the inorganic ions on the TC degradation efficiency. Among the anions, HCO3− exhibits a significant inhibition for the degradation of TC, possibly due to the effective scavenging of HCO3− by the h+, resulting in decreased photocatalytic activity. Other anions viz Cl−, NO3− and PO42−, may undergo scavenging reactions leading to secondary reactions that produce less reactive species as shown in the reaction eqn (8)–(12) mentioned below.29,92,93
| HCO3− + h+ → CO3˙− | (8) |
| Cl− + h+ → Cl˙ | (9) |
| Cl˙ + Cl− → Cl2˙− | (10) |
 | (11) |
| HPO42− + h+ → HPO4˙− + h+ | (12) |
Moreover, humic acid (HA), a natural organic compound frequently detected in aquatic environments, significantly impacts the degradation of the tetracycline (TC) BSO/LED system. HA inhibits the degradation process by several mechanisms. Firstly, HA can adsorb onto the photocatalyst's surface, obstructing its active sites and hindering catalytic activity. Additionally, HA exhibits strong absorption in the UVA region, resulting in an inner filter effect that reduces the penetration of UVA light and limits photon availability for photocatalysis. Moreover, HA may react with reactive species generated in the reaction system, reducing the efficiency of TC degradation.94
 | ||
| Fig. 11 The effect of scavenging agents on the degradation TC [IPA] = [NaN3] = [BQ] = [EDTA] = 100 mM. [TC] = 10 mg L−1, BSO = 0.4 g L−1. | ||
ESR was utilized to identify the radicals generated during the photocatalysis. As illustrated in Fig. 12a, the DMPO-superoxide adduct was observed clearly in the BSO/LED system. The oxidation of TEMPO by h+, causing a decrease in TEMPO signal, was observed. Further, the photocatalysis reaction in the presence of DMPO showed no noticeable signals of DMPO–OH adduct, revealing HO˙ radical was not effectively generated in BSO photocatalysis Fig. 12b. Additionally, TEMP-singlet oxygen adduct single was not appreciably formed under BSO/LED system.96,97 Fig. 12c. These results corroborate the radical scavenging results, holes, and superoxide radical for the degradation of TC.29,82
TC under UVA LED irradiation showed no significant degradation (14.2%) because the energy gap between the HOMO and LUMO of TC is 4.2 eV,98 which is too high for a UVA LED (3.3 eV) to induce direct photochemical excitation and reaction. TC was also not effectively removed by adsorption onto the BSO (Fig. 6). However, 98.42% degradation efficiency was achieved by the BSO/LED system within 45 min. Based on the above results, the BSO/LED system photocatalysis mechanism is proposed as discussed below. BSO, upon light irradiation, generates e− and h+ pairs. A clear photocurrent response was observed for the BSO under LED irradiation, Fig. S9.† Interestingly, in the presence of TC, the photocurrent response was significantly enhanced, revealing that holes generated in under irradiation were scavenged by the TC. The Nyquist plot (Fig. S10a†) and Bode's plot (Fig. S10b†) were obtained, and the fmax (84.64) value was obtained from the Bode plot. The free charge carrier lifetime was determined to be 1.18 ms using eqn (13).99
 | (13) |
This result further corroborates with the effect of pH, scavenging by the EDTA, and the effect of bicarbonate ion, emphasising that the holes play a pivotal role in degradation next to the superoxide radical ion. The position of the conduction band (CB) −1.84 V (RHE) reveals that BSO is thermodynamically favourable to reducing the oxygen to a superoxide radical anion. Superoxide radical anion is known to induce the oxidation of antibiotic pollutants.29 The ECB value has more negative potential (i.e., −1.84 V) than the standard redox potential of O2˙−(E(O2/O2˙−) = −0.33 V), which is favourable for formation of superoxide radicals (O2˙−) by the reaction between O2 and excited photoelectron in CB.100 EVB is 1.36 V which is less compared to the standard potential of HO˙(E(HO−/HO˙) = +1.99 V, and E(H2O/HO˙) = +2.73 V), and hence the formation of hydroxy radical (HO˙) is not favourable, and corroborate well with the ESR, and radical scavenging experiments.101,102 The position of the valence band (VB) at 1.36 V reveals BSO is not efficient in producing hydroxyl radicals by oxidation of the water molecule. Thus, hydroxyl radicals were not observed in the ESR, and in the presence of hydroxyl radical scavenger, the photocatalysis was not retarded significantly. Under acidic conditions, pH = 3, the O2˙− may also go undergo reaction with photogenerated h+ to form less reactive H2O2 which may also contribute to retard the degradation reaction, in addition to electrostatic repulsion between positively charged TC, and BSO as discussed earlier.103 The degradation mechanism of TC by using BSO/LED light is represented by the following eqn (eqn (14)–(17)).104
| BaSnO3 + hν → e− + h+ | (14) |
| e− + O2 → O2˙− | (15) |
| h+ + TC → degradation | (16) |
| O2˙− + TC → degraded TC products | (17) |
3.3 Recyclability and stability studies
The reusability studies of the BSO were studied (Fig. 13). BSO exhibited excellent stability in the reuse study. Importantly, in the fourth cycle, photocatalytic activity was found to be more than 94%. This slight reduction in the activity may be attributed to the loss of the photocatalyst during the washing step. Further, Fig. S2† shows the comparative XRD plots before and after antibiotic degradation. It was observed that there was no significant decrease in the crystallinity of BSO even after use four times. Additionally, ICP-OES analysis was carried out to evaluate the leaching of active components, and the results showed minimal leaching of Ba (0.18%) and Sn (0.1%), indicating excellent chemical stability. Therefore, the slight reduction in degradation efficiency (from 100% to 94%) is primarily attributed to minor catalyst loss during the washing and recovery steps, rather than any significant deactivation. | ||
| Fig. 13 Recyclability test of BSO photocatalyst, experimental conditions: [TC] = 0.01 g L−1, BSO = 0.4 g L−1, and volume: 80 ml. | ||
The FESEM image shows well-separated smooth-surface cubes even after the degradation experiments (Fig. S3c†). The elemental composition of BSO after TC degradation remains largely unchanged and the atomic percentage and weight percentage of the elements are mentioned in the inset of Fig. S3d.† This indicates the stability of BSO following degradation. After the degradation experiment, the TEM image of BSO was also obtained which revealed no morphological changes (Fig. S4a and b†) compared to the fresh BSO. Overall, these results highlight the stability and reusability of BSO.
3.4 Degradation byproducts and toxicity
Degradation byproducts TC-1 and TC-2 were formed due to the cleavage of ring II, with complete ring-opening reactions and bond-breaking reactions, yielding smaller molecular fragments, as shown in Scheme 1. The loss of the side chain amino group in ring IV (deamination) and the amide group in ring IV (deamidation) led to intermediate TC-3 and TC-4, respectively (Fig. S11 and S12†). Similar degradation byproducts were earlier reported in photocatalysis.105,106 | ||
| Scheme 1 Possible degradation mechanism of TC by BSO/UVA-LED system [TC] = 10 mg L−1, BSO = 0.4 g L−1, and volume: 80 ml. | ||
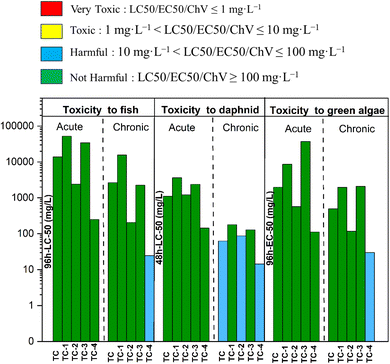
The ecotoxicity of TC and its degradation by-products using the ECOSAR model were presented in Fig. 14. TC and its degradation byproducts were predicted to be not harmful to acute toxicity towards fish, daphnids (LC50 value > 100 mg L−1), and green algae (EC50 value >100 mg L−1). Notably, TC and TC-2 showed chronic toxicity against daphnids (categorised as harmful). TC-4 was predicted chronic toxicity towards fish, daphnids, and green algae (categorised as harmful). Importantly, none of the by-products were predicted as being in the toxic chemical category, revealing the BSO/LED system as a potential system for treating TC without generating very toxic degradation byproducts (Fig. 14).107
3.5 Antibacterial activity assay
The antibacterial activity of TC before and after BSO/LED treatment (at 0–50 min) by BSO photocatalyst was determined (Fig. 15). These results reveal that TC (0.01 g L−1) before treatment showed a zone of inhibition against target E. faecalis and E. coli (Fig. 15A and C). Notably, after treatment (50 min), degraded TC showed no zone of inhibition against E. faecalis and E. coli. Notably, BSO photocatalysts showed no antibacterial activity against E. faecalis and E. coli (Fig. 15A and C). These results reveal that treatment by the BSO/LED system caused the complete is first to demonstrate the degradation of TC by BSO/LED system with complete removal of the antibacterial activity of TC.108 To the best of our knowledge, the present study of antibacterial activity is important to prevent the emergence of ARBs.4. Conclusion
TC was effectively degraded by the proposed BSO/LED system. XRD revealed the cubic phase of BSO. FESEM revealed the cubic morphology of BSO. TEM image showed the aggregation of nanosheets of BSO to form a cubic block-like morphology. The BET analysis reveals the slit-like mesopores in BSO. XPS analysis confirmed the presence of Ba2+, Sn4+ and lattice oxygen and adsorbed hydroxy species in BSO. At the optimised condition (BSO = 0.4 g L−1, TC = 10 mg L−1), the pseudo-first-order rate constant for TC degradation by the BSO/LED system was determined to be 0.0824 min−1. The effects of the initial concentration of TC, dosage of BSO, and pH on TC degradation by the BSO/LED system were presented. The positive Mott–Schottky curve reveals that BSO is an n-type semiconductor with a flat band potential (Efb) of −1.75 V (Vs Ag/AgCl). The radical scavenging results highlight the major role of superoxide (O2−˙) and holes (h+) in the degradation of TC. ESR results confirmed the O2−˙ and h+ were present in the BSO/LED system. The photocurrent experiments revealed the interaction of holes (h+) with TC, thereby increasing the photocurrent compared to BSO. The antibacterial activity of TC against Gram-negative Escherichia coli (E. coli) and Gram-positive Enterococcus faecalis (E. faecalis) was completely lost after BSO/LED treatment. The degradation pathways involved ring opening, deamination and deamidation reactions to produce degradation byproducts, which were identified by high-resolution mass spectrometry. Importantly, ECOSAR analysis predicted these degradation byproducts to be non-toxic. Further, EEo values were determined to be 13.63 kWh per m3 per order, and the cost was estimated to be $ 1 m−3, revealing that the BSO/LED system is energy-efficient and cost-effective in removing TC. Overall, the finding presents the degradation of TC by the proposed stable and energy-efficient BSO/LED system with antibacterial activity removal which is important to prevent the spreading of ARBs.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There is no conflict to declare.Acknowledgements
The authors acknowledge BITS Pilani, KK Birla Goa Campus for the PhD scholarship, and financial assistance for this research effort. Furthermore, author express thanks to the Department of Chemistry at BITS Pilani, KK Birla Goa Campus, provided access to BET, EIS, and UV-visible spectroscopy analysis, LC-MS, CSIF at BITS Pilani, KK Birla Goa Campus, provided FESEM, XRD, and Raman facilities. XPS was performed at CAL, BITS Pilani, Hyderabad Campus. The authors also acknowledge the Anusandhan National Research Foundation (ANRF- formerly DST-SERB) for a recently completed project that provided a desktop server for small-scale computations. Furthermore, the authors also acknowledge the Indian Institute of Technology, Bombay (IIT Bombay), India, for providing access to the ESR facilities and the University of Hyderabad for the TEM analysis.References
- M. Patel, R. Kumar, K. Kishor, T. Mlsna, C. U. Pittman and D. Mohan, Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods, Chem. Rev., 2019, 119(6), 3510–3673 CrossRef CAS PubMed.
- D. O'Flynn, J. Lawler, A. Yusuf, A. Parle-Mcdermott, D. Harold, T. Mc Cloughlin, L. Holland, F. Regan and B. White, A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic, RSC Adv., 2021, 5, 575–594 Search PubMed.
- O. A. H. Jones, N. Voulvoulis and J. N. Lester, Human Pharmaceuticals in Wastewater Treatment Processes, Crit. Rev. Environ. Sci. Technol., 2005, 35(4), 401–427 CrossRef CAS.
- K. Samal, S. Mahapatra and M. Hibzur Ali, Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health, Energy Nexus, 2022, 6, 100076 CrossRef CAS.
- N. Kemper, Veterinary antibiotics in the aquatic and terrestrial environment, Ecol. Indic., 2008, 8(1), 1–13 CrossRef CAS.
- S. M. Zainab, M. Junaid, N. Xu and R. N. Malik, Antibiotics and antibiotic-resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks, Water Res., 2020, 187, 116455 CrossRef CAS PubMed.
- K. O'Malley, W. McDonald and P. McNamara, Antibiotic resistance in urban stormwater: a review of the dissemination of resistance elements, their impact, and management opportunities, RSC Adv., 2023, 9, 2188–2212 Search PubMed.
- L. T. Le, Z. Huang, K. Whiteson and S. Jiang, Water Impact Statement The Occurrence and Diversity of Antibiotic Resistance and Virulence Factor Genes in Wastewater from Four North American Treatment Plants, RSC Adv., 2022, 8, 1650–1664 CAS.
- E. Y. Klein, I. Impalli, S. Poleon, P. Denoel, M. Cipriano, T. P. Van Boeckel, S. Pecetta, D. E. Bloom and A. Nandi, Global trends in antibiotic consumption during 2016–2023 and future projections through 2030, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(49), e2411919121 CrossRef CAS PubMed.
- P. Suyana, P. Ganguly, B. N. Nair, S. C. Pillai and U. S. Hareesh, Structural and compositional tuning in g-C3N4 based systems for photocatalytic antibiotic degradation, Chem. Eng. J. Adv., 2021, 8, 100148 CrossRef CAS.
- I. Chopra and M. Roberts, Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance, Microbiol. Mol. Biol. Rev., 2001, 65, 232–260 CrossRef CAS PubMed.
- D. Dionysiou, L. Puma, J. Ye, J. Schneider, D. Bahnemann, M. G. Antoniou, C. Zhao, K. E. O’shea, G. Zhang, D. D. Dionysiou, C. Zhao, C. Han, M. N. Nadagouda G, H. Choi, T. Fotiou, M. Triantis and A. Hiskia, Photocatalytic Degradation of Organic Contaminants in Water: Process Optimization and Degradation Pathways, RSC Energy Environ. Ser., 2016, 15, 1–34 Search PubMed.
- L. Xu, H. Zhang, P. Xiong, Q. Zhu, C. Liao and G. Jiang, Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A Review, Sci. Total Environ., 2021, 753, 141975 CrossRef CAS PubMed.
- Y. Amangelsin, Y. Semenova, M. Dadar, M. Aljofan and G. Bjørklund, The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies, Antibiotics, 2023, 12, 440 CrossRef CAS PubMed.
- Y. Liu, X. He, X. Duan, Y. Fu and D. D. Dionysiou, Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical, Chem. Eng. J., 2015, 276, 113–121 CrossRef CAS.
- Y. Zhao, Z. Liu, L. Li, S. Jiang and C. Shi, Systematic review of randomized controlled trials of traditional Chinese medicine treatment of non-acute bronchial asthma complicated by gastroesophageal reflux, J. Tradit. Chin. Med., 2012, 32(1), 12–18 CrossRef PubMed.
- H. Sah, Degradation patterns of tetracycline antibiotics in reverse micelles and water, Biomed, Chromatogr., 2006, 20(11), 1142–1149 CrossRef CAS PubMed.
- Y. Lin, S. Xu and J. Li, Fast and highly efficient tetracycline removal from environmental waters by graphene oxide functionalized magnetic particles, Chem. Eng. J., 2013, 225, 679–685 CrossRef CAS.
- A. Onal, Overview on liquid chromatographic analysis of tetracycline residues in food matrices, Food Chem., 2011, 127(1), 197–203 CrossRef.
- M. H. Khan, H. Bae and J. Y. Jung, Tetracycline degradation by ozonation in the aqueous phase: Proposed degradation intermediates and pathway, J. Hazard. Mater., 2010, 181, 659–665 CrossRef CAS PubMed.
- Y. Ding, W. Jiang, B. Liang, J. Han, H. Cheng, M. R. Haider, H. Wang, W. Liu, S. Liu and A. Wang, UV photolysis as an efficient pretreatment method for antibiotics decomposition and their antibacterial activity elimination, J. Hazard. Mater., 2020, 392, 122321 CrossRef CAS PubMed.
- K. Sharma, A. Sudhaik, Sonu, R. Kumar, V. H. Nguyen, Q. Van Le, T. Ahamad, S. Thakur, S. Kaya, L. H. Nguyen, P. Raizada and P. Singh, Advanced photo-Fenton assisted degradation of tetracycline antibiotics using α-Fe2O3/CdS/SiO2 based S-scheme photocatalyst, J. Water Proc. Eng., 2024, 59, 105011 CrossRef.
- Y. Liu, H. Liu, Z. Zhou, T. Wang, C. N. Ong and C. D. Vecitis, Degradation of the Common Aqueous Antibiotic Tetracycline using a Carbon Nanotube Electrochemical Filter, Environ. Sci. Technol., 2015, 49, 7974–7980 CrossRef CAS PubMed.
- K. Wei, Y. Faraj, G. Yao, R. Xie and B. Lai, Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress, Chem. Eng. J., 2021, 414, 128783 CrossRef CAS.
- P. Swaminaathan, A. Saravanan, P. R. Yaashikaa and A. S. Vickram, Recent advances in photocatalytic degradation of persistent organic pollutants: Mechanisms, challenges, and modification strategies, SCENV, 2024, 8, 100171 Search PubMed.
- Z. Yang, H. S. Xie, W. Y. Lin, Y. W. Chen, D. Teng and X. S. Cong, Enhanced Adsorption-Photocatalytic Degradation of Organic Pollutants via a ZIF-67-Derived Co-N Codoped Carbon Matrix Catalyst, ACS Omega, 2022, 7(45), 40882–40891 CrossRef CAS PubMed.
- S. Li, C. Zhang, F. Li, T. Hua, Q. Zhou and S. H. Ho, Technologies towards antibiotic resistance genes (ARGs) removal from aquatic environment: A critical review, J. Hazard. Mater., 2021, 411(6), 125148 CrossRef CAS PubMed.
- X. Hu, X. Hu, Q. Peng, L. Zhou, X. Tan, L. Jiang, C. Tang, H. Wang, S. Liu, Y. Wang and Z. Ning, Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2, Chem. Eng. J., 2020, 380, 122366 CrossRef CAS.
- L. G. Raikar, A. Patel, J. Gandhi, K. V. K. Gupta and H. Prakash, Nano-TiO2 immobilized polyvinylidene fluoride based spongy-spheres for ciprofloxacin photocatalytic degradation: antibacterial activity removal, mechanisms, UVA LED irradiation and easy recovery, Environ. Sci. Nano., 2024, 9, 3729–3743 RSC.
- K. Wang, G. Zhang, J. Li, Y. Li and X. Wu, 0D/2D Z-Scheme Heterojunctions of Bismuth Tantalate Quantum Dots/Ultrathin g-C3N4 Nanosheets for Highly Efficient Visible Light Photocatalytic Degradation of Antibiotics, ACS Appl. Mater. Interfaces, 2017, 9(50), 43704–43715 CrossRef CAS PubMed.
- N. Lin, Y. Gong, R. Wang, Y. Wang and X. Zhang, Critical review of perovskite-based materials in advanced oxidation system for wastewater treatment: Design, applications and mechanisms, J. Hazard. Mater., 2022, 424, 127637 CrossRef CAS PubMed.
- J. Wu, J. Chen, J. Gao, Z. Chen, L. Li and W. Wang, Recent Progress and Perspectives on Nonlead Halide Perovskites in Photocatalytic Applications, Energy Fuels, 2022, 36(24), 14613–14624 CrossRef CAS.
- T. Tabari, H. Tavakkoli, P. Zargaran and D. Beiknejad, Fabrication of Perovskite-type Oxide BaPbO3 Nanoparticles and their Efficiency in Photodegradation of Methylene Blue, S. Afr. J. Chem., 2012, 65, 239–244 CAS.
- J. Li, H. L. Cao, W. Bin Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold, Nat. Commun., 2020, 11, 310 CrossRef CAS PubMed.
- X. Chen, Q. Dong, S. Chen, Z. Zhang, X. Zhang, Y. Di, A. Jiang, D. Zhang and T. Li, Halloysite nanotubes supported BiVO4/BaSnO3 p–n heterojunction photocatalysts for the enhanced degradation of methylene blue under visible light, Colloids Surf., A, 2023, 664, 131143 CrossRef CAS.
- S. Bimli, S. R. Mulani, E. Choudhary, V. Manjunath, P. Shinde, S. R. Jadkar and R. S. Devan, Perovskite BaSnO3 nanoparticles for solar-driven bi-functional photocatalytic activity: PEC water splitting and Wastewater treatment, Int. J. Hydrogen Energy, 2024, 51, 1497–1507 CrossRef CAS.
- Z. M. Wong, H. Cheng, S. W. Yang, T. L. Tan and G. Q. Xu, Computational Design of Perovskite BaxSr1-xSnO3 Alloys as Transparent Conductors and Photocatalysts, J. Phys. Chem. C, 2017, 121(47), 26446–26456 CrossRef CAS.
- A. S. Deepa, S. Vidya, P. C. Manu, S. Solomon, A. John and J. K. Thomas, Structural and optical characterization of BaSnO3 nanopowder synthesized through a novel combustion technique, J. Alloys Compd., 2011, 509(5), 1830–1835 CrossRef CAS.
- S. Tao, F. Gao, X. Liu and O. T. Sùrensen, Ethanol-sensing characteristics of barium stannate prepared by chemical precipitation, Sens. Actuators, B, 2000, 71(3), 223–227 CrossRef CAS.
- Y. H. Ochoa-Muñoz, J. E. Rodríguez-Páez and R. Mejía de Gutiérrez, Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route, Mater. Chem. Phys., 2021, 266, 124557 CrossRef.
- A. Stanulis, S. Sakirzanovas, M. Van Bael and A. Kareiva, Sol-gel (combustion) synthesis and characterization of different alkaline earth metal (Ca, Sr, Ba) stannates, J. Sol-Gel Sci. Technol., 2012, 64(3), 643–652 CrossRef CAS.
- M. Licheron, G. Jouarf and E. Hussona, Characterization of BaSnO3, Powder Obtained by a Modified Sol-Gel Route, J. Eur. Ceram. Soc., 1997, 17, 1453–1457 CrossRef CAS.
- K. Habeeba, T. E. Manjulavalli, D. V. Ezhilarasi Gnanakumari and V. Karthikadevi, Highly crystalline perovskite BaSnO3 nanopowder synthesised using hydrothermal technique, Mater. Res. Express, 2019, 6(9), 094004 CrossRef CAS.
- N. Bader and B. Zimmermann, Co-precipitation as a sample preparation technique for trace element analysis: An overview, Int. J. Chem. Sci., 2014, 12(2), 519–525 CAS.
- Y. Jayavelu, G. Maharana, G. Rajender, R. Muniramaiah, S. Divyadharshini, B. H. Baby, M. Kovendhan, J. M. Fernandes and D. P. Joseph, Defect-mediated time-efficient photocatalytic degradation of methylene blue and ciprofloxacin using tungsten-incorporated ternary perovskite BaSnO3 nanoparticles, Chemosphere, 2024, 351, 141128 CrossRef CAS PubMed.
- X. Zhu, F. Qin, Y. Luo, L. Zhang, D. Yang, W. Feng and S. Chen, Enhanced photocatalytic activity of p (BaSnO3)-n (anatase/rutile/brookite TiO2) heterojunction composites by efficient interfacial charge transfer, J. Mol. Struct., 2023, 1294, 136440 CrossRef CAS.
- T. Li, A. Jiang, Y. Di, D. Zhang, X. Zhu, L. Deng, X. Ding and S. Chen, Novel BaSnO3/TiO2 @HNTs Heterojunction Composites with Highly Enhanced Photocatalytic Activity and Stability, ChemistrySelect, 2021, 6(40), 10817–10826 CrossRef CAS.
- J. E. Yager and C. David Yue, Environmental Chemistry Evaluation of the Xenon Arc Lamp As A Light Source For Aquatic Photodegradation Studies: Comparison With Natural Sunlight, Environ. Chem., 1988, 7(12), 1003–1011 CrossRef CAS.
- W. Aggoune, A. Eljarrat, D. Nabok, K. Irmscher, M. Zupancic, Z. Galazka, M. Albrecht, C. Koch and C. Draxl, A consistent picture of excitations in cubic BaSnO3 revealed by combining theory and experiment, Commun. Mater., 2022, 3(1), 12 CrossRef CAS.
- M. Asisi Janifer, S. Anand, C. Joseph Prabagar and S. Pauline, Structural and optical properties of BaSnO3 ceramics by solid state reaction method, Mater. Today Proc., 2021, 47, 2067–2070 CrossRef CAS.
- I. A. Mkhalid and S. I. El-Hout, S-scheme AgVO3-decorated BaSnO3 heterojunction for efficient photoreduction of Mercury (II) ions under visible light, J. Taiwan Inst. Chem. Eng., 2023, 146, 104896 CrossRef CAS.
- X. Song, W. Jiang, Z. Cai, X. Yue, X. Chen, W. Dai and X. Fu, Visible light-driven deep oxidation of NO and its durability over Fe doped BaSnO3: The NO+ intermediates mechanism and the storage capacity of Ba ions, Chem. Eng. J., 2022, 444, 136709 CrossRef CAS.
- K. A. Alzahrani and A. A. Ismail, Enhanced photocatalytic performances of highly efficient perovskite BaSnO3 nanorods decorated by CuMn2O4 nanoparticles, J. Photochem. Photobiol., A, 2023, 435, 114298 CrossRef CAS.
- M. Figueredo, E. M. Rodríguez, E. M. Cordero and F. J. Beltrán, UVA LEDs and solar light photocatalytic oxidation/ozonation as a tertiary treatment using supported TiO2: With an eye on the photochemical properties of the secondary effluent, J. Environ. Chem. Eng., 2022, 10(2), 107371 CrossRef CAS.
- C. Langford, M. Izadifard, E. Radwan and G. Achari, Some observations on the development of superior photocatalytic systems for application to water purification by the “Adsorb and shuttle” or the interphase charge transfer mechanisms, Molecules, 2014, 19, 19557–19572 CrossRef PubMed.
- W. K. Jo and R. J. Tayade, New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications, Ind. Eng. Chem. Res., 2014, 53, 2073–2084 CrossRef CAS.
- M. Forouzandeh-Malati, F. Ganjali, E. Zamiri, S. Zarei-Shokat, F. Jalali, M. Padervand, R. Taheri-Ledari and A. Maleki, Efficient Photodegradation of Eriochrome Black-T by a Trimetallic Magnetic Self-Synthesized Nanophotocatalyst Based on Zn/Au/Fe-Embedded Poly(vinyl alcohol), Langmuir, 2022, 38, 13728–13743 CrossRef CAS PubMed.
- S. Sharma, R. Shanmugam, R. B. Harikrishna, U. Prasad, G. Ranga Rao and A. M. Kannan, Y2O3 electrodeposited TiO2 nanotube arrays as photoanode for enhanced photoelectrochemical water splitting, Int. J. Hydrogen Energy, 2024, 52, 1415–1427 CrossRef CAS.
- C. Zhang, L. Chen, H. Luo, H. Weng, F. Qin, D. Qin and D. Huang, Selective micropollutants removal in wastewaters by non-radical activation of peroxymonosulfate: Multiparameter analysis of electron-donating capacity, Appl. Catal. B Environ., 2025, 362, 124695 CrossRef CAS.
- L. G. Raikar, J. Gandhi, K. V. K. Gupta and H. Prakash, Degradation of Ampicillin with antibiotic activity removal using persulfate and submersible UVC LED: Kinetics, mechanism, electrical energy and cost analysis, Chemosphere, 2023, 349, 140831 CrossRef PubMed.
- X. Zhang, M. Feng, R. Qu, H. Liu, L. Wang and Z. Wang, Catalytic degradation of diethyl phthalate in aqueous solution by persulfate activated with nano-scaled magnetic CuFe2O4/MWCNTs, Chem. Eng. J., 2016, 301, 1–11 CrossRef CAS.
- A. A. Ioannidi, G. Bampos, M. Antonopoulou, P. Oulego, G. Boczkaj, D. Mantzavinos and Z. Frontistis, Sonocatalytic degradation of Bisphenol A from aquatic matrices over Pd/CeO2 nanoparticles: Kinetics study, transformation products, and toxicity, Sci. Total Environ., 2024, 919 Search PubMed.
- J. H. Jorgensen and M. J. Ferraro, Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices, Clin. Infect. Dis., 2009, 49, 1749–1755 CrossRef CAS PubMed.
- J. Farkas, M. Náfrádi, T. Hlogyik, B. Cora Pravda, K. Schrantz, K. Hernádi and T. Alapi, Comparison of advanced oxidation processes in the decomposition of diuron and monuron-efficiency, intermediates, electrical energy per order and the effect of various matrices, Environ. Sci., 2018, 4, 1345–1360 CAS.
- U. Kumar, M. J. Ansaree and S. Upadhyay, Structural and optical characterizations of BaSnO3 nanopowder synthesized by aqueous sol-gel method, Process. Appl. Ceram., 2017, 11(3), 177–184 CrossRef CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- K. R. Dileep, M. K. Rajbhar, A. Ashina, E. Ramasamy, S. Mallick, T. N. Rao and G. Veerappan, A facile co-precipitation method for synthesis of Zn doped BaSnO3 nanoparticles for photovoltaic application, Mater. Chem. Phys., 2021, 258, 123939 CrossRef.
- T. N. Stanislavchuk, A. A. Sirenko, A. P. Litvinchuk, X. Luo and S. W. Cheong, Electronic band structure and optical phonons of BaSnO3 and Ba0.97La0.03SnO3 single crystals: Theory and experiment, J. Appl. Physiol., 2012, 112(4), 044108 CrossRef.
- N. Rajamanickam, K. Jayakumar and K. Ramachandran, Effect of iron doping on magnetic and electrical properties of BaSnO3 nanostructures, J. Mater. Sci.: Mater. Electron., 2018, 29, 19880–19888 CrossRef CAS.
- J. John, M. Dhananjaya, S. Suresh, S. Savitha Pillai, M. Sahoo, O. M. Hussain, R. Philip and V. P. Mahadevan Pillai, Effect of manganese doping on the structural, morphological, optical, electrical, and magnetic properties of BaSnO3, J. Mater. Sci.: Mater. Electron., 2020, 31, 11159–11176 CrossRef CAS.
- J. John, S. Suresh, S. Pillai.S, R. Philip and V. P. M. Pillai, Effect of Fe doping on the structural, morphological, optical, magnetic and dielectric properties of BaSnO3, J. Mater. Sci.: Mater. Electron., 2021, 32, 11763–11780 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- A. Marikutsa, M. Rumyantseva, A. Baranchikov and A. Gaskov, Nanocrystalline BaSnO3 as an alternative gas sensor material: Surface reactivity and high sensitivity to SO2, Materials, 2015, 8, 6437–6454 CrossRef CAS PubMed.
- W. Zhang, F. A. Guo and G. Li, Barium staminate as semiconductor working electrodes for dye-sensitized solar cells, Int. J. Photoenergy, 2010, 2010, 1–7 CrossRef.
- A. Marikutsa, M. Rumyantseva, A. Baranchikov and A. Gaskov, Nanocrystalline BaSnO3 as an Alternative Gas Sensor Material: Surface Reactivity and High Sensitivity to SO2, Materials, 2015, 8(9), 6437–6454 CrossRef CAS PubMed.
- J. John, S. Suresh, S. Savitha Pillai, R. Philip and V. P. Mahadevan Pillai, Structural, Morphological, Magnetic and Optical Limiting Performance of Ni Doped BaSnO3, J. Electron. Mater., 2021, 50, 5868–5880 CrossRef CAS.
- L. H. Grey, H. Y. Nie and M. C. Biesinger, Defining the nature of adventitious carbon and improving its merit as a charge correction reference for XPS, Appl. Surf. Sci., 2024, 653, 159319 CrossRef CAS.
- S. H. Butt, M. S. Rafique, K. Siraj, A. Latif, A. Afzal, M. S. Awan, S. Bashir and N. Iqbal, Epitaxial thin-film growth of Ruddlesden–Popper-type Ba3Zr2O7 from a BaZrO3 target by pulsed laser deposition, Appl. Phys. A:Mater. Sci. Process., 2016, 122(7), 658 CrossRef.
- J. John, S. R. Chalana, R. Prabhu and V. P. Mahadevan Pillai, Effect of oxygen pressure on the structural and optical properties of BaSnO3 films prepared by pulsed laser deposition method, Appl. Phys. A:Mater. Sci. Process., 2019, 125(3), 155 CrossRef CAS.
- J. C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Systematic XPS studies of metal oxides, hydroxides and peroxides, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
- L. Q. Wu, Y. C. Li, S. Q. Li, Z. Z. Li, G. D. Tang, W. H. Qi, L. C. Xue, X. S. Ge and L. L. Ding, Method for estimating ionicities of oxides using O1s photoelectron spectra, AIP Adv., 2015, 5(9), 097210 CrossRef.
- S. Gupta, J. Gandhi, S. Kokate, L. G. Raikar, V. G. Kopuri and H. Prakash, Augmented photocatalytic degradation of Acetaminophen using hydrothermally treated g-C3N4 and persulfate under LED irradiation, Heliyon, 2023, 9, e16450 CrossRef CAS PubMed.
- S. Kokate, S. Gupta, V. G. Kopuri and H. Prakash, Energy efficient photocatalytic activation of peroxymonosulfate by g-C3N4 under 400 nm LED irradiation for degradation of Acid Orange 7, Chemosphere, 2022, 287, 132099 CrossRef CAS PubMed.
- J. Wang, C. Liu, J. Feng, D. Cheng, C. Zhang, Y. Yao, Z. Gu, W. Hu, J. Wan and C. Yu, MOFs derived Co/Cu bimetallic nanoparticles embedded in graphitised carbon nanocubes as efficient Fenton catalysts, J. Hazard. Mater., 2020, 394, 122567 CrossRef CAS PubMed.
- M. Hasham Firooz, A. Naderi, M. Moradi and R. R. Kalantary, Enhanced tetracycline degradation with TiO2/natural pyrite S-scheme photocatalyst, Sci. Rep., 2024, 14, 4954 CrossRef PubMed.
- S. Sun, Y. Zhou, Y. Chen, Y. Wu and S. Bao, The performance and mechanism of phosphotungstic acid-modified NiFe-LDH heterogeneous photo-Fenton catalyst for efficient degradation of tetracycline, Environ. Sci.: Nano, 2024, 11, 4321–4336 RSC.
- F. Saadati, N. Keramati and M. M. Ghazi, Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review, Crit. Rev. Environ. Sci. Technol., 2016, 46, 757–782 CrossRef CAS.
- S. Wu, X. Li, Y. Tian, Y. Lin and Y. H. Hu, Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light, Chem. Eng. J., 2021, 406, 126747 CrossRef CAS.
- C. Hu, H. Huang, F. Chen, Y. Zhang, H. Yu and T. Ma, Coupling Piezocatalysis and Photocatalysis in Bi4NbO8X (X = Cl, Br) Polar Single Crystals, Adv. Funct. Mater., 2020, 30, 1908168 CrossRef CAS.
- V. Vaiano, G. Iervolino and L. Rizzo, Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light, Appl. Catal. B Environ, 2018, 238, 471–479 CrossRef CAS.
- L. Xu, H. Zhang, P. Xiong, Q. Zhu, C. Liao and G. Jiang, Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review, Sci. Total Environ., 2021, 753, 141975 CrossRef CAS PubMed.
- J. Gandhi and H. Prakash, Photo-disinfection processes for bacterial inactivation and underlying principles for water constituents' impact: A review, Chem. Eng. J. Adv., 2023, 14, 100482 CrossRef CAS.
- N. Li, Y. Wang, X. Cheng, H. Dai, B. Yan, G. Chen, L. Hou and S. Wang, Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review, Water Res., 2022, 222, 118896 CrossRef CAS PubMed.
- S. Li, C. Wang, Y. Liu, M. Cai, Y. Wang, H. Zhang, Y. Guo, W. Zhao, Z. Wang and X. Chen, Photocatalytic degradation of tetracycline antibiotic by a novel Bi2Sn2O7/Bi2MoO6 S-scheme heterojunction: Performance, mechanism insight and toxicity assessment, Chem. Eng. J., 2022, 429, 132519 CrossRef CAS.
- C. Yin, Y. Liu, X. Kang and X. Li, Synergistic degradation of tetracycline by CDs decorated g-C3N4 under LED light irradiation combined with the persulfate-based advanced oxidation process, Appl. Catal. A Gen., 2022, 636, 118571 CrossRef CAS.
- H. Zhang, J. Joseph, C. Felix and B. Kalyanaraman, Bicarbonate Enhances the Hydroxylation, Nitration, and Peroxidation Reactions Catalyzed by Copper, Zinc Superoxide Dismutase Intermediacy Of Carbonate Anion Radical, J. Biol. Chem., 2000, 275, 14038–14045 CrossRef CAS PubMed.
- I. Milošević, S. Guillot, M. Tadić, M. Duttine, E. Duguet, K. Pierzchala, A. Sienkiewicz, L. Forró and M. L. Saboungi, Loading and release of internally self-assembled emulsions embedded in a magnetic hydrogel, Appl. Phys. Lett., 2014, 104, 043701 CrossRef.
- D. Y. Xing, X. D. Zhao, C. S. He and B. Lai, Kinetic study and DFT calculation on the tetracycline abatement by peracetic acid, Chin. Chem. Lett., 2024, 35(9), 109436 CrossRef CAS.
- S. Bimli, S. R. Mulani, E. Choudhary, V. Manjunath, P. Shinde, S. R. Jadkar and R. S. Devan, Perovskite BaSnO3 nanoparticles for solar-driven bi-functional photocatalytic activity: PEC water splitting and Wastewater treatment, Int. J. Hydrog. Energy, 2024, 51, 1497–1507 CrossRef CAS.
- W. Zhang, L. Zhou, J. Shi and H. Deng, Synthesis of Ag3PO4/G-C3N4 Composite with Enhanced Photocatalytic Performance for the Photodegradation of Diclofenac under Visible Light Irradiation, Catalysts, 2018, 8, 45 CrossRef.
- C. Hu, H. Huang, F. Chen, Y. Zhang, H. Yu and T. Ma, Coupling Piezocatalysis and Photocatalysis in Bi4NbO8X (X = Cl, Br) Polar Single Crystals, Adv. Funct. Mater., 2020, 30, 1908168 CrossRef CAS.
- R. Yanagi, T. Zhao, D. Solanki, Z. Pan and S. Hu, Charge Separation in Photocatalysts: Mechanisms, Physical Parameters, and Design Principles, ACS Energy Lett., 2022, 7, 432–452 CrossRef CAS.
- J. Ni, W. Wang, D. Liu, Q. Zhu, J. Jia, J. Tian, Z. Li, X. Wang and Z. Xing, Oxygen vacancy-mediated sandwich-structural TiO2−x/ultrathin g-C3N4/TiO2−x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics, J. Hazard. Mater., 2021, 408, 124432 CrossRef CAS PubMed.
- A. A. Baoum and A. A. Ismail, Enhanced photocatalytic efficiency of highly effective and stable perovskite BaSnO3 with monoclinic Li2MnO3 nanoparticles: Atrazine a case study of herbicide, Ceram. Int., 2023, 49, 23227–23237 CrossRef CAS.
- X. Yu, X. Jin, M. Li, Y. Yu, M. Zhu, S. Tang, H. Zhou, K. Wang, R. Dou and J. Sun, Degradation mechanism of tetracycline using sulfidated nanoscale zerovalent iron driven peroxymonosulfate and metabolomic insights into environmental risk of intermediates products, Chem. Eng. J., 2022, 430, 133141 CrossRef CAS.
- Z. Maa, L. Hub, X. Lia, L. Denga, G. Fana and Y. Heb, A novel nano-sized MoS2 decorated Bi2O3 heterojunction with enhanced photocatalytic performance for methylene blue and tetracycline degradation, Ceram. Int., 2019, 45, 15824–15833 CrossRef.
- H. Liu, J. Qu, T. Zhang, M. Ren, Z. Zhang, F. Cheng, D. He and Y. nan Zhang, Insights into degradation pathways and toxicity changes during electro-catalytic degradation of tetracycline hydrochloride, Environ. Pollut., 2019, 258, 113702 CrossRef PubMed.
- A. Patidar, V. R. Dugyala, S. Chakma, M. N. Galodiya and A. S. Giri, Reactive oxygen species aided photocatalytic degradation of tetracycline using non-metal activated carbon doped TiO2 nanocomposite under UV-light irradiation, Res. Chem. Intermed., 2024, 50, 1035–1063 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02938d |
| This journal is © The Royal Society of Chemistry 2025 |