 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rare earth composite MOFs materials for energy, environmental and medical applications
Haonan Peia,
Guilin Hea,
Shuaibo Jianga,
Bingxin Xuea,
Fangdi Huanga,
Hongqun Tangac,
Nannan Wang *a and
Yanqiu Zhu
*a and
Yanqiu Zhu *ab
*ab
aState Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures, MOE Key Laboratory of New Processing Technology for Nonferrous Metals and Materials, School of Resources, Environment and Materials, Guangxi University, Nanning 530004, China. E-mail: wangnannan@gxu.edu.cn
bFaculty of Environment, Science and Economy, University of Exeter, EX4 4QF, UK. E-mail: Y.zhu@exeter.ac.uk
cKey Laboratory of High Performance Structural Materials and Thermo-surface Processing (Guangxi University), Education Department of Guangxi Zhuang Autonomous Region, Nanning 530004, China
First published on 24th June 2025
Abstract
Rare Earth Elements (REEs) are a group of seventeen specialized elements that have shown irreplaceable roles in energy, environment and medical fields due to their unique electronic structures and chemical properties. Rare Earth Composite Metal–Organic Frameworks (RE-MOFs) have become a hotspot for interdisciplinary research as they combine the excellent properties of REEs with the porous properties of MOFs, which have high specific surface area, adjustable pore size, abundant active sites, and multifunctionality. In this paper, we systematically review the progress of the application of RE-MOFs in the fields of energy storage and conversion, pollution control, and biomedicine, analyze the performance optimization strategies and challenges, and look forward to the future development direction. Through integrated material design, innovation in synthesis methods and interdisciplinary cooperation, RE-MOFs are expected to drive the commercialization of next-generation high-performance materials and help achieve the global sustainable development goals.
1. Introduction
Rare earth elements (REEs) are known as the “vitamins of modern industry” because of their unique 4f electron layer structure and variable oxidation states, which have shown irreplaceable functionality in the fields of catalysis, optics, magnetism and energy conversion. With the growing global demand for clean energy,1,2 environmental governance and precision medicine,3,4 the boundaries of rare earth element applications continue to expand. Meanwhile, metal–organic frameworks, as a class of porous crystalline materials formed by the self-assembly of metal nodes and organic ligands, have become a research hotspot in the field of materials science by virtue of their high specific surface area, adjustable pore size, abundant active sites, and multifunctionality.5–8 In the environmental field, MOFs show great promise as desalination membrane materials for desalination of seawater by virtue of their structural diversity, tunability, and porous voids providing secondary water channels.9 Furthermore, the selection of linkers and metal nodes along with the post-synthesis modifications to tune the pore-functionalized MOFs can also achieve efficient target-specific identification of aquatic inorganic pollutants.10 In the biomedical field, MOFs are an emerging candidate to mitigate environmental pollution and health diseases related to it.11In recent years, the research on the application of RE-MOFs in the fields of energy, environment and medicine has made remarkable progress. In the field of energy, RE-MOFs have significantly improved the electrochemical energy storage efficiency and photoelectrocatalytic activity through the synergistic effect of rare earth ions and organic ligands. Examples include the high capacity properties of rare earth-doped MOFs for lithium-ion batteries,12 and photocatalytic CO2 reduction with a high selectivity.13 In environmental governance, the multistage pore and selective adsorption capabilities of RE-MOFs make them an efficient platform for pollutant detection and removal, such as fluorescent sensing of volatile organic compounds (VOCs),14 targeted adsorption of heavy metal ions and photocatalytic degradation of organic pollutants. In the biomedical field, RE-MOFs, with their luminescent properties, biocompatibility and drug loading capacity, have promoted the development of targeted drug delivery, multimodal imaging and intelligent diagnostic and therapeutic systems, for example, europium (Eu) MOFs-based fluorescent probes in the early diagnosis of cancer.15 Aptamer-guided nanocarriers with mesoporous MOF shells and upconverted luminescent NaYF4:Yb3+/Er3+ NPs for targeted drug delivery and cellular imaging.16
However, the large-scale application of RE-MOFs still faces many challenges. First, the cycling performance of the materials needs to be improved; second, the potential migration risk of rare earth ions and environmental safety need to be systematically evaluated;13 in addition, the problems of high synthesis cost and complex process17 also restrict their industrialization. To address these issues, the researchers have developed a new approach through topological modification,18 defect engineering,19 cross-material composites, and other strategies to optimize performance, to advance RE-MOFs from laboratory exploration to practical applications.
In this paper, we systematically review the latest research progress of RE-MOFs in the fields of energy storage and conversion, environmental remediation, and biomedicine, with a focus on analyzing the mechanism of performance optimization, and looking forward to the future development direction. Through the integration of material design, synthesis method innovation and multidisciplinary cross-collaboration, RE-MOFs are expected to provide transformative solutions to global challenges such as carbon neutrality, pollution control and precision medicine, and contribute to the realization of sustainable development goals.
2. Material design
The design and synthesis of novel functional materials is a common goal for researchers in the fields of science, engineering, and technology. MOFs have gained notable attention as potential functional materials due to their highly tunable synthesis. The introduced reticulation chemistry, which is the basis of MOF synthesis, is defined as the design and formation of ordered network materials with predetermined structures. This means that by understanding the geometry and connectivity of the building blocks, it is possible to build the same structure (or network) with several building blocks in order to generate a specific network structure.Regardless of size, these building blocks can be manipulated to have the same (or similar) geometry and connectivity. These building blocks are inorganic metal nodes (ions, chains, or clusters) and organic connectors that come together to form secondary structural units (SBUs), which are assembled to form the framework material.20 Combining the unique properties of rare earth elements with the structural advantages of MOFs to form Rare Earth Composite Metal Organic Frameworks (RE-MOFs) not only inherits tunable porous structure21 and high adsorption capacity of MOFs, but also endows the materials with a new functional dimension through the optical, electrical, and magnetic properties of rare earth ions, which provides an important interdisciplinary innovation carrier.
The following is a systematic classification summary based on the types, doping mechanisms and functionalization applications of rare earth metal–organic frameworks (RE-MOFs), which provides theoretical references and practical guidelines for the rational design and cross-field applications of novel functional materials (Table 1).
| RE-MOF type | Rare earth doping mechanism | Typical application |
|---|---|---|
| Ce-MOF | In situ coordination substitution: Ce3+/Ce4+ directly acts as a metal node, forming strong coordination bonds with carboxylic acid ligands (Ce–O bond energies >500 kJ mol−1), replacing conventional transition metal nodes | Photocatalytic degradation of pollutants, CO2 reduction |
| Eu-MOF | Ligand-sensitized luminescence: Eu3+ captures the energy of the ligand excited state through the “antenna effect” (ligand → Eu3+ energy transfer efficiency >80%), achieving efficient fluorescence emission | Fluorescence sensing (Hg2+ detection) |
| Tb-MOF | Rigid coordination locking: Tb3+ forms an octa-coordinated structure with a polydentate carboxylic acid ligand, which inhibits ion leakage through spatial site resistance (Tb3+ release rate <0.01%) | Biological microenvironment temperature monitoring |
| Gd-MOF | Rapid nucleation dominance: microwave heating accelerates the coordination kinetics of Gd3+ with carboxylic acid ligands, preferentially forming Gd–O clusters as stable SBUs (secondary structural units) | MRI contrast agent |
| Yb-MOF | Pore-limited domain ion exchange: Yb3+ enters the ZIF-8 pore by diffusion, displacing part of the Zn2+ site (exchange rate ∼30%) to form a Yb–N coordination bond | Tumor diagnosis |
| Nd-MOF | Macrocyclic ligand chelation: the four pyrrole nitrogens of the porphyrin form a planar tetragonal coordination with Nd3+, with a central cavity stabilizing Nd3+ (coordination constant log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K > 15) K > 15) |
Photothermal therapy |
| Er-MOF | Dynamic coordination regulation: Er3+ forms a reversible coordination bond with a flexible fumarate ligand, and humidity change triggers the ligand to rotate, realizing pore switching (pore size change 0.3 → 1.2 nm) | Real-time monitoring of environmental humidity |
3. Applications in the energy sector
3.1 Energy storage
Metal–organic frameworks (MOFs) represent a distinct type of hybrid materials, combining high porosities with diverse properties that arise from their organic and inorganic building units,22 with excellent properties such as morphological diversity, high porosity, large specific surface area, and abundant active sites,23 and thus show significant potential for application in aerospace24 and electrochemical energy storage. However, pristine MOF severely limits its application in electrochemical energy storage due to poor electrical conductivity and low specific capacitance.25 Rare earth elements (REEs) are widely used in energy storage due to their atomic structure with 4f electron layers and abundant electron energy levels, which can be combined with MOF structures to form a variety of new composites with unique properties. In this section, we summarize the application of RE-MOF materials to batteries and supercapacitors.The RE-MOFs materials can provide rich interfaces and efficient mass transfer channels for the electrochemical reactions inside the battery. The introduction of lanthanides, which have high charge density and strong coordination ability, can optimize the electronic structure and pore environment of MOFs, enhance electrostatic interactions with Li+ and selective adsorption, and thus improve the lithium ion storage performance. Therefore, RE-MOFs materials can be a candidate for solving the LIB electrode material problem. Zhao et al. synthesized a series of bimetallic metal–organic frameworks (MOFs) with the same spatial structure using 3,5-pyrazole dicarboxylic acid monohydrate as the organic linker.33 Mn–La MOF doped with lanthanides as an anode material for lithium-ion batteries (LIBs) exhibited the highest capacity (510.67 mAh g−1 after 400 cycles at 100 mA g−1 current density) and the lowest difficulty in ion transport, as shown in Fig. 1.12 Lv et al. prepared a new highly symmetric MOF material with the chemical formula C30H40Fe6LnN6Na2O44P6 (where Ln stands for lanthanum, cerium, and praseodymium) by a new ionothermal synthesis method.34 FLaN-MOF and commercial LiCoO2 were used as negative and positive electrodes, respectively, to assemble the cell for LIBs. The cell provides a reversible capacity of 145 mAh g−1 with a coulombic efficiency of 98.6%. After 100 cycles, the reversible specific capacity was as high as 337 mAh g−1, see Fig. 2.35 Tb-MOF, a rare earth complex, was successfully prepared by solvothermal method by Xia et al. Experiments have shown that the flexible skeleton of Tb-MOF provides efficient channels for lithium ion de-embedding, the abundant carboxyl groups and benzene rings construct chemisorption sites, and the strong Tb–O bond guarantees the reversibility of the electrode structure, which results in the excellent cycling stability of the complex.36
 | ||
| Fig. 1 Current density of Mn–La MOF after 400 cycles. Reproduced from ref. 12 with permission from Elsevier, copyright 2022. | ||
 | ||
| Fig. 2 Reversible capacity of FLaN-MOF after 100 cycles at 100 mA g−1. Reproduced from ref. 35 with permission from Elsevier, copyright 2021. | ||
 | ||
| Fig. 3 Rare earth compounds in lithium–sulfur batteries. Reproduced from ref. 40 with permission from Elsevier, copyright 2023. | ||
The rare earth element yttrium has unique optical, electrical, magnetic and catalytic properties,41 and the composite of yttrium with MOF can endow the material with high specific surface area and excellent catalytic properties. Qian et al. prepared Y2O3–C@CNT composite diaphragm material by growing Y2O3–C nanoparticles in situ on the surface of carbon nanotubes via aqueous method, and then prepared Y2O3–C@CNT composite diaphragm material through high temperature carbonization. The experimental results show that the material is characterized by porous structure, high specific surface area, synergistic distribution of micro- and mesopores and excellent electrical conductivity, and the abundant polar sites on its surface can effectively anchor polysulfides. The PE diaphragm coated with this composite material enables the lithium–sulfur battery to obtain an initial discharge capacity of 900 mAh g−1 at 0.5C and 3 mg cm−2 high sulfur loading, and the capacity retention rate reaches 53.7% (483.85 mAh g−1) after 400 cycles, which is a significant en hancement of the cycling performance for LSBs.
CeO2 nanoparticles have received much attention due to their ability to adsorb and catalyze the transformation of polysulfides well,42 and combining CeO2 nanoparticles with MOF can rapidly adsorb sulfides and promote their rapid catalytic. Hong et al. synthesized MOFs containing Ce(IV)-cluster nodes, which were then combined with CNTs to form Ce-MOFs/CNT composites and used as the diaphragm coatings for LSBs.43 The experimental results show that the initial specific capacity of the Ce-MOF-2/CNT-coated cell is as high as 1021.8 mAh g−1, which slowly decreases to 838.8 mAh g−1 after 800 cycles, with a decay rate of 0.022% and a coulombic efficiency of nearly 100%. This demonstrated that the Ce-MOF-2/CNT porous diaphragm coating material (see Fig. 4 (ref. 43)) could play the dual functional roles of catalytic conversion of polysulfides and blocking of polysulfide transport pathways, thus effectively suppressing the shuttle effect in Li–S batteries. Liu et al. successfully synthesized phosphorus-doped porous CeO2 (P-CeO2) by solvothermal method, calcination and phosphatization treatment, and modified commercial PP diaphragm by coating process.44 The results show that at a sulfur loading of 1.28 mg cm−2, the cell capacity reaches 1180 mA h−1 with a decay rate of 0.10% per turn at 0.5C magnification. When the sulfur loading was increased to 2.0 mg cm−2, the decay rate per turn decreased to 0.048% at 0.5C multiplication. As a result, the P-CeO2 diaphragm promotes chemical uptake and catalytic conversion of polysulfides in lithium–sulfur batteries, leading to high performance and long cycle life.
 | ||
| Fig. 4 Ce-MOF-2/CNT as a diaphragm coating material for Li–S batteries. Reproduced from ref. 43 with permission from ACS Publications, copyright 2019. | ||
In addition to the introduction of Y and Ce, Nd, as an active rare earth element, can be combined with MOF to have a unique pore structure and a large number of active sites, which can block the shuttle effect of LiPs and ensure the rapid penetration of the electrolyte and ion diffusion.43 Hao et al. synthesized Nd-MOF/KB and Nd-MOF/CNT materials by hydrothermal method and obtained Nd2O3–C/KB and Nd2O3–C/CNT composites by high-temperature carbonization treatment.45 The experimental results show that these carbonized composites have a porous structure and the metal oxides in them provide abundant adsorption sites, and these properties can effectively inhibit the migration of lithium polysulfides (LiPSs) and promote the redox transformations in the cathode reaction, and Nd2O3–C/KB exhibits better cycling and multiplication performance than Nd2O3–C/CNT when used as a battery separator performance.
CeO2, as an economical rare earth oxide with both environmental friendliness and excellent intrinsic redox activity,50 is regarded as a promising candidate for application, but its low specific surface area results in a significantly lower intrinsic theoretical specific capacity (∼560 F g−1) than that of transition metal oxides, which restricts its practical application in high-performance supercapacitors. MOF consists of metal ions or clusters and organic joints, which are compounds that can provide the highest surface area. Mutual bonding of CeO2 with MOF enhances its specific surface area and thus facilitates the commercialization of CeO2. Maiti et al. innovatively employed Ce-BTC metal–organic frameworks (cerium 1,3,5-benzenetricarboxylic acid ligand) as a sacrificial template, which were synthesized via solvothermal synthesis combined with a 650 °C/3 h calcination process to successfully prepare nanostructured CeO2 electrode materials.51 The experimental results show that 92% of the theoretical specific capacitance (based on the theoretical value of 560 F g−1) can be achieved in aqueous supercapacitors with this MOF-derived CeO2, and the pseudocapacitance performance occurs through the introduction of the K4Fe(CN)6/KOH composite electrolyte optimization strategy. The measured specific capacitance reaches 1204 F g−1, which exceeds the theoretical value by 115%. This dual-track strategy of “MOF template method + electrolyte engineering” provides a new idea to break the intrinsic capacitance limit of metal oxides. Zeng et al. developed a room-temperature alkali treatment method based on Ce-BTC MOF precursor, and successfully prepared CeO2 electrode materials with a graded dumbbell-like structure.52 The mesoporous template effect of the pristine MOF and the nanoparticle gap work together to give the CeO2 material a bimodal pore system that cuts through the mesopore and the microporous, which enhances the electrolyte wettability and ion diffusion rate. The experimental results show that this material exhibits a higher specific capacitance as a supercapacitor electrode when K4Fe(CN)6 is added to the KOH electrolyte. The maximum capacitance was 779 F g−1 at 1 A g−1, and the capacitance retention was close to 91% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, see Fig. 5.52
000 cycles, see Fig. 5.52
 | ||
Fig. 5 Capacitance of CeO2 electrode material after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Reproduced from ref. 52 with permission from Elsevier, copyright 2016. 000 cycles. Reproduced from ref. 52 with permission from Elsevier, copyright 2016. | ||
Hao et al. successfully prepared unique spherical-rod-like structure (NiCo)Se2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-CeO2 materials by adjusting the ratio of ligands in the precursor MOF and using a one-step solvothermal method followed by a selenization process.25 The electrode material exhibits a specific capacitance of up to 2715 F g−1 (or 1222 mAh g−1) at a current density of 1 A g−1 and shows excellent multiplicative performance at 10 A g−1 with a loss of only 3.43% of the specific capacitance, solving the electrochemical energy storage and harvesting problem to some extent, see Fig. 6.53
7-CeO2 materials by adjusting the ratio of ligands in the precursor MOF and using a one-step solvothermal method followed by a selenization process.25 The electrode material exhibits a specific capacitance of up to 2715 F g−1 (or 1222 mAh g−1) at a current density of 1 A g−1 and shows excellent multiplicative performance at 10 A g−1 with a loss of only 3.43% of the specific capacitance, solving the electrochemical energy storage and harvesting problem to some extent, see Fig. 6.53
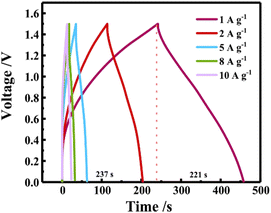 | ||
| Fig. 6 GCD curve for 1 to 10 A g−1. Reproduced from ref. 53 with permission from Elsevier, copyright 2024. | ||
Lanthanides other than Ce also have higher coordination numbers and flexible coordination geometries, which can increase the porosity and size of voids.54 Dezfuli et al. synthesized Eu-MOF by a hydrothermal method using fumarate and oxalate.55 Eu-MOF has a specific capacitance of 468 F g−1 discharge current density of 1 A g−1 and maintains 95.2% capacitance after 4000 cycles. Jafari et al. prepared by hydrothermal method [Tb2(fma)2(ox)(H2O)4·4H2O] rare earth metal–organic framework (Tb-MOF).56 The experimental results show that the Tb-MOF electrode exhibits a high specific capacitance of 510 F g−1 at a current density of 1 A g−1, and maintains a capacity retention of 351 F g−1 (68.8% capacity retention) even at a high current density of 16 A g−1, and a capacity retention of 91.9% after 4000 charge/discharge cycles. The capacity retention rate reaches 91.9% after 4000 charge/discharge cycles, providing a new paradigm for the design and application of rare earth MOFs in energy storage devices.
3.2 Gas storage
3.3 Energy conversion
Hydrogen energy is one of the world's important future energy sources, and hydrogen production from electrolyzed water has been the mainstream industrial hydrogen production technology.61 However, the problems of large overpotential and low electrochemical energy conversion efficiency during the hydrogen precipitation reaction62 have become the main obstacles to the commercialization of hydrogen energy, and the use of RE-MOFs materials can solve the above problems to a certain extent. Shi et al. prepared Er-MOF/NiS catalysts by loading NiS catalysts onto Er-MOF via a modified hot-solvent method.63 The results showed that the catalyst exhibited a significantly lower overpotential (115 mV), smaller Tafel slope (83.48 mV dec−1), smaller charge transfer resistance (11.304 Ω), and larger electrochemically active area (1432.025 cm2). Liao et al. applied the hot solvent method to prepare Er-MOF/MoS2 composites, which were obtained by using Er-MOF as a precursor and compounding MoS2.61 Compared to Er-MOF and MoS2 alone, the Er-MOF/MoS2 composite exhibits the best performance in terms of hydrolysis electrocatalytic activity. Specifically, the composite achieves a current density of 10 mA cm−2 at an overpotential of only 234 mV, while exhibiting excellent long-term durability, highlighting its superior catalytic performance.
Another important reaction in hydrogen production from electrolyzed water is the Oxygen Evolution Reaction (OER). The use of rare-earth composite MOF materials as catalysts can improve the overall electrochemical energy conversion efficiency of electrolyzed water. Shabbir et al. successfully prepared innovative Pr-MOF/Fe2O3 composites by hydrothermal synthesis under controlled conditions. Pr-MOF/Fe2O3 nanocomposites at a current density of 10 mA cm−2 exhibited remarkable OER properties with low onset potential (1.41 V), low overpotential (238 mV) and low Tafel slope value (37 mV dec−1).64 In addition, the composite showed good long-term stability in 1 M alkaline solution (KOH) with no change in current density or performance. Fig. 7 (ref. 65) demonstrates the polarization curves of nanomaterials deposited on NF (nickel foam) in aqueous solution (1 M potassium hydroxide).
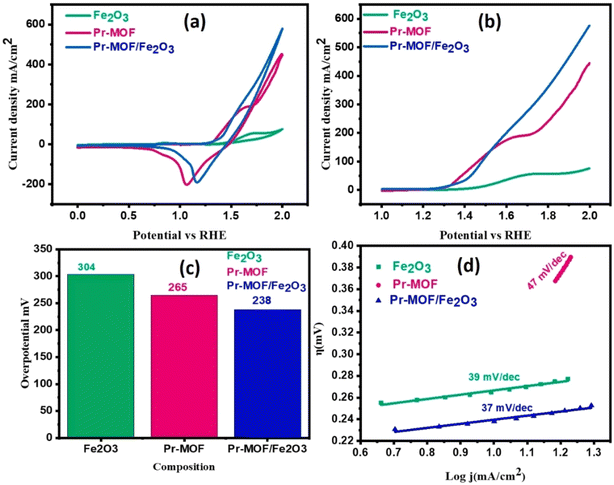 | ||
| Fig. 7 (a) Cyclic voltammograms; (b) linear scanning voltammograms; (c) comparison of overpotentials at a current density of 10 mA cm−2; and (d) Tafel slopes for Fe2O3, Pr-MOF, and Pr-MOF/Fe2O3. Reproduced from ref. 65 with permission from Elsevier, copyright 2023. | ||
Ma et al. successfully prepared OER electrocatalysts with ultra-high activity and stability by one-step hot solvent method with optimized Er doping.66 Compared to the precursor Fe-MOF/NF, the Er0.4Fe-MOF/NF electrode excels in OER performance, being able to achieve a current density of 100 mA cm−2 at an overpotential of 248 mV and exhibiting a long-term electrochemical durability of at least 100 hours. In addition, the electrode is able to achieve high current densities of 500 mA cm−2 and 1000 mA cm−2 at very low overpotentials (297 mV and 326 mV, respectively). Fig. 8 (ref. 67) exhibits the electrochemical performance of Er0.4Fe-MOF/NF.
 | ||
| Fig. 8 (a) Multi-current process curves of Er0.4Fe-MOF/NF (b) current densities of 30–250 mA cm−2 obtained at 3600 s intervals. Time-varying (i–t) curves at a current density of 100 mA cm−2, and the inset is a post-test SEM image. (c) Time-varying curves of Er0.4Fe-MOF/NF and Fe-MOF/NF. (e) ECSA evolution (d) and TOF values of Er0.4Fe-MOF/NF and Fe-MOF/NF. (f) Experimental amount of oxygen produced by the Er0.4Fe-MOF/NF electrode at 100 and 500 mA cm−2. Reproduced from ref. 67 with permission from MDPI, copyright 2021. | ||
Hydrogen, as an efficient and clean energy medium, has the potential to be widely used as a green energy source, and photocatalytic hydrogen production is a potentially sustainable method of converting water to hydrogen using solar energy.71 For efficient photocatalytic hydrogen production, it is necessary to develop photocatalysts with excellent activity and stability. Li et al. successfully synthesized Pr–NO2–TPTC crystals based on binuclear Pr–O clusters by hot solvent reaction, and composited them with Cd0.2Zn0.8S at different mass ratios to prepare a series of stable Pr–NO2–TPTC/CZS composites.72 When the mass ratio of Pr–NO2–TPTC to Cd0.2Zn0.8S was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the composite material showed the best photocatalytic hydrogen production performance with a hydrogen production capacity of 6321 μmol g−1 h−1, which was higher than that of most reported CZS based heterojunction photocatalysts, and Fig. 9 (ref. 72) demonstrates the photocatalytic performance of this compliant material.
1, the composite material showed the best photocatalytic hydrogen production performance with a hydrogen production capacity of 6321 μmol g−1 h−1, which was higher than that of most reported CZS based heterojunction photocatalysts, and Fig. 9 (ref. 72) demonstrates the photocatalytic performance of this compliant material.
 | ||
Fig. 9 (a) and (b) Hydrogen production rate profiles of Pr–NO2–TPTC/CZS photocatalysts under full light conditions ((I) Pr–NO2–TPTC, (II) Cd0.2Zn0.8S, (III) Pr–NO2–TPTC/CZS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (VI) Pr–NO2–TPTC/CZS (1 1), (VI) Pr–NO2–TPTC/CZS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (V) Pr–NO2–TPTC/CZS (1 2), (V) Pr–NO2–TPTC/CZS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), (VI) no catalyst); (c) recovery performance of Pr–NO2–TPTC/CZS (1 4), (VI) no catalyst); (c) recovery performance of Pr–NO2–TPTC/CZS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (d) PXRD patterns of Pr–NO2–TPTC/CZS (1 1). (d) PXRD patterns of Pr–NO2–TPTC/CZS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) before and after photocatalytic reaction. Reproduced from ref. 72 with permission from Elsevier, copyright 2024. 1) before and after photocatalytic reaction. Reproduced from ref. 72 with permission from Elsevier, copyright 2024. | ||
Sun et al. successfully synthesized a Gd-MOF based on a dye analog. Gd3+, as a rare earth metal ion, has a high coordination number and a strong coordination ability, and forms a stable coordination network with the polydentate ligands in the dye analogs, so that the MOF exhibited extremely high stability in different pH solutions.73 When 1.5% Ag was deposited as a co-catalyst on Gd-MOF, its photocatalytic activity was significantly increased to 1.5 times of the original Gd-MOF. Electrochemical Impedance Spectroscopy (EIS) and Photoluminescence (PL) tests also confirmed the fast transfer of photogenerated carriers and low complexation rate in Ag(1.5)/Gd-MOF. This rare-earth MOFs composite provides a new strategy for the construction of MOF photocatalysts for efficient solar hydrogen production.
MOFs show remarkable potential in the field of photocatalytic CO2 reduction reaction (CO2RR) due to their precisely designable metal–oxygen cluster nodes with topologically tunable multilevel pore systems. Meanwhile, rare-earth cations possess rich energy-level structures that can be combined with MOFs to significantly improve their catalytic efficiency. Therefore, it is particularly important to develop efficient catalysts that react under mild conditions, and rare-earth MOFs composites have received extensive attention due to their excellent physicochemical properties. Yan et al. prepared a triangular Ru(phen)3-derived tricarboxylic acid ligand (H3L) by a multistep reaction with Eu(NO3)3·6H2O and 2-fluorobenzoic acid (2-FBA) in dimethylformamide (DMF) to synthesize Eu–Ru(phen)3-MOF.74 Under visible light irradiation (420 nm < λ < 800 nm), the Eu–Ru(phen)3-MOF exhibited efficient CO2 reduction activity, with a formate generation rate of 321.9 μmol per h per mmol MOF, and Fig. 10 (ref. 75) demonstrated the photocatalytic performance. The catalytic properties of the catalytic materials Gd–TPTC–NH2 and Gd–TPTC–NH–[BMIM]Br in solvent-free catalytic cycloaddition reactions were investigated by Bao et al.76 It was found that Gd–TPTC–NH–[BMIM]Br exhibited excellent catalytic activity under optimized conditions, with yields up to 91% of 4-phenyl-1,3-dioxolan-2-one. The catalytic activity of the catalyst remained unchanged after five repeated uses, showing good stability and structural integrity.76 In addition, Gd–TPTC–NH–[BMIM]Br can effectively catalyze the cycloaddition reactions of other epoxides in yields as high as 95%, demonstrating its broad applicability in cycloaddition reactions with different substrates.
 | ||
| Fig. 10 (a) Time profiles of HCOO− production catalyzed by Eu–Ru(phen)3-MOF or H3L with or without catalyst under Xe lamp (420–800 nm) irradiation. (b) 13C NMR spectra of the liquid phase products after reaction with 13CO2 and 12CO2, respectively. (c) Amount of HCOO− produced by three repetitions. (d) PXRD spectra of Eu–Ru(phen)3-MOF before synthesis and after photocatalytic reaction, showing that it maintains a good structure during the catalytic reaction. Reproduced from ref. 75 with permission from Springer Nature, copyright 2018. | ||
4. Applications in the environmental field
4.1 Pollution prevention and control
 | ||
| Fig. 11 The adsorption process of acetaldehyde molecules in the La-BTC MOF passes through three states of the biomarker molecule (a): position 1 outside the MOF (a and b), position 2 close to the frame node (a and c), and position 3 inside the cage near the La3+ cation (a and d). Reproduced from ref. 79 with permission from Elsevier, copyright 2023. | ||
Among all volatile gaseous pollutants, formaldehyde is one of the most harmful gases,80 prolonged inhalation of formaldehyde can cause respiratory dysfunction, long-term exposure can lead to skin necrosis, liver damage, and even a variety of cancers.81–84 Zhu et al. successfully synthesized a new type of Eu-MOF by a solvothermal method, which exhibits a strong red fluorescence and is capable of detecting formaldehyde in both gas and liquid phases.85 This combination of sensitivity and multi-phase detection capability makes Eu-MOF show significant application value in the pollutant monitoring module of intelligent air purification systems.
Tang et al. prepared four structurally novel RE-MOFs by hydrothermal method, confirmed their structures by single-crystal X-ray diffraction, and characterized their physicochemical properties by TG, PXRD and FTIR. Finally, the sensing properties were investigated and the possible mechanisms were hypothesized, see Fig. 12.87 The successful implementation of this project plays an active role in the application of RE-MOFs in environmental protection and human health detection.
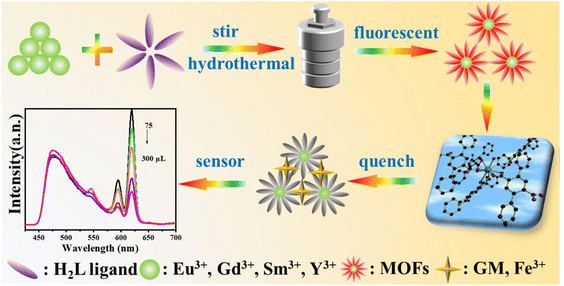 | ||
| Fig. 12 Synthesis and sensing map of 3D RE-MOFs. Reproduced from ref. 87 with permission from Elsevier, copyright 2024. | ||
It has been shown that RE-MOFs have the potential to be applied as thin-film active layers in electrochemical sensors for the detection of hydrogen sulfide (H2S) at room temperature. This unique fcu-based topology (RE-fcu-MOF) has a very high sensitivity for the detection of H2S at concentrations as low as 100 ppb, with a lower detection limit of about 5 ppb.88 In addition, carbendazim (CBZ) poses a serious threat to human health due to food-borne residues, and a smartphone-assisted fluorescent sensor based on a bimetallic organic framework (Eu/Tb-MOF) can be constructed. Eu3+ and Tb3+ emit characteristic red and green light, respectively, through ligand-sensitized luminescence, and the ratio of the fluorescence intensities of the two constitutes the internal reference signal eliminates environmental interference and enables rapid visualization of CBZ in food products.89 Dichlorophenol (Dcp) is known for its resistance to degradation as a highly toxic environmental pollutant, and La-MOF can be used to create novel electrochemical sensors for the detection of Dcp due to its large specific surface area and abundance of electrocatalytically active sites.90 Wang et al. synthesized a pH-stabilized three-dimensional Tb-MOF (JXUST-19) used as a rare switch and blue-shifted MOF sensor for benzaldehyde and salicylaldehyde.91 Guo et al. reported the preparation of a dual-emitting material, Eu–Ca-MOF, which has excellent photoluminescence properties and can be used as a ratiometric fluorescence sensor (I381/I590) for sensitive detection of Hg2+ ions.92 Xiao et al. synthesized a multi-responsive metal–organic framework (MOF), formulated as [Zn(tpbpc)2] solvent which can detect Hg2+, CrO42− and Cr2O72− ions and realize the visual detection of Hg2+.93
For example, Bacillus anthracis, as a Gram-positive aerobic bacillus, has some ability to survive in the natural environment. Soil can be one of the reservoirs of Bacillus anthracis spores. When infected animal carcasses are not handled properly, the spores can penetrate deep into the soil and become enriched in specific environments, threatening ecological security.94 To address this problem, metal ions in RE-MOFs may react with chemical groups on the surface of B. anthracis, and specific functional groups of proteins, polysaccharides, and other components of the cell wall may undergo coordination or ion exchange reactions to form chemical bonds, thus realizing the adsorption and fluorescence detection of B. anthracis.95
In summary, RE-MOFs can optimize soil physical properties, strengthen nutrient cycling and synergize the management of compound pollution through the three-in-one mechanism of “structural improvement-biological activation-pollution blocking and controlling”, which has the dual functions of environmental remediation and agricultural efficiency enhancement, and provides a new material paradigm for sustainable soil management.
4.2 Pollutant removal
 | ||
| Fig. 13 Mechanism of removal of heavy metal ions, dyes and other harmful substances from water by RE-MOFs. Reproduced from ref. 96 with permission from Elsevier, copyright 2022. | ||
As a typical variable rare earth element, cerium (Ce3+/Ce4+) shows significant advantages in the field of environmental adsorption due to its unique electronic structure ([Xe]4f15d16s2) and physicochemical properties. Ce has a relatively small ionic radius, ionic potential, and high affinity for surface hydroxyl groups. Ce has a relatively small ionic radius, ionic potential, and high affinity for surface hydroxyl groups, so Ce doping into the MOF crystal structure will form coordination defects, which provide more active adsorption sites for adsorption of phosphates and arsenates.98 Currently, Ce-MOFs-based adsorbents have been successfully applied to the remediation of eutrophic lake sediments, and their retention of trace arsenate (<10 ppb) can reach more than 90%, which provides a new strategy for the targeted treatment of heavy metal-like metal-contaminated waters. Liu et al. attempted to use microwave-assisted Ce doping in the pristine crystal structure of UiO-66-NH2 to create defects. The 0.75Ce-UiO-66-NH2 material absorbed up to 211.86 mg g−1 of phosphorus, which exceeded that of the undoped Ce-doped UiO-66-NH2, and the model of the phosphorus trapping mechanism of this material is shown in Fig. 14.99
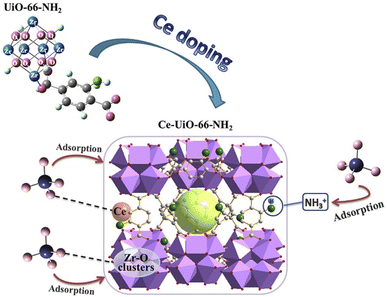 | ||
| Fig. 14 A possible mechanistic model for phosphate trapping on 0.75Ce-UiO-66-NH2. Reproduced from ref. 99 with permission from Elsevier, copyright 2020. | ||
The equilibrium adsorption capacity of Ce3+ introduced with UiO-67 as a carrier was enhanced by 85% compared to the undoped system,100 which was mainly attributed to the electronic modulation of rare earth ions. In contrast, the modification mechanism of MIL-88(Fe) by Ce doping presents different features: more ligand defects and higher Lewis acidity can be introduced into the backbone through a simple Ce doping strategy, which results in the generation of more surface-adsorbed hydroxyl radicals (·OHads) and reactive oxygen species such as single-linear oxygen species (1O2) and the enhancement of ozone conversion catalytic activity.100
Secondly, various pollutants are discharged into water bodies from different sources, resulting in increased water pollution.101 Among them, due to the illegal use of organophosphorus pesticides in agricultural production, they are present in water bodies in the form of residues,102 which are highly toxic and carcinogenic to humans due to their persistence, bioaccumulation and stability,103 and various organophosphorus pesticides can be detected in environmental water, food crops and soils, with widely varying concentrations over time and location, despite the implementation of strict pesticide regulations.104,105 Therefore, there is an urgent need to develop novel materials to remove organophosphorus pesticides to protect the environment and human health.102
Although RE-MOFs, as emerging environmental functional materials, provide revolutionary solutions for the management of pollutants in water bodies, they still have potential limitations. For example, except for La, Ce, and Nd doping, other rare earth elements have rarely been investigated in adsorption frameworks. Meanwhile, most RE-MOFs materials exhibit poor performance after multiple recycling, which limits their practical applications, and the coordination through organic matter or substrate metal ions may lead to the inactivation of free acidic metal sites in RE-MOFs. Future research should focus on the above aspects in order to promote RE-MOFs from theoretical innovation to engineering application, and provide sustainable technical support for global water environment management.
RE-MOFs with zeolite-like topologies maintain backbone stability even after dehydration, and their dynamic structural changes can induce selective adsorption properties. The Eu3+, Tb3+, and Y3+-based fcu-MOF metal–organic skeleton constructed by Xue et al. using a reticulated chemical approach achieved specific recognition and truncation effects on sorbate pollutant molecules by modulating the selective entry of the contracted pores.108 Such pore size engineering endows the materials with unique molecular sieving functions: on the one hand, the domain-limited pores can realize selective adsorption of volatile organic compounds (VOCs) such as formaldehyde and benzene, and efficiently differentiate and separate the coexisting polar/non-polar molecules in the air by using the kinetic separation property; on the other hand, the rare-earth nodes in the rigid skeleton stabilize the adsorbent by strong coordination, and maintain high VOCs capturing efficiency even under dynamic airflow conditions, the design provides a structural paradigm for the development of smart air purification materials.
In the catalytic treatment of VOCs, Ln-MOFs as catalysts or carriers can significantly enhance the reaction activity and selectivity through the electron modulation effect of rare earth ions (e.g., La3+, Ce3+). It has been shown that the presence of rare-earth-doped MOFs can enhance the activity and selectivity of the catalysts and promote the reaction of VOCs with oxygen, which can be converted into harmless substances such as CO2 and H2O at lower temperatures.109 In addition, some Ln-MOFs can generate electron–hole pairs under visible light excitation, which leads to the generation of reactive oxygen species such as hydroxyl radicals (·OH) and superoxide radicals (·O2−), which can decompose the VOCs by the photocatalytic oxidation pathway,110 and improve the degradation efficiency. The unique electronic structure and optical properties of rare-earth metal ions make Ln-MOFs potentially applicable in the photocatalytic degradation of VOCs, and Ln-MOFs can also be synergized with other technologies or materials to improve the treatment effect on VOCs. For example, by combining Ln-MOFs with adsorbents and catalysts, the integrated adsorption-catalytic treatment of VOCs can be realized, which can improve the treatment efficiency and reduce the treatment cost.
However, although RE-MOFs show a broad application prospect in the field of gas adsorption, separation, and purification, their actual industrialization process is still limited by multiple technological bottlenecks. From the perspective of synthetic chemistry, the current preparation strategies of some RE-MOFs are in conflict with the principles of green chemistry, such as their fabrication consumes a large amount of energy,111 which restricts their potential for large-scale applications. Future research needs to focus on low-carbon preparation techniques, combined with topology modulation and rare earth element recycling strategies, to promote the sustainable transformation of RE-MOFs from the laboratory to industrial scenarios.
5. Applications in the medical field
5.1 Drug delivery
Drug delivery systems (DDS) are technological systems that comprehensively regulate the distribution of drugs in an organism in terms of space, time, and dose. The development of DDS has made it possible to introduce therapeutic substances into the body through appropriately formulated devices, and to improve efficiency and safety by controlling the rate, time, and location of drug release in the body.112 To date, several classes of DDSs, such as micelles,113 polymers,114 liposomes,115 carbon nanomaterials,116 gold nanostructures,117 bio ceramics,118 mesoporous silica119–121 and MOFs for cancer therapy.122,123 However these DDS have limitations in cancer therapy, to overcome these barriers we can use smart drug delivery systems (SDDS) with innovative nanocarriers, RE-MOF is considered to be one of the representative categories of innovative porous smart nano biomaterials.124In the field of anticancer, the paclitaxel loading system constructed on the basis of Eu-MOF is able to achieve precise drug release in the micro-acidic environment of tumors (pH about 5.5–6.5) through pH-responsive release mechanism. This intelligent delivery system can not only increase the effective concentration of the drug by 2–3 times, but also reduce the toxicity of the system by about 40%, which significantly enhances the efficacy of anticancer treatment.126
In antimicrobial therapy, rare-earth MOF materials can be used as carriers for the antimicrobial drug vancomycin127 to achieve uniform dispersion and slow release of the drug and to improve the effectiveness of local or systemic anti-infective therapy. Zhang et al. found that the lanthanide salt-based constructed MOF and two ligands reacted solvent-free at mild temperatures to form ternary lanthanide nanoscale CPs at the 10 gram level.128 The in vitro antimicrobial activity of these ternary hybrids was investigated using zone of inhibition method, minimum inhibitory concentration, minimum bactericidal concentration and transmission electron microscopy and was found to have excellent antimicrobial properties. The in vitro antitumor activity was carried out by measuring the absorbance values by CCK-8 (Cell Counting Kit-8). This simple synthetic method has the potential to produce ternary lanthanide CPs on a large scale at room temperature, which may be promising candidates as antimicrobial compounds and antitumor agents.
Through precise design and modification, the rare-earth MOF materials are able to realize controlled release and targeted delivery of drugs, thus prolonging the duration of drug efficacy, reducing side effects, and increasing the cumulative concentration of drugs in the lesion site. At the same time, Eu-MOF materials show good compatibility in biological systems,15 which reduces adverse reactions to the organism and ensures the safety and stability of drug carriers in vivo.
Re-MOFs are ideal for optical imaging due to their excellent optical properties and high stability. Especially as fluorescent markers, Re-MOFs can realize highly sensitive and selective imaging of specific targets in vivo, providing an important basis for precise diagnosis of diseases. Francesca Lo Presti et al. used ultrasound (US) as a promising synthesis method to produce Y-BTC and Eu-doped Y-BTC MOFs with excellent structural stability and luminescence properties, and demonstrated their potential application in diagnostic imaging,129 which provides a new way of thinking about the application of Re-MOFs in fluorescence imaging.
In magnetic resonance imaging, RE-MOFs can significantly enhance the contrast of MRI signals by introducing paramagnetic rare-earth ions such as gadolinium to be used as MRI contrast agents,130 which in turn enhances the tissue differences in the images, enabling physicians to more accurately identify lesion areas, thus improving the accuracy of the disease diagnosis and early detection rate. Compared with conventional MRI contrast agents, RE-MOFs contrast agents have higher stability and lower toxicity, taking gadolinium ions as an example, RE-MOFs contrast agent reduces the toxicity of free gadolinium ions by virtue of its high porosity and large specific surface area, which ensures efficient loading of therapeutic agents and makes it more suitable for clinical applications.
Flunitrazepam is one of the most potent prescription psychotropic drugs in the benzodiazepine family.132 It is soluble in alcohol, colorless and odorless,133 and when combined with alcohol causes medical symptoms such as unconsciousness in the drinker.134 Flunitrazepam has been used maliciously in major criminal cases, especially in drug-related sexual assaults against females, posing a serious threat to public safety and social stability.135–137 Eu-MOF can be used as a highly selective and sensitive luminescent probe for the detection of flunitrazepam, and has shown excellent detection performance in methanol solutions, alcoholic beverages and urine samples.134
Notably, the rare-earth MOF material also enables multimodal imaging capabilities, combining a variety of imaging techniques into one,138 Wei et al. prepared CsLu2F7:Yb/Er/Tm-based nanoparticles for CT/UCL dual-modality imaging based on the high X-ray absorption coefficient of Lu.3 The dual modality of CT and optical imaging realizes real-time imaging and precise diagnosis of brain tumors. It provides more comprehensive information for accurate diagnosis of diseases. This multimodal imaging technology can fully utilize the advantages of various imaging technologies to improve the accuracy and reliability of diagnosis.
5.2 Bioassay
Rare earth composite MOF has high specific surface area, rich pore structure and excellent physicochemical properties, which enable it to be applied as an ideal sensing element in the construction of biosensors.140 Under specific conditions, rare-earth MOFs can specifically recognize and capture target biomolecules, such as proteins, nucleic acids, and small-molecule metabolites,141 thus realizing high-sensitivity and high-selectivity biosensing.
In addition, the unique fluorescence properties of rare earth elements also provide new ideas for the application of rare earth MOFs in biosensors. Eu3+-MOF, as a fluorescent sensing element, is able to eliminate the interference of the background fluorescence by using time-resolved fluorescence technology to realize ultra-sensitive detection of biomolecules.142 This fluorescence sensing technology is not only suitable for in vitro analysis, but can also be extended to in vivo imaging, providing powerful support for early diagnosis of diseases and monitoring of treatment (Fig. 15).
 | ||
| Fig. 15 Number of publications of: (a) MOFs in the period 1994–2017; (b) MOFs used for chemical sensor or biosensor development in the period 2005–2017. Distribution of MOF publications in different fields for the years: (c) 2007; and (d) 2017. Source: Web of Science. Reproduced from ref. 140 with permission from MDPI, copyright 2018. | ||
The application of RE-MOFs in bioimaging is also reflected in the targeted recognition and imaging of specific biomolecules. Through surface modification or functionalized design, RE-MOFs can specifically bind to target biomolecules, such as receptors on the surface of tumor cells, biomarkers, etc., to achieve highly selective imaging detection.141 This targeted imaging technology provides strong support for early diagnosis and precise treatment of diseases.
5.3 Organizational engineering
Functionally, the stent material has a relatively single function, but the introduction of the rare earth composite material MOF can give the stent antibacterial, anti-inflammatory, cell growth promotion and other functions. Determination of antibacterial activity of RE-MOF [Y2(MH)6]n-DMF (1), [Er2(MH)6]n (2), [Yb2(MH)6]n (3) and [La(MH)3]n (4) against Escherichia coli and Staphylococcus aureus by Zhu et al.145 The results showed that the antimicrobial drugs derived from RE-MOFs not only possessed stronger antimicrobial activities, but also exhibited excellent stability and long-lasting antimicrobial effects. In addition, rare earth elements have unique bioactivities in living organisms and can promote cell proliferation and angiogenesis, further enhancing the advantages of rare earth composite MOFs as scaffold materials.
Conventional stent materials may induce a stronger immune response or risk of toxicity due to insufficient biocompatibility, which in turn affects cell growth and tissue regeneration. RE-MOFs exhibit good bio inertness or bioactivity,144 which can reduce the occurrence of foreign body reactions and inflammation, and ensure that the scaffold materials are stable in vivo for a long period of time. Meanwhile, through surface modification and biofunctionalized design, the biocompatibility of rare-earth composite MOFs can be further improved to promote the interaction between cells and scaffold materials and accelerate the tissue regeneration process.
In summary, the rare earth composite MOF, as a biocompatible scaffold material with good biocompatibility, shows a broad application prospect in the field of tissue regeneration. Its unique structure and excellent properties provide new ideas and methods for the development of tissue engineering. In the future, with the in-depth study of the properties of RE-MOFs and their innovative applications in biocompatible scaffold materials, it is believed that they will provide strong support for the realization of efficient and precise tissue regeneration.
6. Conclusion and outlook
Rare-earth composite metal–organic framework materials (RE-MOFs) have realized breakthrough applications in energy, environment and healthcare fields by virtue of their unique ligand tunability, porous structure and multi-functional optical/electrical/magnetic properties of rare-earth elements. In the field of energy, the synergistic effect of rare earth ions and organic ligands significantly improves the efficiency of photocatalytic hydrogen production, CO2 reduction, and electrochemical energy storage; in the field of environmental treatment, the high specific surface area and selective adsorption capacity provide an efficient platform for VOCs capture, fluorescence sensing, and catalytic degradation of ozone; and in the direction of biomedicine, the synergistic effect of the luminescent properties of rare earth elements and the drug-loading advantages of metal–organic frameworks (MOFs) has given rise to new diagnostic and therapeutic platforms, such as precision targeted diagnostics, high-resolution imaging and controlled drug delivery systems. Future research needs to focus on three major dimensions: first, deepen the material design theory, combine machine learning and high-throughput computing to precisely regulate the rare-earth coordination microenvironment, and develop smart materials with dynamic stability (e.g., humidity/heat and acid/alkali resistance) and directional functions (e.g., optical/magnetic response); second, promote cross-scale integration and application, and explore the composite strategy of RE-MOFs with flexible devices, nano-enzymes, or biofilms to build a “detection–purification–repair” platform. Third, to break through the bottleneck of industrialization, reduce the preparation cost through green synthesis technology (mechanochemical method, continuous flow synthesis), and systematically evaluate the risk of rare earth ion migration and develop biodegradable materials, so as to balance the advantages of performance and ecological safety. With the deepening of multidisciplinary crossover, RE-MOFs are expected to move from the “functional exploration” in the laboratory to the “precise regulation” in practical scenarios, and become a transformative solution for global challenges such as carbon neutralization, environmental remediation, and precision medicine, etc., and the core breakthroughs may depend on the development of rare earth-ligands, which can be utilized in the production of rare earths. The core breakthrough may depend on the atomic level analysis of the electron transfer mechanism at the rare earth-ligand interface and the innovative construction of dynamically responsive material systems.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by grants from the National Natural Science Foundation (12205056), and Guangxi Key Laboratory of Manufacturing Systems and Advanced Manufacturing Technology (20-065-40S007), the Interdisciplinary Scientific Research Foundation of Guangxi University (2022JCA002), State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures. This research and funding project is cross-disciplinary.References
- Y. Ghorbani, et al., Rare earth permanent magnets for the green energy transition: Bottlenecks, current developments and cleaner production solutions, Resour., Conserv. Recycl., 2025, 212, 107966 CrossRef CAS.
- B. Zhou, Z. Li and C. Chen, Global potential of rare earth resources and rare earth demand from clean technologies, Minerals, 2017, 7(11), 203 CrossRef.
- Z. Wei, et al., Rare-earth based materials: an effective toolbox for brain imaging, therapy, monitoring and neuromodulation, Light: Sci. Appl., 2022, 11(1), 175 CrossRef CAS.
- H. Li, et al., The role of rare earth elements in biodegradable metals: A review, Acta Biomater., 2021, 129, 33–42 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 673–674 CrossRef CAS PubMed.
- A. R. Silva, et al., The chemistry and applications of metal–organic frameworks (MOFs) as industrial enzyme immobilization systems, Molecules, 2022, 27(14), 4529 CrossRef CAS PubMed.
- L. Jiao, et al., Metal–organic frameworks as platforms for catalytic applications, Adv. Mater., 2018, 30(37), 1703663 CrossRef PubMed.
- S. Dutta, S. Fajal and S. K. Ghosh, Heavy Metal-Based Toxic Oxo-Pollutants Sequestration by Advanced Functional Porous Materials for Safe Drinking Water, Acc. Chem. Res., 2024, 57(17), 2546–2560 CrossRef CAS PubMed.
- S. Dutta, et al., Metal–Organic Frameworks for Water Desalination, Adv. Funct. Mater., 2023, 34(43), 2304790 CrossRef.
- S. Dutta, et al., Recognition and Sequestration of Toxic Inorganic Water Pollutants with Hydrolytically Stable Metal-Organic Frameworks, Chem. Rec., 2021, 21(7), 1666–1680 CrossRef CAS PubMed.
- A. Karmakar, et al., Fluorescent “Turn-on” Sensing Based on Metal–Organic Frameworks (MOFs), Chem.–Asian J., 2019, 14(24), 4506–4519 CrossRef CAS PubMed.
- Z.-Q. Zhao, et al., Cubic Mn–Ln (Ln=La, Ce, Pr) bimetallic metal–organic frameworks for highly stable lithium storage capacity, J. Alloys Compd., 2023, 938, 168446 CrossRef CAS.
- S. Meng, et al., Rare earth-based MOFs for photo/electrocatalysis, Mater. Chem. Front., 2023, 7(5), 806–827 RSC.
- N. Wu, C. Bo and S. Guo, Luminescent Ln-MOFs for chemical sensing application on biomolecules, ACS Sens., 2024, 9(9), 4402–4424 CrossRef CAS PubMed.
- Q. Zhang, et al., One-pot synthesis of Eu-MOFs for bioimaging and drug delivery, Drug Dev. Ind. Pharm., 2021, 47(7), 1175–1182 CrossRef CAS PubMed.
- S. Mourdikoudis, et al., State-of-the-Art, Insights, and Perspectives for MOFs-Nanocomposites and MOF-Derived (Nano)Materials, Adv. Mater., 2025, 2415399 CrossRef PubMed.
- S. Rajeev and U. G. Panicker, Polymer-Metal Organic Frameworks: Recent Advances in Synthesis Strategies and Applications, J. Inorg. Organomet. Polym. Mater., 2025, 1–32 Search PubMed.
- T.-Y. Luo, et al., Rare earth pcu metal–organic framework platform based on RE4 (μ3-OH) 4 (COO) 62+ clusters: rational design, directed synthesis, and deliberate tuning of excitation wavelengths, J. Am. Chem. Soc., 2017, 139(27), 9333–9340 CrossRef CAS PubMed.
- J. So, et al., MOF-derived CeO 2 catalysts with Pr doping: engineering oxygen vacancies for improved CO 2 conversion to dimethyl carbonate, J. Mater. Chem. A, 2024, 12(46), 32281–32297 RSC.
- F. Saraci, et al., Rare-earth metal–organic frameworks: from structure to applications, Chem. Soc. Rev., 2020, 49(22), 7949–7977 RSC.
- S. Dutta, et al., Nanoscience and nanotechnology for water remediation: an earnest hope toward sustainability, Nanoscale Horiz., 2024, 9(6), 885–899 RSC.
- E. Loukopoulos and P. N. Trikalitis, Understanding Structural Dynamics in Flexible Rare-Earth Metal-Organic Frameworks, Eur. J. Inorg. Chem., 2024, 28(2), e202400606 CrossRef.
- M. Prajapati, et al., Recent advancement in metal–organic frameworks and composites for high-performance supercapatteries, Renewable Sustainable Energy Rev., 2023, 183, 113509 CrossRef CAS.
- J. C. Najmon, S. Raeisi, and A. Tovar, Review of additive manufacturing technologies and applications in the aerospace industry, in Additive Manufacturing for the Aerospace Industry, 2019, pp. 7–31 Search PubMed.
- Y. Hao, et al., Heterogeneous nanocomposite of MOF-derived (NiCo)Se2 nanospheres decorated with rare earth oxide nanorods for high-performance electrochemical energy storage, J. Energy Storage, 2024, 91, 112168 CrossRef.
- A. Bhandari, et al., Li nucleation on the graphite anode under potential control in Li-ion batteries, J. Mater. Chem. A, 2022, 10(21), 11426–11436 RSC.
- X. Zhang, et al., Porous α-Fe2O3 nanoparticles encapsulated within reduced graphene oxide as superior anode for lithium-ion battery, Nanotechnology, 2020, 31(14), 145404 CrossRef CAS PubMed.
- M. Canal-Rodríguez, et al., Carbon xerogels graphitized by microwave heating as anode materials in lithium-ion batteries, Carbon, 2018, 137, 384–394 CrossRef.
- M. Trukawka, et al., Hollow carbon spheres loaded with uniform dispersion of copper oxide nanoparticles for anode in lithium-ion batteries, J. Alloys Compd., 2021, 853, 156700 CrossRef CAS.
- M. Sun, et al., Construction of porous CoTiO3 microrods with enhanced performance as lithium-ion battery anode, J. Alloys Compd., 2022, 926, 166809 CrossRef CAS.
- H. Pang, et al., A general strategy for metal oxide nanoparticles embedded into heterogeneous carbon nanosheets as high-rate lithium-ion battery anodes, J. Mater. Chem. A, 2020, 8(47), 25382–25389 RSC.
- G. Ke, et al., Unveiling the reaction mechanism of an Sb 2 S 3–Co 9 S 8/NC anode for high-performance lithium-ion batteries, Nanoscale, 2021, 13(47), 20041–20051 RSC.
- Z.-Q. Zhao, et al., Cubic Mn–Ln (Ln La, Ce, Pr) bimetallic metal–organic frameworks for highly stable lithium storage capacity, J. Alloys Compd., 2023, 938, 168446 CrossRef CAS.
- X.-N. Lv, et al., One pot synthesis of lanthanide-iron-sodium trimetallic metal–organic frameworks as anode materials for lithium-ion batteries, J. Solid State Chem., 2022, 306, 122786 CrossRef CAS.
- X.-N. Lv, et al., One pot synthesis of lanthanide-iron-sodium trimetallic metal–organic frameworks as anode materials for lithium-ion batteries, J. Solid State Chem., 2022, 306, 122786 CrossRef CAS.
- S.-B. Xia, et al., A lanthanide-based coordination polymer as lithium ion battery anode with high cyclic stability, Mater. Lett., 2019, 238, 171–174 CrossRef CAS.
- M. Yousaf, et al., A mechanistic study of electrode materials for rechargeable batteries beyond lithium ions byin situtransmission electron microscopy, Energy Environ. Sci., 2021, 14(5), 2670–2707 RSC.
- M. Yousaf, et al., A mechanistic study of electrode materials for rechargeable batteries beyond lithium ions by in situ transmission electron microscopy, Energy Environ. Sci., 2021, 14(5), 2670–2707 RSC.
- R.-B. LingHu, et al., Concurrent hetero-/homo-geneous electrocatalysts to bi-phasically mediate sulfur species for lithium–sulfur batteries, J. Energy Chem., 2024, 93, 663–668 CrossRef CAS.
- B. Lin, et al., Recent advances in rare earth compounds for lithium–sulfur batteries, eScience, 2024, 4(3), 100180 CrossRef.
- X. Qian, et al., Application of Y-MOF–CNT-Derived Y2O3–C@ CNT Composites in Lithium–Sulfur Battery Separators, Langmuir, 2024, 40(44), 23529–23537 CrossRef CAS PubMed.
- L. Ma, et al., Cerium oxide nanocrystal embedded bimodal micromesoporous nitrogen-rich carbon nanospheres as effective sulfur host for lithium–sulfur batteries, ACS Nano, 2017, 11(7), 7274–7283 CrossRef CAS PubMed.
- X.-J. Hong, et al., Cerium based metal–organic frameworks as an efficient separator coating catalyzing the conversion of polysulfides for high performance lithium–sulfur batteries, ACS Nano, 2019, 13(2), 1923–1931 CAS.
- X. Liu, et al., MOF derived phosphorus doped cerium dioxide nanorods modified separator as efficient polysulfide barrier for advanced lithium-sulfur batteries, Chin. Chem. Lett., 2024, 110369 CrossRef.
- Q. Hao, et al., Application of Nd-MOF derived Nd2O3-C/KB and Nd2O3-C/CNT for lithium-sulfur battery separators, Colloids Surf., A, 2024, 702, 134948 CrossRef CAS.
- M. Tomy, et al., Emergence of novel 2D materials for high-performance supercapacitor electrode applications: a brief review, Energy Fuels, 2021, 35(24), 19881–19900 CrossRef CAS.
- X. Xia, et al., Synthesis of free-standing metal sulfide nanoarrays via anion exchange reaction and their electrochemical energy storage application, Small, 2014, 10(4), 766–773 CrossRef CAS PubMed.
- Y. Kou, et al., Supercapacitive energy storage and electric power supply using an aza-fused π-conjugated microporous framework, Angew. Chem., 2011, 123(37), 8912–8916 CrossRef.
- M. A. A. M. Abdah, et al., Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors, Mater. Des., 2020, 186, 108199 CrossRef.
- Y. Wang, et al., CeO 2 nanoparticles/graphene nanocomposite-based high performance supercapacitor, Dalton Trans., 2011, 40(24), 6388–6391 RSC.
- S. Maiti, A. Pramanik and S. Mahanty, Extraordinarily high pseudocapacitance of metal organic framework derived nanostructured cerium oxide, Chem. Commun., 2014, 50(79), 11717–11720 RSC.
- G. Zeng, et al., Hierarchical cerium oxide derived from metal-organic frameworks for high performance supercapacitor electrodes, Electrochim. Acta, 2016, 222, 773–780 CrossRef CAS.
- Y. Hao, et al., Heterogeneous nanocomposite of MOF-derived (NiCo) Se2 nanospheres decorated with rare earth oxide nanorods for high-performance electrochemical energy storage, J. Energy Storage, 2024, 91, 112168 CrossRef.
- R. Siddiqui, et al., Enhanced electrochemical performance with exceptional capacitive retention in Ce–Co MOFs/Ti3C2Tx nanocomposite for advanced supercapacitor applications, Heliyon, 2024, 10(17), e36540 CrossRef CAS PubMed.
- A. S. Dezfuli, et al., Study of the supercapacitive activity of a Eu-MOF as an electrode material, New J. Chem., 2019, 43(23), 9260–9264 RSC.
- H. Jafari, et al., Terbium metal–organic frameworks as capable electrodes for supercapacitors, New J. Chem., 2020, 44(27), 11615–11621 RSC.
- J. Luo, et al., Hydrogen Adsorption in a Highly Stable Porous Rare-Earth Metal–Organic Framework: Sorption Properties and Neutron Diffraction Studies, J. Am. Chem. Soc., 2008, 130(30), 9626–9627 CrossRef CAS PubMed.
- X. Shi, et al., Rare-Earth-Based Metal-Organic Frameworks as Multifunctional Platforms for Catalytic Conversion, Small, 2021, 17(22), e2005371 CrossRef PubMed.
- Q. Lan, et al., MOF-derived, CeOx-modified CoP/carbon composites for oxygen evolution and hydrogen evolution reactions, J. Mater. Sci., 2018, 53(17), 12123–12131 CrossRef CAS.
- L. Xiong, et al., Improving the electrocatalytic property of CoP for hydrogen evolution by constructing porous ternary CeO2-CoP-C hybrid nanostructure via ionic exchange of MOF, Int. J. Hydrogen Energy, 2018, 43(45), 20372–20381 CrossRef CAS.
- J. Liao, et al., MoS2 supported on Er-MOF as efficient electrocatalysts for hydrogen evolution reaction, J. Alloys Compd., 2022, 898, 162991 CrossRef CAS.
- F. Liu, et al., Rare-Earth-Based Bimetallic Metal–Organic Frameworks Promote Oxygen Electrocatalysis for Rechargeable Zn–Air Batteries, ACS Sustain. Chem. Eng., 2022, 10(33), 10978–10988 CrossRef.
- R. Shi, et al., Er-MOF composite NiS nanomaterials as a highly efficient electrocatalyst for hydrogen evolution reaction, Electrochim. Acta, 2024, 483, 143993 CrossRef CAS.
- B. Shabbir, et al., Solvothermally designed Pr-MOF/Fe(2)O(3) based nanocomposites for efficient electrocatalytic water splitting, Heliyon, 2023, 9(10), e20261 CrossRef CAS PubMed.
- B. Shabbir, et al., Solvothermally designed Pr-MOF/Fe2O3 based nanocomposites for efficient electrocatalytic water splitting, Heliyon, 2023, 9(10), e20261 CrossRef CAS PubMed.
- Y. Ma, et al., Highly Enhanced OER Performance by Er-Doped Fe-MOF Nanoarray at Large Current Densities, Nanomaterials, 2021, 11(7), 1847 CrossRef CAS PubMed.
- Y. Ma, et al., Highly enhanced OER performance by Er-doped Fe-MOF nanoarray at large current densities, Nanomaterials, 2021, 11(7), 1847 CrossRef CAS PubMed.
- B. Pattengale, et al., Metal–Organic Framework Photoconductivity via Time-Resolved Terahertz Spectroscopy, J. Am. Chem. Soc., 2019, 141(25), 9793–9797 CrossRef CAS PubMed.
- X. Sun, K. Yuan and Y. Zhang, Advances and prospects of rare earth metal–organic frameworks in catalytic applications, J. Rare Earths, 2020, 38(8), 801–818 CrossRef CAS.
- Y. Zhang, et al., Advancements in rare earth metal–organic frameworks: Harnessing the power of photonics and beyond, Coord. Chem. Rev., 2024, 514, 215905 CrossRef CAS.
- M. Hesari, X. Mao and P. Chen, Charge Carrier Activity on Single-Particle Photo(electro)catalysts: Toward Function in Solar Energy Conversion, J. Am. Chem. Soc., 2018, 140(22), 6729–6740 CrossRef CAS PubMed.
- T.-T. Li, et al., Synthesis of rare earth MOF/CZS materials derived from aromatic tetracarboxylic acids and photocatalytic hydrogen production, Int. J. Hydrogen Energy, 2024, 65, 225–235 CrossRef CAS.
- X. Sun, et al., A dye-like ligand-based metal–organic framework for efficient photocatalytic hydrogen production from aqueous solution, Catal. Sci. Technol., 2016, 6(11), 3840–3844 RSC.
- Z. H. Yan, et al., Photo-generated dinuclear Eu(II)(2) active sites for selective CO(2) reduction in a photosensitizing metal–organic framework, Nat. Commun., 2018, 9(1), 3353 CrossRef PubMed.
- Z.-H. Yan, et al., Photo-generated dinuclear {Eu (II)} 2 active sites for selective CO2 reduction in a photosensitizing metal–organic framework, Nat. Commun., 2018, 9(1), 3353 CrossRef PubMed.
- W.-L. Bao, et al., Ionic liquid post-modified carboxylate-rich MOFs for efficient catalytic CO 2 cycloaddition under solvent-free conditions, Dalton Trans., 2024, 53(14), 6215–6223 RSC.
- P. Hajivand, et al., Application of metal–organic frameworks for sensing of VOCs and other volatile biomarkers, Coord. Chem. Rev., 2024, 501, 215558 CrossRef CAS.
- Y. Chen, et al., A CuO–ZnO nanostructured p–n junction sensor for enhanced N-butanol detection, RSC Adv., 2016, 6(3), 2504–2511 RSC.
- V. Gaidamavichute, et al., A LA-BTC MOF as a sensor element of an electronic nose for selective adsorption of biomarkers of diseases: Molecular dynamics simulations of adsorption, Mater. Today Commun., 2024, 38, 107787 CrossRef CAS.
- L. Zhou, et al., Single atom Rh-sensitized SnO2 via atomic layer deposition for efficient formaldehyde detection, Chem. Eng. J., 2023, 475, 146300 CrossRef CAS.
- D.-Y. Ryu, et al., Urea/nitric acid co-impregnated pitch-based activated carbon fiber for the effective removal of formaldehyde, J. Ind. Eng. Chem., 2019, 80, 98–105 CrossRef CAS.
- H. Du, et al., A novel fluorescent probe for the detection of formaldehyde in real food samples, animal serum samples and gaseous formaldehyde, Food Chem., 2023, 411, 135483 CrossRef CAS PubMed.
- Z. Min, et al., A near-infrared fluorescence-on fluorescent probe for formaldehyde imaging in Arabidopsis thaliana, Dyes Pigm., 2023, 218, 111446 CrossRef CAS.
- Y. Min, et al., Highly selective detection of formaldehyde and its analogs in clams based on unique and specific conjugation reactions via ultraviolet-visible absorptions, Chin. J. Anal. Chem., 2023, 51(8), 100287 Search PubMed.
- J. Zhu, et al., A novel Eu-MOF ratiometric fluorescent probe for visual detection of Hg2+, Cd2+ and formaldehyde, J. Photochem. Photobiol., A, 2024, 452, 115583 CrossRef CAS.
- E. Gulcay-Ozcan, et al., Airborne Toluene Detection Using Metal–Organic Frameworks, ACS Appl. Mater. Interfaces, 2022, 14(48), 53777–53787 CrossRef CAS PubMed.
- S.-F. Tang, et al., Four three-dimensional rare earth metal–organic framework fluorescent sensor for efficient detection of gentamicin sulfate and Fe3+, Spectrochim. Acta, Part A, 2024, 322, 124765 CrossRef CAS PubMed.
- O. Yassine, et al., H2S Sensors: Fumarate-Based fcu-MOF Thin Film Grown on a Capacitive Interdigitated Electrode, Angew. Chem., Int. Ed., 2016, 55(51), 15879–15883 CrossRef CAS PubMed.
- Y. He, et al., A smartphone-assisted fluorescent sensor using Eu/Tb-MOF nanorods for ultrasensitive and visual monitoring of carbendazim in food samples, Microchem. J., 2025, 209, 112602 CrossRef CAS.
- Y. Xie, et al., A novel electrochemical sensor based on La-MOF@ C60-β-cyclodextrin composite for sensitive detection of dichlorophen in lake and tap water, J. Environ. Chem. Eng., 2025, 13(1), 115388 CrossRef CAS.
- K. Wang, et al., A pH-stable TbIII-based metal–organic framework as a turn-on and blue-shift fluorescence sensor toward benzaldehyde and salicylaldehyde in aqueous solution, Inorg. Chem., 2022, 61(40), 16177–16184 CrossRef CAS PubMed.
- N. W. H. Guo, et al., A novel ratiometric fluorescence sensor based on lanthanide-functionalized MOF for Hg2+ detection, Talanta, 2022, 250, 123710 CrossRef PubMed.
- J. Xiao, et al., A multi-chemosensor based on Zn-MOF: Ratio-dependent color transition detection of Hg (II) and highly sensitive sensor of Cr (VI), Sens. Actuators, B, 2018, 269, 164–172 CrossRef CAS.
- J. P. Wood, Review of techniques for the in-situ sterilization of soil contaminated with Bacillus anthracis spores or other pathogens, Res. Microbiol., 2024, 175(4), 104175 CrossRef CAS PubMed.
- J. Gou, et al., A multi-dimensional anti-counterfeiting nanocomposite based on fluorescent CDs and Eu-MOFs with dual function for continuous detection of Cu2+ and Bacillus anthracis, Mater. Today Chem., 2024, 41, 102270 CrossRef CAS.
- J. Darabdhara and M. Ahmaruzzaman, Recent developments in MOF and MOF based composite as potential adsorbents for removal of aqueous environmental contaminants, Chemosphere, 2022, 304, 135261 CrossRef CAS PubMed.
- M. Liu, et al., Cerium-doped MIL-101-NH2 (Fe) as superior adsorbent for simultaneous capture of phosphate and As (V) from Yangzonghai coastal spring water, J. Hazard. Mater., 2022, 423, 126981 CrossRef CAS PubMed.
- S. Wu, et al., Efficient removal of mercury from flue gases by regenerable cerium-doped functional activated carbon derived from resin made by in situ ion exchange method, Fuel Process. Technol., 2019, 196, 106167 CrossRef CAS.
- M. Liu, et al., Highly efficient capture of phosphate from water via cerium-doped metal–organic frameworks, J. Cleaner Prod., 2020, 265, 121782 CrossRef CAS.
- D. Yu, et al., Tuning Lewis acidity of iron-based metal–organic frameworks for enhanced catalytic ozonation, Chem. Eng. J., 2021, 404, 127075 CrossRef CAS.
- A. Khaleeq, S. R. Tariq and G. A. Chotana, Co-MOF 74 decorated with Eu for UV/Visible assisted photocatalytic removal of lufenuron pesticide from aqueous medium, Appl. Surf. Sci., 2025, 687, 162239 CrossRef CAS.
- Y. Zhao, et al., Hierarchically porous Cu-MOF fiber membrane enables instantaneous and continuous removal of organophosphorus pesticides in water, J. Environ. Chem. Eng., 2025, 115877 CrossRef CAS.
- A. Sharma, et al., Global trends in pesticides: A looming threat and viable alternatives, Ecotoxicol. Environ. Saf., 2020, 201, 110812 CrossRef CAS PubMed.
- E. A. Songa and J. O. Okonkwo, Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review, Talanta, 2016, 155, 289–304 CrossRef CAS PubMed.
- W. Qiu, et al., Determination of OCPs, OPPs, and 21 SVOCs in water and sediment samples in five rivers of Shenzhen, China, during the period of 2017 and 2018, Environ. Sci. Pollut. Res., 2021, 28, 42444–42457 CrossRef CAS PubMed.
- S. Roy, A. Chakraborty and T. K. Maji, Lanthanide–organic frameworks for gas storage and as magneto-luminescent materials, Coord. Chem. Rev., 2014, 273–274, 139–164 CrossRef CAS.
- S. Mukherjee, et al., Advances in adsorptive separation of benzene and cyclohexane by metal–organic framework adsorbents, Coord. Chem. Rev., 2021, 437, 213852 CrossRef CAS.
- D.-X. Xue, et al., Tunable rare earth fcu-MOF platform: access to adsorption kinetics driven gas/vapor separations via pore size contraction, J. Am. Chem. Soc., 2015, 137(15), 5034–5040 CrossRef CAS PubMed.
- S. Liu, et al., Catalytic CO oxidation on CeO2-based materials: Modification strategies, structure-performance relationships, challenges and prospects, Sep. Purif. Technol., 2024, 130556 Search PubMed.
- P. Mazierski, et al., The role of lanthanides in TiO2-based photocatalysis: A review, Appl. Catal., B, 2018, 233, 301–317 CrossRef CAS.
- S. Dutta, et al., Cradle-to-Gate Environmental Impact Assessment of Commercially Available Metal-Organic Frameworks Manufacturing, Adv. Funct. Mater., 2024, 34(52), 2410751 CrossRef CAS.
- B. Sana, A. Finne-Wistrand and D. Pappalardo, Recent development in near infrared light-responsive polymeric materials for smart drug-delivery systems, Mater. Today Chem., 2022, 25, 100963 CrossRef CAS.
- B. Ghosh and S. Biswas, Polymeric micelles in cancer therapy: State of the art, J. Controlled Release, 2021, 332, 127–147 CrossRef CAS PubMed.
- X. Guo, et al., Polymer-based drug delivery systems for cancer treatment, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(22), 3525–3550 CrossRef CAS.
- T. O. Olusanya, et al., Liposomal drug delivery systems and anticancer drugs, Molecules, 2018, 23(4), 907 CrossRef PubMed.
- L. Zhang, et al., Mesoporous carbon/CuS nanocomposites for pH-dependent drug delivery and near-infrared chemo-photothermal therapy, RSC Adv., 2015, 5(113), 93226–93233 RSC.
- Z. Zhang, et al., Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods, J. Am. Chem. Soc., 2014, 136(20), 7317–7326 CrossRef CAS PubMed.
- S. Bhat, et al., Functionalized porous hydroxyapatite scaffolds for tissue engineering applications: a focused review, ACS Biomater. Sci. Eng., 2021, 8(10), 4039–4076 CrossRef PubMed.
- S. Sargazi, et al., Recent trends in mesoporous silica nanoparticles of rode-like morphology for cancer theranostics: A review, J. Mol. Struct., 2022, 1261, 132922 CrossRef CAS.
- G. Yang, et al., Mesoporous silica nanorods intrinsically doped with photosensitizers as a multifunctional drug carrier for combination therapy of cancer, Nano Res., 2015, 8, 751–764 CrossRef CAS.
- M. Mozafarinia, et al., In vitro breast cancer targeting using Trastuzumab-conjugated mesoporous silica nanoparticles: towards the new strategy for decreasing size and high drug loading capacity for drug delivery purposes in MSN synthesis, Microporous Mesoporous Mater., 2021, 316, 110950 CrossRef CAS.
- S. Mallakpour, E. Nikkhoo and C. M. Hussain, Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment, Coord. Chem. Rev., 2022, 451, 214262 CrossRef CAS.
- F. Su, et al., Aptamer-templated silver nanoclusters embedded in zirconium metal–organic framework for targeted antitumor drug delivery, Microporous Mesoporous Mater., 2019, 275, 152–162 CrossRef CAS.
- U. Uthappa, et al., Rare earth derived porous metal-organic-frameworks (RE-MOFs) as a smart nanobiomaterials for cancer therapy: Recent trends, Microporous Mesoporous Mater., 2023, 362, 112795 CrossRef CAS.
- S. A. Younis, et al., Rare earth metal–organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications, Coord. Chem. Rev., 2021, 429, 213620 CrossRef CAS.
- A. Hamedi, et al., A γ-cyclodextrin-based metal–organic framework (γ-CD-MOF): a review of recent advances for drug delivery application, J. Drug Targeting, 2022, 30(4), 381–393 CrossRef CAS PubMed.
- L. Zhang, et al., Ten-Gram-Scale Mechanochemical Synthesis of Ternary Lanthanum Coordination Polymers for Antibacterial and Antitumor Activities, Front. Chem., 2022, 10, 898324 CrossRef CAS PubMed.
- L. Zhang, et al., Ten-gram-scale mechanochemical synthesis of ternary lanthanum coordination polymers for antibacterial and antitumor activities, Front. Chem., 2022, 10, 898324 CrossRef CAS PubMed.
- F. Lo Presti, et al., Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs, Molecules, 2023, 28(16), 6088 CrossRef CAS PubMed.
- Z. Wei, et al., Rare-earth based materials: an effective toolbox for brain imaging, therapy, monitoring and neuromodulation, Light:Sci. Appl., 2022, 11(1), 175 CrossRef CAS PubMed.
- A. H. Valekar, et al., Novel amine-functionalized iron trimesates with enhanced peroxidase-like activity and their applications for the fluorescent assay of choline and acetylcholine, Biosens. Bioelectron., 2018, 100, 161–168 CrossRef CAS PubMed.
- H. Druid, P. Holmgren and J. Ahlner, Flunitrazepam: an evaluation of use, abuse and toxicity, Forensic Sci. Int., 2001, 122(2–3), 136–141 CrossRef CAS PubMed.
- T. Saïas and T. Gallarda, Paradoxical aggressive reactions to benzodiazepine use: a review, L'encephale, 2007, 34(4), 330–336 CrossRef PubMed.
- S. Chen, et al., Eu-MOF-based highly sensitive and selective luminescence probe for trace, in situ and visual detection of flunitrazepam, Spectrochim. Acta, Part A, 2025, 326, 125226 CrossRef CAS PubMed.
- D. Bosone, et al., Effect of flunitrazepam as an oral hypnotic on 24-hour blood pressure in healthy volunteers, Eur. J. Clin. Pharmacol., 2018, 74, 995–1000 CrossRef PubMed.
- E. Bermejo, et al., Voltammetric studies of a psychotropic drug with nitro groups. Determination of flunitrazepam in urine using HMDE, Talanta, 1993, 40(11), 1649–1656 CrossRef CAS PubMed.
- V. Vindenes, et al., Detection of drugs of abuse in simultaneously collected oral fluid, urine and blood from Norwegian drug drivers, Forensic Sci. Int., 2012, 219(1–3), 165–171 CrossRef CAS PubMed.
- M. Zevon, et al., CXCR-4 Targeted, Short Wave Infrared (SWIR) Emitting Nanoprobes for Enhanced Deep Tissue Imaging and Micrometastatic Cancer Lesion Detection, Small, 2015, 11(47), 6347–6357 CrossRef CAS PubMed.
- T. Wang, et al., A cardiomyocyte-based biosensor for antiarrhythmic drug evaluation by simultaneously monitoring cell growth and beating, Biosens. Bioelectron., 2013, 49, 9–13 CrossRef CAS PubMed.
- S. Carrasco, Metal–Organic Frameworks for the Development of Biosensors: A Current Overview, Biosensors, 2018, 8(4), 92 CrossRef CAS PubMed.
- Y. Liu, Y. Zhao and X. Chen, Bioengineering of Metal–organic Frameworks for Nanomedicine, Theranostics, 2019, 9(11), 3122–3133 CrossRef CAS PubMed.
- R. Zhai, et al., Tunable chiroptical application by encapsulating achiral lanthanide complexes into chiral MOF thin films, Nano Res., 2022, 15, 1102–1108 CrossRef CAS.
- R. A. Hamideh, et al., Biodegradable MRI visible drug eluting stent reinforced by metal organic frameworks, Adv. Healthcare Mater., 2020, 9(14), 2000136 CrossRef CAS PubMed.
- N. Rabiee, et al., Bioactive hybrid metal–organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen, Sci. Total Environ., 2022, 825, 153902 CrossRef CAS PubMed.
- L. Zhu, N. Liu, X. Jiang, L. Yu and X. Li, Four novel 3D RE-MOFs based on maleic hydrazide: syntheses, structural diversity, efficient electromagnetic wave absorption and antibacterial activity properties, Inorg. Chim. Acta, 2020, 501, 119291 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |
