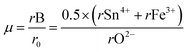Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An analysis of the crystal structure, vibrational properties, and optoelectronic behavior of NdCaFeSnO6
Sondes Chahlaa,
Yaovi Gagoub,
Mimoun El Marssib,
Hanen Chaker *a and
Rached Ben Hassen
*a and
Rached Ben Hassen a
a
aLaboratory of Materials and Environment for Sustainable Development (LR18ES10) ISSBAT, University of Tunis El Manar, Tunis, Tunisia. E-mail: hanen_chaker@yahoo.fr
bLPMC, Université of Picardie Jules Verne, 33 rue Saint Leu, CEDEX 01, 80039 Amiens, France
First published on 4th July 2025
Abstract
The co-substitution of neodymium (Nd) and iron (Fe) into CaSnO3 provides, for the first time, the double perovskite-like structure NdCaFeSnO6. A comprehensive investigation of the structural, microstructural, vibrational (Raman), optical and electrical properties of the resulting NdCaFeSnO6 compound, synthesized via a solid-state method, is reported. Elemental analysis using Energy-Dispersive X-ray spectroscopy (EDX) confirmed the successful incorporation of Nd, Fe, Ca, Sn and O. Semi-quantitative analysis results are consistent with the intended stoichiometry. X-ray diffraction (XRD) investigation revealed a monoclinic P21/c crystal structure, with a minor secondary phase. Rietveld refinement converges to satisfactory factors, indicating an equidistribution of Fe and Sn cations between the octahedral sites. Raman spectroscopy further characterized this symmetry, showing several bands associated with the bending and stretching modes of (Sn/Fe)O6 octahedra. Significant narrowing of the bandgap from 4.27 eV for the pristine CaSnO3 to 2.44 eV in NdCaFeSnO6 supports the presence of structural defects and oxygen vacancies within the (Nd/Ca)–(Fe/Sn)–O framework. The electrical properties were elucidated with emphasis on the ionic conduction mechanism in NdCaFeSnO6with charge carrier hopping at intermediate temperatures with an activation energy of 0.97 eV for grain boundaries and 0.31 eV for grains, based on impedance spectroscopy measurements which permit also to establish a–c and d–c conductivity contributions in this compound through Jonscher analysis. Furthermore, direct current I–V measurements revealed a Poole–Frenkel conduction mechanism at high temperatures with an activation energy of 1.55 eV. A comparison of these conduction processes was conducted to place this compound in the family of best materials for optoelectronic applications.
1. Introduction
The exploration and development of novel materials such as ceramics, nanorods, and composites exhibiting desirable physicochemical properties has remained a fundamental objective within the fields of chemistry and physics. These functional materials demonstrate significant potential for integration into various advanced technological systems, notably in energy storage, optoelectronic devices, photocatalysis, and supercapacitor technologies.1–5 Among them, materials adopting the perovskite crystal structure have been extensively investigated due to their exceptional structural tunability and multifunctionality. Perovskite-type compounds have demonstrated broad applicability in fields ranging from dielectric capacitors and solid oxide fuel cell anodes to heterogeneous catalysis and photovoltaic systems. The chemical flexibility of the perovskite structure allows for the formation of numerous elemental combinations at the A and B sites, resulting in simple (ABO3) or complex compositions (A′A″B2O6, A2B′B″O6, A′A″B′B″O6, etc.), depending on the atomic arrangement within the structure.Among perovskite materials, calcium stannate (CaSnO3) has garnered significant scientific interest due to its noteworthy dielectric behavior and remarkable optical and electrochemical properties.6 One of its most remarkable attributes is its high upconversion efficiency, attributed to its low phonon energy, making it an optimal material for bright white upconversion luminescence. This enables the generation of a broad visible emission spectrum with minimal energy consumption under near-infrared excitation.7–9 Furthermore, CaSnO3 possesses a wide bandgap and a high melting point, making it suitable for various applications, including display matrices, thermally stable capacitors in electronics, ceramic materials, lithium-ion batteries, photocatalysis, and gas sensing.8–10 Sumithra et al. demonstrated that Fe doping in CaSnO3 significantly altered the material's structural, electronic, and magnetic properties.11 The incorporation of Fe ions induced lattice distortions, leading to modifications in bond lengths and lattice parameters. These structural changes facilitated the generation of localized defect states and enhanced magnetic interactions. The resulting alterations in the electronic structure also influenced the material's conductivity and optical characteristics, thereby affecting its overall magnetization and magnetic behavior. In particular, Ca(Fe1−xSnx)O3 (ref. 11) has emerged as a promising candidate for spintronic and optoelectronic applications. Moreover, the incorporation of neodymium into the CaSnO3 structure induces structural modifications and charge compensation effects, enhancing the material's physical properties, making it suitable for high-level nuclear waste containment.12
Similarly, strontium stannate (SrSnO3) has attracted considerable attention due to its potential applications in single quantum flux circuits, lithium batteries, high-temperature humidity sensors, and low thermal capacitance capacitor devices.13,14 As an ideal transparent conducting oxide (TCO),15 SrSnO3 has been the subject of extensive research, focusing on doping and substitution strategies to improve its electro-optical properties.16 Recent studies in our laboratory have demonstrated that Al substitution at the B-site significantly enhances SrSnO3's performance as a p-type TCO material.17 Moreover, it was reported that crystalline structure, electrical conductivity, optical, and dielectric properties of Sr2Sn1.6In0.2Cu0.2O6−δ revealed its suitability for UV filtering, UV detection, and mixed ionic conduction in electronic applications.18 Given the industrial demand for innovative materials, B. Belgacem et al.19 identified Sr2(Sn0.33Sb0.33In0.33)2O6as a promising candidate for high-temperature optoelectronic and thermistor applications. Furthermore, the recently developed semiconductor nanomaterial La0.25Sr0.75Sn0.4In0.25Ru0.35O3, which features a narrow bandgap (1.3 eV) along with remarkable electrical and ferromagnetic properties, has demonstrated potential for applications in optoelectronics, memory devices, photocatalysis,20 and spintronics.21
In addition, double perovskites of the Sr2B′B″O6type, where B′ and B″ represent elements such as Ti, Co, and Mo, exhibit an intriguing p–n switching behavior as a function of temperature. This phenomenon is accompanied by significant variations in thermopower, making these materials suitable for the development of multifunctional devices. Due to their high electrical conductivity across a broad temperature range (300–1300 K), environmental compatibility, thermal stability, oxidation resistance, and lower processing costs compared to conventional chalcogenides and intermetallics, these compounds have been widely explored for thermoelectric applications.22–24
Furthermore, the La2CaFe2SnO9 double perovskite adopts a monoclinic structure (P21/n space group) with an unusual cation ordering at the B-site. This material exhibits magnetic behavior characteristic of an iron-based magnet, with a Curie temperature exceeding room temperature. Notably, the Fe3+ magnetic moment decreases gradually upon heating from 5 K to room temperature.25
Building upon these findings, we propose the synthesis of NdCaFeSnO6(NCFSO) to harness the unique optical properties of rare-earth elements (Nd) while benefiting from the ferromagnetic nature of Fe3+ in its high-spin configuration (t2g3eg2). This study aims to explore the structural, optical, and electrical properties of this material, broadening its potential applications in energy storage technology and optoelectronic devices.
2. Experimental procedure
2.1. Synthesis protocol
The NdCaFeSnO6 sample was synthesized using the conventional solid-state reaction method, which involves high-temperature processing of precursor powders to obtain the desired crystalline phase. The starting materials, CaCO3, Nd2O3, Fe2O3, and SnO2 (purity > 99.95%), were carefully selected to ensure high chemical purity. Prior to synthesis, Nd2O3 was preheated at 1100 °C for 12 hours to eliminate any residual hydrogen carbonate (HCO3−) and moisture. The precursors were then weighed according to the stoichiometric ratio, thoroughly mixed, and ground in an agate mortar for 60 minutes to achieve a fine and homogeneous powder. The resulting powder was placed in an alumina crucible and calcined at 900 °C for 12 hours in a muffle furnace to initiate solid-state diffusion and phase formation. After cooling to room temperature, the powder was finely ground again and pressed into cylindrical pellets (13 mm in diameter, 2 mm in thickness) under an axial pressure of 10 t cm−2, ensuring proper grain boundary contact for enhanced densification. The compacted pellets were then placed in an alumina crucible and subjected to a sintering process at 1050 °C for 12 hours in ambient air to ensure adequate compactness and phase formation. Following this, the furnace was gradually cooled to 800 °C and held for 30 minutes before allowing the sample to return to room temperature. This controlled cooling step helped to minimize internal stresses and structural defects. Initial sintering resulted in a marked decrease in pellet diameter and thickness, confirming effective densification. To further optimize compaction and promote grain growth, a sequence of grinding, granulation, and additional sintering cycles was employed. Four successive sintering cycles at 1050 °C were conducted, with densification progress meticulously tracked via precise measurements of pellet diameter and thickness before and after each cycle. The convergence and subsequent stabilization of these parameters following the fourth cycle evidenced the achievement of a uniform, dense, and stable microstructure.Fig. 1 presents the schematic diagram of the solid-state synthesis process for the NdCaFeSnO6 sample fabrication. The stoichiometric proportions of the reagents used in this synthesis are represented by the following equation:
| 1/2 Nd2O3 + CaCO3 + 1/2 Fe2O3 + SnO2 → NdCaFeSnO6 + CO2(g) |
 | ||
| Fig. 1 Schematical description of the elaboration process of NdCaFeSnO6 ceramics via the solid-state method. | ||
After each heat treatment, the sample undergoes powder X-ray diffraction (XRD) analysis to verify the formation of the desired phase and confirm its purity.
2.2. Characterization techniques
X-ray diffractograms were obtained using an X'Pert3 Powder PANalytical diffractometer in Bragg–Brentano geometry, employing monochromatic Kα radiation with a copper anticathode (λKα1 = 1.54056 Å). The scanning was performed in the 20° ≤ 2θ ≤ 110° range, with a step size of 0.01° and a counting time of 30 seconds per step. The diffraction pattern refinements were carried out using the FULLPROF program.26The morphological analysis of the synthesized sample was carried out using a field emission scanning electron microscope (FE-SEM, FEI Quanta FEG 200) equipped with an energy-dispersive X-ray spectroscopy (EDX) system. SEM observations were performed at an accelerating voltage of 10 kV, providing adequate resolution for detailed examination of the sample's microstructural characteristics. Micrographs were registered at various magnifications ranging from 9195× to 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×, enabling the assessment of grain morphology, distribution, and surface texture.
00×, enabling the assessment of grain morphology, distribution, and surface texture.
Raman vibration modes were identified using a RENISHAW in Via Raman spectroscope (Renishaw UK Sales Ltd, Gloucestershire, UK) with a non-polarized laser (λ = 633 nm). To minimize sample heating and prevent any thermal damage at the surface, a 0.3 neutral density (ND) filter was used, reducing the effective laser power to approximately 5% of its nominal intensity. For each measurement range, five consecutive accumulations were performed, with an exposure time of 30 seconds per accumulation, in order to enhance the signal-to-noise ratio. This procedure was repeated in three independent acquisitions obtain complete acquisition.
The current–voltage (I–V) characteristics were obtained using a KEITHLEY SourceMeter 2611A System (Tektronix/Keithley, Cleveland, OH, USA), with the sample connected to a Linkam THS340-type oven (Linkam Scientific Instruments Ltd, Redhill, UK).
UV-visible diffuse reflectance spectra (DRS) were acquired using a PerkinElmer Lambda 365 spectrophotometer, covering the 200–1100 nm range. By analyzing the DR spectra, key optical properties such as band gap energy and light absorption behavior were determined, contributing to a comprehensive characterization of the material.
Finally, I–V measurements were performed on the NdCaFeSnO6 sample at a specified temperature to investigate the conduction mechanisms involved, using Keithley 2611 SMU Multimeter.
For this purpose, commercial silver electrodes were deposited on both surfaces of the sintered ceramic pellets. The coated samples were air-dried for 30 minutes and then heat-treated at 300 °C for 3 hours to ensure good adhesion and establish stable ohmic contact.
3. Results and discussions
In this section, we will explore the results of our characterization study, beginning with an analysis of the structural properties and concluding with an investigation of the conduction mechanism.3.1. X-ray diffraction study
Several searches conducted on the COD, PDF4+, and highscore databases confirmed the novelty of our synthesized compound. Furthermore, these searches enabled us to determine the most suitable structural model, which is prototyped from the double perovskite compound Ca2CoNbO6,28 exhibiting monoclinic symmetry with space group P21/n.
To further identify and confirm the structure of the NdCaFeSnO6 compound, a slow scan was performed to collect a highly resolved XRD pattern. The most intense peaks were indexed according to monoclinic symmetry (P21/n), although this group is not standard. Therefore, we opted for the P21/c space group, and the reliability factors confirmed this choice.
An important parameter in the ABO3 perovskite-like structure is the Goldschmidt tolerance factor (t), which is related to the likelihood of forming octahedral coordination of the B and B′ cations with the oxygen anion, as well as the tilting of these octahedra. Based on the effective Shannon ionic radii,29 rNd3+ = 1.27 Å, rCa2+ = 1.34 Å (coordination 12), rSn4+ = 0.69 Å, rFe3+ = 0.645 Å (high spin), and r(O2−) = 1.40 Å, the to lerance factor for NdCaFeSnO6 structural organization was determined based on this formula:
The calculated tolerance factor value found equal to 0.925 falls within the stability range of 0.825 to 1.06,30 confirming that this sample exhibit a perovskite-like structural stability. However, the deviation from unity suggests significant distortions from the ideal cubic perovskite structure. These distortions arise due to variations in the ionic radii at sites A and B, leading to minor octahedral tilting and a reduction in symmetry.
Additionally, the octahedral factor µ was determined using the equation:
The obtained value of 0.4767 lies within the stability range (0.414–0.732),31 indicating the formation of stable (Sn/Fe)O6 octahedra. This result, in conjunction with the tolerance factor, supports the prediction that NdCaFeSnO6 adopts a stable double perovskite structure. The structural refinement of the NdCaFeSnO6 sample, performed using the FullProf software with the Rietveld method, confirms that the compound crystallizes in the monoclinic system (space group P21/c). The analysis indicates a predominant phase with a 1:1 ratio of B-site cations. Additionally, a minor secondary phase (5.33%) is identified, which is likely attributed to pyrochlore Nd2Sn2O7. A negligible fraction (0.8%) of Nd3Fe5O12 is also detected. The observed, calculated and difference profiles for the Rietveld refinement of NdCaFeSnO6 compound was shown in Fig. 2. The lattice parameters refined by the Rietveld method are listed in Table 1.
| Atomes | X | Y | Z | Biso (Å2)a | Occb | Site |
|---|---|---|---|---|---|---|
| O1 | 0.153(9) | 0.017(2) | 0.251(8) | 1.517(4) | 1.0 | 4e |
| O2 | 0.208(6) | 0.746(5) | 0.030(4) | 0.068(9) | 1.0 | 4e |
| O3 | 0.310(7) | 0.214(4) | 0.065(4) | 0.556 (8) | 1.0 | 4e |
| Fe1 | 1/2 | 0 | 1/2 | 0.879(0) | 0.5 | 2d |
| Sn1 | 1/2 | 0 | 1/2 | 0.879(0) | 0.5 | 2d |
| Fe2 | 0 | 0 | 0 | 0.565(0) | 0.5 | 2a |
| Sn2 | 0 | 0 | 0 | 0.565(0) | 0.5 | 2a |
| Nd | 0.265(2) | 0.4519(3) | 0.2522(2) | 0.643(7) | 0.5 | 4e |
| Ca | 0.265(2) | 0.4519(3) | 0.2522(2) | 0.643(7) | 0.5 | 4e |
The double perovskite structure of NdCaFeSnO6 refers to a complex oxide material characterized by two interwoven perovskite-like substructures. Based on the Ca2CoNbO6 isotype,28 the structural model of NdCaFeSnO6 presents an ordered distribution of cations at specific lattice sites:
• The 4e site accommodates the larger cations Nd3+ and Ca2+, which are statistically equivalent at the A-site positions. These sites are spacious enough to accommodate large ions.
• The B-site positions (2a (0,0,0) and 2d (1/2,0,1/2)) are occupied by the Fe3+ and Sn4+ cations, which form corner-sharing octahedral coordination with surrounding oxygen atoms.
• The oxygen ions (O2−) fill the three 4e sites, coordinating with metal cations and enhancing the overall stability of the perovskite lattice.
The NdCaFeSnO6 structure presented in Fig. 3 consists of a complex arrangement of rare-earth, alkaline-earth, transition metal, and oxygen ions in a perovskite-related framework. It can be visualized as an alternating stack of two layers:
1. Octahedral layer – composed of rows of (Sn1/Fe1)O6 octahedra (light blue) and (Sn2/Fe2)O6 octahedra (pink), aligned along the b-axis.
2. Rock-salt-type oxide layer – formed by alternating Nd/CaO units along the same axis (see Fig. 4).
This unique arrangement contributes to a range of intriguing physical properties, particularly in terms of conductivity and magnetism, making NdCaFeSnO6 a promising material for various applications. Interatomic distances and angles for polyhedron are listed in Table 2.
| Monoclinic P21/c | Distance (Å) | Angles (°) |
|---|---|---|
| 2 × (Sn1/Fe1)–O1 | 1.84(3) | 1 × O1– (Sn1/Fe1)–O1 180(3) |
| 2 × (Sn1/Fe1)–O2 | 2.27(6) | 2 × O1–(Sn1/Fe1)–O2 87(2) |
| 2 × (Sn1/Fe1)–O3 | 1.948(1) | 2 × O1–(Sn1/Fe1)–O2 93(4) |
| 2 × O1–(Sn1/Fe1)–O3 88.6(1) | ||
| 2 × O1–(Sn1/Fe1)–O3 91.4(1) | ||
| 1 × O2–(Sn1/Fe1)–O2 180(5) | ||
| 2 × O2–(Sn1/Fe1)–O3 98(2) | ||
| 2 × O2–(Sn1/Fe1)–O3 82(3) | ||
| 1× O3–(Sn1/Fe1)–O3 180.0(1) | ||
| Moyen <(Sn1/Fe1)–O> | 2.019(3) | |
| 2 × (Sn2/Fe2)–O1 | 2.22(4) | 1 × O1–(Sn2/Fe2)–O1 180(3) |
| 2 × (Sn2/Fe2)–O3 | 1.78(4) | 2 × O1–(Sn2/Fe2)–O2 86(4) |
| 2.151(1) | 2 × O1–(Sn2/Fe2)–O2 94(3) | |
| 2 × O1–(Sn2/Fe2)–O3 87(2) | ||
| 2 × O1–(Sn2/Fe2)–O3 93.0(1) | ||
| 1 × O2–(Sn2/Fe2)–O2 180(4) | ||
| 2 × O2–(Sn2/Fe2)–O3 79(2) | ||
| 2 × O2–(Sn2/Fe2)–O3 101(3) | ||
| 1 × O3–(Sn2/Fe2)–O3 180.0(1) | ||
| Moyen <(Sn2/Fe2)–O> | 2.050(1) | |
| (Ca/Nd)–O1 | 2.492(2) | |
| (Ca/Nd)–O1 | 3.259(1) | |
| (Ca/Nd)–O1 | 2.36(4) | |
| (Ca/Nd)–O1 | 3.22(4) | |
| (Ca/Nd)–O2 | 2.65(7) | |
| (Ca/Nd)–O2 | 2.29(4) | |
| (Ca/Nd)–O2 | 3.07(4) | |
| (Ca/Nd)–O2 | 3.28(8) | |
| (Ca/Nd)–O3 | 2.61(3) | |
| (Ca/Nd)–O3 | 2.326(1) | |
| (Ca/Nd)–O3 | 3.550(1) | |
| (Ca/Nd)–O3 | 2.79(2) | |
| Moyen <(Ca/Nd)–O> | 2.824(7) |
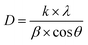 | (1) |
The most significant diffraction peaks (as listed in Table 3) were used for this calculation. The average crystallite size was determined to be 34.106(4) nm, closely matching that of CaSnO3 doped with 1% Fe, which crystallizes in an orthorhombic structure (space group Pnma).11
| h | k | l | 2θ | Iobs | FWHM |
|---|---|---|---|---|---|
| 0 | 0 | 2 | 22.6395 | 5.8 | 0.17773 |
| 1 | 1 | 0 | 25.3098 | 61.7 | 0.17898 |
| 0 | 2 | 0 | 31.7765 | 509.2 | 0.18430 |
| 1 | 1 | 1 | 32.2047 | 1085.7 | 0.18477 |
| 0 | 2 | 1 | 33.8144 | 81.6 | 0.18665 |
| −2 | 0 | 2 | 32.5142 | 522.8 | 0.18511 |
| −2 | 1 | 1 | 38.1511 | 20.4 | 0.19274 |
| −2 | 2 | 2 | 46.1028 | 572.6 | 0.20770 |
| 0 | 0 | 4 | 46.2289 | 309.6 | 0.20798 |
| −2 | 2 | 1 | 47.6261 | 38.6 | 0.21113 |
| 2 | 2 | 0 | 51.9727 | 15.9 | 0.22188 |
| −1 | 1 | 5 | 52.0193 | 3.4 | 0.22201 |
| 1 | 3 | 0 | 52.8268 | 55.5 | 0.22417 |
| −3 | 1 | 1 | 57.8328 | 242.8 | 0.23870 |
| 2 | 2 | 2 | 67.3812 | 151.1 | 0.27196 |
| 1 | 1 | 5 | 76.7363 | 73.3 | 0.31217 |
| 4 | 0 | 0 | 86.3469 | 30.1 | 0.36263 |
| 4 | 2 | 0 | 94.9437 | 30.5 | 0.41748 |
Although the co-substitution of elements with different ionic radii inevitably induces micro-deformations within the lattice, these distortions significantly influence the average crystallite size. To account for both crystallite size effects and lattice strain, Williamson and Hall proposed a method that separates these two contributions to peak broadening in nanocrystals. This approach can be mathematically expressed as:
 | (2) |
By plotting β cos(θ) against 4 sin(θ), the slope of the resulting linear fit provides ε (lattice strain), while the y-intercept yields the inverse of the crystallite size (D−1). This method allows for a more precise assessment of both structural distortions and size-dependent broadening effects in NdCaFeSnO6.
This model assumes a homogeneous lattice strain in all directions, with ε representing the intrinsic strain. To analyze this, a graph was plotted with β cos(θ) on the y-axis and 4 sinθ on the x-axis, incorporating all Bragg peaks. The slope of the fitted line represents the overall lattice strain (ε), while the y-intercept provides an estimate of the crystallite size (D). The resulting fit is illustrated in Fig. 5.
The calculated micro-strain value for the NdCaFeSnO6 compound is 1.21 × 10−3, which is lower than that observed for the parent CaSnO3 and Fe-doped compounds (Fe-1%, Fe-3%, and Fe-5%), recorded at 1.812 × 10−3, 1.631 × 10−3, 1.522 × 10−3, and 1.413 × 10−3, respectively.11 This reduction can be attributed to the differences in ionic radii of the constituent elements in NdCaFeSnO6: r(Nd3+) = 1.27 Å, r(Ca2+) = 1.34 Å (site A), r(Sn4+) = 0.69 Å, and r(Fe3+) = 0.645 Å (site B).
The crystallite size determined using the Williamson–Hall method is 96.972(9) nm, which notably differs from the value obtained via the Scherrer equation. A comparative analysis, as highlighted in a study published in Advances in Natural Sciences: Nanoscience and Nanotechnology (2019),32 demonstrates that the Scherrer method neglects lattice deformations, often underestimating crystallite sizes, as seen in this case. In contrast, the Williamson–Hall approach, which decouples the effects of crystallite size and lattice strain, provides a more accurate estimation, particularly in materials with significant internal stresses.
The substantial discrepancy between the two methods underscores the presence of notable internal strains within the NdCaFeSnO6 double perovskite compound. In summary the crystallite size and micro-strain values determined via Williamson–Hall analysis confirm the nanometric scale of the NdCaFeSnO6 powder. The observed micro-strain, evidenced by XRD peak broadening and corroborated by microstructural features, significantly influences the material's optoelectronic and dielectric behavior. These localized lattice distortions alter orbital interactions, particularly within the (Sn/Fe)O6 octahedra, leading to bond angle deformations and promoting the generation of point defects such as oxygen vacancies. These defects directly affect charge carrier mobility, dielectric relaxation mechanisms, and contribute to the observed bandgap narrowing.
3.2. Scanning electron microscopy (SEM) and EDX analysis
This qualitative and semi-quantitative study enabled the identification and verification of all elements present in the NdCaFeSnO6 double perovskite compound. Fig. 6 presents micrographs registered at magnifications 9195× and 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×, illustrating a homogeneous elemental distribution and a well-organized structural arrangement. The powder demonstrates a high level of densification with uniformly shaped grains indicating consistent morphology across the sample.
00×, illustrating a homogeneous elemental distribution and a well-organized structural arrangement. The powder demonstrates a high level of densification with uniformly shaped grains indicating consistent morphology across the sample.
Additionally, the sample consists of particles of varying sizes, with most having a diameter of approximately 0.15 µm, while a few reached up to 0.4 µm. To determine the elemental composition of the synthesized powder, EDX analysis was performed. The results confirm the presence of all expected chemical elements in their stoichiometric proportions, further validating the successful synthesis of the NdCaFeSnO6 double perovskite compound.
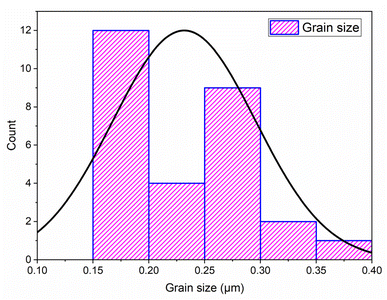
Grain size distribution was quantitatively assessed using Image J software.33 Measurements were conducted on a representative set of at least 28 grains. As shown in the histogram (Fig. 7), the average particle size of the NdCaFeSnO6 powder is approximately 226 nm, which is significantly larger than the values obtained from XRD analysis. This discrepancy arises from the differences in measurement techniques: scanning electron microscopy (SEM) provides an estimation of particle size, often reflecting the size of agglomerates, whereas X-ray diffraction (XRD) determines the crystallite size, representing the individual grains within a particle.
Fig. 8 presents the EDX spectrum for the NdCaFeSnO6 sample. The EDX analysis, which is qualitative and semi-quantitative in nature, enabled the identification of all the constituent elements of the NdCaFeSnO6 compound. However, since this technique is primarily surface-sensitive and has a shallow analysis depth, the results mainly reflect the surface composition. Within these limits, the data confirm the expected stoichiometry. Moreover, any potential interfering phase detected corresponds to a combination of Nd, Ca, Fe, Sn, and O elements.
From EDX (Energy Dispersive X-ray Spectroscopy) analysis, we use separate individual color to distinguish different elements present in a sample. The software assigns specific colors to different elements based on their characteristic X-ray emissions. This visualization technique is crucial for identifying the spatial distribution of elements within a material. Fig. 8 shows the presence of the element present in the studied compound.
This verification confirms the successful synthesis of the NdCaFeSnO6 double perovskite compound with the expected stoichiometry. The EDX analysis conducted at four different locations ensures that the elemental distribution is homogeneous across the sample. Any minor deviations from the theoretical composition could be attributed to instrumental limitations or localized variations in elemental concentration. To evaluate the synthesis quality, the percentage deviations from the theoretical stoichiometric values were calculated, as shown in Table 4. The low absolute errors confirm the compositional accuracy of the synthesized NdCaFeSnO6 compound.
| Element | Mass percentage (wt%) | Experimental composition | Theoretical composition | Absolute error (%) |
|---|---|---|---|---|
| O | 21.34 | 6.29 | 6.00 | 0.05 |
| Ca | 8.5 | 1 | 1.00 | 0 |
| Nd | 32.16 | 1.05 | 1.00 | 0.05 |
| Fe | 12 | 1.01 | 1.00 | 0.01 |
| Sn | 26 | 1.03 | 1.00 | 0.03 |
3.3. Raman spectroscopy
Raman spectroscopy is a powerful, non-destructive technique widely used to investigate various material properties, including crystalline quality, the structure of inorganic compounds, and the presence of disorder and defects in semiconductor oxides. This technique relies on the inelastic scattering of light, known as the Raman effect. The number of bands observed in a Raman spectrum depends on the crystal symmetry of the studied compound and corresponds to the vibrational modes of the atoms within the structure.In this study, the crystal symmetry and local distortion of the synthesized compound NdCaFeSnO6 were examined through Raman spectroscopy at room temperature. Spectra were recorded in the range of 50 to 900 cm−1 using an excitation wavelength of 633 nm. To accurately distinguish between vibrational and translational lattice modes, a polarized Raman spectrum was required.34 For a monoclinic structure with space group P21/c (C52h), the Raman-active modes are given by: Γ (P21/c) = 6T (3Ag + 3Bg) + 6L (3Ag + 3Bg) + 2ν1 (Ag + Bg) + 4ν2(2Ag + 2Bg) + 6ν5 (3Ag + 3Bg).32
The translational lattice modes (T) associated with non-degenerate bands exhibit minimal Raman activity in the low-frequency range of 50–200 cm−1. The vibrational lattice modes (L), also referred to as silent modes, were observed in the 200–400 cm−1 range. Meanwhile, the internal modes ν1, ν2, and ν5 displayed relatively high Raman activity above 400 cm−1.
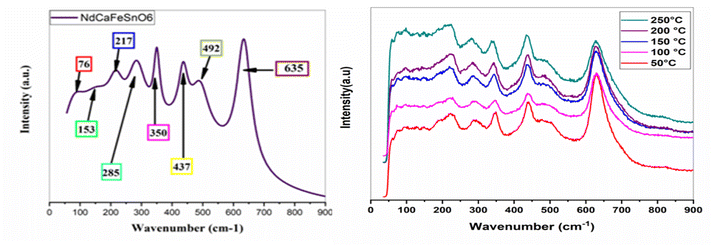
The co-substitution of Nd and Fe in the CaSnO3 compound may induce shifts in the Raman peak frequencies, particularly in the low-to intermediate-frequency ranges, due to changes in crystal symmetry. However, the overall vibrational characteristics and the Raman mode pattern are expected to remain consistent with those of other double perovskite-type compounds exhibiting P21/c symmetry.35–37 The experimental Raman spectrum recorded at room temperature on NdCaFeSnO6 ceramic, presented in Fig. 9, provides precise frequency values for the observed vibrational modes. This spectrum measured in the range 50–900 cm−1 using a 633 nm excitation wavelength, reveals several characteristic vibrational modes of the P21/c monoclinic structure. In the low-frequency region (50–200 cm−1), the translational modes (T) associated with Nd and Ca cations at the A-site exhibit weak Raman activity. Two distinct bands are observed at 76 cm−1 and 153 cm−1, corresponding to the translation of these cations and the slight tilting of the (Sn/Fe)O6 octahedra, respectively. The vibrational modes (L), also known as silent modes, appear in the 200–400 cm−1 range. Although these modes are typically Raman-inactive, they become detectable due to the symmetry reduction induced by the monoclinic structure. Three main bands are identified in thisregion:
• 217 cm−1: attributed to bending vibrations involving oxygen displacement.38
• 285 cm−1: associated with Nd–O scattering, in agreement with previous studies.12
• 350 cm−1: linked to the distortion of the octahedral coordination around Sn and Fe cations.11
In the high-frequency region (>400 cm−1), the internal modes ν1, ν2, and ν5 exhibit strong Raman activity:
• 437 cm−1: assigned to the ν5 mode, corresponding to the bending motion of O–(Sn/Fe)–O bonds.
• 492 cm−1 and 635 cm−1: attributed to the ν2 and ν1 modes, corresponding to the symmetric and asymmetric stretching vibrations of the (Sn/Fe)O6 octahedra, respectively. These results are consistent with previous Raman studies.39–41
Fig. 9 shows the Raman spectra at different temperature. We note an expansion of the crystal lattice as a function of temperature resulting in the progressive displacement of the modes, in particular the one located at 350 cm−1. No mode appears or disappears, proving that there is no clear phase transition in the material (no symmetry breaking).
Table 5 summarizes the experimentally observed vibrational frequencies, their possible assignments, and their correlation with the crystal structure and characteristic spectral signatures of the material.
| Raman wavenumber (cm−1) | Modes | Attributes |
|---|---|---|
| 76 | T | •Translation of Nd and Ca cations from A-site |
| 153 | •Low tilt of (Sn/Fe)O6 octahedra | |
| 217 | L | •Bending vibrations |
| 285 | •Nd–O scattering | |
| 350 | •Distortion of the octahedral coordination around the Sn and Fe cations | |
| 437 | ν5 | •The internal oxygen bending motion of the O–(Sn/Fe)–O |
| 492 | ν2 | •Totally symmetric and antisymmetric stretching of the (Sn/Fe)O6 octahedron |
| 35 | ν1 |
3.4. UV-visible analysis
To investigate the optical properties of our compound, we performed UV-vis spectroscopy using a solid-state UV-visible spectrophotometer.The reflectance spectrum of the NdCaFeSnO6 sample, presented in Fig. 10, exhibits an optical response characteristic of a wide bandgap semiconductor, with strong absorption occurring in the 350–450 nm region, peaking at approximately 391 nm.
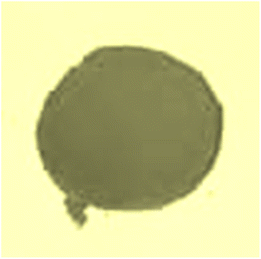
The sample demonstrates intense optical absorption in the 380–430 nm wavelength range, which corresponds to the violet region of the visible spectrum. As a result, the sample appears green, the complementary color of violet. Fig. 11 depicts the actual color of the NdCaFeSnO6 pellet, consistent with its absorption characteristics.
The reflectance spectra were further analyzed using the Kubelka–Munk theory to determine the maximum absorbance of the sample.42 The Kubelka–Munk function was calculated based on the measured reflectance values using the following equation:
| F(R) = (1 − R)2/2R ≈ K/S | (3) |
The optical band gap (Eg) of NdCaFeSnO6 was determined using the Tauc plot method. The Tauc equation is given by:
| (F(R) × hν)p = A(hν − Eg) | (4) |
• p = 2 for an allowed direct transition.
• p = 1/2 for an allowed indirect transition.
In Fig. 12, we plot (F(R) hν)2 as a function of hν (incident photon energy). The band gap energy values are obtained from the intersection of the extrapolated linear region with the photon energy axis (hν).
Fig. 12 presents the Tauc plot corresponding to the direct electronic transitions since the obtained Tauc parameter is 2 for NdCaFeSnO6 nanoparticles. The corresponding gap energy was 2.44 eV. These values are relatively low compared to the parent compound CaSnO3,43 which exhibits an ultrawide band gap of 4.2–4.4 eV, as well as Fe-doped CaSnO3 compounds,11 which have band gap values ranging from 3.04 to 3.78 eV.
The optical band gap is primarily influenced by intermediate energy levels within the band gap, as well as the degree of order and disorder in the compound's structural composition. In this case, the band gap reduction (Eg) can be attributed to the presence of oxygen vacancies  , lattice defects, and local bond distortions.44 As reported in the literature,11 substituting Sn with Fe leads to the formation of stable holes at the top of the valence band and increases the covalency of the Sn–O bond. Moreover, optical absorption studies confirm the incorporation of Fe into our structure.45
, lattice defects, and local bond distortions.44 As reported in the literature,11 substituting Sn with Fe leads to the formation of stable holes at the top of the valence band and increases the covalency of the Sn–O bond. Moreover, optical absorption studies confirm the incorporation of Fe into our structure.45
3.5. Dielectric properties, impedance spectroscopy and I–V measurements
Measurements were performed using wire connecting the samples to Solartron SI-1200 apparatus. Fig. 13(a) illustrates the behavior of the real part of the dielectric permittivity during the cooling process as a function of temperature, for various frequencies. The onset of a conductivity regime is observed to depend on the source frequency. At higher frequencies, the onset of this regime is delayed to higher temperatures. A noticeable bump appears in the permittivity curve, followed by a sharp increase in permittivity, when temperature increases, which is attributed to the electrical conductivity in the material. This behavior is further confirmed by the high dielectric loss observed in the material (Fig. 13(b)), which is significantly pronounced at lower frequencies and lower temperatures. This can be attributed to ionic conductivity and thermal charge carrier activation in the material. Dielectric permittivity was found around 120 at room temperature and 3 kHz.
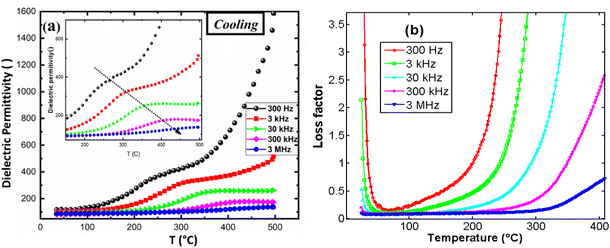 | ||
| Fig. 13 (a) Real dielectric permittivity, (b) dielectric loss, versus temperature for several frequencies. | ||
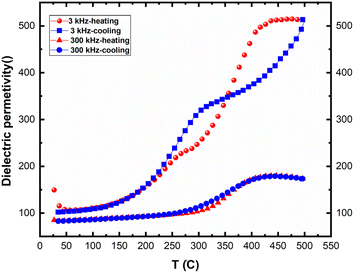
Fig. 14 presents the variation of real permittivity during heating and cooling at 3 kHz and 300 kHz. Two distinct dynamic behaviors are observed at these frequencies, with the presence of two thermal hysteresis loops at the lower frequency (30 kHz). At heating, two separated anomalies were observed for the frequency 3 kHz. However, only one anomaly is observed for the frequency 30 kHz. The second anomaly (at high temperature) disappeared at cooling. All these behaviors are in favor of electrical conductivity in this compound.
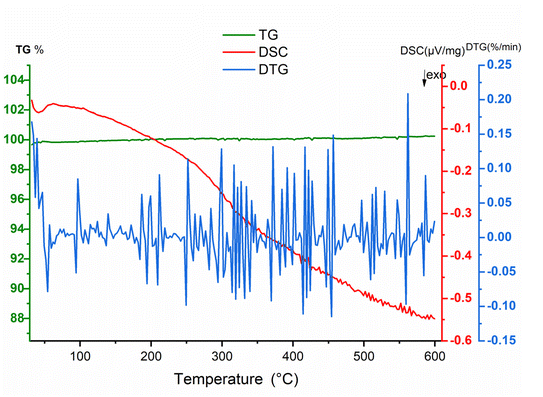
Thermal analysis was performed under air. TGA curve did not indicate a clear weight lost characterized by the flat curve where the DTA curve showed no significative variation versus temperature (Fig. 15). The absence of a sharp pic on the DSC curve indicates also no phase transition in the material.
In the absence of a phase transition, these behaviors could be attributed to different electrical conductivity regimes within the material, influenced by both temperature and frequency.
At low temperatures, charge carriers may become trapped in localized states (e.g., defects or impurities), temporarily increasing impedance until thermal activation enhances conduction. Additionally, slight temperature variations can modify defect distributions at grain boundaries initially, leading to higher resistance. In this temperature range an imbalance in oxygen ion mobility may further contribute to a transient increase in impedance before conductivity further improves. When increasing temperatures, charge polarization effects can intensify, that led to increase the resistive component of impedance before dominant conduction mechanisms take over.48,49
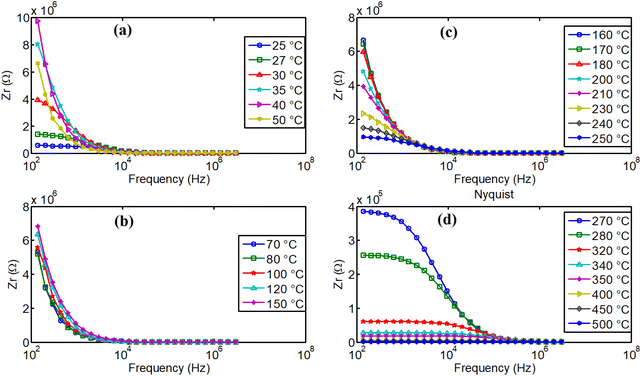
From 80 °C, we can remark the progressive decrease of real part of the impedance when temperature increases and the material exhibits normal electrical conductivity that increases with the temperature. This conductivity is governed by various mechanisms, including grain conductivity and grain boundary conductivity, facilitated for example, by hopping or oxygen vacancies.50 Fig. 16(b–d) illustrate high dispersion of the material's real part of impedance, for several temperatures. Distinct relaxation frequencies were observed in the low frequency region in the temperature range 270–500 °C, that correspond also to high conduction region. Conduction mechanism is further analyzed using impedance spectroscopy data for a more comprehensive understanding.
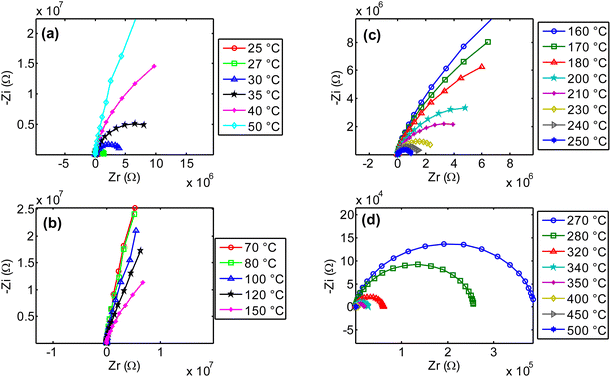
Fig. 17(a–d) presents the Nyquist diagrams across four different temperature ranges. At low temperatures (Fig. 17(a)), as expected, in agreement with previous observations, the radius of the semicircle increases abnormally with temperature. This unusual behavior was explained in previous paragraphs. In contrast, for temperatures above 80 °C (Fig. 17(b–d)), the opposite effect is observed: the radius of the imperfect semicircles decreases as temperature increases. This confirms the earlier findings regarding the activation of ionic conductivity in this compound, as well as the possible contribution of other charge transient mechanisms. It is important to note that the observed relaxation phenomena do not follow a pure Debye-type behavior due to the distribution of relaxation times and the complex nature of the conduction mechanism. The electrical properties of the bulk ceramic material can be distinguished from those of the grains, grain boundaries, and electrode contributions by employing frequency-dependent impedance spectroscopy at various temperatures. A clear reduction in grain and grain boundary resistance is observed, along with a negative temperature coefficient of resistance (NTCR) behavior, like that of semiconductors,51,52 characterized by imperfect semicircles in the Nyquist plots.
Using an equivalent circuit model, the experimental data (in Nyquist representation) were fitted, allowing us to determine the contributions of grain, grain boundary, and electrode effects to the overall dielectric response of the ceramic material. An equivalent circuit analysis (Randle circuit) was conducted to fit the Nyquist plots for each temperature. Among the multiple circuit configurations tested, satisfactory results were achieved with a model consisting of a series connection of three parallel R-CPE branches and a very low conducting resistance for full satisfactory results. The equivalent circuit diagram is presented below in Fig. 18.
 | ||
| Fig. 18 Equivalent electrical circuit (EEC) use to fit impedance data to determine conductivity mechanism in the material. | ||
Recall that the CPE (Constant Phase Element) is a charge phase element characterized by an impedance Z given by the formula:
The constant phase element (CPE) corresponds to an ideal capacitor with C = Q for p = 1 and to an idealresistor with  for p = 0. Therefore, p can be used to represent the degree of imperfection of the capacitor, and represents a measure of arc distortion below the real impedance axis.53 The parameter p is related to the depression angle αd given as following expression:
for p = 0. Therefore, p can be used to represent the degree of imperfection of the capacitor, and represents a measure of arc distortion below the real impedance axis.53 The parameter p is related to the depression angle αd given as following expression:
From this analysis, we determined the resistances of the grain (R3), grain boundary (R2), defects and space charge effects at electrodes (R3) and system wiring resistance (R4). Then, we calculated the conductivity through the material separating clearly the contribution of grain and grain boundary. We report on Fig. 19 (a–d) the experimental data and fitted curves for several temperatures chosen in between the previous defined temperature ranges.
 | ||
| Fig. 19 Experimental and fitted curves of NdCaFeSnO6 impedance spectral responses in Nyquist plots for some temperatures chosen in the 3 regions. | ||
Data are fitted satisfactorily in accordance of the Randle circuit shown in Fig. 19. The calculated values of the resistances of the materials at different temperatures are summarized in Table 6. In this table we can see clearly, the decrease of all the resistance when temperature increases. This is in favor of high conductivity at higher temperatures.
| T (°C) | R3 (Ω) | Q (F) | p | R3 (Ω) | Q (F) | p | R3 (Ω) | Q (F) | p | R4 (Ω) |
|---|---|---|---|---|---|---|---|---|---|---|
| 27 | 6.67 × 105 | 8.11 × 10−10 | 8.60 × 10−1 | 9.35 × 104 | 2.13 × 10−10 | 9.28 × 10−1 | 6.32 × 105 | 8.84 × 10−11 | 9.83 × 10−1 | 1.23 × 102 |
| 30 | 1.32 × 106 | 2.61 × 10−10 | 1.10 × 10 | 2.77 × 106 | 7.45 × 10−11 | 9.72 × 10−1 | 2.59 × 105 | 2.83 × 10−10 | 8.93 × 10−1 | 1.08 × 102 |
| 35 | 8.39 × 106 | 1.07 × 10−10 | 1.01 × 10 | 3.10 × 106 | 9.51 × 10−11 | 9.81 × 10−1 | 4.47 × 105 | 4.30 × 10−10 | 8.65 × 10−1 | 2.52 × 102 |
| 70 | 2.84 × 108 | 6.01 × 10−11 | 9.55 ×10−1 | 1.37 × 106 | 8.00 × 10−10 | 9.41 ×10−1 | 2.79 × 105 | 2.17 × 10−9 | 8.81 ×10−1 | 1.11 × 102 |
| 80 | 2.99 × 108 | 6.46 × 10−11 | 9.59 ×10−1 | 2.42 × 106 | 4.86 × 10−10 | 9.04 ×10−1 | 2.76 × 105 | 3.11 × 10−10 | 1.42 × 10 | 5.23 × 101 |
| 120 | 1.35 × 108 | 9.26 × 10−11 | 9.57 ×10−1 | 4.02 × 106 | 2.17 × 10−10 | 9.23 ×1 0−1 | 7.72 × 104 | 6.12 × 10−10 | 1.00 × 10 | 1.21 × 102 |
| 180 | 1.54 × 107 | 2.41 × 10−10 | 9.42 ×10−1 | 3.03 × 106 | 2.30 × 10−10 | 9.27 × 10−1 | 4.76 × 105 | 3.72 × 10−10 | 8.87 ×10−1 | 1.83 × 103 |
| 210 | 4.59 × 106 | 3.43 × 10−10 | 9.18 ×10−1 | 1.15 × 106 | 1.88 × 10−10 | 9.43 ×10−1 | 1.65 × 105 | 2.73 × 10−10 | 9.19 ×10−1 | 4.74 × 101 |
| 250 | 3.69 × 107 | 1.64 × 10−10 | 9.78 × 10−1 | 5.54 × 105 | 8.16 × 10−10 | 8.90 × 10−1 | 4.10 × 105 | 2.26 × 10−10 | 9.04 ×10−1 | 1.01 × 102 |
| 270 | 1.65 × 105 | 1.10 × 10−9 | 8.88 × 10−1 | 2.59 × 104 | 3.00 × 10−10 | 9.32 × 10−1 | 2.00 × 105 | 2.33 × 10−10 | 9.01 × 10−1 | 1.83 × 102 |
| 320 | 4.11 × 103 | 3.88 × 10−11 | 1.07 × 10 | 4.75 × 104 | 2.86 × 10−10 | 8.85 × 10−1 | 8.93 × 103 | 8.91 × 10−9 | 8.34 × 10−1 | 2.55 × 10 |
| 450 | 2.37 × 103 | 2.94 × 10−9 | 7.27 × 10−1 | 4.65 × 102 | 1.26 × 10−4 | 1.12 × 10−2 | 2.27 × 104 | 1.21 × 10−13 | 4.24 × 10 | 3.70 × 102 |
| 500 | 1.03 × 103 | 5.57 × 10−10 | 8.82 × 10−1 | 1.39 × 102 | 3.98 × 10−5 | 2.24 × 10−1 | 1.49 × 106 | 4.24 × 10−12 | 7.38 × 10 | 4.88 × 102 |
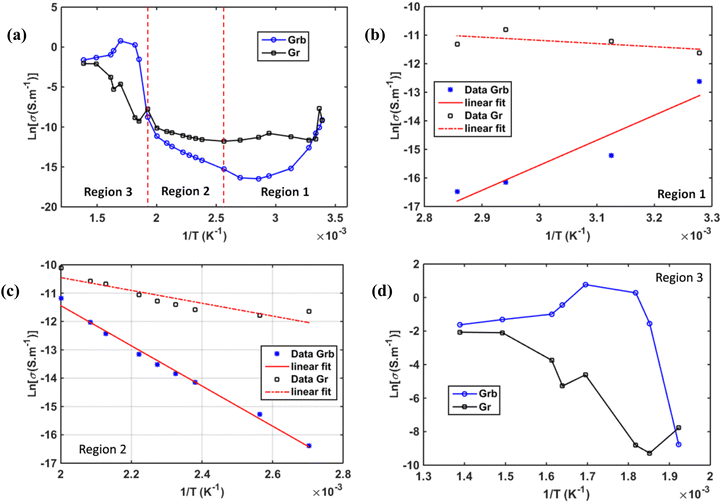
In Fig. 20, we report the results of the conductivity analysis for this material. Fig. 20(a) shows the overall temperature evolution of conductivity in this compound for the global temperature range. The conductivity evolution is quite different according to temperature ranges separated in three regions: Region 1 corresponds to [30–70 °C] (Fig. 20(b)), Region 2 to [80–250 °C] (Fig. 20(c)), and Region 3 to [270–500 °C] (Fig. 20(d)). As mentioned previously, the dynamics of conductivity in these separated regions are distinct. Only Region-2 can be described by a normal thermally activated ionic conductivity, which followed Arrhenius-type law54 with activation energies of 0.31 eV for the grains and 0.97 eV for the grain boundaries. The conductivity in Region 1 is unstable but can be described with Arrhenius plot with positive slop traducing the increase of resistivity that limits electrical conductivity. This phenomenon was attributed to atomic reordering and charge carrier trapping as described in previous paragraph. In Region 3, another mechanism was clearly observable that is different to that observed in region 1 and 2. This region corresponds to the high temperature range where electrical conductivity was maximal. The mechanism of conduction does not follow Arrhenius law. That is why to better understand the mechanism of conductivity we performed data adjustment using Jonscher type law in this region.55
Fig. 21(a–d) illustrate the evolution of the ac conductivity calculated from impedance modulus as a function of temperature across the three regions. At low temperatures, a decrease in conductivity is observed with temperature, displaying a plateau-like behavior in the low-frequency region relative to DC contribution. This is followed by a positive slope at higher frequencies, beyond 104 Hz, where the conductivity increases linearly, characteristic of the Jonscher effect.55 At intermediate temperatures (70–250 °C), a linear increase of the conductivity curves is observed for all frequencies (Fig. 21(b and c)) while a plateau began to be observed 250 °C, that was amplified by thermo-activated ionic conductivity, strongly dependent on the frequency at high temperatures. At high temperatures (270–500 °C), the impedance behavior exhibits clearly a low-frequency plateau (DC) followed by a linear increase at higher frequencies, with a more accelerated dynamic. The plateau enlarged more and more when temperature increased signature of DC predominance that can be attributed to electron appearance. This progression is observed in the correct temperature order, as shown in Fig. 21(d). This behavior is supported by Jonscher law described by the equation:55
| σac = σdc + Aωα. |
 | ||
| Fig. 21 AC conductivity variation as function of frequency in several temperature regions. These curves were fitted with Jonscher law satisfactorily. | ||
Basing on this expression, all conductivity curves were fitted to determine all the coefficients (σac: ac conductivity, σdc: dc conductivity, A: the pre-exponential factor intrinsic to the material and α: exponent of the frequency, the Jonscher coefficient). The obtained values were summarized in Table 7 and some fitted curves were plotted on Fig. 21(d) in Region 3.
| Region-1 | Region-2 | Region-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | σdc (µS m−1) | A (ng) | α | T (°C) | σdc (µS m−1) | A (ng) | α | T (°C) | σdc (mS m−1) | A (ng) | α |
| 25 | 47.5 | 1.62 | 0.953 | 80 | 0.130 | 0.78 | 0.999 | 270 | 0.077 | 0.40 | 1.046 |
| 27 | 19.8 | 1.07 | 0.979 | 100 | 0.132 | 0.79 | 0.999 | 280 | 0.115 | 0.43 | 1.042 |
| 30 | 5.16 | 1.70 | 0.950 | 120 | 0.633 | 0.79 | 0.999 | 320 | 0.455 | 0.39 | 1.048 |
| 35 | 2.72 | 0.75 | 1.000 | 150 | 0.985 | 0.81 | 0.999 | 340 | 1.047 | 0.40 | 1.048 |
| 40 | 1.59 | 1.23 | 0.970 | 160 | 2.203 | 0.77 | 1.002 | 350 | 1.512 | 0.48 | 1.037 |
| 50 | 0.09 | 1.24 | 0.970 | 170 | 2.191 | 0.78 | 1.002 | 400 | 5.439 | 0.45 | 1.046 |
| 70 | 0.12 | 0.78 | 0.999 | 180 | 2.437 | 0.79 | 1.002 | 450 | 11.890 | 0.07 | 1.159 |
| n = nano, g = [(S m−1) × (rad s−1)−α] is the pre-factor Aunity in the Jonscher law | 200 | 3.121 | 2.86 | 0.923 | 500 | 19.730 | 0.01 | 1.263 | |||
| 210 | 6.790 | 0.81 | 1.001 | ||||||||
| 230 | 12.060 | 0.83 | 1.000 | ||||||||
| 240 | 18.860 | 2.02 | 0.946 | ||||||||
| 250 | 25.470 | 0.61 | 1.019 | ||||||||
The results in Table 7 showed the increase of DC conductivity as a function of temperature. Enlargement of DC current plateau is observed in Region 3, the high temperature region. In Region 1 the plateau is almost shrined when temperature increased by the charge trapping phenomenon, while intermediate temperature phase (Region 2) exhibited almost linear behavior. The fitted curves, using Jonscher law, are added Fig. 21(d) which demonstrates accurate results obtained in region 3. This figure showed more and more DC current creation when temperature increases.
This method enables the identification of the field dependence of charge carrier transport across the compound. Additionally, to gain deeper insight into the underlying conduction mechanisms, I–V measurements were carried out at various temperatures. Analyzing the temperature dependence of the electrical response allows for the differentiation of transport phenomena such as thermally activated conduction, hopping mechanisms, defect-assisted charge movement, Schottky and Poole–Frenkel50,55–58 conduction, thereby providing a comprehensive understanding of the material's electrical properties. In the present case Poole–Frenkel conduction brought more accurate response of simulation particularly in the region 3 corresponding to the conductivity at high temperature range comparatively to those reported earlier in ref. 59, 60 and 61. Poole–Frenkel model was preferred after tested Schottky and Mott models.62,63 Fig. 22 displays the evolution of current density as a function of the applied voltage in Region 3. A diode-like behavior is observed, with absence of hysteresis, indicating that there is no resistive switching effect and no rigid formation of a Schottky barrier at the interfaces.64 The threshold voltage is observed around ∼2 V, which corresponds to ∼0.036 kV cm−1 applied electric field.
It can be concluded that the conduction mechanism of the NdCaFeSnO6 compound follows a Poole–Frenkel (P–F) type mechanism, in Region 3 as the curve fitting results at high temperatures are satisfactory for the analysis of this material.57,65 Recall that the P–F effect describes the field-enhanced thermal ionization of trapped charge carriers in an insulating or semiconducting material. The characteristic equation for P–F conductivity is given by the emission current density, which can be expressed as follows:
It is worth to recall that  , where: q is the charge of electron (1.6 × 10−19 C), ε0 the vacuum permittivity (8.85 × 10−12 F m−1) and εrthe relative permittivity of the material.
, where: q is the charge of electron (1.6 × 10−19 C), ε0 the vacuum permittivity (8.85 × 10−12 F m−1) and εrthe relative permittivity of the material.
To determine the activation energy of the NdCaFeSnO6 compound at high temperatures, we first plotted Ln (J) as a function of √E. The intercept values on the y-axis were then extracted for each temperature and subsequently plotted as a function of 1/T. The slope of the resulting linear fit enabled the calculation of the activation energy.
The upper branches of these curves were modeled based on the Poole–Frenkel (P–F) relation, leading to the construction of Fig. 23(a), which illustrates the current density as a function of √E, exhibiting linear behavior. The linear fit of these curves provided the intercept on the y-axis, which, when plotted against 1/T, allowed for the determination of the activation energy in this temperature range.
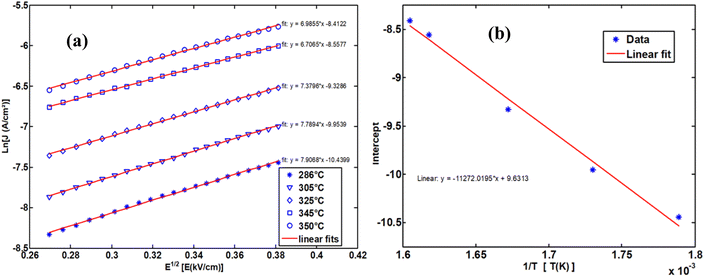 | ||
| Fig. 23 The Poole–Frenkel model employs a variety of representations to ascertain the activation energy. | ||
The obtained activation energy was 1.55 eV, a relatively high value compared to that derived from impedance spectroscopy measurements. In the latter case, excitation in a frequency dynamic mode facilitates faster charge carrier motion, thereby reducing the activation energy. Fig. 23(a) presents the DC current density as a function of √E, while Fig. 23(b) depicts the variation of the intercept as a function of 1/T. The slope of this second plot was used to extract the P–F activation energy.
Finally, at higher temperatures (from 270–500 °C), a transition occurs, characterized by a conduction mechanism that follows Jonscher's law and exhibits a wide frequency range. In this high-temperature range, grain boundary conductivity surpasses that of the grains, with a crossing point observed around 280 °C. This transition occurs without a structural phase change, yet it reflects different thermoactive conduction regimes in the presence of the diffect chemical element such us Fe and Sn. Additionally, a Poole–Frenkel (P–F) type conduction mechanism dominates, that was evidenced by I–V measurements. Overall, this material demonstrates promising optoelectronic properties, making it suitable for various technologie applications.
4. Conclusion
The polycrystalline NdCaFeSnO6 powder was successfully synthesized via the solid-state method, and its structural and optoelectronic properties were extensively studied. The results revealed a complex arrangement of rare-earth, alkaline-earth, and metal oxides within a distorted double perovskite-related framework, contributing to a range of interesting physical properties, particularly in optical absorption and electrical conductivity across various temperature regions.Structural analysis via Rietveld refinement of X-ray diffraction data confirmed a monoclinic crystal system with the P21/c space group. Crystallographic analysis revealed nanocrystalline domains with sizes ranging from 30 to 90 nm, as determined by Scherrer and Williamson–Hall methods. SEM observations indicated larger particle sizes (226 nm) due to agglomeration, while SEM and EDS confirmed the material's crystallinity, morphological homogeneity, and elemental composition. These complementary techniques together provide a comprehensive understanding of the structural and chemical characteristics of the synthesized powder. Furthermore, Raman spectroscopy validated the XRD results, revealing the vibrational modes of Ca, Nd, Fe, Sn, and O ions and highlighting the connectivity of the (Sn/Fe)O6 octahedra in the monoclinic double perovskite structure. UV-visible spectrophotometry demonstrated strong optical absorption around 391 nm in the UV region, giving the sample a distinct green color. The determination of direct optical band gap yielded 2.44 eV value, notably lower than those reported for other calcium-stannate-based perovskites, establishing NdCaFeSnO6 as a promising low-bandgap semiconductor. The material's electrical properties were thoroughly analyzed with a focus on ionic conduction mechanisms. Impedance spectroscopy revealed charge carrier hopping at intermediate temperatures, with activation energies of 0.97 eV for grain boundaries and 0.31 eV for grains. Additionally, I–V measurements indicated a Poole–Frenkel conduction mechanism at higher temperatures, with an activation energy of 1.55 eV. The comparison of these conduction processes underscores the potential of NdCaFeSnO6 as a promising candidate for optoelectronic applications.
Data availability
This statement serves to certify that all authors affirm the availability of the data underpinning the findings of this study. These data may be obtained from the corresponding author upon justified request.Author contributions
Sondes CHAHLA: carried out the preparation of the material, collection and data analysis and paper draft. Yaovi GAGOU: participated in physical measurements, results interpretation, and manuscript revision, impedance spectroscopy analysis. Hanen CHAKER: was responsible for the methodology, data curation, writing, visualization, validation and supervision. Mimoun EL MARSSI and Rached Ben Hassen participates in XRD, Raman. All authors have read and approved the final version of the manuscript.Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Acknowledgements
The authors would like to thank Prof. Hamadi KHEMAKHEM and Prof. Chiheb CHAKER from the University of Sfax, Faculty of Science of Sfax, LaMMA, for performing UV–visible measurements. The authors sincerely acknowledge Prof. Ramzi Maalej, the principal investigator of the QNRF-MME Food Security (REF No : 04/2023/MME03-1226-210042) project, for his valuable collaboration. Financial support from the Ministry of higher education and scientific research of Tunisia is gratefully acknowledged.References
- M. Nadeen Madni, F. Ahmed, M. Danish, M. Jahangeer, M. U. Islam, M. Adnan and A. Ahmed, Divalent ion doping in CaFe2O4: A strategy for enhancing electrical conductivity in energy storage materials, Solid State Ionics, 2025, 420, 116782 CrossRef.
- M. Shahzad, F. Ahmad, M. Ibraheem, A. Shakoor, S. M. Ramay, M. Rafi Raza and S. Atiq, Tunnig diffusion coefficient, ionic conductivity and transference number in rGo/BaCoO3 electrode material for optimized supercapacitor energy storage, R. Soc. Chem. Adv., 2025, 15, 6308–6323 CAS.
- M. Usman, M. Waqas, F. Ahmad, M. Danish, A. M. Rana, I. Sadiq, R. Qaiser, S. Atiq and A. Ahmed, Investigating Al-doped Y-type hexaferrite polyaniline composites: synthesis, structural and dielectric properties, Journal of Ionics, 2025, 31, 2319–2335 CrossRef CAS.
- M. Usama, M. Waqas, F. Ahmad, M. E. Mazhar, S. Atiq, R. Qaiser, M. Usman, M. Danish, N. bano and R. Ahmad, Optimizing ZnSe nanorods with La doping for structural, electrical, and dielectric properties in device applications, J. Surface. Interfac. Mater, 2024, 54, 105275 CrossRef CAS.
- M. Ahmed Khan, K. Shahbaz, G. M. Mustafa, S. M. Ramay, F. Ahmad and S. Atiq, Elucidating efficient magnetoelectric coupling mediated by effective ferroelectric efficiency in BiFeO3 based tri-phasic composites for designing ultra-fasttunable gadgets, J. Environ. Sci. Eng. B, 2025, 313, 117959 Search PubMed.
- J. M. Henriques, E. W. S. Caetano, V. N. Freire, J. A. P. da Costa and E. L. Albuquerque, Structural, electronic, and optical absorption properties of orthorhombic CaSnO3 through ab initio calculations, J. Phys.: Condens. Matter, 2007, 19(10), 106214–106223 CrossRef.
- A. M. Azad, L. L. Shyan and M. A. Alim, Immittance response of CaSnO3 prepared by self-heat-sustained reaction, J. Mater. Sci., 1999, 34, 1175–1187 CrossRef CAS.
- N. Sharma, K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Sol–gel derived nano-crystalline CaSnO3 as high capacity anode material for Li-ion batteries, Electrochem. Commun., 2002, 4, 947–952 CrossRef CAS.
- J. Cerda, J. Arbiol, G. Dezanneau, R. Dıaz and J. R. Morante, Perovskite-type BaSnO3 powders for high temperature gas sensor applications, Sensor. Actuator. B Chem., 2002, 84, 21–25 CrossRef CAS.
- X. L. Pang, C. H. Jia, G. Q. Li and W. F. Zhang, Bright white up conversion luminescence from Er3+ –Tm3+ –Yb3+ doped CaSnO3 powders, Opt. Mater., 2011, 34, 234–238 CrossRef CAS.
- S. Sumithra and N. V. Jaya, Structural, optical and magnetization studies of Fe-doped CaSnO3 nanoparticles via hydrothermal route, J. Mater. Sci.: Mater. Electron., 2018, 29, 4048–4057 CrossRef CAS.
- J. Goethals, C. Fourdrin, M. Tarrida, A. Bedidi, F. Hatert and S. Rossano, Structural investigations of neodymium incorporation in calcium stannate perovskite CaSnO3, Phys. Chem. Miner., 2019, 46, 143–155 CrossRef CAS.
- E. H. Mountstevens, J. P. Attfield and S. A. T. Redfern, Cation-size control of structural phase transitions in tin perovskites, J. Phys. Condens. Matter., 2003, 15, 8315–8326 CrossRef CAS.
- W. Hironori, A. Seiji, K. Ai, N. Kouhei, I. Yoshihiro, O. Yasuo, T. Yoshinobu and T. Keiichi, Improvement in reproducibility of multilayer and junction process for HTS SFQ circuits, IEEE Trans. Appl. Supercond., 2005, 15, 153–156 CrossRef.
- K. P. Ong, X. Fan, A. Subedi, M. B. Sullivan and D. J. Singh, Transparent conducting properties of SrSnO3 and ZnSnO3, APL Mater., 2015, 3, 062505 CrossRef.
- F. Liu, Z. Yang, D. Abramovitch, S. Guo, K. Andre Mkhoyan, M. Bernardi and B. Jalan, Deep ultraviolet-transparent conducting SrSnO3 via heterostructure design, J. Adv. Sci., 2024, 10, 7892 CrossRef.
- L. B. Amor, B. Belgacem, J. S. Filhol, M. L. Doublet, M. B. Yahia and R. Ben Hassen, New p-type Al-substituted SrSnO3 perovskites for TCO applications, Chem. Commun., 2020, 56, 2566–2569 RSC.
- A. Zouaghi, B. Belgacem, A. alhussein, A. Brahmia and R. Ben Hassen, Study of the structural, optical, and electrical characteristics of Sr2Sn1.6In0.2Cu0.2O6-δ mixed ionic-electronic conductor, J. Solid State Chem., 2023, 328, 124303 CrossRef CAS.
- B. Belgacem, N. Nasri, M. Ben Yahia, A. Oueslati and R. Ben Hassen, First principles approach and experimental exploration of a new double perovskite phase Sr2(In0.33Sn0.33Sb0.33)2O6: evaluation of structural, optical, and dielectric properties, R. Soc. Chem. Adv., 2024, 14, 32292–32303 CAS.
- A. Q. Jiang, C. Wang, K. J. Jin, X. B. Liu, J. F. Scott, C. S. Hwang, T. A. Tang, H. B. Lu and G. Z. Yang, A resistive memory in semiconducting BiFeO3 thin-film capacitors, Adv. Mater., 2011, 23, 1277–1281 CrossRef CAS.
- S. Barouni, A. Brahmia, H. Chaker, M. M. Maslov, A. Alhusseind and R. Ben Hassen, First-principles prediction of half metallic-ferromagnetism in La0.25Sr0.75Sn0.4In0.25Ru0.35O3 and enhanced experimental electrical and magnetic behaviours, Phys. Chem. Chem. Phys., 2024, 26, 18102–18112 RSC.
- K. Tanwar, M. Saxena and T. Maiti, Enhancement of thermoelectric power factor of Sr2CoMoO6 double perovskite by annealing in reducing atmosphere, J. Appl. Phys., 2017, 122, 164902 CrossRef.
- M. Saxena, K. Tanwar and T. Maiti, Environmental friendly Sr2TiMoO6 double perovskite for high temperature thermoelectric applications, Scr. Mater., 2017, 130, 205–209 CrossRef CAS.
- M. Saxena and T. Maiti, Compositional modification of Sr2TiCoO6 double perovskites by Mo and La for high temperature thermoelectric applications, Ceram. Int., 2018, 44(3), 2732–2737 CrossRef CAS.
- Y. Qian, Q. Ruan, M. Xue and L. Chen, Emerging perovskite materials for supercapacitors: Structure, synthesis, modification, advanced characterization, theoretical calculation and electrochemical performance, J. Energy Chem., 2024, 89, 41–70 CrossRef CAS.
- J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Phys. B, 1993, 192, 55–69 CrossRef.
- I. Elgargouri, I. Raies, H. Kadiri, A. Alhussein, G. Leronde and R. Ben Hassen, Emerging mixed electronic-ionic conductivity in double perovskite (La2/3Sr1/3)2(Sn1/3Fe1/3Cu1/3)2O6-δ : Phase transitions, structural, optical and dielectric study, J. Alloys Compd., 2025, 1010, 177543 CrossRef CAS.
- R. Shaheen and J. Bashir, Ca2CoNbO6: A new monoclinically distorted double perovskite, Solid State Sci., 2010, 12(8), 1496–1499 CrossRef CAS.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides, Acta Crystallogr., Sect. A, 1972, 32, 751–767 CrossRef.
- V. M. Goldschmidt, Die Gesetze der Krystallochemie, Naturwissenschaften, 1926, 14, 477–485 CrossRef CAS.
- W. Li, E. Ionescu, R. Riedel and A. Gurlo, can we predict the formability of perovskite oxynitrides from tolerance and octahedral factors, J. Mater. Chem. A, 2013, 1(39), 12239–12245 RSC.
- S. Mustapha, M. M. Ndamitso, A. S. Abdulkareem, J. O. Tijani, D. T. Shuaib, A. K. Mohammed and A. Sumaila, Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles, Adv. Nat. Sci. Nanosci. Nanotechnol., 2019, 10, 045013 CrossRef.
- C. Schneider, W. Rasband and K. Eliceiri, NIH Image to Image J: 25 years of image analysis, Nat. Methods, 2012, 9, 671–675 CrossRef CAS.
- R. L. Andrews, A. M. Heynsa and P. M. Woodward, Raman studies of A2MWO6 tungstate double perovskites, Dalton Trans., 2015, 23, 1–7 Search PubMed.
- A. P. Ayala, I. Guedes, E. N. Silva, M. S. Augsburger, M. delC. Viola and J. C. Pedregosa, Raman investigation of A2CoBO6 (A=Sr and Ca, B=Te and W) double perovskites, J. Appl. Phys., 2007, 101, 123511 CrossRef.
- R. Mukherjee, S. Saha, A. Dutta and T. P. Sinha, Dielectric and Raman spectroscopic studies of A2ErSbO6 (A= Ba, Sr and Ca), J. Alloys Compd., 2015, 651, 222–229 CrossRef CAS.
- A. Zaraq, B. Orayech, J. M. Igartua and A. El Bouari, Synthesis, crystal structure, and vibrational studies of the monoclinically distorted double perovskite Sr2Mn1−xNixTeO6 with (x = 0.25, 0.5, and 0.75), Powder Diffr., 2019, 34, 48–58 CrossRef.
- S. A. T Redfern, C. J. Chen, J. Kung, O. Chaix-Pluchery, J. Kreisel and E. K. H. Salje, Raman spectroscopy of CaSnO3 at high temperature: A highly quasi-harmonic perovskite Journal of Physics, Condens. Matter, 2011, 23, 425401–425405 CrossRef.
- M. Kumar, S. K. Roy, R. K. Singh and K. Prasad, Double perovskite Nd2FeTiO6 ceramics: Structural and electrical Properties, Processing and Application of, Ceramics, 2023, 17, 428–435 CAS.
- K. Ji, J. R. Bedward, Q. Li, a P. Manuel, C. Ritter and J. Paul Attfield, CaFeFeNbO6 an iron-based double, Perovskite, Chem. Commun., 2023, 59, 6371–6374 RSC.
- D. Wlodarczyk, M. Amilusik, K. M. Kosyl, M. Chrunik, K. Lawniczak-Jablonska, H. Przybylinska, P. Kosmela, M. Strankowski, L. I. Bulyk, V. Tsiumra, R. Islam, C. Autieri, F. Xue, M. Zajac, A. Lysak, R. Minikayev, M. Bockowski and A. Suchocki, Synthesis and Properties of the Ba2PrWO6 Double Perovskite, Inorg. Chem., 2024, 63, 10194–10206 CrossRef CAS.
- P. Makuła, M. Pacia and W. Macyk, How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-vis Spectra, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef.
- H. Mizoguchi and P. M. Woodward, Electronic Structure Studies of Main Group Oxides Possessing Edge-Sharing Octahedra: Implications for the Design of Transparent Conducting Oxides, Chem. Mater., 2004, 16, 5233–5248 CrossRef CAS.
- U. Kumar, D. Yadav, A. K. Thakur, K. K. Srivastav and S. Upadhyay, Investigation on phase formation of Sr2SnO4 and effect of La-doping on its structural and optical properties, J. Therm. Anal. Calorim., 2019, 135, 1987–1999 CrossRef CAS.
- K. J. Kim and Y. R. Park, Optical investigation of Zn1−xFexO films grown on Al2O3 (0001) by radio-frequency sputtering, J. Appl. Phys., 2004, 96, 4150–4153 CrossRef CAS.
- M. Karouchi, A. Ejjabli, O. Bajjou, J. Guerroum, M. Al-Hattab, M. Basyooni, M. A. Kabatas, K. Rahmani and Y. Lachtioui, Investigating the structural, electronic and optical properties of the novel double perovskite K2AgBiI6 using DFT, Front. Mater., 2024, 11, 1448400 CrossRef.
- M. Baskurt, P. Erhart and J. Wiktor, Direct, Indirect and Self-Trapped Excitons in Cs2AgBiBr6, J. Phys. Chem. Lett., 2024, 15, 8549–8554 CrossRef CAS.
- M. A. Sheikh and S. Chakraborty, Spectroscopic approach to understanding complex impedance in sodium silicate, Phys. Open, 2025, 23, 100255 CrossRef CAS.
- S. Singh, A. Kaur, P. Kaur and L. Singh, High-Temperature Dielectric Relaxation and Electric Conduction Mechanisms in a LaCoO3-Modified Na0.5Bi0.5TiO3 System, ACS Omega, 2023, 8, 25623–25638 CrossRef CAS.
- K. Souifi, O. Rejaiba and M. Nasri, et al., Hopping conduction mechanism and impedance spectroscopy analyses of the half-doped perovsikte Nd0.5Ba0.5FeO3 prepared by sol–gel method, Appl. Phys. A, 2022, 128, 981 CrossRef CAS.
- A. Bendahhou, K. Chourti, R. El Bouayadi, S. El Barkany and M. Abou-Salama, Structural, dielectric and impedance spectroscopy analysis of Ba 5 CaTi1.94Zn0.06Nb8O3 ferroelectric ceramic, Chem. Adv. Mater., 2020, 10, 28007–28018 CAS.
- R. Gupta, A. Khan, R. Gupta and A. Khan, Investigation of dielectric and electrical behaviour of rare-earth doped chlorapatites synthesized via hydrothermal method based on liquid solid solution, J. Mater. Sci.: Mater. Electron., 2025, 36, 98 CrossRef CAS.
- A. Ch. Lazanas and M. I. Prodromidis, Electrochemical Impedance Spectroscopy–A Tutorial, ACS Meas. Sci. Au, 2023, 3, 162–193 CrossRef CAS.
- S. Arrhenius, Über die Reaktionsgeschwindigkeitbei der Inversion von RohrzuckerdurchSäuren, Z. Phys. Chem., 4U(1889), 226–248 Search PubMed.
- A. K. Jonscher, The Universal Dielectric Response, Nature Publishing Group, 1977, vol. 267, pp. 673–679 Search PubMed.
- R. B. Hall, The Poole-Frenkel effect, Thin Solid Films, 1971, 8, 263–271 CrossRef CAS.
- J. Frenkel, On Pre-Breakdown Phenomena in Insulators and Electronic Semi-Conductors, Phys. Rev., 1938, 54, 647–648 CrossRef.
- J. G. Simmons, Poole-Frenkel Effect and Schottky Effect in Metal-Insulator-Metal Systems, Phys. Rev., 1967, 155, 657–660 CrossRef CAS.
- I. Farooq, M. U. Islam, M. Danish, F. Ahmad, I. H. Gul and M. Azhar Khan, Synergistic effects of Li-based ferrite and graphene oxide in microwave absorption applications, Synth. Met., 2024, 307, 117674 CrossRef CAS.
- R. Perveen, M. U. Islam, M. Danish, F. Ahmad, L. Zaffar and M. Nadeem Madni, Ayesha,Innovative Nanostructuring of Li–Zn ferrite/graphene composites with tunable properties, Ceram. Int., 2024, 50, 39564–39573 CrossRef CAS.
- M. Danish, M. ul Islam, F. Ahmad, M. Nadeem Madni and M. Jahangeer, Synthesis of M-type hexaferrite reinforced graphene oxide composites for electromagnetic interference shielding, J. Phys. Chem. Solids, 2024, 185, 111783 CrossRef CAS.
- A. Grillo and A. Di Bartolomeo, A Current–Voltage Model for Double Schottky Barrier Devices, Adv. Electron. Mater., 2021, 7, 2000979 CrossRef CAS.
- D. Belitz and T. R. Kirkpatrick, The Anderson-Mott transition, Rev. Mod. Phys., 1994, 66, 261 CrossRef CAS.
- M. A. Rafiq, Carrier transport mechanisms in semiconductor nanostructures and devices, J. Semicond., 2018, 39, 061002 CrossRef.
- A. Sawa, Resistive switching in transition metal oxides, Mater. Today, 2008, 11, 28–36 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |