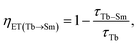DOI:
10.1039/D5RA02624E
(Paper)
RSC Adv., 2025,
15, 19348-19360
Tunable color, optical properties, and energy transfer of Tb3+–Sm3+–Yb3+ tri-doped lithium–niobium–tellurite glass for applications in color display devices and WLEDs†
Received
15th April 2025
, Accepted 4th May 2025
First published on 9th June 2025
Abstract
A TeO2–Nb2O5–LiO2–CaO (TNLC) lithium–niobium–tellurite glass single-doped and co-doped with Tb3+, Sm3+, and Yb3+ ions was synthesized via a conventional melt-quenching method. The Tb3+–Sm3+ co-doped TNLC glass could be tuned to emit white light effectively by controlling the ratio of Tb3+ and Sm3+ in the glass. The fluorescence lifetime of the Tb3+–Sm3+ co-doped TNLC glass indicated the existence of multiple energy transfer channels, including from Tb3+ to Sm3+ ions and the reverse energy transfer from Sm3+ to Tb3+ ions. Taking advantage of these energy transfer channels, the color coordinates of the material could be changed from yellowish-pink and yellowish-green emissions to white emission by controlling the ratio of ions doped in TNLC glasses. The optimal molar concentration ratio between Tb3+ and Sm3+ ions for the best white light emission was 0.83 for the TNLC-0.5Tb0.6Sm sample. Changing the ratio of these rare-earth (RE) ions allowed tuning the color temperature of the material from 5616 to 7699 K. Thus, Tb3+–Sm3+ co-doped and Tb3+–Sm3+–Yb3+ tri-doped TNLC glasses are promising materials for color display applications and white light-emitting diodes (WLEDs).
1. Introduction
In recent years, with the rapid development and high demand of optoelectronic materials for color display technologies and devices,1,2 light-emitting diodes (LEDs)3,4 that emit in the visible region, display full colors and are color-tunable are in high demand, promoting extensive research on rare-earth (RE)-ion doping in various host matrices,5–7 such as phosphors, glasses, and glass-ceramic containing nanocrystals. Among various host matrices, tellurite-based glasses have emerged as promising candidates owing to their unique physicochemical and optical properties.5,6 Tellurite-based glasses have a relatively low melting point, high thermal stability, good RE ion solubility, a wide transmission window (covering the visible (VIS) to near-infrared (NIR) region), and low phonon energy (which minimizes non-radiative (NR) losses and enhances emission efficiency).7,8 Co-doping RE ions in tellurite glasses facilitates sharp, well-defined intra-4f electronic transitions that result in highly stable and efficient luminescence. The incorporation of RE ions in tellurite glasses further opens up pathways for energy transfer (ET) interactions, enabling the tuning of emission intensity and chromaticity of color in the visible wavelength range.9,10 The combination of Sm3+, Tb3+, and Yb3+ ions produces a cooperative, energy-transferring, and tunable emission intensity and colorimetry for visible and upconversion (UC) emissions when excited at different wavelengths.11 Sm3+ ions exhibit prominent orange-red emission around 600–650 nm, corresponding to 4G5/2 → 6H7/2 and 4G5/2 → 6H5/2 transitions;12,13 Tb3+ ions produce strong green emission near 546 nm, corresponding to 5D4 → 7F5 transition,14,15 and Yb3+ ions exhibit only one 2F5/2 → 2F7/2 transition and acts as a sensitizer owing to their strong absorption at ∼980 nm and the ability to participate in UC and cross-relaxation (CR) mechanisms.16,17 Some works have explored the color tunability, luminescence properties, and ET mechanisms in RE ion co-doped glasses/glass ceramics to develop materials suitable for color display and LED applications.18–20 F. Nawaz et al.21 investigated and reported the influence of Yb3+ co-doping on the optical properties of Sm3+-doped sodium tellurite glasses. They found that Yb3+ co-doping affected the absorption and emission characteristics of Sm3+ ions, indicating ET between the Sm3+ and Yb3+ ions.21 J. Li et al.22 reported a multi-color afterglow in Tb3+–Sm3+ co-doped gallo-silicate glass ceramics. By adjusting the concentrations of Tb3+ and Sm3+ ions, the afterglow emission could be tuned from green to orange and then to yellow.22 This study highlighted ET and trap-sharing mechanisms between the dopants, which are significant for optical anti-counterfeiting applications. In general, these studies collectively enhance our understanding of ET dynamics and luminescence tuning in RE co-doped tellurite glass systems, contributing to the development of materials for color displays, LEDs, and other photonic applications.21,22
Lithium–niobium–tellurite (Li–Nb–Te) glass is particularly advantageous as a host because the incorporation of lithium and niobium oxides enhances the structural network,23 improves RE ion dispersion, and increases the optical bandgap and nonlinear optical properties. The presence of Nb5+ ions, which act as a glass modifier, can influence local field symmetry and aid the creation of non-centrosymmetric sites that are beneficial for RE ion emission.23,24 Furthermore, the addition of Li2O serves to improve glass formability and optical clarity while also aiding ET dynamics by modifying the local coordination environment of the RE ions.25 Although several studies have explored tellurite glasses single-doped and co-doped with Tb3+, Sm3+, and Yb3+ ions, as well as other optical materials containing these RE ions, most of them have focused on ET mechanisms from Tb3+ to Sm3+ ions. However, reverse ET mechanisms, such as from Sm3+ to Tb3+ ions or from Yb3+ to both Tb3+ and Sm3+ ions, have not been reported. These mechanisms are crucial for enabling strong visible emission under 980 nm infrared excitation, corresponding to the transition of Yb3+ from the 2F7/2 to 2F5/2 state. In this study, TeO2–Nb2O5–Li2O–CaO (TNLC) glasses with various doping schemes, including Tb3+-doped, Sm3+-doped, Tb3+–Sm3+ co-doped, and Tb3+–Sm3+–Yb3+ tri-doped glasses, were synthesized via melt-quenching. We investigated the effect of varying the concentrations of these ions on the emission color. A detailed analysis of the ET processes from Tb3+ to Sm3+, Sm3+ to Tb3+, and Yb3+ to both Tb3+ and Sm3+ ions was conducted. By adjusting the ion ratios, white-light emission was achieved. These findings suggest that TNLC glasses co-doped with Tb3+, Sm3+, and Yb3+ ions are promising candidates for color display technologies and white light-emitting diodes (LEDs).
2. Experimental details
The lithium–niobium–tellurite glasses used in this work were synthesized using a conventional melt-quenching technique. High-purity laboratory-grade reagents (99.99%), including TeO2, Nb2O5, Li2O, CaO, TbF3, Sm2O3, and Yb2O3, were used as raw materials. Specific compositions, molar ratios, and corresponding sample abbreviations are summarized in Table 1.
Table 1 Specific glass compositions and molar concentration ratios of the as-synthesized TeO2–Nb2O5–LiO2–CaO–TbF3–Sm2O3–Yb2O3 lithium–niobium–tellurite glasses
| Glass sample |
Molar concentration ratio of the components |
| TeO2 |
Nb2O5 |
LiO2 |
CaO |
TbF3 |
Sm2O3 |
Yb2O3 |
| TNLC-0.5Tb |
60 |
18 |
12 |
9.5 |
0.5 |
0 |
0 |
| TNLC-0.5Sm |
60 |
18 |
12 |
9.5 |
0 |
0.5 |
0 |
| TNLC-0.5Tb0.5Sm |
60 |
18 |
12 |
9.0 |
0.5 |
0.5 |
0 |
| TNLC-0.6Tb0.5Sm |
60 |
18 |
12 |
8.9 |
0.6 |
0.5 |
0 |
| TNLC-0.7Tb0.5Sm |
60 |
18 |
12 |
8.8 |
0.7 |
0.5 |
0 |
| TNLC-0.8Tb0.5Sm |
60 |
18 |
12 |
8.7 |
0.8 |
0.5 |
0 |
| TNLC-0.9Tb0.5Sm |
60 |
18 |
12 |
8.6 |
0.9 |
0.5 |
0 |
| TNLC-1.0Tb0.5Sm |
60 |
18 |
12 |
8.5 |
1.0 |
0.5 |
0 |
| TNLC-0.5Tb0.6Sm |
60 |
18 |
12 |
8.9 |
0.5 |
0.6 |
0 |
| TNLC-0.5Tb0.7Sm |
60 |
18 |
12 |
8.8 |
0.5 |
0.7 |
0 |
| TNLC-0.5Tb0.8Sm |
60 |
18 |
12 |
8.7 |
0.5 |
0.8 |
0 |
| TNLC-0.5Tb0.9Sm |
60 |
18 |
12 |
8.6 |
0.5 |
0.9 |
0 |
| TNLC-0.5Tb1.0Sm |
60 |
18 |
12 |
8.5 |
0.5 |
1.0 |
0 |
| TNLC-0.5Tb0.5Sm2Yb |
60 |
18 |
12 |
7.0 |
0.5 |
0.5 |
2 |
| TNLC-0.6Tb0.5Sm2Yb |
60 |
18 |
12 |
6.9 |
0.6 |
0.5 |
2 |
| TNLC-0.7Tb0.5Sm2Yb |
60 |
18 |
12 |
6.8 |
0.7 |
0.5 |
2 |
| TNLC-0.8Tb0.5Sm2Yb |
60 |
18 |
12 |
6.7 |
0.8 |
0.5 |
2 |
| TNLC-1.0Tb0.5Sm2Yb |
60 |
18 |
12 |
6.5 |
1.0 |
0.5 |
2 |
Approximately 12 grams of each glass batch was prepared by accurately weighing the required mixtures of raw materials using an electronic analytical balance. After finely grinding them using an onyx mortar and agate pestle, the mixtures were compacted, placed in a platinum crucible and then heated in a Nabertherm electric furnace (Germany) at 1150 °C for 45 minutes under an air atmosphere.16,26,27 Following the melting process, the molten materials were cast into molds and rapidly cooled on a stainless-steel plate to form the initial glass samples. To enhance the mechanical strength and eliminate residual thermal stresses, the glass samples were annealed at approximately 342 °C for 10 hours.26,27 The TNLC glass materials used for optical measurements were cut into samples with approximate dimensions of 10 mm × 10 mm × 2 mm. These TNLC glass samples were then thoroughly polished on the edges and surfaces. Differential thermal analysis (DTA) was performed on a Shimadzu DTG-60H TG/DTA. The absorption spectra of the TNLC glasses in the wavelength range of 350–2000 nm were recorded using a Hitachi U-4100 UV/VIS/NIR spectrophotometer. The excitation, visible, and UC emission spectra of the Tb3+-doped, Sm3+-doped, Tb3+–Sm3+ co-doped, Tb3+–Sm3+–Yb3+ tri-doped TNLC glasses were measured in the wavelength range of 200 to 750 nm using an Edinburgh Instruments FLS-1000 photoluminescence spectrometer. All the absorption, excitation, visible, and UC emission spectral measurements, and decay lifetime analyses of the TNLC glass samples were performed at ambient temperature.26,27
3. Results and discussion
DTA was conducted to investigate the thermal stability and glass-forming ability of the lithium–niobium–tellurite glass using the TNLC-0.5Tb0.5Sm2Yb glass sample. The DTA curve was recorded in the 100 to 1000 °C temperature range at a constant heating rate (typically 10 °C min−1) under a nitrogen atmosphere. The DTA curve revealed typical thermal parameters. The onset of endothermic deviation in the DTA curve corresponded with the glass transition temperature (Tg), which was observed at approximately 342 °C. This Tg value indicates the temperature at which the amorphous glass matrix transitions from a rigid, glassy state to a more flexible, rubbery state. A relatively low Tg is characteristic of tellurite-based glasses and is attributed to the weak Te–O bonds and the open network structure of the tellurite matrix. An exothermic peak was observed at around 493 °C, corresponding to the onset of crystallization (Tx). This temperature indicates the initiation of structural reorganization and phase separation within the glass matrix, leading to the formation of crystalline phases.16,28,29 The difference between Tx and Tg was ΔT = Tx − Tg = (493 − 342)°C = 151 °C; often referred to as the thermal stability window, it is an important parameter to evaluate glass stability against devitrification.16,29,30 For the TNLC-0.5Tb0.5Sm2Yb sample, the ΔT of approximately 151 °C indicates reasonably good thermal stability and suggests that the material is suitable for optical applications that require thermal processing.16 Besides, the values of crystallization temperature (Tc) and crystallization peak temperatures Tp (including Tp1, Tp2, and Tp3 temperatures)16 were also determined to be ∼598, 637, 786, and 851 °C, respectively, as depicted in Fig. 1.
 |
| | Fig. 1 DTA analysis of the TNLC-0.5Tb0.5Sm2Yb lithium–niobium–tellurite glass sample. | |
The absorption spectra of TNLC-0.5Tb, TNLC-0.5Sm, TNLC-0.5Tb0.5Sm, and TNLC-0.5Tb0.5Sm2Yb lithium–niobium–tellurite glass samples are shown in Fig. 2. The absorption spectra characterize the Tb3+ ions due to f–f transitions. These transitions typically occur in the ultraviolet (UV) and visible wavelength regions, primarily at ∼398 and 480 nm, corresponding to the 7F6 → 5D3 and 7F6 → 5D4 transitions of Tb3+ ions.18,31 Sm3+ has different absorption characteristics from Tb3+, primarily because of the 4f states. The absorption peaks of Sm3+ are typically broader than those of the Tb3+ ions due to the nature of its electronic transitions. The absorption spectrum of Sm3+ in the wavelength range of 350–2000 nm includes peaks at ∼360, 374, 402, 473, 939, 1076, 1224, 1369, 1472, and 1536 nm attributed to the 6H5/2 → −6P7/2, −4F5/2, −4F7/2, −4I11/2, −6F11/2, −6F9/2, −6F7/2, −6F5/2, −6F3/2 and 6H15/2 transitions, respectively.4,22,32 The absorption spectrum of the Tb3+–Sm3+ co-doped TNLC-0.5Tb0.5Sm glass sample in the wavelength range of 350–2000 nm contains all the absorption peaks of Tb3+ and Sm3+ ions.4,22,32–34 The absorption spectrum of the Tb3+–Sm3+–Yb3+ tri-doped TNLC-0.5Tb0.5Sm2Yb glass sample in the wavelength range of 350–2000 nm includes all the absorption peaks of Tb3+, Sm3+ ions and the absorption peak of Yb3+ ions at ∼975 nm (2F7/2 → 2F5/2).
 |
| | Fig. 2 Absorption spectra of TNLC-0.5Tb, TNLC-0.5Sm, TNLC-0.5Tb0.5Sm, and TNLC-0.5Tb0.5Sm2Yb lithium–niobium–tellurite glass samples. | |
The direct optical bandgaps (DOB) and indirect optical bandgaps (IOB) of the TNLC-0.5Sm, TNLC-0.5Tb, TNLC-0.5Sm2Yb, and TNLC-0.5Tb0.5Sm2Yb glass samples were calculated and analyzed based on their absorption spectra using the Tauc formula:19,35,36
| |
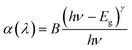 | (1) |
Here,
α(
λ) is the absorption coefficient;
λ is the wavelength;
ν is the frequency;
h is the Planck constant;
B is the energy-independent constant;
19,35,36 Eg is the energy gap of the glass samples;
γ = 2 for DOB;
γ = ½ for IOB.
19,35,36 The calculated direct optical bandgaps (DOB) of the TNLC-0.5Sm, TNLC-0.5Tb, TNLC-0.5Sm2Yb, and TNLC-0.5Tb0.5Sm2Yb glass samples are shown in
Fig. 3(a).
 |
| | Fig. 3 (a) Direct optical bandgaps of the TNLC-0.5Sm, TNLC-0.5Tb, TNLC-0.5Sm2Yb, and TNLC-0.5Tb0.5Sm2Yb glass samples. (b) Indirect optical bandgaps of the TNLC-0.5Sm, TNLC-0.5Tb, TNLC-0.5Sm2Yb, and TNLC-0.5Tb0.5Sm2Yb glass samples. | |
Similarly, the indirect optical bandgaps of the TNLC-0.5Sm, TNLC-0.5Tb, TNLC-0.5Sm2Yb, and TNLC-0.5Tb0.5Sm2Yb glass samples were calculated based on their absorption spectra using the Tauc formula, as shown in Fig. 3(b). A summary of the energy indirect/direct bandgap values of the TNLC-0.5Tb, TNLC-0.5Sm, TNLC-0.5Tb0.5Sm, and TNLC-0.5Tb0.5Sm2Yb glass samples is presented in Table 2.
Table 2 Indirect/direct energy bandgap values of the TNLC-0.5Tb, TNLC-0.5Sm, TNLC-0.5Tb0.5Sm, and TNLC-0.5Tb0.5Sm2Yb glass samples
| Glass sample |
Direct bandgap values |
Indirect bandgap values |
| EDg1 (eV) |
EDg2 (eV) |
ΔED = EDg2 – EDg1 (eV) |
EIg1 (eV) |
EIg2 (eV) |
ΔEI = EIg2 – EIg1 (eV) |
| TNLC-0.5Sm |
3.02 |
3.23 |
0.21 |
2.98 |
3.19 |
0.21 |
| TNLC-0.5Tb |
2.34 |
2.95 |
0.61 |
2.84 |
— |
— |
| TNLC-0.5Sm2Yb |
2.36 |
2.74 |
0.38 |
2.75 |
2.82 |
0.07 |
| TNLC-0.5Tb0.5Sm2Yb |
2.28 |
2.59 |
0.31 |
2.72 |
2.79 |
0.07 |
As shown in Table 2, all the glass samples exhibited significantly smaller bandgaps than pure TeO2 glass. The incorporation of Nb2O5, CaO, and Li2O introduces electronic states within the TeO2 bandgap, resulting in a notable reduction in the band gap. Among the samples, the TNLC-0.5Sm glass sample had a larger bandgap than the TNLC-0.5Tb glass sample, though both remain smaller than that of pure TeO2. This reduction is attributed to the addition of RE ions, which alter the glass network by modifying oxygen bonding, increasing non-bridging oxygen content, and affecting light absorption. Furthermore, the TNLC-0.5Sm2Yb and TNLC-0.5Tb0.5Sm2Yb glass samples showed even lower bandgaps owing to the higher RE ion concentrations, which introduced additional intermediate energy levels and further enhanced non-bridging oxygen states. The increasing concentration of RE ions in the glass matrix also increases the non-bridging oxygen bonding state, causing the band gap of the glass to decrease.
The excitation spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm glass samples were obtained, as shown in Fig. 4, to determine the excitation wavelengths for the TNLC glass samples containing both Tb3+ and Sm3+ ions. Based on the spectral data, we chose 374 nm as the excitation wavelength for the Tb3+-Sm3+ co-doped TNLC glass sample.37,38
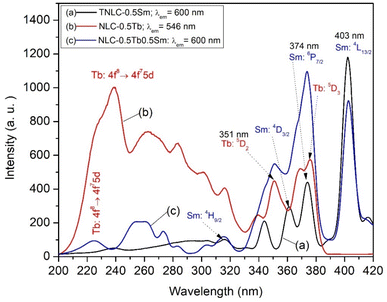 |
| | Fig. 4 Excitation spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm lithium–niobium–tellurite glass samples. | |
The visible emission spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm lithium–niobium–tellurite glass samples under 374 nm are shown in Fig. 5. For the TNLC-0.5Tb glass sample, the visible emission of Tb3+ under 374 nm excitation revealed weaker peaks at around 415 and 438 nm due to the 3D5 → 7F5 and 3D5 → 7F4 transitions.4,16,32 This result is intriguing because these emission peaks have not been reported in most previous works. For the TNLC-0.5Sm glass sample, the visible emission peaks of Sm3+ under 374 nm excitation at around 440, 564, 601, and 645 nm could be attributed to the 4G7/2 → 6H9/2, 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, and 4G5/2 → 6H9/2 transitions, respectively.32–34 For the TNLC-0.5Tb0.5Sm glass sample, the visible emission of peaks co-doped Tb3+ and Sm3+ under 374 nm excitation were found at around 415, 438, 490, 546, 587, 622, and 645 nm due to transitions from Tb3+, Sm3+ ions or the combination of transitions from both Tb3+ and Sm3+ions corresponding to (Tb3+: 3D5 → 7F5), (Tb3+: 3D5 → 7F4 + Sm3+: 4G7/2 → 6H9/2), (Tb3+: 3D4 → 7FJ(J=6, 5, 4, and 3)) and (Sm3+: 4G5/2 → 6H9/2) transitions, respectively.4,32–34
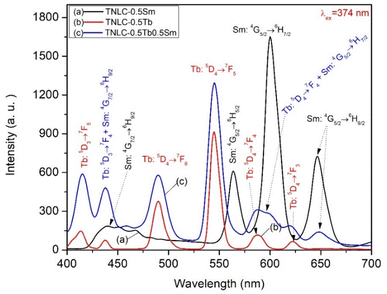 |
| | Fig. 5 Visible emission spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm lithium–niobium–tellurite glass samples under 374 nm excitation. | |
The Commission Internationale de L'Eclairage (CIE) 1931 (x; y) chromaticity coordinates19,39 for the visible emission spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm glass samples were determined to be PTb (0.2840; 0.5303), PTb–Sm (0.2901; 0.3728), and PSm (0.4906; 0.3537) located in the yellow-pink, yellowish-green and black body curve regions, respectively, as shown in Fig. 6.
 |
| | Fig. 6 CIE 1931 (x; y) color coordinates for the visible emission spectra of the TNLC-0.5Tb, TNLC-0.5Sm, and TNLC-0.5Tb0.5Sm glass samples under 374 nm excitation. | |
The visible emission spectra of the TNLC-pTb0.5Sm (p = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples under 374 nm are shown in Fig. 7. With the increase in molar concentration of Tb3+ from 0.6 to 1.0 mol%, the visible emission intensity of the peaks of Tb3+ ions at ∼415, 438, 490, 546, and 622 nm increased significantly.32,33 Similarly, the visible emission intensity of the Sm3+ ion peaks at around 645 nm, which is attributed to the 4G5/2 → 6H9/2 transition, was also increased. These findings confirm that energy from the neighboring states of Tb3+ ions were transferred to the 4G5/2 → 6H9/2 transition of Sm3+ ions.33,34,37–40
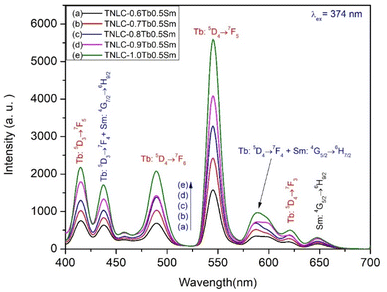 |
| | Fig. 7 Visible emission spectra of the TNLC-pTb0.5Sm (p = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
Fig. 8 shows the CIE1931 (x; y) color coordinates for the visible emission spectra of the TNLC-pTb0.5Sm (p = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples under excitation at 374 nm. With the increase in the molar concentration of Tb3+ ions from 0.6 to 1.0 mol%, the color points of the visible emission spectra of the Tb3+–Sm3+ co-doped glass shifted in the order of P1 (0.2838; 0.3610), P2 (0.2864; 0.3801), P3 (0.2878; 0.3912), P4 (0.2857; 0.3834) and P5 (0.2861; 0.4041) in the CIE1931 (x; y) color coordinate chart, respectively,19,33,34 as shown in Fig. 8. From the results in Fig. 8, we can see that the TNLC-0.6Tb0.5Sm sample corresponding to the Tb3+/Sm3+ concentration ratio of 1.2 has the color point closest to the white light region.33,34
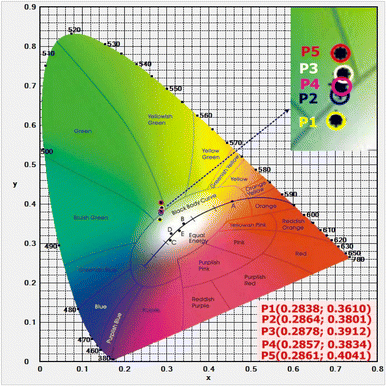 |
| | Fig. 8 CIE 1931 (x; y) color coordinates for the visible emission spectra of the TNLC-pTb0.5Sm (p = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
To further investigate the optimal concentration ratio between Tb3+ and Sm3+ ions for the color point to move closer to the white light region, which is necessary for application to WLEDs, the Sm3+ concentration was changed while keeping the Tb3+ concentration constant to investigate the emission of the Tb3+–Sm3+ co-doped glass sample. The visible emission spectra of the TNLC-0.5TbqSm (q = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples under 374 nm are shown in Fig. 9.
 |
| | Fig. 9 Visible emission spectra of the TNLC-0.5TbqSm (q = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
When excited at 374 nm, with an increase in Sm3+ concentration from 0.6 to 1.0 mol%, most of the emission peaks of both Sm3+ and Tb3+ ions increased in intensity. This result affirms that energy from the neighboring states of Sm3+ ions is transferred to the Tb3+ ions. Simultaneously, with the increase in molar concentration of Sm3+ ions from 0.6 to 1.0 mol%, the tunable color for the visible emission spectra of the Tb3+–Sm3+ co-doped glass samples shifted to color points 1 (0.3118; 0.3821), 2 (0.3093; 0.3827), 3 (0.3095; 0.3875), 4 (0.3169; 0.3937) and 5 (0.3263; 0.4049) on the CIE 1931 (x; y) coordinate diagram,19,33,34 as shown in Fig. 10. From the visible emission spectra results of the Tb3+–Sm3+ co-doped glass samples under 374 nm excitation (Fig. 7 and 9), as well as the color coordinates for their visible emission spectra (Fig. 8 and 10), we determined the optimal concentration ratio of Tb3+ and Sm3+ ions to be 0.83 for the color point to move the closest to the white light region, corresponding to the TNLC-0.5Tb0.6Sm glass sample.
 |
| | Fig. 10 CIE1931 (x; y) color coordinates for the visible emission spectra of the TNLC-0.5TbqSm (q = 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
During the analysis of the absorption capacity of the materials in the infrared region, we observed that the material strongly absorbed in the range from 940 to 1070 nm, with a maximum peak at about 980 nm. We surveyed the UC emission process of the Tb3+–Sm3+–Yb3+ co-doped material samples to investigate the ET process between Yb3+ ions and Tb3+/Sm3+ ions. In addition to investigating the VIS emission spectra of Tb3+–Sm3+ co-doped glass samples, we conducted further investigations using the UC emission spectra of these samples. Fig. 11 shows the UC emission spectra of the TNLC-pTb0.5Sm2Yb (p = 0.5, 0.6, 0.7, 0.8, and 1.0 mol%) lithium–niobium–tellurite glass samples under a 980 nm laser diode (LD). With the increase in molar concentration of Tb3+ from 0.5 to 1.0 mol%, the UC emission intensity of the Tb3+ ion peaks at around 415, 438, 490, 546, and 622 nm increased for the Tb3+–Sm3+–Yb3+ co-doped glass samples.41 Meanwhile, the UC emission intensity of the peaks at around 645 nm, attributed to the 4G5/2 → 6H9/2 transition of Sm3+ ions, was also increased. This result confirms that energy from the neighboring states of Tb3+ ions was transferred to the 4G5/2 → 6H9/2 transition of Sm3+ ions.39,40
 |
| | Fig. 11 UC emission spectra of the TNLC-pTb0.5Sm2Yb (p = 0.5, 0.6, 0.7, 0.8, and 1.0 mol%) glass samples under 980 nm LD excitation. | |
Fig. 12 shows the CIE 1931 (x; y) color coordinates for the UC emission spectra of the TNLC-pTb0.5Sm (p = 0.5, 0.6, 0.7, 0.8, and 1.0 mol%) lithium–niobium–tellurite glass samples under a 980 nm LD. With the increase in molar concentration of Tb3+ ions from 0.5 to 1.0 mol%, the color points shifted from in the order of points A (0.3277; 0.4847), B (0.2974; 0.5169), C (0.2997; 0.5441), D (0.3050; 0.5676), and E (0.3167; 0.5782) on the CIE 1931 (x; y) color coordinate diagram and were mainly located in the yellowish-green region.
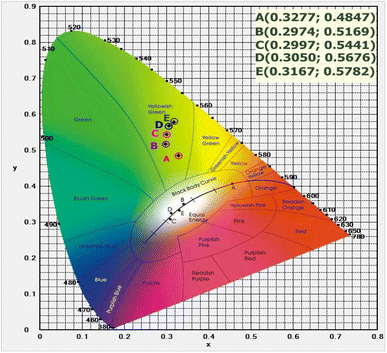 |
| | Fig. 12 CIE 1931 (x; y) color coordinates for the UC emission spectra of the TNLC-pTb0.5Sm2Yb (p = 0.5, 0.6, 0.7, 0.8, and 1.0 mol%) glass samples excited under a 980 nm LD. | |
To further determine the roles of excitation wavelength and the molar concentration of the doped RE ions on the CIE 1931 (x; y) color coordinates and color region, we compared the results obtained in this study with previously published reports, as listed in Table 3.
Table 3 Compare the energy transfer processes, color coordinates, and excitation wavelengths of RE ions in this study with those reported in previously published papers
| Host material |
RE co-doping ratio |
λex (nm) |
Energy transfer |
CIE 1931 coordinates (x; y) |
Color region |
References |
| Tellurite glass |
1Ce3+–1Tb3+–1Sm3+ |
350 |
Ce3+ → Tb3+ |
0.3750; 0.3245 |
Pure white |
T. T. Hong et al.42 |
| LiYF4 single crystals |
1.24Tb3+–1.63Sm3+–0.25Ce3+ |
374 |
— |
0.3084; 0.3612 |
Yellowish-green |
Y. Z. Jiang et al.43 |
| Ba3MgSi2O8:phosphors |
0.12Tb3+–0.05Sm3+ |
233 |
Tb3+ → Sm3+ |
0.555; 0.425 |
Orange yellow |
X. K. Sun et al.44 |
| Phosphate glasses |
0.5Sm3+–0.1Tb3+ |
374 |
Tb3+ → Sm3+ |
0.541; 0.300 |
Reddish-orange |
K. A. Kumar et al.37 |
| 1.0Sm3+–0.1Tb3+ |
0.551; 0.312 |
| Zinc phosphate glasses |
0.5Sm3+–1.0Tb3+ |
361 |
Tb3+ → Sm3+ |
0.448; 0.459 |
Greenish-yellow |
A. N. Meza-Rocha et al.45 |
| 374 |
0.407; 0.485 |
Yellow |
| CaWO4 nanoparticles |
0.5Sm3+–0.5Tb3+ |
355 |
— |
0.3098; 0.4033 |
White |
N. F. Andrade Neto et al.46 |
| 1.0Sm3+–1.0Tb3+ |
355 |
|
0.3002; 0.3997 |
Blue |
| Ca2La3(SiO4)3F phosphor |
0.15Tb3+,0.04Sm3+ |
377 |
Tb3+ → Sm3+ |
0.4175; 0.5658 |
Yellow-green |
K. Nie et al.47 |
| 0.15Tb3+–0.18Sm3+ |
377 |
0.4691; 0.5160 |
Greenish-yellow |
| CaLa4(SiO4)3O:phosphors |
9%Tb3+–11%Sm3+ |
377 |
Tb3+ → Sm3+ |
0.393; 0.387 |
Bright white |
B. Yuan et al.48 |
| K3Gd(PO4)2 crystalline glass ceramics |
0.3%Tb3+–0.4%Sm3+ |
376 |
Tb3+ → Sm3+ |
0.3201; 0.3297 |
White |
Y. N. Guo et al.49 |
| K2Y(WO4)(PO4) phosphors |
2%Sm3+–5%Tb3+ |
377 |
Sm3+ → Tb3+ |
0.37; 0.57 |
Yellow-green |
E. J. Ansari et al.50 |
| Potassium-zinc phosphate glasses |
1.0Sm3+–1.0Tb3+ |
344 |
Tb3+ → Sm3+ |
0.529; 0.447 |
Orange |
O. Soriano-Romero et al.51 |
| 1.0Sm3+–1.0Tb3+ |
360 |
Tb3+ → Sm3+ |
0.534; 0.442 |
Yellow |
| 1.0Sm3+–1.0Tb3+ |
377 |
Tb3+→ Sm3+ |
0.442; 0.507 |
Yellow |
| Lithium–niobium–tellurite glass |
0.5Tb3+–0.6Sm3+ |
374 |
Tb3+ ↔ Sm3+ |
0.3118; 0.3821 |
White (near black body curve) |
This study |
| 0.5Tb3+–0.5Sm3+–2Yb3+ |
980 |
Yb3+ → Tb3+ |
0.3277; 0.4847 |
Yellowish-green |
| Yb3+ → Sm3+ |
Investigation and calculation of correlated color temperature (CCT) are essential for optimizing the output light quality. From the CIE 1931 (x; y) results, the CCT of the TNLC glass samples was calculated using the McCamy formula (often called McCamy's approximation):50,52
| | |
CCT = an3 + bn2 + cn + d ≈ −449n3 + 3525n2 − 6823.3n + 5520.33
| (3) |
where:
| |
 | (4) |
Here,
x and
y are the CIE 1931 (
x;
y) chromaticity coordinates of the light source;
xe = 0.3320 and
ye = 0.1858 are the chromaticity coordinates of the Planckian locus.
50,52 The CCT calculation results of the as-prepared glass samples are described in detail in
Table 4.
Table 4 CCT values and CIE 1931 (x; y) chromaticity coordinates of the TNLC glass samples
| Glass samples |
λex |
Color point |
CIE 1931 x |
CIE 1931 y |
CCT (K) |
Color region |
| TNLC-0.5Tb |
374 nm |
PTb |
0.2840 |
0.5303 |
6544 |
Yellowish green |
| TNLC-0.5Tb0.5Sm |
374 nm |
PTb–Sm |
0.2901 |
0.3728 |
7208 |
White |
| TNLC-0.5Sm |
374 nm |
PSm |
0.4906 |
0.3537 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489 489 |
Yellowish pink |
| TNLC-0.6Tb0.5Sm |
374 nm |
P1 |
0.2838 |
0.3601 |
7662 |
Green |
| TNLC-0.7Tb0.5Sm |
374 nm |
P2 |
0.2864 |
0.3801 |
7294 |
Green |
| TNLC-0.8Tb0.5Sm |
374 nm |
P3 |
0.2878 |
0.3912 |
7129 |
Green |
| TNLC-0.9Tb0.5Sm |
374 nm |
P4 |
0.2857 |
0.3834 |
7290 |
Green |
| TNLC-1.0Tb0.5Sm |
374 nm |
P5 |
0.2861 |
0.4041 |
7085 |
Green |
| TNLC-0.5Tb0.6Sm |
374 nm |
1 |
0.3118 |
0.3821 |
6254 |
White |
| TNLC-0.5Tb0.7Sm |
374 nm |
2 |
0.3093 |
0.3827 |
6347 |
White |
| TNLC-0.5Tb0.8Sm |
374 nm |
3 |
0.3095 |
0.3875 |
6318 |
White |
| TNLC-0.5Tb0.9Sm |
374 nm |
4 |
0.3169 |
0.3937 |
6033 |
White |
| TNLC-0.5Tb1.0Sm |
374 nm |
5 |
0.3263 |
0.4049 |
5706 |
White |
| TNLC-0.5Tb0.5Sm2Yb |
980 nm |
A |
0.3277 |
0.4847 |
5616 |
Yellowish green |
| TNLC-0.6Tb0.5Sm2Yb |
980 nm |
B |
0.2974 |
0.5169 |
6274 |
Yellowish green |
| TNLC-0.7Tb0.5Sm2Yb |
980 nm |
C |
0.2997 |
0.5441 |
6165 |
Yellowish green |
| TNLC-0.8Tb0.5Sm2Yb |
980 nm |
D |
0.3050 |
0.5676 |
6020 |
Yellow |
| TNLC-1.0Tb0.5Sm2Yb |
980 nm |
E |
0.3167 |
0.5782 |
5790 |
Yellowish green |
The energy level diagram of the visible and UC emissions corresponding to the transitions of the Tb3+–Sm3+ and Tb3+–Sm3+–Yb3+ co-doped glass materials under excitation wavelengths of 374 nm and 980 nm is shown in Fig. 13. The Tb3+ ions are excited from the ground state 3H6 to the higher-energy state 3D5. These ions show characteristic emission in the visible region due to 3D5 and 4 → 5FJ (J = 3, 4, 5, and 6) transitions.39 Under 374 nm excitation, the visible emission of Tb3+ peaks at around 490, 546, 587, and 622 nm, which can be attributed to the 3D4 → 5FJ (J = 6, 5, 4, and 3) transitions. The Sm3+ ions, when excited at 374 nm, move from the 6H5/2 ground state to a higher-energy 6P7/2 state.40 From this 6P7/2 state, energy is transferred to 4G5/2 lower-energy states through non-radiative and ET processes. The ET1, ET2, CET1, and CET2 processes shown in Fig. 13 can be described in detail as follows:41,51,53
| ET1: 5D4(Tb3+) + 4G7/2(Sm3+) → 5F6(Tb3+) + 6H5/2(Sm3+). |
| ET2: 4G5/2(Sm3+) + 5D4(Tb3+) → 6H7/2(Sm3+) + 7F6(Tb3+). |
| CET1: 22F5/2(Yb3+) + 7F6(Tb3+) → 22F7/2(Yb3+) + 5D4(Tb3+). |
| CET2: 22F5/2(Yb3+) + 6H5/2(Sm3+) → 22F7/2(Yb3+) + 4I11/2(Sm3+). |
 |
| | Fig. 13 Energy levels of the Tb3+, Sm3+, and Yb3+ ions and the mechanisms of visible and UC emissions via CET1, CET2, ET1, and ET2 processes in the TNLC glass system. | |
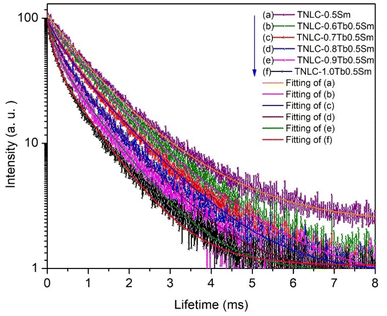
The charge transfer process between the Tb3+ and Sm3+ ions depends on the concentration of optical centers in the material. To further investigate the influence of Tb3+ ion concentration on the ET process, we analyzed the decay lifetimes of Tb3+ and Sm3+ ions in the synthesized TNLC glass materials. We measured the time-resolved fluorescence spectra of TNLC-0.5TbqSm (q = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples using an excitation wavelength of 374 nm and emission at 546 nm corresponding to the 5D4 → 5F6 transition of Tb3+;4,32–34 the obtained results are presented in Fig. 14. Through the fitting process, we observed that the time-resolved fluorescence spectra of the materials followed the equation:16,50
| |
 | (5) |
where
τ1 and
τ2 are the decay lifetime components, and
A1 and
A2 are constants. The average decay lifetime
τ was calculated as follows:
16,51,54,55| |
 | (6) |
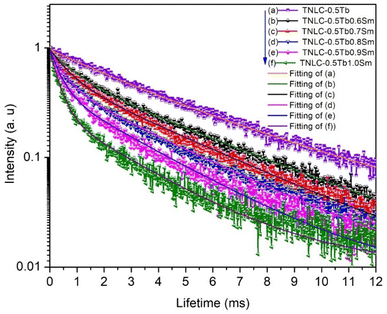 |
| | Fig. 14 Decay lifetimes of Tb3+ at the 5D4 → 5F6 transition in TNLC-0.5TbqSm (q = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
The decay lifetimes of TNLC-0.5TbqSm (q = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples calculated according to formula (6) are listed in Table 5. The decay lifetimes at 546 nm, corresponding to the 5D4 → 5F6 transition of Tb3+ ions in the TNLC-0.5TbqSm (q = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples, were found to decrease with increasing Sm3+ concentration. This is strong evidence for ET from Tb3+ to Sm3+ ions.4,32–34,44 Similarly, the decay lifetimes of Sm3+ ions at 601 nm, corresponding to the 4G5/2 → 6H7/2 transition of Sm3+ ions4,32–34 in the TNLC-pTb0.5Sm (p = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples, were measured under 374 nm excitation, as shown in Fig. 15. The average decay lifetimes of TNLC-pTb0.5Sm (p = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) lithium–niobium–tellurite glass samples were calculated, as presented in Table 5. The decay lifetimes of Sm3+ at 601 nm, corresponding to 4G5/2 → 6H7/2 transition of Sm3+ ions32,33 in these glass samples under 374 nm excitation were found to decrease with increasing Tb3+ concentration in the glass samples, further proving ET from Sm3+ to Tb3+ ions.33,34,39,40
Table 5 Decay lifetimes of Tb3+ ions at 546 nm (5D4 → 5F6) and Sm3+ ions at 564 nm (4G5/2 → 6H5/2) under 374 nm excitation
| Glass sample |
Decay lifetime (ms) |
| TNLC-0.5Tb |
4.62 |
| TNLC-0.5Tb0.6Sm |
3.97 |
| TNLC-0.5Tb0.7Sm |
3.48 |
| TNLC-0.5Tb0.8Sm |
3.13 |
| TNLC-0.5Tb0.9Sm |
2.63 |
| TNLC-0.5Tb1.0Sm |
2.21 |
| TNLC-0.5Sm |
7.86 |
| TNLC-0.6Tb0.5Sm |
6.91 |
| TNLC-0.7Tb0.5Sm |
5.63 |
| TNLC-0.8Tb0.5Sm |
4.24 |
| TNLC-0.9Tb0.5Sm |
3.18 |
| TNLC-1.0Tb0.5Sm |
2.56 |
 |
| | Fig. 15 Decay lifetimes of Sm3+ at the 4G5/2 → 6H5/2 transition in TNLC-pTb0.5Sm (p = 0, 0.6, 0.7, 0.8, 0.9, and 1.0 mol%) glass samples under 374 nm excitation. | |
To evaluate the energy transfer efficiency (ETE) between Tb3+ and Sm3+ ions, we carried out ETE calculations for these ET processes. The ETE from Tb3+ to Sm3+ ions is denoted as ηET(Tb → Sm) and was estimated using the luminescence lifetimes of the donor (Tb3+) in the presence and absence of the acceptor (Sm3+), according to the following equation:
| |
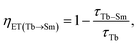 | (7) |
where
τTb is the luminescence lifetime of Tb
3+ ions in the absence of Sm
3+ ions, and
τTb–Sm is the luminescence lifetime of Tb
3+ ions in the presence of Sm
3+ ions (
i.e. when energy transfer occurs).
Similarly, the ETE from Sm3+ to Tb3+ ions is denoted as ηET(Sm → Tb) and was estimated using the luminescence lifetimes of the donor (Sm3+) in the presence and absence of the acceptor (Tb3+), according to the following equation:
| |
 | (8) |
where
τSm is the luminescence lifetime of Sm
3+ ions in the absence of Tb
3+ ions, and
τSm–Tb is the luminescence lifetime of Sm
3+ ions in the presence of Tb
3+ ions. Based on the results presented in
Table 5, we calculated ETE values to be 52.26% and 67.43% for the ET processes from Tb
3+ to Sm
3+ ions and from Sm
3+ to Tb
3+ ions, respectively.
4. Conclusions
In this study, a series of Tb3+-doped, Sm3+-doped, Tb3+-Sm3+ co-doped, and Tb3+–Sm3+–Yb3+ tri-doped lithium–niobium–tellurite TeO2–Nb2O5–LiO2–CaO glasses were synthesized via conventional melt-quenching. These glasses exhibited tunable multicolor emissions under excitation at 374 nm and 980 nm, which could be attributed to Sm3+ (yellow-pink), Tb3+ (yellowish-green), and Yb3+-sensitized UC emissions of Tb3+–Sm3+–Yb3+ tri-doped glass materials. The DTA analysis showed the high thermal stability of the tri-doped glass, with ΔT = 151 °C, suitable for heat treatment and mechanical durability. The emission color could be effectively controlled by varying the Sm3+–Tb3+ concentrations and excitation wavelengths. The optimal Tb3+/Sm3+ molar ratio was 0.83 in the TNLC-0.5Tb0.6Sm sample for achieving CIE coordinates closest to white light emission. Spectral and lifetime measurements confirmed ETE between Tb3+ and Sm3+ ions. The combination of low phonon energy, high stability, emission tunability, and efficient ET highlights the potential of Tb3+–Sm3+ co-doped and Tb3+–Sm3+–Yb3+ tri-doped TNLC glasses for application in advanced color display and solid-state lighting devices, including WLEDs.
Data availability
The authors confirm that the data supporting the findings of this study are available from the corresponding author upon request.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
The corresponding author (Ho Kim Dan) would like to express his gratitude to Van Lang University.
References
- G. J. Li, M.-C. Tseng, Y. Chen, F. S.-Y. Yeung, H. Y. He, Y. C. Cheng, J. H. Cai and E. G. Chen, Color-conversion displays: current status and future outlook, Light:Sci. Appl., 2024, 13, 301, DOI:10.1038/s41377-024-01618-8.
- J. C. Chen, Q. L. Zhao, B. H. Yu and U. Lemmer, A review on quantum dot-based color conversion layers for mini/micro-LED displays: Packaging, light management, and pixelation, Adv. Opt. Mater., 2024, 12, 2300873, DOI:10.1002/adom.202300873.
- M. Vasilopoulou, A. R. B. M. Yusoff, M. Daboczi, J. Conforto, A. E. X. Gavim, W. J. D. Silva, A. G. Macedo, A. Soultati, G. Pistolis, F. K. Schneider, Y. F. Dong, P. Jacoutot, G. Rotas and J. Jang, High efficiency blue organic light-emitting diodes with below-bandgap electroluminescence, Nat. Commun., 2021, 12, 4868, DOI:10.1038/s41467-021-25135-z.
- T. Q. Sheng, Z. L. Fu, X. J. Wang, S. H. Zhou, S. Y. Zhang and Z. W. Dai, Solvothermal synthesis and luminescence properties of BaCeF5, and BaCeF5: Tb3+, Sm3+ Nanocrystals: An approach for white light emission, J. Phys. Chem. C, 2012, 36(116), 19597–19603, DOI:10.1021/jp306935k.
- J. S. Alzahrani, Z. A. Alrowaili, A. V. Lebedev, I. O. Olarinoye, A. Hammoud, L. V. Vasileva and M. S. Al-Buriahi, Optical properties and radiation shielding performance of tellurite glassy composites: role of rare earth oxides, Radiat. Phys. Chem., 2023, 212, 111168, DOI:10.1016/j.radphyschem.2023.111168.
- J. E. Stanworth, Tellurite glasses, Nature, 1952, 169, 581–582, DOI:10.1038/169581b0.
- R. El-Mallawany, The optical properties of tellurite glasses, J. Appl. Phys., 1992, 72, 1774–1777, DOI:10.1063/1.351649.
- J. Lousteau, D. Milanese, S. Abrate, N. Boetti, M. Pittarelli, S. Barbero and M. Ferrari, Exploring the influence of ZnF2 on zinc-tellurite glass: unveiling changes in OH content, structure, and optical properties, ACS Omega, 2023, 8(38), 35266–35274, DOI:10.1021/acsomega.3c05010.
- J. Biswas, S. Jana and S. Ghosh, Investigation on luminescence characteristics of Dy3+–Tb3+ co-doped phospho-tellurite glasses for tunable green/white LED applications, Spectrochim. Acta, Part A, 2025, 331, 125756, DOI:10.1016/j.saa.2025.125756.
- V. K. Rai, S. B. Rai and D. K. Rai, Optical properties of Tb3+ doped tellurite glass, J. Mater. Sci., 2004, 39, 4971–4975, DOI:10.1023/B:JMSC.0000035348.82074.0a.
- K. S. Rudramamba, S. K. Taherunnisa, D. V. K. Reddy, N. Veeraiah and M. R. Reddy, The structural and warm light emission properties of Sm3+/Tb3+ doubly doped strontium bismuth borosilicate glasses for LED applications, Spectrochim. Acta, Part A, 2019, 220, 117097, DOI:10.1016/j.saa.2019.05.002.
- M. Vijayakumar and K. Marimuthu, Effect of Tb3+ concentration on Sm3+ doped leadfluoro-borophosphate glasses for WLED applications, J. Non-Cryst. Solids, 2016, 447, 45–54, DOI:10.1016/j.jnoncrysol.2016.05.028.
- G. Lakshminarayana, A. N. Meza-Rocha, O. Soriano-Romero, E. F. Huerta, U. Caldiño, A. Lira, D.-E. Lee, J. H. Yoon and T. J. Park, Analysis of fluorescence characteristics of Sm3+-doped B2O3-rich glasses for Orange-light-emitting diodes, J. Alloys Compd., 2021, 884, 161076, DOI:10.1016/j.jallcom.2021.161076.
- A. U. Trápala-Ramírez, J. L. N. Gálvez-Sandoval, A. Lira, I. Camarillo, E. Alvarez-Ramos, A. N. Meza-Rocha and U. Caldiño, Calcium-zinc phosphate glasses activated with Tb3+/Eu3+ for laser and white LED applications, J. Lumin., 2019, 215, 116621, DOI:10.1016/j.jlumin.2019.116621.
- H. K. Dan, D. C. Zhou, R. F. Wang, Q. Jiao, Z. W. Yang, Z. G. Song, X. Yu and J. B. Qiu, Effect of Mn2+ ions on the enhancement upconversion emission and energy transfer of Mn2+/Tb3+/Yb3+ tri-doped transparent glass-ceramics, Mater. Lett., 2015, 150, 76–80, DOI:10.1016/j.matlet.2015.03.005.
- P. X. Le, N. M. Ty, J. B. Qiu, D. C. Zhou and H. K. Dan, Enhanced upconversion and near-infrared emissions of co-doped Ho3+/Yb3+ in TeO2–ZnO–Na2CO3–La2O3 tellurite glasses, Opt. Mater. Express, 2019, 9, 3998–4008, DOI:10.1364/OME.9.003998.
- A. Roy, A. Dwivedi, H. Mishra, A. K. Rai and S. B. Rai, Generation of color tunable emissions from Ho3+/Tm3+/Yb3+ co-doped YTaO4 phosphors through NIR excitation under different conditions (variation of concentration, excitation pump power and the external temperature), J. Alloys Compd., 2021, 865, 158938, DOI:10.1016/j.jallcom.2021.158938.
- P. Dharmaiah, C. S. Dwaraka Viswanath, C. Basavapoornima, K. V. Krishnaiah, C. K. Jayasankar and S.-J. Hong, Luminescence and energy transfer in Dy3+/Tb3+ co-doped transparent oxyfluorosilicate glass-ceramics for green emitting applications, Mater. Res. Bull., 2016, 83, 507–514, DOI:10.1016/j.materresbull.2016.06.044.
- H. K. Dan, N. D. Trung, T. H. Le, N. L. Thai, N. M. Ty, D. C. Zhou and J. B. Qiu, Influence of F− on the reduction process of Eu3+ to Eu2+ and optical properties of Eu3+/Eu2+–Er3+–Yb3+ co-doped niobate silicate glasses, J. Non-Cryst. Solids, 2022, 581, 121417, DOI:10.1016/j.jnoncrysol.2022.121417.
- J. Dahiya, A. Hooda, A. Agarwal and S. Khasa, Effect of Dysprosium and Samarium RE ion co-doping on photoluminescence behaviour of novel alkali fluoride bismuth borate glasses: A white LED material, Opt. Mater., 2022, 134, 113162, DOI:10.1016/j.optmat.2022.113162.
- F. Nawaz, M. R. Sahar and S. K. Ghoshal, The influence of Yb co-doping on optical properties of Sm-doped sodium tellurite glasses, Adv. Mater. Res., 2014, 895, 359–362, DOI:10.4028/www.scientific.net/AMR.895.
- J. Li, D. Zhang, Y. X. Wu, L. L. Li, X. L. Zhang, S. Q. Xu and J. J. Zhang, Multi-color afterglow of the LiGa5O8: Tb3+/Sm3+ co-doped gallosilicate glass via energy transfer and trap sharing for optical anti-counterfeiting, J. Mater. Chem. C, 2024, 12, 15733–15738, 10.1039/D4TC03171G.
- N. Elkhoshkhany, R. Essam and E. Sayed Yousef, Influence of La2O3 on the structural, optical and thermal properties of TeO2–ZnO–Li2O–Nb2O5 glass, J. Non-Cryst. Solids, 2020, 536, 119994, DOI:10.1016/j.jnoncrysol.2020.119994.
- N. Elkhoshkhany, S. Y. Marzouk, N. Moataz and S. H. Kandil, Structural and optical properties of TeO2–Li2O–ZnO–Nb2O5–Er2O3 glass system, J. Non-Cryst. Solids, 2018, 500, 289–301, DOI:10.1016/j.jnoncrysol.2018.08.011.
- H. Benrejeb, K. Soler-Carracedo, A. D. Lozano-Gorrín, S. Hraiech and I. R. Martin, Energy transfer studies in Tb3+–Yb3+ co-doped phosphate glasses, Mater., 2021, 14(22), 6782, DOI:10.3390/ma14226782.
- H. K. Dan, N. M. Ty, V. H. Nga, D. T. Phuc, A.-L. Phan, D. C. Zhou and J. B. Qiu, Broadband flat near-infrared emission and energy transfer of Pr3+–Er3+–Yb3+ tri-doped niobate tellurite glasses, J. Non-Cryst. Solids, 2020, 549, 120335, DOI:10.1016/j.jnoncrysol.2020.120335.
- D. C. Zhou, R. F. Wang, Z. W. Yang, Z. G. Song, Z. Y. Yin and J. B. Qiu, Spectroscopic properties of Tm3+ doped TeO2–R2O–La2O3 glasses for 1.47 μm optical amplifiers, J. Non-Cryst. Solids, 2011, 357, 2409–2412, DOI:10.1016/j.jnoncrysol.2010.12.027.
- R. El-Mallawany, Tellurite glasses Part 2. Anelastic, phase separation, Debye temperature and thermal properties, Mater. Chem. Phys., 1999, 60, 103–131, DOI:10.1016/S0254-0584(99)00082-6.
- G. V. Prakash, D. N. Rao and A. K. Bhatnagar, Linear optical properties of niobium-based tellurite glass, Solid State Commun., 2001, 119(1), 39–44, DOI:10.1016/S0038-1098(01)00195-8.
- H. K. Dan, N. M. Ty, T. D. Tap, D.-N. Le, L. T. Vinh, D. C. Zhou and J. B. Qiu, Energy transfer and spectroscopic properties of Cr3+/Yb3+ co-doped TeO2–ZnO–La2O3 tellurite glasses under different wavelength excitation lights, Opt. Mater., 2020, 100, 109662, DOI:10.1016/j.optmat.2020.109662.
- H. K. Dan, D. C. Zhou, R. F. Wang, T. M. Hau, Q. Jiao, Z. W. Yang, Z. G. Song, X. Yu and J. B. Qiu, Energy transfer and upconversion emission of Tm3+/Tb3+/Yb3+ co-doped transparent glass-ceramics containing Ba2LaF7 nanocrystals, J. Non-Cryst. Solids, 2013, 378, 181–185, DOI:10.1016/j.jnoncrysol.2013.07.006.
- C. Jin, J. Zhang, W. D. Lu and Y. T. Fei, Tunable luminescence of LiY9(SiO4)6O2: Ce3+–Tb3+–Sm3+ phosphors for LED and temperature-sensing applications, J. Lumin., 2019, 214, 116581, DOI:10.1016/j.jlumin.2019.116581.
- Y. N. Guo, Q. W. Wang, X. Y. Zhao, L. P. Wang, Y. Wan, H. Zhang and C. Su, Preparation and luminescence properties of Tb3+–Sm3+ co-doped K3Gd(PO4)2 crystalline glass ceramics, Opt. Mater., 2024, 148, 114830, DOI:10.1016/j.optmat.2024.114830.
- Le-Q. Yao, G.-H. Chen, T. Yang, Y. Luo and Y. Yang, Optical properties and energy transfer in Tb3+/Sm3+ co-doped Na2O–CaO–P2O5–B2O3–ZrO2 glasses, J. Alloys Compd., 2017, 692, 346–350, DOI:10.1016/j.jallcom.2016.09.053.
- H. K. Dan, A. L. Phan, N. M. Ty, D. C. Zhou and J. B. Qiu, Optical bandgaps and visible/near-infrared emissions of Bin+-doped (n = 1, 2, and 3) fluoroaluminosilicate glasses via Ag+–K+ ions exchange process, Opt. Mater., 2021, 112, 110762, DOI:10.1016/j.optmat.2020.110762.
- P. T. Phong, P. H. Nam, N. X. Phuc, B. T. Huy, L. T. Lu, D. H. Manh and I.-J. Lee, Effect of zinc concentration on the structural, optical, and magnetic properties of mixed Co–Zn ferrites nanoparticles synthesized by low-temperature hydrothermal method, Metall. Mater. Trans. A, 2019, 50, 1571–1581, DOI:10.1007/s11661-018-5096-z.
- K. A. Kumar, S. Babu, V. R. Prasad, S. Damodaraiah and Y. C. Ratnakaram, Optical response and luminescence characteristics of Sm3+ and Tb3+/Sm3+ co-doped potassium-fluoro-phosphate glasses for reddish-orange lighting applications, Mater. Res. Bull., 2017, 90, 31–40, DOI:10.1016/j.materresbull.2017.01.046.
- V. Lakkamraju, K. N. Kumar and H. Pyung, Unravelling the process of energy transfer and color tuning from warm to cool white light emission in (Tb/Sm): lithium borate glasses, Opt. Lett., 2019, 44(20), 5029–5032, DOI:10.1364/OL.44.005029.
- C. G. Pérez-Hernández, R. Sánchez-Zeferino, U. Salazar-Kuri and M. E. Álvarez-Ramos, Fabrication, structural properties, and tunable light emission of Sm3+, Tb3+ co-doped SrSnO3 perovskite nanoparticles, Chem. Phys., 2021, 551, 111324, DOI:10.1016/j.chemphys.2021.111324.
- A. Bahadur, R. S. Yadav, R. V. Yadav and S. B. Rai, Multimodal emissions from Tb3+/Yb3+ co-doped lithium borate glass: upconversion, downshifting and quantum cutting, J. Solid State Chem., 2017, 246, 81–86, DOI:10.1016/j.jssc.2016.11.004.
- S. Pandey, A. Pandey and V. K. Rai, Synthesis and Characterization of Sm3+–Yb3+ codoped Y2O3 Phosphor, J. Disp. Technol., 2013, 9(12), 989–994 CAS . https://doi:10.1016/s0038-1098(01)00195-8.
- T. T. Hong, P. D. H. Yen, V. X. Quang and P. T. Dung, Luminescence properties of Ce/Tb/Sm
co-doped tellurite glass for white LEDS application, Mater. Trans., 2015, 56(9), 1419–1421, DOI:10.2320/matertrans.MA201509.
- Y. Z. Jiang, H. P. Xia, L. Fu, J. Z. Zhang, S. Yang, X. M. Gu, J. L. Zhang, Y. P. Zhang, D. J. Wang, H. C. Jiang and B. J. Chen, Growth and Optical Spectra of Tb3+/Sm3+/Ce3+ Triply doped LiYF4 single crystals for White-Light LEDs, Nanosci. Nanotechnol., 2016, 16, 1532–1536, DOI:10.1166/jnn.2016.10803.
- X. K. Sun, Z. Z. Huang, Y. H. Guo, S. H. Zhou and K. L. Liu, Tb3+ → Sm3+ energy transfer induced tunable luminescence in Ba3MgSi2O8:Tb3+/Sm3+ phosphors, J. Lumin., 2020, 219, 116925, DOI:10.1016/j.jlumin.2019.116925.
- A. N. Meza-Rocha, G. Muñoz H, A. Speghini, M. Bettinelli and U. Caldiño, Neutral and warm white light emission in Tb3+/Sm3+ zinc phosphate glasses, Opt. Mater., 2015, 47, 537–542, DOI:10.1016/j.optmat.2015.06.035.
- N. F. Andrade Neto, J. M. P. Silva, R. L. Tranquilin, E. Longo, J. F. M. Domenegueti, M. R. D. Bomio and F. V. Motta, Photoluminescent properties of Sm3+ and Tb3+ codoped CaWO4 nanoparticles obtained by a one-step sonochemical method, J. Mater. Sci.: Mater. Electron., 2020, 31, 13261–13272, DOI:10.1007/s10854-020-03878-7.
- K. Nie, R. R. Zhou, C.-A. Cheng, X. Q. Duan, Z. Y. Hu, L. F. Mei, H. K. Liu, L. X. Wang, H. Wang and X. X. Ma, Tb3+ and Sm3+ co-doped Ca2La3(SiO4)3F phosphor: Synthesis, color regulation, and luminescence properties, RSC Adv., 2022, 12, 33200–33206, 10.1039/D2RA06648C.
- B. Yuan, J. X. Gou, C. C. Qi, L. Kong, M. Y. Qu, G. Y. Luan and X. T. Zhang, Energy transfer for Ce3+/Tb3+/Sm3+ induced bright white emission in single-phase CaLa4(SiO4)3O: Ce3+, Tb3+, Sm3+ phosphors and their application in white-light-emitting diodes, RSC Adv., 2024, 14, 7061–7072, 10.1039/D4RA00443D.
- Y. N. Guo, Q. W. Wang, X. Y. Zhao, L. P. Wang, Y. C. Wan, H. B. Zhang and C. H. Su, Preparation and luminescence properties of Tb3+-Sm3+ co-doped K3Gd(PO4)2 crystalline glass ceramics, Opt. Mater., 2024, 148, 114830, DOI:10.1016/j.optmat.2024.114830.
- E. J. Ansari, S. Patle, A. R. Pusdekar, N. S. Ugemuge and A. Selokar, Structural and spectroscopic analysis of K2Y(WO4)(PO4): Sm3+, Tb3+ phosphors for optical applications, Spectrochim. Acta Part A Mol. Spectrosc., 2025, 126269, DOI:10.1016/j.saa.2025.126269 ,https://www.sciencedirect.com/journal/spectrochimica-acta-part-a-molecular-and-biomolecular-spectroscopy/vol/339/suppl/C339.
- O. Soriano-Romero, E. F. Huerta, A. N. Meza-Rocha and U. Caldiño, Orange and yellow emissions through Sm3+ and Tb3+/Sm3+ doped potassium-zinc phosphate glasses for WLED applications, Ceram. Interfaces, 2023, 49, 36353–36359, DOI:10.1016/j.ceramint.2023.08.319.
- J. Hernández-Andrés, R. L. Lee and J. Romero, Calculating correlated color temperatures across the entire gamut of daylight and skylight chromaticities, Appl. Opt., 1999, 38(27), 5703–5709, DOI:10.1364/AO.38.005703.
- M. E. Alvarez-Ramos, J. Alvarado-Rivera, M. E. Zayas, U. Caldiño and J. Hernández-Paredes, Yellow to orange-reddish glass phosphors: Sm3+, Tb3+ and Sm3+/Tb3+ in zinc tellurite-germanate glasses, Opt. Mater., 2018, 112, 88–93, DOI:10.1016/j.optmat.2017.09.033.
- X. S. Qiao, X. P. Fan, Z. Xue and X. H. Xu, Short-wavelength upconversion luminescence of Yb3+/Tm3+ co-doped glass ceramic containing SrF2 nanocrystals, J. Non-Cryst. Solids, 2011, 357(1), 83–87, DOI:10.1016/j.jnoncrysol.2010.09.008.
- D. T. Khan, N. T. Dang, S. H. Jabarov, T. G. Naghiyev, R. M. Rzayev, T. Q. Nguyen, H. V. Tuyen, N. T. Thanh and L. V. T. Son, Study on luminescent properties of Tb3+ and Sm3+ co-doped CaSiO3 phosphors for white light emitting diodes, Mater. Res. Express, 2020, 7, 016507, DOI:10.1088/2053-1591/ab5ab8.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab,
Nguyen Dinh Trungc,
Nguyen Minh Tam
*ab,
Nguyen Dinh Trungc,
Nguyen Minh Tam d,
L. T. Hae,
Vu Thi Kim Lien*fg,
D. T. Khanh,
Nguyen Le Thaii,
Dacheng Zhouj and
Jianbei Qiuj
d,
L. T. Hae,
Vu Thi Kim Lien*fg,
D. T. Khanh,
Nguyen Le Thaii,
Dacheng Zhouj and
Jianbei Qiuj

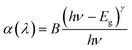










![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489
489