 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Up-conversion luminescence and temperature sensing based on Ba3Y(BO3)3:Er3+,Yb3+ phosphors†
Lei Zhang‡
a,
You Zhang‡b,
Cuilin Jina,
Chunhao Wanga,
Qiongyu Baia,
Yibo Zheng *a and
Xu Li
*a and
Xu Li *b
*b
aHebei Key Laboratory of Optoelectronic Information and Geo-detection Technology, College of Gems and Materials, Hebei GEO University, Shijiazhuang 050031, China. E-mail: yibo_zheng@hgu.edu.cn
bHebei Key Laboratory of Photo-Electricity Information and Materials, College of Physics Science and Technology, Hebei University, Baoding 071002, China. E-mail: lixcn@sina.com
First published on 9th June 2025
Abstract
Noncontact fluorescence temperature detection systems have become a significant research focus. In this study, a new Ba3Y(BO3)3 phosphor co-doped with Er3+/Yb3+ was successfully synthesized using a high-temperature solid phase method. The introduction of Yb3+ as a sensitizer enhanced the luminescence properties of the Ba3Y(BO3)3 phosphor. The occupation of Er3+ and Yb3+ ions in the crystal lattice was analyzed in detail. The up-conversion luminescence and underlying mechanisms were explained by the double logarithmic relationship between the luminescence intensity and pump power. The temperature sensitivity of the phosphors was explored in the range of 333–513 K using fluorescence intensity ratio technology based on the two green peaks (thermal coupling level Er3+). The temperature sensing Sr reached 1.44 × 10−4 K−1 at 333 K, indicating that Ba3Y(BO3)3:Er3+,Yb3+ phosphors have potential applications as temperature sensors.
1. Introduction
Highly sensitive temperature detection is crucial for enhancing both production quality and daily life.1–3 Noncontact temperature sensing using luminescence thermometry offers advantages such as fast response, high sensitivity, and versatility in various environments, including high temperatures, high pressures and biological systems, making it a topic of significant interest.4–8 Luminescence thermometry measures changes in the luminescence properties of a system with respect to temperature, enabling temperature characterization. These properties include single-emission peak intensity, emission peak position, fluorescence intensity ratio of two emission peaks, fluorescence lifetime, and emission peak half-width.9–13 Among these techniques, the fluorescence intensity ratio is the most resistant to interference. Therefore, researchers often focus on developing systems based on fluorescence intensity ratios for temperature measurement.14–16 Inorganic up-conversion luminescent materials, which have low background noise and excitation bands in the infrared range, have garnered widespread attention in temperature sensing and bioimaging.17–20 Up-conversion temperature-detecting phosphors are usually doped with Er3+/Yb3+ ions, utilizing the two thermally coupled energy levels of Er3+ as the temperature measurement energy level.21–24 Although Yb3+ has a larger absorption cross-section and wider absorption band for near-infrared energies, Yb3+ ions are often used as sensitizers.25–28Borates, which are known for their diverse crystal structures, good physical stability,29,30 and low synthesis temperatures,31,32 are expected to become mainstream phosphors.33,34 Shangke Pan et al. synthesized Nd3+-doped Ba3Y(BO3)3 phosphors using the lift-off method, achieving a crystalline phase transition temperature of 1148 °C.35 Zhao et al. explored the luminescence mechanism of Ce3+/Nd3+ co-doped Ba3Y(BO3)3 phosphors and proposed their application as solar spectral converters in Si-based solar cells.36 Li et al. successfully prepared Ba3Y(BO3)3 doped with 24 mol% Eu3+ and 1 mol% Bi3+ phosphors and demonstrated their potential for application in white LEDs.37
To the best of our knowledge, Er3+/Yb3+-doped Ba3Y(BO3)3 phosphors have not been extensively studied. In this study, we prepared Er3+/Yb3+ co-doped Ba3Y(BO3)3 phosphors and thoroughly investigated their structure, morphology, luminescence properties, and energy transfer mechanism between Yb3+ and Er3+ ions. The two-photon green emission of Er3+ was confirmed through the double logarithmic relationship between the power and luminescence intensity. Additionally, we examined the upconversion optical temperature-sensing properties of Er3+/Yb3+-co-doped Ba3Y(BO3)3 phosphors using the FIR technique.
2. Experimental section
2.1 Sample synthesis
BaCO3, Y2O3, H3BO3, Er2O3 (99.99%), Yb2O3 (99.99%), deionized water, and anhydrous ethanol were used to prepare the phosphors. Ba3Y1−x(BO3)3:xEr3+ and Ba3Y0.93−y(BO3)3:0.07Er3+, yYb3+ phosphors were synthesized using a high-temperature solid-state method with molar fractions x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, and 0.09, and y = 0.07, 0.14, 0.21, 0.28, 0.35, 0.42, 0.49, and 0.56, respectively. The corresponding number of precursors was weighed and ground in an agate mortar and pestle for 10 min. The mixture was then placed in an alumina crucible and pre-annealed in a high-temperature muffle furnace at 1250 °C for 6 h. After cooling to room temperature, the samples were ground into a powder for further analysis.2.2 Sample characterization details
We used a Bruker D8 Advanced Diffractometer to determine the profile of the synthesized materials through X-ray diffraction (XRD) analysis using Cu Kα radiation (1.5406 Å) over a range of 15–80°. The emission spectra of the samples were obtained using a Pro-FL spectrometer (F-7000, Hitachi, Japan) under 980 nm excitation from a semiconductor laser. Temperature-dependent emission spectra were obtained using the same equipment and a TCB1402C temperature controller (China).3. Results and discussion
3.1 Structure and morphology of samples
Fig. 1(a) and (b) show the XRD patterns of Ba3Y(BO3)3:xEr3+ (x = 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09) and Ba3Y(BO3)3:0.07Er3+,yYb3+ (y = 0.07, 0.14, 0.21, 0.28, 0.35, 0.42, 0.95, 0.56) samples. The diffraction patterns are consistent with the standard card PDF#51-1849. No significant impurity peaks were observed, indicating that the Er3+ and Yb3+ did not change the lattice structure because Er3+ and Yb3+ ions have similar radii to Y3+ ions.38 The synthesized material was in a pure hexagonal crystalline phase with space group P63 cm (185), with cell parameters a = b = 9.419 Å, c = 17.598 Å, and a cell volume of 1352.2 Å3.39 As shown in Fig. 1c, every B atom is coordinated with three O atoms to form triangles [BO3]. For the Ba atom, two different coordinate methods are coordinated with nine and six O atoms to form polyhedral [Ba1O9] and [Ba2O6]. Although all the Y atoms are coordinated with six O atoms, there exist two different kinds of polyhedra owing to the difference in the distortion of [Y1O6] and [Y2O6].40,41The SEM patterns of the Ba3Y(BO3)3:0.07Er3+,0.21Yb3+ phosphor are presented in Fig. 2a. It can be observed that the sample exhibits better crystallization, with a size of approximately 3–8 μm. Meanwhile, the element mapping in Fig. 2b–f shows that the main elements Ba, Y, O, Er, and Yb are uniformly distributed in the sample. The EDS measure in Table S1† indicates that the Er and Yb ions are doped in the matrix.
 | ||
| Fig. 2 (a) SEM patterns of Ba3Y(BO3)3:0.07Er3+,0.21Yb3+. (b)–(f) Elemental mapping of Ba, O, Er, Yb, and Y in Ba3Y(BO3)3:0.07Er3+,0.21Yb3+. | ||
3.2 Optical properties of samples
The diffuse reflection spectrum of 0.07Er3+, 0.21Yb3+ phosphor is shown in Fig. S1.† It can be observed that there are some absorption peaks at 297 nm, 379 nm, 488 nm, 521 nm, 651 nm, 970 nm and 1416 nm, respectively. In particular, the strongest band is located at 870–1040 nm, which indicates that it can well absorb light at 980 nm. To investigate the up-conversion luminescence performance of the samples, we tested the emission spectra of samples doped with different concentrations of Er3+ ions under 980 nm excitation at room temperature. Fig. 3(a) shows the upconverted emission spectra of Ba3Y(BO3)3:xEr3+ (x = 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09). The spectra reveal the same emission peaks across the samples, consisting of two groups of peaks in the green domain and one group of peaks in the red domain. Generally, different numbers of peaks are in one group of emissions according to the splitting degree of the ground state and excited state in different crystal fields. The peaks at 520 nm, 525 nm and 538 nm are attributed to the transition of 2H11/2 → 4I15/2 while the peaks at 549 nm, 553 nm and 563 nm are attributed to the transition of 4S3/2 → 4I15/2. The group of red emission peaks at 656 nm, 662 nm, 670 nm, 677 nm, and 684 nm is caused by the 4F9/2 → 4I15/2 transition of Er3+ ions. All the results confirm the successful incorporation of Er3+ ions into the Ba3Y(BO3)3 lattice.42,43 The intensities of the green and red emissions increased consistently with increasing Er3+ concentration from 0.01 (x = 0.01). The luminescence intensity reaches a maximum at x = 0.07, after which phosphor concentration quenching occurs, leading to a gradual decrease in luminescence intensity with further Er3+ doping. | ||
| Fig. 3 (a) UCL spectra of (a) Ba3Y(BO3)3:xEr3+ samples and (b) Ba3Y(BO3)3:7mol% Er3+,yYb3+ samples under 980 nm laser excitation. | ||
Fig. 3(b) shows the up-conversion emission spectra of phosphor Ba3Y(BO3)3:7 mol% Er3+,yYb3+ (y = 7, 14, 21, 28, 35, 42, 49, and 0.56). When the Yb3+ doping concentration was less than 21 mol%, the red emission intensity significantly increased with increasing Yb3+ concentration, while the green emission showed no significant relative change; however, the trend in green emission was similar to that of the red emission. At an Yb3+ doping concentration of 0.21, the luminous intensities of both the green and red emission bands reached their maximum values. Beyond this concentration, further Yb3+ doping resulted in decreased luminescence intensity, indicating the onset of concentration quenching at y = 0.21. Additionally, with increased Yb3+ doping, the EBT process from Er3+ to Yb3+ was enhanced, as described by the following equation: [Er3+(4S3/2) + Yb3+(2F7/2) → Er3+(4I13/2) + Yb3+(2F5/2)]. On the one hand, the defect produced in the synthesis process provides an absorption site that promotes the doping of Yb3+ ions. On the other hand, energy transfer occurs between the Yb3+ ions and non-bridging oxygen defects, which limits the amount of Yb3+ doping. However, the doping content of Yb3+ and the defect concentration have a nonlinear relationship.44
Generally, the luminescence center grows in number, and the distance between the dopant ions decreases with an increase in activator doping concentration, which increases the competition between the radiative transition and the nonradiative (NR) process and influences PL performance. The critical distance (Rc) is an important parameter for expressing the average distance between dopants when the concentration quenching effect occurs and can be expressed as follows:45
 | (1) |
To further explain the concentration quenching mechanism of the dopant in the sample, the following equation is used:46
 | (2) |
To further investigate the luminescence mechanism of the Er3+/Yb3+ co-doped Ba3Y(BO3)3 phosphor, we examined the up-conversion emission spectra of Ba3Y(BO3)3:0.07Er3+,0.21Yb3+ samples under different excitation powers. As shown in Fig. 4a, as the excitation power of the 980 nm laser increased, the up-conversion emission intensity of the samples also increased gradually. When the up-conversion emission is in a non-saturated excitation state, the luminescence intensity I is related to the excitation power P using the following equation:47
| I = Pn, | (3) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = n I = n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P + C, P + C,
| (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I/log
I/log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P indicates that the upconversion process can be attributed to n-photon absorption. A double logarithmic plot of the up-conversion luminescence intensity versus excitation power for Ba3Y(BO3)3:0.07Er3+ and 0.21Yb3+ under different excitation powers of a 980 nm laser is shown in Fig. 4(b), where we can quantitatively analyze the relationship between light intensity and excitation power. The fitted curves for the three emission peaks are shown in the figure and closely match the experimental curves with minimal errors. The 530 nm green emission has a fitted slope of 1.46, the 550 nm green emission has a slope of 1.62, and the 660 nm red emission has a slope of 1.59. The values of n were close to 2, suggesting that the emission of the samples arose from a two-photon process.
P indicates that the upconversion process can be attributed to n-photon absorption. A double logarithmic plot of the up-conversion luminescence intensity versus excitation power for Ba3Y(BO3)3:0.07Er3+ and 0.21Yb3+ under different excitation powers of a 980 nm laser is shown in Fig. 4(b), where we can quantitatively analyze the relationship between light intensity and excitation power. The fitted curves for the three emission peaks are shown in the figure and closely match the experimental curves with minimal errors. The 530 nm green emission has a fitted slope of 1.46, the 550 nm green emission has a slope of 1.62, and the 660 nm red emission has a slope of 1.59. The values of n were close to 2, suggesting that the emission of the samples arose from a two-photon process.
To investigate the luminescence mechanism of Ba3Y(BO3)3:0.07 Er3+,0.21Yb3+ phosphor, possible up-conversion emission processes are depicted in energy-level leap diagrams, as shown in Fig. 4c. Under excitation at 980 nm, Er3+ ions single doped or Er3+–Yb3+ ions co-doped Ba3Y(BO3)3 have a similar energy-transfer (ET) mechanism. Er3+ ions absorb a photon (980 nm) through a ground-state absorption (GSA) process, transitioning from 4I15/2 to 4I11/2, and Yb3+ ions absorb a photon (980 nm) through a GSA process, moving from 2F7/2 to 2F5/2. Compared to Er3+, Yb3+ has a larger absorption cross-section, and its introduction improves energy transfer efficiency. For the Er3+, Yb3+ co-doped Ba3Y(BO3)3 phosphor, energy transfer (ET I) from Yb3+ to Er3+ can be expressed as follows: 2F5/2 (Yb3+) + 4I15/2 (Er3+) → 2F7/2 (Yb3+) + 4I11/2 (Er3+). Some ions located at 4I11/2 are transferred to the 4I13/2 energy level by applying a non-radiative transition (NRI) process. Then, the ions at the 4I13/2 energy level can absorb a photon to reach the 4F9/2 energy level by excited-state absorption (ESA I). However, some Er3+ ions at the 4I11/2 excited-state energy level is further excited to 4F7/2 (ESA II) via energy transfer (ET II) from Yb3+ ions. The ions at the 4F7/2 (Er3+) energy level can gradually relax to the 2H11/2, 4S3/2 and 4F9/2 energy levels by the radiation-less relaxation of NR II, NR III, NR IV, and NR V. The transitions between 2H11/2 → 4I15/2 energy levels correspond to green emission at 530 nm, the transitions between the 4S3/2 → 4I15/2 energy levels correspond to green emission at 550 nm, and the transitions between the 4F9/2 → 4I15/2 energy levels correspond to 660 nm. Furthermore, compared with the green emission, the red emission violently increases with the Yb3+ ion concentration, which is attributed to the decrease in the distance between the ions improving the efficiency of the cross-relaxation (CR): [4F7/2 (Er3+) + 4I11/2 (Er3+) → 4F9/2 (Er3+) + 4I9/2 (Er3+) ] (CRI); [2H11/2 (Er3+) + 4I15/2 (Er3+) → 4I9/2 (Er3+) + 4I13/2 (Er3+)] (CRII); and [2H11/2 (Er3+) + 4I13/2 (Er3+) → 4F9/2 (Er3+) + 4I11/2 (Er3+)] (CRIII).
3.3 Optical temperature sensing performance of the samples
We investigated the temperature sensing performance of Ba3Y(BO3)3:0.07Er3+,0.21Yb3+ phosphors. To reduce the heating effect of the laser, we used a low excitation power of 1.0 W. The variable-temperature emission spectra of the samples, measured between 90 K and 279 K, are shown in Fig. S4,† in which the PL intensity undergoes irregular changes with temperature. Under high temperature conditions, the variable-temperature emission spectra of the samples, measured between 333 and 513 K, are shown in Fig. 5(a). As the temperature increased, the intensity of the red emission decreased; however, the 530 nm emission intensity increased and the 550 nm emission initially increased before decreasing with temperature. Fig. 5(a) clearly shows the variations in green emission intensity. The increase in emission intensity with temperature may result from enhanced thermal mobility of ions from the lower to higher energy levels.Er3+ ions have two thermally coupled energy levels, 2H11/2 and 4S3/2, with the number of ions at these levels following the Boltzmann distribution. Therefore, the intensities of the 2H11/2 → 4I15/2 (530 nm) and 4S3/2 → 4I15/2 (550 nm) emissions change with temperature. The fluorescence intensity ratio (FIR) of the two green emissions can be expressed as follows:48
 | (5) |
 | (6) |
The absolute sensitivity (Sa) and relative sensitivity (Sr) are crucial parameters for evaluating temperature sensing and can be defined as eqn (7) and (8), respectively:49
 | (7) |
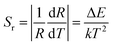 | (8) |
The fitted curve of the linear relationship between Ln (FIR) and 1/T is shown in Fig. 5(b), where the linear relationship between ln(FIR) and 1/T is consistent with the linear relationship in eqn (4). The slope ΔE/k is 602.16, and the intercept ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is 0.56. The calculated ΔE for the thermal coupling energy for this phosphor is consistent with previously reported values. The corresponding sensitivities Sa and Sr of Ba3Y(BO3)3:0.07Er3+ and 0.21Yb3+ at different temperatures are shown in Fig. 5(c) and (d), respectively. It can be observed that the Sa and Sr of the phosphor reach their maximum values of 1.44 × 10−3 K−1 and 4.68 × 10−3 K−1 at 333 K, respectively. Table S2† shows some published results and compares them with the results of this study, which indicates that this material can be used as a candidate for temperature sensing.
C is 0.56. The calculated ΔE for the thermal coupling energy for this phosphor is consistent with previously reported values. The corresponding sensitivities Sa and Sr of Ba3Y(BO3)3:0.07Er3+ and 0.21Yb3+ at different temperatures are shown in Fig. 5(c) and (d), respectively. It can be observed that the Sa and Sr of the phosphor reach their maximum values of 1.44 × 10−3 K−1 and 4.68 × 10−3 K−1 at 333 K, respectively. Table S2† shows some published results and compares them with the results of this study, which indicates that this material can be used as a candidate for temperature sensing.
4. Conclusion
In this study, novel up-conversion red phosphors of Ba3Y(BO3)3 co-doped with Er3+ and Yb3+ were successfully synthesized using a high-temperature solid-phase method. Three emission bands at 530, 550, and 660 nm, corresponding to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 of Er3+ ions, respectively, were observed under 980 nm excitation. The luminescence of Ba3Y(BO3)3 maintains a high-purity red emission by modulating the doping concentration of Yb3+. The introduction of Yb3+ ions enhanced the emission intensity of the samples. The luminescence mechanism was explained by the double logarithmic relationship between luminescence intensity and pump power. Additionally, the temperature sensitivity from 333 K to 513 K was explored using the FIR technique with two green emissions, achieving a maximum Sr of 1.44 × 10−3 K−1 at 333 K. These results demonstrate the potential applications of Ba3Y(BO3)3:Er3+/Yb3+ phosphors in luminescence and optical temperature sensing.Data availability
The data supporting the findings of this study will be made available from the corresponding author upon request.Author contributions
Lei Zhang and You Zhang: writing–original draft and investigation. Cuilin Jin: supervision, software, and investigation. Chunhao Wang: supervision, software, and investigation. Chunhao Wang and Qiongyu Bai: supervision, software, and investigation. Xu Li: writing–review & editing, supervision, and investigation. Yibo Zheng: writing–review & editing, supervision, software, and funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was partly funded by the S&T Program of Hebei, China (No. 22371701D), the Hebei Province Department of Natural Resources (No. 2024043) and the Hebei Province Optoelectronic Information Materials Laboratory Performance Subsidy Fund Project (No. 22567634H). We also appreciate the Excellent Going Abroad Experts' Training Program in Hebei Province.References
- J. Zhou, B. Del Rosal, D. Jaque, S. Uchiyama and D. Jin, Advances and challenges for fluorescence nanothermometry, Nat. Methods, 2020, 17(10), 967–980 Search PubMed.
- A. Bednarkiewicz, J. Drabik, K. Trejgis, D. Jaque, E. Ximenes and L. Marciniak, Lanthanide-based nanothermometers for bioapplications: excitation and temperature sensing in optical transparency windows, Appl. Phys. Rev., 2021, 8(1), 011317 CAS.
- C. D. S. Brites, R. Marin, M. Suta, A. N. C. Neto, E. Ximendes, D. Jaque and L. D. Carlo, Spotlight on luminescence thermometry: basics, challenges, and cutting-edge applications, Adv. Mater., 2023, 35(36), 2302749 CrossRef CAS PubMed.
- J. Chen, W. N. Zhang, S. F. Cui, X. S. Peng, F. F. Hu, R. F. Wei, H. Guo and D. X. Huang, Up-conversion luminescence properties and temperature sensing performances of Ba5Y8Zn4O21: Yb3+, Er3+ phosphors, J. Alloys Compd., 2021, 875, 159922 CrossRef CAS.
- X. W. Liu, B. C. Mei and G. L. Tan, Fabrication, microstructure and upconversion luminescence properties of Er:Sr5(PO4)3F transparent nanostructured ceramics, J. Eur. Ceram. Soc., 2024, 44(13), 7855–7866 CrossRef CAS.
- Y. Zhao, X. Wang, Y. Zhang, Y. Li and X. Yao, Optical temperature sensing of up-conversion luminescent materials: Fundamentals and progress, J. Alloys Compd., 2020, 817, 152691 CrossRef CAS.
- P. Du, L. Luo, H. K. Park and J. S. Yu, Citric-assisted sol-gel based Er3+/Yb3+-codoped Na0.5Gd0.5MoO4: A novel highly-efficient infrared-to-visible upconversion material for optical temperature sensors and optical heaters, Chem. Eng. J., 2016, 306, 840–848 CrossRef CAS.
- X. Li, P. Gao, J. Li, L. Guan, X. Li, F. Wang, D. Wang, Z. Li and X. Li, Enhancing upconversion emission and temperature sensing modulation of the La2(MoO4)3: Er3+, Yb3+ phosphor by adding alkali metal ions, Ceram. Int., 2020, 46(13), 20664–20671 CrossRef CAS.
- Q. Wang, H. Wu, L. Zhang, H. Wu, Y. Luo, G. Pan, Z. Hao, Q. Mu and J. Zhang, Green upconversion luminescence of Er3+ and Yb3+ codoped Gd2Mo4O15 for optical temperature sensing, J. Alloys Compd., 2022, 895, 162516 CrossRef CAS.
- S. L. Ye and L. Lei, Controlled synthesis of cubic NaYF4:Yb/Er@NaYF4 core/shell nanocrystals for ratiometric fluorescence temperature sensing, Chem. Phys. Lett., 2023, 833, 140935 CrossRef CAS.
- L. Li, F. Qin, Y. Zhou, Y. Zheng, J. Miao and Z. Zhang, Three-energy-level-cascaded strategy for a more sensitive luminescence ratiometric thermometry, Sens. Actuators, A, 2020, 304, 111864 CrossRef CAS.
- G. Xiang, Q. Xia, X. Liu and X. Wang, Optical thermometry based on the thermally coupled energy levels of Er3+ in upconversion materials, Dalton Trans., 2020, 49, 17115–17120 Search PubMed.
- H. Suo, X. Zhao, Z. Zhang, Y. Wang, J. Sun, M. Jin and C. Guo, Rational design of ratiometric luminescence thermometry based on thermally coupled levels for bioapplications, Laser Photonics Rev., 2020, 15(1), 2000319 CrossRef.
- N. Zhang, M. S. Molokeev, Q. Liu and Z. Xia, Pure red upconversion luminescence and optical thermometry of Er3+ doped sensitizer-rich SrYbInO4 phosphors, J. Mater. Chem. C, 2018, 6(27), 7361–7366 RSC.
- Q. Xiao, X. M. Yin, L. Lv, X. Y. Dong, N. Zhou, K. C. Liu and X. X. Luo, White up-conversion luminescence and highly-sensitive optical temperature sensing in Na3La(VO4)2: Yb, Er, Tm, Ho phosphors, J. Rare Earths, 2023, 41(7), 981–988 CrossRef CAS.
- X. Li, B. Bao, X. He, G. Wang, Y. Huang, L. Li and Y. Yu, Optical temperature sensing with an Er3+, Yb3+ co-doped LaBMoO6 single crystal, J. Mater. Chem. C, 2023, 11, 2494–2504 RSC.
- H. L. Gong, X. S. Peng, G. A. Ashraf, F. F. Hu, R. F. Wei and H. Guo, Dual-mode optical thermometry based on transparent NaY2F7: Er3+, Yb3+ glass-ceramics, Ceram. Int., 2022, 48(3), 4023–4030 CrossRef CAS.
- F. Lu, L. Wang, F. Hu, X. Tian, H. Guo and R. Wei, Multipath luminescent thermometry inCs3GdGe3O9: Yb3+, Er3+ phosphor, Ceram. Int., 2022, 48(24), 37186–37193 CrossRef CAS.
- Y. Tong, W. N. Zhang, R. F. Wei, L. P. Chen and H. Guo, Na2YMg2(VO4)3: Er3+, Yb3+ phosphors: up-conversion and optical thermometry, Ceram. Int., 2021, 47(2), 2600–2606 CrossRef CAS.
- X. Yang, Y. Zhu, T. Li, S. Long and B. Wang, High-accuracy dual-mode optical thermometry based on up-conversion luminescence in Er3+/Ho3+-Yb3+ doped LaNbO4 phosphors, Ceram. Int., 2023, 49(13), 21932–21940 CrossRef CAS.
- N. M. Bhiri, M. Dammak, M. Aguiló, F. Díaz, J. J. Carvajal and M. C. Pujol, Stokes and anti-Stokes operating conditions dependent luminescence thermometric performance of Er3+-doped and Er3+, Yb3+ co-doped GdVO4 microparticles in the non-saturation regime, J. Alloys Compd., 2020, 814, 152197 CrossRef CAS.
- X. Li, X. Wang, H. Zhong, L. Cheng, S. Xu, J. Sun, J. Zhang, X. Li, L. Tong and B. Chen, Effects of Er3+ concentration on down-/up-conversion luminescence and temperature sensing properties in NaGdTiO4: Er3+/Yb3+ phosphors, Ceram. Int., 2016, 42(13), 14710–14715 Search PubMed.
- J. Liao, M. Wang, F. Lin, Z. Han, B. Fu, D. Tu, X. Chen, B. Qiu and H. R. Wen, Thermally boosted upconversion and downshifting luminescence in Sc2(MoO4)3:Yb/Er with two-dimensional negative thermal expansion, Nat. Commun., 2022, 13(1), 1–11 Search PubMed.
- J. Hu, X. Bian, R. Wang, L. Liu, N. Ilyas, F. Wang, Z. Song and H. Fu, Giant Enhancement in Upconversion Luminescence of β-Ba2ScAlO5:Yb3+/Er3+ Phosphor by the Intermediate Band through Ca2+ Doping, Chem. Mater., 2022, 34(7), 3089–3098 CrossRef CAS.
- X. L. Yan, Y. Z. Cao, X. K. Wang, J. S. Zhang, S. Xu, G. J. Li and B. J. Chen, Molten salt synthesized LaTa7O19:Er3+/Yb3+ with superior upconversion luminescence using KCl flux, J. Mater. Chem. C, 2024, 12, 13875–13883 RSC.
- H. J. Zhang, X. B. Dong, L. Y. Jiang, Y. Yang, X. R. Cheng and H. M. Zhao, Comparative analysis of upconversion emission of LaF3: Er/Yb and LaOF: Er/Yb for temperature sensing, J. Mol. Struct., 2020, 1206, 127665 CrossRef CAS.
- L. C. V. Rodrigues, H. F. Brito and A. Meijerink, Highly luminescent Gd2O2S: Er3+, Yb3+ upconversion microcrystals obtained by a time-and energy-saving microwave-assisted solid-state synthesis, J. Alloys Compd., 2023, 942, 169083 CrossRef.
- K. Maciejewska, A. Bednarkiewicz and L. Marciniak, NIR luminescence lifetime nanothermometry based on phonon assisted Yb3+–Nd3+ energy transfer, Nanoscale Adv., 2021, 3, 4918–4925 RSC.
- L. Yue, X. Zheng, P. Xia, C. Wang, Y. Lei, M. Xu and W. Dai, Structure and optical properties of stable Bi/Eu codoped borophosphate La7O6(BO3)(PO4)2 phosphors for application in wLEDs, Ceram. Int., 2024, 50(7), 10947–10958 CrossRef.
- K. Elzbieciak-Piecka and L. Marciniak, Optical heating and luminescence thermometry combined in a Cr3+-doped YAl3(BO3)4, Sci. Rep., 2022, 12, 16364 CrossRef CAS.
- P. Xia, W. Li, K. Wu, Y. Lei and W. Dai, Crystal Structure, Photoluminescence, and “Abnormal” High Stability of Ca3Y(GaO)3(BO3)4: Dy3+/Sm3+/La3+ for n-UV wLEDs, Cryst. Growth Des., 2024, 24(8), 3355–3369 CrossRef CAS.
- M. Oglakci, S. Akça-Özalp, Z. G. Portakal-Uçar, V. Correcher, J. F. Benavente, M. Sonsuz, N. Can, Y. Z. Halefoglu and M. Topaksu, Thermoluminescence study of Nd3+ doped lanthanum tri-borate phosphor, J. Alloys Compd., 2025, 1013, 178570 CrossRef CAS.
- X. Li, W. Dai, K. Nie, S. Li and M. Xu, Investigation on optical properties of borate Sr3Y2B4O12: Ce/Tb/Sm and its application in wLEDs, J. Lumin., 2024, 53, 14153–14162 Search PubMed.
- C. G. Ma, H. L. Chen, M. Luo, F. Y. Duan, Y. Ding, Y. H. Han, T. X. Zheng, X. Yang and Y. Xiao, A novel borate phosphor Lu5Ba6B9O27:Ce3+ codoped with Sr2+/Tb3+ for NUV-white light emitting diode application, Dalton Trans., 2023, 11(6), 2202550 Search PubMed.
- S. Pan, Z. Hu, Z. Lin and G. Wang, Growth and X-ray diffraction of Nd3+-doped Ba3Y(BO3)3 crystal, J. Cryst. Growth, 2003, 247, 452–456 CrossRef CAS.
- J. Zhao, X. Wang, Q. Pang and A. P. Zhang, Enhanced Near-Infrared Luminescence in Ba3Y(BO3)3: Nd3+ by Codoping with Ce3+, ECS J. Solid State Sci. Technol., 2021, 10, 016004 CrossRef CAS.
- Q. Li, C. Chen, B. Shen, B. Yu and Y. P. Zhang, Enhanced red emission and high thermal stability from Bi3+/Eu3+ co-doped Ba3Y(BO3)3 phosphors for WLEDs application, J. Lumin., 2021, 237, 118196 CrossRef CAS.
- X. L. Wu, J. L. Zheng, Q. Ren, W. N. Bai, Y. H. Ren and O. Hai, Synthesis and luminescent properties of a novel orange-red Ba3Y(BO3)3:Sm3+ phosphors for white LEDs, Polyhedron, 2019, 164, 17–22 CrossRef CAS.
- X. B. Qiao and H. J. Seo, Phase transition, structural and spectroscopic properties of Ba3Y(BO3)3 phosphor, J. Alloys Compd., 2015, 637, 504–508 CrossRef CAS.
- W. W. Wu, Y. P. Zhang, Y. P. Zhang and J. X. Hu, Ba3YB3O9 based phosphor ceramic plates withexcellent thermal stability for wLED applications, Dalton Trans., 2021, 50, 5287 RSC.
- X. Z. Li, X. L. Chen, L. Wu, Y. G. Cao, T. Zhou and Y. P. Xu, Ba3YB3O9: phase transition and crystal structure, J. Alloys Compd., 2004, 370, 53–58 CrossRef CAS.
- W. Liu, S. Q. Xu and L. Lei, Enhancing upconversion of Sc2Mo3O12:Yb/Ln (Ln = Er, Ho) phosphors by doping Ca2+ ions, Opt. Mater., 2023, 143, 114166 CrossRef CAS.
- K. Saidi, C. Hernández-Álvarez, M. Runowski, M. Dammak and I. R. M. Benenzuela, Temperature and Pressure Sensing Using an Optical Platform Based on Upconversion Luminescence in NaSrY(MoO4)3 Codoped with Er3+ and Yb3+ Nanophosphors, ACS Appl. Nano Mater., 2023, 6, 19431–19442 CrossRef CAS.
- J. P. Ma, Y. M. Chen, L. M. Zhang, S. Q. Guo, J. D. Liu, H. Li, B. J. Ye, Z. Y. Li, Y. Zhou, B. B. Zhang, O. M. Zhang and H. T. Sun, Insights into the local structure of dopants, doping efficiency, and luminescence properties of lanthanide-doped CsPbCl3 perovskite nanocrystals, J. Mater. Chem. C, 2019, 7(10), 3037–3048 RSC.
- K. M. Zhu, Z. Y. Wang, H. Y. Xu and Z. L. Fu, Development of Multifunctional Materials Based on Heavy Concentration Er3+-Activated Lead-free Double Perovskite Cs2NaBiCl6, Adv. Opt. Mater., 2022, 10, 2201182 CrossRef CAS.
- H. Jin, N. Fu, C. H. Wang, C. X. Qi, Z. Y. Liu, D. W. Wang, L. Guan, F. H. Wang and X. Li, Sr/Ba substitution induced higher thermal stability far red-emitting Ba1-ySryLaLiWO6:Mn4+ phosphors for plant growth applications, Dalton Trans., 2023, 52, 787–795 Search PubMed.
- S. M. Wei, Y. Q. Yang and Z. H. Li, The Mn/Yb/Er triple-doped CeO2 nanozyme with enhanced oxidase-like activity for highly sensitive ratiometric detection of nitrite, Chin. Chem. Lett., 2024, 35(6), 109114 CrossRef CAS.
- J. X. Jiao, Y. W. Liu, H. Wang, X. M. Yin, M. M. Xing, X. X. Luo and Y. Tian, Enhancing upconversion luminescence and thermal sensing properties of Er/Yb co-doped oxysulfide core-shell nanocrystals, J. Am. Ceram. Soc., 2021, 104(2), 985–994 CrossRef CAS.
- K. He, L. Zhang, Y. Liu, B. Xu, L. Chen and G. Bai, Lanthanide ions doped nonhygroscopic La2Mo3O12 microcrystals based on multimode luminescence for optical thermometry, J. Alloys Compd., 2022, 890, 161918 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01277e |
| ‡ Lei Zhang and Meitong Guo are co-first authors of this paper. |
| This journal is © The Royal Society of Chemistry 2025 |



