Open Access Article
Open Access ArticleDesign of a magnetic Fenton-like catalyst by decorating diamond-shaped MIL-88A with Chenopodium-derived biochar for nitrophenol degradation: optimization and mechanistic insights†
Abdelazeem S. Eltaweil *a,
Mohammed Salah Ayoupb,
Jawaher Y. Al Nawah
*a,
Mohammed Salah Ayoupb,
Jawaher Y. Al Nawah *b and
Eman M. Abd El-Monaemc
*b and
Eman M. Abd El-Monaemc
aChemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt. E-mail: abdelazeemeltaweil@alexu.edu.eg
bDepartment of Chemistry, College of Science, King Faisal University, Al-Ahsa 31982, Saudi Arabia. E-mail: jalnawah@kfu.edu.sa; mayoup@kfu.edu.sa
cAdvanced Technology Innovation, Borg El-Arab, Alexandria, Egypt. E-mail: emanabdelmonaem5925@yahoo.com
First published on 13th June 2025
Abstract
An effective Fenton-like Fe3O4/MIL-88A/BC catalyst was fabricated by combining magnetite nanoparticles (Fe3O4) with diamond-shaped MIL-88A and Chenopodium-derived biochar for the degradation of 2-NP. The elemental composition, morphology, functional groups, surface net charge, and crystallographic phase of the Fe3O4/MIL-88A/BC catalyst were examined using various characterization techniques, including XPS, SEM, FTIR, ZP, and XRD. Optimization experiments were conducted to determine the optimal Fenton-like degradation conditions for 2-NP using H2O2/Fe3O4/MIL-88A/BC. Laboratory experiments showed that the 2-NP degradation efficiency by H2O2/Fe3O4/MIL-88A/BC reached 91.04% within 120 min at pH = 5, Fe3O4/MIL-88A/BC = 10 mg, and H2O2 concentration = 500 mg L−1. Kinetic studies indicated that the Fenton-like degradation of 2-NP followed a second-order model, while H2O2 decomposition was best described by a first-order model. Quenching tests indicated that the Fenton-like reaction of 2-NP proceeded via a radical mechanism and confirmed that the ˙OH radicals are the controlling reactive O-species. The degradation mechanism of 2-NP was proposed based on the XPS spectra of the neat and used Fe3O4/MIL-88A/BC catalysts. The intermediates obtained from the Fenton-like degradation of 2-NP by the Fe3O4/MIL-88A/BC catalyst were predicted from the GC-MS spectrum.
1. Introduction
Recently, the world has delved deeply into the issue of water pollution, which threatens the survival of human beings and the entire planet. Scientists and environmental experts are challenged by this dilemma because most water contaminants are cogs in the wheel of vital industries. For instance, 2-NP is widely used in numerous industries, including fungicide, petrochemical, pigment, explosive, pharmaceutical, insecticide, chemical intermediate, and herbicide industries. The total annual production of 2-NP ranges from 10 to 15 million pounds.1 Nonetheless, the leakage of even a relatively low concentration of 2-NP into water bodies could lead to harmful effects on human health, such as blurred vision, liver and kidney damage, drowsiness, mouth irritation, and cyanosis.2 In addition, 2-NP has a disruptive impact on the environment, with its nitro-containing group contributing to its stability in water and soil.3 Therefore, there is an essential need to investigate effective measures for controlling the presence of 2-NP in water bodies. Techniques such as adsorption, membrane filtration, electrolysis, coagulation, and Fenton degradation have progressed as promising approaches to tackle such hazardous compounds.4,5The non-toxicity, plentiful iron sources, absence of secondary contaminant formation, and use of green oxidants make Fenton degradation a felicitous strategy to outdo 2-NP from wastewater.6 Fenton reaction is one of the most efficacious hydroxyl radical-based advanced oxidation processes and has exhibited preeminent performance in degrading the notorious 2-NP. Generally, Fenton degradation of such persistent nitro-aromatic compounds proceeds via the formation of hydroxyl radicals (˙OH) by the activation of hydrogen peroxide (H2O2) using iron-based catalysts.7,8 However, iron-based catalysts still require further development to enhance the production of ˙OH radicals and accelerate the Fe2+/Fe3+ cycle.
Metal–organic frameworks (MOFs) are considered a recent breakthrough in material science revolution. The discernible characteristics of MOFs, such as excellent thermal and mechanical properties, high surface area, and high porosity, render them appropriate for numerous applications, including drug delivery, sensors, gas separation, wastewater purification, and solar cells.9,10 For wastewater purification, MOFs have demonstrated outstanding results in removing persistent pollutants via many remediation strategies. In particular, Fenton degradation is due to the individual chemical structures of the ligands, which contain unsaturated coordination species and abundant oxygen functional groups.11 Iron-based MOFs, including MIL-101, MIL-88A, MIL-100, MIL-88B, and MIL-53, have demonstrated remarkable performance in degrading diverse contaminants by the Fenton degradation process. In particular, MIL-88A has gained exceptional interest as a remarkable Fenton catalyst owing to its high recyclability, efficient catalytic activity, and chemical stability.12
Biochar (BC) is a dust-carbon-rich substance derived from biowaste via thermal decomposition under pyrolysis temperatures in the range of 300–900 °C.13,14 Interestingly, the conversion of biowaste, like agricultural waste, to BC could provide a better solution for removing this waste instead of burning it. Reported studies have demonstrated the hazardous impact of burning agricultural waste on human health, especially the respiratory system.15 Thanks to the remarkable properties of BC, such as good mechanical strength, porous structure, high surface area, and possession of oxygenated groups, it makes it a superb catalyst for degrading pollutants by Fenton/Fenton-like reactions.16 In addition, environmentally persistent free radicals (EPFRs)-containing BC can activate H2O2 to generate ˙OH radicals during Fenton/Fenton-like reactions.17
Magnetite (Fe3O4) is a bright iron-based nanocatalyst that has demonstrated remarkable performance in Fenton degradation reactions of many harmful pollutants.18 This auspicious catalytic performance of Fe3O4 stems from its Fe2+ ions, which activate H2O2 via the Haber–Weiss reaction and produce ˙OH radicals.19 Fe3O4 possesses an excellent ferromagnetic character, resulting in easy separation features that prevent mass loss during regeneration and save time during the separation step.20 Additionally, Fe3O4 is a non-toxic substance with a wide surface area, good chemical stability, intrinsic peroxidase, and propitious recyclability.21,22 Notably, the octahedral site in the Fe3O4 matrix allows it to accommodate Fe2+ and Fe3+; therefore, the Fe2+ moieties can be oxidized reversibly by transferring electrons between Fe2+ and Fe3+.23,24
The MIL-88A and/or BC-based heterogeneous catalysts exhibit eminent catalytic activity in Fenton degradation reactions of various organic contaminants. For instance, Ren et al. fabricated ZnO-decorated MIL-88A rods to degrade methylene blue, clarifying that the degradation percentage attained 96.15% after a reaction time of 40 min.25 Abd El-Monaem et al. developed rod-shaped MIL-88A with SnFe2O4 and supported them on MXene sheets for degrading the anionic Congo red dye, achieving a promising degradation percentage of 100% after 60 min. In addition, the synergistic effect of the CR adsorption and the Fenton-like process was monitored, where the adsorption percentage of CR was 47.25%.26 Liao and his coworkers compared the catalytic activity of rod, diamond, and spindle-shaped MIL-88A toward the Fenton degradation of phenol. The experimental findings demonstrated that the catalytic degradation efficiency of the rod-like MIL-88A toward phenol was higher than that of the other forms, where it fulfilled 100% after 15 min.27 Moreover, Sang et al. study involved investigating the Fenton-like degradation of ciprofloxacin by natural pyrite/rice straw-derived biochar, revealing that the degradation percentage achieved 96.8% after 20 minutes.28 Park and his coauthors fabricated Fe-impregnated sugarcane biochar for degrading Orange G throughout a Fenton-like reaction. The degradation percentage of Orange G reached 99.70% after 120 minutes using 0.5 g L−1 of the Fe-impregnated biochar at pH = 5.5.29
In light of previous studies, diamond-shaped MIL-88A and BC were not used to degrade o-NP by the Fenton-like reaction. Meanwhile, MIL-88A and BC exhibit high dispersity, which hinders their separation from the catalytic media after Fenton reactions. To overcome the high dispersity of MIL-88A and BC, they can be incorporated into polymeric materials and shaped into beads, hydrogels, or membranes. However, the degradation efficacy may decline because of the lower catalytic activity of the polymers. On the other hand, adding a magnetic substance to MIL-88A and BC results in a magnetic catalyst that can be separated perfectly from the catalytic media by an external magnet. Magnetic substances contain transition metals that exhibit remarkable activity toward degrading organic pollutants via Fenton/Fenton-like reactions.
Accordingly, our investigation aimed to take the merits of MIL-88A, Fe3O4, and BC to fabricate a Fenton-like catalyst with eminent catalytic activity, excellent chemical stability, and outstanding recyclability. Consequently, an Fe3O4/MIL-88A/BC composite was prepared, and its chemical and physical properties were studied using varied characterization tools, including XPS, FTIR, VSM, ZP measurements, SEM, and XRD. The optimum Fenton-like parameters for degrading 2-NP by Fe3O4/MIL-88A/BC were investigated after a series of degradation reactions at different pH values, catalyst doses, 2-NP and H2O2 concentrations, and temperatures. The kinetic models were applied to the experimental results obtained by degrading 2-NP and H2O2 over time. The chemical stability of the Fenton-like Fe3O4/MIL-88A/BC catalyst was confirmed by cycling tests and metal leaching studies after each recycling run. The Fenton-like degradation mechanism of o-NP by H2O2 activation using the Fe3O4/MIL-88A/BC catalyst was proposed based on the quenching test results and XPS spectra of fresh and used Fe3O4/MIL-88A/BC. The intermediates yielded from 2-NP degradation by Fe3O4/MIL-88A/BC were determined by GC-MS.
2. Materials and methods
2.1. Materials
The chemicals used in the preparation stage of the Fe3O4/MIL-88A/BC catalyst were Ferric chloride hexahydrate (FeCl3·6H2O) and fumaric acid (FA), which were purchased from Aladdin Industrial Corporation (China). Ferrous chloride tetrahydrate was obtained from Sigma-Aldrich (USA). Ammonium solution (NH4OH) and 2-NP were obtained from Sinopharm Chemical Reagent (China). N,N dimethyl formamide (DMF) and ethanol (C2H5OH) were obtained from Shanghai Chemical Reagent Co., Ltd (China).2.2. Preparation of Fe3O4/MIL-88A/BC catalyst
2.3. Fenton-like degradation experiment
The Fenton-like reaction of 2-NP by activating H2O2 using an Fe3O4/MIL-88A/BC catalyst was studied as follows: (i) a comparison test between Fe3O4, MIL-88A, BC, and Fe3O4/MIL-88A/BC composites for evincing the synergistic effect between the composite's components and selecting the optimal BC proportion. (ii) Proceeding with the Fenton-like degradation of 2-NP at a pH range of 3–11 to pick out the optimum pH of the catalytic medium. (iii) Determine the economical dose of Fe3O4/MIL-88A/BC by proceeding with Fenton-like degradation of 2-NP using varied doses of the catalyst (5–20 mg). (iv) Studying the effect of oxidant concentration on the degradation aptitude of 2-NP at H2O2 concentrations ranging from 100 to 1000 mg L−1. (v) Identifying the effect of the temperature of the Fe3O4/MIL-88A/BC-H2O2 catalytic system on the degradation efficacy of 2-NP. (vi) Investigating the influence of the 2-NP concentration (50–300 mg L−1) on the degradation efficiency at the constant weight of Fe3O4/MIL-88A/BC. The undegraded 2-NP concentration was determined using a spectrophotometer at a maximum wavelength of 344 nm, and the degradation efficiency of 2-NP was estimated using eqn (1).
 | (1) |
2.4. Cycling test
The regeneration potential of the Fe3O4/MIL-88A/BC catalyst was assessed by performing the cycling test for five cycles. After the Fenton-like degradation of 2-NP was completed, the Fe3O4/MIL-88A/BC catalyst was collected using a magnet, washed with an aqueous solution of NaOH (1 M) and distilled water, and dried at 50 °C in an oven. Subsequently, the regenerated Fe3O4/MIL-88A/BC catalyst was tested in the next Fenton-like degradation cycle of 2-NP.2.5. Extraction of the intermediate compounds
The intermediate compounds from degrading 2-NP by Fe3O4/MIL-88A/BC were extracted from the Fenton-like catalytic medium by a liquid–liquid extraction procedure as follows: adding 20 mL of the 2-NP solution after the Fenton-like degradation reaction and 20 mL of C4H8O2 in a separatory funnel. After extracting the organic compounds, they were dried with Na2SO4 using rotary evaporation. Next, the extracted sample was dissolved in MeOH and injected into the GC-MS (PE-5MS column; 0.18 mm film thickness, 0.18 mm diameter, and 20 m length) at 80 °C with the temperature increasing by 10 °C min−1 until reaching 280 °C.323. Results and discussion
3.1. Studying the Fe3O4/MIL-88A/BC characteristics
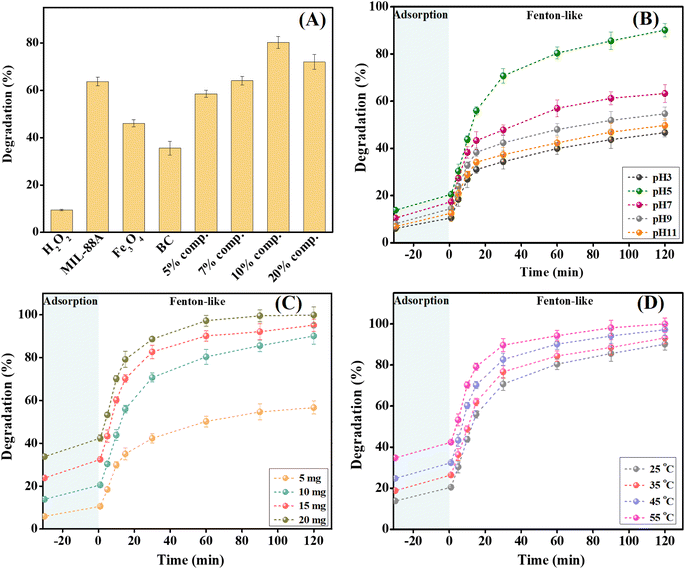
Based on the experimental results, the Fe3O4/MIL-88A/BC composite containing 10 wt% BC exhibited the highest catalytic activity toward o-NP. Therefore, a characterization analysis was conducted on the catalyst containing 10 wt% BC.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks of the carboxylate groups of FA appeared at wavenumbers of 1551 cm−1 (asymmetric C
O peaks of the carboxylate groups of FA appeared at wavenumbers of 1551 cm−1 (asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1398 cm−1 (symmetric C
O) and 1398 cm−1 (symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The wide-broad band between 3354 and 3514 cm−1 is attributed to OH groups.23–25 The FTIR spectrum of Fe3O4 demonstrated the chemical functional groups of Fe3O4, which revealed the accredited peaks to Fe–O–Fe at wavenumbers of 559 and 1402 cm−1. In addition, the FTIR peaks of bending OH appeared at 1634 cm−1, and vibrating OH appeared at 891 cm−1.26,27 The spectrum of BC shows FTIR peaks at 458 and 1088 cm−1, which are assigned to Si–O and C–O, respectively. The C–H peak appeared at 797 cm−1, and the peak at 1593 cm−1 corresponded to C
O). The wide-broad band between 3354 and 3514 cm−1 is attributed to OH groups.23–25 The FTIR spectrum of Fe3O4 demonstrated the chemical functional groups of Fe3O4, which revealed the accredited peaks to Fe–O–Fe at wavenumbers of 559 and 1402 cm−1. In addition, the FTIR peaks of bending OH appeared at 1634 cm−1, and vibrating OH appeared at 891 cm−1.26,27 The spectrum of BC shows FTIR peaks at 458 and 1088 cm−1, which are assigned to Si–O and C–O, respectively. The C–H peak appeared at 797 cm−1, and the peak at 1593 cm−1 corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.28 The FTIR spectrum of the Fe3O4/MIL-88A/BC composite denoted the well-blending of MIL-88A, Fe3O4, and BC, where their distinguishing bands are obvious.
C.28 The FTIR spectrum of the Fe3O4/MIL-88A/BC composite denoted the well-blending of MIL-88A, Fe3O4, and BC, where their distinguishing bands are obvious.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, and the peak at 284.67 eV corresponds to C–H and C–C. The peaks located in the Fe 2P spectrum (Fig. 3C) at 710.35 and 712.47 eV are assigned to Fe2+ and Fe3+ of Fe2p3/2, respectively, and their satellite peaks exist at 716.01 and 719.36 eV. The XPS peaks of Fe2p1/2 at 724.16 and 727.42 eV belong to Fe2+ and Fe3+, respectively, whereas the satellite peak appeared at 732.16 eV.
O and C–O, and the peak at 284.67 eV corresponds to C–H and C–C. The peaks located in the Fe 2P spectrum (Fig. 3C) at 710.35 and 712.47 eV are assigned to Fe2+ and Fe3+ of Fe2p3/2, respectively, and their satellite peaks exist at 716.01 and 719.36 eV. The XPS peaks of Fe2p1/2 at 724.16 and 727.42 eV belong to Fe2+ and Fe3+, respectively, whereas the satellite peak appeared at 732.16 eV.
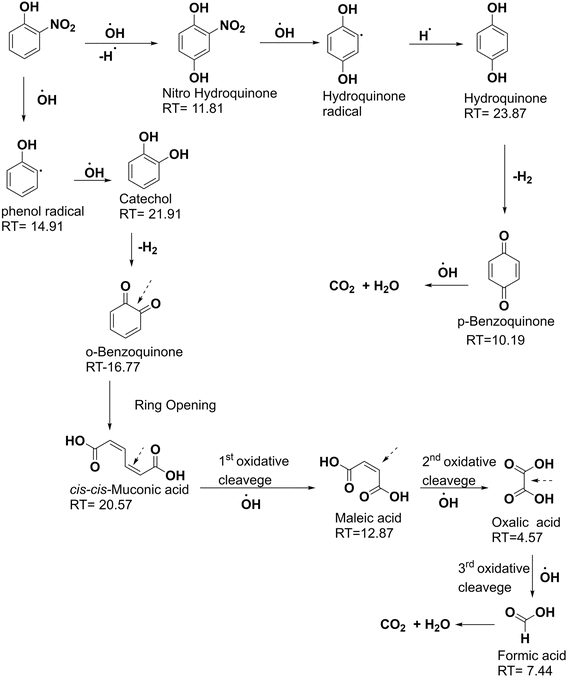
3.2. Optimizing the Fenton-like degradation process of 2NP
| H2O2 + OH− → OOH− + H2O | (2) |
| M + OOH− → M⋯˙OOH | (3) |
| 2H2O2 → O2 + 2H2O | (4) |
| H2O2 + ˙OH → HOO˙ + H2O | (5) |
| HOO˙ + ˙OH → H2O + O2 | (6) |
3.3. Kinetics study
Regression analyses for the obtained results from the Fenton-like degradation reaction of 2-NP by the H2O2/Fe3O4/MIL-88A/BC system were performed based on First-order and Second-order (eqn (7) and (8)).
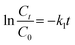 | (7) |
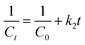 | (8) |
As illustrated in Fig. 7A, B and Table 1, the Fenton-like degradation reaction of 2-NP by the H2O2/Fe3O4/MIL-88A/BC system fits the Second-order more than the First-order, where the derived R2 values from the Second-order are higher at varied 2-NP concentrations.40 Notably, k1 and k2 are the slopes of the First-order plot (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct/C0 vs. time) and Second-order plot (1/Ct vs. time). The rate constants of the Fenton-like degradation of 2-NP based on the Second-order model were 0.0785, 0.0263, 0.0139, and 0.0093 mg L−1 min−1 when the initial 2-NP concentrations were 50, 100, 200, and 300 mg L−1, respectively. The rate constant of the degradation reaction of 2-NP decreased as the initial 2-NP concentration increased, which may be due to the governing of some factors, including ˙OH production and consumption; in addition, the competition between 2-NP and its degradation intermediates.41,42 The kinetic study of H2O2 decomposition was conducted by analyzing the experimental data using first-order and second-order models (Fig. 7C and D). The derived kinetic parameters clarified the suitability of the first-order model for H2O2 decomposition by Fe3O4/MIL-88A/BC, where the R2 values of the first-order and second-order models were 0.977 and 0.692, respectively. Furthermore, the decomposition rate constant was determined as 0.032 min−1 using first-order kinetics calculations.
Ct/C0 vs. time) and Second-order plot (1/Ct vs. time). The rate constants of the Fenton-like degradation of 2-NP based on the Second-order model were 0.0785, 0.0263, 0.0139, and 0.0093 mg L−1 min−1 when the initial 2-NP concentrations were 50, 100, 200, and 300 mg L−1, respectively. The rate constant of the degradation reaction of 2-NP decreased as the initial 2-NP concentration increased, which may be due to the governing of some factors, including ˙OH production and consumption; in addition, the competition between 2-NP and its degradation intermediates.41,42 The kinetic study of H2O2 decomposition was conducted by analyzing the experimental data using first-order and second-order models (Fig. 7C and D). The derived kinetic parameters clarified the suitability of the first-order model for H2O2 decomposition by Fe3O4/MIL-88A/BC, where the R2 values of the first-order and second-order models were 0.977 and 0.692, respectively. Furthermore, the decomposition rate constant was determined as 0.032 min−1 using first-order kinetics calculations.
| Kinetic model | Concentrations (mg L−1) | |||
|---|---|---|---|---|
| 50 | 100 | 200 | 300 | |
| First order | ||||
| k1 | 0.0185 | 0.0111 | 0.0083 | 0.0067 |
| R2 | 0.950 | 0.947 | 0.973 | 0.975 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Second order | ||||
| k2 | 0.0785 | 0.0263 | 0.0139 | 0.0093 |
| R2 | 0.985 | 0.990 | 0.992 | 0.985 |
3.4. Fenton-like degradation mechanism
Second, the Fenton-like degradation reaction of 2-NP by Fe3O4/MIL-88A/BC was based on the XPS spectra that implied governing the Haber–Weiss mechanism in the degradation reaction as follows: The Fe 2p spectra (Fig. 8C) of the used and fresh Fe3O4/MIL-88A/BC catalysts showed a significant increment in the Fe3+/Fe2+ ratio from 0.35 to 0.63, reflecting the consumption of Fe2+ in the H2O2 activation to produce ˙OH as represented in eqn (9). Furthermore, the XPS peaks of Fe2+ shifted from 710.35 and 724.16 eV to 710.30 and 723.85 eV; in addition, the peaks of Fe3+ also shifted from 712.24 and 727.24 eV to 713.05 and 727.03 eV. The continuous Fenton-like reaction of 2-NP requires a sustained and fast Fe3+→ Fe2+ redox cycle; thus, the electron-rich BC can supply the redox cycle with high amounts of electrons that facilitate the reduction of Fe3+ to Fe2+ (eqn (10)).
| Fe2+ + H2O2 → Fe3+ + ˙OH + −OH | (9) |
| Fe3+ + e− → Fe2+ | (10) |
Furthermore, oxygen-centered EPFRs activate H2O2, generating ˙OH radicals (eqn (11)). The O 1s spectrum (Fig. 8D) of the Fe3O4/MIL-88A/BC exhibited a peak shifting of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and Fe–O–Fe from 531.15, 531.18, and 529.56 eV to 531.08, 532.31, and 529.45 eV, respectively. Furthermore, the intensity of C–O and Fe–O–Fe peaks decreased, whereas the peak intensity of C
O, C–O, and Fe–O–Fe from 531.15, 531.18, and 529.56 eV to 531.08, 532.31, and 529.45 eV, respectively. Furthermore, the intensity of C–O and Fe–O–Fe peaks decreased, whereas the peak intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increased after the Fenton-like degradation of 2-NP. These observations implied the participation of C
O increased after the Fenton-like degradation of 2-NP. These observations implied the participation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and Fe–O–Fe in the Fenton-like reaction of 2-NP using the H2O2/Fe3O4/MIL-88A/BC catalytic system. Hence, we could conclude the significant role of BC that is not only responsible for half of the redox cycle (Fe3+ → Fe2+) but also contributes to H2O2 activation and ˙OH radical production. Finally, the produced ˙OH, even by the Haber–Weiss mechanism or EPFRs-based BC, can attack o-NP molecules and degrade them to CO2 and H2O, as clarified in eqn (12).
O, C–O, and Fe–O–Fe in the Fenton-like reaction of 2-NP using the H2O2/Fe3O4/MIL-88A/BC catalytic system. Hence, we could conclude the significant role of BC that is not only responsible for half of the redox cycle (Fe3+ → Fe2+) but also contributes to H2O2 activation and ˙OH radical production. Finally, the produced ˙OH, even by the Haber–Weiss mechanism or EPFRs-based BC, can attack o-NP molecules and degrade them to CO2 and H2O, as clarified in eqn (12).
| EPFRs + H2O2 → ˙OH + −OH | (11) |
| 2-NP + ˙OH → byproducts → CO2 + H2O | (12) |
3.5. Degradation pathway
The suggested degradation pathway and the intermediates formed during the Fenton-like degradation of 2-NP onto the Fe3O4/MIL-88A/BC composite were analyzed using GC-MS (Fig. 9). The ˙OH radicals attacked 2-NP via two distinct pathways. Fig. 10 illustrates the possible Fenton-like degradation pathway of 2-NP on the Fe3O4/MIL-88A/BC composite.In the first pathway, the hydrogen atom of the phenyl ring at position 4 was substituted, leading to the formation of nitrohydroquinone, which appeared at a retention time (RT) of 11.81 min. Subsequently, the nitro group was replaced by OH, forming a hydroquinone radical that was converted into hydroquinone (RT = 23.87 min). Hydroquinone was further oxidized to p-benzoquinone (RT = 10.19 min), whose oxidative cleavage ultimately yielded CO2 and water. The second pathway involved the direct substitution of the NO2 group by OH via a phenol radical intermediate to form catechol (RT = 21.91 min). Catechol was then oxidized to o-benzoquinone (RT = 16.77 min) and underwent ring opening to produce cis–cis-muconic acid (RT = 20.57 min). The successive oxidative cleavage of muconic acid by ˙OH radicals resulted in the formation of maleic acid (RT = 12.87 min), followed by oxalic acid (RT = 4.57 min) and formic acid (RT = 7.44 min), which were eventually degraded into carbon dioxide and water. Fig. 10 illustrates the possible Fenton-like degradation pathway of 2-NP on the Fe3O4/MIL-88A/BC composite.
3.6. Stability of the Fe3O4/MIL-88A/BC catalyst
To confirm the stability of Fe3O4/MIL-88A/BC, it was crucial to examine the metal leaching after the Fenton-like reaction and study the recyclability of the catalyst.4. Conclusion
Fe3O4/MIL-88A/BC was successfully fabricated, and its physical and chemical characteristics were identified by various characterization analyses, including SEM, ZP, XRD, XPS, and FTIR. The SEM image revealed the diamond-shaped MIL-88A fabricated using the solvothermal approach. The FTIR and XRD patterns of Fe3O4/MIL-88A/BC demonstrated a good combination of Fe3O4, MIL-88A, and BC inside the composite structure. The results of the laboratory experiments showed that the DE of 2-NP by the H2O2/Fe3O4/MIL-88A/BC catalytic system reached 91.04% at pH = 5, H2O2 concentration = 500 mg L−1, Fe3O4/MIL-88A/BC = 10 mg, and 120 min. The quenching test deduced 2-NP degradation via the radical pathway, with ˙OH as the main reactive O-species. The XPS analysis of pristine and used Fe3O4/MIL-88A/BC suggested the occurrence of the Fenton-like degradation of 2-NP by the Haber–Weiss mechanism and EPFRs-based BC. Notably, the ability of BC to donate electrons enabled it to be the controller in the half of the redox cycle (Fe3+ → Fe2+) and the continuity of the Fenton-like degradation of 2-NP.Based on our in-depth study, we recommend our catalyst as a superb catalyst for Fenton-like reactions, taking into consideration increasing the number of transition metals in the catalyst to expand the redox cycle by doping metal species, fabricating bimetallic/binary MOF, or adding layered double/triple hydroxide to the catalyst matrix. These transition metals boost the concentration of the generated ˙OH radicals and work together with BC to recover the Fe2+ ions, thereby further improving the degradation efficacy and catalyst recyclability. Furthermore, the Fenton/Fenton-like degradation of 2-NP requires further investigation because there is a shortage of published research highlighting this topic. Excessive studies are essential to propose other degradation pathways for 2-NP and to examine the toxicity of the produced intermediates.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. KFU252061).Acknowledgements
JA and MSA acknowledge “the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, for financial support under the annual funding track [KFU252061].References
- B. S. Marques, K. Dalmagro, K. S. Moreira, M. L. Oliveira, S. L. Jahn, T. A. de Lima Burgo and G. L. Dotto, J. Alloys Compd., 2020, 838, 155628 CrossRef CAS.
- A. M. Omer, A. S. Eltaweil, A. M. Abdelhamed, E. M. Abd El-Monaem and G. M. El-Subruiti, Sci. Rep., 2024, 14, 14463 CrossRef CAS.
- D. Ewis, M. M. Ba-Abbad, A. Benamor, N. Mahmud, M. Nasser, M. El-Naas and A. W. Mohammad, Int. J. Environ. Res., 2022, 16, 23 CrossRef CAS.
- D. Khan, J. Kuntail and I. Sinha, J. Mol. Graphics Modell., 2022, 116, 108251 CrossRef CAS PubMed.
- Y. Yang, Y. Gu, H. Lin, B. Jie, Z. Zheng and X. Zhang, J. Colloid Interface Sci., 2022, 608, 2884–2895 CrossRef CAS PubMed.
- A. S. Eltaweil, A. M. Galal, E. M. Abd El-Monaem, N. Al Harby and M. El Batouti, Nanomaterials, 2024, 14 Search PubMed.
- J. Lu, M. Yue, W. Cui, C. Sun and L. Liu, Colloids Surf., A, 2022, 655, 130222 CrossRef CAS.
- Y. Liu and J. Wang, Chem. Eng. J., 2023, 466, 143147 CrossRef CAS.
- E. M. Abd El-Monaem, A. M. Omer and A. S. Eltaweil, J. Polym. Environ., 2024, 32, 2075–2090 CrossRef CAS.
- J. Tang and J. Wang, Environ. Sci. Technol., 2018, 52, 5367–5377 CrossRef CAS PubMed.
- V. P. Viswanathan, S. V. Mathew, D. P. Dubal, N. N. Adarsh and S. Mathew, ChemistrySelect, 2020, 5, 7534–7542 CrossRef CAS.
- H. Fu, X.-X. Song, L. Wu, C. Zhao, P. Wang and C.-C. Wang, Mater. Res. Bull., 2020, 125, 110806 CrossRef CAS.
- S. M. Shaheen, N. K. Niazi, N. E. Hassan, I. Bibi, H. Wang, D. C. Tsang, Y. S. Ok, N. Bolan and J. Rinklebe, Int. Mater. Rev., 2019, 64, 216–247 CrossRef CAS.
- J. Wang and S. Wang, J. Cleaner Prod., 2019, 227, 1002–1022 CrossRef CAS.
- E. M. Abd El-Monaem, A. M. Omer, G. M. El-Subruiti, M. S. Mohy-Eldin and A. S. Eltaweil, Biomass Convers. Biorefin., 2024, 14, 1697–1709 CrossRef CAS.
- R. Deng, H. Luo, D. Huang and C. Zhang, Chemosphere, 2020, 255, 126975 CrossRef CAS.
- X. Ruan, Y. Sun, W. Du, Y. Tang, Q. Liu, Z. Zhang, W. Doherty, R. L. Frost, G. Qian and D. C. Tsang, Bioresour. Technol., 2019, 281, 457–468 CrossRef CAS.
- M. Jin, M. Long, H. Su, Y. Pan, Q. Zhang, J. Wang, B. Zhou and Y. Zhang, Environ. Sci. Pollut. Res., 2017, 24, 1926–1937 CrossRef CAS PubMed.
- R. C. Costa, F. C. Moura, J. Ardisson, J. Fabris and R. Lago, Appl. Catal., B, 2008, 83, 131–139 CrossRef CAS.
- G. Fang, J. Gao, C. Liu, D. D. Dionysiou, Y. Wang and D. Zhou, Environ. Sci. Technol., 2014, 48, 1902–1910 CrossRef CAS.
- Y. Pan, M. Zhou, X. Li, L. Xu, Z. Tang and M. Liu, Sep. Purif. Technol., 2016, 169, 83–92 CrossRef CAS.
- L. Yu, X. Yang, Y. Ye and D. Wang, RSC Adv., 2015, 5, 46059–46066 RSC.
- L. Xu and J. Wang, Environ. Sci. Technol., 2012, 46, 10145–10153 CrossRef CAS PubMed.
- G. Fang, C. Zhu, D. D. Dionysiou, J. Gao and D. Zhou, Bioresour. Technol., 2015, 176, 210–217 CrossRef CAS.
- G. Ren, K. Zhao and L. Zhao, RSC Adv., 2020, 10, 39973–39980 RSC.
- E. M. Abd El-Monaem, N. Al Harby, M. E. Batouti and A. S. Eltaweil, Nanomaterials, 2023, 14, 54 CrossRef.
- X. Liao, F. Wang, F. Wang, Y. Cai, Y. Yao, B.-T. Teng, Q. Hao and L. Shuxiang, Appl. Catal., B, 2019, 259, 118064 CrossRef CAS.
- F. Sang, Z. Yin, W. Wang, E. Almatrafi, Y. Wang, B. Zhao, J. Gong, C. Zhou, C. Zhang and G. Zeng, J. Cleaner Prod., 2022, 378, 134459 CrossRef CAS.
- J.-H. Park, J. J. Wang, R. Xiao, N. Tafti, R. D. DeLaune and D.-C. Seo, Bioresour. Technol., 2018, 249, 368–376 CrossRef CAS PubMed.
- S. Zhao, Y. Li, M. Wang, B. Chen, Y. Zhang, Y. Sun, K. Chen, Q. Du, Z. Jing and Y. Jin, Microporous Mesoporous Mater., 2022, 345, 112241 CrossRef CAS.
- A. M. Omer, M. El-Sayed, E. M. Abd El-Monaem, G. M. El-Subruiti and A. S. Eltaweil, Int. J. Biol. Macromol., 2023, 253, 127437 CrossRef CAS.
- A. S. Eltaweil, S. S. Bakr, E. M. Abd El-Monaem and G. M. El-Subruiti, Environ. Sci. Pollut. Res., 2023, 30, 75332–75348 CrossRef CAS.
- E. M. Abd El-Monaem, M. Hosny and A. S. Eltaweil, Chem. Eng. Sci., 2024, 287, 119707 CrossRef CAS.
- B. S. Marques, K. Dalmagro, K. S. Moreira, M. L. Oliveira, S. L. Jahn, T. A. De Lima Burgo and G. L. Dotto, 2020.
- H. Ma, Z. Xu, W. Wang, X. Gao and H. Ma, RSC Adv., 2019, 9, 39282–39293 RSC.
- J. Chen, X. Sun, L. Lin, X. Dong and Y. He, Chin. J. Chem. Eng., 2017, 25, 775–781 CrossRef CAS.
- A. Jawad, J. Lang, Z. Liao, A. Khan, J. Ifthikar, Z. Lv, S. Long, Z. Chen and Z. Chen, Chem. Eng. J., 2018, 335, 548–559 CrossRef CAS.
- X. Li, K. Cui, Z. Guo, T. Yang, Y. Cao, Y. Xiang, H. Chen and M. Xi, Chem. Eng. J., 2020, 379, 122324 CrossRef CAS.
- A. S. Eltaweil, N. Al Harby, A. I. Osman, M. Alrasheedi, Y. Su and E. M. Abd El-Monaem, J. Ind. Eng. Chem., 2025, 143, 704–716 CrossRef CAS.
- N. A. Youssef, S. A. Shaban, F. A. Ibrahim and A. S. Mahmoud, Egypt. J. Pet., 2016, 25, 317–321 CrossRef.
- T. Geng, J. Yan, B. Li, H. Yan, L. Guo, Q. Sun, Z. Guan, C. Zhao, J. Xu and W. Wang, Results Chem., 2024, 11, 101795 CrossRef CAS.
- J. Singh, J. Yang, Y. Chang and J. Koduru, Chem. Eng. J., 2017, 307, 74–84 CrossRef.
- G. Rózsa, M. Náfrádi, T. Alapi, K. Schrantz, L. Szabó, L. Wojnárovits, E. Takács and A. Tungler, Appl. Catal., B, 2019, 250, 429–439 CrossRef.
- Y. Wu, X. Li, H. Zhao, F. Yao, J. Cao, Z. Chen, F. Ma, D. Wang and Q. Yang, Chemosphere, 2022, 287, 132061 CrossRef CAS PubMed.
- X. Li, H. Zhou, R. Qian, X. Zhang and L. Yu, Chin. Chem. Lett., 2025, 36, 110036 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02562a |
| This journal is © The Royal Society of Chemistry 2025 |