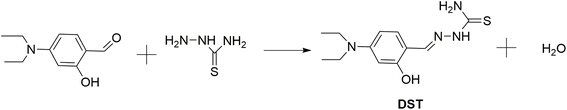Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A mercury complex-based fluorescent sensor for biological thiols†
Nguyen Khoa Hien a,
Trinh Thi Giao Chaua,
Nguyen Dinh Luyenb,
Quan V. Vo
a,
Trinh Thi Giao Chaua,
Nguyen Dinh Luyenb,
Quan V. Vo c,
Mai Van Bayd,
Son Tung Ngoef,
Pham Cam Nam
c,
Mai Van Bayd,
Son Tung Ngoef,
Pham Cam Nam *g and
Duong Tuan Quang*b
*g and
Duong Tuan Quang*b
aMientrung Institute for Scientific Research, Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, Hue 49000, Vietnam
bDepartment of Chemistry, Hue University, Hue 49000, Vietnam. E-mail: duongtuanquang@dhsphue.edu.vn
cFaculty of Chemical Technology-Environment, The University of Danang - University of Technology and Education, 48 Cao Thang, Danang 50000, Vietnam
dThe University of Danang, University of Science and Education, Danang 50000, Vietnam
eLaboratory of Biophysics, Institute for Advanced Study in Technology, Ton Duc Thang University, Ho Chi Minh City 72915, Vietnam
fFaculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City 72915, Vietnam
gThe University of Danang, University of Science and Technology, Danang 50000, Vietnam. E-mail: pcnam@dut.udn.vn
First published on 13th June 2025
Abstract
A novel fluorescent sensor, Hg(DST)2, was developed for the selective detection of biological thiols, including glutathione (GSH), cysteine (Cys), and homocysteine (Hcy), in fully aqueous solutions at pH 7.2. The sensor exhibited significant fluorescence quenching upon coordination with Hg2+, which was reversibly restored in the presence of thiols due to the formation of thermodynamically favored Hg-thiol complexes. The OFF–ON fluorescence mechanism of the sensor was elucidated using DFT calculations. Fluorescence titration experiments revealed a strong linear correlation (R2 ≈ 0.998) between fluorescence intensity and thiol concentrations within the ranges of 0.34–8.00 μM for GSH, 0.47–10.00 μM for Cys, and 0.26–8.00 μM for Hcy, with corresponding limits of detection (LOD) of 0.34, 0.47, and 0.26 μM, respectively. The sensor demonstrated high selectivity toward thiols in the presence of common amino acids, metal ions, and anions, with interference from Ag+, Cu2+, Co2+, and Ni2+ mitigated using 1,10-phenanthroline (PHEN). Owing to its high sensitivity, selectivity, and water solubility, Hg(DST)2 represents a promising tool for thiol quantification in biological and environmental matrices.
1. Introduction
Cysteine (Cys), glutathione (GSH), and homocysteine (Hcy) are non-protein biological thiols that play crucial roles in various physiological and biochemical functions in humans.1–3 In particular, Cys is a precursor for protein synthesis and plays a vital role in protecting cells from damage caused by free radicals and oxidative agents. Additionally, Cys is involved in detoxification processes by binding to heavy metals and other toxic substances, aiding in their removal from the body.4 The intracellular concentration of Cys ranges from 30 to 200 μM.5 The highest concentration of Cys in plasma has been found to reach up to 250 μM.1 Changes in Cys levels have been shown to be associated with several diseases such as Alzheimer's and Parkinson's disease, autoimmune deficiency syndrome, and hyperhomocysteinemia.1Glutathione is a tripeptide made up of three amino acids: L-glutamate, L-cysteine, and L-glycine. It is the most abundant non-protein thiol in cells, with intracellular concentrations ranging from 1 to 15 mM, primarily in its reduced form.6 GSH is one of the body's most powerful antioxidants, protecting cells from oxidative damage by neutralizing free radicals.6,7 It plays a critical role in detoxification, particularly in the liver, where it helps eliminate harmful substances and convert them into less toxic forms.8 Additionally, GSH is essential for maintaining and regulating the immune system.6 It is also believed to be associated with diseases, including cancer, stroke, heart disease, pancreatic and kidney disorders, diabetes, Alzheimer's, Parkinson's, gastritis, peptic ulcers, and atherosclerosis diseases.6,7
Homocysteine (Hcy) is a metabolic intermediate formed during the catabolism of methionine in the one-carbon metabolism cycle. It participates in methylation processes, methionine regeneration, and cysteine biosynthesis, with its homeostasis maintained through pathways that either regenerate methionine or convert it to cysteine.9 Typical blood Hcy levels range from 5 to 13 μM.10 Elevated Hcy levels, a condition known as hyperhomocysteinemia, are linked to cardiovascular, neurological, and bone-related disorders, as well as fertility issues, including recurrent miscarriages and infertility in both genders.11–14
Given the important roles of Cys, GSH, and Hcy, various methods for their determination have been developed, including high-performance liquid chromatography,1,15 gas chromatography-mass spectrometry,16 electrochemistry,17 and UV-Vis absorption and fluorescence spectroscopy.18–20 Among these, fluorescent sensors have garnered significant interest from scientists due to their high sensitivity, simple analysis methods, and ability to monitor in living cells.21–24
Some recently reported fluorescent sensors for biothiol detection based on metal ion complexes are summarized in Table 1. Most reported fluorescent sensors do not selectively detect individual biothiols because Cys, GSH, and Hcy have similar structures.25 Nevertheless, this limitation does not diminish the efforts to develop new fluorescent sensors for biothiols. This is because, in practice, there are cases where only the concentration change of a specific biothiol related to a particular disease is of interest, such as monitoring glutathione levels in the liver to assess liver function or monitoring homocysteine levels in the blood to evaluate the risk of cardiovascular diseases and stroke.26–28 Additionally, different biothiols are distributed differently in various body parts. For example, the concentrations of cysteine and glutathione are highest in tissues, particularly in the liver, while homocysteine is usually undetectable in tissues and is primarily found in plasma.29–31 Notably, fluorescent sensors often interact reversibly with biothiols, which is very useful for monitoring real-time changes in biothiol concentrations, and providing detailed information about pathological conditions.10
| Complexes of metal ions | Detectable biothiols | LOD (μM) | Solvent/pH | The influence of other thiols | Ref. |
|---|---|---|---|---|---|
| Cu2+ | GSH | 0.16 | HEPES/DMSO (95/5, v/v), pH 7.4 | Affected by Cys and Hcy | 32 |
| Cu2+ | GSH | 0.80 | DMF/HEPES (3/7, v/v), pH 7.4 | Cannot detect individual thiols separately | 33 |
| Cys | 1.00 | ||||
| Hcy | 1.50 | ||||
| Cu2+ | GSH | 0.30 | Ethanol/HEPES (1/1, v/v), pH 7.4 | Cannot detect individual thiols separately | 19 |
| Cys | |||||
| Hcy | |||||
| Cu2+ | Cys | 0.17 | CH3CN/HEPES (1/1, v/v), pH 7.4 | - Cannot detect individual thiols separately | 34 |
| Hcy | 0.25 | - The influence of GSH has not been reported | |||
| Cu2+ | GSH | 0.20 | CH3OH/HEPES (9/1, v/v), pH 7.2 | The influence of Cys and Hcy has not been reported | 35 |
| Cu2+ | GSH | 0.44 | DMF/HEPES (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), pH 7.4 3, v/v), pH 7.4 |
Cannot detect individual thiols separately | 36 |
| Cys | 0.96 | ||||
| Hcy | 0.68 | ||||
| Cu2+ | Cys | 0.015 | CH3CN/HEPES (7/3, v/v), pH 7.4 | The influence of GSH and Hcy has not been reported | 37 |
| Hg2+ | Cys | 0.20 | Ethanol/HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v), pH 7.4 9, v/v), pH 7.4 |
Affected by GSH and Hcy | 20 |
| Hg2+ | Cys | 0.016 | CH3CN | The influence of GSH and Hcy has not been reported | 38 |
| Ag+ | GSH | 0.208 | Dioxane/Tris–HClO4 (3/7, v/v), pH 7.4 | Cannot detect individual thiols separately | 39 |
| Cys | 0.089 | ||||
| Hcy | 0.174 | ||||
| Fe3+ | Cys | 0.45 | Water (1% DMSO) pH: 2–11 | Not affected by GSH and Hcy | 40 |
In this study, we report a fluorescent sensor (Hg(DST)2) that can detect the real-time concentrations of Cys, GSH, and Hcy, based on the complex of a fluorescent compound DST with Hg2+ ions. The Hg(DST)2 complex interacts reversibly with Cys, GSH, and Hcy, releasing DST, accompanied by a fluorescence signal change from OFF to ON at 445 nm. This process can be reversed at least five times by alternating the addition of Hg2+ and biothiols.
2. Materials and methods
2.1. Instruments
The structural characteristics of the compounds were determined through the study of 1H NMR, 13C NMR, and mass spectra, using a Bruker Ascend 600 spectrometer for NMR and an Agilent 6200 series TOF LC/MS system for mass spectrometry. The absorption and fluorescence properties of the compounds were studied using Shimadzu spectrometers, with the UV-1800 for UV-Vis absorption spectra and the RF-5301 PC Series for fluorescence spectra.2.2. Reagents
All chemicals used were purchased from Merck. Among them, 4-(diethylamino)salicylaldehyde and aminothiourea were of synthesis grade. The solvents used were of HPLC grade. The metal salts, amino acids, and biothiols (Cys, GSH, and Hcy) were of analytical purity grade.2.3. Computational methodology
Density Functional Theory (DFT) calculations were performed using the Gaussian 16 software at the PBE0/Lanl2dz level of theory.41–45 Time-Dependent Density Functional Theory (TD-DFT) method was employed to investigate the excited states.45,46 Solvent calculations were carried out using the Polarizable Continuum Model (PCM).473. Results and Discussion
3.1. Synthesis and characterization of the Hg(DST)2 sensor
The DST fluorescent compound was synthesized according to the reaction shown in Fig. 1, as previously reported.48 Briefly, equimolar solutions of aminothiourea and 4-(diethylamino)salicylaldehyde were refluxed in ethanol with anhydrous sodium sulfate at 85 °C under a nitrogen atmosphere for 8 hours, followed by stirring at ambient temperature for 12 hours. The reaction mixture was filtered to remove sodium sulfate, purified via silica gel column chromatography using a chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (70
methanol (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) eluent, and the solvent was evaporated under reduced pressure, yielding DST with an approximate efficiency of 71.4% (13C NMR, 1H NMR, and MS spectra are provided in Fig. S1–S3 of the ESI data†).
1, v/v) eluent, and the solvent was evaporated under reduced pressure, yielding DST with an approximate efficiency of 71.4% (13C NMR, 1H NMR, and MS spectra are provided in Fig. S1–S3 of the ESI data†).
Experimental results in Fig. 2 showed that DST exhibited a fluorescence spectrum with a maximum wavelength at 445 nm, with an excitation wavelength at 368 nm. DST reacted with Hg2+ in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, accompanied by approximately 95% fluorescence quenching. The Stern–Volmer plot for the fluorescence quenching of DST by Hg2+ (Fig. S4†) showed that the relationship between the ratio F0/F and the concentration Q of Hg2+ (where F0 and F were the fluorescence intensities of the DST solution in the absence and presence of Hg2+ at concentration Q, respectively) was not linear but exhibited an upward (positive) deviation. This suggested that both static and dynamic quenching mechanisms were responsible for the observed decrease in fluorescence intensity.49,50
1 molar ratio, accompanied by approximately 95% fluorescence quenching. The Stern–Volmer plot for the fluorescence quenching of DST by Hg2+ (Fig. S4†) showed that the relationship between the ratio F0/F and the concentration Q of Hg2+ (where F0 and F were the fluorescence intensities of the DST solution in the absence and presence of Hg2+ at concentration Q, respectively) was not linear but exhibited an upward (positive) deviation. This suggested that both static and dynamic quenching mechanisms were responsible for the observed decrease in fluorescence intensity.49,50
The stable structure of the complex between DST and Hg2+ in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
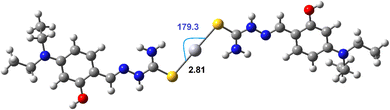
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was determined at the DFT/PBE0/Lanl2dz and is presented in Fig. 3. The calculation results show that the Hg(DST)2 complex is stabilized by two Hg⋯S interactions, with a contact distance of 2.81 Å, which is significantly smaller than the van der Waals radius sum of Hg and S atoms, which is 3.35 Å. The Hg⋯S⋯Hg bond angle is nearly linear, with a value of 179.3°.51 The two DST molecules in the complex are not in the same plane but lie in two parallel planes (considering only the π-conjugated system plane).
1 molar ratio was determined at the DFT/PBE0/Lanl2dz and is presented in Fig. 3. The calculation results show that the Hg(DST)2 complex is stabilized by two Hg⋯S interactions, with a contact distance of 2.81 Å, which is significantly smaller than the van der Waals radius sum of Hg and S atoms, which is 3.35 Å. The Hg⋯S⋯Hg bond angle is nearly linear, with a value of 179.3°.51 The two DST molecules in the complex are not in the same plane but lie in two parallel planes (considering only the π-conjugated system plane).
The results of the study on the singlet electron transition process from the ground state S0 to the excited state S1 (Fig. 4) indicated that in the DST compound, this process was determined by the electron transition from HOMO to LUMO (oscillator strength f = 1.2441). Meanwhile, in the Hg(DST)2 complex, this process was determined by the electron transition from HOMO − 1 to LUMO (f = 0.5515). Therefore, in the excited state, the Hg(DST)2 complex underwent a photoinduced electron transfer (PET) process with the transfer of an electron from HOMO to HOMO − 1, leading to the absence of an excited electron transition from LUMO to HOMO − 1 (which would normally result in energy emission in the form of fluorescence).51–54 Instead, there was an internal conversion process from LUMO to HOMO, resulting in the quenching of fluorescence in the Hg(DST)2 complex.
 | ||
| Fig. 4 Diagram illustrating the singlet electron transition processes in the excited state for DST and Hg(DST)2. | ||
3.2. Application of the Hg(DST)2 sensor for detecting biothiols
| Hg2+ + 2DST = Hg(DST)2 | (1) |
 | (2) |
If the initial concentration of DST is denoted as CL, the initial fluorescence intensity of the DST solution as F0, and the fluorescence intensity of the DST solution at equilibrium as F, then let x = F/F0.The concentration of Hg2+ used is denoted as y = CM. At equilibrium, the concentrations are expressed as follows:
 | (3) |
 | (4) |
 | (5) |
Substituting into eqn (2) yields (6) as follows:
 | (6) |
 | (7) |
 | (8) |
 | (9) |
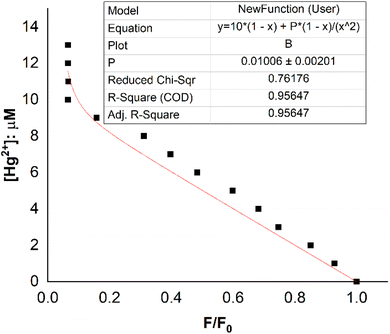
The fluorescence titration results of the DST solution (CL = 20 μM) with Hg2+ (concentrations of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 13 μM) and the nonlinear curve fitted to eqn (8) are presented in Fig. 5. The P value obtained from this process is 0.01006 ± 0.00201. From eqn (9), the calculated stability constant βex is 2.458 μM−1 (or 100.395 μM−1, equivalent to 1012.395 M−1).
3.3. Application of the Hg(DST)2 sensor for the detection of biothiols
The potential application of the Hg(DST)2 complex as a fluorescence sensor for detecting GSH, Cys, and Hcy is based on the complex exchange reaction, which depends on the stability constants of the complexes between Hg2+ and the ligands GSH, Cys, Hcy, and DST. For GSH (written as the ligand H3L), the complexes with Hg2+ are detected as ML, MLH, MLH2, ML2, ML2H, ML2H2, and ML2H3, with corresponding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βex values of 26.04, 32.49, 35.68, 41.58, 42.40, 52.29, and 55.28, respectively.56,57 For Cys (written as the ligand H2L′), the complexes with Hg2+ are detected as
βex values of 26.04, 32.49, 35.68, 41.58, 42.40, 52.29, and 55.28, respectively.56,57 For Cys (written as the ligand H2L′), the complexes with Hg2+ are detected as 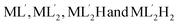 , with corresponding log
, with corresponding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βex values of 14.21, 43.57, 54.37, and 61.79, respectively.56,58–60 For Hcy (written as the ligand H2L′′), there are still very few published studies on the composition and stability constants of its complexes with Hg2+. One study has identified the existence of the
βex values of 14.21, 43.57, 54.37, and 61.79, respectively.56,58–60 For Hcy (written as the ligand H2L′′), there are still very few published studies on the composition and stability constants of its complexes with Hg2+. One study has identified the existence of the 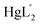 complex, with a stability constant log
complex, with a stability constant log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βex determined to be 39.4.61 In general, previous studies have shown that Hg2+ complexes with GSH, Cys, and Hcy exist in various forms, depending on the environment and their concentration ratios. At neutral pH, the predominant species are
βex determined to be 39.4.61 In general, previous studies have shown that Hg2+ complexes with GSH, Cys, and Hcy exist in various forms, depending on the environment and their concentration ratios. At neutral pH, the predominant species are  . Specifically, for GSH, even at a GSH
. Specifically, for GSH, even at a GSH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ ratio of up to 22
Hg2+ ratio of up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the ML2 form still accounts for 95%. For Cys, the proportions of other complexes increase, but
1, the ML2 form still accounts for 95%. For Cys, the proportions of other complexes increase, but  remains the dominant species.61
remains the dominant species.61
According to the calculations above, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βexof the Hg(DST)2 complex is 12.395, much smaller than the log
βexof the Hg(DST)2 complex is 12.395, much smaller than the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βex of the
βex of the  complexes, which are 41.58, 43.57, and 39.4, respectively. Therefore, theoretically, GSH, Cys, and Hcy can react with Hg(DST)2 to form corresponding complexes and release free DST, altering the fluorescence intensity of the solution. In other words, Hg(DST)2 can be used as a fluorescence sensor to detect GSH, Cys, and Hcy. One noteworthy point is that since the log
complexes, which are 41.58, 43.57, and 39.4, respectively. Therefore, theoretically, GSH, Cys, and Hcy can react with Hg(DST)2 to form corresponding complexes and release free DST, altering the fluorescence intensity of the solution. In other words, Hg(DST)2 can be used as a fluorescence sensor to detect GSH, Cys, and Hcy. One noteworthy point is that since the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βex values of the
βex values of the  complexes are approximately similar, it is unlikely that Hg(DST)2 can selectively detect each thiol in the GSH, Cys, and Hcy group.
complexes are approximately similar, it is unlikely that Hg(DST)2 can selectively detect each thiol in the GSH, Cys, and Hcy group.
Indeed, the experimental results on the fluorescence titration of the Hg(DST)2 sensor solution by the thiols GSH, Cys, and Hcy, as presented in Fig. 6, are entirely consistent with the aforementioned observations. All three thiols – GSH, Cys, and Hcy – react with Hg(DST)2, releasing DST and leading to an increase in the fluorescence intensity of the solution (The images showing the fluorescence color changes of the Hg(DST)2 solution in the presence of biothiols, taken inside the fluorescence spectrophotometer chamber, are presented in Fig. S5†). When the concentration of the thiols approaches approximately twice the concentration of Hg(DST)2 used, as well as beyond this point, the fluorescence intensity of the solution remains nearly unchanged. This indicates that the complexes formed between Hg2+ and GSH, Cys, and Hcy are predominantly in the  forms, as previously noted.
forms, as previously noted.
The survey results indicate that the Hg(DST)2 complex sensor can quantitatively detect GSH, Cys, and Hcy, as evidenced by the excellent linear relationship between the fluorescence intensity of the solution and the concentrations of GSH, Cys, and Hcy within a certain range. Accordingly, the linear ranges for determining GSH, Cys, and Hcy using the Hg(DST)2 sensor are 0.34–8.00 μM, 0.47–10.00 μM, and 0.26–8.0 μM, respectively, with corresponding linear correlation coefficients (R) of approximately 0.998. Among these, the values of 0.34, 0.47, and 0.26 μM represent the detection limits of GSH, Cys, and Hcy, respectively, using the Hg(DST)2 sensor, as determined from the calibration curve equation at low concentrations (see Fig. S6 and Table. S1 of the ESI†).62 Compared to some fluorescent sensors for thiol detection based on metal ion complexes reported in recent times (Table 1), the Hg(DST)2 sensor had a comparable LOD for thiols but had the advantage of operating in a fully aqueous environment.
The influence of amino acids on the use of the Hg(DST)2 sensor for thiol detection was also investigated and presented in Fig. 7. The results showed that, except for Lys (lysine), which altered the fluorescence spectrum of the Hg(DST)2 sensor solution, the other amino acids, including Ala (alanine), Arg (arginine), Asp (aspartic acid), Glu (glutamic acid), Gly (glycine), His (histidine), Ile (isoleucine), Leu (leucine), Met (methionine), Ser (serine), Thr (threonine), Trp (tryptophan), Tyr (tyrosine), and Val (valine), had little to no effect on the fluorescence spectrum of the Hg(DST)2 sensor solution. These results indicated that the Hg(DST)2 sensor could detect the thiols GSH, Cys, and Hcy in the presence of the above amino acids, except for Lys. Additionally, the investigation results showed that the Hg(DST)2 sensor was unable to detect individual thiols within the GSH, Cys, and Hcy groups separately. This finding was consistent with previous reports on fluorescent sensors for thiol detection based on metal ion complexes. However, this limitation did not diminish the applicability of such sensors, as thiols typically do not coexist in equal concentrations in real samples. For instance, in human whole blood samples, GSH is significantly higher than other thiols (sometimes up to 1 mM), whereas, in human plasma samples, Cys is much more abundant than other thiols (sometimes reaching up to 250 μM).63–65
The use of the Hg(DST)2 complex fluorescent sensor for detecting the thiols GSH, Cys, and Hcy was also investigated for its susceptibility to interference by various ions, including alkali metal ions (Na+, K+), alkaline earth metal ions (Ca2+, Ba2+, Mg2+), transition metal ions (Ag+, Cu2+, Co2+, Ni2+, Mn2+, Fe2+, Fe3+, Cr3+), other metal ions (Zn2+, Pb2+, Cd2+, Al3+), as well as common anions such as sulfate (SO42−), carbonate (CO32−), chloride (Cl−), bromide (Br−), iodide (I−), and cyanide (CN−). The results, presented in Fig. 8, S7, and S8† (in the ESI†), demonstrated that, except for Ag+, Cu2+, Co2+, and Ni2+, the presence of the other ions did not affect the thiol detection method using the Hg(DST)2 sensor, as evidenced by the absence of significant fluorescence intensity changes in (Hg(DST)2 + thiol) solutions. The interference caused by Cu2+, Co2+, and Ni2+ could be eliminated by the complexing agent 1,10-phenanthroline (PHEN). Meanwhile, a suitable complexing agent to mitigate the interference of Ag+ has not yet been identified. However, since Ag+ is not a biological metal, its interference in thiol detection in biological samples is not a major concern.
The reaction time between the Hg(DST)2 complex and the biothiols GSH, Cys, and Hcy was almost instantaneous. After 1 minute, the fluorescence intensity of the solution had nearly stabilized. The sensing mechanism is summarized in Scheme 1.
4. Conclusions
This study presents the successful development and validation of the Hg(DST)2 fluorescent sensor for the detection of thiols (GSH, Cys, and Hcy) in aqueous media, achieving detection limits of 0.34, 0.47, and 0.26 μM, respectively. The sensor exhibited robust selectivity against most common amino acids, metal ions, and anions, with interference from Cu2+, Co2+, and Ni2+ effectively neutralized using 1,10-phenanthroline (PHEN), while Ag+ interference remains negligible in biological contexts due to its non-physiological relevance. Compared to existing metal-complex-based fluorescent sensors, Hg(DST)2 offers comparable sensitivity and the distinct advantage of operating in a fully aqueous environment. These attributes position Hg(DST)2 as a highly effective and practical tool for real-time thiol monitoring in biomedical diagnostics (e.g., oxidative stress assessment) and environmental analysis.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.06-2021.59 (Nguyen Khoa Hien).References
- G. Chwatko and E. Bald, Talanta, 2000, 52, 509–515 CrossRef CAS PubMed.
- C. X. Yin, K. M. Xiong, F. J. Huo, J. C. Salamanca and R. M. Strongin, Angew. Chem., Int. Ed., 2017, 56, 13188–13198 CrossRef CAS PubMed.
- D. Chen and Y. Feng, Crit. Rev. Anal. Chem., 2022, 52, 649–666 CrossRef CAS PubMed.
- S. M. Nabavi and A. S. Silva, Nonvitamin and Nonmineral Nutritional Supplements, Academic Press, 2018 Search PubMed.
- Y. Yue, F. Huo, P. Ning, Y. Zhang, J. Chao, X. Meng and C. Yin, J. Am. Chem. Soc., 2017, 139, 3181–3185 CrossRef CAS PubMed.
- D. Matuz-Mares, H. Riveros-Rosas, M. M. Vilchis-Landeros and H. Vázquez-Meza, Antioxidants, 2021, 10, 1220 CrossRef CAS PubMed.
- R. Vona, L. Pallotta, M. Cappelletti, C. Severi and P. Matarrese, Antioxidants, 2021, 10, 201 CrossRef CAS PubMed.
- J. Pizzorno, Integr. Med., 2014, 13, 8 Search PubMed.
- J. Selhub, Annu. Rev. Nutr., 1999, 19, 217–246 CrossRef CAS PubMed.
- Y. Li, L. Chen, Y. Zhu, L. Chen, X. Yu, J. Li and D. Chen, RSC Adv., 2021, 11, 21116–21126 RSC.
- I. M. Graham, L. E. Daly, H. M. Refsum, K. Robinson, L. E. Brattström, P. M. Ueland, R. J. Palma-Reis, G. H. Boers, R. G. Sheahan and B. Israelsson, JAMA, 1997, 277, 1775–1781 CrossRef CAS PubMed.
- D. S. Wald, M. Law and J. K. Morris, BMJ, 2002, 325, 1202 CrossRef PubMed.
- K. S. McCully, Am. J. Pathol., 1969, 56, 111 CAS.
- A. D. Smith, S. M. Smith, C. A. De Jager, P. Whitbread, C. Johnston, G. Agacinski, A. Oulhaj, K. M. Bradley, R. Jacoby and H. Refsum, PLoS One, 2010, 5, e12244 CrossRef PubMed.
- M. Isokawa, T. Funatsu and M. Tsunoda, Analyst, 2013, 138, 3802–3808 RSC.
- C.-J. Tsai, F.-Y. Liao, J.-R. Weng and C.-H. Feng, J. Chromatogr. A, 2017, 1524, 29–36 CrossRef CAS PubMed.
- I. M. Mostafa, H. Liu, S. Hanif, M. R. H. S. Gilani, Y. Guan and G. Xu, Anal. Chem., 2022, 94, 6853–6859 CrossRef CAS PubMed.
- P. Li, S. M. Lee, H. Y. Kim, S. Kim, S. Park, K. S. Park and H. G. Park, Sci. Rep., 2021, 11, 3937 CrossRef CAS PubMed.
- N. K. Hien, M. Van Bay, P. D. Tran, N. T. Khanh, N. D. Luyen, Q. V. Vo, D. U. Van, P. C. Nam and D. T. Quang, RSC Adv., 2020, 10, 36265–36274 RSC.
- D. T. Nhan, N. K. Hien, H. Van Duc, N. T. A. Nhung, N. T. Trung, D. U. Van, W. S. Shin, J. S. Kim and D. T. Quang, Dyes Pigments, 2016, 131, 301–306 CrossRef.
- H. J. Park, C. W. Song, S. Sarkar, Y. W. Jun, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Commun., 2020, 56, 7025–7028 RSC.
- L. Chen, Y. Feng, Y. Dang, C. Zhong and D. Chen, Anal. Bioanal. Chem., 2020, 412, 7819–7826 CrossRef CAS PubMed.
- D. Chen, Z. Long, Y. Sun, Z. Luo and X. Lou, J. Photochem. Photobiol., A, 2019, 368, 90–96 CrossRef CAS.
- K. Dou, W. Huang, Y. Xiang, S. Li and Z. Liu, Anal. Chem., 2020, 92, 4177–4181 CrossRef CAS PubMed.
- L. Chen, L. Sun, L. Ye and Y. Wang, Opt. Mater., 2020, 106, 109961 CrossRef CAS.
- L. Flohé, Glutathione, CRC Press, 2018 Search PubMed.
- L. Yuan and N. Kaplowitz, Mol. Aspects Med., 2009, 30, 29–41 CrossRef CAS PubMed.
- P. Ganguly and S. F. Alam, Nutr. J., 2015, 14, 1–10 CrossRef PubMed.
- L. Zhou, J. Liu, Y. An, Y. Wang and G. Wang, Front. Cardiovasc. Med., 2022, 9, 898305 CrossRef CAS PubMed.
- B. Sameem, F. Khan and K. Niaz, in Nonvitamin and Nonmineral Nutritional Supplements, Elsevier, 2019, pp. 53–58 Search PubMed.
- M. Vairetti, L. G. Di Pasqua, M. Cagna, P. Richelmi, A. Ferrigno and C. Berardo, Antioxidants, 2021, 10, 364 CrossRef CAS PubMed.
- Y. Hu, C. H. Heo, G. Kim, E. J. Jun, J. Yin, H. M. Kim and J. Yoon, Anal. Chem., 2015, 87, 3308–3313 CrossRef CAS PubMed.
- H. Jia, M. Yang, Q. Meng, G. He, Y. Wang, Z. Hu, R. Zhang and Z. Zhang, Sensors, 2016, 16, 79 CrossRef CAS PubMed.
- Q. Li, Y. Guo and S. Shao, Sens. Actuators, B, 2012, 171, 872–877 CrossRef.
- S. Li, D. Cao, X. Meng, Z. Hu, Z. Li, C. Yuan, T. Zhou, X. Han and W. Ma, Bioorg. Chem., 2020, 100, 103923 CrossRef CAS PubMed.
- Y. Wang, H. Feng, H. Li, X. Yang, H. Jia, W. Kang, Q. Meng, Z. Zhang and R. Zhang, Sensors, 2020, 20, 1331 CrossRef CAS PubMed.
- Y. Wang, Q. Meng, Q. Han, G. He, Y. Hu, H. Feng, H. Jia, R. Zhang and Z. Zhang, New J. Chem., 2018, 42, 15839–15846 RSC.
- N. Kaur, P. Kaur and K. Singh, RSC Adv., 2014, 4, 29340–29343 RSC.
- C. Zhao, X. Kong, S. Shuang, Y. Wang and C. Dong, Analyst, 2020, 145, 3029–3037 RSC.
- Y. Hu, L. Lu, S. Guo, X. Wu, J. Zhang, C. Zhou, H. Fu and Y. She, Sens. Actuators, B, 2023, 382, 133534 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision A. 03. 2016, Gaussian inc, Wallingford CT, 2016 Search PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- A. D. Laurent and D. Jacquemin, Int. J. Quantum Chem., 2013, 113, 2019–2039 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- D. Jacquemin, I. Duchemin and X. Blase, J. Chem. Theory Comput., 2015, 11, 5340–5359 CrossRef CAS PubMed.
- C. Adamo and D. Jacquemin, Chem. Soc. Rev., 2013, 42, 845–856 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- N. K. Hien, M. Van Bay, Q. V. Vo, D. T. Quang and P. C. Nam, J. Fluids, 2024, 1–13 Search PubMed.
- K.-W. Ding, T.-Q. Li, Z.-X. Ge, J.-H. Bu and Y. Liu, RSC Adv., 2018, 8, 35759–35767 RSC.
- K. Fenner, G. Reynolds and S. Basu, Spectrochim. Acta, Part A, 2020, 239, 118473 CrossRef CAS PubMed.
- N. K. Hien, N. C. Bao, N. T. A. Nhung, N. T. Trung, P. C. Nam, T. Duong, J. S. Kim and D. T. Quang, Dyes Pigments, 2015, 116, 89–96 CrossRef CAS.
- M. V. Bay, N. K. Hien, S. Son, N. D. Trinh, N. T. Trung, P. C. Nam, J. S. Kim and D. T. Quang, Sensors, 2019, 19, 128 CrossRef PubMed.
- N. K. Hien, D. T. Nhan, W. Y. Kim, M. Van Bay, P. C. Nam, D. U. Van, I.-T. Lim, J. S. Kim and D. T. Quang, Dyes Pigments, 2018, 152, 118–126 CrossRef.
- D. T. Nhan, N. T. A. Nhung, V. Vien, N. T. Trung, N. D. Cuong, N. C. Bao, D. Q. Huong, N. K. Hien and D. T. Quang, Chem. Lett., 2017, 46, 135–138 CrossRef CAS.
- J.-S. Wu, F. Wang, W.-M. Liu, P.-F. Wang, S.-K. Wu, X. Wu and X.-H. Zhang, Sens. Actuators, B, 2007, 125, 447–452 CrossRef CAS.
- W. Stricks and I. Kolthoff, J. Am. Chem. Soc., 1953, 75, 5673–5681 CrossRef CAS.
- P. D. Oram, X. Fang, Q. Fernando, P. Letkeman and D. Letkeman, Chem. Res. Toxicol., 1996, 9, 709–712 Search PubMed.
- G. Lenz and A. Martell, Biochemistry, 1964, 3, 745–750 CrossRef CAS PubMed.
- D. Perkins, Biochem. J., 1953, 55, 649 CrossRef CAS PubMed.
- K. Dubey and M. Qazi, Proc. Natl. Acad. Sci., India, 1983, 53, 342–346 CAS.
- V. Liem-Nguyen, U. Skyllberg, K. Nam and E. Björn, Environ. Chem., 2017, 14, 243–253 CrossRef CAS.
- Y. Liu, T. B. Ren, D. Cheng, J. Hou, D. Su and L. Yuan, ChemistryOpen, 2019, 8, 1251–1257 CrossRef CAS PubMed.
- X. Fu, S. A. Cate, M. Dominguez, W. Osborn, T. Özpolat, B. A. Konkle, J. Chen and J. A. López, Sci. Rep., 2019, 9, 115 CrossRef PubMed.
- P. Hallman, D. Perrin and A. E. Watt, Biochem. J., 1971, 121, 549–555 CrossRef CAS PubMed.
- P. M. Ueland, H. Refsum, S. P. Stabler, M. R. Malinow, A. Andersson and R. H. Allen, Clin. Chem., 1993, 39, 1764–1779 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02268a |
| This journal is © The Royal Society of Chemistry 2025 |