Open Access Article
Open Access ArticleElectron donor–acceptor complex-driven photocatalyst-free synthesis of nitrocyclopropanes†
Paula Pérez-Ramos,
Patrícia I. C. Godinho,
Raquel G. Soengas * and
Humberto Rodríguez-Solla
* and
Humberto Rodríguez-Solla *
*
Department of Organic and Inorganic Chemistry, Instituto Universitario de Química Organometálica Enrique Moles, University of Oviedo, Julián Clavería 8, 33006, Oviedo, Spain. E-mail: rsoengas@uniovi.es; hrsolla@uniovi.es
First published on 8th May 2025
Abstract
Herein, a visible light-promoted metal-free protocol for the synthesis of nitrocyclopropanes under mild conditions is reported. Specifically, the process is driven by the photochemical activity of ternary EDA complexes formed upon complexation of α-bromonitrostyrenes and DIPEA in the presence of benzaldehyde. This reaction provides a variety of densely functionalized cyclopropanes with good selectivity under mild reaction conditions. Mechanistic investigations on the aspects of the process also demonstrate formation of the hypothesized EDA complex.
Introduction
Cyclopropanes, with their high strain energy and unique structure, have been fascinating organic chemists for decades. The cyclopropane motif is present in a broad range of natural products and bioactive compounds1 and has widespread application in modern drug discovery owing to its ability to influence the physico-chemical and pharmacological properties of small molecules.2 Moreover, owing to their strained ring, cyclopropanes possess higher reactivity than other alkanes, making them important precursors or key intermediates in synthetic chemistry.3 Therefore, the preparation of cyclopropanes has attracted much attention over the past decades.4Traditional strategies for the construction of the cyclopropane ring involve the reaction of alkenes with carbenoids. These carbenoids are usually generated from organozinc, samarium or chromium reagents and halomethanes5 or by the transition-metal-catalyzed decomposition of diazo compounds.6 Another common cyclopropanation strategy relies on the Michael-initiated ring closure (MIRC) reaction of electron-deficient alkenes.7 A relevant example is the Corey–Chaykovsky reaction, initiated by the reaction of sulfur ylides with enones.8
As with every other field in organic synthesis, the chemistry of cyclopropanes is evolving, with a greater concern on the environmental impact caused by the hazardous chemical wastes generated by industries and laboratories.9 In this regard, visible light-mediated cyclopropanation recently emerged as a promising alternative strategy to build cyclopropane backbones in a more sustainable fashion.10 The vast majority of these cyclopropanation strategies typically involve the generation of carbenes from diazo precursors and their reaction with olefins.11 As an alternative strategy, Suero's group developed an alkene cyclopropanation reaction of olefins based on the photocatalytic generation of radical carbenoid species.12 Using this concept, several cyclopropane derivatives were prepared from diverse Michael acceptors via photocatalytic cascade radical carbenoid addition/cyclopropanation reactions (Scheme 1a).13
Although the direct intermolecular construction of cyclopropanes via photocatalysis was a remarkable development, the synthesis of nitrocyclopropanes remained unattainable, as nitroolefins were not suitable Michael acceptors for carbenoid radicals.13a In fact, to the best of our knowledge, the photocatalytic synthesis of nitrocyclopropanes is still unknown and has never been the focus of systematic investigation. Nitrocyclopropanes are a unique class of cycloalkanes that combine the conformational rigidity of the cyclopropane ring and the reactivity associated with the presence of a nitro group.14 Therefore, there have been significant efforts toward the synthesis of nitro-functionalized cyclopropanes.15
Inspired by the work of Suero's group on the photocatalytic cyclopropanation of Michael acceptors, we herein report a photocatalyst-free strategy for the synthesis of nitrocyclopropanes from highly versatile α-bromonitrostyrenes,16 in which the initiating radical is generated within the Michael acceptor via the formation of a ternary electron donor–acceptor (EDA) complex between α-bromonitrostyrenes and DIPEA in the presence of benzaldehyde (Scheme 1b).
In the past few years, EDA complex photochemistry has emerged as a powerful strategy for expanding the potential of visible-light-promoted radical chemistry.17 The strategy differs from catalysis in that no external photocatalyst is added, but rather an advantage is taken of the formation of an electron donor–acceptor (EDA) complex that absorbs visible light from two or more compounds that do not.18 Visible light irradiation then triggers an intramolecular single-electron-transfer (SET) event, able to generate radical intermediates under mild conditions.19 The most common synthetic application of the EDA complex activation strategy involves the light-driven coupling of two substrates, which are also involved in the EDA complex formation, the donor and the acceptor.20 In this regard, the chemical diversity of the reaction products is limited by the required donor and acceptor properties on the two molecular scaffolds that finally end up in the products. A recent strategy developed to overcome this limitation is to introduce sacrificial donor compounds that aggregates with the electron donor and electron acceptor partners and increase productive light absorption without directly participating in the process.21 This approach improved the synthetic versatility of the photochemistry of EDA complexes, since the electronic properties of the reactants can be tuned by the addition of a suitable additive.
Results and discussion
Initially, we evaluated the cyclopropanation of α-bromonitrostyrene 1a as model substrate in the presence of DIPEA and stoichiometric benzaldehyde as a sacrificial electron donor, in 1,4-dioxane as solvent at 20 °C, under irradiation with 465 nm blue LED, and an air atmosphere. We were delighted to find that the reaction led to the expected 2-nitrocyclopropane-1-carbaldehydes 2a/2a′ in good yield and moderate diastereoselection (74%, d. r. 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32). The relative stereochemistry of the obtained products was confirmed by NOE experiments on 2a/2a′ and from the coupling constants of the ring protons.
32). The relative stereochemistry of the obtained products was confirmed by NOE experiments on 2a/2a′ and from the coupling constants of the ring protons.
We evaluated other solvents, donors and visible-light sources and the results are depicted in Table 1. 1,4-Dioxane was the most effective solvent for promoting the reaction, while other commonly used solvents gave less favorable results (entries 2–6). Poorer efficiency was also observed when reactions were irradiated at other blue LED wavelengths (entries 8, 9). Under irradiation with either purple or green LED (entries 7, 10) the reaction failed, as both light sources are unable to activate the EDA complex. We further identified benzaldehyde as the most effective sacrificial donor.
| Entry | Deviation from the standard conditionsa | Yieldb (%) | d.rc |
|---|---|---|---|
| a Standard reaction conditions: α-bromonitrostyrene 1a (0.3 mmol), benzaldehyde (0.3 mmol) and DIPEA (1.5 mmol) in 2 mL of 1,4-dioxane under air, irradiation with a 465 nm lamp (52 W) at 20 °C for 12 hours.b Isolated yield after column chromatography.c Diastereomeric ratio 2a/2a′ determined using 1H NMR (300 MHz) analysis.d Not determined. | |||
| 1 | None | 74 | 68/32 |
| 2 | DMF as solvent | 25 | n.dd |
| 3 | CH3CN as solvent | 34 | 59/41 |
| 4 | CH2Cl2 as solvent | 37 | 52/48 |
| 5 | Toluene as solvent | 42 | 57/43 |
| 6 | DME as solvent | 58 | 61/39 |
| 7 | 390 nm purple LED | 0 | n.d |
| 8 | 440 nm blue LED | 16 | n.d |
| 9 | 456 nm blue LED | 29 | n.d |
| 10 | 525 nm green LED | 0 | n.d |
| 11 | p-MeOC6H4CHO as donor | 10 | n.d |
| 12 | p-ClC6H4CHO as donor | 34 | n.d |
| 13 | PhCOPh as donor | 26 | n.d |
| 14 | 0 °C | 44 | 42/58 |
| 15 | 40 °C | 68 | 70/30 |
| 16 | 60 °C | 0 | n.d |
| 17 | 6 h reaction time | 49 | 59/41 |
| 18 | 24 h reaction time | 71 | 66/34 |
| 19 | Without PhCHO | 0 | n.d |
| 20 | 2 equiv. PhCHO | 73 | 68/32 |
| 21 | In the dark | 0 | n.d |
| 22 | Under N2 | Traces | n.d |
Electron-donating and electron-withdrawing substituents in the benzylic ring alter the electron donor properties, affecting the formation of the EDA complex and deactivating the desired radical process (entries 11, 12). In addition, aromatic ketones were not suitable for efficiently forming the required EDA complex (entry 13).
One particularly relevant parameter in photoredox chemistry is the reaction temperature. Although this parameter has often been neglected, recent studies highlight that efficient control of the temperature is crucial to achieve reproducible results.22 In fact, an increased number of photoredox reactions have been described as requiring a defined temperature range to obtain optimal results.23 To avoid reproducibility issues and ensure optimal temperature control, we developed a simple and cost-effective set-up that can overcome the limitations of the configurations in current use for controlling the temperature in photoredox reactions. Moreover, as a thermostatic fluid from a recirculating chiller/heater unit is used, the arrangement permits temperature control over a wide range of temperatures. This possibility encouraged us to explore the effect that temperature has on the yield and selectivity of the cyclopropanation process. Surprisingly, when the cyclopropanation of α-bromonitrostyrene 1a was performed at 0 °C, the selectivity was reversed and 2-nitro-cyclopropane-1-carbaldehyde 2a′ was the major isomer isolated from the reaction mixture. However, the process was less efficient both in terms of yield and selectivity (entry 14). On the contrary, when the temperature of the process was raised to 40 °C, the diastereoisomeric ratio of the cyclopropanation products 2a/2a′ was similar to that of the reaction performed at 20 °C, whereas the yield slightly decreased (entry 15). Attempts to further increase the temperature of the process resulted in a complex reaction mixture in which the desired cyclopropanes could not be detected (entry 16). Shorter times led to lower product yields (entry 17) and no significant differences were observed, in terms of yield, when the reaction was carried out at longer reaction times (entry 18).
Control experiments further confirmed that benzaldehyde (entry 19) and light (entry 21) are essential components for this process. The reaction is not significantly affected by a higher concentration of benzaldehyde (entry 20). Furthermore, when the reaction mixture was degassed and irradiated under nitrogen atmosphere, only traces of the cyclopropanation were detected by 1H NMR (entry 22). This result underscores the essential role of oxygen in this transformation.
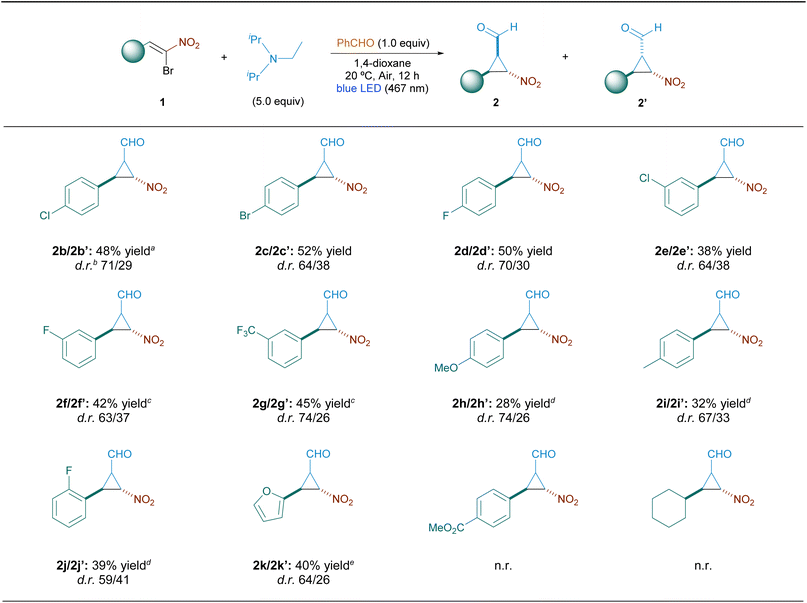
To investigate the substrate scope of this cyclopropanation strategy, α-bromonitroalkenes 1 containing various substitutions were reacted under the optimal conditions (Table 2). The substituent group on the aryl moiety of the bromonitrostyrene had a significant effect on the reactivity and efficiency of the cyclopropanation reaction. Specifically, bromonitroalkenes bearing halogenated electron-withdrawing groups on the aromatic ring (fluoro, trifluoromethyl, chloro, and bromo) were well tolerated, leading to cyclopropanation products with moderate to good yields. In contrast, the cyclopropanation of bromonitrostyrenes bearing electron-donating groups (methyl and methoxy) required longer reaction times and gave rise to the cyclization product in comparatively lower yields. The cyclopropanation process was also sensitive to steric bulk, and the position of the substituents on the phenyl moiety markedly affected the reaction efficiency. Furanyl bromonitroalkene was also amenable for this photo-transformation, affording the target cyclopropanes 2k/2k′ in moderate yields. However, these latter products are unstable and significantly degrade upon purification, even in neutral alumina. In all cases, except for 2k/k′, the remaining mass balance comprised mainly unreacted starting material, along with other minor non-identified byproducts. 4-(Methoxycarbonyl)-substituted bromonitrostyrene was inapplicable to the cyclopropanation protocol, probably due to destabilizing interactions of the carbonyl group. Aliphatic 2-bromo-2-nitrovinyl cyclohexane also failed to produce the desired cyclopropanes, pointing to the need of a conjugated π-system to obtain satisfactory results.
We considered that alternative Michael acceptors could be cyclopropanated by using the process developed for α-bromonitrostyrenes. However, α-bromo-α,β-unsaturated esters and amides and α-bromocyanostryrene were not suitable for this radical cyclopropanation reaction. Furthermore, the use of α-iodonitrostyrene as a starting material also failed to produce the desired nitrocyclopropanes 2a/2a′.
To gain further insight into the mechanism underlying this visible light-promoted cyclopropanation, a series of control experiments and spectroscopy assays were carried out. First, the addition of the radical scavenger TEMPO under the standard reaction conditions completely inhibited the formation of the desired product, confirming the intermediacy of the radical species. To investigate the possibility of a propagative mechanism occurring within this system, we conducted a ‘‘light on/light off’’ experiment, which showed that no radical chain propagation events took place (see the ESI† for details). In order to confirm the formation of a ground EDA complex between α-bromonitroalkene, DIPEA and benzaldehyde, the system was examined by UV-Vis spectroscopy. Specifically, upon mixing bromonitrostyrene and DIPEA in 1,4-dioxane, a weak but detectable bathochromic shift was observed in the UV-Vis spectrum. Subsequent addition of benzaldehyde to the mixture and further measurement of the UV-Vis spectrum indicated a significant red shift and increased absorption of visible light, corresponding to the charge-transfer absorption of a ternary EDA complex.
This observation is consistent with the expected charge redistribution among these three components to form a ternary EDA complex, which enables more productive light absorption (see the ESI† for details).
Based on the above experiments, a plausible mechanism for this new cyclopropanation reaction is proposed in Scheme 2. Initially, the molecular interactions of α-bromonitrostyrene, DIPEA and benzaldehyde lead to the formation of a ternary EDA complex. Under excitation by visible light, a single electron transfer (SET) process takes place. The electron-accepting intermediate, the α-bromonitrostyrene radical anion, loses the bromine leaving group, leading to the intermediate nitrovinyl radical A. On the other hand, the electron-donor DIPEA forms the corresponding amine cation radical I, while benzaldehyde is recovered without any changes.
The amine radical cation I in the presence of DIPEA as H atom acceptor affords α-amino radical II.24 Subsequently, a dioxygen molecule captures the electron, generating a superoxide radical, which undergoes hydrogen atom transfer (HAT) with the generated imine III to form enamine IV.25 Formation of enamines from trialkylamines in visible light photoredox processes has been widely reported, as the competitive alkylation of enamines has been one of the major problems facing successful implementation of intermolecular radical coupling reactions.26 The addition of the α-nitro vinyl radical to the enamine forms a C–C bond and generates the strongly reducing α-amino radical B, which can be rapidly oxidized with the [HOO˙] radical species to the corresponding iminium cation C.27 Subsequent abstraction of a hydrogen atom α to the iminium ion, followed by intramolecular nucleophilic Michael-addition affords cyclopropane D, which tautomerizes into enamine E. Upon hydrolysis, nitrocyclopropane 2′ is formed, which can be epimerized through the enolate intermediate E to provide 2. Due to the electrostatic repulsion between the nitro group and the carbonyl group, 2′ is less stable, and the balance favors the formation of 2.28 The observed dependence of the diastereoselectivity on the temperature is in agreement with the proposed mechanism. Thus, at lower temperatures, the reaction proceeds under kinetic control, where a faster-forming isomer 2′ is favoured. When the temperature is increased, this reaction shifts to thermodynamic control, where the stereoisomeric ratio is enriched in the more stable isomer 2.
In order to investigate the synthetic utility of the photoinduced nitrocyclopropanation reaction and based on the pharmacological importance of GABA (γ-amino butyric acid) derivatives, the chemoselective ring-opening of the 1-formyl-2-nitrocyclopropane ring was carried out under heterocyclic carbene catalysis.29 As depicted in Scheme 3, reaction of nitrocyclopropanes 2a/2a′ and methanol in the presence of both DIPEA and catalytic 3-benzyl-4,5-dimethylthiazol-3-ium chloride, afforded in good yield the corresponding methyl 3-nitro-3-phenylpropanoate 3, precursor of anxiolytic drug phenibut (3-amino-3-phenylpropanoic acid).30
Conclusions
In summary, this study describes a simple and effective metal-free, visible light-mediated method for the direct formation of nitrocyclopropanes from α-bromonitrostyrenes. This approach exploits the photochemical activity of electron donor–acceptor (EDA) complexes formed between α-bromonitrostyrenes, DIPEA and benzaldehyde, which acts as a sacrificial donor.Selective photolysis to the EDA complex leads to the generation of the nitrovinyl radicals, which are suitably trapped by an enamine radical trap in situ generated from DIPEA. The subsequent intramolecular Michael reaction, followed by hydrolysis, provides a potent and versatile synthetic method for the effective formation of 2-nitrocyclopropane-1-carbaldehydes. Mechanistic studies support the proposed formation and photolysis of an EDA complex.
The synthetic utility of the 1-formyl-2-nitrocyclopropane products was demonstrated by conversion of cyclopropane 2a to the corresponding γ-nitromethylester 3, precursor of the GABA analogue phenibut.
Experimental section
General methods
All the bromonitrostyrenes 1 were prepared following previously reported methodologies.31 The different reagents employed during the development of this work are commercially available, and were purchased from Chemosapiens S.L. Dry 1,4-dioxane stored over molecular sieves commercially available from Sigma Aldrich Chemical Co. was used for the photochemical reactions. NMR spectra were recorded in CDCl3 at 300 MHz for 1H and 75 MHz for 13C, with tetramethylsilane as an internal standard for 1H and the residual solvent signals as the standard for 13C. The data are reported as s = singlet, bs = broad singlet, d = doublet, dd = double doublet, t = triplet, dt = double triplet, q = quadruplet, p = quintuplet and m = multiplet or unresolved, with chemical shifts in ppm and coupling constant(s) in Hz. The values of the chemical shift of the signals in the NMR reports are in ppm. HRMS were measured in APCI negative mode, and the mass analyzer of the HRMS was TOF (Bruker model Impact II).Photochemical reactions setup
A Kessil® PR160 Rig equipped with different lamps (PR160-366 nm, PR160-390 nm, PR160-427 nm, PR160-440 nm and PR160-456 nm) fan was used for the photochemistry setup. A glassware reactor developed in our group was used to run the photochemical reactions under controlled temperature (see the ESI† for details). The reactor was placed at a distance of approximately 5 cm away from the lamp prior to irradiation at maximum intensity (100% power) of the Kessil lamp (see the ESI† for details).General procedure for the cyclopropanation reaction
To a solution of α-bromonitrostyrene (0.3 mmol) in 1,4-dioxane (2 mL), DIPEA (1.5 mmol) and benzaldehyde (0.3 mmol) were added. The mixture was stirred at 20 °C for 12 hours under irradiation with a blue LED light (467 nm). After completion of the reaction, the solvent was removed under vacuum and the crude products were purified by column chromatography.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H9NO3, 191.0588; found: 191.0581. 1H NMR (300 MHz, CDCl3): δ 9.71 (d, J = 5.0 Hz, 1H, CHO-2a′), 9.30 (d, J = 3.5 Hz, 1H, CHO-2a), 7.45–7.13 (m, 10H, ArH), 5.33 (dd, J = 4.9, 3.5 Hz, 1H, H2-2a), 4.83 (dd, J = 8.2, 4.7 Hz, 1H, H2-2a′), 4.03 (dd, J = 7.8, 4.7 Hz, 1H, H3-2a′), 3.88 (dd, J = 11.1, 4.8 Hz, 1H, H3-2a), 3.44 (dt, J = 11.1, 3.5 Hz, 1H, H1-2a), 2.73 (dt, J = 8.0, 5.0 Hz, 1H, H1-2a′); 13C NMR (75 MHz, CDCl3): δ 193.3, 193.1, 130.2, 129.2, 128.97, 129.03, 128.8, 128.6, 128.7, 66.6, 62.4, 39.3, 38.6, 36.3, 32.6.
1); HRMS (APCI−) [M]− calcd for C10H9NO3, 191.0588; found: 191.0581. 1H NMR (300 MHz, CDCl3): δ 9.71 (d, J = 5.0 Hz, 1H, CHO-2a′), 9.30 (d, J = 3.5 Hz, 1H, CHO-2a), 7.45–7.13 (m, 10H, ArH), 5.33 (dd, J = 4.9, 3.5 Hz, 1H, H2-2a), 4.83 (dd, J = 8.2, 4.7 Hz, 1H, H2-2a′), 4.03 (dd, J = 7.8, 4.7 Hz, 1H, H3-2a′), 3.88 (dd, J = 11.1, 4.8 Hz, 1H, H3-2a), 3.44 (dt, J = 11.1, 3.5 Hz, 1H, H1-2a), 2.73 (dt, J = 8.0, 5.0 Hz, 1H, H1-2a′); 13C NMR (75 MHz, CDCl3): δ 193.3, 193.1, 130.2, 129.2, 128.97, 129.03, 128.8, 128.6, 128.7, 66.6, 62.4, 39.3, 38.6, 36.3, 32.6.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H835ClNO3: 225.0198; found: 225.0197; calcd for C10H837ClNO3: 227.0172; found: 227.0167. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.8 Hz, 1H, CHO-2b′), 9.39 (d, J = 3.5 Hz, 1H, CHO-2b), 7.44–7.06 (m, 8H, ArH), 5.30 (dd, J = 4.9, 3.5 Hz, 1H, H2-2b), 4.81 (dd, J = 4.8, 3.7 Hz, 1H, H2-2b′), 3.99 (dd, J = 8.0, 4.8 Hz, 1H, H3-2b′), 3.83 (dd, J = 11.1, 4.8 Hz, 1H, H3-2b), 3.49 (dt, J = 11.1, 3.5 Hz, 1H, H1-2b), 2.70 (dt, J = 8.0, 4.8 Hz, 1H, H1-2b′); 13C NMR (75 MHz, CDCl3): δ 192.9, 192.7, 134.6, 130.2, 129.0, 129.4, 129.2, 128.6, 128.2, 127.1, 66.3, 62.5, 39.1, 38.4, 35.8, 31.8.
1); HRMS (APCI−) [M]− calcd for C10H835ClNO3: 225.0198; found: 225.0197; calcd for C10H837ClNO3: 227.0172; found: 227.0167. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.8 Hz, 1H, CHO-2b′), 9.39 (d, J = 3.5 Hz, 1H, CHO-2b), 7.44–7.06 (m, 8H, ArH), 5.30 (dd, J = 4.9, 3.5 Hz, 1H, H2-2b), 4.81 (dd, J = 4.8, 3.7 Hz, 1H, H2-2b′), 3.99 (dd, J = 8.0, 4.8 Hz, 1H, H3-2b′), 3.83 (dd, J = 11.1, 4.8 Hz, 1H, H3-2b), 3.49 (dt, J = 11.1, 3.5 Hz, 1H, H1-2b), 2.70 (dt, J = 8.0, 4.8 Hz, 1H, H1-2b′); 13C NMR (75 MHz, CDCl3): δ 192.9, 192.7, 134.6, 130.2, 129.0, 129.4, 129.2, 128.6, 128.2, 127.1, 66.3, 62.5, 39.1, 38.4, 35.8, 31.8.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H879BrNO3, 268.9693; found: 268.9700; calcd for C10H881BrNO3, 270.9673; found: 270.9671. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.8 Hz, 1H, CHO-2c′), 9.39 (d, J = 3.0 Hz, 1H, CHO-2c), 7.61–7.48 (m, 4H, ArH), 7.17–7.00 (m, 4H, ArH), 5.29 (dd, J = 4.9, 3.6 Hz, 1H, H2-2c), 4.80 (dd, J = 4.8, 3.7 Hz, 1H, H2-2c′), 3.98 (dd, J = 8.0, 4.8 Hz, 1H, H3-2c′), 3.80 (dd, J = 11.1, 4.8 Hz, 1H, H3-2c), 3.49 (dt, J = 11.1, 3.0 Hz, 1H, H1-2c), 2.69 (dt, J = 8.0, 4.8 Hz, 1H, H1-2c′); 13C NMR (75 MHz, CDCl3): δ 192.9, 192.7, 132.4, 132.2, 131.8, 130.5, 129.2, 128.5, 127.5, 122.7, 66.2, 62.4, 39.0, 38.3, 35.8, 31.9.
1); HRMS (APCI−) [M]− calcd for C10H879BrNO3, 268.9693; found: 268.9700; calcd for C10H881BrNO3, 270.9673; found: 270.9671. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.8 Hz, 1H, CHO-2c′), 9.39 (d, J = 3.0 Hz, 1H, CHO-2c), 7.61–7.48 (m, 4H, ArH), 7.17–7.00 (m, 4H, ArH), 5.29 (dd, J = 4.9, 3.6 Hz, 1H, H2-2c), 4.80 (dd, J = 4.8, 3.7 Hz, 1H, H2-2c′), 3.98 (dd, J = 8.0, 4.8 Hz, 1H, H3-2c′), 3.80 (dd, J = 11.1, 4.8 Hz, 1H, H3-2c), 3.49 (dt, J = 11.1, 3.0 Hz, 1H, H1-2c), 2.69 (dt, J = 8.0, 4.8 Hz, 1H, H1-2c′); 13C NMR (75 MHz, CDCl3): δ 192.9, 192.7, 132.4, 132.2, 131.8, 130.5, 129.2, 128.5, 127.5, 122.7, 66.2, 62.4, 39.0, 38.3, 35.8, 31.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0504. 1H NMR (300 MHz, CDCl3): δ 9.70 (d, J = 4.8 Hz, 1H, CHO-2d′), 9.38 (d, J = 3.0 Hz, 1H, CHO-2d), 7.30–7.01 (m, 8H, ArH), 5.34–5.25 (m, 1H, H2-2d), 4.79 (dd, J = 8.0, 4.8 Hz, 1H, H2-2d′), 3.98 (dd, J = 8.0, 4.8 Hz, 1H, H3-2d′), 3.83 (dd, J = 11.1, 4.8 Hz, 1H, H3-2d), 3.48 (dt, J = 11.1, 3.1 Hz, 1H, H1-2d), 2.69 (dt, J = 8.0, 4.8 Hz, 1H, H1-2d′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2d): δ 189.8, 162.7 (d, J = 248.4 Hz), 130.7 (d, J = 8.3 Hz), 126.1, 116.2 (d, J = 21.9 Hz), 62.0, 38.6, 35.8.
1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0504. 1H NMR (300 MHz, CDCl3): δ 9.70 (d, J = 4.8 Hz, 1H, CHO-2d′), 9.38 (d, J = 3.0 Hz, 1H, CHO-2d), 7.30–7.01 (m, 8H, ArH), 5.34–5.25 (m, 1H, H2-2d), 4.79 (dd, J = 8.0, 4.8 Hz, 1H, H2-2d′), 3.98 (dd, J = 8.0, 4.8 Hz, 1H, H3-2d′), 3.83 (dd, J = 11.1, 4.8 Hz, 1H, H3-2d), 3.48 (dt, J = 11.1, 3.1 Hz, 1H, H1-2d), 2.69 (dt, J = 8.0, 4.8 Hz, 1H, H1-2d′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2d): δ 189.8, 162.7 (d, J = 248.4 Hz), 130.7 (d, J = 8.3 Hz), 126.1, 116.2 (d, J = 21.9 Hz), 62.0, 38.6, 35.8.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H835ClNO3: 225.0198; found: 225.0198. calcd for C10H837ClNO3: 227.0172; found: 227.0170. 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2e′), 9.37 (d, J = 2.8 Hz, 1H, CHO-2e), 7.22–7.01 (m, 8H, ArH), 5.29 (dd, J = 4.9, 3.6 Hz, 1H, H2-2e), 4.80 (dd, J = 8.0, 4.6 Hz, 1H, H2-2e′), 3.97 (dd, J = 8.0, 4.6 Hz, 1H, H3-2e′), 3.85–3.79 (m, 1H, H3-2e), 3.50–3.47 (m, 1H, H1-2e), 2.70 (td, J = 8.0, 4.8 Hz, 1H, H1-2e′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2e): δ 192.5, 134.3, 132.2, 130.2, 129.1, 128.2, 127.0, 62.5, 38.2, 35.6.
1); HRMS (APCI−) [M]− calcd for C10H835ClNO3: 225.0198; found: 225.0198. calcd for C10H837ClNO3: 227.0172; found: 227.0170. 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2e′), 9.37 (d, J = 2.8 Hz, 1H, CHO-2e), 7.22–7.01 (m, 8H, ArH), 5.29 (dd, J = 4.9, 3.6 Hz, 1H, H2-2e), 4.80 (dd, J = 8.0, 4.6 Hz, 1H, H2-2e′), 3.97 (dd, J = 8.0, 4.6 Hz, 1H, H3-2e′), 3.85–3.79 (m, 1H, H3-2e), 3.50–3.47 (m, 1H, H1-2e), 2.70 (td, J = 8.0, 4.8 Hz, 1H, H1-2e′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2e): δ 192.5, 134.3, 132.2, 130.2, 129.1, 128.2, 127.0, 62.5, 38.2, 35.6.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0484. 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2f′), 9.36 (d, J = 2.9 Hz, 1H, CHO-2f), 7.42–7.29 (m, 6H, ArH), 7.06–6.95 (m, 2H, ArH), 5.28 (dd, J = 4.9, 3.5 Hz, 1H, H2-2f), 4.79 (dd, J = 8.3, 4.7 Hz, 1H, H2-2f′), 3.98 (dd, J = 7.7, 4.7 Hz, 1H, H3-2f′), 3.82 (dd, J = 11.1, 4.9 Hz, 1H, H3-2f), 3.46 (dt, J = 11.1, 3.5 Hz, 1H, H1-2f), 2.69 (dt, J = 8.0, 4.7 Hz, 1H, H1-2f′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2f): δ 192.6, 162.8 (d, J = 247.2 Hz), 132.5 (d, J = 8.3 Hz), 130.6 (d, J = 8.5 Hz), 124.5, 116.1 (d, J = 22.5 Hz), 115.8 (d, J = 20.9 Hz), 62.3, 38.3, 29.7.
1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0484. 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2f′), 9.36 (d, J = 2.9 Hz, 1H, CHO-2f), 7.42–7.29 (m, 6H, ArH), 7.06–6.95 (m, 2H, ArH), 5.28 (dd, J = 4.9, 3.5 Hz, 1H, H2-2f), 4.79 (dd, J = 8.3, 4.7 Hz, 1H, H2-2f′), 3.98 (dd, J = 7.7, 4.7 Hz, 1H, H3-2f′), 3.82 (dd, J = 11.1, 4.9 Hz, 1H, H3-2f), 3.46 (dt, J = 11.1, 3.5 Hz, 1H, H1-2f), 2.69 (dt, J = 8.0, 4.7 Hz, 1H, H1-2f′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2f): δ 192.6, 162.8 (d, J = 247.2 Hz), 132.5 (d, J = 8.3 Hz), 130.6 (d, J = 8.5 Hz), 124.5, 116.1 (d, J = 22.5 Hz), 115.8 (d, J = 20.9 Hz), 62.3, 38.3, 29.7.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C11H8F3NO3, 259.0458; found: 259.0469. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.7 Hz, 1H, CHO-2g′), 9.44 (d, J = 2.5 Hz, 1H, CHO-2g), 7.65–7.36 (m, 8H, ArH), 5.33 (dd, J = 4.8, 3.8 Hz, 1H, H2-2g), 4.84 (dd, J = 8.3, 4.8 Hz, 1H, H2-2g′), 4.05 (dd, J = 7.8, 4.7 Hz, 1H, H3-2g′), 3.88 (dd, J = 11.1, 5.0 Hz, 1H, H3-2g), 3.54 (dt, J = 11.1, 5.0 Hz, 1H, H1-2g), 2.74 (dt, J = 8.0, 4.8 Hz, 1H, H1-2g′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2g): δ 192.4, 134.3, 132.1, 131.5 (d, J = 32.6 Hz), 129.5, 129.2 (q, J = 232.6 Hz), 128.8 (d, J = 3.7 Hz), 125.4 (d, J = 3.7 Hz), 62.3, 38.2, 35.9.
1); HRMS (APCI−) [M]− calcd for C11H8F3NO3, 259.0458; found: 259.0469. 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 4.7 Hz, 1H, CHO-2g′), 9.44 (d, J = 2.5 Hz, 1H, CHO-2g), 7.65–7.36 (m, 8H, ArH), 5.33 (dd, J = 4.8, 3.8 Hz, 1H, H2-2g), 4.84 (dd, J = 8.3, 4.8 Hz, 1H, H2-2g′), 4.05 (dd, J = 7.8, 4.7 Hz, 1H, H3-2g′), 3.88 (dd, J = 11.1, 5.0 Hz, 1H, H3-2g), 3.54 (dt, J = 11.1, 5.0 Hz, 1H, H1-2g), 2.74 (dt, J = 8.0, 4.8 Hz, 1H, H1-2g′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2g): δ 192.4, 134.3, 132.1, 131.5 (d, J = 32.6 Hz), 129.5, 129.2 (q, J = 232.6 Hz), 128.8 (d, J = 3.7 Hz), 125.4 (d, J = 3.7 Hz), 62.3, 38.2, 35.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C11H11NO4, 221.0694; found: 221.0697. 1H NMR (300 MHz, CDCl3): δ 9.70 (d, J = 5.0 Hz, 1H, CHO-2h′), 9.31 (d, J = 3.5 Hz, 1H, CHO-2h), 7.24–7.11 (m, 4H, ArH), 6.93–6.81 (m, 2H, ArH), 5.29 (dd, J = 4.8, 3.5 Hz, 1H, H2-2h), 4.77 (dd, J = 8.1, 4.8 Hz, 1H, H2-2h′), 3.98 (dd, J = 8.0, 4.6 Hz, 1H, H3-2h′), 3.89 (q, J = 11.1, 4.8 Hz, 1H, H3-2h), 3.83 (s, 3H, CH3), 3.81 (s, 3H, CH3), 3.41 (dt, J = 11.0, 3.5 Hz, 1H, H1-2h), 2.67 (td, J = 8.0, 5.0 Hz, 1H, H1-2h′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2h): δ 192.9, 157.6, 136.9, 130.4, 114.4, 114.2, 62.7, 55.3, 38.3, 34.9.
1); HRMS (APCI−) [M]− calcd for C11H11NO4, 221.0694; found: 221.0697. 1H NMR (300 MHz, CDCl3): δ 9.70 (d, J = 5.0 Hz, 1H, CHO-2h′), 9.31 (d, J = 3.5 Hz, 1H, CHO-2h), 7.24–7.11 (m, 4H, ArH), 6.93–6.81 (m, 2H, ArH), 5.29 (dd, J = 4.8, 3.5 Hz, 1H, H2-2h), 4.77 (dd, J = 8.1, 4.8 Hz, 1H, H2-2h′), 3.98 (dd, J = 8.0, 4.6 Hz, 1H, H3-2h′), 3.89 (q, J = 11.1, 4.8 Hz, 1H, H3-2h), 3.83 (s, 3H, CH3), 3.81 (s, 3H, CH3), 3.41 (dt, J = 11.0, 3.5 Hz, 1H, H1-2h), 2.67 (td, J = 8.0, 5.0 Hz, 1H, H1-2h′); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2h): δ 192.9, 157.6, 136.9, 130.4, 114.4, 114.2, 62.7, 55.3, 38.3, 34.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C11H11NO3, 205.0744; found: 205.0740. 1H NMR (300 MHz, CDCl3): δ 9.83 (d, J = 5.0 Hz, 1H, CHO-2i′), 9.42 (d, J = 3.6 Hz, 1H, CHO-2i), 7.26–7.15 (m, 8H, ArH), 5.28 (dd, J = 4.8, 3.6 Hz, 1H, H2-2i), 4.92 (dd, J = 8.0, 4.5 Hz, 1H, H2-2i′), 3.77–3.70 (m, 1H, H3-2i′), 3.81 (dd, J = 11.1, 4.8 Hz, 1H, H3-2i), 3.39 (dt, J = 11.1, 3.6 Hz, 1H, H1-2i), 2.85–2.78 (m, 1H, H1-2i′), 2.50 (s, 3H, CH3), 2.48 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2i): δ 190.0, 138.3, 129.69, 129.67, 128.7, 62.6, 36.1, 29.7, 21.1.
1); HRMS (APCI−) [M]− calcd for C11H11NO3, 205.0744; found: 205.0740. 1H NMR (300 MHz, CDCl3): δ 9.83 (d, J = 5.0 Hz, 1H, CHO-2i′), 9.42 (d, J = 3.6 Hz, 1H, CHO-2i), 7.26–7.15 (m, 8H, ArH), 5.28 (dd, J = 4.8, 3.6 Hz, 1H, H2-2i), 4.92 (dd, J = 8.0, 4.5 Hz, 1H, H2-2i′), 3.77–3.70 (m, 1H, H3-2i′), 3.81 (dd, J = 11.1, 4.8 Hz, 1H, H3-2i), 3.39 (dt, J = 11.1, 3.6 Hz, 1H, H1-2i), 2.85–2.78 (m, 1H, H1-2i′), 2.50 (s, 3H, CH3), 2.48 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2i): δ 190.0, 138.3, 129.69, 129.67, 128.7, 62.6, 36.1, 29.7, 21.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0486. 1H NMR (300 MHz, CDCl3) (data for the major isomer-2j): δ 9.49 (d, J = 2.5 Hz, 1H, CHO), 7.42–7.02 (m, 4H, ArH), 5.25 (dd, J = 4.9, 3.6 Hz, 1H2), 3.77 (dd, J = 11.0, 4.9 Hz, 1H, H3), 3.56 (dd, J = 11.0, 4.9, 2.5 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2j): δ 192.6, 163.7 (d, J = 276.4 Hz), 130.6 (d, J = 8.3 Hz), 130.4 (d, J = 2.5 Hz), 124.5 (d, J = 2.9 Hz), 115.9 (d, J = 21.2 Hz), 62.1, 37.2, 30.5 (d, J = 3.6 Hz).
1); HRMS (APCI−) [M]− calcd for C10H8FNO3, 209.0494; found: 209.0486. 1H NMR (300 MHz, CDCl3) (data for the major isomer-2j): δ 9.49 (d, J = 2.5 Hz, 1H, CHO), 7.42–7.02 (m, 4H, ArH), 5.25 (dd, J = 4.9, 3.6 Hz, 1H2), 3.77 (dd, J = 11.0, 4.9 Hz, 1H, H3), 3.56 (dd, J = 11.0, 4.9, 2.5 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3) (data for the major isomer-2j): δ 192.6, 163.7 (d, J = 276.4 Hz), 130.6 (d, J = 8.3 Hz), 130.4 (d, J = 2.5 Hz), 124.5 (d, J = 2.9 Hz), 115.9 (d, J = 21.2 Hz), 62.1, 37.2, 30.5 (d, J = 3.6 Hz).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2k′), 9.43 (d, J = 4.0 Hz, 1H, CHO-2k), 7.36 (bs, 2H), 6.38–6.36 (m, 4H), 5.29 (dd, J = 4.3, 4.0 Hz, 1H, H2-2k), 4.92 (dd, J = 8.3, 4.5 Hz, 1H, H2-2k′), 3.99 (dd, J = 7.6, 4.5 Hz, 1H, H3-2k′), 3.70 (dd, J = 10.8, 4.7 Hz, 1H, H3-2k), 3.35 (dt, J = 10.8, 4.0 Hz, 1H, H1-2k), 2.82 (td, J = 7.9, 4.8 Hz, 1H, H1-2k′).
1); 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 4.8 Hz, 1H, CHO-2k′), 9.43 (d, J = 4.0 Hz, 1H, CHO-2k), 7.36 (bs, 2H), 6.38–6.36 (m, 4H), 5.29 (dd, J = 4.3, 4.0 Hz, 1H, H2-2k), 4.92 (dd, J = 8.3, 4.5 Hz, 1H, H2-2k′), 3.99 (dd, J = 7.6, 4.5 Hz, 1H, H3-2k′), 3.70 (dd, J = 10.8, 4.7 Hz, 1H, H3-2k), 3.35 (dt, J = 10.8, 4.0 Hz, 1H, H1-2k), 2.82 (td, J = 7.9, 4.8 Hz, 1H, H1-2k′).Ring opening of nitrocyclopropane 2a
To a solution of nitrocyclopropane 2a (0.13 mmol) in dichloromethane (0.5 mL) was added 3-benzyl-4,5-dimethylthiazol-3-ium (6 mg, 20 mol%). To this solution, DIPEA (20 μL, 40 mol%) and MeOH (20 μL, 0.26 mmol, 3 equiv.) were added. The mixture was stirred at r. t. for 15 hours, and then treated with sat. aq. NH4Cl (1 mL) and extracted with EtOAc (2 × 5 mL). The combined organic extracts were washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography (Hex/EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford nitroester 3 (24 mg, 86%).
1) to afford nitroester 3 (24 mg, 86%).
Methyl 4-nitro-3-phenylbutanoate 3 (ref. 32)
1H NMR (300 MHz, CDCl3): δ 7.38–7.18 (m, 5H, C7–C9), 4.74 (dd, J = 12.6, 7.0 Hz, 1H, C5), 4.64 (dd, J = 12.6, 7.9 Hz, 1H, C5), 3.99 (p, J = 7.4 Hz, 1H, C4), 3.63 (s, 3H, C1), 2.78 (d, J = 7.4 Hz, 2H, C3); 13C NMR (75 MHz, CDCl3) δ 171.2, 138.4, 129.2, 128.2, 127.4, 79.5, 52.0, 40.3, 37.6.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization, R. G. S. and H. R. S.; investigation, P. P. R. and P. I. C. G; writing—original draft preparation, R. G. S.; writing—review and editing, H. R. S.; supervision, R. G. S. and H. R. S.; funding acquisition, R. G. S. and H. R. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received financial support from MCI (PID2022-137893OB-I00), Principado de Asturias (SEK-25-GRU-GIC-24-054) and INGENIUM Alliance of European Universities (ERASMUS-EDU-2022-EUR-UNIV-2, Project number 101090042). P.P.R. thanks “Programa Investigo” for the predoctoral contract (AYUD/2022/9313, EU Next Generation), and P.I.C.G. thanks FCT for providing the PhD grant (2022.11294. BD).References
- (a) D. Y.-K. Chen, R. H. Pouwer and J.-A. Richard, Chem. Soc. Rev., 2012, 41, 4631 RSC; (b) J. Salaün, Top. Curr. Chem., 2000, 207, 1 CrossRef; (c) R. Munir, A. F. Zahoor, S. Javed, B. Parveen, A. Mansha, A. Irfan, S. G. Khan, A. Irfan, K. Kotwica-Mojzych and M. Mojzych, Molecules, 2023, 28, 5651 CrossRef CAS.
- (a) Z. Časar, Synthesis, 2020, 1315 CrossRef; (b) T. T. Talele, J. Med. Chem., 2016, 59, 8712 CrossRef CAS PubMed.
- (a) C. Ebne and E. M. Carreira, Chem. Rev., 2017, 117, 11651 CrossRef PubMed; (b) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS; (c) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051 RSC; (d) C. Apel and M. Christmann, Tetrahedron, 2021, 82, 131760 CrossRef CAS; (e) A. L. Gabbey, K. Scotchburn and S. A. L. Rousseaux, Nat. Rev. Chem, 2023, 7, 548 CrossRef CAS PubMed.
- (a) W. Wu, Z. Lin and H. Jiang, Org. Biomol. Chem., 2018, 16, 7315 RSC; (b) M. Mato, A. Franchino, C. García-Morales and A. Echavarren, Chem. Rev., 2021, 121, 8613 CrossRef CAS; (c) D. Nam, V. Steck, R. J. Potenzino and R. Fasan, J. Am. Chem. Soc., 2021, 143, 2221 CrossRef CAS; (d) J. D. Johnson, C. R. Teeples, N. R. Akkawi and S. M. Wilkerson-Hill, J. Am. Chem. Soc., 2022, 144, 14471 CrossRef CAS; (e) J. L. Crompton, J. R. Frost, S. M. Rowe, K. E. Christensen and T. J. Donohoe, Org. Lett., 2023, 25, 5253 CrossRef CAS PubMed; (f) E. Sansinenea and A. Ortíz, Eur. J. Org Chem., 2022, e202200210 CrossRef CAS; (g) M. J. Kim, D. J. Wang, K. Targos, U. A. García, A. F. Harris, I. A. Guzei and Z. K. Wickens, Angew. Chem., Int. Ed., 2023, 62, e202303032 CrossRef CAS; (h) P. W. Seavill, Nat. Synth., 2024, 3, 280 CrossRef CAS; (i) A. Menzek, S. Sirtbasi, E. Sahin, C. Bayrak, M. E. Olgun, A. Yavari, S. Bayram and M. Rezaei, J. Mol. Struct., 2025, 1324, 140807 CrossRef CAS.
- (a) H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1958, 80, 5323 CrossRef CAS; (b) H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1959, 81, 4256 CrossRef CAS; (c) J. M. Concellón, H. Rodríguez-Solla, C. Concellón and V. del Amo, Chem. Soc. Rev., 2010, 39, 4103 RSC; (d) H. Rodríguez-Solla, C. Concellón, V. del Amo, A. Díaz-Pardo, E. G. Blanco, S. García-Granda, M. R. Díaz, R. Llavona and R. G. Soengas, Eur. J. Org Chem., 2013, 4953 CrossRef.
- (a) S. E. Denmark, R. A. Stavenger, A. M. Faucher and J. P. Edwards, J. Org. Chem., 1997, 62, 3375 CrossRef CAS; (b) M. Honma, H. Takeda, M. Takano and M. Nakada, Synlett, 2009, 1695 CAS; (c) B. Morandi and E. M. Carreira, Science, 2012, 335, 1471 CrossRef CAS PubMed; (d) E. M. D. Allouche and A. B. Charette, Synthesis, 2019, 3947 CAS; (e) L. G. Menchikov, E. V. Shulishov and Y. V. Tomilov, Russ. Chem. Rev., 2021, 90, 199 CrossRef CAS.
- R. Moorthy, W. Bio-Sawe, S. S. Thorat and M. P. Sibi, Org. Chem. Front., 2024, 11, 4560 RSC.
- (a) E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 1962, 84, 3782 CrossRef CAS; (b) E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 1965, 87, 1353 CrossRef CAS; (c) Y. G. Gololobov, A. N. Nesmeyanov, V. P. Lysenko and I. E. Boldeskul, Tetrahedron, 1987, 43, 4057 CrossRef; (d) A. Mamai and J. S. Madalengoitia, Tetrahedron Lett., 2000, 41, 9009 CrossRef CAS; (e) G. L. Beutner and D. T. George, Org. Process Res. Dev., 2023, 27, 10 CrossRef CAS.
- P. I. C. Godinho, R. G. Soengas and A. M. S. Silva, Synthesis, 2025 DOI:10.1055/a-2557-7569.
- For recent reviews see: (a) Z. Chen, Y. Xie and J. Xuan, Eur. J. Org Chem., 2022, e202201066 CrossRef CAS; (b) X. Han, N. Zhang, Q. Li, Y. Zhang and D. Das, Chem. Sci., 2024, 15, 13576 RSC.
- (a) Z. Wang, A. G. Herraiz, A. M. del Hoyo and M. G. Suero, Nature, 2018, 554, 86 CrossRef CAS; (b) Y. Zhang, G. Zhou, X. Gong, Z. Guo, X. Qi and X. Shen, Angew. Chem., Int. Ed., 2022, 61, e202202175 CrossRef CAS; (c) D. Xia, R. Wu, J. Wang, X. Han, Y. Li, Q. Li, X. Luan, X. Hong, Y. Zhang and W. D. Zhang, ACS Catal., 2023, 13, 9806 CrossRef CAS.
- (a) C. Shu, R. S. Mega, B. J. Andreassen, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2018, 57, 15430 CrossRef CAS PubMed; (b) J. P. Phelan, S. B. Lang, J. S. Compton, C. B. Kelly, R. Dykstra, O. Gutierrez and G. A. Molander, J. Am. Chem. Soc., 2018, 140, 8037 CrossRef CAS; (c) A. G. Herraiz and M. G. Suero, Synthesis, 2019, 2821 CAS; (d) T. Chidley, I. Jameel, S. Rizwan, P. A. Peixoto, L. Pouységu, S. Quideau and G. K. Murphy, Angew. Chem., Int. Ed., 2019, 58, 16959 CrossRef CAS; (e) D. P. Poudel, A. Pokhrel, R. K. Tak, M. Shankarv and R. Giri, Science, 2023, 381, 545 CrossRef CAS PubMed.
- (a) A. M. del Hoyo and M. García-Suero, Eur. J. Org Chem., 2017, 2122 CrossRef CAS; (b) T. Guo, L. Zhang, X. Liu, Y. Fang, X. Jin, Y. Yang, Y. Li, B. Chen and M. Ouyang, Adv. Synth. Catal., 2018, 360, 4459 CrossRef CAS.
- (a) E. B. Averina, N. V. Yashin, T. S. Kuznetsova and N. S. Zefirov, Russ. Chem. Rev., 2009, 78, 887 CrossRef CAS; (b) R. Ballini and A. Palmieri, Nitro Cyclopropanes: Synthesis and Applications in Nitroalkanes: Synthesis, Reactivity, and Applications, Wiley-VHC, 2021, pp. 239–262 Search PubMed; (c) Z.-Y. Xu, J.-S. Wei, L. Liu, Q.-B. Hu, J.-Y. Zhu, Z.-Y. Zhou, A.-B. Xia and D.-Q. Xu, J. Org. Chem., 2024, 89, 13868 CrossRef CAS; (d) S. R. Jeny, S. Selvi, M. Deerkadharshini and K. Srinivasan, RSC Adv., 2024, 14, 33587 RSC.
- (a) Y. Xuan, S. Nie, L. Dong, J. Zhang and M. Yan, Org. Lett., 2009, 11, 1583 CrossRef CAS; (b) H. J. Lee, S. M. Kim and D. Y. Kim, Tetrahedron Lett., 2012, 53, 3437 CrossRef CAS; (c) X.-D. Zhang, J. Song, N. Gao, Z. Guan and Y.-H. He, J. Mol. Cat. B., 2016, 134, 1 CrossRef CAS; (d) K. Iwai, R. Kamidate, K. Wada, H. Asahara and N. Nishiwaki, Beilstein J. Org. Chem., 2023, 19, 892 CrossRef CAS; (e) D. Kim, H.-J. Jeon, Y. Kwak, S. J. Lee, T.-G. Nam, J. H. Yu, H. Anand and K. B. Hong, RSC Adv., 2024, 14, 831 RSC; (f) P. Y. Ushakov, I. S. Golovanov, S. L. Ioffea and A. A. Tabolin, Org. Chem. Front., 2024, 11, 315 RSC; (g) B. Wang, J. Liu, H. Jin and L. Zhang, Adv. Synth. Catal., 2024, 366, 2742 CrossRef CAS.
- F. Doraghi, B. Larijani and M. Mahdavi, RSC Adv., 2024, 14, 14835 RSC.
- For reviews see: (a) G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142, 5461 CrossRef CAS PubMed; (b) A. K. Wortman and C. R. J. Stephenson, Chem, 2023, 9, 2390 CrossRef CAS; (c) A. A. Volkov, D. I. Bugaenko and A. V. Karchava, ChemCatChem, 2024, 16, e202301526 CrossRef CAS.
- R. Foster, J. Phys. Chem., 1980, 84, 2135 CrossRef CAS.
- (a) T. M. Bockman, S. M. Hubig and J. K. Kochi, J. Org. Chem., 1997, 62, 2210 CrossRef CAS PubMed; (b) S. V. Rosokha and J. K. Kochi, Acc. Chem. Res., 2008, 41, 641 CrossRef CAS.
- (a) Z. Yang, Y. Liu, K. Cao, X. Zhang, H. Jiang and J. Li, Beilstein J. Org. Chem., 2021, 17, 771 CrossRef CAS; (b) M. Balletti, T. Wachsmuth, A. Di Sabato, W. C. Hartley and P. Melchiorre, Chem. Sci., 2023, 14, 4923 RSC; (c) H. F. Piedra and M. Plaza, Chem. Sci., 2024, 15, 19077 RSC; (d) Y.-Z. Liu, Y. Chen, A. Wang, Z. Shen, X. Zhou, J. Zhang, Y. Jian and X. Ma, Green Chem., 2024, 26, 2280 RSC.
- (a) Z. Q. Zhu, W. Y. Zhang, X. L. Huang, Q. Li, Z. Z. Xu and H. Y. Rao, J. Org. Chem., 2025, 90, 2842 CrossRef CAS; (b) J. Gu, Y. Meng, Z. Cheng, G. Lu, M. Xin, J. Tang, Z. Du, X. Zhang, M. Deng and Y. Zou, Cell Rep. Phys. Sci., 2024, 5, 102230 CrossRef CAS.
- B. Pijper, L. M. Saavedra, M. Lanzi, M. Alonso, A. Fontana, M. Serrano, J. E. Gómez, A. W. Kleij, J. Alcázar and S. Cañellas, JACS Au, 2024, 4, 2585 CrossRef CAS.
- T. D. Svejstrup, A. Chatterjee, D. Schekin, T. Wagner, J. Zach, M. J. Johansson, G. Bergonzini and B. König, ChemPhotoChem, 2021, 5, 808 CrossRef CAS.
- (a) H. J. Wang, T. H. Nguyen and N. Zheng, Beilstein J. Org. Chem., 2013, 9, 1977 CrossRef; (b) J. P. Dinnocenzo and T. E. Banach, J. Am. Chem. Soc., 1989, 111, 8646 CrossRef CAS.
- M. Chen, Z.-T. Huang and Q. Zheng, Org. Biomol. Chem., 2014, 12, 9337 RSC.
- (a) L. Furst, B. S. Matsuura, J. M. R. Narayanam, J. W. Tucker and C. R. J. Stephenson, Org. Lett., 2010, 12, 3104 CrossRef CAS; (b) J. W. Beatty and C. R. J. Stephenson, Acc. Chem. Res., 2015, 48, 1474 CrossRef CAS.
- Q. Liu, Y.-N. Li, H.-H. Zhang, B. Chen, C.-H. Tung and L.-Z. Wu, Chem.–Eur. J., 2012, 18, 620 CrossRef CAS.
- J. Zhang, Z. Hu, S. Zhao and M. Yan, Tetrahedron, 2009, 65, 802 CrossRef CAS.
- S. S. Sohn and J. W. Bode, Angew. Chem., Int. Ed., 2006, 45, 6021 CrossRef CAS.
- I. Lapin, CNS Drug Rev., 2001, 7, 471 CrossRef CAS PubMed.
- Z. D. Susam, B. D. Özcan, E. Kurtkaya, E. Yilcrim and C. Tanyeli, Org. Biomol. Chem., 2022, 20, 8725 RSC.
- S. Patra and D. Katayev, Chem.–Eur. J., 2024, 30, e202403654 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02540k |
| This journal is © The Royal Society of Chemistry 2025 |